Abstract
We previously found that endoplasmic reticulum stress (ERS) might be exhibited in the conventional protocol of the primary culture of neonate rat myocardial cells (NRMCs) and that the high glucose concentration (25 mmol/L) in the culture medium might be the cause. Here, we investigated if the high concentration of glucose might influence ERS in myocardial cells during culture. GRP78 expression (ERS marker) was similar in groups with tunicamycin (TM) and without TM in high glucose cultured cells (p > 0.01). Different glucose concentrations elicited different GRP78 expressions according to analyses of protein and RNA levels, which showed ERS in H/H groups. Finally, we found that GRP78 expression was higher in TM groups compared with M/M groups (p < 0.01). The conventional high-glucose culture media during primary culture of NRMCs induced ERS. We propose that medium-glucose culture media should be used and describe an improved protocol for the primary culture of NRMCs.
Keywords: Endoplasmic reticulum stress, Primary culture of neonate rat myocardial cells, Culture technique
Introduction
There have been considerable improvements in the methods used for the primary culture of the myocardial cells (MCs) of neonatal rats. Hence, appreciable quantities of cardiomyocytes with good survival rates can be cultured effectively and rapidly (Simpson and Savion 1982; Kasten 1972). In addition, the proliferation of nonmyocardial cells (NMCs) can be inhibited to a considerable degree. NMCs eventually comprise the major fraction of the culture population and may physically overgrow the MCs.
Primary culture of neonate rat myocardial cells (NRMCs) has been used widely to study the mechanisms of cardiovascular disease (CVD). It has recently been proposed that heart cells in primary cultures may be useful models for studies on endoplasmic reticulum stress (ERS) (Kasten 1972). In preliminary experiments, we found that ERS might be exhibited in the conventional protocol of the primary culture of NRMCs. A hallmark of ERS, expression of glucose-regulated protein 78 (GRP78), was greatly increased. As a general protocol, a high glucose concentration (25 mmol/L) is applied in the culture medium (Simpson and Savion 1982; Kasten 1972; Shen et al. 1987). We wondered if the high concentration of glucose may influence ERS in MCs.
In the present study, we investigated the influence of a high glucose concentration in the culture medium on GRP78 expression in primary cultures of NRMCs. We wished to improve upon the conventional protocol to ensure that the use of such cultures in studies on CVD do not produce erroneous results.
Materials and methods
The Animal Care and Use Committee of Zhejiang University (Hangzhou, China) approved the study protocol. All animals and procedures were handled strictly in accordance with guidelines by the National Institutes of Health (Bethesda, MD, USA).
Primary culture of NRMCs
One-day-old Sprague–Dawley rats were provided by the Shanghai Laboratory Animal Center, (Chinese Academy of Sciences, Shanghai, China). They were anesthetized by inhalation of isoflourane and sterilized by 75 % alcohol for 30 s at room temperature. Digestion buffer containing 0.125 % trypsinase and 0.05 % type-I collagenase (Sigma-Aldrich, St. Louis, MO, USA) was diluted with D-Hanks solution and the resulting solution adjusted to pH 7.4, sterilized by filtration and stored at 4 °C for use.
The chest wall was cut and the heart isolated. The heart was washed thrice in ice-cold D-Hanks solution. Atria were removed and residual ventricles cut into small pieces (1 mm3) on an iced surgical plate. Six hearts were dissected at one time, being careful not to press down on the hearts while cutting and to cut as quickly as possible. Heart tissue was minced and placed into sterilized flasks. Ten microliters of digestion buffer was added to heart tissue and the mixture incubated at 37 °C for 10 min using a magnetic stirrer at 80 rpm. After stirring, the supernatant was removed. Another 10 mL of digestion buffer was added and the supernatant transferred to 50-mL tubes containing 5 mL of 10 % fetal calf serum (FCS; Gibco, Billings, MT, USA): This process was repeated four times to release single cells. Once digestion was complete, the 50-mL tubes containing the supernatant were transferred to the Sorvall TJ-25 Table Top Centrifuge (Sorvall, Newton, CT, USA) set at 4 °C. Tubes are centrifuged at 1,000 rpm for 10 min at 4 °C.
