Abstract
Purpose
Metal-on-metal total disc replacement is a recent alternative treatment for degenerative disc disease. Wear and corrosion of these implants can lead to local and systemic transport of metal debris. This prospective longitudinal study examined the serum chromium and cobalt levels in 24 patients with cobalt–chromium alloy metal-on-metal lumbar disc replacements.
Methods
Serum was assayed for chromium (Cr) and cobalt (Co) using high-resolution inductively-coupled plasma-mass spectrometry. Detection limits were 0.015 ng/mL for Cr and 0.04 ng/mL for Co.
Results
Median serum Co levels at pre-op, 3, 6, 12, 24, and 36-months post-op were 0.10, 1.03, 0.96, 0.98, 0.67, and 0.52 ng/mL, respectively. Median serum Cr levels were 0.06, 0.49, 0.65, 0.43, 0.52, and 0.50 ng/mL, respectively.
Conclusion
In general, these results indicated that serum Co and Cr levels are elevated at all postoperative time points and are of the same order of magnitude as those observed in well-functioning metal-on-metal surface replacements of the hip and in metal-on-metal total hip replacements at similar postoperative time points.
Keywords: Lumbar degenerative disc disease, Disc arthroplasty, Metal ions, Chromium, Cobalt, Metal-on-metal
Introduction
Lumbar disc arthroplasty (LDA) is one of several surgical techniques used to relieve the painful symptoms of degenerative lumbar disc disease when extended conservative measures have failed. For appropriately selected patients, LDA is a motion-preserving alternative to arthrodesis designed to stabilize the diseased intervertebral segment by restoring and maintaining disc height, sagittal alignment and physiologic motion, while potentially preventing degenerative disease onset at adjacent levels [1–5].
Implants for lumbar disc arthroplasty are commonly designed with two or more articulating surfaces (the bearing surfaces). Like hip and knee replacements, implants for LDA with components that move relative to one another will generate wear to some extent. The strategy for managing the extent of wear particles generated (and the subsequent potential solubility of wear particles) is by the proper selection of material combinations for articulating components. Based on decades of design and clinical experience from large joint arthroplasty, common materials used for spinal bearing applications include metal-on-polyethylene and metal-on-metal combinations. In large joints, ultra high molecular weight polyethylene (UHMWPE) has been an excellent option for the high service conditions of the hip and knee. Historically, however, some embodiments of UHMWPE have been associated with peri-implant osteolysis secondary to wear particle-induced inflammatory reactions [6]. Osteolysis has also recently been reported in UHMWPE total disc arthroplasty [7]. Because osteolysis is believed to be partially dependent on wear particle dose, the use of lower-wearing metal-on-metal bearing articulations has been pursued. In large joint arthroplasty, multiple cobalt–chromium (CoCr) alloy metal-on-metal total hip and surface replacement arthroplasty systems have resulted in good clinical outcomes demonstrating high survivorships [8, 9], low wear on retrieval studies [10] and relatively low rates of osteolysis. Other clinical reports, however, have not been as favorable, reporting adverse local tissue responses [11, 12]. In addition, elevated levels of cobalt and chromium in the serum, erythrocytes, and urine have been reported [13–16] and the long-term clinical implication of the systemic distribution of implant-derived particles and soluble metal ions remains unclear.
Techniques for metal ion analysis are well-established in the total hip literature [17]. Studies of metal ion levels in patients with spinal implants are less numerous. There have been only a few retrospective reports of metal ion levels in patients with stainless steel [18–22] and titanium [23, 24] posterior spinal instrumentation. Less is known for metal-on-metal total disc arthroplasty [25–27].
Zeh et al. [25] reported no statistically significant difference between LDA patients receiving Maverick implants at one versus two levels. Kim et al. [18] measured serum levels of nickel and chromium after posterior spinal arthrodesis using stainless steel implants and found that levels diminish rapidly with time but remained above normal levels after surgery suggesting that ion levels decrease as fusion occurred. A study by del Rio et al. [19] described similarly elevated serum nickel and chromium levels in patients with instrumented spinal arthrodesis, noting the correlation between significantly higher metal ion levels and radiological signs of device corrosion. Rackham et al. [21] determined that the number of metal connections/interfaces was positively correlated with serum chromium levels in a group of 30 patients undergoing posterior spinal arthrodesis.
