Abstract
Introduction
Long-acting beta-agonists (LABA) and/or inhaled corticosteroids (ICS) have been shown to reduce COPD exacerbation risk. Using data from a large integrated health-care system, we sought to examine whether these medication classes were initiated after an exacerbation of COPD.
Methods
We identified patients who experienced an inpatient or outpatient COPD exacerbation within the Veterans Affairs Integrated Service Network (VISN)-20. We assessed the addition of a new inhaled therapy (an ICS, LABA or both) within 180 days after the exacerbation. We assessed independent predictors of adding treatment using logistic regression.
Results
We identified 45,780 patients with COPD, of whom 2,760 patients experienced an exacerbation of COPD. Of these individuals, 2,570 (93.1 %) were on either none or only one long-acting medication studied (LABA or ICS). In the subsequent 180-day period after their exacerbation, only 875 (34.1 %) patients had at least one of these additional therapies dispensed from a VA pharmacy. Among patients who were treated in the outpatient setting, older age [OR 0.98/year, 95 % CI (0.97–0.99)], current tobacco use [OR 0.74, 95 % CI (0.60–0.90)], greater use of ipratropium bromide [OR 0.97/canister, 95 % CI (0.96–0.98)], prior COPD exacerbation [OR 0.55, 95 % CI (0.46–0.67)], depression [OR 0.77, 95 % CI (0.61–0.98)], CHF [OR 0.74, 95 % CI (0.57–0.97)], and diabetes (OR 0.77 (0.60–0.99)] were associated with lower odds of additional therapy. Patients who were treated in the hospital had similar associated predictors.
Conclusion
Among patients treated for an exacerbation of COPD, we found relatively few were subsequently prescribed inhaled therapies known to reduce exacerbations.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2276-1) contains supplementary material, which is available to authorized users.
KEY WORDS: COPD, exacerbation, inhaled medication use
INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a highly prevalent disease affecting up to 10 % of people over the age of 40.1 Patients who suffer from COPD exacerbations have lower health-care related quality of life compared to those with similar disease severity who do not,2 and each exacerbation causes a decline in lung function.3 Multiple randomized controlled trials have demonstrated that long-acting beta agonists (LABAs) and inhaled corticosteroids (ICSs) improve symptoms and decrease the frequency of COPD exacerbations.4–6 The risk of a subsequent COPD exacerbation is increased in the 8-week period immediately following an episode,7 suggesting that the period immediately following an exacerbation represents a potential opportunity for disease modifying interventions.
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines were first published in 2001 and have been updated frequently.8,9 This guideline suggests adding inhaled therapies in a step-wise fashion based on clinical history, presence of COPD exacerbations, and pulmonary function testing. The severity of COPD is characterized as stage I, mild (FEV1 ≥ 80 % predicted); stage II, moderate (50 % ≤ FEV1 <80 % predicted); stage III, severe (30 % ≤ FEV1 <50 % predicted); and stage IV, very severe (FEV1 < 30 % predicted or <50 % predicted with respiratory failure). For all disease stages a short-acting beta agonist (SABA) as needed is recommended for symptoms, with the addition of a LABA or long-acting muscarinic antagonist (LAMA) at stage II or greater. ICSs are recommended for stage III or greater if the patient has recurrent exacerbations.10,11
Other guidelines like the 2004 American Thoracic Society guidelines recommend adding further medication classes such as LABA or LAMA based on uncontrolled symptoms, with the addition of an ICS for any patient with FEV1 < 50 % predicted with an exacerbation in the past year.12 The Towards a Revolution in COPD Health (TORCH) trial studied the effect of LABA, ICS, and the combination on a number of factors, and it included patients with FEV1 <60 %, overlapping with GOLD stage II and III. It did not demonstrate a survival benefit for inhaled therapies, but did show that fluticasone alone, salmeterol alone, and both used in combination decreased the yearly exacerbation rate,13 and this result has been replicated in other studies.14
While these guidelines and data are available to clinicians, it is unknown to what extent clinicians adhere to these recommendations after a COPD exacerbation. Studies of guideline adherence in other disease states such as congestive heart failure have shown moderate rates of adherence of about 60 %,15 with improved guideline adherence associated with decreased hospitalizations and cost.16
The goal of this study was to examine the variability in the prescription of additional inhaled therapies for COPD in the period following a COPD exacerbation.
