ABSTRACT
BACKGROUND
Patients at risk for generating high health care expenditures often receive fragmented, low-quality, inefficient health care. Guided Care is designed to provide proactive, coordinated, comprehensive care for such patients.
OBJECTIVE
We hypothesized that Guided Care, compared to usual care, produces better functional health and quality of care, while reducing the use of expensive health services.
DESIGN
32-month, single-blind, matched-pair, cluster-randomized controlled trial of Guided Care, conducted in eight community-based primary care practices.
PATIENTS
The “Hierarchical Condition Category” (HCC) predictive model was used to identify high-risk older patients who were insured by fee-for-service Medicare, a Medicare Advantage plan or Tricare. Patients with HCC scores in the highest quartile (at risk for generating high health care expenditures during the coming year) were eligible to participate.
INTERVENTION
A registered nurse collaborated with two to five primary care physicians in providing eight services to participants: comprehensive assessment, evidence-based care planning, proactive monitoring, care coordination, transitional care, coaching for self-management, caregiver support, and access to community-based services.
MAIN MEASURES
Functional health was measured using the Short Form–36. Quality of care and health services utilization were measured using the Patient Assessment of Chronic Illness Care and health insurance claims, respectively.
KEY RESULTS
Of the eligible patients, 904 (37.8 %) gave written consent to participate; of these, 477 (52.8 %) completed the final interview, and 848 (93.8 %) provided complete claims data. In intention-to-treat analyses, Guided Care did not significantly improve participants’ functional health, but it was associated with significantly higher participant ratings of the quality of care (difference = 0.27, 95 % CI = 0.08–0.45) and 29 % lower use of home care (95 % CI = 3–48 %).
CONCLUSIONS
Guided Care improves high-risk older patients’ ratings of the quality of their care, and it reduces their use of home care, but it does not appear to improve their functional health.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-012-2287-y) contains supplementary material, which is available to authorized users.
KEY WORDS: multi-morbidity, primary care, care management, randomized controlled trial, transitional care
INTRODUCTION
One quarter (24 %) of Americans have two or more chronic conditions.1 Their health care is often fragmented, low-quality, inefficient and unsatisfactory to them and their physicians.2 The Institute of Medicine has described chronic care in America as “a nightmare to navigate.”3 People with multi-morbidity are also at high risk for generating high health care expenditures: 96 % of the Medicare budget is spent on beneficiaries with multiple chronic conditions.4
Several flaws in the infrastructure of the U.S. health care system underlie these problems: inadequate professional education,5 inconsistent use of information technology, payment incentives that drive high-volume care, and lack of financial support for inter-professional communication and patient engagement in self-care.6
Correcting these flaws will require both long-term and short-term initiatives. Reforming health professional education, implementing interoperable health information technology, and migrating health insurance from fee-for-service to “value-based” will take many years. In the meantime, however, as millions of baby-boomers are reaching retirement age each year, near-term improvements may be achievable by developing and adopting clinical models that improve outcomes for high-risk people with multiple chronic conditions. Some such models have shown promise,7–12 while others have either failed13 or have not yet been tested rigorously.14
Guided Care is a new model of comprehensive, interdisciplinary care that comprises primary care-based care management, transitional care, and support for self-management and family caregiving. An interdisciplinary team developed this model by identifying from the scientific literature several successful complementary chronic care innovations and combining them into a single model of care that could be adopted widely by primary care practices.
In Guided Care, a registered nurse partners with two to five physicians in a primary care practice to provide 50–60 high-risk multi-morbid patients with eight services: home-based assessment of patients’ needs and goals, evidence-based care planning, proactive monitoring, care coordination, transitional care, coaching for self-management, caregiver support, and access to community-based services.15,16
The present paper reports the final results of a 32-month study designed to test the hypothesis that Guided Care teams, compared to usual care teams, produce better functional health (primary outcome) and quality of care for their patients with less use of expensive health services (secondary outcomes). Patients were randomized by cluster (i.e., by team of physicians), to acknowledge that Guided Care is a team-level intervention and to minimize contamination across groups.
METHODS
During 2006–2009, we conducted a matched-pair, cluster-randomized controlled trial of Guided Care versus usual care in eight community-based primary care practices operated by three large delivery systems in urban and suburban Baltimore, MD and Washington DC. Six of the practices housed two teams apiece (2–5 physicians per team); two of the practices, selected for their similarities (in panel size, geography, and payer mix), housed one team apiece. Three of the practices relied on capitated payments, while five received primarily fee-for-service payments. Additional study details have been published previously.15,17 The study was approved by the Institutional Review Boards of the Johns Hopkins Bloomberg School of Public Health, Kaiser Permanente Mid-Atlantic States, and MedStar Physician Partners.
Selection of Physician Teams
Within the three health care delivery systems, teams of primary care physicians with aggregate panels of at least 400 patients aged 65 years or older and on-site office space for a Guided Care nurse were eligible for the study. Physicians within these teams were eligible to participate if they were board-certified general internists or family physicians who provided patient care at least 28 h per week. All 49 eligible physicians within the 14 eligible teams agreed to participate.
Recruitment of Nurses
We used traditional media to solicit applications from local registered nurses with at least 3 years of clinical experience. Applicants with experience in geriatric nursing, interest in counseling patients in self-management, and comfort with interdisciplinary team practice and information technology were given preference. Among the seven nurses hired, all were female, three were African-American, and four were white. The average age was 45 years (range = 32–57 years); the average nursing practice experience was 16 years (range = 4–31 years).
Recruitment of Patients
Patients of the participating physicians were selected for initial screening if they were age 65 years or older and covered by fee-for-service Medicare Parts A and B, a Kaiser Permanente Medicare health plan, or TriCare insurance (for retired military personnel and their dependents). Patients’ health insurance claims from the previous 12 months were analyzed using the Hierarchical Condition Category (HCC) predictive model, which uses diagnosis codes to estimate a person’s risk for generating high health care expenditures during the coming year.18 Patients were potentially eligible if their HCC risk ratios were in the highest quartile of the population of older patients covered by their health care insurer.
High-risk patients were initially contacted by mail. A professional interviewer then called those who had not “opted out” to describe the study, answer questions, and offer an in-home enrollment meeting. Interviewers then visited the homes of receptive patients to describe the study further, answer questions and obtain written informed consent. Potential participants were deemed ineligible if they did not have a telephone, did not speak English, were planning extended travel, or failed a brief cognitive screen and did not have a proxy who could provide consent.