The purpose of the current prospective longitudinal study was to measure the concentrations of cobalt (Co) and chromium (Cr) in the serum of patients who underwent a single level lumbar total disc replacement with a CoCr metal-on-metal implant. In this study, the following hypotheses were investigated: (1) Co and Cr ion levels will be higher at all postoperative time periods when compared to preoperative levels and (2) Co and Cr ion levels will be lower at long-term (24 and 36 months) when compared to short-term (3 and 6 months) follow-up.
Materials and methods
A prospective longitudinal study was performed consisting of a group of 24 patients implanted with a single-level MAVERICKTM Lumbar Disc (Medtronic, Memphis, TN). This system [5] consists of a metal-on-metal ball-in-socket articulation with both components manufactured from a high carbon-wrought cobalt–chromium alloy matching the specifications of American Society for Testing and Materials (ASTM) F1537 [28]. All implant sizes contain the same articulation. This prospective, non-randomized longitudinal study was conducted under a Food and Drug Administration (FDA)-approved investigational device exemption (IDE) continued access arm of the MAVERICK® total disc replacement trial. The study was approved by the institutional review board of each participating site and all the participants signed informed consents prior to their inclusion in the study.
Patient selection
Twenty-four patients were enrolled at two investigational sites within the United States and underwent surgery between October 2005 and July 2006. The patient group consisted of 13 males and 11 females with an average age at implantation of 41.0 years (range 22–63 years). Eight patients were treated at L4/L5 and 16 patients at L5/S1. Patient demographic data are shown in Table 1.
Table 1.
Demographic data
| Variable | Lumbar disc group |
|---|---|
| Number of patients | 24 |
| Age (years) | 41.0 (range 22–63) |
| Height (in) | 68.6 (range 61–76) |
| Weight (lbs) | 182.2 (range 133–235) |
| Male (%) | 54.2 |
| Workers’ compensation (%) | 62.5 |
| Litigation (%) | 66.7 |
| Alcohol use (%) | 37.5 |
| Tobacco use (%) | 41.7 |
All patients participating in this study were required to be eligible and to sign consent for participation in the continued access arm of the MAVERICK Total Disc Replacement trial, meeting all of the protocol-specified inclusion and exclusion criteria [29]. In the continued access arm for the metal ion study, the exclusion criteria were expanded in an attempt to minimize secondary sources of metal ion exposure. The patients signed an additional consent form for the metal ion study and were screened for eligibility. Patients were excluded from the study if they had undergone a prior surgical procedure that used permanent metal implants (i.e., coronary stents, large joint replacement, fracture fixation plates, and dental implants); if they currently worked in a profession that had increased exposure to metal particles (i.e., jewelry making, construction, iron working, metal grinding, welding); if they were currently receiving B12 injections and/or taking nutritional supplements with metal-containing compounds such as chromium picolinate; and if they were taking or had a history of chronic usage of certain medications (e.g., Cloxacillin or Clotrimazole, which are known metal corrosion inhibitors) [30, 31].
Specimen collection
Samples were collected preoperatively and at 3, 6, 12, 24 and 36 months postoperatively. Blood was collected by venipuncture using a butterfly needle in triplicate s-monovette syringes. The first syringe was used to rinse the system and the metal ion analysis was performed on the second and third vials. Blood was allowed to clot at room temperature and centrifuged to separate into serum and cell fraction. The serum was stored at −80 °C until analysis. Storage tubes, pipette tips, and vessels coming in contact with the sample were acid-washed and verified to be free of trace metals. All manipulations of the specimens following collection were carried out in a class-100 environment to minimize atmospheric and manual contamination. The methodology has been standardized in the Rush University Medical Center Trace Metal Analysis Laboratory for nearly two decades [32].
The serum was assayed for cobalt and chromium using a high-resolution inductively-coupled plasma-mass spectrometer (ICP-MS) (Element 2, Finnigan MAT, Bremen, Germany) as described previously. All samples were tested using the method of additions. The detection limits for cobalt and chromium in serum were 0.04 and 0.015 ng/mL, respectively. Quality control was assured by comparison with Seronorm Trace Elements (Sero, Billingstad, Norway) in Serum reference standard, which is certified for cobalt and chromium.
Concentration values below the detection limit were assigned by convention a value of one-half the detection limit for each element. Longitudinal statistical comparisons were made using the Friedman test. Statistical significance was determined at p < 0.05. Correlations were established with the use of Spearman rank-order correlation test.
