ABSTRACT
BACKGROUND
While there has been extensive research into patient-specific predictors of medication adherence and patient-specific interventions to improve adherence, there has been little examination of variation in clinic-level medication adherence.
OBJECTIVE
We examined the clinic-level variation of oral hypoglycemic agent (OHA) medication adherence among patients with diabetes treated in the Department of Veterans Affairs (VA) primary care clinics. We hypothesized that there would be systematic variation in clinic-level adherence measures, and that adherence within organizationally-affiliated clinics, such as those sharing local management and support, would be more highly correlated than adherence between unaffiliated clinics.
DESIGN
Retrospective cohort study.
SETTING
VA hospital and VA community-based primary care clinics in the contiguous 48 states.
PATIENTS
444,418 patients with diabetes treated with OHAs and seen in 158 hospital-based clinics and 401 affiliated community primary care clinics during fiscal years 2006 and 2007.
MAIN MEASURES
Refill-based medication adherence to OHA.
KEY RESULTS
Adjusting for patient characteristics, the proportion of patients adherent to OHAs ranged from 57 % to 81 % across clinics. Adherence between organizationally affiliated clinics was high (Pearson Correlation = 0.82), and adherence between unaffiliated clinics was low (Pearson Correlation = 0.04).
CONCLUSION
The proportion of patients adherent to OHAs varied widely across VA primary care clinics. Clinic-level adherence was highly correlated to other clinics in the same organizational unit. Further research should identify which factors common to affiliated clinics influence medication adherence.
KEY WORDS: pharmacoepidemiology, health services research, diabetes, veterans, primary care
INTRODUCTION
Oral hypoglycemic agent (OHA) drug therapy is a major component of medical management for most patients with type 2 diabetes. Prior research suggests that adherence is highly variable across patients, although often poor, with studies indicating that patients take only 7–64 % of their anti-diabetic drug doses.1
Although some patient-level predictors of medication adherence have been identified, patient-level interventions such as removing copayments result in a uniformly small improvement in adherence, typically in the range of 4–6 %.2 Organizational barriers to medication adherence have received far less attention than patient-level predictors, despite studies showing that system-level interventions (e.g., provision of pillboxes, care management, or facilitated refilling) can improve adherence.3
The Department of Veterans Affairs (VA) has an integrated primary care clinic system that is embracing a patient-centered medical home model.4 This system is integrated not only locally through a shared electronic record, but also by the centralized collection of information on all utilization and medication use for research, performance measurement, and quality improvement. A VA hospital serves as parent facility to its affiliated community clinics and can have multiple affiliated community clinics.5 A veteran receives primary care through a primary care clinic based at a VA hospital or a community clinic. A VA hospital and its affiliated community clinics share the management structure and support, such as administration, feedback regarding treatment quality, the same electronic health record (EHR), and centralized support for care management. The extent to which there are measurable differences in clinic-level adherence among these different facilities is unknown.
We examined variation in clinic-level medication adherence to oral hypoglycemic medications in order to characterize the extent of variation throughout the VA overall. It is possible to examine variation in clinic-level medication adherence in VA because it is the largest health care system and unlike any other in the US, with approximately 160 hospitals and over 800 community clinics in 2008. By identifying primary care clinics where patients are more adherent to medications, a health system could identify organizational characteristics and practices that facilitate better adherence. This information could be used to improve adherence at lower performing clinics, with additional efforts to improve chronic disease management, provision of decision-support and other intervention strategies.6–9
