ABSTRACT
BACKGROUND
Even though medications can greatly reduce the risk of recurrent stroke, medication adherence is suboptimal in stroke survivors.
OBJECTIVE
To identify key barriers to medication adherence in a predominantly low-income, minority group of stroke and transient ischemic attack (TIA) survivors.
DESIGN
Cross-sectional study.
PARTICIPANTS
Six hundred stroke or TIA survivors, age ≥ 40 years old, recruited from underserved communities in New York City.
MAIN MEASURES
Medication adherence was measured using the 8-item Morisky Medication Adherence Questionnaire. Potential barriers to adherence were assessed using validated instruments. Logistic regression was used to test which barriers were independently associated with adherence. Models were additionally controlled for age, race/ethnicity, income, and comorbidity.
KEY RESULTS
Forty percent of participants had poor self-reported medication adherence. In unadjusted analyses, compared to adherent participants, non-adherent participants had increased concerns about medications (26 % versus 7 %, p < 0.001), low trust in their personal doctor (42 % versus 29 %, p = 0.001), problems communicating with their doctor due to language (19 % versus 12 %, p = 0.02), perceived discrimination from the health system (42 % versus 22 %, p < 0.001), difficulty accessing health care (16 % versus 8 %, p = 0.002), and inadequate continuity of care (27 % versus 20 %, p = 0.05). In the fully adjusted model, only increased concerns about medications [OR 5.02 (95 % CI 2.76, 9.11); p < 0.001] and perceived discrimination [OR 1.85 (95 % CI 1.18, 2.90); p = 0.008] remained significant barriers.
CONCLUSIONS
Increased concerns about medications (related to worry, disruption, long-term effects, and medication dependence) and perceived discrimination were the most important barriers to medication adherence in this group. Interventions that reduce medication concerns have the greatest potential to improve medication adherence in low-income stroke/TIA survivors.
KEY WORDS: medication adherence, stroke, transient ischemic attack, barriers
INTRODUCTION
Stroke is the fourth leading cause of death and a leading cause of severe physical disability in US adults.1 Medications from several classes, including antiplatelet agents, antihypertensives, and statins, substantially reduce the risk of both incident and recurrent stroke.2, 3 Yet, adherence to medications is suboptimal in stroke survivors. In one study of 2,598 patients discharged from the hospital after stroke or transient ischemic attack (TIA), approximately one in four participants discontinued one or more of their stroke prevention medications within 3 months of discharge,4 and in another study of 3,571 stroke survivors, approximately one-third of patients had suboptimal adherence to antihypertensive medications in the year following their stroke.5
Although researchers have begun to identify predictors of non-adherence to medications for cardiovascular disease,6, 7 comparatively few have assessed barriers to medication adherence, particularly in stroke survivors.4, 5, 8, 9 Predictors of adherence encompass patient factors such as age, income level, or years of education that can be used to identify patients most in need of adherence interventions, but that cannot necessarily be directly modified. Barriers to adherence, in contrast, are restricted to potentially modifiable factors that physicians and/or the health care system can attempt to overcome to reduce medication non-adherence.6 An understanding of the key barriers to medication adherence in stroke survivors has great potential to inform novel approaches to reducing non-adherence in this population.
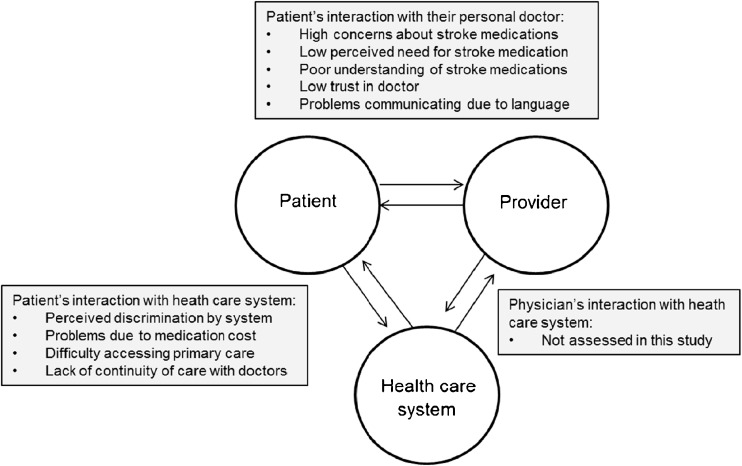
Stroke disproportionately affects patients from low income and minority groups,10, 11 and members of these groups have traditionally been under-represented in research studies.12 Accordingly, we assessed barriers to medication adherence in a predominantly low-income, minority sample of stroke and TIA survivors. The conceptual model used to identify potential barriers to medication adherence in the present study was adapted from a model by Osterberg and Blaschke.6 According to this model, barriers are organized into those that stem from interactions between: 1) the patient and their health providers, 2) the patient and the health care system, and 3) health providers and the health care system (Fig. 1). The current study focuses on patients’ interactions with health providers and systems.
Figure 1.