Randomization
Each team of physicians and their participating patients comprised a “pod.” The study’s statistician, blinded to the identities of the pods, used a random number generator to assign one pod from each pair (matched by practice) to the Guided Care group and the other to the “usual care” control group. Group assignments were concealed until patient recruitment was completed.
Intervention
Before beginning practice, the seven nurses took a Guided Care preparatory course,19 and each completed a structured orientation to her assigned practice.20 At the rate of two patients per week, each nurse built a case load of 50–60 patients, each of whom received the eight Guided Care services throughout the study.15 The nurses received feedback on defined elements of their performance at monthly meetings with the research team. Participating physicians received an orientation to the nurses’ role and to the nature of the study, but no compensation for participating or incentives for attaining specific outcomes for their patients. Patients in the control pods continued to receive “usual care” from their physicians throughout the study.
Measurements
To evaluate the effects of Guided Care from the perspectives of all of the stakeholders involved in chronic care, we used a broad range of measures. Before randomization, in early 2006, professional interviewers conducted face-to-face, in-home interviews to assess participants’ functional health, quality of health care, sociodemographic characteristics and chronic medical conditions. During April to June of 2009, professional interviewers, who were masked to group assignment, conducted final computer-assisted telephone interviews that incorporated these same measures of functional health (the Short Form [SF]-36, version 221 and quality of care (the Patient Assessment of Chronic Illness Care22 and components of the Primary Care Assessment Survey (PCAS)23). Participants’ health insurers provided data on health services utilization. The National Death Index provided mortality status and dates of death.
Statistical Power
The pre-recruitment power analysis, based on simplifying assumptions, indicated that 580 participants would need to complete the final interview to provide greater than 80 % power to detect clinically significant, three-point differences on the physical and mental health components of the SF-36.24
Analysis
Missing baseline interview responses were addressed by generating five imputed data sets25 and combining inferences across them. We computed all scale scores as recommended by the scales’ originators, and analyzed all data according to the “intention-to-treat” principle.
We compared study groups’ baseline covariates within pairs of pods by testing jointly the significance of interaction terms in regression models with pair-specific intercepts and interaction terms that included pair and group assignment. We also compared the baseline covariates of the study completers and non-completers by testing jointly the significance of interaction terms in regression models with pod-specific intercepts and interaction terms that included pod and completion status.
To estimate the effect of Guided Care on the quality of care and on patients’ functional health (the study’s primary endpoint), use of health services and satisfaction with primary care, we used a two-stage analytic approach that accounts for clustering and imbalances in covariates at baseline. In the first stage, we used a model-based standardization procedure to estimate the pod-specific mean outcomes, setting the distribution of baseline covariates for each pod equal to the overall distribution of covariates for its matched pair. In the second stage, these pair-specific means (and their estimated variances) were used to estimate (using the Bayesian formalism) the overall treatment effects using two-level hierarchical models, a technique used in meta-analysis.26 This two-stage approach was fit for each of the five imputed baseline data sets. The posterior samples for the five analyses were combined. Based on the combined samples, the posterior mean and the 95 % posterior percentile interval for the overall treatment effect are reported for each endpoint. The two-stage approach was implemented using the statistical software packages R (Version 2.14.0, R Foundation for Statistical Computing, Vienna, Austria), Stata (Version 10.0, StataCorp LP, College Station, Texas) and OpenBUGS.27 Additional methodological details are available in the online appendix.
RESULTS
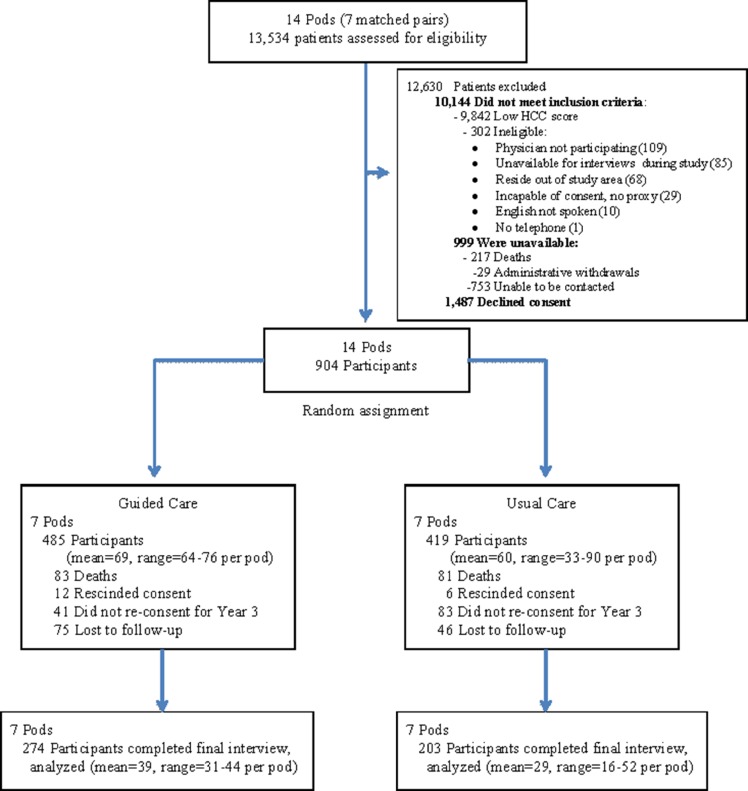
As shown in Fig. 1, we screened 13,534 patients in 14 pods and offered study participation to the 2,391 (17.7 %) who were eligible and available. Of these, 904 (37.8 %) gave informed consent and were allocated to receive either Guided Care (n = 485) or usual care (n = 419). The study participants’ baseline characteristics are shown in Table 1. Participants’ responses to each of the baseline interview items were more than 99 % complete, except for the question about finances (96 % complete). Pair-stratified analyses of the participants’ baseline characteristics showed that the two study groups were similar, except for statistically significant differences in finances (p = 0.005), HCC score (p < 0.001), difficulty with two or more instrumental activities of daily living (IADLs) (p = 0.03), SF-36 physical health (p < 0.001) and mental health (p = 0.005) scores, PCAS integration scores (p = 0.03) and satisfaction with their regular health care team (p = 0.008).