METHODS
Participants and Setting
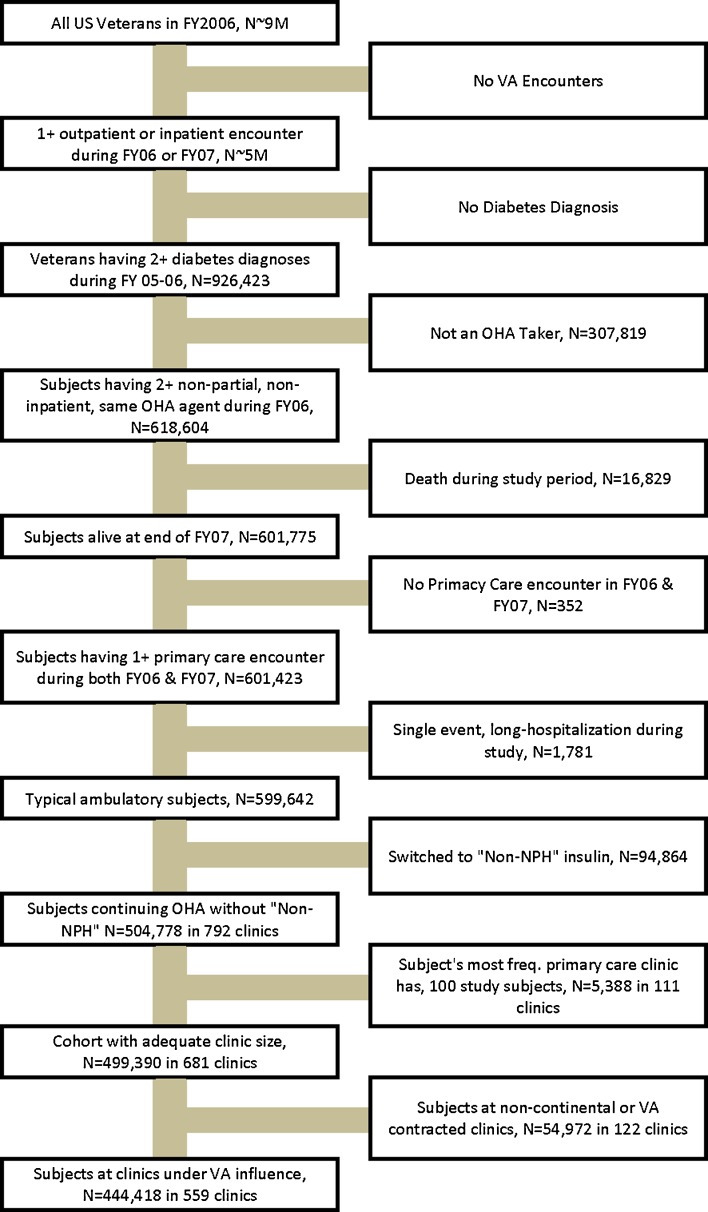
We assessed medication adherence in fiscal year (FY) 2007 (October 1, 2006-September 30, 2007) among VA diabetic patients who were prevalent users of OHAs. Diagnosed diabetes was identified using a validated algorithm based on at least two ICD-9 diagnoses of diabetes from inpatient and outpatient data in FY2005 or FY2006.10 Patients were assigned to the VA clinic where they had the most primary care visits during FY2006, with one clinic randomly selected in the case of a tie. To be eligible, patients had to have at least two medication fills for an oral hypoglycemic agent (metformin, sulfonylureas, and glitazones) in FY2006.11 Patients also had to have at least one primary care visit per year in FY2006 and FY2007, and be alive at the end of FY2007. We excluded patients with a prescription for any insulin except neutral protamine Hagedorn (NPH), which is commonly used to supplement oral therapy in the VA. Other insulins may indicate patients were switched from an oral agent and there are no currently accepted methods for determining when oral agents are discontinued. We also excluded patients in nursing homes or who had extended hospital stays in FY2006, because the focus of this work is outpatient, non-institutionalized medication adherence. Finally, clinics with fewer than 100 patients were excluded (111 clinics excluded with 5,388 subjects), to ensure stable clinic-level adherence estimates (See Fig. 1).
Figure 1.
Cohort selection flow diagram.
Data Sources and Measures
Outpatient pharmacy records were obtained to construct the primary outcome of medication refill adherence, based on the date the prescription was dispensed, the drug name, and the days supply. We used VA inpatient and outpatient administrative data sets to construct demographic characteristics and utilization measures. VA vital status data were obtained to identify mortality.
Medication Adherence
We estimated patients’ adherence to OHAs for FY2006 and FY2007 using a validated algorithm that produces a proportion of days covered for the interval of interest.12–14 We constructed two adherence measures, one using a 90-day interval at the beginning of FY2007, and a sensitivity analysis using a 1-year interval of FY2007, for two separate analyses examining variation in these measures. Our primary analysis used an assessment period of 90 days because adherence for this period has been correlated with blood pressure control related to antihypertensive use, and low-density lipoprotein (LDL) response related to statin use.12 Other work has demonstrated a relationship between OHA refill adherence and A1c control.12,15 The 90-day and 1-year analyses were similar, and so only the primary analysis is presented.
For patients on a single OHA, after calculating the proportion of days covered, we classified patients as adherent if they had medication available ≥80 % of the period.13,14,16 A threshold of 80 % is chosen because it is common in prior literature,13,14 and has reasonable properties to identify adherent versus nonadherent patients.16 Additionally, this dichotomous outcome may be more significant and interpretable for clinic level adherence than small changes or averages of a linear measurement of adherence across an entire clinic, which would assume the same increase in clinical effectiveness across the adherence scale. In other words, the dichotomous measure allows us to count and adjust for the proportion of patients adherent at a clinic level, rather than trying to compare an average linear adherence between two clinics.