Conceptual model of barriers to medication adherence in stroke survivors. Model adapted from Osterberg and Blaschke.6
The hypothesis regarding the barriers that would be most strongly associated with poor adherence was informed by Leventhal’s theory of self-regulation.13 According to this theory, patients’ health behaviors are determined by patients’ common sense understanding of their illness. When patients’ understanding fits a model that is consistent with adherence to recommended treatments, then it is predicted that patients will be more adherent. Horne and Weinman expanded this theory to incorporate the notion that patients’ common-sense beliefs about medications are key to determining whether patients will adhere to medications.14 Accordingly, we hypothesized that barriers related to medication beliefs would be most strongly associated with medication adherence. Although patients’ beliefs about medications come from many sources, including personal experience, the experiences of friends and family members, or the media, barriers related to medication beliefs fall within patient–health provider interactions in the Osterberg and Blaschke model, as clinicians also have the potential to strongly influence these beliefs. As an exploratory analysis, we also tested whether the pattern of barriers differed by race or ethnicity.
METHODS
Recruitment
Participants were recruited as part of a clinical trial, Preventing Recurrence of All Inner City Strokes (PRAISE), which tests an intervention to improve adherence to risk-reducing behaviors in stroke survivors. Details of the study design have been published elsewhere,15 but briefly, participants were eligible if they were at least 40 years of age and if they self-reported a history of stroke or TIA in the prior 5 years. Participants were excluded if they had significant aphasia, cognitive impairment, or other neurologic deficits that would preclude them from providing informed consent or from meaningfully participating in classes teaching self-management skills. Proxies could not provide consent on behalf of participants. Additionally, participants were excluded if they had a terminal illness, were pregnant, spoke languages other than Spanish or English, or resided in institutionalized settings. Spanish speaking participants were interviewed by bilingual study personnel, and were surveyed using materials that were translated into Spanish and then back-translated into English. All data presented in this manuscript come from the baseline interviews that took place prior to randomization into the clinical trial. Ethics approval was obtained from the Institutional Review Board of the Mount Sinai School of Medicine. All participants provided written informed consent.
Medication Adherence
Adherence to medications was measured using the 8-item Morisky Medication Adherence Questionnaire (Morisky).16 Sample items include “Do you sometimes forget to take your medicine?” and “Have you ever cut back or stopped taking your medicine without telling your doctor because you felt worse when you took it?” The questionnaire has been validated against an objective measure of adherence17 and has been used in racially diverse and elderly patient samples.18 Scores on the questionnaire can be used to classify patients into low and high adherence groups. Consistent with standard cut points, participants who scored less than 6 points on the Morisky were categorized as non-adherent to medications and participants who scored 6 to 8 points were categorized as adherent.16
Adherence Barriers Related to Interactions Among Health Care Providers and Patients
Concerns about medications were measured by adapting four items from the Concerns subscale of the Beliefs about Medicines Questionnaire (BMQ).19 Three items ask participants to rate the degree to which they worry about “having to take your medicines”, “long-term effects of your medicines”, and “becoming too dependent on your medicines.” The fourth item asks how much “medicines disrupt your life.” Questions were modified from the original instrument to make them more understandable for a study population at risk for mild cognitive impairment and low health literacy. Specifically, instead of asking participants to read each item and then rate their agreement with it, participants were directly asked each question by the interviewer. In addition, the response scale for the included items was reduced from the usual five options (“strongly disagree” to “strongly agree”) to four options (1-“not at all”, 2-“a little bit”, 3-“somewhat” and 4-“very much”). Finally, one of the five items on the original scale (“My medicines are a mystery to me” was omitted as participants had difficulty understanding this item, even in the adapted format. The four items were averaged for a mean Concerns score. A cut-point of 2.5, the midpoint of the scale, was used to dichotomize participants into having high versus low concerns. Cronbach’s α for the scale in this sample was 0.71.
Perceived necessity of medications was measured by adapting the Necessity subscale of the BMQ.19 The five items ask participants to rate how much “your health depends on your medications”, they would be “ill without your medications”, “your health in the future depends on your medications”, “your life would be impossible without your medications”, and “your medicines protect you from becoming worse.” Participants were given the same response options as for the Concerns items, and the five items were averaged for a mean Necessity score. A cut-point of 3 was used to dichotomize participants into having high versus low perceived benefits of medications. Cronbach’s α for the scale in this sample was 0.80.
Knowledge of their disease was assessed by asking participants to state the three most important things they would recommend to others to lower the risk of having a stroke. Participants who could not name more than one of the three most important risk-reducing behaviors20 (i.e., taking antihypertensive medications or controlling blood pressure; taking cholesterol-lowering medications for controlling cholesterol; or taking aspirin or other medications to thin the blood) were considered to have poor understanding of how to prevent recurrent stroke.
Trust in doctors was assessed using three items adapted from the Trust in Doctors scale.21 Specifically, participants were asked how much they felt “you could tell your doctor anything”, how often they thought their doctor “put your best interests first”, and “all things considered, how much do you trust your personal doctor?” Response options for the first two items were scored 1-“never”, 2-“sometimes”, 3-“always”, and the third item was scored -1-“not at all”, 2-“a little bit”, 3-“somewhat”, and 4-“very much”, with higher scores signifying increased trust. The median score was used to dichotomize participants into having low versus high trust in their personal doctor.
