Abstract
Introduction
To better understand cervical kinematics following cervical disc replacement (CDR), the in vivo behavior of a minimally constrained CDR was assessed.
Methods
Radiographic analysis of 19 patients undergoing a 1-level CDR from C4–5 to C6–7 (DISCOVER, Depuy-Spine, USA) was performed. Neutral–lateral and flexion–extension radiographs obtained at preop, postop and late follow-up were analyzed for segmental angle and global angle (GA C2–7). Flexion–extension range of motion was analyzed using validated quantitative motion analysis software (QMA®, Medical Metrics, USA). The FSU motion parameters measured at the index and adjacent levels were angular range of motion (ROM), translation and center of rotation (COR). Translation and COR were normalized to the AP dimension of the inferior endplate of the caudal vertebra. All motion parameters, including COR, were compared with normative reference data.
Results
The average patient age was 43.5 ± 7.3 years. The mean follow-up was 15.3 ± 7.2 months. C2–7 ROM was 35.9° ± 15.7° at preop and 45.4° ± 13.6° at follow-up (∆p < .01). Based on the QMA at follow-up, angular ROM at the CDR level measured 9.8° ± 5.9° and translation was 10.1 ± 7.8 %. Individuals with higher ROM at the CDR level had increased translation at that level (p < .001, r = 0.97), increased translation and ROM at the supra-adjacent level (p < .001, r = .8; p = .005, r = .6). There was a strong interrelation between angular ROM and translation at the supra-adjacent level (p < .001, r = .9) and caudal-adjacent level (p < .001, r = .9). The location of the COR at the CDR- and supra-adjacent levels was significantly different for the COR-X (p < .001). Notably, the COR-Y at the CDR level was significantly correlated with the extent of CDR-level translation (p = .02, r = .6). Shell angle, which may be influenced by implant size and positioning had no impact on angular ROM but was correlated with COR-X (p = .05, r = −.6) and COR-Y (p = .04, r = −.5).
Conclusion
The COR is an important parameter for assessing the ability of non-constrained CDRs to replicate the normal kinematics of a FSU. CDR size and location, both of which can impact shell angle, may influence the amount of translation by affecting the location of the COR. Future research is needed to show how much translation is beneficial concerning clinical outcomes and facet loading.
Keywords: Cervical disc replacement, Center of rotation analysis, Cervical arthroplasty, In-vivo kinematics
Introduction
Anterior cervical decompression and fusion (ACDF) is a successful technique to treat symptoms derived from cervical stenosis [12, 23]. Cervical disc replacement (CDR) is designed to overcome limitations of cervical fusion [28] to restore physiologic segmental function and prevent accelerated adjacent segment degeneration [31]. To achieve these goals a CDR must closely simulate the physiologic kinematics at the operated and adjacent segments [27]. Clinical studies have shown encouraging results echoing those of ACDF but with maintenance of motion [12, 23, 32]. Biomechanical studies have shown increased intradiscal pressure and segmental range of motion (ROM) at the adjacent level to ACDF during normal ROM [9, 37] that was not found in CDR patients [37]. However, concerns exist regarding indications, costs and long-term benefits of CDR [5, 39]. The early enthusiasm with CDR has waned [36]. Others have reported experience with cervical revision surgery for failed CDRs [6, 19, 40] and a significant rate of ossifications at the CDR-level in the long-term run [13, 35]. Biomechanical studies have suggested that function of the CDR and motion characteristics are related not only to the quality of insertion of the device but also to the segmental release created during decompression and selection of implant size [34, 38]. Accordingly, due to conflicting information, surgeons face difficulties with the selection of appropriate indications and the type and size of implants [20, 21]. Ex vivo motion studies and finite element studies have provided insight into the function of different CDR designs [4, 38]. Unfortunately, laboratory studies are limited and cannot completely simulate the complex coupled in vivo motions during cervical spine activities, so patient-based research is required.
Typical designs of CDRs include a two-piece or three-piece articulation. In the three-piece family, a mobile nucleus (e.g., polymer core) is intercalated between two metal endplates (e.g., Bryan Disc, M6-C) [30]. In the two-piece family, most CDRs use a ball-and-socket design that constrains the center of rotation (COR) to the center of the radius of curvature of the prosthesis (e.g., Discover, CerviCore, PCM, Discocerv, ProDisc-C) [30]. The location and geometry of the articular surfaces of the ball-and-socket design influence the position of the COR in the AP and cranial-caudal directions. CDRs offer a varying degree of mobility and can be further stratified into constrained, semi-constrained and minimally/non-constrained (Fig. 1) [4, 20, 21, 25, 30, 31]. With constrained devices motion is less than that seen physiologically and may theoretically cause high-stress concentrations at the implant–vertebra interface. Devices are considered semi-constrained in certain planes if they allow motion similar to that seen physiologically with motion being restrained by design of the CDR and muscololigamentous restraints [21]. A minimally/non-constrained CDR, as analyzed in the current study, relies on ligament integrity to maintain segmental balance during cervical motion thereby minimizing bone-implant interface stresses [21]. There is no mechanical stop and the motion is restricted by the design of the CDR and merely by the musculoligamentous restraints [20, 21].
Fig. 1.

Example of the device analyzed in the current study
An analysis of COR and its changes after CDR may be important for assessing the ability of a CDR to replicate the kinematics of a patient’s functional spinal unit (FSU). To improve understanding of the in vivo cervical kinematics after implantation of a minimally constrained CDR, the authors conducted a radiographic study of 20 patients using a high-precision measurement tool, focusing on the postoperative position of the COR [29].
Materials and methods
Patient sample
A prospective database assessment of 20 consecutive patients treated with a minimally constrained 1-level CDR was conducted. Radiographs and outcomes were assessed retrospectively. One patient was excluded because of early conversion to an ACDF. The age of the remaining 19 patients (3 males, 16 females) at index surgery was 43.5 ± 7.3 years (range, 27–58 years). The CDR was implanted at C4–5 (1), C5–6 (12) and C6–7 (6). The mean follow-up was 15.3 ± 7.2 months (range, 6–26 months). Patients had surgery for 1-level cervical stenosis due to disc herniation with radiculopathy. Standard diagnostics included preoperative biplanar radiographs, flexion–extension radiographs, MRI, reconstructed CT-scans and neurologist consultation. The inclusion/exclusion age range was 18–60 years. The common indications and contraindications were similar to those used in other US IDE clinical studies [3, 26]. Postoperatively, patients were mobilized with a soft-collar for 3 days under surveillance of a physical therapist. Radiographic follow-up was scheduled at 6 weeks postoperatively, at 6 months, 12 months and after 2 years [27]. For statistical analysis, demographics, follow-up length and gender were recorded. Any medical or surgical complications were recorded.