METHODS
Design, Setting and Participants
We conducted a nested cohort study of patients experiencing an exacerbation among a larger cohort of patients with COPD receiving care within the VA Integrated Service Network (VISN)-20.
Data Source
We used clinical information from the VISN-20 data warehouse that routinely collects data using the VA electronic medical record. This includes demographics, prescription medications, office visits, inpatient admissions, inpatient and outpatient diagnoses, and pulmonary function testing when available.
Cohort Development: Inclusion and Exclusion Criterion
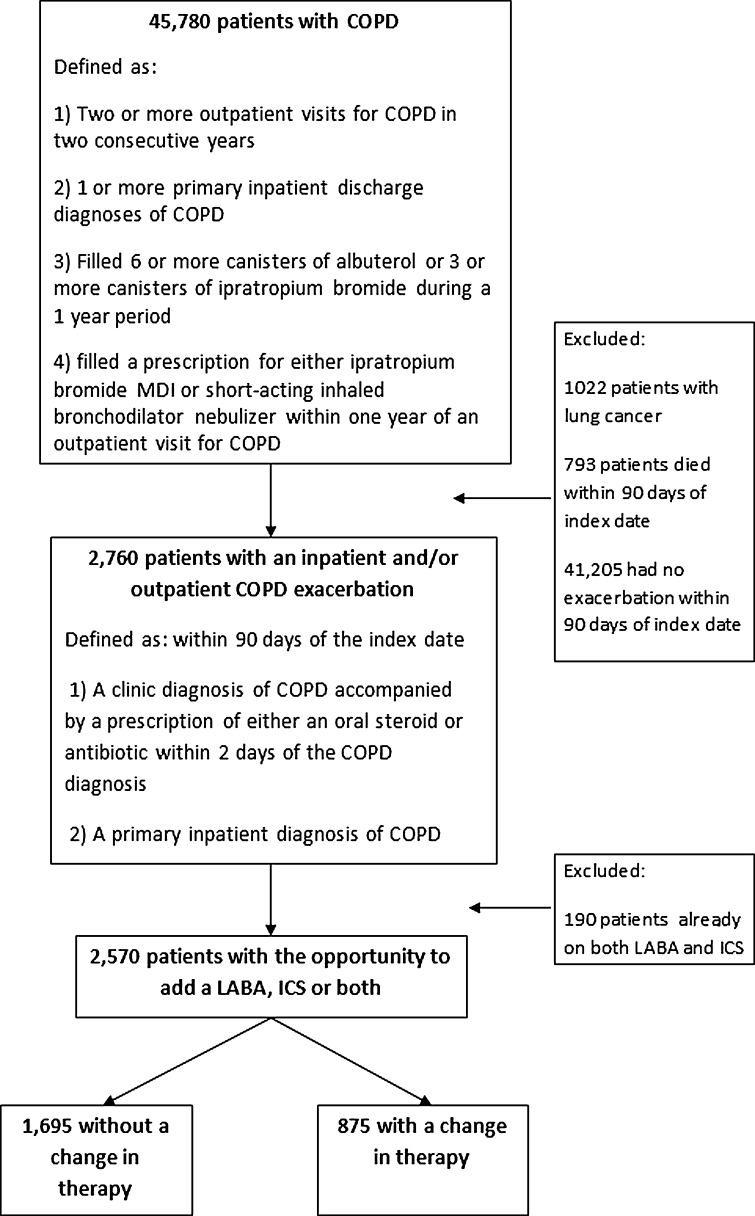
We developed our cohort in multiple steps by first defining a parent cohort of patients with COPD and then identifying those participants who had an exacerbation within the first 90 days after entry into the cohort (Fig. 1). To define the parent cohort, we used a validated algorithm combining ICD-9 diagnosis codes (491.X, 492.X , 493.2, 496.X) and inhaled medication use to identify patients with a diagnosis of COPD receiving care at any of the VISN-20 medical centers between January 2001 and December 2006.17 Specifically, to be included in the cohort, patients were required to have one or more of the following criteria: two or more outpatient visits for COPD in two consecutive years, one or more primary inpatient discharge diagnoses of COPD, filled six or more canisters of albuterol or three or more canisters of ipratropium bromide during a 1-year period, or filled a prescription for either ipratropium bromide MDI or short-acting inhaled bronchodilator nebulizer within 1 year of an outpatient visit for COPD. A similar method has been used with good success using the VA electronic health record to identify COPD registries for quality improvement and research.18 We defined entry into the cohort (index date) as the date when the patient met entry criteria.