Difficulty communicating with doctors due to language was assessed by asking participants how often they had a hard time speaking or understanding their health providers because they spoke different languages. Participants were given the options “always”, “sometimes”, or “never” and were categorized as having language problems if they responded “always” or “sometimes”. This item was adapted from the CAHPS 4.0 survey.
Barriers Related to Interactions Among Patients and the Health Care System
Perceived discrimination was assessed by asking participants how often (“always”, “often”, “sometimes”, “rarely”, “never”) they felt discriminated against by doctors or staff at their clinic because of their 1) race or ethnicity or 2) education or income.22 Participants who answered “sometimes”, “often”, or “always” on either item were categorized as perceiving discrimination from the health system.
Difficulty accessing medical care was assessed by asking participants how difficult it was for them to get medical care when they needed it. Participants who answered “somewhat”, or “very much” were categorized as having problems accessing medical care.23
Poor continuity of care was assessed by asking participants if they usually got to see the same doctor when they went to their primary care visit. Participants who answered “no” or who reported not having a primary care doctor were categorized as having poor continuity of care. This item was adapted from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) 4.0 survey.
Problems due to medication cost were assessed by asking participants if they were worried about the cost of their medicines, and if they ever skipped a medicine or took a smaller dose to make the medicine last longer because they were concerned about cost.24 If participants answered “yes” to either item they were categorized as having problems due to medication cost.
Additional factors assessed to describe the participants included demographic factors (age, gender, race/ethnicity, annual household income, and health insurance type) and medical history. Stroke severity was measured at the time of enrollment into the study using the modified Rankin scale;25 a score of 3 or higher on this scale signifies at least moderate functional disability. When answering questions pertinent to the Rankin scale, participants were asked to report on functional problems that occurred as a result of symptoms from their stroke. Stroke timing was measured by asking the year of the most recent stroke or TIA.25 Comorbidity was measured using the Charlson comorbidity index.26
Analysis Plan
Chi-squared and t-tests were used to compare characteristics of participants according to whether they were or were not adherent to medications. Spearman’s correlation was used to measure the correlation between number of barriers and adherence. Logistic regression was used to calculate the unadjusted and adjusted odds ratios and 95 % confidence intervals for the association between barriers to adherence and non-adherence. For the primary analysis, barriers were entered as categorical variables (e.g., high versus low trust in physicians). The adjusted model was repeated by entering barriers as continuous measures in cases where barriers were measured using a scaled instrument (e.g., total score on trust in personal doctor scale). Adjusted models were additionally controlled for demographics (age, gender, race/ethnicity, income, years of education) and medical history (modified Rankin, Charlson) as these factors have been associated with medication adherence in prior studies.4 Adjusted analyses were repeated for African American and Hispanic subgroups. Analyses were performed using SPSS statistical software (version 18; SPSS Inc, Chicago, IL).
RESULTS
The mean age of participants was 63 years, about 60 % were women, and a majority of participants were non-white and had low income (Table 1). Spanish was the preferred language in 24 % of participants. Overall, 40 % of participants were non-adherent to medications. Younger age, non-white race/ethnicity, and increasing comorbidity were all associated with medication non-adherence.
Table 1.
Characteristics of Participants According to Medication Adherence Status
