Abstract
Purpose
Modic type 1 changes/bone edema in the vertebrae are present in 6 % of the general population and 35–40 % of the low back pain population. It is strongly associated with low back pain. The aim was to test the efficacy of antibiotic treatment in patients with chronic low back pain (>6 months) and Modic type 1 changes (bone edema).
Methods
The study was a double-blind RCT with 162 patients whose only known illness was chronic LBP of greater than 6 months duration occurring after a previous disc herniation and who also had bone edema demonstrated as Modic type 1 changes in the vertebrae adjacent to the previous herniation. Patients were randomized to either 100 days of antibiotic treatment (Bioclavid) or placebo and were blindly evaluated at baseline, end of treatment and at 1-year follow-up.
Outcome measures
Primary outcome, disease-specific disability, lumbar pain. Secondary outcome leg pain, number of hours with pain last 4 weeks, global perceived health, EQ-5D thermometer, days with sick leave, bothersomeness, constant pain, magnetic resonance image (MRI).
Results
144 of the 162 original patients were evaluated at 1-year follow-up. The two groups were similar at baseline. The antibiotic group improved highly statistically significantly on all outcome measures and improvement continued from 100 days follow-up until 1-year follow-up. At baseline, 100 days follow-up, 1-year follow-up the disease-specific disability-RMDQ changed: antibiotic 15, 11, 5.7; placebo 15, 14, 14. Leg pain: antibiotics 5.3, 3.0, 1.4; placebo 4.0, 4.3, 4.3. Lumbar pain: antibiotics 6.7, 5.0, 3.7; placebo 6.3, 6.3, 6.3. For the outcome measures, where a clinically important effect size was defined, improvements exceeded the thresholds, and a trend towards a dose–response relationship with double dose antibiotics being more efficacious.
Conclusions
The antibiotic protocol in this study was significantly more effective for this group of patients (CLBP associated with Modic I) than placebo in all the primary and secondary outcomes.
Keywords: Modic changes, Antibiotics, Chronic low back pain, End plate changes, LBP
Introduction
Modic type 1 changes are bone edema in vertebrae and have been shown to be both commonly observed in and tightly associated with, low back pain [1, 2]. A recent systematic review showed that the prevalence for any type of Modic change in patients with non-specific low back pain (LBP) was 46 % as opposed to 6 % in the general population [1]. A positive association between Modic type and non-specific LBP was found with a mean odds ratios of 4.5 [4, 5]. These findings are relevant as chronic lower back pain (CLBP) is seldom reliably attributable to specific pathoanatomical causes [3].
Modic changes are only visible on magnetic resonance images (MRI) and three types have been identified (Types 1–3) [4]. Histological studies of material harvested during surgery, show that Type 1 involve disruption and fissuring of the endplate with regions of degeneration, regeneration, reactive bone formation, endplate edema and vascular granulation tissue [4, 5]. Several studies have examined the reliability of reporting Modic changes and all have shown excellent inter and intra-observer reliability [6, 7]. In comparative studies, Modic changes have demonstrated a higher reliability than other MRI findings, such as disc herniations [8].
Infection is one of the hypothetical causes of the bone edema underlying Modic type 1 changes [9]. Stirling found nuclear tissue removed under strict sterile conditions during surgery for lumbar herniated discs, to be infected with low virulent anaerobic organisms (Proprionibacterium acnes and Corynebacterium propinquum) in 53 % of the patients [10]. To investigate if skin contamination was the cause of this surprising result, Stirling et al. conducted another study with 207 patients with lumbar disc herniation and 27 patients with other spinal disorders such as scoliosis, fracture and tumors, all patients had nucleus material removed. In 37 % of the patients with lumbar disc herniation bacteria were indentified, mainly P. acnes. Conversely, no (0 %) bacteria were found in the extracted nuclear material in the group with other spinal disorders. If skin contamination was the cause of the bacterial presence in the nucleus material the percentage of infected patients should be very similar [11]. Corsia et al. [12] replicated this and evacuated extruded disc material in 30 lumbar disc herniations: 71 % were infected, 36 % with Staphylococcus and 18 % with P. acnes; and in 30 cervical disc herniations they found 59 % were infected, 37 % with P. acnes. Agarwal et al. [12] cultured material from 52 patients, 10 (19 %) of them were infected and in 7 (70 %) of those, P. acnes was the sole organism isolated. It is thought that these anaerobic mouth and skin commensal organisms gain access to the disc during normal bacteremias as a result of the neovascularisation associated with disc degeneration or herniation [13–21]. Local inflammation in the adjacent bone may be a secondary effect due to cytokine and propionic acid production, i.e., the infection is in the disc and the Modic change is a “side effect” manifest in the bone. P. acnes cannot live in the highly vascularised/aerobic bone and is not present there [22].
To evaluate the role of P. acnes in the occurrence of later Modic type 1 changes after a disc herniation, a cohort study of 61 immunocompetent patients undergoing surgery for a lumbar disc herniation underwent an MRI examination before surgery and 1–2 years afterwards. Evacuation of nuclear material took place during surgery under strict sterile conditions. The study revealed that 46 % of the patients had infected nuclear material, 84 % of these with P. acnes. The possible role of bacteria in developing Modic changes after a disc herniation was confirmed hence, 20 of the 25 (80 %) with anaerobic cultures developed new Modic changes in the vertebrae adjacent to the previous disc herniation, compared to 16 of the 36 (44 %) with no identified infection or aerobic bacterial infection (n = 2). Fisher’s exact test p < 0.0079 [23].
In a recently published uncontrolled pilot study, 32 patients with chronic low back pain, following a lumbar disc herniation and of up to 2 years duration associated with Modic type 1 changes/bone edema, were treated with Amoxicillin–clavulanate (500 mg/125 mg) 3 × day for 90 days [24]. Twenty-nine patients (90.6 %) completed the treatment and three patients dropped out due to diarrhea. At the end of treatment and at long-term follow-up (mean 10.8 months) there was both a clinically important and statistically significant (p ≤ 0.001) improvement in all outcome measures [24]. These results provided additional tentative support for the hypothesis that bacterial infection may play a role in LBP with Modic changes (bone edema).