Figure 1.
Randomization of Pods and Flow of Participants through the Study
Table 1.
Characteristics of Participants at Baseline
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Pair 6 | Pair 7 | Pairs 1–7 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | GC 64 | UC 90 | GC 64 | UC 48 | GC 76 | UC 61 | GC 69 | UC 33 | GC 67 | UC 42 | GC 70 | UC 82 | GC 75 | UC 63 | GC 485 | UC 419 |
| Age (mean) | 79.3 | 78.9 | 77.3 | 78.8 | 78.3 | 77.7 | 75.1 | 76.7 | 77.0 | 77.0 | 77.7 | 78.2 | 76.3 | 78.3 | 77.2 | 78.1 |
| Sex (% female) | 43.8 | 42.2 | 51.6 | 64.6 | 65.8 | 63.9 | 44.9 | 66.7 | 62.7 | 57.1 | 44.3 | 50.0 | 64.0 | 58.7 | 54.2 | 55.4 |
| Race (%) | ||||||||||||||||
| Caucasian | 76.6 | 76.7 | 4.7 | 6.3 | 22.4 | 15.0 | 81.2 | 78.8 | 92.5 | 85.7 | 41.4 | 46.3 | 42.7 | 37.1 | 51.1 | 48.9 |
| African-American | 17.2 | 13.3 | 93.8 | 93.8 | 72.4 | 76.7 | 15.9 | 18.2 | 6.0 | 7.1 | 54.3 | 52.4 | 56.0 | 61.3 | 45.6 | 46.3 |
| Other | 6.3 | 10.0 | 1.6 | 0.0 | 5.3 | 8.3 | 2.9 | 3.0 | 1.5 | 7.1 | 4.3 | 1.2 | 1.3 | 1.6 | 3.3 | 4.8 |
| Marital status (% married) | 59.4 | 62.2 | 39.1 | 39.6 | 31.6 | 41.0 | 62.3 | 42.4 | 44.8 | 40.5 | 52.9 | 53.7 | 34.7 | 44.4 | 46.0 | 48.5 |
| Education (% > 12 years) | 82.8 | 77.8 | 32.8 | 29.2 | 51.3 | 47.5 | 44.9 | 21.2 | 44.8 | 28.6 | 45.7 | 41.5 | 25.3 | 25.4 | 46.4 | 43.4 |
| Finances each month (%)*** | ||||||||||||||||
| Some money left | 66.6 | 73.8 | 46.3 | 37.1 | 51.8 | 44.6 | 75.4 | 23.0 | 74.6 | 64.3 | 53.1 | 56.6 | 40.0 | 34.6 | 57.9 | 51.1 |
| Just enough money | 29.4 | 22.4 | 40.3 | 45.8 | 39.7 | 45.3 | 20.3 | 52.7 | 22.4 | 26.2 | 42.0 | 28.3 | 34.7 | 34.6 | 32.8 | 34.2 |
| Not enough money | 4.1 | 3.8 | 13.4 | 17.1 | 8.4 | 10.2 | 4.4 | 24.2 | 3.0 | 9.5 | 4.9 | 15.1 | 25.3 | 30.8 | 9.3 | 14.7 |
| Living alone (%) | 29.7 | 28.9 | 23.4 | 20.8 | 42.1 | 34.4 | 24.6 | 33.3 | 29.9 | 26.2 | 27.1 | 29.3 | 44.0 | 39.7 | 32.0 | 30.6 |
| HCC score (mean) *** | 2.4 | 1.7 | 2.0 | 1.8 | 1.6 | 2.1 | 2.1 | 2.3 | 2.3 | 2.0 | 2.0 | 2.0 | 2.2 | 2.1 | 2.1 | 2.0 |
| Self-rated health (%) | ||||||||||||||||
| Excellent/Very good | 20.3 | 25.5 | 10.9 | 12.5 | 25.1 | 16.4 | 34.8 | 6.1 | 31.4 | 4.8 | 21.5 | 24.4 | 13.3 | 11.1 | 22.5 | 16.7 |
| Good | 39.1 | 50.0 | 39.1 | 37.5 | 44.7 | 37.7 | 33.3 | 27.3 | 38.8 | 40.5 | 35.7 | 29.3 | 33.3 | 27.0 | 37.7 | 36.5 |
| Fair | 28.1 | 18.9 | 39.1 | 35.4 | 26.3 | 36.1 | 26.1 | 39.4 | 25.4 | 45.2 | 34.3 | 26.8 | 32.0 | 39.7 | 30.9 | 32.2 |
| Poor | 12.5 | 5.6 | 10.9 | 14.6 | 4.0 | 9.8 | 5.8 | 27.3 | 4.5 | 9.5 | 8.6 | 19.5 | 21.3 | 22.2 | 9.7 | 14.6 |
| Health conditions (mean) | 4.3 | 3.9 | 4.2 | 4.4 | 4.0 | 4.4 | 4.5 | 5.0 | 4.4 | 4.9 | 4.2 | 3.6 | 4.4 | 4.7 | 4.3 | 4.3 |
| Difficulty 1+ ADL (%) | 43.8 | 24.4 | 32.8 | 41.7 | 31.6 | 27.9 | 27.5 | 33.3 | 23.9 | 19.1 | 35.7 | 32.9 | 30.7 | 36.5 | 32.2 | 30.6 |
| Difficulty 2+ IADL (%)* | 26.6 | 16.7 | 28.1 | 45.8 | 17.1 | 31.2 | 33.3 | 17.4 | 16.4 | 28.6 | 22.9 | 25.6 | 36.0 | 38.1 | 23.5 | 29.6 |
| SF-36 scores (mean) | ||||||||||||||||
| Physical health*** | 36.5 | 41.8 | 37.6 | 34.5 | 42.4 | 39.4 | 39.1 | 38.9 | 40.4 | 32.3 | 38.6 | 38.6 | 35.7 | 36.8 | 38.7 | 38.1 |
| Mental health** | 49.8 | 52.3 | 52.3 | 46.3 | 51.2 | 47.1 | 49.9 | 43.0 | 49.8 | 48.5 | 50.4 | 50.5 | 48.6 | 47.5 | 50.3 | 48.7 |
GC Guided Care; UC Usual Care; HCC hierarchical condition category; ADL activities of daily living; IADL instrumental activities of daily living; SF-36 Short Form–36
*p < 0.05; **p < 0.01; ***p < 0.001
As shown in Fig. 1, 274 (56.5 %) of all Guided Care recipients and 203 (48.4 %) of all usual care recipients completed the final interview. Pod-specific completion rates ranged from 44.0 % to 65.7 % across Guided Care pods, and from 38.1 to 57.8 % across control pods. Pod-stratified comparisons of baseline characteristics showed that, compared to participants who completed the study, non-completers were significantly older (p = 0.01), had worse SF-36 physical health (p = 0.03) and mental health (p = 0.003) scores, and were more likely to be African-American (p < 0.001), to use health services heavily in the coming year (p = 0.001), and to have difficulty with two or more IADLs (p = 0.001).
