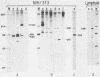
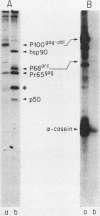
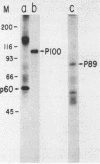
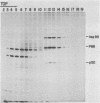
Abstract
The v-abl and v-src oncogenes encode protein-tyrosine kinases that possess different biological properties in spite of their high degree of amino acid conservation. To correlate functional differences with structural domains of the two oncogenes, we recombined v-abl and v-src just downstream of the lysines in their ATP-binding sites, within the kinase domain. The biological activity of the chimeric genes was studied and compared with that of v-src and v-abl. The v-src/v-abl recombinant shared with v-src and v-abl the ability to transform fibroblasts. In addition, like v-abl, it transformed lymphoid cells and relieved a hematopoietic cell line of its interleukin 3 requirement. In contrast, the reciprocal construct, v-abl/v-src, was transformation defective. Lack of biological activity correlated with formation of a stable complex between the chimeric protein and two cellular proteins and with low kinase activity. We conclude that the specificity within the kinase domain determines the particular biological behavior of protein-tyrosine kinase oncogenes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brugge J. S., Darrow D. Analysis of the catalytic domain of phosphotransferase activity of two avian sarcoma virus-transforming proteins. J Biol Chem. 1984 Apr 10;259(7):4550–4557. [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Brugge J., Yonemoto W., Darrow D. Interaction between the Rous sarcoma virus transforming protein and two cellular phosphoproteins: analysis of the turnover and distribution of this complex. Mol Cell Biol. 1983 Jan;3(1):9–19. doi: 10.1128/mcb.3.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook W. D., Metcalf D., Nicola N. A., Burgess A. W., Walker F. Malignant transformation of a growth factor-dependent myeloid cell line by Abelson virus without evidence of an autocrine mechanism. Cell. 1985 Jul;41(3):677–683. doi: 10.1016/s0092-8674(85)80048-9. [DOI] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross F. R., Garber E. A., Hanafusa H. N-terminal deletions in Rous sarcoma virus p60src: effects on tyrosine kinase and biological activities and on recombination in tissue culture with the cellular src gene. Mol Cell Biol. 1985 Oct;5(10):2789–2795. doi: 10.1128/mcb.5.10.2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePinho R., Kruger K., Andrews N., Lutzker S., Baltimore D., Alt F. W. Molecular basis of heavy-chain class switching and switch region deletion in an Abelson virus-transformed cell line. Mol Cell Biol. 1984 Dec;4(12):2905–2910. doi: 10.1128/mcb.4.12.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garber E. A., Mayer B. J., Jove R., Hanafusa H. Analysis of p60v-src mutants carrying lesions involved in temperature sensitivity. J Virol. 1987 Feb;61(2):354–360. doi: 10.1128/jvi.61.2.354-360.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Cooper J. A. Protein-tyrosine kinases. Annu Rev Biochem. 1985;54:897–930. doi: 10.1146/annurev.bi.54.070185.004341. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Taylor S. S., Sefton B. M. Direct evidence that oncogenic tyrosine kinases and cyclic AMP-dependent protein kinase have homologous ATP-binding sites. Nature. 1984 Aug 16;310(5978):589–592. doi: 10.1038/310589a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leof E. B., Van Wyk J. J., O'Keefe E. J., Pledger W. J. Epidermal growth factor (EGF) is required only during the traverse of early G1 in PDGF stimulated density-arrested BALB/c-3T3 cells. Exp Cell Res. 1983 Aug;147(1):202–208. doi: 10.1016/0014-4827(83)90285-9. [DOI] [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Baltimore D. Specific transforming potential of oncogenes encoding protein-tyrosine kinases. EMBO J. 1985 Jul;4(7):1769–1774. doi: 10.1002/j.1460-2075.1985.tb03849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Nabel G., Palacios R., Baltimore D. Abelson virus abrogation of interleukin-3 dependence in a lymphoid cell line. Mol Cell Biol. 1986 Nov;6(11):4133–4135. doi: 10.1128/mcb.6.