Figure 1.
Study cohort.
Second, because we were interested in examining clinician prescribing practice after exacerbation, we identified those patients who experienced an inpatient and/or outpatient COPD exacerbation within the first 90 days after entry into the parent cohort. We defined an inpatient COPD exacerbation as a primary ICD-9 discharge diagnosis of COPD (an approach that has been shown to have an extremely high specificity)16 and an outpatient COPD exacerbation as a visit with a diagnosis of COPD accompanied by a prescription of either an oral steroid or an antibiotic within 2 days of the COPD diagnosis. This combination of ICD-9 codes and pharmacy data to identify COPD exacerbations is similar to that used in previous studies.14 Third, since we were interested in newly prescribed medications, we excluded patients who were previously dispensed both a LABA and an ICS. We did not exclude patients on tiotroprium, but did not include this medication in our analysis because of its limited use within the VA during the study period. Lastly, we excluded patients with comorbid lung cancer or death within 90 days of the index date.
Outcome Assessment
Our outcome measure was whether patients were dispensed new prescriptions for ICS, LABA or both in the 6-month period after their COPD exacerbation.
Potential Predictors of Change in Therapy
In the 1 year prior to their exacerbation, we collected patient information that we conceptually grouped into one of three categories: (1) demographics and health behaviors (age, BMI, race, smoking status within the past year, and the number of missed appointments); (2) comorbid conditions (congestive heart failure, hypertension, acute coronary syndrome, diabetes, and depression), and (3) markers of disease severity (history of COPD exacerbation within the past year, canisters of SABA filled, and canisters of ipratroprium filled).
Statistical Analysis
Bivariate analysis was performed using the appropriate parametric or non-parametric analyses. We developed multivariable logistic regression models to assess predictors of change in care, with separate models for each group of potential patient predictors described above. To create a parsimonious final adjusted model, variables that achieved p ≤ 0.1 in the preliminary models were added to the final model. We performed separate analyses based on whether patients had had an inpatient or outpatient exacerbation.
Sensitivity Analysis
A priori, we defined a second cohort of patients undergoing pulmonary function testing at one of three VISN 20 medical centers located throughout the Pacific Northwest between January 2003 and December 2007. For this second cohort, we identified patients with a diagnosis of COPD based upon a post-bronchodilator FEV1/FVC ratio of <0.70.19 We defined entry (index date) as the date on which the results of the first pulmonary function test (PFT) were recorded. We followed the same steps as above to further define the cohort of interest, outcome and exposure assessment, and analysis plan.
RESULTS
We identified 45,780 patients who met our definition of COPD, 10,728 (23.4 %) of whom were on a LABA and/or ICS in the year prior to cohort entry. Of these patients, 2,760 experienced an inpatient and/or outpatient COPD exacerbation; 190 (6.9 %) were already treated with both a LABA and ICS and were excluded. The remaining 2,570 (93.1 %) were on one or no long-acting medications (Fig. 1). Of these 2,570 patients, 644 (25.1 %) were treated with one long-acting agent, and 1,926 (74.9 %) were on neither LABA nor ICS. Of these 2,570 patients, 1,913 patients had an outpatient exacerbation only, 478 patients had an inpatient exacerbation only, and 179 had both an inpatient and outpatient exacerbation, resulting in a total of 2,092 outpatient exacerbations and 657 inpatient exacerbations. Of those 2,092 patients treated in the outpatient setting, in the year prior to study entry 1,520 (72.7 %) patients had received neither ICS nor LABA, 511 patients (24.4 %) had received a prescription for an ICS, and 61 (2.9 %) had received a prescription for a LABA. Likewise, among the 657 patients who experienced an inpatient exacerbation, in the year prior to study entry 532 (81.0 %) of patients had received neither an ICS nor a LABA, 109 (16.6 %) had received an ICS, and 16 (2.4 %) had received a LABA prescription.
As seen in Table 1, our cohort of patients experiencing a COPD exacerbation consisted of older, predominantly white males with a significant history of tobacco use within the past year and significant number of comorbid illnesses. Among all patients with an exacerbation and the opportunity for an additional long-acting medication to be added, 875 (34.0 %) had a change in therapy in the 6 months following their exacerbation. In bivariate analyses (Table 1), patients were less likely to have medication added to their regimen if they were older, used greater amounts of short-acting bronchodilators, had an exacerbation in the year prior, and had a greater number of comorbid conditions, specifically hypertension, depression, diabetes, and congestive heart failure.