If patients were receiving more than one OHA, the proportion of days covered during the period for each medication was averaged. For example, a person defined as a user of both metformin and glyburide, but during follow-up had perfect adherence (100 %) for glyburide but no fills (0 %) of metformin, would have an average adherence of 50 %, and would be considered nonadherent to the overall regimen. Finally, we estimated the adherence at the clinic level, defined as the proportion of patients who had adherence ≥80 % for their regimen within each clinic. We further risk adjusted the clinic-level adherence as described below.
Statistical Analysis
Our outcome variable of interest is the risk adjusted proportion of patients in a clinic who are adherent to their oral diabetes regimen. We created risk adjusted clinic-level adherence using hierarchical logistic regression. This accounts for the characteristics of the patient population receiving care at a clinic. Specifically, we fit a patient-level model with adherence as the outcome and predictors including patient demographics, comorbidities, prior utilization, and copay status, described in detail along with the adjustment model in Wong, Piette et al. (2012).11 The variables used for adjustment are included in Table 1.
Table 1.
Descriptive Statistics for the Cohort of Diabetic Patients Taking Oral Hypoglycemic Agents During the First Quarter of FY2007 and the VA Primary Care Clinics Where Patients Were Seen
| Measure | Overall | Hospital clinics | Community clinics |
|---|---|---|---|
| Facility characteristics | |||
| Number of facilities (N) | 559 | 158 | 401 |
| Cohort subjects per facility (SD) | 795 (782) | 1,623 (904) | 469 (392) |
| Maximum (N) | 7,399 | 7,399 | 2,296 |
| Minimum (N) | 104 | 253 | 104 |
| Patient characteristics | |||
| Subjects (N) | 444,418 | 256,520 | 187,898 |
| Age (SD) | 67.9 (11.0) | 67.2 (11.0) | 68.9 (10.9) |
| Female (%) | 2.1 | 2.4 | 1.7 |
| Married (%) | 65.6 | 63.1 | 69.1 |
| Service connected percentage (SD)* | 17.9 (30.2) | 19.2 (31.2) | 16.0 (28.8) |
| Primary reason received free care: | |||
| Disability (%) | 34.6 | 27.7 | 35.1 |
| Low income (%) | 34.6 | 35.7 | 33.2 |
| No free care (%) | 30.8 | 36.6 | 31.7 |
| FY06 Total VA care encounters (SD) | 7.1 (8.8) | 8.0 (9.9) | 5.9 (7.0) |
| First quartile (%)* | 37.2 | 31.7 | 44.8 |
| Second quartile (%)* | 19.8 | 19.4 | 20.3 |
| Third quartile (%)* | 21.3 | 23.2 | 18.6 |
| Four quartile (%)* | 21.7 | 25.7 | 16.3 |
| FY06 VA Primary Care encounters (SD)* | 3.8 (2.9) | 3.8 (2.9) | 3.7 (2.9) |
| Total FY06 Drug Classes (SD) | 7.5 (3.5) | 7.7 (3.6) | 7.1 (3.3) |
| Distance to main VA care facility (mi) (SD) | 24.3 (29.3) | 28.1 (31.5) | 19.2 (25.2) |
| Hospitalized during Q4 FY06 (%) | 2.4 | 3.1 | 1.4 |
| Inpatient days if in hospital (SD) | 6.8 (9.9) | 2.1 (7.3) | 2.2 (8.0) |
| Diagnosis Cost Group Score (SD) | 0.86 (0.59) | 0.89 (0.63) | 0.82 (0.52) |
| Diabetes Complication Severity Index (SD) | 3.8 (1.2) | 3.8 (1.2) | 3.8 (1.1) |
| Comorbid conditions—Vascular disease: | |||
| Atrial fibrilation or flutter (%) | 6.0 | 6.2 | 5.6 |
| Cerebrovascular: stroke or TIA (%) | 2.4 | 2.5 | 2.2 |
| Cerebrovascular: Non-stroke, non-TIA (%) | < 1 | 1.1 | 0.8 |
| Congestive heart failure (%) | 3.9 | 4.2 | 3.3 |
| Hypertension (%) | 63.5 | 65.3 | 61.0 |
| Ischemic heart disease (%) | 19.4 | 19.4 | 19.3 |
| Myocardial infarction (%) | 1.2 | 1.4 | 1.0 |
| Peripheral Vascular disease (%) | 1.2 | 1.4 | 1.0 |
| Mental or neurological illness: | |||
| Alcohol abuse (%) | 1.9 | 2.2 | 1.4 |
| Dementia (%) | < 1 | 1.0 | < 1 |
| Depression (%) | 8.3 | 8.8 | 7.6 |
| Post-traumatic stress disorder (%) | 6.8 | 7.2 | 6.2 |
| Schizophrenia (%) | 1.5 | 1.8 | 1.1 |
| Substance abuse, non-alcohol (%) | 6.9 | 7.5 | 6.2 |
| Other serious mental illness (%) | 1.6 | 1.7 | 1.3 |
| Other conditions: | |||
| Chronic lung disease (%) | 7.4 | 8.0 | 6.7 |
| Chronic renal disease (%) | 2.3 | 2.8 | 1.7 |
| NPH insulin prescribed FY06- FY07 (%) | 8.8 | 9.6 | 7.8 |