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtagh K., Skladany G., Hoag J., Rosenberg N. Abelson murine leukemia virus variants with increased oncogenic potential. J Virol. 1986 Nov;60(2):599–606. doi: 10.1128/jvi.60.2.599-606.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliff A., Agranovsky O., McKinney M. D., Murty V. V., Bauchwitz R. Friend murine leukemia virus-immortalized myeloid cells are converted into tumorigenic cell lines by Abelson leukemia virus. Proc Natl Acad Sci U S A. 1985 May;82(10):3306–3310. doi: 10.1073/pnas.82.10.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A., Anderson S. M. Hematopoietic cell transformation by a murine recombinant retrovirus containing the src gene of Rous sarcoma virus. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2374–2378. doi: 10.1073/pnas.81.8.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce J. H., Di Fiore P. P., Aaronson S. A., Potter M., Pumphrey J., Scott A., Ihle J. N. Neoplastic transformation of mast cells by Abelson-MuLV: abrogation of IL-3 dependence by a nonautocrine mechanism. Cell. 1985 Jul;41(3):685–693. doi: 10.1016/s0092-8674(85)80049-0. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L. Creation of a chimeric oncogene: analysis of the biochemical and biological properties of v-erbB/src fusion polypeptide. J Virol. 1987 Jun;61(6):1938–1948. doi: 10.1128/jvi.61.6.1938-1948.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prywes R., Hoag J., Rosenberg N., Baltimore D. Protein stabilization explains the gag requirement for transformation of lymphoid cells by Abelson murine leukemia virus. J Virol. 1985 Apr;54(1):123–132. doi: 10.1128/jvi.54.1.123-132.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N. E., Clark D. R., Witte O. N. Abelson murine leukemia virus mutants deficient in kinase activity and lymphoid cell transformation. J Virol. 1980 Dec;36(3):766–774. doi: 10.1128/jvi.36.3.766-774.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D., Scher C. D. In vitro transformation of lymphoid cells by Abelson murine leukemia virus. Proc Natl Acad Sci U S A. 1975 May;72(5):1932–1936. doi: 10.1073/pnas.72.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scher C. D., Siegler R. Direct transformation of 3T3 cells by Abelson murine leukaemia virus. Nature. 1975 Feb 27;253(5494):729–731. doi: 10.1038/253729a0. [DOI] [PubMed] [Google Scholar]
- Schwartz D. E., Tizard R., Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983 Mar;32(3):853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Takeya T., Feldman R. A., Hanafusa H. DNA sequence of the viral and cellular src gene of chickens. 1. Complete nucleotide sequence of an EcoRI fragment of recovered avian sarcoma virus which codes for gp37 and pp60src. J Virol. 1982 Oct;44(1):1–11. doi: 10.1128/jvi.44.1.1-11.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. Y., Baltimore D. Localization of tyrosine kinase-coding region in v-abl oncogene by the expression of v-abl-encoded proteins in bacteria. J Biol Chem. 1985 Jan 10;260(1):64–71. [PubMed] [Google Scholar]
- Wang J. Y. Isolation of antibodies for phosphotyrosine by immunization with a v-abl oncogene-encoded protein. Mol Cell Biol. 1985 Dec;5(12):3640–3643. doi: 10.1128/mcb.5.12.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S. M., Rosenberg N. E., Witte O. N. A membrane-associated, carbohydrate-modified form of the v-abl protein that cannot be phosphorylated in vivo or in vitro. J Virol. 1984 Sep;51(3):620–627. doi: 10.1128/jvi.51.3.620-627.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler S. F., Whitlock C. A., Goff S. P., Gifford A., Witte O. N. Lethal effect of the Abelson murine leukemia virus transforming gene product. Cell. 1981 Dec;27(3 Pt 2):477–486. doi: 10.1016/0092-8674(81)90389-5. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis: a simple method using two oligonucleotide primers and a single-stranded DNA template. DNA. 1984 Dec;3(6):479–488. doi: 10.1089/dna.1.1984.3.479. [DOI] [PubMed] [Google Scholar]