Table 1.
Characteristics of 2,570 Patients with an Exacerbation of COPD by Whether or Not They Had the Addition of an ICS and/or LABA in the Subsequent 6 Months
| Characteristic | Patients without a change in therapy (N = 1695) | Patients with a change in therapy (N = 875) | P-value |
|---|---|---|---|
| Age (years, μ, SD) | 66.6 (11.2) | 64.6 (10.4) | p < 0.01 |
| Body mass index (μ, SD) | 28.2 (6.4) | 28.3 (6.4) | p = 0.71 |
| Number of missed appointments (μ, SD) | 0.2 (0.7) | 0.2 (0.6) | p = 0.09 |
| Number of ipratroprium canisters (μ, SD) | 4.6 (9.3) | 3.1 (6.3) | p < 0.01 |
| Number of SABA canisters (μ, SD) | 4.9 (8.9) | 4.0 (7.4) | p < 0.01 |
| Race (%) | |||
| White | 71.0 | 69.4 | p = 0.64 |
| Other | 3.5 | 3.4 | |
| Unknown/not provided | 25.5 | 27.2 | |
| Male (%) | 96.9 | 96.8 | p = 0.92 |
| Smoker in the previous year (%) | 38.9 | 36.0 | p = 0.15 |
| History of CHF (%) | 24.1 | 15.2 | p < 0.01 |
| History of ACS (%) | 4.5 | 3.5 | p = 0.26 |
| History of HTN (%) | 54.6 | 47.3 | p < 0.01 |
| History of diabetes (%) | 22.1 | 16.3 | p < 0.01 |
| History of depression (%) | 23.5 | 18.1 | p < 0.01 |
| History of exacerbation in year prior (%) | 65.0 | 52.8 | p < 0.01 |
ICS inhaled corticosteroid; LABA long-acting beta-agonist; SABA short acting beta agonists; CHF congestive heart failure; ACS acute coronary syndrome; HTN hypertension
Change in Therapy after COPD Exacerbation
Among the 2,092 patients who experienced an outpatient exacerbation with the opportunity to add at least one medication, relatively few patients had the addition of one or more therapies in the subsequent 6 months. In total, 739 (35.3 %) patients were prescribed new medication. Of those patients, 148 (20 %) had the addition of LABA, 359 (48.6 %) had the addition of ICS, and 232 (31.4 %) had both medications added.
We found similar results for patients treated in the inpatient setting. Among the 657 patients, 227 (34.6 %) had the addition of an ICS, LABA, or both. Of those patients, 45 (19.8 %) had the addition of a LABA, 111 (48.9 %) had the addition of an ICS, and 71 (31.3 %) had both medications added.
Because the literature and guidelines recommend adding LABA and then ICS specifically for recurrent exacerbations, we repeated the analysis for both groups stratified by the occurrence of an exacerbation in the year prior to index date and found the same prescribing patterns (Appendix Table 3 available online).
Predictors of Receiving Additional Therapies
We did not find any predictors associated with increased odds of receiving additional long-acting inhaled therapy among either group of patients. Among patients treated for an outpatient exacerbation, older age, current tobacco use, higher use of ipratropium bromide, history of a prior COPD exacerbation in the past year, depression, or diabetes were associated with lower odds of receiving an ICS, LABA, or both in our final model (see Table 2). Base models for the individual groups of predictors can be found in Appendix Table 1 (available online).
Table 2.