| Warfarin prescribed FY06- FY07 (%) | 6.3 | 6.2 | 6.3 |
| Adherent to prescribed OHA regimen (%) | 70.6 | 69.9 | 71.4 |
| Adherent to any single OHA (%) | 79.6 | 79.0 | 80.5 |
SD standard deviation; mi mile; FY fiscal year; Q4 fourth quarter; TIA transient ischemic attack; NPH neutral protamine hagedorn; OHA oral hypoglycemic agent
*These additional variables were included for descriptive purposes, but were not included in the risk adjustment model
In addition to looking at variation in the interquartile ranges, we compared variation in the top and bottom 25 clinics after examining the distribution and ranking in adherence in Wong, Piette et al. (2012).11 These ends were chosen as extremes that still contained a substantial number of clinics and patients, yet were outside a number of clinics having similar, average clinic-level adherence.
Statistical Evidence of Organizational Relationships
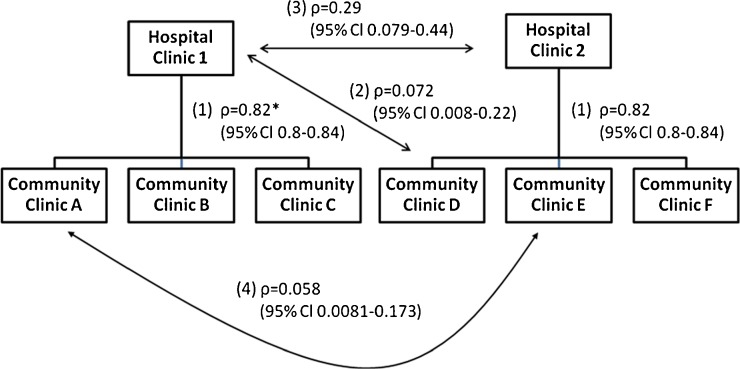
For the purposes of these analyses, we defined an organizational relationship as statistical correlation in adherence, not due to similarities in patient population, and between the affiliated hospital and community based clinics. To examine the correlation in adjusted adherence among organizationally affiliated clinics, we performed several analyses. First, we plotted the proportion of adherent patients in each hospital clinic against the proportion of adherent patients in their affiliated community clinics. Each point represents the level of adherence for one community clinic, which is plotted over the adherence for its parent hospital clinic; each vertical cluster of points represents a group of affiliated community clinics that lie over the associated parent hospital clinic on the x-axis. Second, we also calculated Pearson correlations between adherence scores for the following groups: 1) parent hospital clinics and their affiliated community clinics; 2) hospital clinics and unrelated, randomly selected, community clinics; 3) hospital clinics and other unrelated hospital clinics; and 4) different community clinics which are not affiliated with the same hospital. Figure 4 illustrates these relationships. The confidence interval around each of the point estimates for the correlations was determined by bootstrapping.17
Figure 4.
Correlation in clinic level adherence between different levels of the VA health care system. Hospital 1 and Hospital 2 are hospital-based primary care clinics; Hospital 1 has three community primary care clinics that are affiliated. *Numbers 1 through 4 in parentheses refer to relationships defined in the methods section.
The Pearson correlation coefficient assumes a linear relationship, the absence of outliers and normally distributed variables.18 We verified the absence of outliers graphically. However, the respective distributions adherence measures among community-based and hospital-based clinics were slightly skewed. Thus, we also assessed correlations using Spearman’s rho, which produced similar results.
The overall study was reviewed and approved by Institution Review Boards at the University of Washington and the Seattle, Ann Arbor, and Durham VA Medical Centers.
RESULTS
We identified a total of 401 community-based and 158 hospital-based primary care clinics treating at least 100 diabetes patients each, resulting in a sample size of 444,418 diabetic patients treated with at least one OHA. On average, community clinics had fewer patients with diabetes than hospital clinics (496 vs. 1,624, p < 0.0001). Community clinic patients were older (69 years vs. 67 years, p < 0.0001), had lower service-connected disability (16 % vs. 19 %, p < 0.0001), and were less likely to be taking NPH insulin as an adjunct to their oral therapy (8 % vs. 10 %, p = 0.97) (Table 1). There were no significant differences among patients in adherence to OHA regimen or individual OHA medications by clinic type (any regimen 69.9 vs. 71.4, p = 0.98, sulfonylureas 69.5 vs. 70.9, p = 0.99, metformin 69.3 vs. 70.9, p = 0.988, and glitazones 64.4 vs. 66.4, p = 0.97).