| Characteristic | Overall (N = 600) | Adherent; N = 358 (59.7 %) | Non-adherent; N = 242 (40.3 %) | P-value |
|---|---|---|---|---|
| Age, mean (SD), in years | 63.4 (11.2) | 65.1 (11.4) | 60.9 (10.5) | < 0.001 |
| Female gender | 356 (59.4) | 203 (56.7) | 153 (63.5) | 0.10 |
| Race/ethnicity* | ||||
| White | 96 (16.0) | 67 (18.7) | 29 (12.0) | 0.03 |
| Black | 280 (46.7) | 164 (45.8) | 116 (47.9) | 0.61 |
| Hispanic | 216 (36.0) | 122 (34.1) | 94 (38.8) | 0.23 |
| Other | 42 (7.0) | 25 (7.0) | 17 (6.0) | 0.98 |
| Income < $15,000 | 341 (58.6) | 194 (59.4) | 147 (62.6) | 0.11 |
| Less than high school education | 182 (30.5) | 109 (30.6) | 73 (30.4) | 0.96 |
| Prescription insurance coverage | 573 (96.6) | 348 (97.5) | 225 (95.3) | 0.16 |
| Preferred language Spanish | 141 (23.5) | 89 (24.9) | 52 (21.6) | 0.35 |
| Modified Rankin score > 2 | 280 (46.7) | 160 (44.7) | 120 (49.6) | 0.24 |
| Charlson score, mean (SD) | 3.6 (2.1) | 3.4 (2.0) | 4.0 (2.3) | 0.005 |
Data are presented as number (%) unless otherwise specified in the Table. Numbers do not always add up to 100 % due to missing cases. Fewer than 3 % missing for each variable
*Percentages add up to more than 100 %, as participants could choose more than one category
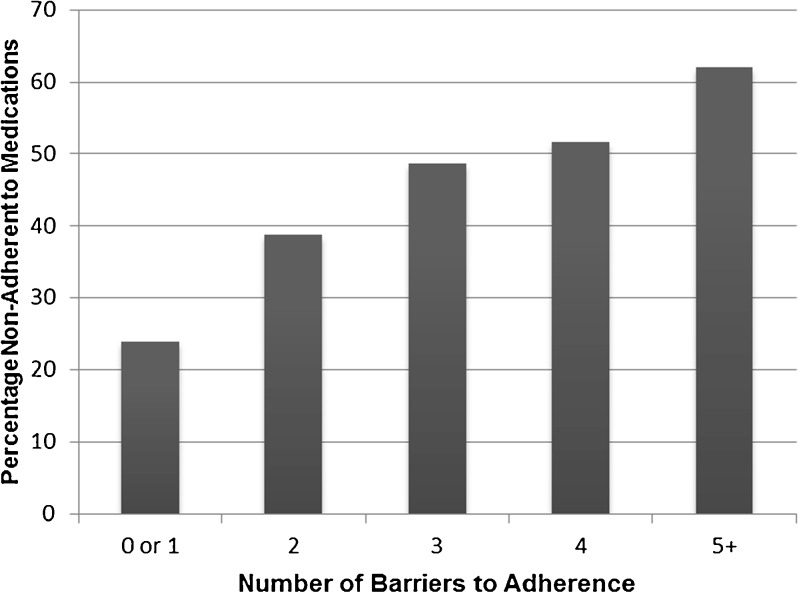
Among the possible barriers to medication adherence, participants most commonly reported poor knowledge of stroke prevention therapies (77 %), low trust in their personal doctor (34 %), problems due to medication cost (32 %), and perceived discrimination by the health system (30 %) (Table 2). A similar number of participants perceived discrimination due to race/ethnicity (25 %), as compared to discrimination due to education or income (22 %). There was a modest correlation between increasing number of barriers and non-adherence to medications (Spearman’s rho 0.25, p < 0.001; Fig. 2).
Table 2.
Prevalence of Barriers to Medication Adherence in Patients Who Were and Were Not Adherent to Medications
| Potential barriers to medication adherence | Overall; N = 600 | Adherent; N = 358; 59.7 % | Non-adherent; N = 242; 40.3 % | P-value |
|---|---|---|---|---|
| Barriers Related to Interactions between Patients and their Personal Doctor | ||||
| Increased concerns about medications | 85 (14.4) | 23 (6.5) | 62 (26.4) | < 0.001 |
| Low perceived necessity of medications | 150 (26.6) | 87 (25.7) | 63 (28.0) | 0.55 |
| Poor understanding of stroke prevention | 466 (77.7) | 272 (76.0) | 194 (80.2) | 0.23 |
| Low trust in personal doctor | 205 (34.2) | 103 (28.8) | 102 (42.1) | 0.001 |
| Problems communicating with doctor due to language | 90 (15.1) | 44 (12.3) | 46 (19.3) | 0.02 |
| Barriers Related to Interactions between Patients and the Health System | ||||
| Perceive discrimination due to race/ethnicity or education/income | 175 (30.0) | 75 (21.6) | 100 (42.2) | < 0.001 |
| Problems due to medication cost | 196 (32.8) | 110 (30.7) | 86 (36.0) | 0.18 |
| Difficulty accessing health care | 68 (11.4) | 29 (8.1) | 39 (16.3) | 0.002 |
| Lack continuity of care | 132 (22.6) | 69 (19.8) | 63 (26.7) | 0.05 |
Data presented as number (%). Numbers do not always add up to 100 % due to missing cases; Fewer than 3% missing for all variables other than perceived necessity of medication, for which 6.3 % of responses were missing
Figure 2.
Association between number of barriers and adherence to medications in survivors of strokes and transient ischemic attacks.
In unadjusted analyses (Table 2), the following barriers were significantly (p < 0.05) more common in non-adherent participants compared to those who were adherent: increased concerns about medications, low trust in doctor, problems communicating with doctors due to language problems, perceived discrimination by the health care system, difficulty accessing health care, and poor continuity of medical care.
In the fully adjusted model (Table 3), the only barriers that remained significantly associated with medication non-adherence included increased concerns about medications [OR 5.1 (95 % CI 2.8 to 9.2)] and perceived discrimination on account of race, ethnicity, education or income [OR 1.8 (95 % CI 1.1 to 2.8)]. Age was also associated with adherence; older patients were less likely to be non-adherent.
Table 3.