Final Model: Predictors of Receiving New Inhaled Therapies (LABA and/or ICS) Among 2,092 Patients with an Outpatient Exacerbation
| Variable | Patients without change in therapy N = 1353 | Patients with change in therapy N = 739 | Odds ratio to obtain new drug (95 % CI) | P-value |
|---|---|---|---|---|
| Age (each year older), μ (SD) | 66.4 (11.0) | 64.5 (10.4) | 0.98 (0.97, 0.99) | <0.01 |
| Smoker within the past year, n | n = 546 | n = 270 | 0.74 (0.60,0.90) | <0.01 |
| Prior COPD exacerbation, n | n = 818 | n = 353 | 0.55 (0.46, 0.67) | <0.01 |
| No. of ipratroprium canisters, μ (SD) | 5.1 (9.6) | 3.4 (6.2) | 0.97 (0.96,0.98) | <0.01 |
| Congestive heart failure, n | n = 269 | n = 99 | 0.74 (0.57,0.97) | 0.03 |
| Depression, n | n = 306 | n = 139 | 0.77 (0.61,0.98) | 0.03 |
| Diabetes, n | n = 281 | n = 113 | 0.77 (0.60, 0.99) | 0.04 |
| Hypertension, n | n = 725 | n = 347 | 0.92 (0.76,1.11) | 0.37 |
The three models used to construct the final model were categorized by demographics and health behaviors, comorbid conditions, and markers of disease severity; these included age, BMI, race, smoking status within the past year, number of missed appointments, history of congestive heart failure, hypertension, acute coronary syndrome, diabetes, or depression, history of COPD exacerbation within the past year, canisters of short-acting beta-agonists (SABA) filled, and canisters of ipratroprium filled. See Appendix Table 1 (available online) for details
Among individuals who had an inpatient exacerbation and the opportunity for escalation in therapy, several similar factors were associated with a decreased odds of receiving an ICS, LABA, or both: age, history of prior exacerbation, CHF, or depression (see Table 3). Base models for the individual groups of predictors can be found in Appendix Table 2 (available online).
Table 3.
Final Model: Predictors of Receiving New Inhaled Therapies (LABA and/or ICS) Among 657 Patients with an Inpatient Exacerbation
| Variable | Patients without change in therapy N = 430 | Patients with change in therapy N = 227 | Odds ratio to obtain new drug (95 % CI) | P-value |
|---|---|---|---|---|
| Age (each year older), μ (SD) | 67.7 (11.3) | 64.6 (10.5) | 0.97 (0.96, 0.99) | <0.01 |
| Race, n | ||||
| White | n = 316 | n = 159 | ||
| Other race | n = 16 | n = 6 | 0.61 (0.23, 1.64) | 0.33 |
| Unknown/not provided | n = 98 | n = 62 | 1.19 (0.81, 1.75) | 0.38 |
| Prior COPD exacerbation, n | n = 347 | n = 167 | 0.60 (0.40, 0.91) | 0.02 |
| No. of ipratroprium canisters, μ (sd) | 3.9 (9.2) | 3.0 (7.5) | 0.99 (0.96, 1.01) | 0.22 |
| Congestive heart failure, n | n = 172 | n = 50 | 0.46 (0.31,0.67) | <0.01 |
| Depression, n | n = 116 | n = 32 | 0.38 (0.24, 0.60) | <0.01 |
| Missed appointments >1 | n = 24 | n = 7 | 0.53 (0.21,1.30) | 0.16 |
The three models used to construct the final model were categorized by demographics and health behaviors, comorbid conditions, and markers of disease severity; these included age, BMI, race, smoking status within the past year, number of missed appointments, history of congestive heart failure, hypertension, acute coronary syndrome, diabetes, or depression, history of COPD exacerbation within the past year, canisters of short-acting beta-agonists (SABA) filled, and canisters of ipratroprium filled. See Appendix Table 2 (available online) for details
Sensitivity Analysis
In order to assess whether our cohort definition may have selected for patients at risk for lower acquisition of medications, we analyzed an additional cohort of patients who had spirometry as part of routine clinical care. Among our validation cohort of 333 patients who had undergone pulmonary function testing and experienced an outpatient exacerbation, 82 (25 %) were treated with both a LABA and ICS in the year prior to study entry and therefore excluded. We identified 251 (75 %) patients with the opportunity for the addition of at least one long-acting agent. Among these 251 patients, at baseline 138 (55.0 %) had not received an ICS or LABA, 80 (31.9 %) had been dispensed an ICS, and 33 (13.1 %) had been dispensed a LABA. Similar to our primary analysis, in the 6 months after the exacerbation, 103 (41.0 %) had the addition of an ICS, LABA, or both, with 24 (9.6 %) of patients receiving an ICS, 9 (3.6 %) a LABA, and 70 (27.9 %) both drugs. There were insufficient inpatient exacerbations to perform similar analyses.
We performed multivariate analysis of our sensitivity cohort which was very similar to our overall cohort, and notable for the fact that lower severity of airflow limitation (defined by FEV1 percent predicted) was associated with decreased odds of being prescribed ICS, LABA or both drugs.