Implant and surgical procedure
All subjects were implanted with the DISCOVER disc. It is a three-piece, biarticulating, metal-on-hard polyethylene minimally constrained device (Depuy Spine, Inc., Raynham, MA, USA; Fig. 1). It consists of two titanium endplates with an ultra-high molecular weight polyethylene insert on the caudal plate. The CDR allows for 21° of lateral motion. Axial rotation and flexion–extension are limited by the articulating surfaces and musculoligamentous restraints. The hard polymer core on the caudal endplate articulates with the cephalad metal endplate to form a ball-and-socket type joint. The implant is available in five sizes and five heights (5–9 mm).The DISCOVER disc has been CE marked (04-2006) and has received premarket approval for an IDE study in the USA. Related clinical outcomes have been previously reported [2, 8, 11]. The mean disc height was 6.7 ± 0.7 mm (range, 6–8 mm). Eight patients had CDR height of 6 and 7 mm, respectively, and two patients had a height of 8 mm.
The CDR was implanted according to the manufacturer’s manual and instruments. The posterior longitudinal ligament was resected for thorough decompression. The uncus was resected in patients with spondylosis using a Kerrison rongeur. Sufficient ligament balancing and disc height was assessed manually with a Codmann distractor.
Radiographic analysis
All digital radiographs were subjected to manual review in a PACS-Viewer using a digital caliper (Infinitt, Infinitt-Europe, Frankfurt/Germany). In addition, the latest follow-up digital radiographs were analyzed using quantitative motion analysis software (QMA®, Medical Metrics, Inc., Houston, TX, USA).
Preoperative lateral and flexion–extension radiographs, postoperative lateral radiographs and follow-up lateral and flexion–extension radiographs were included. Minimum follow-up was 6 months. Antero-posterior radiographs at follow-up were analyzed to rule out malalignment of the device.
Standard cervical alignment measurements
Measurements on preoperative and follow-up radiographs included the global angle from C2 to C7 (GA C2–7) and the segmental angle (SA) at the CDR-level (Fig. 2). Angular measurements were performed using the Harrison posterior tangent method [14]. A positive measurement indicates kyphotic alignment. Sagittal plane range of motion from C2 to C7 (ROM C2–7) and range of motion at the CDR-level (ROM CDR) were calculated from the GA C2–7 and the SA on flexion–extension films. On the postoperative lateral and follow-up lateral radiographs the shell angle was measured as defined by the angle formed between the two tangents of the adjacent titanium endplates of the implanted CDR (Fig. 2).
Fig. 2.
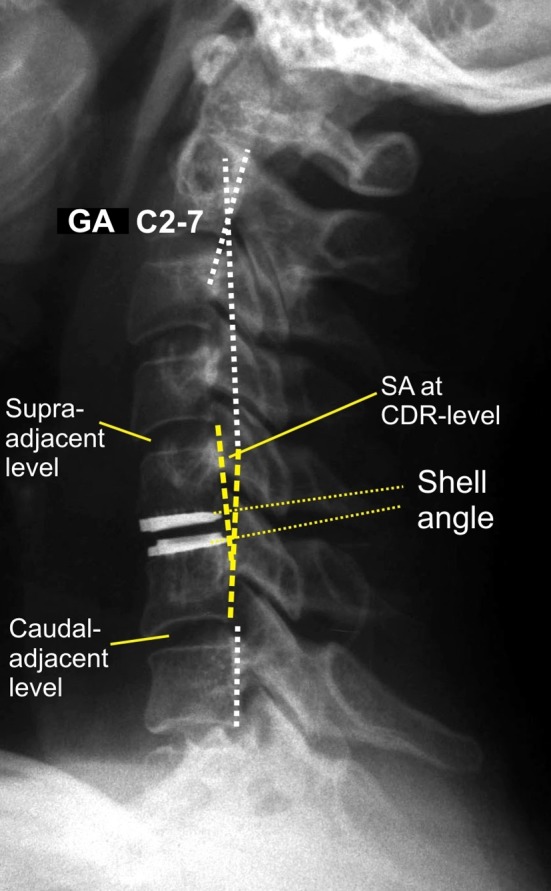
Postoperative radiograph illustrating the manual measurements performed at C2–C7. SA Segmental rotation angle, GA global angle C2–7 according to the Harris-tangent method
Quantitative motion analysis
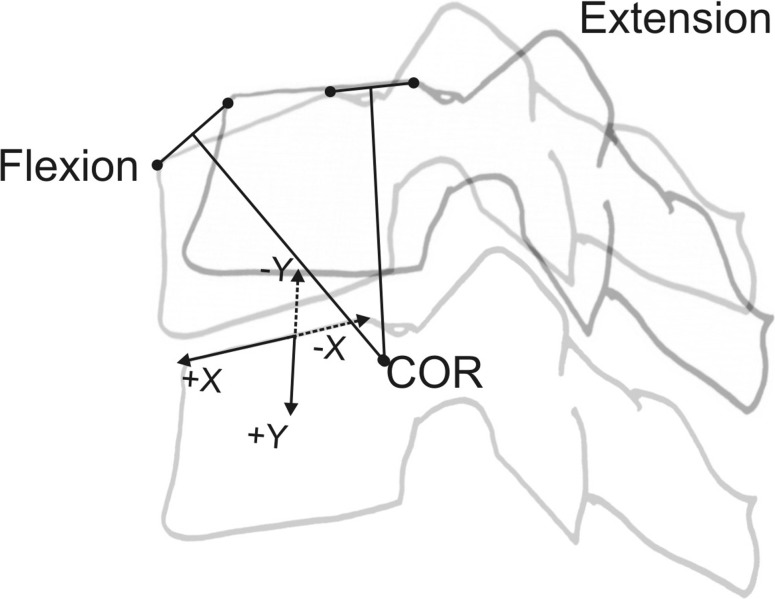
The flexion–extension radiographs at follow-up were processed using the QMA® software to calculate the FSU motion parameters at the index and supra-adjacent and caudal-adjacent levels. QMA® uses computer-assisted image overlay methods to track the motion of spinal vertebrae. The software has been shown to substantially improve accuracy and reproducibility compared with manual techniques [28, 29]. The accuracy of QMA for cervical intervertebral motion measurements was previously determined to be <0.5° for rotation and <0.3 mm for translation [40]. The bony features of the cervical vertebra are identified in the flexion image and then digitally superimposed on the extension image. If the osseous landmarks are selected in flexion, the new position of these landmarks can be calculated in extension based on the image registration. This avoids the reproducibility errors associated with picking landmarks on multiple images in serial radiographs and assures that the relative position of vertebral landmarks remains constant between images [29]. In the current study, the FSU motion parameters assessed were the angular range of intervertebral motion (ROMQMA), translation and the position of the center of rotation COR. Translation was measured in mm as displacement of the cephalad vertebral body along a line parallel to the superior endplate of the caudal vertebral body and also reported as percent of the superior endplate of the caudal vertebra. The position of the COR was measured in mm and also reported normalized to the AP diameter of the superior endplate of the caudal vertebra (Fig. 3). A negative COR-X value means the COR is posterior to the mid-point of the endplate in the sagittal plane. A negative COR-Y value means the COR is above the endplate.