Results
Twenty-four patients were enrolled in the study. One patient withdrew 6 months postoperatively and another patient withdrew 24 months postoperatively. An additional three patients missed their 24-month appointment, two patients missed their 36-month appointment and one 36-month sample has yet to be shipped for analysis. This resulted in a sample size of 23 at 12 months, 20 at 24 months, and 19 at 36 months.
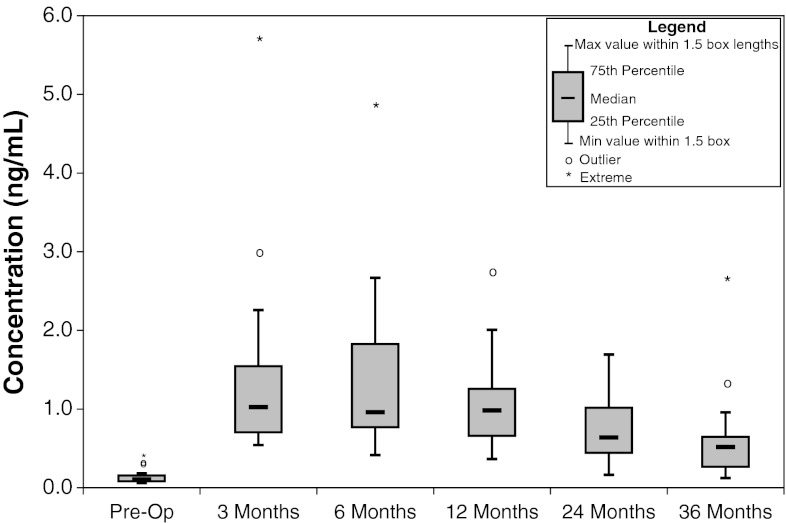
The median serum Co levels at pre-op, 3, 6, 12, 24, and 36 months post-op were 0.10, 1.03, 0.96, 0.98, 0.64, and 0.52 ng/mL, respectively (Fig. 1; Table 2). Serum Co levels were statistically higher at the 3, 6, 12, 24, and 36 month time periods compared with preoperative levels (Friedman p < 0.01). In addition the 24-month levels were lower than at the 3- (Friedman p < 0.01), 6- (Friedman p = 0.025), and 12-month (Friedman p = 0.025) time periods. There were four cases which were identified as having outlier1 levels and three cases which had extreme2 levels at various time points. All of the post-operative levels were higher than the preoperative levels. Of the 23 cases which had 12-month values, 14 had lower levels at 12 months than at 6 months. At the 24-month post-operative time period, 9 of the 20 cases had lower levels at 24 months than at 12 months. At the 36-month postoperative time, 13 of the 18 cases had lower levels at 36 months than at 24 months.
Fig. 1.
Concentration of cobalt serum metal ion levels
Table 2.
Serum chromium and cobalt concentrations (ng/mL)
| Pre-op n = 24 | 3 months n = 24 | 6 months n = 24 | 12 months n = 23 | 24 months n = 20 | 36 months n = 19 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cr | Co | Cr | Co | Cr | Co | Cr | Co | Cr | Co | Cr | Co | |
| Mean | 0.06 | 0.14 | 0.74 | 1.35 | 0.75 | 1.35 | 0.66 | 1.06 | 0.64 | 0.73 | 0.57 | 0.62 |
| St Dev Mean | 0.01 | 0.017 | 0.15 | 0.22 | 0.11 | 0.20 | 0.09 | 0.20 | 0.08 | 0.09 | 0.10 | 0.13 |
| Median | 0.05 | 0.10 | 0.49 | 1.03 | 0.65 | 0.96 | 0.43 | 0.98 | 0.52 | 0.64 | 0.50 | 0.52 |
| Minimum | 0.04 | 0.06 | 0.14 | 0.54 | 0.11 | 0.41 | 0.26 | 0.36 | 0.17 | 0.16 | 0.14 | 0.12 |
| Maximum | 0.16 | 0.39 | 3.38 | 5.65 | 2.52 | 4.81 | 1.92 | 2.72 | 1.36 | 1.69 | 1.85 | 2.59 |
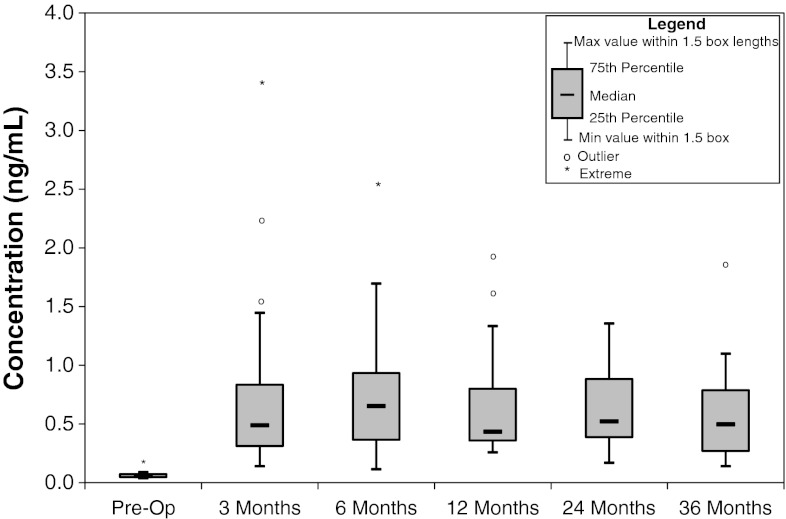
The median serum Cr levels at pre-op, 3, 6, 12, 24, and 36 months post-op were 0.05, 0.49, 0.65, 0.43, 0.52, and 0.50 ng/mL, respectively (Fig. 2; Table 2). Cr levels were statistically higher (p < 0.01) at the 3-, 6-, 12-, 24-, and 36-month time periods compared with preoperative levels. No other statistically significant differences were observed for Cr. Examining the data revealed four cases identified as having outlier levels and one case as having extreme levels. All of the postoperative levels were higher than the preoperative levels. Of the 23 cases which had 12-month values, 14 had lower levels at 12 months than at 6 months. At the 24-month postoperative time, 9 of the 20 cases had lower levels at 24 months than at 12 months and of the 18 cases at the 36-month postoperative time, 13 had lower levels than at their 24-month postoperative time period.