Clinic-Level Variation
At the clinic level, the unadjusted proportion of patients adherent to their OHAs ranged from 48 % to 84 %. Interquartile range was 48–69 %, and 76–84 % for the first and fourth quartile, respectively.
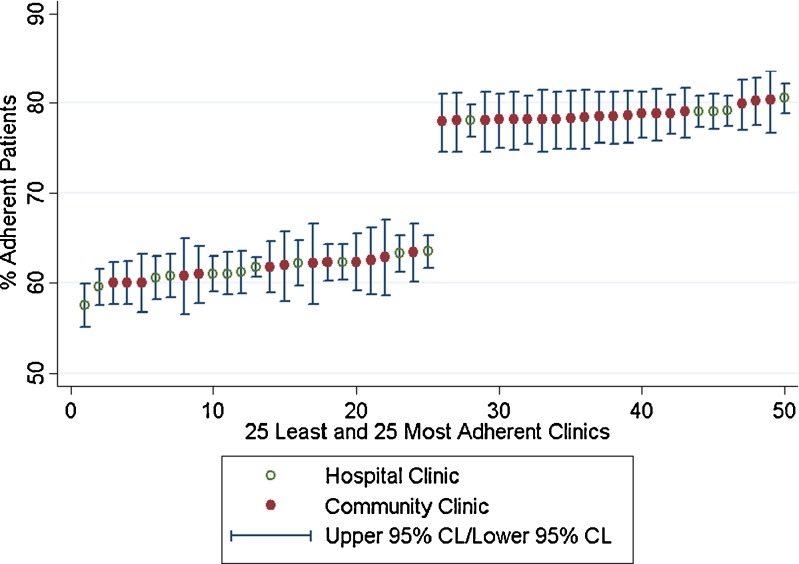
After adjusting for patient characteristics, the proportion of adherent patients ranged from 57 % (95 % Confidence Interval [CI]: 55–60 %) to 81 % (95 % CI: 79–82 %) across all 559 primary care clinics. The magnitude of the difference in adjusted clinic-level adherence when comparing the least to most adherent clinic is an absolute difference of approximately 24 % in the proportion of diabetic patients who are adherent to their medication. The interquartile range for adherence was 58–69 % and 75–81 % for the first and fourth quartile, respectively. The top 25 clinics had an average adherence of 79 % and the bottom 25 had an average of 62 % (Fig. 2). Adjusted clinic-level adherence was higher in community clinics compared to hospital clinics (hospital adherence = 70.8 % (95 % CL 70.1–71.5 %), clinic adherence 72.0 % (95 % CL 71.6–72.4 %), p = 0.0021), although the difference was small in absolute terms.
Figure 2.
Variation in adherence for the 25 least and the 25 most adherent clinics.
Organizational Relationship and Clinic-Level Adherence
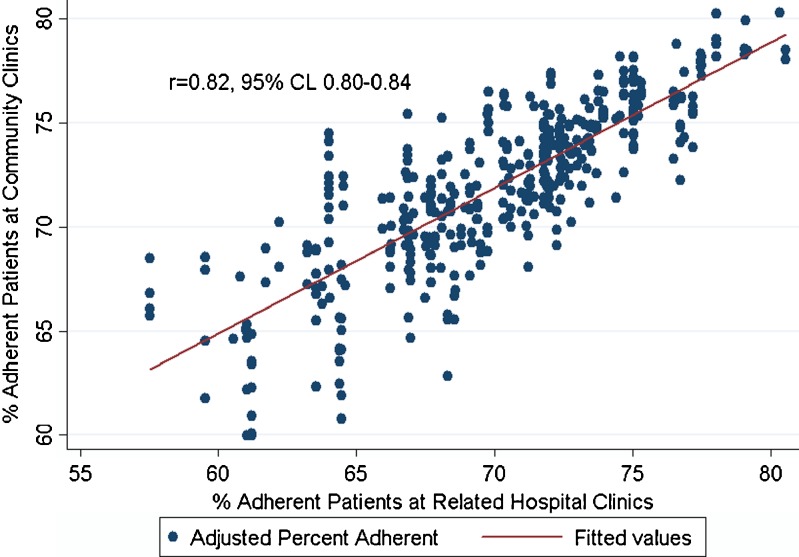
Figure 3 demonstrates the relationship between the proportion of adherent patients in each parent hospital’s primary care clinic and the proportions of adherent patients in affiliated community-based clinics. The leftmost cluster of points on the graph represents a cluster of four community clinics with adherence levels ranging from 66 % to 68 %, plotted over the affiliated parent hospital clinic adherence of 57 %. The correlation between the proportion of adherent patients in parent hospital clinics compared to the affiliated community clinics in the same organizational unit was high (R = 0.82, 95 % CI 0.80–0.84).