Fig. 3.
Technique of reconstructing the center of rotation (COR), defining and measuring its position in relation to the vertebral body’s height and width. See text for further explanations
At follow-up, the ROM at the CDR-level was defined as the ROMQMA. The results of the study population were compared with that of normals [15, 29] and the proportion of patients with their COR-X and COR-Y within normal limits was evaluated.
Statistical analysis
The descriptive data are presented as mean ± standard deviations and ranges. The continuous variables were assessed for normal distribution using Shapiro–Wilk tests. Paired Student’s t tests and Pearson’s correlation coefficients were used to test for significant differences and correlations. Fisher’s exact test and Pearson’s test were used to analyze cross-tabulation tables. A p value less than 5 % indicated a statistical significance. With reporting of significant correlations, a stratification was performed and only those with r ≥ 0.5 were reported, indicating at least moderate correlation. All analyses were performed using SPSS software (SPSS, Version18, Chicago, IL, USA), Statistica 6.1 (StatSoft, Tulsa, OK, USA) and StatXact (Cytel Software Corp, Cambridge, MA, USA). Study results and motion parameters were compared with published normative reference data for asymptomatic, radiographically normal volunteers [15, 29].
Results
Standard measurements
The results obtained from standard manual measurements are summarized in Table 1.
Table 1.
Results of standard manual measurements
| Preoperative mean ± SD (range) | Postoperative Mean ± SD (range) | Follow-up Mean ± SD (range) | Difference (p value) | |
|---|---|---|---|---|
| GA C2–7 | −10° ± 12.5° (−37° to 14°) | −13.8° ± 11.1° (−38° to 4.5°) | – | |
| SA at CDR-level in neutral position | −1.8° ± 4.4° (−10.5° to 4.5°) | −3.5° ± 3.9° (−12.5° to 3°) | – | |
| GA C2–7 in flexion | −7.1° ± 10.6° (−19° to 22°) | – | 7.0° ± 8.0° (−14° to 20°) | .036 |
| GA C2–7 in extension | −22.6° ± 24.5° (−59° to 4.5°) | – | −38.4° ± 14.4° (−12° to 60°) | .017 |
| Shell angle | – | −5.5° ± 4.9° (−17° to 0°) | −7.1° ± 6.8° (−20° to 4°) | ns |
| SA at the CDR-level in flexion | 3.4° ± 6.4° (−13° to 11.5°) | – | 1.8° ± 4.3° (−4° to 13°) | ns |
| SA at the CDR-level in extension | −1.0° ± 6.5° (−11° to 11°) | – | −9.3° ± 5.5° (−16° to 4°) | <.001 |
| ROM at the CDR-level* | 5.9° ± 5.1° (0°–18°) | – | 11.5° ± 6.3° (3°–26°) | .006 |
| ROM C2–7 | 35.9° ± 15.7° (1°–63°) | – | 45.4° ± 13.6° (22°–65°) | <.01 |
Data of measurements in the table refer to standard manual measurements
GA global alignment, SA segmental angle, ROM range of motion, CDR cervical disc replacement
Quantitative motion analysis
Rotation
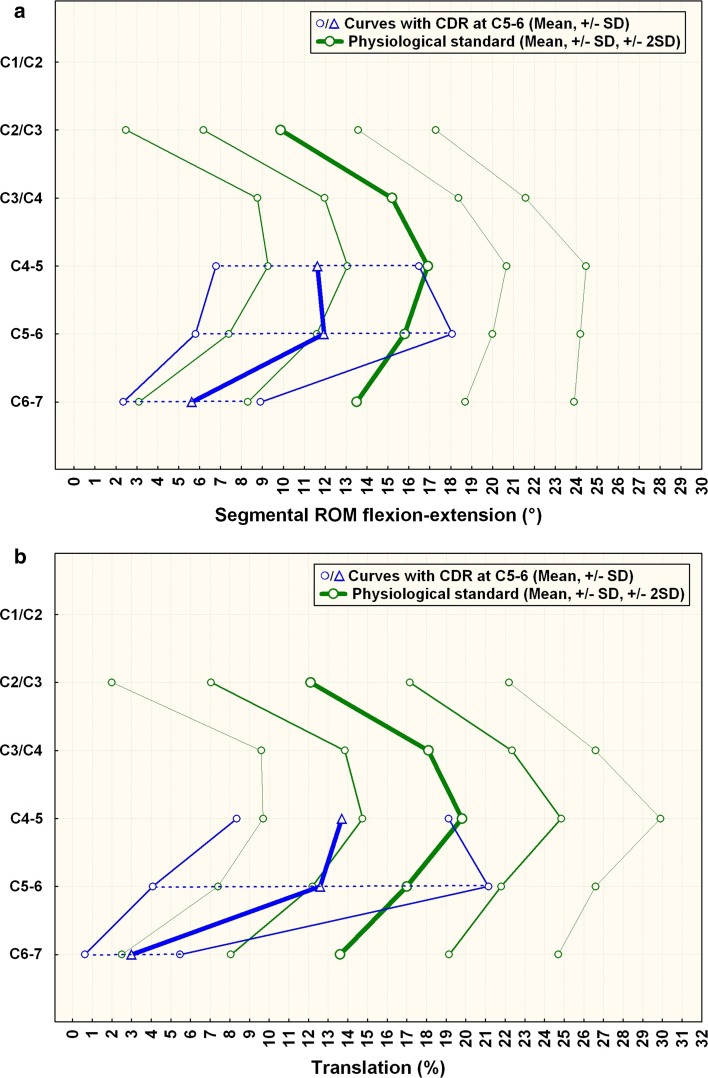
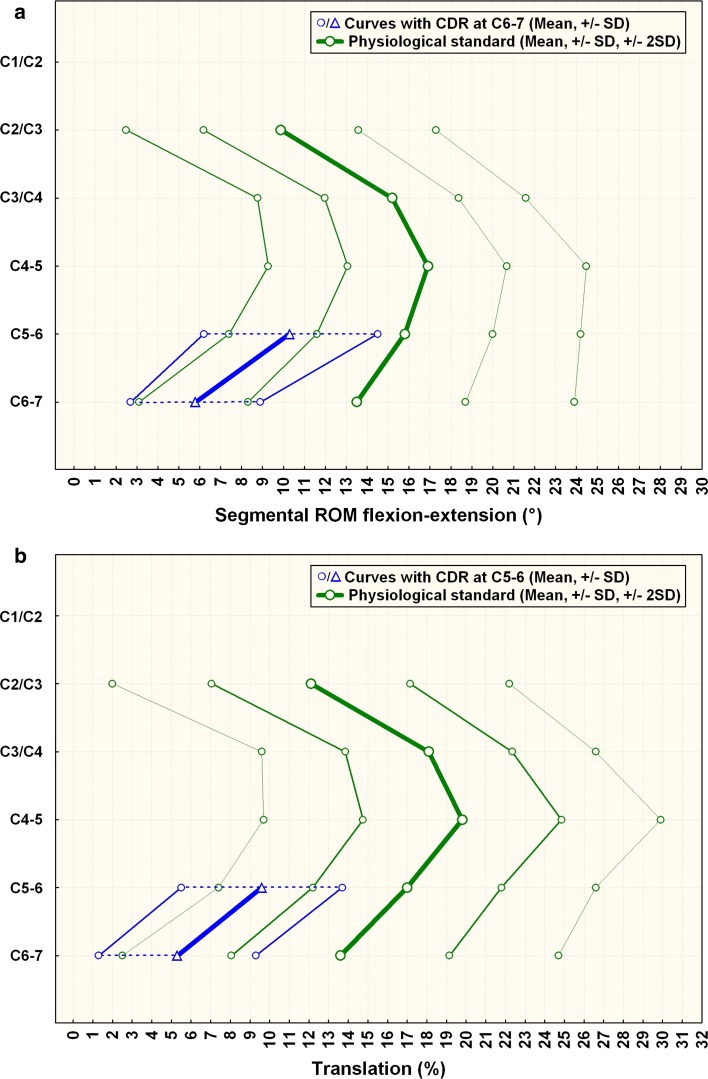
The results for rotation per the QMA method are summarized in Table 2. Figures 4a and 5a illustrate the results compared with normals.
Table 2.
Results of quantitative motion analysis at follow-up: rotation
![]()
Dashed lines denote corresponding parameters and level of significance
Fig. 4.
a Comparison of segmental ROM in patients with CDR at C5–6 compared to normals. C6–7 is the caudal-adjacent segment, C4–5 the supra-adjacent segment. The graph illustrates sagittal segmental rotation of C2–C7. The results of the CDR-group at the instrumented level, above and below are plotted against the physiological standard [10] ±1 SD (green smaller lines) and ±2 SD (green outer lines). b Comparison of segmental translation in patients with CDR at C5–6 (n = 12) compared to normals. C6–7 is the caudal adjacent segment, C4–5 the supra-adjacent segment. The graph illustrates sagittal segmental translation of C2–C7. The results of the CDR-group at the instrumented level, above and below are plotted against the physiological standard [10] ±1 SD (greensmaller lines) and ±2 SD (greenouter lines)
Fig. 5.