DISCUSSION
We found that despite efficacy data supporting the addition of ICS and/or LABA, relatively few patients had the addition of one of these medications known to moderate exacerbation risk, and the pattern of prescribing was not guideline adherent. COPD exacerbations are common,20 adversely affect the quality of life among patients with COPD, and are an important driver of health-care costs. In the 12 months following COPD exacerbations, exacerbations are known to reoccur in up to 50 % of patients, making this an important opportunity to intervene with therapies known to reduce exacerbation risk. Our overall results were robust to cohort definitions and persisted regardless of whether the COPD exacerbation required hospital admission. These results suggest an opportunity to improve processes of care that may reduce the burden associated with COPD exacerbations.
Our findings are consistent with other studies that have shown poor adherence to treatment guidelines, although none had examined an extended period immediately after a COPD exacerbation. In a Swiss study of 615 outpatient clinic patients with a diagnosis of COPD, only 50–66 % of patients were prescribed inhaled therapies as recommended by GOLD guidelines.21 In a separate study of over 2,000 patients admitted for COPD exacerbations, only 58 % were prescribed short-acting bronchodilators at discharge, and 24 % were not prescribed any inhaled therapy at all.22 Our study adds to this literature by demonstrating that not only are these medications not regularly prescribed at discharge, but in the 6-month period after an inpatient or outpatient exacerbation, a substantial number of patients still do not receive these therapies.
We identified a number of predictors with reduced odds of receiving additional therapy. The decreased odds associated with greater age and comorbid conditions such as depression or chronic heart failure may represent ageism or the effect of competing illnesses that shifts attention away from the need for additional COPD treatment. There is sometimes a perception that older patients may benefit less from therapies, though there are no data to support this, and it can lead to health disparities for older patients. Clinicians may also attribute symptoms of COPD to alternate diseases such as heart failure. Likewise, among current smokers, providers may be correctly focusing first on having patients stop tobacco use, although there is little evidence to suggest that smoking modifies the benefits of inhaled therapies for COPD. Nonetheless, these risk factors merit further investigation to explain these disparities.
We were surprised that markers of COPD severity, including previous exacerbation were strongly associated with not receiving additional controller therapy, although in sensitivity analyses, milder severity of airflow obstruction was associated with being less likely to receive additional medications. There are a number of potential explanations. Patients with exacerbations in the year prior to enrollment may have had previous exposure to these medications, and the medications were discontinued more than 1 year prior to entry into the cohort. In addition, patients with exacerbations in the past may have returned to baseline health status, and therefore their clinicians were not prompted to prescribe additional therapy by ongoing symptoms. Clinicians may not be aware of the role of these therapies specifically in decreasing the exacerbation rate. Providers may have been unaware that an exacerbation occurred if it was treated by a different practitioner, one who may have been reluctant to change chronic medications on another physician’s patient. Patient preferences in the use of chronic medications and their perceptions of the benefit of these inhalers may have also had an effect.
This study had limitations. First, the data were obtained administratively and reflected care that was received within the VA system, limiting some of the generalizeability outside of integrated health-care systems. Second, it is possible that some of the patients received these longer acting therapies from outside pharmacies. We feel this is unlikely to be common as the majority of patients receiving care within the VA use it as their primary pharmacy resource, and these medications are typically not low copayment medications available through large retail outlets.23 Third, there were relatively few women and minorities in our cohort, limiting the ability to generalize to these groups. Finally, although we have identified patient-level factors for providing guideline-adherent care, we did not have systems and provider variables, which are key components to enhancing the possibility of providing high quality care.
We defined COPD exacerbations administratively by receipt of specific medical care with the possibility that in the outpatient setting, steroids and/or antibiotics were given to self-treat in case of future exacerbations. However, we found no difference in prescribing practices when we examined only those who were admitted for exacerbations or those who had pulmonary function testing.
This study also had a number of important strengths. We utilized data from an entire integrated network of care, therefore increasing the likelihood of complete ascertainment of measures that are not commonly available in settings where care is delivered by multiple independent providers. In addition, we had a large number of patients from a diverse set of VA settings including academically and non-academically affiliated centers. This would decrease the possibility of idiosyncratic prescribing patterns leading to our results. We were able to identify important health behaviors that influence care, including tobacco exposure. We also had the opportunity to perform sensitivity analyses for those subjects who had previous PFTs. It is notable that more of the patients with airflow obstruction on PFTs were on both LABA and ICS in the year prior to the index date. It is possible that this group of patients was referred for PFTs because of being more symptomatic and was therefore on more medications. The presence of airflow obstruction on the PFTs may also have prompted the addition of inhaled therapies. Finally, because our results are within an integrated health-care system, interventions can be targeted to the direct population from which it was derived, thus making the results more easily actionable than if these results were derived from many separate systems of care.