The aim of this study was to test the efficacy of Modic antibiotic spine therapy (MAST) in patients with chronic low back pain, new Modic type 1 changes in the vertebrae adjacent to a previously herniated disc. Additionally, we investigated whether a dose–response relationship could be identified.
Methods
This clinical trial was conducted at the Spine Centre of Southern Denmark and the participants were recruited from two secondary spine centers. The initial inclusion criteria were: age between 18 and 65 years, MRI-confirmed disc herniation L3/L4 or L4/L5 or L5/S1 within the preceding 6–24 months, lower back pain of >6 months duration, both conservative and surgically treated patients were included, regardless of sciatica, neuropathic pain or not. Individuals who fulfilled these criteria were posted a letter describing the project as well as a questionnaire containing pain drawings and the exclusion criteria: allergy to antibiotics, current pregnancy or lactation, any kidney disease or pending litigation. Subjects who fulfilled final criteria and were interested in participation underwent a repeat MRI; all performed at the Spine Center of Southern Denmark (T1- and T2-weighted images). Individuals were then consecutively recruited into the study if their repeat MRI showed Modic type 1 changes adjacent to the previously herniated disc regardless of size of the Modic change. Modic findings were coded by an experienced research radiologist. With regard to the presence and coding of Modic changes, this radiologist demonstrated a perfect intra-tester reliability (kappa = 1.0) and an excellent inter-tester reliability (kappa = 0.93) [25]. The size and volume were graded according to the Nordic Modic Protocol [8]: 1 = endplate only, 2 = up to 25 %, 3 = up to 50 % 4 = above 50 % of the vertebrae. Patients also had to have LBP in the area from L1 to L5 with a Numerical Pain Rating Scale score of ≥6 after adding the current LBP (0–10); the mean LBP during the last 2 weeks (0–10) and worst LBP during the last 2 weeks (0–10) and then dividing by three.
Randomization was performed by the Central Pharmacy, Odense University Hospital using a computer-generated randomization list retained at the pharmacy until after the last patient’s 1-year follow-up. Placebo tablets were calcium carbonate and identical in size, color, coating and aluminum packing to the active tablets. All tablets were packed in sealed white cardboard boxes on which only the patient number was printed (1–162). Patients and the assessor were blinded to randomization until after completion of the patient’s 1-year follow-up. Patients were recommended not to seek any other treatment during the 1-year follow-up period. At both follow-up treatments by doctors, physiotherapists and alternative treatment providers were registered.
Three independent experts in infectious diseases were presented with the bacterial culture results of Stirling’s study [10] and all three recommended amoxicillin–clavulanate. This drug is known to be able to penetrate into the discs [26]. Therefore, treatment consisted of amoxicillin–clavulanate (500 mg/125 mg) (Bioclavid®) tablets three times a day, at 8 h intervals, for 100 days. This long duration of antibiotic treatment is commonly prescribed for post-operative discitis.
The randomization divided patients into four dosage groups: A (n = 45) one Bioclavid tablet, B (n = 36) one placebo tablet, C (n = 45) two Bioclavid tablets, D (n = 36) two placebo tablets. Compliance was measured in daily medication diaries. During their 12-month participation in this trial patients were not provided with any other treatment except mild analgesics, if required.
Study flow
At both baseline and at 1-year follow-up each participant underwent a physical examination and completed self-reported questionnaires. An MRI and blood samples were also taken. The MRI protocol at baseline and at the 1-year follow-up were uniform and performed by the same staff, blinded to treatment, and in the same MRI machine. At the end of the treatment period, (100 days), patients were also mailed a questionnaire. The patients and the observer were blinded to the allocation and all previous measurements. All physical examinations were performed by the same blinded observer.
At baseline, all patients had a 1.5 h thorough patient education session including important information about; what are Modic changes? Why are they so painful? What is the presumed pathoanatomical pathway for the link between the previous herniation and the present Modic type 1 changes? Why they should not do exercise during the treatment period and so forth. All patients were allowed to take their usual anti-inflammatory and pain relieving medication (treatment as usual).
To avoid a later need for imputation, the project secretary checked every questionnaire for completion, if missing data, the patient was asked to fill in before leaving the center. A project secretary, also blinded to treatment allocation, entered all data in EpiData v3.1 (The EpiData Association, Odense, Denmark), coded under the participant’s project number.
Outcome measures
All patients underwent clinical examination and blood tests (serum analysis) at baseline and at end of treatment. Self-reported questionnaires were completed at baseline; end of antibiotic treatment (100 days), and at 12-month follow-up. The outcome measures are presented in Table 1.
Table 1.
Outcome measures
| Self-reported questionnaires | Questionnaire characteristics |
|---|---|
| Global perceived effect | The patients compare their baseline status with their status at follow-up, measured on a 7-point Likert scale |
| Roland Morris Disability Questionnaire (RMDQ) | A disease-specific disability questionnaire in which the patient answers 23 yes/no questions. The scale width is 0–23, where high scores are worst [27] |
| LBP pain rating scale | Three 11-point box scales measuring current pain, the worst within the last 2 weeks and usual pain within the last 2 weeks. These three scores are measured and averaged for both leg and lumbar pain independently [28] |
| Hours with LBP during the last 4 weeks | Number of days during the last 28 days (4 weeks) the participant had experienced LBP (0–28 days), and, on an typical day, how many of the hours awake they experienced LBP (0–16 h). The number of days and hours are multiplied (a 0–448 scale) |
| EQ-5D: Thermometer | Quality-adjusted health status EQ-5D [40] on a vertical Thermometer (1–100), 100 is best |
| Days with sick leave | The number of days within the last year the participant was on sick-leave support from the government |
| Bothersomeness | Measured on a 11-point box scale, where 0 = none, 10 = my life is worthless due to my back pain [41] |
| Constant pain | Pain that can vary during the day but is always present |
| MRI | Volume of the vertebrae with Modic type 1 change, (1–4) |
| Serum analysis | Leukocytes, neutrophils, eosinophils, basophils, lymphocytes, monocytes, P/S creatinine, lactate dehydrogenase, alkaline phosphatase, C-reactive protein |
The primary outcome measures were disease-specific disability Roland Morris Questionnaire [27] (RMDQ) and lumbar pain [28] (LBP Rating Scale). A clinically important change was defined as a 30 % reduction of the individual’s baseline score and 2 LBP rating scale points [29] (Table 1). Secondary outcome measures were global perceived effect, leg pain, hours with LBP during the last 4 weeks, EQ-5D Thermometer, days with sick leave, bothersomeness, constant pain, MRI Modic grading, serum analysis, four test at the physical examination.