Cells were preplated for 1 h in Dulbecco’s modified Eagle medium (DMEM, Jinuo Biotechnology, Hangzhou, China) with 10 % FCS, and attached cells discarded. Supernatants were aspirated and, this myocyte-contained media diluted up to 200 mL. Ten microliters of myocyte-contained media were placed in a hemocytometer, and the number of cells in a 5 × 5 square in the hemocytometer was counted under a light microscope twice. The concentration of cardiomyocytes was adjusted to 1 × 106/mL. Cardiomyocytes were plated onto gelatin-coated culture dishes. Myocytes are cultured with DMEM with 10 % FCS and 100 μM 5-bromo-2-deoxyuridine (BrdU; Sigma–Aldrich). Thirty-six hours after plating, the medium was changed to DMEM without BrdU. Cells were incubated at 37 °C in an incubator containing 5 % CO2. DMEM was then changed every 3 days. On day 5, cells were harvested and their morphology and phenotypic markers determined (Piper et al. 1990; Mitcheson et al. 1998).
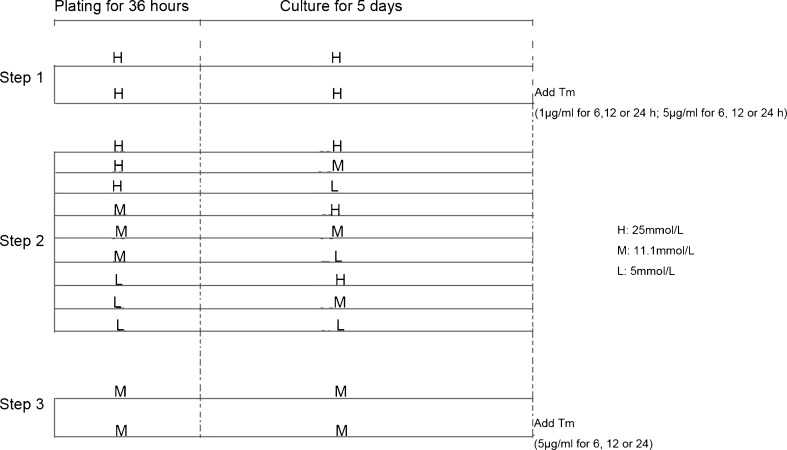
Protocol
-
Step 1Effect of the positive control of ERS with tunicamycin (TM) in high-glucose (25 mmol/L) DMEM
- Normal group: After 36 h of plating and 5 days of cell culture in high-glucose normoxic DMEM, the culture medium was changed to high-glucose (25 mmol/L) normoxic DMEM without TM, and incubated for a further 24 h.
- TM group: After 36 h of plating and 5 days of cell culture in high-glucose (25 mmol/L) normoxic DMEM, TM (1 or 5 μg/mL) was added and incubated for 6, 12, and 24 h to induce ERS.
-
Step 2
Effect of different glucose concentrations of DMEM during plate/culture on GRP78 expression
According to the different glucose concentration in the plate medium (36 h) and culture medium (5 days), cells were divided into nine groups. That is, during plating/culture, DMEM with different glucose concentrations was applied [high (H), 25 mmol/L; medium (M), 11.1 mmol/L; low (L), 5 mmol/L]. Hence, the groups were H/H, H/M, H/L, M/H, M/M, M/L, L/H, L/M, and L/L.
-
Step 3Effect of the positive control of ERS with TM in medium-concentration glucose (11.1 mmol/L) DMEM
- Normal group: After 36 h of plating and 5 days of cell culture in medium-concentration glucose normoxic DMEM, the culture medium was changed to medium-concentration glucose normoxic DMEM without TM, and incubated for a further 24 h.
- TM group: After 36 h of plating and 5 days of cell culture in medium-concentration glucose normoxic DMEM, TM (5 μg/mL) was added and incubated for 6, 12, and 24 h to induce ERS (Fig. 1).
Fig. 1.
Protocol
Pathological study of cultured MCs
The cultured MCs were stained by hematoxylin and eosin (H&E) and examined by an observer blinded to the study protocol. The following morphological criteria (Zingarelli et al. 1998) were used to determine histopathological changes:
Normal: There are abundant cardiomyocytes, they are contoured with several antennae, the nuclei are of identical size, and the cytoplasm is enriched and well stained.
Injured: There are few cardiomyocytes, they are contoured with few antennae, the nuclei are small, and the cytoplasm is not enriched and show acidophilic staining.
The factors associated with group A have a score of 1 and those associated with group B have a score of 0. The score (per high-magnification view) was used as the basis for assessing lesion severity on this semiquantitative scoring scale.
Assessment of the viabilities of MCs using a cell counting kit
Cell viability was assessed using Cell Counting Kit-8 (CCK-8 Tongren, Japan) according to the manufacturer’s instructions (Ishiyama et al. 1997). CCK-8 test is a dehydrogenase-dependent colorimetric assay that indirectly measures cellular metabolic activity.