a Comparison of segmental ROM of patients with CDR at C6–7 compared (n = 6) to normals. C5–6 is the supra-adjacent segment. The caudal-adjacent segment motion C7–T1 is not shown. The graph illustrates sagittal segmental rotation of C2–C7. The results of the CDR-group at the instrumented level and above are plotted against the physiological standard [10] ±1 SD (green smaller lines) and ±2 SD (green outer lines). b Comparison of segmental translation of patients with CDR at C6–7 compared with normals. C5–6 is the supra-adjacent segment. The caudal-adjacent segment motion C7–T1 is not shown. The graph illustrates sagittal segmental rotation of C2–C7. The results of the CDR-group at the instrumented level and above are plotted against the physiological standard [10] ±1 SD (green smaller lines) and ±2 SD (green outer lines)
Translation
The results for translation per the QMA method are summarized in Table 3. Figures 4b and 5b illustrate the results compared to normals.
Table 3.
Results of quantitative motion analysis at follow-up: translation

Dashed lines denote corresponding parameters and level of significance
COR-analysis
The results for the position of the COR on the X–Y axis per the QMA method are summarized in Table 4. Compared with level-specific data for normals [15, 29], the COR-X at the CDR-level was within normal limits (defined by the 95 % CI) in 9 patients (47.4 %), and the COR-Y was within normal limits in 16 patients (84.2 %). The COR was within normal limits in both the X and Y directions in eight patients (42.1 %). The COR-X and the COR-Y at the supra-adjacent level were within normal limits in 18 patients (94.7 %). The COR was within normal limits in both axes in 17 patients (89.5 %). In the caudal-adjacent segments the COR was within normal limits in all cases assessed.
Table 4.
Results of quantitative motion analysis at follow-up: COR location

Dashed lines denote corresponding parameters and level of significance
It was not possible to analyze COR in two patients at the CDR-level and in one patient at the supra-adjacent segment. QMA requires a minimum ROM of 3° [27] for accurate assessment of the COR, which was not present in two cases. For the caudal-adjacent segment, rotation, translation and COR could be accurately analyzed in 13, 13 and 10 patients, respectively. Missing data points are related to the most common level of surgery being located at C5–C7, and the related caudal-adjacent segment being C6–T1, which is sometimes difficult to visualize on radiographs.
Interrelation of segmental rotation, translation and position of the COR
Statistical analysis showed that manual measurements of the preoperative ROM at the CDR-level and the GA C2–7 had no correlation with the ROMQMA at the CDR-level and the GA C2–7 at follow-up. The preoperative ROM C2–7 as well as the ROM at CDR-level was not correlated with the ROM C2–7 or the ROM at CDR-level at follow-up, respectively, when comparing the manual measurements. At follow-up, the individuals with higher ROMQMA and translation at the CDR-level had an increased ROM C2–7 (p = .01, r = .6; p = .04, r = .5). Likewise, at the supra-adjacent level, with higher ROMQMA at follow-up the translation also increased significantly (p < .001, r = .9). The same was true for the caudal-adjacent level with the ROMQMA strongly correlating with the amount of translation (p < .001, r = .9).
Notably, consistent with the design of the disc prosthesis, there was a high correlation between the ROMQMA and the translation at the CDR-level at follow-up (p < .001, r = .97). There was also a significant interrelation between the ROMQMA at the CDR-level and the ROMQMA and translation at the supra-adjacent level (p = .005, r = .6; p < .001, r = .8). The amount of translation at the CDR-level was significantly correlated with the ROMQMA and the translation of the supra-adjacent level (p = .02, r = .5; p = .001, r = .7). Finally, individuals with increased ROMQMA at the caudal-adjacent segment also had significantly increased translation (p < .001, r = .9).