Sample size
Power calculations were based on the results of a pilot study, where the patients receiving antibiotics had a median reduction of RMDQ of 3.7 [24]. A sample size of 65 in each group was calculated as necessary to detect a reduction of minimum 3.7 in the antibiotic group on the RMDQ, with a SD of the difference of four and a placebo effect of 1.4 on the RMDQ, for an alpha of 0.05 and a power of 0.90. Dropout due to side effects was estimated to be 10 %. The sample size calculation is based on a two-group comparison hence comparing antibiotics with placebo is the main aim. To fulfill a late request from the Danish Medical authorities a dose–response part was included, but the study is not designed for this tertiary purpose and therefore this is not formally tested.
Statistics
All statistics were performed by an independent professor in statistics at Aalborg University who was blinded to group allocation. Data were analyzed in STATA version 16.
Differences in binary variables were tested with Fisher’s exact test. Continuous data were analyzed with three tests. Firstly, crude an unpaired T-tests; secondly, adjusted for gender and age. Thirdly, if the distribution was not normally distributed, with the Mann–Whitney test. No interim analysis was performed. Analyses were preformed on all patients who participated at the 1-year follow-up and all patients were analyzed in their ‘intention to treat’ group, regardless of their compliance to the medicine protocol.
Ethics
The study was approved by and registered in: the Danish Medicine Agency and the Scientific Ethics Committee of the Region of Southern Denmark (no. S-VF-20050112), the Danish Data registry, Euro-dract (2005-005500-17), Government Clinical trial registry (NCT00302796) and monitored by the Unit of Good Clinical Practice (GCP), University of Southern Denmark. All patients signed a written informed consent prior to participation.
Results
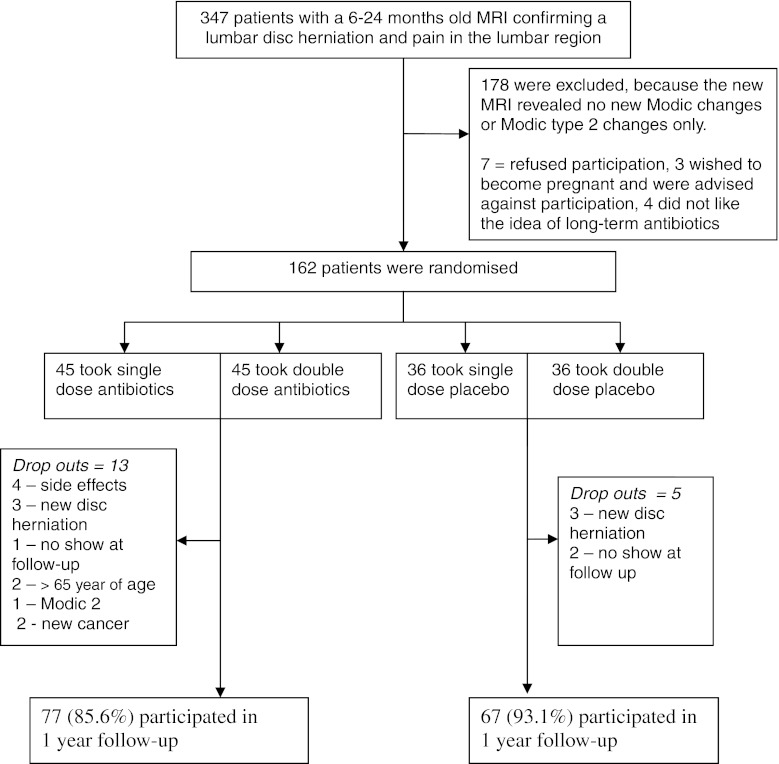
The study flow chart is shown in Fig. 1. Of the 162 patients that entered the study 147 (90.7 %) completed the end of treatment questionnaires, 144 (88.9 %) completed the 1-year follow-up (MRI, questionnaires, physical examination).
Fig. 1.
Study flow chart
All baseline variables were equally distributed in the placebo and antibiotic groups (Table 2). The only exception was the volume of Modic changes, where the placebo group had significantly more minute changes (grade 1).
Table 2.