Fig. 2.
Concentration of chromium serum metal ion levels
Correlation between Cr and Co was statistically significant at all postoperative time periods (Spearman: p < 0.01, r = 0.78, 0.84, 0.75, 0.87, and 0.84 for the 3-, 6-, 12-, 24-, and 36-month time periods, respectively).
Discussion
The median serum metal levels presented in this study at 12 and 24 months after surgery are half the values for Cr and less than one-third the values for Co presented by Zeh et al. [25, 26] for either single- or two-level LDA implants at an average postoperative period of 14.8 and 36.7 months. Bisseling et al.[33] reported lower serum metal levels for a retrospective study of ten patients with a single-level LDA at one postoperative time point averaging 34.5 months (13–61 months). Both Zeh et al. and Bisseling et al. reported on the same device studied in the present series with either one or two time periods postoperatively, unlike the present series in which each patient was studied preoperatively and followed longitudinally with serial metal ion levels obtained at 3, 6, 12, 24, and 36 months postoperatively. Whereas the Bisseling study measured whole blood and serum with ICP-MS, and reported a detection limit of 0.5 μg/L, the present series analyzed only serum using HR-ICP-MS, with a detection limit over one order of magnitude lower. In comparison, the Zeh study measured serum with atomic absorption spectrometry, with detection limits not reported. Such inter-laboratory differences are not uncommon in the metal ion literature [34]. These reported differences again underscore the difficulty in comparing metal ion results across different published studies using different patient selection, specimen collection and analytical techniques.
The median serum metal levels presented in this study were equal to or lower for Cr than those associated with stainless steel posterior spinal instrumentation reported by Kim et al. [16], del Rio et al. [11], and McPhee et al. [26]. It is important to note that stainless steel alloy actually contains less Cr (approximately 18 % by mass) than CoCr alloy (approximately 30 % Cr by mass). A major driver for the difference in serum metal levels is the fundamental mechanism of wear associated with both implant types. Wear generated by posterior spinal instrumentation is primarily from fretting (unintended microscopic movement between two parts in contact) [35] compared with the microscopic surface fatigue and abrasion associated with articulating metal-on-metal TDRs [36]. This comparison is particularly relevant as a direct comparison of the serum metal burden associated with two peri-spinal metallic implant systems.