Western blot analyses
Proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to a nitrocellulose membrane, and incubated with monoclonal antibodies to GRP-78 (diluted 1:500, sc-1050, Sant Cruz Biotechnology, Santa Cruz, CA,USA) at 4 °C overnight. Membranes were incubated with secondary antibody (diluted 1:5,000, Lianke Biosciences, China) for 2 h and developed with BeyoECL reagent (Biyuntian Biosciences, China). The blots were exposed for development (Chomczynski and Sacchin 1987).
RNA extraction and reverse transcription PCR analyses
Chemicals and reagents were purchased from TaKaRa Biotechnology (Shiga, Japan) unless specified otherwise.
RNA isolation and complementary DNA syntheses
Total RNA was extracted from the cultured NRMCs using RNAiso Plus (TaKaRa) in accordance with the manufacturer’s instructions. RNA concentrations were determined by absorbance readings at 260 nm with a SmartSpec Plus spectrophotometer (Bio-Rad, Tokyo, Japan). Reverse transcription of RNA was undertaken with PrimeScript® Reverse Transcriptase (RNase Hˉ) (TaKaRa) as outlined in the instruction manual. Complementary DNA served as a template in the PCR.
Quantitative PCR
The primer sequences were as follows: 5′-CTCCACGGCTTCCGATAATCA-3′ and 5′-TCCAGTCAGATCAAATGTACCCAGA-3′ for GRP-78; and 5′-AAATGGTGAAGGTCGGTGTGAAC-3′ and 5′-CAACAATCTCCACTTTGCCACTG-3′ for GAPDH. Using the reverse transcriptase (RNase Hˉ) kit (TaKaRa), real-time PCR was carried out in a ABI 7500 Fast System (Applied Biosystems, Foster City, CA, USA) with the following universal cycling conditions: denaturation for 30 s at 95 °C, amplification for 40 cycles, with denaturation for 5 s at 95 °C, annealing for 60 s at 60 °C, and extension for 34 s at 72 °C. Samples were tested in triplicate and mean values used for quantification. Messenger RNA (mRNA) expression of different specimens was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Relative mRNA levels are shown as unit values of 2−ΔΔCt, where Ct is the threshold cycle value (defined as the fractional cycle number at which the target fluorescent signal passes a fixed threshold above the baseline).