Baseline characteristics of participants
| Antibiotics group n = 90 | Placebo group n = 72 | p value of difference | |
|---|---|---|---|
| Age (years) | 44.7 SD 10.3 | 45.5 SD 9.2 | NS |
| Gender (female) | 58.2 % | 58.2 % | NS |
| Smokers | 33 % | 32.8 % | NS |
| Number of possible endplates with Modic type one changes, adjacent to the previous herniation | 97 % | 92.2 % | NS |
| Distribution of size of Modic changes minute changes = size 1 |
10.4 % | 28.8 % | 0.007 |
| Continuous pain for more than 2 years | 52.6 % | 52.2 % | NS |
| Disease-specific disability-RMDQ Median, [lower; upper quartile] | 15 [11; 18] |
15 [12; 18] |
NS |
| Back pain (0–10) Median [lower; upper quartile] |
6.7 [5.3; 7.7] |
6.3 [4.7; 8] |
NS |
| Leg pain (0–10) Median [lower; upper quartile] |
5.3 [2.3; 7] |
4.0 [1; 7] |
NS |
| Number of hours with back pain Median [lower; upper quartile] |
448 [364; 448] |
448 [392; 448] |
NS |
| Previous disc herniation surgery | 51.9 % | 40.3 % | NS |
| Days with sick leave Mean |
51 SD 92 |
42 SD 80 |
NS |
| Bothersomeness Median, [lower; upper quartile] |
7 [6; 8] |
8 [5; 9] |
NS |
| Number with constant pain | 75.3 % | 73.1 % | NS |
| EQ-5D, thermometer Median, [lower; upper quartile] |
59 [40; 70] |
60 [40; 75] |
NS |
| Last 2 weeks pain unchanged | 75.3 % | 68.7 % | NS |
| Last 2 weeks pain worsening | 19.5 % | 23.9 % | NS |
There were no significant differences in age and gender between the 1-year participants and those lost to follow-up; however, there were significantly more smokers amongst those lost to follow-up.
The antibiotic group improved on all primary outcome measures and improvement continued from 100 days follow-up until 1-year follow-up. In comparison to the placebo group, the 1-year improvement was both statistically significant on all outcome measures and clinically important in terms of the relative magnitude of improvement for the primary outcome measures (Tables 3, 4). There were no difference between the groups in the serum analysis at baseline or follow-up (Table 5).
Table 3.
Outcome measures at baseline, 100 days follow-up and 1-year follow-up
| Antibiotic baseline n = 90 | Antibiotic 100 days n = 76 | Antibiotic 1-year follow-up n = 77 | Placebo baseline n = 72 | Placebo 100 days n = 67 | Placebo 1-year follow-up n = 67 | p value for difference between placebo and antibiotic groups at 1-year follow-up | |
|---|---|---|---|---|---|---|---|
| Disease-specific disability-RMDQ Median [lower; upper quartile] |
15.0 [11; 18] |
11.5 [7; 14] |
7.0 [4; 11] |
15.0 [12; 18] |
14.0 [11; 18] |
14.0 [8; 18] |
0.0001 |
| Back pain (0–10) Median [lower; upper quartile] |
6.7 [5.3; 7.7] |
5.0 [2.7; 6.7] |
3.7 [1.3; 5.8] |
6.3 [4.7; 8] |
6.3 [3.7; 7.7] |
6.3 [4; 7.7] |
0.0001 |
| Leg pain (0–10) Median [lower; upper quartile] |
5.3 [2.3; 7] |
3.0 [1; 5.7] |
1.7 [0; 4.2] |
4.0 [1; 7] |
4.3 [1; 7] |
4.3 [1; 6.3] |
0.0004 |
| Number of hours with back pain Median [lower; upper quartile] |
448 [364; 448] |
180 [16; 136] |
64 [4; 280] |
448 [392; 448] |
200 [28; 392] |
448 [224; 448] |
0.0001 |
| Days with sick leave last year Mean |
51.0 SD 92 |
* | 18.9 SD 61 |
42.0 SD 80 |
* | 45.4 SD 90 |
0.064 |
| Bothersomeness scale (0–10) Median [lower; upper quartile] |
7 [6; 8] |
* | 3 [2; 5] |
8 [5; 9] |
* | 6 [4; 8] |
0.0001 |
| EQ-5D (1–100) 100 is best Thermometer Median [lower; upper quartile] |
59 [40; 70] |
65 [40; 79] |
75 [54; 90] |
60 [40; 75] |
60 [40; 75] |
60 [39; 74] |
0.0014 |
| General improvement in % | * | * | 39 SD 38.4 |
* | * | 1.8 SD 31.7 |
0.0001 |
* The measurement was not obtained at 100 days follow-up
Table 4.
Outcome measures at baseline and 1-year follow-up
| Antibiotic baseline n = 90 | Antibiotic 1-year follow-up n = 77 | Placebo baseline n = 72 | Placebo 1-year follow-up n = 67 | P value for difference between placebo and antibiotic groups at 1-year follow-up | |
|---|---|---|---|---|---|
| Had low back pain | 100 % | 67.5 % | 100 % | 94.0 % | 0.0001 |
| Had constant pain | 75.3 % | 19.5 % | 73.1 % | 67.2 % | 0.0001 |
| Had disturbed sleep at night due to pain | 74.0 % | 29.9 % | 76.1 % | 61.2 % | 0.001 |
| Had pain during the Valsalva maneuver | 75.3 % | 41.6 % | 71.6 % | 56.7 % | 0.05 |
| Had pain during active flexion of the lumbar spine | 96.1 % | 49.4 % | 100 % | 83.6 % | 0.0001 |
| Had pain during active extension of the lumbar spine | 87.0 % | 51.9 % | 86.6 % | 74.6 % | 0.005 |
| Positive cranial compression test | 36.4 % | 19.5 % | 35.8 % | 34.3 % | 0.044 |
| Had pain during springing test | 92.2 % | 55.8 % | 94.0 % | 77.6 % | 0.006 |
| Consulted a doctor the follow-up year due to back pain | 23.4 % | 41.8 % | 0.002 | ||
| Compliance consuming 95–100 % of all tablets | 94.8 % | 94.0 % | NS | ||
| Observed volume volume 1, minute size | 16 | 29 | 31 | 24 | 0.05 |
| Observed volume volume 2–4, moderate/large size | 126 | 113 | 99 | 96 | 0.07 |
Table 5.