While the COR-Y at the CDR-level was significantly correlated with the amount of translation at the CDR-level (p = .02, r = 0.6), the COR-X was not, even though, on average, the COR-X was slightly anterior to the superior endplate midpoint of the caudal vertebra if compared to that of normals [7, 15]. A relationship between the coordinates of the COR and translation is expected [7]. Segmental translation increased as the COR moved caudally. The positions of both the COR-Y and COR-X had no significant impact on the amount of segmental ROMQMA. Statistics also revealed that the magnitude of the shell-angle at follow-up, representing a gross estimate for the CDR-position in the sagittal plane, had no impact on the amount of the ROMQMA or the translation at the CDR-level. However, the shell angle and the position of the CDR at follow-up were significantly correlated with the position of the COR-X (p < .05, r = −.6) and the COR-Y (p = .04, r = −.5) at follow-up. With increasing shell angle, the COR was located more posteriorly and cephalad. A shift in the position of the COR on the X-axis beyond normal limits was correlated with a shift on the Y-axis (p < .05, r = −.5; p = .04, r = −.5). Statistics indicated that a deviation from normalcy in one axis was accompanied by a deviation in the other axis.
Analysis of the variances in selected CDR-heights showed only a slight trend towards more individuals having their COR-X located within normal limits if a larger-sized CDR was inserted (p = .076). The level of surgery and patient age had no impact on the motion parameters. However, there was an inverse correlation between the follow-up length and the ROMQMA (p = .047, r = −.5) and the caudal-adjacent level ROMQMA (p = .002, r = –.8) as well as the caudal-adjacent translation (p = .004, r = −.7) which indicated some loss of motion over time.
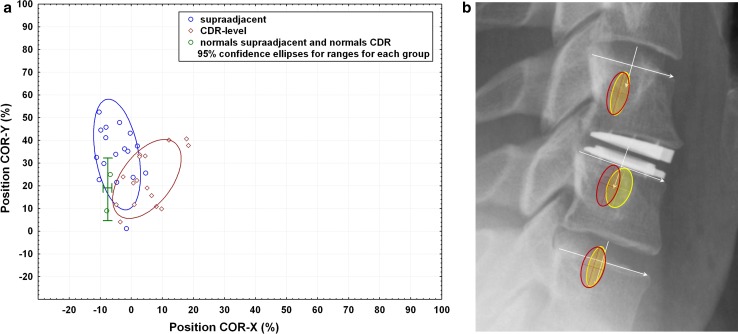
Figure 6 provides a graphical illustration of the range of positions of the COR along the X and Y axes at the CDR-level and supra- and caudal-adjacent levels compared with normative reference data for asymptomatic volunteers.
Fig. 6.
a Position of the center of rotation (COR) during flexion–extension neck motion of normals at the level of the CDR and at the supra-adjacent level (green) in comparison with the position of the COR in patients with a CDR at their level of the prothesis (red) and the supra-adjacent level (blue). A positive COR-X value means the COR is anterior to the mid-point of the vertebral body endplate in the sagittal plane. A positive COR-Y value means the COR is below the endplate. b Visualization of the COR superimposed on a clinical example. Position of the center of rotation (COR) during flexion–extension neck motion of cervical normals (red ellipses) versus patients implanted with the CDR (yellow ellipses). Normal COR data are from Hipp and Wharton, 2008 [15]. The red ellipses represent the 95 % confidence interval for an asymptomatic, radiographically normal population. The cross-hairs represent the mean location of the COR. Data are reported on a level-specific basis (n = 125 at C4–5; n = 121 at C5–6; n = 76 at C6–7). The yellow ellipses represent the 95 % confidence interval for the CDR patients. All CDR-level data (n = 17 implantations at C4–5 through C6–7) are pooled and overlaid on the C5–6 level in the figure. All supra-adjacent data (n = 18, C3–4 through C5–6) are pooled and overlaid on C4–5. All caudal-adjacent data (n = 10, C5–6 and C6–7) are pooled and overlaid on C6–7. (Data are pooled due to low N. Ideally, if N were larger, data would be reported on a level-specific basis in the same manner as the normative reference data in red.). There is near-perfect overlap of the COR data at the superior and inferior adjacent (untreated) levels. Roughly 50 % of subjects have a COR-X that is shifted anteriorly outside of normal limits (as defined by the 95 % confidence interval for an asymptomatic population)
Discussion
Data on the in vivo kinematics of CDR are scant, although needed [25, 31]. Using a minimally constrained CDR, the study showed that motion was restored at the CDR-level yielding significance for the gain in segmental ROM at the CDR-level and C2–C7 at follow-up. Also, the shell angle, which is a measure of CDR alignment, did not show a significant change between the postoperative and follow-up neutral radiographs, remaining at 7º of lordosis. Summarizing the interrelations of the ROMQMA, translation and COR-positions, individuals with greater translation and rotation at the CDR-level at follow-up also had greater ROM C2–7 as well as larger ROMQMA and translation at the supra-adjacent and the caudal-adjacent levels. Individuals with a higher ROMQMA at the CDR-level also had greater translation at the CDR-level as well as larger ROMQMA and translation at the supra-adjacent segment. Increased translation at the CDR-level was strongly related to larger translation at the supra-adjacent level, but the impact of the ROMQMA at the supra-adjacent level on translation was statistically stronger. Interrelations observed in the CDR patients reflected physiologic interdependencies also observed in normals [29].
Keeping in mind that most surgeries were performed at C5–C7, the observation that ROMQMA at the CDR-level was statistically not significantly different to that of the supra-adjacent level (mean: 9.8° vs. 10°) is noteworthy. Compared with normals, ROM at C4–5 is generally larger than that at C5–6 and C6–7 [10, 29]. The differences regarding ROMQMA seen between the CDR-level and the caudal-adjacent level must be interpreted from the perspective that ROM at C6–7 is usually less than that of C5–6 [10, 29]. Of the patients where ROMQMA could be measured at both the CDR and supra-adjacent levels (n = 13), 12 had the caudal-adjacent segment at C6–7 and 1 at C5–6. In general, segmental ROMQMA and translation is reduced in descending order from C4–5 to C7–T1 [10, 29]. Accordingly, with translation, the same relations existed as discussed for the ROM data. Notably, even though segmental ROM on flexion–extension views did not achieve mean values as high as normals, the current ROM at the CDR-level was larger than in benchmark studies also using QMA for assessment of CDR at C5–C7 [31] (9.8° vs. 6.7°). In the study of Sasso [31] comparing nine Bryan discs against ten fusion patients, the adjacent-level motion was not statistically different compared with the CDR-level. Translation was not different to the fusion group. In the study of Rousseau [30] the ROM at CDR level was significantly less than in normals (13.4°) using the Prestige LP (5.1°) or the Prodisc-C disc (3.6°). Finally, in a study by Picket [27] using QMA in 20 patients with 1- or 2-level surgery with the Bryan disc at C5–C7, follow-up 6–24 months, ROM was 8.9° at the CDR-level and not significantly different to the preoperative measures. Neither adjacent segment ROM nor translation increased compared with the preoperative state.