Statistical analyses
Results are the mean ± SD data were analyzed by analysis of variance followed by the Scheffe’s test for multiple comparisons. p< 0.05 was considered significant.
Results
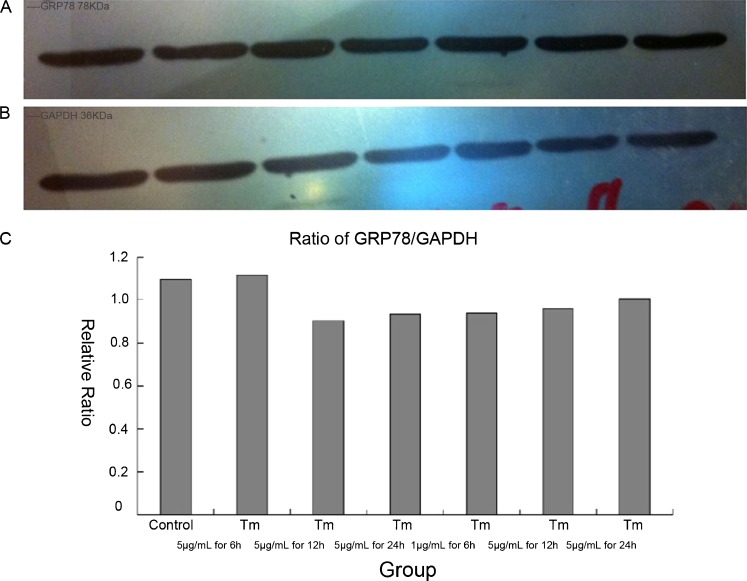
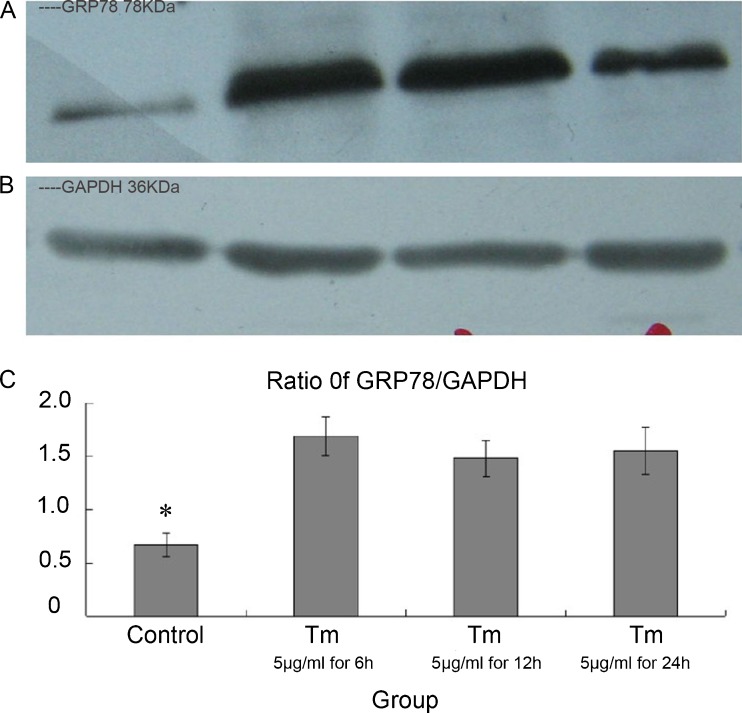
Effect of different concentrations of GRP78 and time-course of TM action in high-glucose plate/culture DMEM
We initially planned to establish a positive control of ERS in cardiomyocytes according to conventional methods. However, we found out that GRP78 expression was similar in groups containing TM and those not containing TM. We subsequently demonstrated that the high glucose concentration in DMEM could influence ERS during cell plate and culture (Fig. 2).
Fig. 2.
Effect of different concentrations of GRP78 and time-course of tunicamycin action in high glucose culture DMEM. aGRP78 expression by western blotting (left to right control; 5 μg/mL TM for 6 h; 5 μg/mL TM for 12 h; 5 μg/mL TM for 24 h; 1 μg/mL TM for 6 h; 1 μg/mL TM for 12 h; 1 μg/mL TM for 24 h). b GAPDH expression by Western blotting. c Relative expression of GRP78 at different times and concentration. It was similar at different times and concentrations
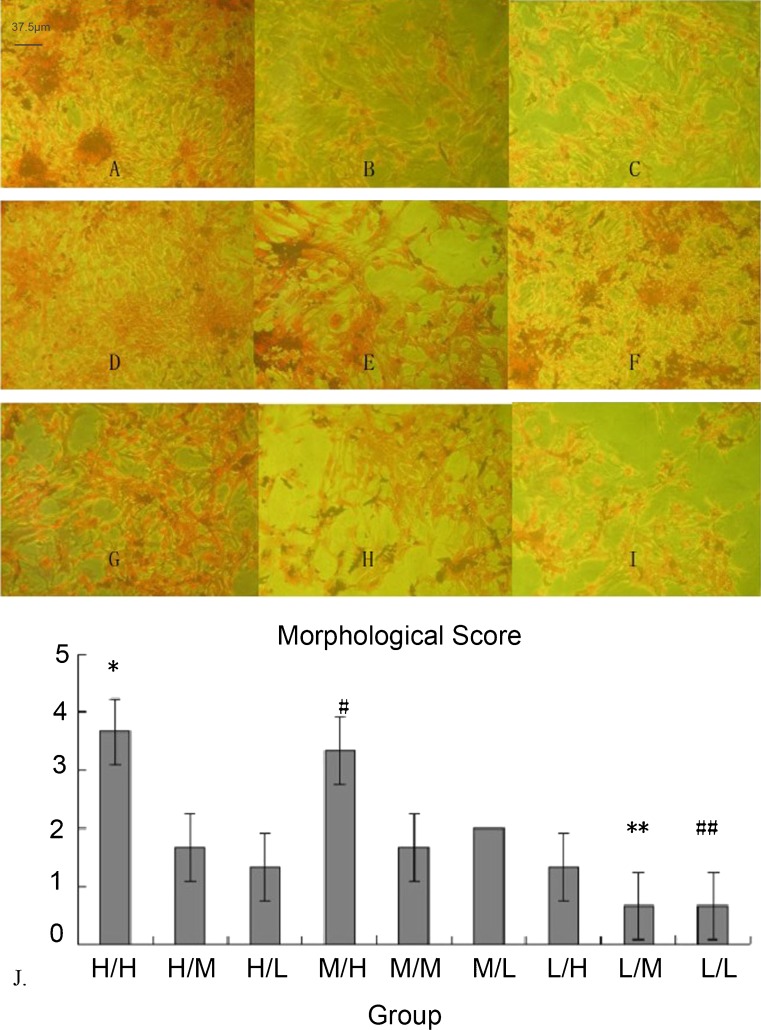
Pathological results using different concentrations of glucose
In low glucose groups (L/H, L/M, and L/L), cell counts at high magnification (×400) were low. Cells were dispersed, the cytoplasm was stained lightly, and nuclei were small. In high and medium glucose groups, cells had more antennae and connected with each other. The cytoplasm was enriched, and nuclei were of equal size. The morphological score also disclosed that low glucose DMEM could have an appreciable effect on cell morphology (Fig. 3).