The serum Cr levels seen here in this cobalt–chromium metal-on-metal disc replacement are generally lower (up to an order of magnitude) than those seen in cobalt–chromium metal-on-metal surface hip replacements and cobalt–chromium metal-on-metal total hips [13, 37, 38]. Serum Co levels reported in this study are either in the same range or as low as one-fifth of the levels measured in cobalt–chromium metal-on-metal surface hip replacements and cobalt–chromium metal-on-metal total hip replacements at 2 years postoperatively [37–40].
When considering a discussion of metal-on-metal bearings for disc arthroplasty applications, it is instructive to refer to the literature concerning metal-on-metal bearings in the hip, including both total hip arthroplasty and surface replacement arthroplasty. Over the past several years, there have been numerous reports of adverse local tissue responses (ALTRs) in metal-on-metal hip replacements leading to poor clinical outcomes [41]. Recently, there have been reports of ALTRs associated with metal-on-metal disc arthroplasty devices as well [42–44]. While the true incidence of this phenomenon in metal-on-metal disc arthroplasty has yet to be defined, as is the case for hip arthroplasty, it is likely that patients with higher Co and Cr levels by virtue of greater implant wear and/or corrosion are at greater risk of developing an ALTR. An advantage of metal-on-metal bearings is the substantially lower volumetric wear debris when compared with conventional metal-on-polyethylene bearing couples. However, patients with metal-on-metal articulating prosthetic implants experience increased serum metal ion concentrations following surgery, and the toxicological sequelae of these chronic elevated local and systemic metal levels have not been determined. Despite continuing concerns about the biologic consequences of metal release, there are currently no reliable threshold values for the toxicity of systemic, circulating metal debris following implantation of articulating orthopedic implants. As such, the clinical implications of elevated local tissue and systemic ionic concentrations are uncertain. Surgeons and their patients should be fully informed as to the benefits as well as the risks of any technology, including metal-on-metal LDA. Clinical judgement, after assessing the overall risk–benefit vis-a-vis indications, patient selection, and the appropriateness of a particular implant, will determine the final treatment plan for each individual patient. Continued observation of patients with metallic LDA implants will provide important intermediate to long-term data regarding the biological effects of particulate debris over a patient’s lifetime.
Acknowledgments
This investigational device exemption study was sponsored by Medtronic, Memphis, TN.
Conflict of interest
Gornet—Royalties and Royalty payments with Medtronic. Burkus—Patent Holder with Medtronic and Royalties and Royalty payments with Medtronic. Harper—Employee of Medtronic. Chan—None Skipor—Institutional Funds are received from Medtronic Spinal and Biologics. Jacobs—Consultant: Zimmer, Medtronic, Johnson and Johnson, Smith and Nephew. Spinal Motion Research Funding: Medtronic, Zimmer and Spinal Motion.
Footnotes
Cases with values between 1.5 and 3 lengths from the upper or lower edge of the box are defined as outlier. The box length is the interquartile range (25–75 %), (SPSS ver. 10.05).
Cases with values more than three box lengths from the upper or lower edge of the box are defined as extreme. The box length is the interquartile range (25–75 %), (SPSS ver. 10.05).
References
- 1.Gamradt SC, Wang JC. Lumbar disc arthroplasty. Spine J. 2005;51:95–103. doi: 10.1016/j.spinee.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Bao QB, McCullen GM, Higham PA, Dumbleton JH, Yuan HA. The artificial disc: theory, design and materials. Biomaterials. 1996;1712:1157–1167. doi: 10.1016/0142-9612(96)84936-2. [DOI] [PubMed] [Google Scholar]