Figure 3.
Correlation between clinic-level adherence for hospital-based primary care clinics and community-based clinics within the same organizational unit.
Figure 4 illustrates the four relationships described in the methods section. Correlation between hospital primary care clinics and community clinics was high, as was the correlation between community clinics with the same hospital affiliation. However, the correlations were similar and much lower between unaffiliated hospital clinics (R = 0.29, 95 % CI = 0.079–0.44), between unaffiliated community clinics (R = 0.058, 95 % CI = 0.008–0.173) and between unaffiliated community and hospital clinics (R = 0.072, 95 % CI = 0.008–0.22).
DISCUSSION
This study examined the variation in adherence to OHA medications at the clinic level across the VA system. We also estimated the extent to which adherence was correlated among organizationally affiliated clinics after accounting for unobserved clinic heterogeneity and the nesting of clinics with the VA. Our results demonstrate marked variation in OHA medication adherence at the primary care clinic level. This pattern of relationships across clinics is somewhat surprising, given that there are features that are consistent across VA clinics nationally—including an integrated electronic medical record system for ordering medications, nationally centralized mail order pharmacies for delivering these medications, national guidelines for patient care, a national VA organizational culture promoted through national leadership meetings, and frequent cyber seminars for sharing information about best practices.
There was a nearly 20 % adjusted absolute difference in the proportion of patients who were adherent to their medications between the top and the bottom clinics. These system-level differences dwarf variation associated with patient-level factors. For example, in the recent Post-Myocardial Free Rx Event and Evaluation Trial (MI FREEE), there was only a 4–6 % improvement associated with copayment eliminations after a myocardial infarction.2 Among diabetic patients in a Northwest health maintenance organization, depression as assessed by the Patient Health Questionnaire was associated with a decreased adherence of 3.6 %.19 A variety of interventions specific for improving the medication adherence among patients with diabetes is described in a 2009 Cochrane review, with no intervention approaching the magnitude of the observed differences between the top and bottom clinics demonstrated here.19,20 This suggests that clinic-level factors may play an important and underappreciated role in medication adherence by patients.
Our results are consistent with a recent study documenting wide variation in the success of anticoagulation clinics to keep patients within target levels of anticoagulation. Rose et al. found that the average observed percent of time in therapeutic range for oral anticoagulation across 100 VA sites ranged from 38 % to 69 %.21 Risk adjustment made only a small difference in ranking for most sites. These parallel findings for a different metric not only point out that the observed variation seen in both studies is real, but also that such large variation within a relatively uniform health care system is meaningful, offering an opportunity to identify and spread best practices from successful sites to improve outcomes at low performing sites. Positive deviant studies would be suited to help answer these questions. These studies use mixed methods to generate and test hypotheses about the characteristics of high performers with practices that are currently in use, as opposed to developing, testing, and implementing entirely new systems of care.22
We found high correlations between parent hospital VA primary care clinics and their related community primary care clinics. While we used the hospital clinic to act as a primary node or identifier, there is no hierarchy implied. However, in most VA settings, all of these clinics generally fall under the same primary care and pharmacy administrative and support structures (service lines) that are frequently located in the hospital, which we broadly considered to be an organizational unit. Despite inherent differences in staffing and patient populations, there are a number of potential reasons why clinics in the same organizational unit may exhibit similar outcomes, including intra-network communication,23 common practices24 and leadership.25,26 For example, Lammers and colleagues found that the level of commitment to quality improvement in VA medical centers was correlated among employees in adjacent levels of the medical center hierarchy (i.e. staff physicians and chiefs of services).26,27 Overall commitment to total quality management at various levels was correlated with observed quality improvement. It is unknown whether these shared structures include things like easier access to medications, common strategies for encouraging patients to obtain and take their medications, the roles of pharmacists in primary care clinics, ease of access by patients to their provider and the pharmacy, or other facility or patient-directed interventions. Future work should be conducted to understand and characterize these organizational structures or processes that may explain the differences between clinics with high and low adherence.