The serum analyses at baseline and end of treatment
| n = 144 | Reference values | Mean baseline value antibiotic | No. patients exceeding reference values antibiotic | Mean baseline value placebo | No. patients exceeding reference values placebo | Mean values at 1-year follow-up treatment antibiotic | No. of patients exceeding reference values antibiotic | Mean values at 1-year follow-up treatment antibiotic | No. of patients exceeding reference values antibiotic |
|---|---|---|---|---|---|---|---|---|---|
| Hemoglobin (if below 7.0) | 7.0–10.0 mmol/l | 8.8 | 0 | 8.8 | 0 | 8.7 | 0 | 8.9 | 0 |
| Erythrocytes | 3.6–5.1 | 4.6 | 6 | 4.6 | 11 | 4.5 | 5 | 4.6 | 9 |
| Erythrocytes; vol.fr | 0.34–0.44 | 0.42 | 12 | 0.42 | 19 | 0.42 | 12 | 0.42 | 13 |
| Erythrocytes; vol | 80–100 | 91 | 3 | 92 | 2 | 87 | 2 | 91 | 2 |
| Ercs (B)-Hemoglobin (fe) | 1.7–2.2 fmol | 1.9 | 10 | 2.0 | 9 | 1.9 | 1 | 1.9 | 3 |
| Leukocytes | 3.0–10.0 10E9/l | 7.0 | 3 | 7.3 | 9 | 7.4 | 25 | 7.4 | 8 |
| Neutrophils | 1.5–7.5 10E9/l | 4.2 | 1 | 4.4 | 4 | 4.5 | 4 | 4.3 | 3 |
| Eosinophils | 0.04–0.5 10E9/l | 0.15 | 1 | 0.16 | 1 | 0.2 | 4 | 0.2 | 5 |
| Basophils | <0.2 10E9/l | 0.04 | 0 | 0.03 | 0 | 0.07 | 2 | 0.04 | 0 |
| Lymphocytes | 1.0–3.5 10E9/l | 2.1 | 2 | 2.1 | 2 | 2.1 | 4 | 2.2 | 2 |
| Monocytes | 0.2–0.8 10E9/l | 0.5 | 2 | 0.5 | 4 | 0.6 | 5 | 0.6 | 8 |
| P/S Creatinine | 62–134 μmol/l | 84 | 0 | 81 | 0 | 82 | 0 | 83 | 0 |
| Lactate dehydrogenase, new method (n = 16) | 105–205 U/l | 152 | 2 | 155 | 0 | 153 | 3 | 152 | 2 |
| Alkaline phosphatase, original method (n = 13) | g 80–275 U/l | 65 | 0 | 64 | 0 | 74 | 1 | 63 | 0 |
Patients reported that pain relief and improvement in disability commenced gradually, for most patients 6–8 weeks after start of the antibiotic tablets and for some at the end of the treatment period. Improvements reportedly continued long after end of the treatment period, at least for another 6 months, and some patients reported continuing improvement at 1-year follow-up.
Modic changes
Comparing the MRI of all patients at baseline and 1-year follow-up. Out of a possible 134 adjacent endplates, 130 (97 %) of the placebo group demonstrated Modic changes and 142 out of 154 (92.2 %) in the antibiotic group. The percentage of patients with grade 1 Modic changes (minute) 28.8 % of the placebo group and 10.4 % of the antibiotic group were noted, p = 0.006. At 1-year follow-up 10 patients in both groups demonstrated no Modic changes. A significant decrease in volume was observed in the antibiotic group, where changes of volume 2–4 were reduced to volume 1 (p = 0.05). This reduction was not observed in the placebo group.
Dose response
There was a trend towards a positive dose–response relationship with double dose antibiotics appearing to be more efficacious; however, this was not statistically significant as the trial was not powered for this comparison (results not shown). For a 30 % or more reduction [29] in baseline disease-specific disability (RMDQ), the ‘number needed to treat’ in the single-dose group was 4.0 (95 % CI 2.1–11.8) and for the double dose group was 3.0 (95 % CI 1.9–5.8). For the arbitrary threshold of a 100 h reduction in ‘painful hours in the last 28 days’ the number needed to treat in the single-dose group was 3.0 (95 % CI 1.6–3.9) and for double dose group was 2.0 (95 % CI 1.4 to 2.7).
Unintended effects
Adverse events were more common in the antibiotic group (65 % of participants) compared to the placebo group (23 %). These were mainly low-grade gastroenterological complaints such as loose bowel movements, increased flatus or burping. Middle-grade events were defined as loose bowl movements lasting more than 3 weeks and were reported by 27 % in the antibiotics group and 11 % of those in the placebo group, considerable side effects in 21 and 6 %, respectively. One case of a serious adverse (severe vomiting resulting in blood in the vomit) event was reported in the placebo group and none in the antibiotic group. No difference in the number of adverse effects was observed between the double and single-dose antibiotics.
Discussion
This double-blinded RCT investigating the efficacy of Modic antibiotic spine therapy (MAST) for CLBP patients with Modic type 1 changes in the adjacent vertebral endplates, demonstrated statistically and clinically significant improvements in all outcome measures while patients receiving placebo treatment experienced no or minimal improvement. For the primary outcome measures, disease-specific disability and lumbar pain, the effect magnitude was also clinically significant. The comparative effect was highly statistically significant for all other secondary outcome measures including: leg pain, global perceived health, number of days with pain, reduced number with chronic pain, physical examination tests, and MRI Modic grading There was a trend towards a dose–response relationship, with double dose antibiotics being more efficacious. The decrease in volume of the lesions in the antibiotic group seen in the follow-up MRI scans perhaps suggests resolution.
Perhaps most encouraging for further work in this area has been the finding that the improvements obtained with the current MAST protocol, in this group of traditionally resistant chronic low back pain patients, has been substantially greater than those described with all other established conservative treatments [3].
On all outcome measures the improvement seen in the antibiotic group at 1-year follow-up was approximately twice that observed at the end of the 100-day treatment period. This could be interpreted as reflecting a biological healing process that starts only when and after the bacteria have been killed.
Proprionibacterium acnes bacteria secrete propionic acid, which has the capacity to dissolve fatty bone marrow and bone. We hypothesize that diffusion of propionic acid from the disc into the vertebrae causes the Modic changes. Similarly, as increased TNF-alfa and the growth of PGP-5 unmyelinated nerve fibers have been reported in Type 1 Modic changes [30], with the inherent slowness of these pathological processes perhaps explaining the delayed onset of improvement observed in this study.