This study demonstrated that among patients treated for COPD exacerbations, there were missed opportunities to potentially reduce subsequent exacerbations by adding treatments known to modify exacerbation risk. We have identified potentially important disparities or targets that deserve greater attention. COPD is the deadliest and most costly respiratory illness in the US, with exacerbations accounting for a significant proportion of symptom burden and health-care costs.24 Given the frequency of exacerbations, even modest reductions in rates may produce significant benefits in symptom burden, disability, and health-care expenditures. Moreover, in integrated health-care systems, such as the Department of Veterans Affairs, our results suggest a potential opportunity to use advanced informatics systems to address these opportunities by proactively identifying and recommending treatment to mitigate future COPD exacerbation risk.
The VA uses Quality Enhancement Research Initiative (QUERI) programs designed to improve delivery of care for some chronic conditions, but not others, which can lead to less robust adherence for other diseases. Practitioners could benefit from a program such as this to specifically improve COPD treatment. In the future, it may also be useful to use specialists, such as pulmonologists, to identify patients who might qualify for an escalation in therapy, for example, at the time of pulmonary function testing or after COPD exacerbations. Another potential intervention could involve prompting physicians to refer for PFTs at the time of diagnosing a COPD exacerbation to enable disease staging and guideline-adherent therapy. Our results suggest that such interventions need to be tested to assess whether they improve processes, reduce subsequent exacerbations, and improve quality of life among patients with COPD.
Electronic supplementary material
(DOCX 26 kb)
Acknowledgments
This material is based upon work supported by the Department of Veterans Affairs, Health Services Research and Development (HSR&D), and American Lung Association grant CI-51755N. Dr. Cecere is supported by a VA HSR&D fellowship (TPM 61-037). The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. Dr. Au had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
Dr. Au is co-investigator on a grant from Gilead Sciences for work that is unrelated to this manuscript and is a research consultant for Bosch LLC. None of the other authors has any conflicts of interest to disclose.
References
- 1.Buist AS, McBurnie MA, Vollmer WM, et al. International variation in the prevalence of COPD (The BOLD study): a population-based prevalence study. Lancet. 2007;370:741–750. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 2.Seemungal TA DG, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1998;157:1418–1422. doi: 10.1164/ajrccm.157.5.9709032. [DOI] [PubMed] [Google Scholar]
- 3.Decramer MNL, Nardini S, Reardon J, Rochester CL, Sanguinetti CM, Troosters T. Targeting the COPD exacerbation. Respir Med. 2008;102:S3–S15. doi: 10.1016/S0954-6111(08)70003-9. [DOI] [PubMed] [Google Scholar]
- 4.Alsaeedi A, Sin DD, McAlister FA. The effects of inhaled corticosteroids in chronic obstructive pulmonary disease: a systematic review of randomized placebo-controlled trials. Am J Med. 2002;113:59–65. doi: 10.1016/S0002-9343(02)01143-9. [DOI] [PubMed] [Google Scholar]
- 5.Boyd G, Morice A, Pounsford JC, Siebert M, Peslis N, Crawford C. An evaluation of salmeterol in the treatment of chronic obstructive pulmonary disease (COPD) Eur Respir J. 1997;10:815–821. [PubMed] [Google Scholar]
- 6.Burge PS, Calverley P, Jones PW, Spencer S, Anderson JA, Maslen TK. Randomised, double blind, placebo controlled study of fluticasone propionate in patients with moderate to severe chronic obstructive pulmonary disease: the ISOLDE trial. BMJ. 2000;320:1297–1303. doi: 10.1136/bmj.320.7245.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurst JR, Donaldson G, Quint JK, Goldring JJ, Baghai-Ravary R, Wedzicha JA. Temporal clustering of exacerbations in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;179:369–374. doi: 10.1164/rccm.200807-1067OC. [DOI] [PubMed] [Google Scholar]
- 8.Global Initiative for Chronic Obstructive Lung Disease Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease NHLBI/WHO workshop report Bethesda, National Heart, Lung and Blood Institute April 2001; NIH Publication No. 270:1–100.