This study has several limitations. Measures of refill adherence will not capture nonadherence if the patient obtains the medication on a regular basis, but does not consume the drug and diverts or stockpiles it instead. In addition, patients who obtain their medications outside the VA but continue to be seen in VA clinics may also be misclassified as nonadherent. To minimize this bias, as described in the methods section, we selected patients who visited the primary care clinic at least once a year for 2 years, and who had at least two medication fills for a candidate OHA to have medication adherence measured. This was to help insure that subjects were active primary care patients at the VA, and that we measured adherence within a reasonable period of time after the cohort was identified. Large regional variations in the availability of non-VA drugs, such as variable market penetration of discount formularies by retailers such as Wal-Mart, may also influence clinic-level measures by this same mechanism. At the time of the acquisition of the study data, all VA medications were available at a copay cost of $8 per 30 days, or $24 for 90 days for patients not exempt from the payment. A comparable formulary prescription at Wal-Mart would have cost $10 for 90 days.28 The use of non-VA discount pharmacies is difficult to address with VA administrative data and without additional detailed information from other payers, such as Medicare Part D or private insurance drug benefit data, which was not available for this study. However, nearly all patients in this sample would have been receiving at least one drug from the VA (e.g., simvastatin) that still was not available on discount formularies, which may have encouraged patients to obtain medications from the VA. Finally, and perhaps most importantly, the majority of patients (69.2 %) in this sample were exempt from copayments, providing further incentive for them to fill their OHA prescriptions in VA pharmacies.
These analyses also have several notable strengths. The refill adherence measure was developed and validated for VA patients, and is correlated with clinical outcomes.12 The sample was drawn from the largest, most geographically dispersed integrated health care system in the United States, with a sample size of patients, clinics and networks that would be difficult to reproduce in other settings. Despite the fact that the VA is a large integrated health care system, there are significant differences in budgetary and administrative priorities between networks, and even clinics, which could contribute to the variation in adherence reported here. Additionally, there are different management styles and organizational contexts, all of which likely lead to significant organizational variation that warrant further study.
In summary, we found large variations in the proportion of primary care patients who were adherent to OHAs across VA clinics, and this marked variation depended on the affiliation between clinics. This analysis suggests that organization of care at the facility level may play a large and underappreciated role in medication adherence by patients. These results suggest that organizational interventions should be considered as targets of intervention studies. Additional research is needed to identify and test organizational implementation strategies for improving adherence to OHA, and whether such interventions delay or avoid complications of diabetes.
Acknowledgements
This research was supported by an Investigator Initiated Research Award (IIR 07-068-2) from the Department of Veterans Affairs, Health Services Research and Development. Dr. Bryson was supported by VA Career Development Award 03-177. Dr. Jackson was supported for a portion of this project by a VA Career Development Award. Dr. Piette is a VA Senior Research Career Scientist; Dr. Maciejewski and Dr. Yano are VA Research Career Scientists. Dr. Wong was supported by VA Health Services Research and Development Postdoctoral Fellowship TPP 61-024. An earlier version of this manuscript was presented as a poster at the Society of General Internal Medicine 32nd Annual Meeting in Miami, FL in 2009. Dr. Bryson takes responsibility for the integrity of the data and the accuracy of the data analysis. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs.
All authors are federal employees for the Department of Veterans Affairs. Dr. Au has received consultation funds from Bosch Inc. and a grant from Gilead Sciences. Dr. Maciejewski has received consultation funds from Takeda Pharmaceuticals, Novartis and the Surgical Review Corporation. Dr. Maciejewski also owns stock in Amgen.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–24. doi: 10.2337/diacare.27.5.1218. [DOI] [PubMed] [Google Scholar]
- 2.Choudhry NK, Avorn J, Glynn RJ, et al. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365(22):2088–97. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 3.Haynes RB, Yao X, Degani A, Kripalani S, Garg A, McDonald HP. Interventions to enhance medication adherence. Cochrane Database Syst Rev. 2005(4):CD000011. [DOI] [PubMed]
- 4.http://www.va.gov/primarycare/pcmh/. Accessed 12/24/12