Complete claims data were available for 92.0 % and 95.9 % of the Guided Care and usual care participants, respectively. The pod-specific rates ranged from 80.3 % to 98.6 % for Guided Care pods, and 91.7 % to 98.8 % for control pods.
As shown in Table 2, Guided Care had no statistically significant effects on self-rated health or on scores on the SF-36 mental health or physical health subscales. There were also no significant differences in mortality (OR = 0.88: 95 % CI: 0.59–1.31).
Table 2.
Effects on Functional Health
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Pair 6 | Pair 7 | Overall Treatment Effect* (95 % CI) | |
|---|---|---|---|---|---|---|---|---|
| SF-36 summary scores, adjusted means | ||||||||
| Physical Health | ||||||||
| Guided Care (n) | 35.47 (38) | 33.61 (44) | 38.39 (43) | 34.68 (33) | 36.03 (42) | 35.22 (31) | 39.37 (43) | |
| Usual Care (n) | 36.34 (17) | 36.64 (16) | 38.46 (42) | 36.61 (23) | 38.35 (52) | 35.68 (23) | 40.19 (28) | |
| Difference: GC–UC (standard error) | −0.86 (2.32) | −3.03 (2.31) | −0.07 (1.69) | −1.94 (2.12) | −2.32 (1.67) | −0.47 (2.13) | −0.82 (1.88) | −1.31 (−3.02, 0.41) |
| Mental Health | ||||||||
| Guided Care (n) | 48.26 (38) | 47.02 (44) | 51.15 (43) | 45.95 (33) | 50.68 (42) | 45.31 (31) | 50.91 (43) | |
| Usual Care (n) | 47.91 (17) | 48.05 (16) | 47.38 (41) | 46.49 (23) | 48.61 (52) | 45.98 (23) | 49.43 (28) | |
| Difference: GC–UC (standard error) | 0.36 (2.73) | −1.04 (2.72) | 3.78 (2.01) | −0.55 (2.50) | 2.07 (1.96) | −0.67 (2.51) | 1.48 (2.22) | 1.05 (−1.08, 3.12) |
| “Excellent, very good or good” self-rated health, adjusted percentages | ||||||||
| Guided Care (n) | 54.22 (38) | 53.53 (44) | 57.22 (43) | 39.17 (33) | 46.08 (42) | 44.21 (31) | 60.50 (43) | |
| Usual Care (n) | 48.57 (17) | 53.91 (16) | 63.26 (42) | 46.37 (23) | 52.53 (52) | 53.90 (23) | 55.19 (28) | |
| Odds ratio GC:UC (standard error) | 1.25 (0.63) | 1.03 (0.51) | 0.78 (0.31) | 0.74 (0.36) | 0.77 (0.30) | 0.69 (0.33) | 1.24 (0.54) | 0.89 (0.61–1.33) |
| Mortality, adjusted percentages | ||||||||
| Guided Care (n) | 13.53 (69) | 14.53 (67) | 17.14 (70) | 16.68 (75) | 21.23 (60) | 22.69 (64) | 16.26 (76) | |
| Usual Care (n) | 19.99 (33) | 24.06 (42) | 15.85 (82) | 20.00 (62) | 17.88 (90) | 27.93 (48) | 13.09 (60) | |
| Odds ratio GC:UC (standard error) | 0.63 (0.34) | 0.54 (0.26) | 1.10 (0.47) | 0.80 (0.34) | 1.24 (0.49) | 0.76 (0.32) | 1.29 (0.60) | 0.88 (0.59, 1.31) |
* Adjusted for baseline age, race, sex, education level, financial status, habitation status, HCC score, SF-36 physical and mental health subscales, and satisfaction with health care.
SF-36Short Form–36; GC Guided Care; UC Usual Care
As shown in Table 3, after 32 months, the adjusted aggregate quality of chronic care was significantly higher with Guided Care than with usual care (difference = 0.27; 95 % CI: 0.08–0.45). Guided Care recipients were also more likely to report “excellent or very good” access to telephone advice (OR = 1.66; 95 % CI: 1.02–2.73). The differences between the study groups on the other two quality of care subscales (PCAS communication and integration) and the other three individual quality of care items (access to “same day” appointments, satisfaction with primary care, and “wait time” for appointments) all favored the Guided Care group, but these difference were not statistically significant.
Table 3.