- 3.Guyer RD, McAfee PC, Hochschuler SH, Blumenthal SL, Fedder IL, Ohnmeiss DD, Cunningham BW. Prospective randomized study of the charite artificial disc: data from two investigational centers. Spine J. 2004;46(Suppl):252S–259S. doi: 10.1016/j.spinee.2004.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Zigler J, Delamarter R, Spivak JM, Linovitz RJ, Danielson GO, III, Haider TT, Cammisa F, Zuchermann J, Balderston R, Kitchel S, Foley K, Watkins R, Bradford D, Yue J, Yuan H, Herkowitz H, Geiger D, Bendo J, Peppers T, Sachs B, Girardi F, Kropf M, Goldstein J. Results of the prospective, randomized, multicenter food and drug administration investigational device exemption study of the prodisc-l total disc replacement versus circumferential fusion for the treatment of 1-level degenerative disc disease. Spine (Phila Pa 1976) 2007;3211:1155–1162. doi: 10.1097/BRS.0b013e318054e377. [DOI] [PubMed] [Google Scholar]
- 5.Mathews HH, Lehuec J, Friesem T, Zdeblick T, Eisermann L. Design rationale and biomechanics of maverick total disc arthroplasty with early clinical results. Spine J. 2004;46(Suppl):268S–275S. doi: 10.1016/j.spinee.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Harris WH. Wear and periprosthetic osteolysis: the problem. Clin Orthop Relat Res. 2001;393:66–70. doi: 10.1097/00003086-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 7.van Ooij A, Kurtz SM, Stessels F, Noten H, van Rhijn L. Polyethylene wear debris and long-term clinical failure of the charite disc prosthesis: a study of 4 patients. Spine (Phila Pa 1976) 2007;322:223–229. doi: 10.1097/01.brs.0000251370.56327.c6. [DOI] [PubMed] [Google Scholar]
- 8.Amstutz HC, Le Duff MJ, Campbell PA, Gruen TA, Wisk LE. Clinical and radiographic results of metal-on-metal hip resurfacing with a minimum ten-year follow-up. J Bone Joint Surg Am. 2010;9216:2663–2671. doi: 10.2106/JBJS.I.01715. [DOI] [PubMed] [Google Scholar]
- 9.Treacy RBC, McBryde CW, Shears E, Pynsent PB. Birmingham hip resurfacing: a minimum follow-up of ten years. J Bone Joint Surg Br. 2011;931:27–33. doi: 10.1302/0301-620X.93B1.24134. [DOI] [PubMed] [Google Scholar]
- 10.Sieber HP, Rieker CB, Kottig P. Analysis of 118 second-generation metal-on-metal retrieved hip implants. J Bone Joint Surg Br. 1999;811:46–50. doi: 10.1302/0301-620X.81B1.9047. [DOI] [PubMed] [Google Scholar]
- 11.Pandit H, Glyn-Jones S, McLardy-Smith P, Gundle R, Whitwell D, Gibbons CLM, Ostlere S, Athanasou N, Gill HS, Murray DW. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg Br. 2008;907:847–851. doi: 10.1302/0301-620X.90B7.20213. [DOI] [PubMed] [Google Scholar]
- 12.Korovessis P, Petsinis G, Repanti M, Repantis T. Metallosis after contemporary metal-on-metal total hip arthroplasty: five to nine-year follow-up. J Bone Joint Surg Am. 2006;886:1183–1191. doi: 10.2106/JBJS.D.02916. [DOI] [PubMed] [Google Scholar]
- 13.Skipor AK, Campbell PA, Patterson LM, Anstutz HC, Schmalzried TP, Jacobs JJ. Serum and urine metal levels in patients with metal-on-metal surface arthroplasty. J Mater Sci Mater Med. 2002;1312:1227–1234. doi: 10.1023/A:1021179029640. [DOI] [PubMed] [Google Scholar]
- 14.MacDonald SJ, McCalden RW, Chess DG, Bourne RB, Rorabeck CH, Cleland D, Leung F. Metal-on-metal versus polyethylene in hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2003;406:282–296. doi: 10.1097/00003086-200301000-00039. [DOI] [PubMed] [Google Scholar]
- 15.Garbuz DS, Tanzer M, Greidanus NV, Masri BA, Duncan CP. The john charnley award: metal-on-metal hip resurfacing versus large-diameter head metal-on-metal total hip arthroplasty: a randomized clinical trial. Clin Orthop Relat Res. 2010;4682:318–325. doi: 10.1007/s11999-009-1029-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langton DJ, Sprowson AP, Joyce TJ, Reed M, Carluke I, Partington P, Nargol AVF. Blood metal ion concentrations after hip resurfacing arthroplasty: a comparative study of articular surface replacement and birmingham hip resurfacing arthroplasties. J Bone Joint Surg Br. 2009;9110:1287–1295. doi: 10.1302/0301-620X.91B10.22308. [DOI] [PubMed] [Google Scholar]