Figures 4 and 5 illustrating segmental ROM at the CDR-level and adjacent levels depict that the ROM and translation at the treated levels was about one SD smaller compared with that of normals [29]. Differences might be due to variability in the level of patient effort during flexion–extension imaging, discomfort, or coping with pain during motion after cervical surgery. The figures also illustrate that it is still difficult to replicate the motion characteristics of normals using a CDR. The motion parameters usually used to benchmark different CDR designs were close to normal limits in the current study. Analyses of the COR showed (Fig. 6) that at the CDR-level, the COR in both the X and the Y axes was within normal limits in 42 % of the patients. In comparison, at the supra-adjacent segment, the COR-X was within normal limits in 90 % of the patients, indicating that normal physiologic kinematics at the adjacent segment were not altered in most patients. At the caudal-adjacent segment, the COR was within normal limits in all patients with radiographs suitable for analysis with QMA. In normals, the location of the COR is reported to be significantly more cranial as the level progresses from C3 to C7 [17, 30]. The average COR location in the AP direction, however, does not change significantly with level [17, 30]. When we compared the COR at the CDR-level with the supra-adjacent normal levels, our study showed that only the COR-X was significantly different, while the COR-Y was not. This indicates that, although device placement was correct, an anterior shift of the COR with the minimally constrained CDR occurred compared with the normal supra-adjacent level.
The current study echoed difficulties of previous authors in replicating the natural COR with CDR. Rousseau [30] subjected 51 ball-and-socket type CDRs in 30 patients to an analysis of ROM and COR similar to QMA. The study included 1- and 2-level surgeries using 25 Prodisc-C (Synthes, caudal type geometric COR) and 26 Prestige-LP prostheses (Medtronic Sofamor Danek, cranial type geometric COR). Generally speaking, with these prostheses, the COR tended to be located more anterior and superior than in normals. Neither the cranial (Prestige-LP) nor the caudal type of ball-and-socket type design (Prodisc-C) did fully restore the normal kinematics in terms of ROM and COR position. The average COR-X for the control group was 33.1, 44.3 % in the Prestige-LP group and 54 % in the Prodisc-C group. The COR-X was significantly more posterior in the control group without differences between the CDRs, as was observed in our. In the Prestige-LP group (n = 14) the COR-X was within normal limits in 8 of 14 cases and anterior to it in 6. In the Prodisc-C group (n = 15), the COR-X was within normal limits in five cases, anterior to it in eight and posterior to it in two cases. Using our coordinate system (Fig. 3), the mean COR-Y was 25 % in the control group, −9.6 % in the Prestige-LP group, and 7.6 % in the Prodisc-C group and significantly different between all groups. In the Prestige-LP group the COR-Y was within normal limits in ten cases and outside normal limits in four. In the Prodisc-C group, the COR-Y was within the normal range in 12 cases, below in 1 and above in 2. Neither the cranial nor caudal geometric center fully restored the normal kinematics in terms of ROM and COR position. Patwardhan [25] performed a laboratory COR analysis with the M6 disc at C5–6 (Spinal kinetics, Sunnyvale,CA, USA), which is a 3-piece CDR with polymer core. Using QMA, the COR-X was shown to be located at −1.6 ± 0.5 mm, posterior to the endplate midpoint. The COR-Y was 1.6 ± 1.5 mm caudal to the endplate. Notably the COR-Y at the implanted level was more cranial compared with the intact control by 2.9 ± 1.3 mm (p < .05). It is known that the COR-Y will move cranially as the ratio of translation per degree of rotation decreases [7]. Variability in the CDR positioning in the disc space was shown to significantly affect the location of the COR and the ROM. This in turn might influence the relative motions and contact forces at the facet and uncovertebral joints [30, 31, 33]. Summarizing current data available in literature, the COR of an artificially reconstructed disc depends on the variability of positioning of the CDR and balancing of the ligaments, the extent of the decompression (release effect) and the selected implant size. In our study, there was a significant relationship between shell angle and the position of the COR in the X-axis and Y-axis. In return, the amount of translation at the CDR-level increased with a more caudal position of the COR-Y. Usually, the shell angle depends on the physiologic shape of the segment, trimming of the endplates by the surgeon and is modulated by the design and the size of the CDR. In the current study, shell angle was −7º postoperatively and did not change significantly during follow-up, echoing data of Anakwenze et al. [1] where the disc angle was −6º at 2-year follow-up. Hence, implant dimensions and positioning, both of which may impact the shell angle, can influence the amount of translation seen by affecting the COR-X and the COR-Y location.
Using a minimally constrained device as in the current study with an inferior spheric ball-and-socket type joint, changes in the CDR size, e.g. selecting a smaller model, causes the COR to be located at and below the vertebral endplate. Selecting a larger model and, thus, a greater radius of curvature of the articulation, locates the COR closer to the disc space, which still enables sufficient motion while reducing loads on the facets and increasing implant loads. Likewise, with selection of a smaller than ideal sized implant, increased facet loads were revealed in finite element studies during flexion–extension testing, which might cause pain and late facet arthrosis [38]. Using a finite element model, Sears [33] showed that the likelihood of facet apposition increases with a more caudal location of the COR. By limiting AP-translation as some CDR designs do (e.g., Discocerv, Scientx, USA), sagittal ROM might be limited while more segmental stability and reduced facet loading can be assumed during normal ranges of motion [16]. CDRs with a ball-and-socket design provide a fixed COR located at the center of the ball; therefore, they require precise device placement with the center of the ball at the location of the physiologic COR if they are to reproduce normal cervical kinematics. Otherwise, a fixed COR might lead to facet impingement and nonphysiologic stress to the facets and thus might not be the ideal solution in terms of replicating physiologic kinematics.
Compared with CDRs with a fixed COR, those with a mobile COR, such as the Bryan disc, have a theoretical advantage because they provide normal kinematics over a range of device positions. With the physiologic COR preserved, the facets and ligaments would not be subjected to abnormal stress, thus reducing the potential for pain derived from facet and ligament overloading. However, detailed data on the behavior of the COR have not been published yet, e.g. for the Bryan disc [31], leaving open the hypothesis that following disc level preparation, selection of size and ligament balancing replication of the physiologic COR is too difficult when using such protheses.