The reduction of leg pain observed in the antibiotic group was surprising as leg pain is usually ascribed to the compression of the nerve root of the lumbar region not inflammatory changes in the vertebrae. Explanations for this pain reduction include: an amelioration of somatic pain referred from the disc or a diminution of infectious by products from the disc capable of irritating the nerve roots.
The placebo group demonstrated minimal or no improvement on most outcome measures in this trial. This reinforces our clinical impression, gained from years of working with CLBP patients with Modic changes that ‘Modic pain’ is difficult to treat effectively with conservative treatment methods [31]. In the pilot study [24], participants receiving antibiotics expressed appreciation that their hours with pain were reduced. Similarly, although many still had morning pain, it was of reduced intensity. In the current study, participants commonly reported 16 waking hours of pain at the beginning of the trial and 2–3 waking hours of pain 1 year later, if they had received antibiotics.
Many antibiotics have an anti-inflammatory effect, via TNFα-inhibition. However, amoxicillin–clavulanate (Bioclavid) has been shown to have a very small anti-inflammatory effect comparable to other antibiotics [32, 33] and only an inhibitory effect on IL-1 and IL-8, not TNFα which is present in the Modic changes. This was an additional reason for the selection of amoxicillin–clavulanate (Bioclavid) in this study. Normally, anti-inflammatory effects are rapid quite fast-acting, whereas in this study the effect took 6–8 weeks to manifest, more consistent with the clinical course of resolving infection in poorly vascularised infected tissue, i.e., an antibiotic effect.
A possible bias could be that the improvement in the antibiotic group could be a result of additional helpful treatment by the GP or specialists doctors. However. the results demonstrated the opposite. At 1-year follow-up 23.4 % of the patients in the Bioclavid group had consulted a doctor for back pain compared to 41.8 % in the placebo group. This likely indicates that the placebo group was less satisfied with their result and therefore sought additional help.
A significantly larger percentage of patients in the placebo group demonstrated small volume grade 1 Modic changes at baseline which would lead one to expect a more favorable outcome at follow-up. This further emphasizes the power of the effect seen in the antibiotic group. It has been shown that the minute volume 1 Modic changes often disappear. 52 % were no longer visible in comparison to 15 % of grade 2–4 over a 4-year period [34]. For practical reasons the patients had MRI shortly before the 1-year follow-up. The patients had taken the antibiotics for 100 days and this meant the period from the end of treatment to follow-up MRI was only 8–8.5 months. Ideally follow-up MRI evaluation should be carried out at a much later date. Several studies have shown that after discitis or osteomyelitis treated with antibiotics even though the patients improve on all clinical outcome measures there were no improvements observed in the bone edema, indeed, some appeared to worsen [35–37]. Carragee wrote “Follow-up magnetic resonance images often gave impressions of progressive disease, where the clinical picture appeared to improve” [35]. Studies have shown even 25 months after treatment the bone edema had not healed [36]. To substitute conventional MRI new research has shown promising results with of DCE-MRI (dynamic contrast enhanced MRI), where an observed steep gadolinium contrast curve is believed to represent active inflammation (active bone edema), compared to a flat gadolinium uptake curve which represents non-inflammation (passive bone edema) [38]. The changes seen in the follow-up MRI scans in this study are compatible with the conclusion that the clinical gains reflect a true antimicrobial effect.
Only a very limited number of patients had increased serum inflammatory markers. This was expected, as the infection has a low virulence and the disc is an avascular structure. The result is in accordance with the pilot study [24] and others concerning spondylodiscitis due to infections caused by P. acne bacteria [39].
The trend favoring double dose antibiotics not reaching significance is likely to reflect a classical type two error, comparing two very active treatments. Such a study requires greater numbers in each group.
To the best of our knowledge, this is the first RCT studying the effect of antibiotic treatment in CLBP. The effects reported here in Modic type 1 (bone edema) patients with CLBP are large and have been observed in patients following lumbar disc herniation with and without surgery. The extent to which the results can be generalized to Modic changes types 2 and 3 is not presently known, though it is hypothesized that Type 1 and Type 2 Modic changes are different stages in the same pathological process [5].
High-dose long-term antibiotics should not be prescribed without due consideration. Clearly in a condition as common as CLBP there is a potential community as well as individual hazard if used indiscriminately. However, as many patients, as in this trial, are on sick leave, at risk of losing their jobs and have a high analgesic intake, we suggest that antibiotics, when applied along the lines of this MAST protocol may be appropriate in this subgroup, i.e., CLBP with Modic type 1 changes. We do not support the proposition that all patients with lumbar pain should have a trial course of antibiotics. The criteria in this study were very clear: CLBP for more than 6 months, Modic type 1 changes in the adjacent vertebrae following a previous disc herniation. As we do with other drugs, we rely on our fellow colleagues to use clear evidence-based criteria and to avoid excessive antibiotic use. We also suggest more confirmatory work in other populations, for example, Modic type 2 changes, and studies on improved protocols should be supported.
Conclusions
In this double-blind RCT patients with CLBP and Modic type 1 change (bone edema) following a lumbar disc herniation, who were treated with antibiotics obtained statistically significant improvements compared to the placebo group in all measured parameters, including: the primary outcome of disease-specific disability, back pain intensity, and the secondary outcomes of, leg pain intensity, general improvement, number of hours with pain, reduced number with chronic pain, physical examination tests, and MRI Modic grading. Only four participants (2.8 %) stopped treatment due to side effects, which were mostly gastroenterointestinal.
For the primary outcome measures, the effect size was clinically important in magnitude and substantially greater than all currently established treatments.
Antibiotics could be considered as a treatment option for this special subgroup of patients with CLBP and Modic type 1 changes after a lumbar disc herniation when all other treatment options have failed. More confirmatory work in other populations and studies on improved protocols as well as the background science should be encouraged.