- 17.MacDonald SJ, Brodner W, Jacobs JJ. A consensus paper on metal ions in metal-on-metal hip arthroplasties. J Arthroplasty. 2004;198(Suppl 3):12–16. doi: 10.1016/j.arth.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 18.Kim Y, Kassab F, Berven SH, Zurakowski D, Hresko MT, Emans JB, Kasser JR. Serum levels of nickel and chromium after instrumented posterior spinal arthrodesis. Spine (Phila Pa 1976) 2005;308:923–926. doi: 10.1097/01.brs.0000158872.42802.be. [DOI] [PubMed] [Google Scholar]
- 19.del Rio J, Beguiristain J, Duart J. Metal levels in corrosion of spinal implants. Eur Spine J. 2007;167:1055–1061. doi: 10.1007/s00586-007-0311-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McPhee IB, Swanson CE. Metal ion levels in patients with stainless steel spinal instrumentation. Spine (Phila Pa 1976) 2007;3218:1963–1968. doi: 10.1097/BRS.0b013e318133aa0d. [DOI] [PubMed] [Google Scholar]
- 21.Rackham MD, Cundy TP, Antoniou G, Freeman BJC, Sutherland LM, Cundy PJ. Predictors of serum chromium levels after stainless steel posterior spinal instrumentation for adolescent idiopathic scoliosis. Spine (Phila Pa 1976) 2010;359:975–982. doi: 10.1097/BRS.0b013e3181d7a197. [DOI] [PubMed] [Google Scholar]
- 22.Cundy TP, Delaney CL, Rackham MD, Antoniou G, Oakley AP, Freeman BJC, Sutherland LM, Cundy PJ. Chromium ion release from stainless steel pediatric scoliosis instrumentation. Spine (Phila Pa 1976) 2010;359:967–974. doi: 10.1097/BRS.0b013e3181d53724. [DOI] [PubMed] [Google Scholar]
- 23.Kasai Y, Iida R, Uchida A. Metal concentrations in the serum and hair of patients with titanium alloy spinal implants. Spine (Phila Pa 1976) 2003;2812:1320–1326. doi: 10.1097/01.BRS.0000065482.41115.B4. [DOI] [PubMed] [Google Scholar]
- 24.Richardson TD, Pineda SJ, Strenge KB, Van Fleet TA, MacGregor M, Milbrandt JC, Espinosa JA, Freitag P. Serum titanium levels after instrumented spinal arthrodesis. Spine (Phila Pa 1976) 2008;337:792–796. doi: 10.1097/BRS.0b013e318169574d. [DOI] [PubMed] [Google Scholar]
- 25.Zeh A, Planert M, Siegert G, Lattke P, Held A, Hein W. Release of cobalt and chromium ions into the serum following implantation of the metal-on-metal maverick-type artificial lumbar disc (medtronic sofamor danek) Spine (Phila Pa 1976) 2007;323:348–352. doi: 10.1097/01.brs.0000253599.89694.c0. [DOI] [PubMed] [Google Scholar]
- 26.Zeh A, Becker C, Planert M, Lattke P, Wohlrab D. Time-dependent release of cobalt and chromium ions into the serum following implantation of the metal-on-metal maverick type artificial lumbar disc (medtronic sofamor danek) Arch Orthop Trauma Surg. 2009;1296:741–746. doi: 10.1007/s00402-008-0677-8. [DOI] [PubMed] [Google Scholar]
- 27.Stieber JR, Errico TJ, Bauer T, Whitaker C, Miz G, Sasso R (2010) Blood metal ion levels following implantation of the all-metal flexicore lumbar intervertebral disc replacement, 24–36 month follow-up. In: 10th Annual Global Symposium on Motion Preservation Technology,Spine Arthroplasty Society, New Orleans, LA, April 2010
- 28.ASTM 1537-08: Standard specification for wrought cobalt-28 chromium-6 molybdenum alloys for surgical implants
- 29.Gornet MF, Burkus JK, Dryer RF, Peloza JH. Lumbar disc arthroplasty with MAVERICK disc versus stand-alone interbody fusion: a prospective, randomized, controlled, multicenter investigational device exemption trial. Spine (Phila Pa 1976) 2011;3625:E1600–E1611. doi: 10.1097/BRS.0b013e318217668f. [DOI] [PubMed] [Google Scholar]
- 30.Obot IB, Obi-Egbedi NO, Umoren SA. Adsorption characteristics and corrosion inhibitive properties of Clotrimazole for aluminium corrosion in hydrochloric acid. Int J Electrochem Sci. 2009;4:863–877. [Google Scholar]