Effects on the Quality of Chronic Care
| Pair 1 | Pair 2 | Pair 3 | Pair 4 | Pair 5 | Pair 6 | Pair 7 | Overall Treatment Effect* (95 % CI) | |
|---|---|---|---|---|---|---|---|---|
| Patient Assessment of Chronic Illness Care, adjusted means | ||||||||
| Guided Care (n) | 3.19 (37) | 3.20 (42) | 3.40 (43) | 3.16 (32) | 3.18 (39) | 3.33 (31) | 3.76 (40) | |
| Usual Care (n) | 2.60 (17) | 3.13 (16) | 3.20 (41) | 3.08 (21) | 2.72 (50) | 3.11 (22) | 3.52 (27) | |
| Difference GC–UC (standard error) | 0.57 (0.24) | 0.08 (0.24) | 0.20 (0.17) | 0.08 (0.22) | 0.47 (0.17) | 0.22 (0.22) | 0.24 (0.20) | 0.27 (0.08, 0.45) |
| Primary Care Assessment Survey subscale scores, adjusted means | ||||||||
| Communication subscale score | ||||||||
| Guided Care (n) | 72.48 (37) | 72.95 (43) | 75.57 (43) | 63.80 (32) | 79.03 (41) | 71.04 (30) | 76.19 (43) | |
| Usual Care (n) | 63.23 (17) | 69.49 (16) | 75.28 (42) | 63.25 (23) | 71.16 (49) | 72.41 (22) | 75.08 (28) | |
| Difference GC–UC (standard error) | 9.26 (4.64) | 3.46 (4.61) | 0.30 (3.36) | 0.56 (4.23) | 7.87 (3.36) | −1.37 (4.32) | 1.11 (3.74) | 2.97 (−0.68, 6.61) |
| Integration subscale score | ||||||||
| Guided Care (n) | 76.42 (37) | 77.35 (43) | 74.09 (42) | 68.05 (32) | 78.49 (38) | 73.50 (30) | 75.97 (41) | |
| Usual Care (n) | 71.50 (17) | 69.12 (16) | 76.34 (42) | 66.98 (22) | 70.71 (44) | 71.76 (22) | 76.03 (27) | |
| Difference GC–UC (standard error) | 4.93 (4.59) | 8.23 (4.66) | −2.25 (3.38) | 1.07 (4.26) | 7.79 (3.52) | 1.74 (4.31) | −0.06 (3.78) | 2.79 (−0.97, 6.60) |
| Primary Care Assessment Survey individual items, adjusted percentages | ||||||||
| “Excellent or very good” access to telephone advice | ||||||||
| Guided Care (n) | 48.45 (36) | 57.77 (41) | 52.10 (43) | 33.89 (31) | 62.64 (41) | 68.81 (30) | 57.58 (43) | |
| Usual Care (n) | 13.71 (17) | 55.10 (16) | 39.89 (40) | 38.95 (23) | 37.46 (49) | 53.42 (22) | 53.40 (26) | |
| Odds ratio GC:UC (standard error) | 5.92 (4.51) | 1.11 (0.65) | 1.64 (0.71) | 0.80 (0.44) | 2.80 (1.24) | 1.92 (1.10) | 1.18 (0.57) | 1.66 (1.02, 2.73) |
| “Excellent or very good” wait time for doctor’s appointment when sick | ||||||||
| Guided Care (n) | 56.22 (37) | 52.80 (41) | 47.55 (42) | 26.19 (30) | 54.76 (40) | 30.79 (31) | 47.24 (38) | |
| Usual Care (n) | 18.63 (15) | 30.80 (16) | 44.29 (38) | 47.10 (20) | 47.76 (47) | 50.08 (21) | 54.96 (27) | |
| Odds ratio GC:UC (standard error) | 5.61 (3.95) | 2.51 (1.53) | 1.14 (0.48) | 0.40 (0.23) | 1.32 (0.56) | 0.44 (0.24) | 0.73 (0.35) | 1.09 (0.61, 2.04) |
| Access to doctor’s appointment “the same day” when sick | ||||||||
| Guided Care (n) | 30.03 (37) | 28.29 (39) | 29.39 (39) | 9.93 (30) | 18.46 (37) | 20.46 (30) | 19.86 (35) | |
| Usual Care (n) | 12.34 (14) | 12.50 (16) | 19.42 (34) | 10.58 (17) | 22.45 (44) | 19.16 (21) | 31.71 (25) | |
| Odds ratio (standard error) | 3.05 (2.61) | 2.76 (2.30) | 1.73 (0.96) | 0.93 (0.90) | 0.78 (0.44) | 1.09 (0.78) | 0.53 (0.32) | 1.20 (0.65, 2.29) |
| “Very satisfied” with regular health care | ||||||||
| Guided Care (n) | 61.13 (37) | 75.85 (43) | 62.48 (43) | 38.69 (31) | 72.11 (41) | 55.15 (30) | 70.31 (43) | |
| Usual Care (n) | 47.45 (17) | 42.05 (16) | 63.03 (42) | 49.11 (22) | 36.14 (50) | 69.61 (22) | 62.47 (28) | |
| Odds ratio GC:UC (standard error) | 1.74 (1.02) | 4.33 (2.64) | 0.98 (0.43) | 0.65 (0.35) | 4.57 (2.08) | 0.54 (0.31) | 1.42 (0.70) | 1.50 (0.77, 2.82) |
*Adjusted for baseline age, race, sex, education level, financial status, habitation status, HCC score, SF-36 physical and mental health subscales, and satisfaction with health care.
GC Guided Care; UC Usual Care
Table 4 shows the participants’ adjusted mean annual per capita rates of use of health services, as well as the adjusted “Guided Care: usual care” ratios of service use. Compared to the usual care group, the Guided Care group used home health care at a 29 % lower rate (ratio = 0.71; 95 % CI: 0.51–0.97). The Guided Care group had fewer hospital admissions (6 % reduction), 30-day hospital re-admissions (13 % reduction), skilled nursing facility days (26 % reduction), and primary care visits (1 % reduction), as well as 2 % more specialist visits and emergency department visits, but none of these differences were statistically significant.
Table 4.