Unadjusted and Adjusted Associations between Barriers to Adherence and Non-Adherence to Medications in Survivors of Strokes and Transient Ischemic Attacks
| Potential barriers to medication adherence | Unadjusted | P-Value | Adjusted* | P-Value |
|---|---|---|---|---|
| Odds ratio (95 % CI) | Odds ratio (95 % CI) | |||
| High concerns about medications | 5.19 (3.11, 8.66) | < 0.001 | 5.09 (2.81, 9.24) | < 0.001 |
| Low perceived need for medications | 1.12 (0.77, 1.64) | 0.55 | 1.23 (0.79, 1.91) | 0.36 |
| Low knowledge of stroke risk factors | 1.28 (0.86, 1.90) | 0.23 | 1.22 (0.76, 1.96) | 0.42 |
| Low trust in personal doctor | 1.80 (1.28, 2.54) | 0.001 | 1.30 (0.84, 2.01) | 0.23 |
| Problems due to language | 1.71 (1.09, 2.68) | 0.02 | 1.32 (0.76, 2.29) | 0.32 |
| Perceive discrimination due to race, ethnicity, education, or income | 2.65 (1.84, 3.81) | < 0.001 | 1.79 (1.14, 2.81) | 0.01 |
| Problems due to medication cost | 1.27 (0.90, 1.79) | 0.18 | 0.87 (0.57, 1.32) | 0.50 |
| Difficulty accessing health care | 2.20 (1.32, 3.66) | 0.003 | 1.32 (0.70, 2.48) | 0.40 |
| Lack continuity of care | 1.47 (1.00, 2.18) | 0.05 | 1.09 (0.68, 1.74) | 0.73 |
*This model adjusted for all nine barriers and the following additional covariates: age, gender, race/ethnicity, income, education, Charlson. The only additional covariate associated with non-adherence was age [OR 0.97 (95 % CI 0.96, 0.99); p < 0.001]
In a subgroup analysis restricted to Hispanics, both increased concerns about medications [OR 6.9 (95 % CI 2.7 to 17.1)] and perceived discrimination [OR 3.1 (95 % CI 1.4 to 7.0)] were associated with non-adherence. In a subgroup analysis of African American participants, only increased concerns about medications [OR 7.2 (95 % CI 2.7 to 19.3] were associated with non-adherence, although perceived discrimination had a similar direction of association that did not reach statistical significance [OR 1.6 (95 % CI 0.8 to 3.1)].
DISCUSSION
In our sample of community-dwelling survivors of strokes and TIAs without substantial cognitive impairment, we found that among the nine barriers assessed, increased concerns about medications—present in nearly 25 % of non-adherent participants—was by far the most important barrier to medication adherence. Participants with increased concerns about medications had five times increased odds of non-adherence as compared to those without increased concerns. Perceived discrimination by the health care system due to race, ethnicity, education, or income was another significant barrier to adherence. None of the other barriers in the model proposed by Osterberg and Blaschke,6 at least as measured by this study, were significant independent predictors of non-adherence.
There is a large body of research demonstrating that beliefs about medications in general, and concerns in particular, are associated with adherence to medications prescribed for chronic illnesses.8, 14, 27–29 This analysis adds to the literature by showing that in comparison with other possible barriers to medication adherence, concerns about medications, such as becoming dependent on them or worrying about their long-term consequences, have the strongest association with non-adherence and hence represent a key barrier to overcoming non-adherence, at least in our group of stroke and TIA survivors.
The association we found between perceived discrimination and non-adherence has been demonstrated in other patient populations such as HIV-infected individuals, and in studies of adherence to other types of recommended health behaviors.30–33 Amongst certain minorities, there is a history of discrimination that continues to inform current perceptions about health care and can lead to distrust in treatments espoused by the health care system.34 Interestingly, in our subgroup analysis, we found that perceived discrimination was a barrier to medication adherence in Hispanics, but not in African Americans. This was surprising as, in some studies, African Americans are equally or more likely to report distress from perceived discrimination on account of minority status as compared to Hispanics.35 This finding reinforces the importance of training clinicians and staff to understand these perceptions, particularly among Hispanics, and to try to overcome them through improved communication and trust. Further, interventions might aim to empower stroke survivors and their caregivers to learn how to advocate for the best treatments available, irrespective of how others in the health care system treat them.