A CDR must adequately mimic the in vivo function and biomechanics of an intervertebral disc if it is to restore the function spinal unit. Currently, the quantity of motion in terms of angular ROM and translation is usually restored using CDR. However, the quality of motion in terms of COR might not be perfectly maintained or restored and might explain why clinical and radiographic results in terms of adjacent segment degeneration do not differ between CDR and ACDF in selected studies [22]. A mismatch between the physiologic kinematics and that after a CDR implantation might also explain why several studies using QMA did not identify differences regarding adjacent segment motion comparing ACDF and a variety of CDRs used [18, 24, 28].
With a minimally constrained CDR used in this study, we showed that replicating the physiological COR is difficult, while angular and translation motion can be maintained or restored. In addition, if one considers that cervical discs are interconnected with complementary joints, such as the zygapophyseal and uncovertebral joints, and that 80 % of stability results from muscular stability with 20 % from ligamentous support, a more freely articulating minimally constrained type of artificial disc such as that used in the current study might be preferable [21]. Nevertheless, changes in kinematics and the COR in particular should be the subject of future long-term outcome studies identifying whether an altered COR might be associated with clinical consequences. Also, if one accepts that replication of the physiologic COR is an important design feature of a CDR prosthesis, then our data indicate that new designs should allow for ideal preparation of the surgical level and ideal placement of the prosthesis to preserve the patient’s segmental COR and ligament balancing.
Acknowledgments
Conflict of interest
None.
References
- 1.Anakwenze OA, Auerbach JD, Milby AH, Lonner BS, Balderston RA. Sagittal cervical alignment after cervical disc arthroplasty and anterior cervical discectomy and fusion. Spine. 2009;34:2001–2007. doi: 10.1097/BRS.0b013e3181b03fe6. [DOI] [PubMed] [Google Scholar]
- 2.Aretz K, Lamos N, Boyaci B, Melcher R, Harms J. Discover vs PCM—comparison of the cervical prosthesis in 1-year-follow-up: global and segmental alignment, intervertebral mobility, location of prosthesis. Eur Spine J. 2008;17:1540–1633. doi: 10.1007/s00586-008-0803-x. [DOI] [Google Scholar]
- 3.Auerbach JD, Jones KJ, Fras CI, Balderston JR, Rushton SA, Chin KR. The prevalence of indications and contraindications to cervical total disc replacement. Spine J. 2008;8:711–716. doi: 10.1016/j.spinee.2007.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Barrey C, Mosnier T, Jund J, Perrin G, Skalli W. In vitro evaluation of a ball-and-socket cervical disc prothesis with cranial geometric center. J Neurosurg Spine. 2009;11:538–546. doi: 10.3171/2009.6.SPINE0949. [DOI] [PubMed] [Google Scholar]
- 5.Bartels R, Donk R, Verbeek ALM. No justification for cervical disc protheses in clinical practice: a meta-analysis of randomized controlled trials. Neurosurgery. 2010;66:1–8. doi: 10.1227/01.NEU.0000369189.09182.5F. [DOI] [PubMed] [Google Scholar]
- 6.Barth M, Brenke C, Schmieder K (2010) Radiological outcome and intraoperative findings following explantation of 20 cervical disc prothesis. Eur Spine J 19:1963–2074 [DOI] [PubMed]
- 7.Bogduk N, Mercer S. Biomechanics of the cervical spine. I: normal kinematics. Clin Biomech. 2000;15:633–648. doi: 10.1016/S0268-0033(00)00034-6. [DOI] [PubMed] [Google Scholar]
- 8.Du J, Li M, Liu H, Meng H, He Q, Luo Z. Early follow-up outcomes after treatment of degenerative disc disease with the discover cervical disc prosthesis. Spine J. 2011;11:281–289. doi: 10.1016/j.spinee.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 9.Eck JC, Humphreys SC, Lim T-H. Biomechanical study on the effect of cervical spine fusion on adjacent-level intradiscal pressure and segmental motion. Spine. 2002;27:2431–2434. doi: 10.1097/00007632-200211150-00003. [DOI] [PubMed] [Google Scholar]
- 10.Frobin W, Leivseth G, Biggemann M, Brinckmann P. Sagittal plane segmental motion of the cervical spine. A new precision measurement protocol and normal motion data of healthy adults. Clin Biomech. 2002;17:21–31. doi: 10.1016/S0268-0033(01)00105-X. [DOI] [PubMed] [Google Scholar]
- 11.Greiner-Perth R, Allam Y, Silbermann J, Simank H-G. First experience and preliminary clinical results with the cervical disc replacement DISCOVER. Z Orthop Unfall. 2009;147:582–587. doi: 10.1055/s-0029-1185897. [DOI] [PubMed] [Google Scholar]
- 12.Grob D, Porchet F, Kleinstück FS, Lattig F, Jeszenszky D, Luca A, Mutter U, Mannion AF (2009) A comparison of outcomes of cervical disc arthroplasty and fusion in everyday clinical practice: surgical and methodological aspects. Eur Spine J (E-Pub) [DOI] [PMC free article] [PubMed]
- 13.Guerin P, Luc S, Bourghli A, Gille O, Obeid I, Verdier N, Vital JM (2011) Heterotopic ossification after cervical disc arthroplasty. A prospective study. Annual meeting of the CSRS-E, Istanbul
- 14.Harrison DE, Harrison DD, Cailliet R, Troyanovic SJ, Janik TJ, Holland B. Cobb method or Harrison posterior tangent method. Which to chose for lateral cervical radiographic analysis. Spine. 2000;25:2072–2078. doi: 10.1097/00007632-200008150-00011. [DOI] [PubMed] [Google Scholar]
- 15.Hipp JA, Wharton ND (2008) Qunatitative motion analysis (QMA) of Motion preserving and fusion technologies for the Spine. In: Yue J, Bertagnoli R, McAfee P, An H (eds) Motion Preservation Surgery of the Spine: advanced techniques and controversies, New York, Elsevier
- 16.Huang RC, Wright TM, Panjabi MM, Lipman JD. Biomechanics of nonfusion implants. Orthop Clin North Am. 2005;36:271–280. doi: 10.1016/j.ocl.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 17.Kang JD, Baillargeon EM, Donaldson WF, Lee JY, Anderst WJ (2010) Motion path of the intervertebral center of rotation in single-level fusion patients and asymptomatic controls during dynamic flexion-extension. Annual meeting of the CSRS-A, Charlotte