Effects on Use of Health Services
| n (GC/UC) | Pair 1 (68/32) | Pair 2 (64/41) | Pair 3 (63/81) | Pair 4 (59/86) | Pair 5 (58/44) | Pair 6 (61/55) | Pair 7 (73/61) | Overall Treatment Effect* (95 % CI) |
|---|---|---|---|---|---|---|---|---|
| Hospital admissions | ||||||||
| Guided Care | 1.23 | 0.90 | 0.86 | 0.43 | 0.95 | 0.52 | 1.21 | |
| Usual Care | 1.56 | 0.92 | 0.65 | 0.65 | 0.73 | 0.63 | 1.32 | |
| GC:UC ratio (standard error) | 0.78 (0.20) | 0.98 (0.28) | 1.31 (0.36) | 0.67 (0.18) | 1.30 (0.36) | 0.83 (0.23) | 0.92 (0.21) | 0.94 (0.74, 1.19) |
| 30-day hospital re-admissions | ||||||||
| Guided Care | 0.45 | 0.23 | 0.17 | 0.07 | 0.21 | 0.07 | 0.20 | |
| Usual Care | 0.47 | 0.12 | 0.09 | 0.16 | 0.24 | 0.15 | 0.35 | |
| GC:UC ratio (standard error) | 0.97 (0.45) | 1.90 (1.19) | 1.80 (0.95) | 0.47 (0.27) | 0.87 (0.39) | 0.50 (0.33) | 0.57 (0.26) | 0.87 (0.53, 1.41) |
| Skilled nursing facility days | ||||||||
| Guided Care | 5.07 | 3.65 | 1.04 | 1.91 | 2.31 | 2.62 | 13.37 | |
| Usual Care | 10.41 | 1.30 | 3.14 | 6.31 | 4.63 | 2.98 | 5.72 | |
| GC:UC ratio (standard error) | 0.49 (0.25) | 2.81 (1.75) | 0.33 (0.18) | 0.30 (0.17) | 0.50 (0.26) | 0.88 (0.48) | 2.34 (1.20) | 0.74 (0.38, 1.44) |
| Home health care episodes | ||||||||
| Guided Care | 1.84 | 0.67 | 0.60 | 0.50 | 0.68 | 0.24 | 1.58 | |
| Usual Care | 2.52 | 1.86 | 0.93 | 0.37 | 0.80 | 0.50 | 1.74 | |
| GC:UC ratio (standard error) | 0.73 (0.21) | 0.36 (0.12) | 0.64 (0.18) | 1.35 (0.51) | 0.85 (0.32) | 0.48 (0.18) | 0.91 (0.21) | 0.71 (0.51, 0.97) |
| Primary care visits | ||||||||
| Guided Care | 13.70 | 13.18 | 10.23 | 9.16 | 10.71 | 9.58 | 12.42 | |
| Usual Care | 20.06 | 9.76 | 12.27 | 9.61 | 9.06 | 8.32 | 13.63 | |
| GC:UC ratio (standard error) | 0.68 (0.12) | 1.35 (0.19) | 0.83 (0.11) | 0.95 (0.18) | 1.18 (0.21) | 1.15 (0.19) | 0.91 (0.14) | 0.99 (0.82–1.18) |
| Specialist visits | ||||||||
| Guided Care | 12.60 | 11.00 | 6.42 | 9.60 | 10.14 | 9.65 | 13.61 | |
| Usual Care | 10.42 | 11.02 | 6.15 | 9.69 | 12.17 | 11.35 | 10.69 | |
| GC:UC ratio (standard error) | 1.21 (0.24) | 1.00 (0.20) | 1.04 (0.18) | 0.99 (0.19) | 0.83 (0.18) | 0.85 (0.16) | 1.27 (0.24) | 1.02 (0.86–1.22) |
| Emergency department visits | ||||||||
| Guided Care | 0.82 | 0.31 | 0.51 | 0.37 | 0.45 | 0.46 | 0.69 | |
| Usual Care | 0.52 | 0.48 | 0.44 | 0.40 | 0.61 | 0.39 | 0.63 | |
| GC:UC ratio (standard error) | 1.58 (0.44) | 0.65 (0.19) | 1.17 (0.30) | 0.94 (0.23) | 0.73 (0.23) | 1.17 (0.46) | 1.10 (0.29) | 1.02 (0.78–1.33) |
*Adjusted for baseline age, race, sex, education level, financial status, habitation status, HCC score, SF-36 physical and mental health subscales, and period of observation.
GC Guided Care; UC Usual Care
DISCUSSION
Data from this 32-month, matched-pair, cluster-randomized study do not support the hypothesis that Guided Care improves high-risk, older patients’ functional health. The data do support the hypotheses that Guided Care improves such patients’ perceptions of the quality of their health care, as well as their access to telephone advice. These data also indicate that Guided Care significantly reduces such patients’ use of home health care.
Previously published data from this study showed Guided Care’s initial improvements in patients’ perceptions of the quality of their care were sustained through 18 months.28 Other analyses have shown that Guided Care improves primary care physicians’ satisfaction with some aspects of chronic care,29,30 and family caregivers’ ratings of the quality of chronic care.31
Several factors may underlie the observed lack of significant effect on patients’ functional health and use of some health services: inadequate potency of the initial version of the Guided Care model tested, the considerable heterogeneity in the implementation of the model by the individual nurses and physicians on the seven different intervention teams, and inadequate statistical power to draw inferences about the intervention’s effects on health care utilization. A much larger number of pods would have been needed to determine whether Guided Care accounted for the observed reductions in hospital admissions, 30-day hospital re-admissions and skilled nursing facility days. Contamination of the control group is unlikely, because the services provided by the nurses were not available to the control pods.
This study has several other limitations. The generalizability of its findings is limited by its geographic location (urban and suburban mid-Atlantic U.S.) and the relatively low consent rate (37.8 %), which is common in randomized clinical studies of health care for frail older people.32 Privacy protections prevented access to data about the characteristics of the study’s non-consenters, but similar studies suggest that the consenters were probably younger, healthier and better educated than non-consenters.32 Finally, incomplete follow-up (52.5 % of the randomized participants completed the 32-month interview) could have biased the study’s results if there were unmeasured differences between completers and non-completers that we could not account for in our regression models. Furthermore, the higher percentage of non-completers in the control group, coupled with the tendency for non-completers to be older, sicker, more disabled and more likely to use health services heavily, may have biased the study’s final results in favor of the control group.
Allowing for these limitations, Guided Care appears to improve the quality of chronic care, but its potential for controlling the utilization and costs of health care remains uncertain. The significant savings from reductions in the use of home health care would help to offset the costs of the intervention, but concomitant reductions in the use of hospitals and skilled nursing facilities (suggested, but not statistically significant in this small sample) would probably be necessary for the model to show cost-neutrality or reduce high-risk patients’ net health care costs.