Even though our sample had a large number of low-income participants and nearly one-third reported at least some problems due to medication cost, concerns about medication costs were not an important barrier to medication adherence in this sample. This may be partially explained by the fact that almost all participants, including low-income ones, had at least some prescription insurance and New York State provides low out-of-pocket costs for Medicaid beneficiaries. As such, copays for beneficiaries are rarely prohibitive. Furthermore, an increasing number of pharmacies now offer a wide variety of generic prescriptions at reasonable prices, even for those without good coverage. Even when patients have some trouble affording medications, they may be willing to find a way to pay for the medications if they believe the medications are important enough.36 Interestingly, in a recent randomized clinical trial, providing full prescription coverage for cardiovascular medications to patients enrolled in a health maintenance organization who had suffered a myocardial infarction resulted in modest improvements in medication adherence.37
There are several limitations that should be considered in the interpretation of our findings. First, adherence was measured using self-report, and objective measures of adherence were not available to confirm responses. Further, the scale used to measure adherence did not specifically ask about adherence to stroke medications, and has not previously been validated in stroke survivors. Nevertheless, self-report measures of adherence are often highly correlated with objective measures,17 and have been reliable predictors of poor outcomes in multiple studies.38, 39 If anything, self-reports may have underestimated the true prevalence of non-adherence in our sample.40 Stroke/TIA history was also based on self-report and was not confirmed by review of medical records; this may have led to the inclusion of some participants who did not have a cerebrovascular event. Another limitation is that the inclusion of a large number of low income African Americans and Hispanic participants may limit the generalizability of our findings to other study populations. However, given the fact that there are unexplained disparities in stroke outcomes, the diversity of our sample can also be viewed as a strength of our study, especially since these groups are often understudied and may be at increased risk for non-adherence.41 Some of our measures of barriers to adherence, such as concerns about medications, were modified from previously validated scales (e.g., BMQ). Nevertheless, we carefully adapted these questions to be more easily understood by our study population. Our choices for cut-points for categorizing participants as having increased concerns about medications, low perceived necessity of medications, and low trust in doctors were made empirically, and hence, estimates of the proportion of participants with each of these barriers should be interpreted with caution. Finally, additional potential barriers to medication adherence such as medication regimen complexity, low quality of provider communication, and lack of social support were not assessed by the study. In particular, lack of collaborative, patient-centered communication has been associated with non-adherence in prior studies.42 Caregiver support may also be particularly important for maintaining medication adherence in stroke survivors who have physical and cognitive limitations as a result of their stroke. Nevertheless, our selection of barriers was guided by a widely referenced conceptual model and we assessed nearly all of the patient-related barriers in this model.6 Finally, one should extrapolate our results to cognitively impaired stroke survivors with caution, as only cognitively intact stroke survivors were included in this analysis.
These limitations notwithstanding, our results should be helpful for informing the design of interventions to help stroke and TIA survivors improve their adherence to medications through modifying concerns about medications. In fact, the intervention that is being tested in the parent study15 may be particularly well suited to overcoming the barriers identified in this analysis. In this intervention, peer-leaders lead community-based workshops that teach stroke and TIA survivors how to better self-manage their health in order to decrease their risk of recurrent cerebrovascular events. This format may be particularly well-suited to providing patients with an opportunity to discuss concerns about medications and hear from respected peers as to whether these concerns are valid. Generally, clinicians also have the potential to modify or overcome concerns in individual patients through open-ended and collaborative communication.42, 43 Counseling strategies, such as motivational interviewing in particular, have been successful at increasing medication adherence in some studies conducted in minority populations.44 However, in order to be successful, clinicians are encouraged to assess patients’ concerns about medications.
Acknowledgements
We gratefully acknowledge the East and Central Harlem Community Action Board and the study participants for their generous contributions. Dr. Kronish received support from the National Heart, Lung and Blood Institute (K23 HL098359). Dr. Horowitz, Dr. Goldfinger and Ms. Fei received support from the National Institute of Minority Health and Health Disparities (P60MD00270), and Dr. Horowitz received funding from the National Center for Research Resources (UL1RR029887).
Conflict of Interest