Acknowledgments
Grant support was received from IMK general foundation, The Danish Rheumatism Association, Svend Hansen and Ina Hansens Foundation, Ib Henriksen Foundation, Dagmar Marshalls Foundation, Karen Hansen Memory Foundation, Ing. K.A. Rohde and Wife’s foundation. The funders had no role in the study design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, approval of, or decision to publish the manuscript. We would like to thank Alan Jordan Ph.D. for editorial assistance.
Conflict of interest
None.
References
- 1.Jensen TS, Karppinen J, Sorensen JS, Niinimäki J, Leboeuf-Yde C. Prevalence of vertebral endplate signal (Modic) changes and their association with non-specific low back pain—A systematic literature review. Eur Spine J. 2008;17:1407–1422. doi: 10.1007/s00586-008-0770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert HB, Manniche C. Modic changes following lumbar disc herniation. Eur Spine J. 2007;16:977–982. doi: 10.1007/s00586-007-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Airaksinen O, Brox JI, Cedraschi C, et al. European guidelines: COST B13 working group on guidelines for chronic low back pain. Eur Spine J. 2006;15(Suppl 2):S192–S300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Modic MT, Masaryk TJ, Ross JS, Carter JR. Imaging of degenerative disk disease. Radiology. 1988;168:177–186. doi: 10.1148/radiology.168.1.3289089. [DOI] [PubMed] [Google Scholar]
- 5.Modic MT, Steinberg PM, Ross JS, Masaryk TJ, Carter JR. Degenerative disk disease: assessment of changes in vertebral body marrow with MR imaging. Radiology. 1988;166:193–199. doi: 10.1148/radiology.166.1.3336678. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, Videman T, Niemeläinen R, Battié MC. Quantitative measures of Modic changes in lumbar spine magnetic resonance imaging: intra- and inter-rater reliability. Spine. 2011;36:1236–1243. doi: 10.1097/BRS.0b013e3181ecf283. [DOI] [PubMed] [Google Scholar]
- 7.Peterson CK, Gatterman B, Carter JC, Humphreys BK, Weibel A. Inter- and intraexaminer reliability in identifying and classifying degenerative marrow (Modic) changes on lumbar spine magnetic resonance scans. J Manipulative Physiol Ther. 2007;30:85–90. doi: 10.1016/j.jmpt.2006.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TS, Sorensen JS, Kjaer P. Intra- and interobserver reproducibility of vertebral endplate signal (modic) changes in the lumbar spine: the Nordic Modic Consensus Group classification. Acta Radiol. 2007;48:748–754. doi: 10.1080/02841850701422112. [DOI] [PubMed] [Google Scholar]
- 9.Albert HB, Kjaer P, Jensen TS, Sorensen JS, Bendix T, Manniche C. Modic changes, possible causes and relation to low back pain. Med Hypotheses. 2008;70:361–368. doi: 10.1016/j.mehy.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 10.Stirling A, Worthington T, Rafiq M, Lambert PA, Elliott TS. Association between sciatica and Propionibacterium acnes. Lancet. 2001;357:2024–2025. doi: 10.1016/S0140-6736(00)05109-6. [DOI] [PubMed] [Google Scholar]
- 11.Stirling AJ, Jiggins M. Association between Sciatica and Skin Commensals. Cleveland: International Society for the Study of the Lumbar Spine; 2002. [Google Scholar]
- 12.Corsia MF, Wack M, Denys G (2003) Low vitulence Bacterial infections of intervertebral discs and the resultant spinal disease processes. Abstract from Scoliosis Research Society (SRS) annual meeting
- 13.Agarwal VJ, Golish R, Kondrashov D, Alamin TF. Results of bacterial culture from surgically excised intervertebral disc in 52 patients undergoing primary lumbar disc microdiscectomy at a single level. Spine J. 2010;10:S45–S46. doi: 10.1016/j.spinee.2010.07.126. [DOI] [Google Scholar]
- 14.Bhanji S, Williams B, Sheller B, Elwood T, Mancl L. Transient bacteremia induced by tooth brushing a comparison of the Sonicare toothbrush with a conventional toothbrush. Pediatr Dent. 2002;24:295–299. [PubMed] [Google Scholar]
- 15.Roberts GJ, Holzel HS, Sury MR. Dental bacteremia in children. Pediatr Cardiol. 1997;18:24–27. doi: 10.1007/s002469900103. [DOI] [PubMed] [Google Scholar]
- 16.Farrar MD, Ingham E. Acne: inflammation. Clin Dermatol. 2004;22:380–384. doi: 10.1016/j.clindermatol.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 17.Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235–241. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 18.Hirabayashi S, Kumano K, Tsuiki T, Eguchi M, Ikeda S. A dorsally displaced free fragment of lumbar disc herniation and its interesting histologic findings. A case report. Spine. 1990;15:1231–1233. doi: 10.1097/00007632-199011010-00026. [DOI] [PubMed] [Google Scholar]
- 19.Ito T, Yamada M, Ikuta F, et al. Histologic evidence of absorption of sequestration-type herniated disc. Spine. 1996;21:230–234. doi: 10.1097/00007632-199601150-00014. [DOI] [PubMed] [Google Scholar]
- 20.Lindblom K, Hultquist G. Absorption of protruded disc tissue. J Bone Joint Surg. 1950;32:557–560. [PubMed] [Google Scholar]
- 21.Gronblad M, Virri J, Tolonen J, et al. A controlled immunohistochemical study of inflammatory cells in disc herniation tissue. Spine. 1994;19:2744–2751. doi: 10.1097/00007632-199412150-00002. [DOI] [PubMed] [Google Scholar]