The results from the present study are consistent with the modest findings from other recent controlled trials of alternative models of comprehensive, interdisciplinary primary care for high-risk patients. For example, during the second year of a two-year, cluster-randomized trial, the more intensive GRACE (Geriatric Resources for Assessment and Care of Elders) model significantly reduced hospital admissions and emergency department visits (but did not affect overall health care costs) among high-risk, low-income seniors.7,8 Similarly, recent studies of other primary care-based interventions for high-risk patients have reported significant improvement in one or two, but not all three, of the components of “the triple aim:” quality of care, health, and health care costs.12,33 High-quality studies of various versions of the “patient-centered medical home” have shown improvement in the quality of care or in some clinical outcomes, but none has shown a significant reduction in net health care costs.33 The national Medicare Care Coordination Demonstration34 showed few improvements in the quality of care;13 four of its 15 demonstration sites showed significant reductions in hospital admissions for high-risk patients, but the costs of the interventions at these sites were equal to the savings generated by the reductions in admissions over six years.35
What lessons can we learn from this body of recent research that will help inform the next generation of comprehensive, interdisciplinary primary care for high-risk patients? Certain core features are common to many of the more successful models, including: systematic identification and intensive care management (including frequent face-to-face contact) of high-risk patients; primary care physicians collaborating with on-site registered nurses and other staff (all working in redefined roles “at the tops of their licenses”); health information technology that facilitates coordinated care; engagement of patients and their family caregivers in evidence-based health education and self-management; easy 24/7/365 access to primary care for emerging problems; well-coordinated transitional care following hospital discharges; comprehensive medication management; and the integration of community-based support services into health care.
Unfortunately, even models that have provided various combinations of these features have produced only modest improvements in clinical and financial outcomes. A fatalistic interpretation of these results could conclude that even more powerful models for providing chronic care will not be able to produce better outcomes for patients with multiple chronic illnesses. An alternative, more optimistic view holds that the research completed so far represents a solid foundation that has included some success and has provided valuable insights for the development and testing of new and better chronic care models for the future.
Supplemental features which have not yet been well tested empirically, but which could facilitate better outcomes in care models of the future include: judicious use of home telemonitoring; close supervision of nurse care managers’ activities to ensure adherence to established priorities; aggressive quality improvement processes that focus on the care of high-risk patients in primary care practices; and meaningful, risk-adjusted financial incentives for providers who deliver high-quality care and achieve above-average outcomes with high-risk patients.
In the near term, accountable care organizations, comprehensive primary care providers, medical homes, and other health care delivery organizations seeking to provide efficient, high-quality care for high-risk patients are positioned to test next-generation chronic care models that integrate the core features of the more successful models with the supplemental features listed above. Careful, pragmatic studies of such new models, technologies and payment schemes will be essential in addressing the clinical and economic challenges of an aging society.
Electronic supplementary matrials
(DOCX 14 kb)
Acknowledgements
We thank: Wade Kramer, MHSA, and Susan Kim, BS, CPA, of Kaiser Permanente; Paula Norman, BS, of Johns Hopkins HealthCare; and Taneka Lee, BS, of the Lipitz Center for the expert technical assistance they provided in the course of their regular employment. We also thank Constantine Frangakis, PhD, and Thomas Louis, PhD, of the Department of Biostatistics at the Johns Hopkins Bloomberg School of Public Health for contributing their assistance with the statistical analysis.
This study was supported by grants from the Agency for Healthcare Research and Quality, the National Institute on Aging, the John A. Hartford Foundation, and the Jacob and Valeria Langeloth Foundation—and by in-kind contributions from Johns Hopkins HealthCare (administrative and information technology support), Johns Hopkins Community Physicians (clinical office space), Kaiser Permanente Mid-Atlantic States (administrative support and clinical office space), MedStar Physician Partners (clinical office space), and the Roger C. Lipitz Center for Integrated Health Care (administrative support).
None of the supporting organizations had any role in: the design and conduct of the study; the collection, management, analysis or interpretation of the data; or the preparation, review, or approval of the manuscript. Drs. Boult and Scharfstein had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest
The authors declare that they do not have a conflict of interest.
Disclaimer
The views expressed in this article are Dr. Boult’s and do not necessarily reflect those of PCORI.
Footnotes
Supported by grants from the Agency for Healthcare Research and Quality, the National Institute on Aging, the John A. Hartford Foundation, and the Jacob and Valeria Langeloth Foundation—and by in-kind contributions from Johns Hopkins HealthCare, Johns Hopkins Community Physicians, Kaiser Permanente Mid-Atlantic States, MedStar Physician Partners, and the Roger C. Lipitz Center for Integrated Health Care.
Clinical Trials.gov ID# NCT0012194
REFERENCES
- 1.Paez KA, Zhao L, Hwang W. Rising out-of-pocket spending for chronic conditions: a ten-year trend. Health Aff (Millwood). 2009;28(1):15–25. doi: 10.1377/hlthaff.28.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Davis K, Schoen C, Stremikis K. Mirror, Mirror on the Wall: How the Performance on the U. S. Health Care System Compares Internationally. New York, New York: The Commonwealth Fund; 2010. http://www.commonwealthfund.org/~/media/Files/Publications/Fund%20Report/2010/Jun/1400_Davis_Mirror_Mirror_on_the_wall_2010.pdf. Accessed October 5, 2012.
- 3.Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001. [PubMed] [Google Scholar]
- 4.Anderson GF Chronic conditions: making the case for ongoing care. Partnership for Solutions; 2004. http://www.partnershipforsolutions.org/DMS/files/chronicbook2004.pdf. Accessed October 5, 2012.