- 9.Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2010. Available from: http://www.goldcopdorg/.
- 10.Rabe K, Hurd S, Anzueto A, Barnes P, Buist S, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;6:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 11.Celli B, MacNee W, Force AET Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23:932–946. doi: 10.1183/09031936.04.00014304. [DOI] [PubMed] [Google Scholar]
- 12.American Thoracic Society / European Respiratory Society Task Force. Standards for the diagnosis and management of patients with COPD [Internet]. Version 1.2. New York: American Thoracic Society; 2004 [updated 2005 September 8]. Available from: http://www.thoracic.org/go/copd. 2004.
- 13.Calverley PMA, Anderson JA, Celli B, Ferguson GT, Jenkins C, Jones PW, Yates JC, Vestbo J, for the TORCH investigators Salmeterol and fluticasone propionate and survival in chronic obstructive pulmonary disease. N Engl J Med. 2007;356:775–789. doi: 10.1056/NEJMoa063070. [DOI] [PubMed] [Google Scholar]
- 14.Mills E, Druyts E, Ghement I, Puhan MA. Pharmacotherapies for chronic obstructive pulmonary disease: a multiple treatment comparison meta-analysis. Clin Epidemiol. 2011;3:107–129. [DOI] [PMC free article] [PubMed]
- 15.Calvin JE, Shanbhag S, Avery E, Kane J, Richardson D, Powell L. Adherence to evidence-based guidelines for heart failure in physicians and their patients: lessons from the Heart Failure Adherence Retention Trial (HART) Congest Heart Fail. 2012;18:73–78. doi: 10.1111/j.1751-7133.2011.00263.x. [DOI] [PubMed] [Google Scholar]
- 16.Komajda M, Lapuerta P, Hermans N, Gonzalez-Juanatey JR, Van Veldhuisen DJ, Erdmann E, Tavazzi L, Poole-Wilson P, Le Pen C. Adherence to guidelines is a predictor of outcome in chronic heart failure: the MAHLER survey. Eur Heart J. 2005;26:1653–1659. doi: 10.1093/eurheartj/ehi251. [DOI] [PubMed] [Google Scholar]
- 17.Cooke CR, Joo MJ, Anderson SM, Lee TA, Udris EM, Johnson E, Au DH. The validity of using ICD-9 codes and pharmacy records to identify patients with chronic obstructive pulmonary disease. BMC Health Serv Res. 2011;11:37. [DOI] [PMC free article] [PubMed]
- 18.Sharafkhaneh AP, Petersen N, Yu H, et al. Burden of COPD in a government health care system: a retrospective observational study using data from the US Veterans Affairs population. Int J Chron Obstruct Pulmon Dis. 2010;5:125–132. doi: 10.2147/copd.s8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabe K, Hurd S, Anzueto A, Barnes P, Buist S, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;176:532–555. doi: 10.1164/rccm.200703-456SO. [DOI] [PubMed] [Google Scholar]
- 20.Seemungal TA. Exacerbation rate, health status and mortality in COPD—a review of potential interventions. Int J Chron Obstruct Pulmon Dis. 2009;4:203–223. doi: 10.2147/COPD.S3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jochmann A, Neubauer F, Miedinger D, Schafroth TS, Chhajed PN, Tamm M, Leuppi J. General practitioners’ adherence to the COPD GOLD guidelines: baseline data from the Swiss COPD Cohort Study. Swiss Med Wkly. 2010;140:13053. doi: 10.4414/smw.2010.13053. [DOI] [PubMed] [Google Scholar]
- 22.Yip NH, Yuen G, Lazar EJ, Regan BK, Brinson MD, Taylor B, George L, Karbowitz SR, Stumacher R, Schluger NW, Thomashow BM. Analysis of hospitalizations for COPD exacerbation: opportunities for improving care. COPD. 2010;7:85–92. doi: 10.3109/15412551003631683. [DOI] [PubMed] [Google Scholar]
- 23.Shen Y, Hendricks A, Zhang S, Kazis L. VHA enrollees’ health care coverage and use of care. Med Care Res Rev. 2003;60:253–267. [DOI] [PubMed]
- 24.Foster TS, Miller J, Marton JP, Caloyeras JP, Russell MW, Menzin J. Assessment of the economic burden of COPD in the US: a review and synthesis of the literature. COPD. 2006;3:177–178. doi: 10.1080/15412550601009396. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 26 kb)