- 5.Chapko MK, Borowsky SJ, Fortney JC, et al. Evaluation of the Department of Veterans Affairs community-based outpatient clinics. Med Care. 2002;40(7):555–60. doi: 10.1097/00005650-200207000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Piette JD, Schillinger D, Potter MB, Heisler M. Dimensions of patient-provider communication and diabetes self-care in an ethnically diverse population. J Gen Intern Med. 2003;18(8):624–33. doi: 10.1046/j.1525-1497.2003.31968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piette JD, Bibbins-Domingo K, Schillinger D. Health care discrimination, processes of care, and diabetes patients’ health status. Patient Educ Couns. 2006;60(1):41–8. doi: 10.1016/j.pec.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 8.Piette JD, Heisler M, Horne R, Caleb AG. A conceptually based approach to understanding chronically ill patients’ responses to medication cost pressures. Soc Sci Med. 2006;62(4):846–57. doi: 10.1016/j.socscimed.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 9.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–15. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller DR, Safford MM, Pogach LM. Who has diabetes? Best estimates of diabetes prevalence in the Department of Veterans Affairs based on computerized patient data. Diabetes Care. 2004;27(Suppl 2):B10–21. doi: 10.2337/diacare.27.suppl_2.B10. [DOI] [PubMed] [Google Scholar]
- 11.Wong ES, Piette JD, Liu CF, et al. Measures of adherence to oral hypoglycemic agents at the primary care clinic level: the role of risk adjustment. Med Care. 2012;50(7):591–8. [DOI] [PubMed]
- 12.Bryson CL, Au DH, Young B, McDonell MB, Fihn SD. A refill adherence algorithm for multiple short intervals to estimate refill compliance (ReComp) Med Care. 2007;45:497–504. doi: 10.1097/MLR.0b013e3180329368. [DOI] [PubMed] [Google Scholar]
- 13.Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–16. doi: 10.1016/S0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 14.Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–74. doi: 10.1002/pds.1230. [DOI] [PubMed] [Google Scholar]
- 15.Cohen HW, Shmukler C, Ullman R, Rivera CM, Walker EA. Measurements of medication adherence in diabetic patients with poorly controlled HbA(1c) Diabet Med. 2010;27(2):210–6. doi: 10.1111/j.1464-5491.2009.02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–22. doi: 10.1345/aph.1L496. [DOI] [PubMed] [Google Scholar]
- 17.Efron BT. R. bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Stat Sci. 1986;1(1):54–75. doi: 10.1214/ss/1177013815. [DOI] [Google Scholar]
- 18.Crawford SL. Correlation and regression. Circulation. 2006;114(19):2083–8. doi: 10.1161/CIRCULATIONAHA.105.586495. [DOI] [PubMed] [Google Scholar]
- 19.Lin EH, Katon W, Von Korff M, et al. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–60. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- 20.Vermeire E, Wens J, Van Royen P, Biot Y, Hearnshaw H, Lindenmeyer A. Interventions for improving adherence to treatment recommendations in people with type 2 diabetes mellitus. Cochrane Database Syst Rev. 2005;(2):CD003638. [DOI] [PMC free article] [PubMed]
- 21.Rose AJ, Hylek EM, Ozonoff A, Ash AS, Reisman JI, Berlowitz DR. Risk-adjusted percent time in therapeutic range as a quality indicator for outpatient oral anticoagulation: results of the Veterans Affairs Study to Improve Anticoagulation (VARIA) Circ Cardiovasc Qual Outcomes. 2011;4(1):22–9. doi: 10.1161/CIRCOUTCOMES.110.957738. [DOI] [PubMed] [Google Scholar]
- 22.Krumholz HM, Curry LA, Bradley EH. Survival after acute myocardial infarction (SAMI) study: the design and implementation of a positive deviance study. Am Heart J. 2011;162(6):981–7. doi: 10.1016/j.ahj.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anderson MA, Helms LB. Comparison of continuing care communication. Image J Nurs Sch. 1998;30(3):255–60. doi: 10.1111/j.1547-5069.1998.tb01301.x. [DOI] [PubMed] [Google Scholar]
- 24.Risser DT, Rice MM, Salisbury ML, Simon R, Jay GD, Berns SD. The potential for improved teamwork to reduce medical errors in the emergency department. The MedTeams Research Consortium. Ann Emerg Med. 1999;34(3):373–83. doi: 10.1016/S0196-0644(99)70134-4. [DOI] [PubMed] [Google Scholar]
- 25.Weiner BJ, Shortell SM, Alexander J. Promoting clinical involvement in hospital quality improvement efforts: the effects of top management, board, and physician leadership. Health Serv Res. 1997;32(4):491–510. [PMC free article] [PubMed] [Google Scholar]
- 26.Lammers JC, Cretin S, Gilman S, Calingo E. Total quality management in hospitals: the contributions of commitment, quality councils, teams, budgets, and training to perceived improvement at Veterans Health Administration hospitals. Med Care. 1996;34(5):463–78. doi: 10.1097/00005650-199605000-00008. [DOI] [PubMed] [Google Scholar]
- 27.Jackson D, White I, Kostis JB, et al. Systematically missing confounders in individual participant data meta-analysis of observational cohort studies. Stat Med. 2009;28(8):1218–37. doi: 10.1002/sim.3540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blundell R, Windmeijer F. Identifying demand for health resources using waiting times information. Health Econ. 2000;9(6):465–74. doi: 10.1002/1099-1050(200009)9:6<465::AID-HEC525>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]