- 5.Darer JD, Hwang W, Pham HH, Bass EB, Anderson G. More training needed in chronic care: a survey of U.S. physicians. Acad Med. 2004;79(6):541–8. doi: 10.1097/00001888-200406000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Benjamin RM. Multiple chronic conditions: a public health challenge. Public Health Rep. 2010;125(5):626–7. doi: 10.1177/003335491012500502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Counsell SR, Callahan CM, Clark DO, et al. Geriatric care management for low-income seniors: a randomized controlled trial. JAMA. 2007;298(22):2623–33. doi: 10.1001/jama.298.22.2623. [DOI] [PubMed] [Google Scholar]
- 8.Counsell SR, Callahan CM, Tu W, Stump TE, Arling GW. Cost analysis of the Geriatric Resources for Assessment and Care of Elders care management intervention. J Am Geriatr Soc. 2009;57(8):1420–6. doi: 10.1111/j.1532-5415.2009.02383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reid RJ, Coleman K, Johnson EA, et al. The group health medical home at year two: cost savings, higher patient satisfaction, and less burnout for providers. Health Aff (Millwood) 2010;29(5):835–43. doi: 10.1377/hlthaff.2010.0158. [DOI] [PubMed] [Google Scholar]
- 10.Gilfillan RJ, Tomcavage J, Rosenthal MB, et al. Value and the medical home: effects of transformed primary care. Am J Manag Care. 2010;16(8):607–14. [PubMed] [Google Scholar]
- 11.Wennberg DE, Marr A, Lang L, O’Malley S, Bennett G. A randomized trial of a telephone care-management strategy. N Engl J Med. 2010;363(13):1245–55. doi: 10.1056/NEJMsa0902321. [DOI] [PubMed] [Google Scholar]
- 12.Boult C, Frank A, Boult L, Pacala J, Snyder C, Leff B. Successful models of comprehensive care for older adults with chronic conditions: evidence for the Institute of Medicine’s “Retooling for an Aging America” report. J Am Geriatr Soc. 2009;57:2328–37. doi: 10.1111/j.1532-5415.2009.02571.x. [DOI] [PubMed] [Google Scholar]
- 13.Peikes D, Chen A, Schore J, Brown R. Effects of care coordination on hospitalization, quality of care, and health care expenditures among Medicare beneficiaries: 15 randomized trials. JAMA. 2009;301(6):603–18. doi: 10.1001/jama.2009.126. [DOI] [PubMed] [Google Scholar]
- 14.Berenson R, Devers K, Burton R. Will the Patient-Centered Medical Home Transform the Delivery of Health Care? Timely Analysis of Immediate Health Policy Issues. Washington, DC: Urban Institute; 2011. [Google Scholar]
- 15.Boyd CM, Boult C, Shadmi E, et al. Guided Care for multi-morbid older adults. Gerontologist. 2007;47(5):697–704. doi: 10.1093/geront/47.5.697. [DOI] [PubMed] [Google Scholar]
- 16.Wolff JL, Rand-Giovannetti E, Palmer S, et al. Caregiving and chronic care: the Guided Care Program for Families and Friends. J Gerontol A Biol Sci Med Sci. 2009;64(7):785–91. doi: 10.1093/gerona/glp030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boult C, Reider L, Frey K, et al. The early effects of “Guided Care” on the quality of health care for multi-morbid older persons: a cluster-randomized controlled trial. J Gerontol A Biol Sci Med Sci. 2008;63(A):321–7. doi: 10.1093/gerona/63.3.321. [DOI] [PubMed] [Google Scholar]
- 18.Pope GC, Kautter J, Ellis RP, et al. Risk adjustment of Medicare capitation payments using the CMS-HCC model. Health Care Financ Rev. 2004;25(4):119–41. [PMC free article] [PubMed] [Google Scholar]
- 19.Giddens JF, Tanner E, Frey K, Reider L, Boult C. Expanding the gerontological nursing role in Guided Care. Geriatr Nurs. 2009;30(5):358–64. doi: 10.1016/j.gerinurse.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 20.Boult C, Giddens JF, Frey K, Reider L, Novak T. Guided Care: A new nurse-physician partnership in chronic care. New York: Springer; 2009. [Google Scholar]
- 21.Ware JE, Kosinski M. Interpreting SF-36 summary health measures: a response. Qual Life Res. 2001;10(5):405–20. doi: 10.1023/A:1012588218728. [DOI] [PubMed] [Google Scholar]
- 22.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005;43(5):436–44. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 23.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728–39. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Angst F, Aeschlimann A, Stucki G. Smallest detectable and minimal clinically important differences of rehabilitation intervention with their implications for required sample sizes using WOMAC and SF-36 quality of life measurement instruments in patients with osteoarthritis of the lower extremities. Arthritis Rheum. 2001;45(4):384–91. doi: 10.1002/1529-0131(200108)45:4<384::AID-ART352>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 25.Royston P. Multiple imputation of missing values: update of ICE. Stata Journal. 2005;5(4):527–36. [Google Scholar]
- 26.Thompson SG, Pyke SD, Hardy RJ. The design and analysis of paired cluster randomized trials: an application of meta-analysis techniques. Stat Med. 1997;16(18):2063–79. doi: 10.1002/(SICI)1097-0258(19970930)16:18<2063::AID-SIM642>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Lunn D, Spiegelhalter D, Thomas A, Best N. The BUGS project: Evolution, critique and future directions. Stat Med. 2009;28(25):3049–67. doi: 10.1002/sim.3680. [DOI] [PubMed] [Google Scholar]
- 28.Boyd CM, Reider L, Frey K, et al. The effects of Guided Care on the perceived quality of health care for multi-morbid older persons: 18-month outcomes from a cluster-randomized controlled trial. J Gen Intern Med. 2010;25(3):235–42. doi: 10.1007/s11606-009-1192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marsteller JA, Hsu YJ, Reider L, et al. Physician satisfaction with chronic care processes: a cluster-randomized trial of guided care. Ann Fam Med. 2010;8(4):308–15. doi: 10.1370/afm.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marteller JA, Hsu YJ, Wen M, et al. Effects of Guided Care on Providers’ Satisfaction with Care: A Three-Year Matched-Pair Cluster-Randomized Trial. Population Health Management 2013 (in press). [DOI] [PMC free article] [PubMed]
- 31.Wolff JL, Giovannetti ER, Boyd CM, et al. Effects of Guided Care on family caregivers. Gerontologist. 2010;50(4):459–70. doi: 10.1093/geront/gnp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boult C, Boult L, Morishita L, Pirie P. Soliciting defined populations to recruit samples of high-risk older adults. J Gerontol A Biol Sci Med Sci. 1998;53(5):M379–84. doi: 10.1093/gerona/53A.5.M379. [DOI] [PubMed] [Google Scholar]
- 33.Peikes D, Zutshi A, Genevro J, Parchman ML, Meyers D. Early Evaluations of the Medical Home: Building on a Promising Start. Am J Manag Care. 2012;18(2):105–16. [PubMed] [Google Scholar]
- 34.Centers for Medicare & Medicaid Services (CMS). Medicare coordinated care demonstration. 2012. Available at http://www.cms.gov/Medicare/Demonstration-Projects/DemoProjectsEvalRpts/Medicare-Demonstrations-Items/CMS1198864.html. Accessed October 5, 2012.
- 35.Brown RS, Peikes D, Peterson G, Schore J, Razafindrakoto CM. Six features of Medicare Coordinated Care Demonstration programs that cut hospital admissions of high-risk patients. Health Aff (Millwood) 2012;31(6):1156–66. doi: 10.1377/hlthaff.2012.0393. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 14 kb)