The authors declare that they do not have a conflict of interest.
REFERENCES
- 1.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 2.Sacco RL, Adams R, Albers G, Alberts MJ, Benavente O, Furie K, Goldstein LB, Gorelick P, Halperin J, Harbaugh R, Johnston SC, Katzan I, Kelly-Hayes M, Kenton EJ, Marks M, Schwamm LH, Tomsick T. Guidelines for prevention of stroke in patients with ischemic stroke or transient ischemic attack: a statement for healthcare professionals from the american heart association/american stroke association council on stroke: co-sponsored by the council on cardiovascular radiology and intervention: the American Academy of Neurology affirms the value of this guideline. Circulation. 2006;113:e409–449. [PubMed] [Google Scholar]
- 3.Rothwell PM, Algra A, Amarenco P. Medical treatment in acute and long-term secondary prevention after transient ischaemic attack and ischaemic stroke. Lancet. 2011;377:1681–1692. doi: 10.1016/S0140-6736(11)60516-3. [DOI] [PubMed] [Google Scholar]
- 4.Bushnell CD, Zimmer LO, Pan W, Olson DM, Zhao X, Meteleva T, Schwamm L, Ovbiagele B, Williams L, Labresh KA, Peterson ED. Persistence with stroke prevention medications 3 months after hospitalization. Arch Neurol. 2010;67:1456–1463. doi: 10.1001/archneurol.2010.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan NA, Yun L, Humphries K, Kapral M. Antihypertensive drug use and adherence after stroke: are there sex differences? Stroke. 2010;41:1445–1449. doi: 10.1161/STROKEAHA.110.579375. [DOI] [PubMed] [Google Scholar]
- 6.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 7.Mann DM, Woodward M, Muntner P, Falzon L, Kronish I. Predictors of nonadherence to statins: a systematic review and meta-analysis. Ann Pharmacother. 2010;44:1410–1421. doi: 10.1345/aph.1P150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Carroll R, Whittaker J, Hamilton B, Johnston M, Sudlow C, Dennis M. Predictors of adherence to secondary preventive medication in stroke patients. Ann Behav Med. 2011;41:383–390. doi: 10.1007/s12160-010-9257-6. [DOI] [PubMed] [Google Scholar]
- 9.Chambers JA, O’Carroll RE, Hamilton B, Whittaker J, Johnston M, Sudlow C, Dennis M. Adherence to medication in stroke survivors: a qualitative comparison of low and high adherers. Br J Health Psychol. 2011;16:592–609. doi: 10.1348/2044-8287.002000. [DOI] [PubMed] [Google Scholar]
- 10.Cox AM, McKevitt C, Rudd AG, Wolfe CD. Socioeconomic status and stroke. Lancet Neurol. 2006;5:181–188. doi: 10.1016/S1474-4422(06)70351-9. [DOI] [PubMed] [Google Scholar]
- 11.Stansbury JP, Jia H, Williams LS, Vogel WB, Duncan PW. Ethnic disparities in stroke: epidemiology, acute care, and post-acute outcomes. Stroke. 2005;36:374–386. doi: 10.1161/01.STR.0000153065.39325.fd. [DOI] [PubMed] [Google Scholar]
- 12.Sacco RL, Boden-Albala B, Abel G, Lin IF, Elkind M, Hauser WA, Paik MC, Shea S. Race-ethnic disparities in the impact of stroke risk factors: the northern Manhattan stroke study. Stroke. 2001;32:1725–1731. doi: 10.1161/01.STR.32.8.1725. [DOI] [PubMed] [Google Scholar]
- 13.Leventhalm H, Benyamini Y, Brownlee S, et al. Illness Perceptions: Theoretical Foundations. In: Petrie KJ, Weinman J, et al., editors. Perceptions of Health and Illness. Amsterdam: Harwood; 1997. pp. 19–45. [Google Scholar]
- 14.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/S0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 15.Goldfinger JZ, Kronish IM, Fei K, Graciani A, Rosenfeld P, Lorig K, Horowitz CR. Peer education for secondary stroke prevention in inner-city minorities: Design and methods of the prevent recurrence of all inner-city strokes through education randomized controlled trial. Contemp Clin Trials. 2012 [DOI] [PMC free article] [PubMed]
- 16.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich). 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 17.Krousel-Wood M, Islam T, Webber LS, Re RN, Morisky DE, Muntner P. New medication adherence scale versus pharmacy fill rates in seniors with hypertension. Am J Manag Care. 2009;15:59–66. [PMC free article] [PubMed] [Google Scholar]
- 18.Holt EW, Muntner P, Joyce C, Morisky DE, Webber LS, Krousel-Wood M. Life events, coping, and antihypertensive medication adherence among older adults: the cohort study of medication adherence among older adults. Am J Epidemiol. 2012;176(Suppl 7):S64–71. doi: 10.1093/aje/kws233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Svarstad BL, Chewning BA, Sleath BL, Claesson C. The brief medication questionnaire: a tool for screening patient adherence and barriers to adherence. Patient Educ Couns. 1999;37:113–124. doi: 10.1016/S0738-3991(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 20.Furie KL, Kasner SE, Adams RJ, Albers GW, Bush RL, Fagan SC, Halperin JL, Johnston SC, Katzan I, Kernan WN, Mitchell PH, Ovbiagele B, Palesch YY, Sacco RL, Schwamm LH, Wassertheil-Smoller S, Turan TN, Wentworth D. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:227–276. doi: 10.1161/STR.0b013e3181f7d043. [DOI] [PubMed] [Google Scholar]
- 21.Safran DG, Kosinski M, Tarlov AR, Rogers WH, Taira DH, Lieberman N, Ware JE. The primary care assessment survey: Tests of data quality and measurement performance. Med Care. 1998;36:728–739. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Stewart AL, Napoles-Springer A, Perez-Stable EJ. Interpersonal processes of care in diverse populations. Milbank Q. 1999;77:305–339. doi: 10.1111/1468-0009.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bindman AB, Grumbach K, Osmond D, Komaromy M, Vranizan K, Lurie N, Billings J, Stewart A. Preventable hospitalizations and access to health care. JAMA. 1995;274:305–311. doi: 10.1001/jama.1995.03530040033037. [DOI] [PubMed] [Google Scholar]