- 22.Wedderkopp N, Thomsen K, Manniche C, Kolmos HJ, Secher Jensen T, Leboeuf Yde C. No evidence for presence of bacteria in Modic type I changes. Acta Radiol. 2009;50:65–70. doi: 10.1080/02841850802524485. [DOI] [PubMed] [Google Scholar]
- 23.Albert HB, Rollason J, Lambert P et al. Is the herniated nucleus material in lumbar disc herniations infected with bacteria, and does the infection cause Modic changes in the surrounding vertebrae? (Submitted to European Spine)
- 24.Albert HB, Manniche C, Sorensen JS, Deleuran BW. Antibiotic treatment in patients with low-back pain associated with Modic changes Type 1 (bone oedema): a pilot study. Br J Sports Med. 2008;42:969–973. doi: 10.1136/bjsm.2008.050369. [DOI] [PubMed] [Google Scholar]
- 25.Solgaard Sorensen J, Kjaer P, Jensen ST, Andersen P. Low-field magnetic resonance imaging of the lumbar spine: reliability of qualitative evaluation of disc and muscle parameters. Acta Radiol. 2006;47:947–953. doi: 10.1080/02841850600965062. [DOI] [PubMed] [Google Scholar]
- 26.Housden PL, Sullivan MF. Do augmentin or cefuroxime reach effective levels in lumbar vertebral discs when used prophylactically for discectomy? A preliminary report. Eur Spine J. 1993;2:145–148. doi: 10.1007/BF00301412. [DOI] [PubMed] [Google Scholar]
- 27.Albert HB, Jensen AM, Dahl D, et al. Criteria validation of the Roland Morris questionnaire. A Danish translation of the international scale for the assessment of functional level in patients with low back pain and sciatica. Ugeskr Laeger. 2003;165:1875–1880. [PubMed] [Google Scholar]
- 28.Manniche C, Asmussen K, Lauritsen B, et al. Low back pain rating scale: validation of a tool for assessment of low back pain. Pain. 1994;57:317–326. doi: 10.1016/0304-3959(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 29.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain towards international consensus regarding minimal important change. Spine. 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 30.Ohtori S, Inoue G, Ito T, Koshi T, et al. Tumor necrosis factor-immunoreactive cells and PGP 9.5-immunoreactive nerve fibers in vertebral endplates of patients with discogenic low back Pain and Modic Type 1 or Type 2 changes on MRI. Spine. 2006;31:1026–1031. doi: 10.1097/01.brs.0000215027.87102.7c. [DOI] [PubMed] [Google Scholar]
- 31.Jensen RK, Leboeuf-Yde C, Wedderkopp N, Sorensen JS, Manniche C. Rest versus exercise as treatment for patients with low back pain and Modic changes. A randomized controlled clinical trial. BMC Med. 2012;10:22. doi: 10.1186/1741-7015-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hahm KB, Lee KJ, Kim YS, et al. Quantitative and qualitative usefulness of reamipide in eradication regimen of Helicobacter pylori. Dig Dis Sci. 1998;43:192S–197S. doi: 10.1023/A:1018825532059. [DOI] [PubMed] [Google Scholar]
- 33.Ziegeler S, Raddatz A, Hoff G, et al. Antibiotics modulate the stimulated cytokine response to endotoxin in a human ex vivo, in vitro model. Acta Anaesthesiol Scand. 2006;50:1103–1110. doi: 10.1111/j.1399-6576.2006.01112.x. [DOI] [PubMed] [Google Scholar]
- 34.Jensen TS, Bendix T, Sorensen JS, Manniche C, Korsholm L, Kjaer P. Characteristics and natural course of vertebral endplate signal (Modic) changes in the Danish general population. BMC Musculoskelet Disord. 2009;3(10):81. doi: 10.1186/1471-2474-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Carragee EJ. The clinical use of magnetic resonance imaging in pyogenic vertebral osteomyelitis. Spine. 1997;22:780–785. doi: 10.1097/00007632-199704010-00015. [DOI] [PubMed] [Google Scholar]
- 36.Kowalski TJ, Layton KF, Berbari EF, Steckelberg JM, Huddleston PM, Wald JT, Osmon DRAJNR. Follow-up MR imaging in patients with pyogenic spine infections: lack of correlation with clinical features. Am J Neuroradiol. 2007;28:693–699. [PMC free article] [PubMed] [Google Scholar]
- 37.Hsu CY, Yu CW, Wu MZ, Chen BB, Huang KM, Shih TT. Unusual manifestations of vertebral osteomyelitis: intraosseous lesions mimicking metastases. AJNR Am J Neuroradiol. 2008;29:1104–1110. doi: 10.3174/ajnr.A1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boesen M, Kubassova O, Bouert R, Axelsen MB, Ostergaard M, Cimmino MA, Danneskiold-Samsøe B, Hørslev-Petersen K, Bliddal H (2011) Correlation between computer-aided dynamic gadolinium-enhanced MRI assessment of inflammation and semi-quantitative synovitis and bone marrow oedema scores of the wrist in patients with rheumatoid arthritis–a cohort study. Rheumatology (epub ahead) [DOI] [PubMed]
- 39.Uçkay I, Dinh A, Vauthey L, Asseray N, Passuti N, Rottman M, Biziragusenyuka J, Riché A, Rohner P, Wendling D, Mammou S, Stern R, Hoffmeyer P, Bernard L. Spondylodiscitis due to Propionibacterium acnes: report of twenty-nine cases and a review of the literature. Clin Microbiol Infect. 2010;16:353–358. doi: 10.1111/j.1469-0691.2009.02801.x. [DOI] [PubMed] [Google Scholar]
- 40.Møller Pedersen K, Wittrup-Jensen K, Brooks R, Gudex C. Vaerdisaetning af sundhed. Odense: University of Southern Denmark Publishing; 2003. p. 256. [Google Scholar]
- 41.Dunn KM, Croft PR. Classification of low back pain in primary care: using “bothersomeness” to identify the most severe cases. Spine. 2005;30:1887–1892. doi: 10.1097/01.brs.0000173900.46863.02. [DOI] [PubMed] [Google Scholar]