- 31.Eddy NO, Ebenso EE. Adsorption and quantum chemical studies on cloxacillin and halides for the corrosion of mild steel in acidic medium Int. J. Electrochem. Sci. 2010;5:731–750. doi: 10.3390/ijms11062473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jacobs JJ, Skipor AK, Black J, Urban R, Galante JO. Release and excretion of metal in patients who have a total hip-replacement component made of titanium-base alloy. J Bone Joint Surg Am. 1991;7310:1475–1486. [PubMed] [Google Scholar]
- 33.Bisseling P, Zeilstra DJ, Hol AM, van Susante JLC. Metal ion levels in patients with a lumbar metal-on-metal total disc replacement: should we be concerned? J Bone Joint Surg Br. 2011;937:949–954. doi: 10.1302/0301-620X.93B7.26392. [DOI] [PubMed] [Google Scholar]
- 34.Clark M, Prentice J, Hoggard N, Stockley I, Jacobs JJ, Wilkinson JM (2012) Effect of laboratory analysis on metal levels after momhr and potential impact on patient management and interpretation of research datasets. In: Transactions of the 58th Annual Meeting of the Orthopaedic Research Society, Poster #1928
- 35.Villarraga ML, Cripton PA, Teti SD, Steffey DL, Krisnamuthy S, Albert T, Hilibrand A, Vaccaro A. Wear and corrosion in retrieved thoracolumbar posterior internal fixation. Spine (Phila Pa 1976) 2006;31:2454–2462. doi: 10.1097/01.brs.0000239132.16484.be. [DOI] [PubMed] [Google Scholar]
- 36.Harper ML, Dooris A, Pare PE. The fundamentals of biotribology and its application to spine arthroplasty. SAS J. 2009;3:125–132. doi: 10.1016/j.esas.2009.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Witzleb W, Ziegler J, Krummenauer F, Neumeister V, Guenther K. Exposure to chromium, cobalt and molybdenum from metal-on-metal total hip replacement and hip resurfacing arthroplasty. Acta Orthop. 2006;775:697–705. doi: 10.1080/17453670610012863. [DOI] [PubMed] [Google Scholar]
- 38.Back DL, Young DA, Shimmin AJ. How do serum cobalt and chromium levels change after metal-on-metal hip resurfacing? Clin Orthop Relat Res. 2005;438:177–181. doi: 10.1097/01.blo.0000166901.84323.5d. [DOI] [PubMed] [Google Scholar]
- 39.Skipor AK, Campbell PA, Gitelis S, Berger RA, Amstutz HC & Jacobs JJ (2004) Three year prospective study of serum and urine metal levels in patients with metal-on-metal total hip and surface arthroplasty. In: Transactions of the 50th Annual Meeting of the Orthopaedic Research Society, Paper 124
- 40.Brodner W, Grubl A, Jankovsky R, Meisinger V, Lehr S, Gottsauner-Wolf F. Cup inclination and serum concentration of cobalt and chromium after metal-on-metal total hip arthroplasty. J Arthroplasty. 2004;198(Suppl 3):66–70. doi: 10.1016/j.arth.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 41.Kwon YM, Thomas P, Summer B, Pandit H, Taylor A, Beard D, Murray DW, Gill HS. Lymphocyte proliferation responses in patients with pseudotumors following metal-on-metal hip resurfacing arthroplasty. J Orthop Res. 2010;284:444–450. doi: 10.1002/jor.21015. [DOI] [PubMed] [Google Scholar]
- 42.Guyer RD, Shellock J, MacLennan B, Hanscom D, Knight RQ, McCombe P, Jacobs JJ, Urban RM, Bradford D, Ohnmeiss DD. Early failure of metal-on-metal artificial disc prostheses associated with lymphocytic reaction: diagnosis and treatment experience in four cases. Spine (Phila Pa 1976) 2011;367:E492–E497. doi: 10.1097/BRS.0b013e31820ea9a2. [DOI] [PubMed] [Google Scholar]
- 43.Berry MR, Peterson BG, Alander DH. A granulomatous mass surrounding a maverick total disc replacement causing iliac vein occlusion and spinal stenosis: a case report. J Bone Joint Surg Am. 2010;925:1242–1245. doi: 10.2106/JBJS.H.01625. [DOI] [PubMed] [Google Scholar]
- 44.Francois J, Coessens R, Lauweryns P. Early removal of a maverick disc prosthesis: surgical findings and morphological changes. Acta Orthop Belg. 2007;731:122–127. [PubMed] [Google Scholar]