- 18.Kelly MP, Mok JM, Frisch RF, Tay BK. Adjacent segment motion after anterior cervical discectomy and fusion versus ProDisc-C cervical disk arthroplasty. Analysis from a randomized, controlled trial. Spine. 2011;36:1171–1179. doi: 10.1097/BRS.0b013e3181ec5c7d. [DOI] [PubMed] [Google Scholar]
- 19.Lebl DR, Cammisa F, Girardi FP, Lee SM, Wright T, Abjornson C (2011) Retrieval analysis of cervical total disc replacements—a study of in vivo wear, surface properties, and fixation. 18th IMAST, Copenhagen
- 20.Lee K, Goel VK. Artificial disc prosthesis: design concepts and criteria. Spine J. 2004;4:209S–218S. doi: 10.1016/j.spinee.2004.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Link HD, McAfee PC, Pimenta L. Choosing a cervical disc replacement. Spine J. 2004;4:294–302. doi: 10.1016/j.spinee.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado CV, Paz RD, Martin CB. Adjacent-level degeneration after cervical disc arthroplasty versus fusion. Eur Spine J. 2011;20:403–407. doi: 10.1007/s00586-011-1916-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murrey D, Janssen M, Delamarter R, Goldstein J, Zigler J, Tay B, Darden B. Results of the prospective, randomized, controlled multicenter Food and Drug Administration investigational device exemption study of the ProDisc-C total disc replacement versus anterior discectomy and fusion for the treatment of 1-level symptomatic cervical disc disease. Spine J. 2009;9:275–286. doi: 10.1016/j.spinee.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Nabhan A, Ishak B, Steudel WI, Ramadhan S, Steimer O (2011) Assessment of adjacent-segment mobility after cervical disc replacement versus fusion: RCT with 1 year’s results. Eur Spine J (E-pub) [DOI] [PMC free article] [PubMed]
- 25.Patwardhan AG, Tzermiadianos MN, Tsitsopoulos PP, Voronov LI, Renner SM, Reo ML, Carandang G, Ritter-Lang, Harvey RM (2010) Primary and coupled motions after cervical total disc replacement using a compressible six-degree-of-freedom prosthesis. Eur Spine J Suppl 5:S618–S629 [DOI] [PMC free article] [PubMed]
- 26.Philipps FM, Allen TR, Regan JJ, Albert TJ, Cappucino A, Devine KG, Ahrens JE, Hipp JA, McAfee Pc. Cervical disc replacement in patients with and without previous adjacent level fusion surgery. Spine. 2009;34:556–565. doi: 10.1097/BRS.0b013e31819b061c. [DOI] [PubMed] [Google Scholar]
- 27.Picket GE, Rouleau JP, Duggal N. Kinematic analyis of the cervical spine following implantation of an artificial cervical disc. Spine. 2005;30:1949–1954. doi: 10.1097/01.brs.0000176320.82079.ce. [DOI] [PubMed] [Google Scholar]
- 28.Reitman CA, Hipp JA, Nguyen L, Esses SI. Changes in segmental intervertebral motion adjacent to cervical arthrodesis: a prospective study. Spine. 2004;29:E221–E226. doi: 10.1097/00007632-200406010-00022. [DOI] [PubMed] [Google Scholar]
- 29.Reitman CA, Mauro KM, Nguyen L, Ziegler JM, Hipp JA. Intervertebral motion between flexion and extension in asymptomatic individuals. Spine. 2004;29:2832–2843. doi: 10.1097/01.brs.0000147740.69525.58. [DOI] [PubMed] [Google Scholar]
- 30.Rousseau MA, Cottin P, Levante S, Alexis N, Lazannec J-Y, Skalli W. In vivo-kinematics of two types of ball-and-socket cervical disc replacements in the sagittal plane. Cranial versus caudal geometric center. Spine. 2008;33:E6–E9. doi: 10.1097/BRS.0b013e31815e5dce. [DOI] [PubMed] [Google Scholar]
- 31.Sasso RC, Best NM. Cervical kinematics after fusion and Bryan disc arthroplasty. J Spinal Disord. 2008;21:19–22. doi: 10.1097/BSD.0b013e3180500778. [DOI] [PubMed] [Google Scholar]
- 32.Sasso RC, Smucker JD, Hacker RJ. Artifical disc versus fusion. A prospective, randomized study with 2-year follow-up on 99 patients. Spine. 2007;32:2933–2940. doi: 10.1097/BRS.0b013e31815d0034. [DOI] [PubMed] [Google Scholar]
- 33.Sears W, McCombe P, Sasso R. Kinematics of cervical and lumbar total disc replacement. Seminar Spine Surg. 2006;18:117–129. doi: 10.1053/j.semss.2006.03.013. [DOI] [Google Scholar]
- 34.Snyder JT, Tzermiadianos MN, Ghanayem AJ, Voronov LI, Rinella A, Doortis A, Carandang G, Renner SM, Havey RM, Patwardhan AG. Effect of uncovertebral joint excision on the motion response of the cervical spine after total disc replacement. Spine. 2007;32:2965–2969. doi: 10.1097/BRS.0b013e31815cd482. [DOI] [PubMed] [Google Scholar]
- 35.Suchomel P, Jurak L, Benes V, Brabec R R, Brada O, Elgawhary S (2009) Clinical results and development of heterotopic ossification in total cervical disc replacement during a 4-year follow-up. Eur Spine J (E-Pub) [DOI] [PMC free article] [PubMed]
- 36.Whang PG, Simpson AK, Rechtine G, Gauer JN. Current trends in spinal arthroplasty. J Spinal Disord. 2009;22:26–33. doi: 10.1097/BSD.0b013e3181659804. [DOI] [PubMed] [Google Scholar]
- 37.Wigfield C, Gill S, Nelson R. Influence of an artifical cervical joint compared with fusion on adjacent-level motion in the treatment of degenerative cervical disc disease. J Neurosurg Spine. 2002;96:17–21. doi: 10.3171/spi.2002.96.1.0017. [DOI] [PubMed] [Google Scholar]
- 38.Womack W, Leahy PD, Patel VV, Puttlitz CM. Finite element modeling of kinematic and load transmission alterations due to cervical intervertebral disc replacement. Spine. 2011;36:E1126–E1133. doi: 10.1097/BRS.0b013e31820e3dd1. [DOI] [PubMed] [Google Scholar]
- 39.Zechmeister I, Winkler R, Mad P. Artifical total disc replacement versus fusion for the cervical spine: a systematic review. Eur Spine J. 2011;20:177–184. doi: 10.1007/s00586-010-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zenner J, Meier O, Ferraris L, Koller H (2010) Revision and retrieval of failed cervical disc replacements. Report on characteristics and early outcomes. Annual meeting of the CSRS-A, Charlotte