- 24.Madden JM, Graves AJ, Zhang F, Adams AS, Briesacher BA, Ross-Degnan D, Gurwitz JH, Pierre-Jacques M, Safran DG, Adler GS, Soumerai SB. Cost-related medication nonadherence and spending on basic needs following implementation of medicare part d. JAMA. 2008;299:1922–1928. doi: 10.1001/jama.299.16.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banks JL, Marotta CA. Outcomes validity and reliability of the modified rankin scale: Implications for stroke clinical trials: A literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 26.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 27.Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res. 1999;47:555–567. doi: 10.1016/S0022-3999(99)00057-4. [DOI] [PubMed] [Google Scholar]
- 28.Sud A, Kline-Rogers EM, Eagle KA, Fang J, Armstrong DF, Rangarajan K, Otten RF, Stafkey-Mailey DR, Taylor SD, Erickson SR. Adherence to medications by patients after acute coronary syndromes. Ann Pharmacother. 2005;39:1792–1797. doi: 10.1345/aph.1G249. [DOI] [PubMed] [Google Scholar]
- 29.Phatak HM, Thomas J., 3rd Relationships between beliefs about medications and nonadherence to prescribed chronic medications. Ann Pharmacother. 2006;40:1737–1742. doi: 10.1345/aph.1H153. [DOI] [PubMed] [Google Scholar]
- 30.Bird ST, Bogart LM, Delahanty DL. Health-related correlates of perceived discrimination in hiv care. AIDS Patient Care STDS. 2004;18:19–26. doi: 10.1089/108729104322740884. [DOI] [PubMed] [Google Scholar]
- 31.Boarts JM, Bogart LM, Tabak MA, Armelie AP, Delahanty DL. Relationship of race-, sexual orientation-, and hiv-related discrimination with adherence to hiv treatment: a pilot study. J Behav Med. 2008;31:445–451. doi: 10.1007/s10865-008-9169-0. [DOI] [PubMed] [Google Scholar]
- 32.Casagrande SS, Gary TL, LaVeist TA, Gaskin DJ, Cooper LA. Perceived discrimination and adherence to medical care in a racially integrated community. J Gen Intern Med. 2007;22:389–395. doi: 10.1007/s11606-006-0057-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dailey AB, Kasl SV, Holford TR, Jones BA. Perceived racial discrimination and nonadherence to screening mammography guidelines: results from the race differences in the screening mammography process study. Am J Epidemiol. 2007;165:1287–1295. doi: 10.1093/aje/kwm004. [DOI] [PubMed] [Google Scholar]
- 34.Kronish IM, Leventhal H, Horowitz CR. Understanding minority patients’ beliefs about hypertension to reduce gaps in communication between patients and clinicians. J Clin Hypertens (Greenwich). 2012;14:38–44. doi: 10.1111/j.1751-7176.2011.00558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnson RL, Saha S, Arbelaez JJ, Beach MC, Cooper LA. Racial and ethnic differences in patient perceptions of bias and cultural competence in health care. J Gen Intern Med. 2004;19:101–110. doi: 10.1111/j.1525-1497.2004.30262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Piette JD, Heisler M, Horne R, Caleb Alexander G. A conceptually based approach to understanding chronically ill patients’ responses to medication cost pressures. Soc Sci Med. 2006;62:846–857. doi: 10.1016/j.socscimed.2005.06.045. [DOI] [PubMed] [Google Scholar]
- 37.Choudhry NK, Avorn J, Glynn RJ, Antman EM, Schneeweiss S, Toscano M, Reisman L, Fernandes J, Spettell C, Lee JL, Levin R, Brennan T, Shrank WH. Post-myocardial infarction free Rx event and economic evaluation (MI FREEE) trial. Full coverage for preventive medications after myocardial infarction. N Engl J Med. 2011;365:2088–2097. doi: 10.1056/NEJMsa1107913. [DOI] [PubMed] [Google Scholar]
- 38.Gehi AK, Ali S, Na B, Whooley MA. Self-reported medication adherence and cardiovascular events in patients with stable coronary heart disease: The heart and soul study. Arch Intern Med. 2007;167:1798–1803. doi: 10.1001/archinte.167.16.1798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rasmussen JN, Chong A, Alter DA. Relationship between adherence to evidence-based pharmacotherapy and long-term mortality after acute myocardial infarction. JAMA. 2007;297:177–186. doi: 10.1001/jama.297.2.177. [DOI] [PubMed] [Google Scholar]
- 40.Shi L, Liu J, Koleva Y, Fonseca V, Kalsekar A, Pawaskar M. Concordance of adherence measurement using self-reported adherence questionnaires and medication monitoring devices. PharmacoEconomics. 2010;28:1097–1107. doi: 10.2165/11537400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Y, Baik SH, Chang CC, Kaplan CM, Lave JR. Disability, race/ethnicity, and medication adherence among medicare myocardial infarction survivors. Am Heart J. 2012;164:425–433. doi: 10.1016/j.ahj.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schoenthaler A, Chaplin WF, Allegrante JP, Fernandez S, Diaz-Gloster M, Tobin JN, Ogedegbe G. Provider communication effects medication adherence in hypertensive African Americans. Patient Educ Couns. 2009;75:185–191. doi: 10.1016/j.pec.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cutrona SL, Choudhry NK, Stedman M, Servi A, Liberman JN, Brennan T, Fischer MA, Brookhart MA, Shrank WH. Physician effectiveness in interventions to improve cardiovascular medication adherence: a systematic review. J Gen Intern Med. 2010;25:1090–1096. doi: 10.1007/s11606-010-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogedegbe G, Chaplin W, Schoenthaler A, Statman D, Berger D, Richardson T, Phillips E, Spencer J, Allegrante JP. A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens. 2008;21:1137–1143. doi: 10.1038/ajh.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]