Fig. 3.
Pathological results at different concentrations of glucose and morphological score. a–i H/H, H/M, H/L, M/H, M/M, M/L, L/H, L/M, and L/L group, respectively (×400 magnification). j H/H and M/H groups had high scores. L/M and L/L groups had a low score. *p < 0.01 compared with the other groups. #p < 0.01 compared with the other groups. **p < 0.01 compared with H/H, H/M, H/L, M/H, M/M, M/L, and L/H groups. ##p < 0.01 compared with H/H, H/M, H/L, M/H, M/M, M/L, and L/H groups
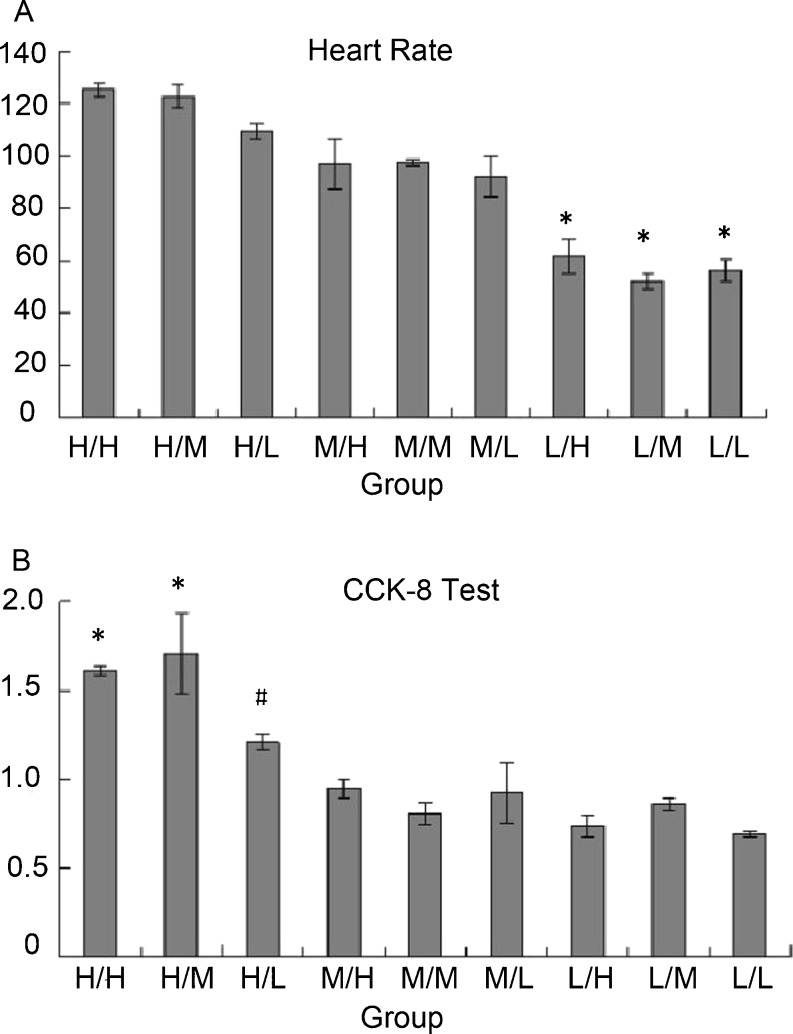
Heart rate and CCK-8 test at different concentrations of glucose
We observed that high glucose during adherence (H/H, H/M, and H/L groups) could influence heart rate. Heart rate was slower in the low glucose-concentration groups (L/H, L/M, and L/L). Cells with medium-concentration glucose during adherence (M/H, M/M, and M/L) had a similar heart rate compared with those in high glucose-concentration groups. The CCK-8 test disclosed that low glucose concentrations during adherence might be associated with lower cell viability (Fig. 4).
Fig. 4.
Heart rate and CCK-8 test at different concentrations of glucose. a Heart rate: *p < 0.01 compared with H/H, H/M, H/L, M/H, M/M, and M/L groups. b CCK-8 test: *p < 0.01 compared with H/L, M/H, M/M, M/L, L/H, L/M, and L/L groups. #p < 0.05 compared with L/H and L/L group
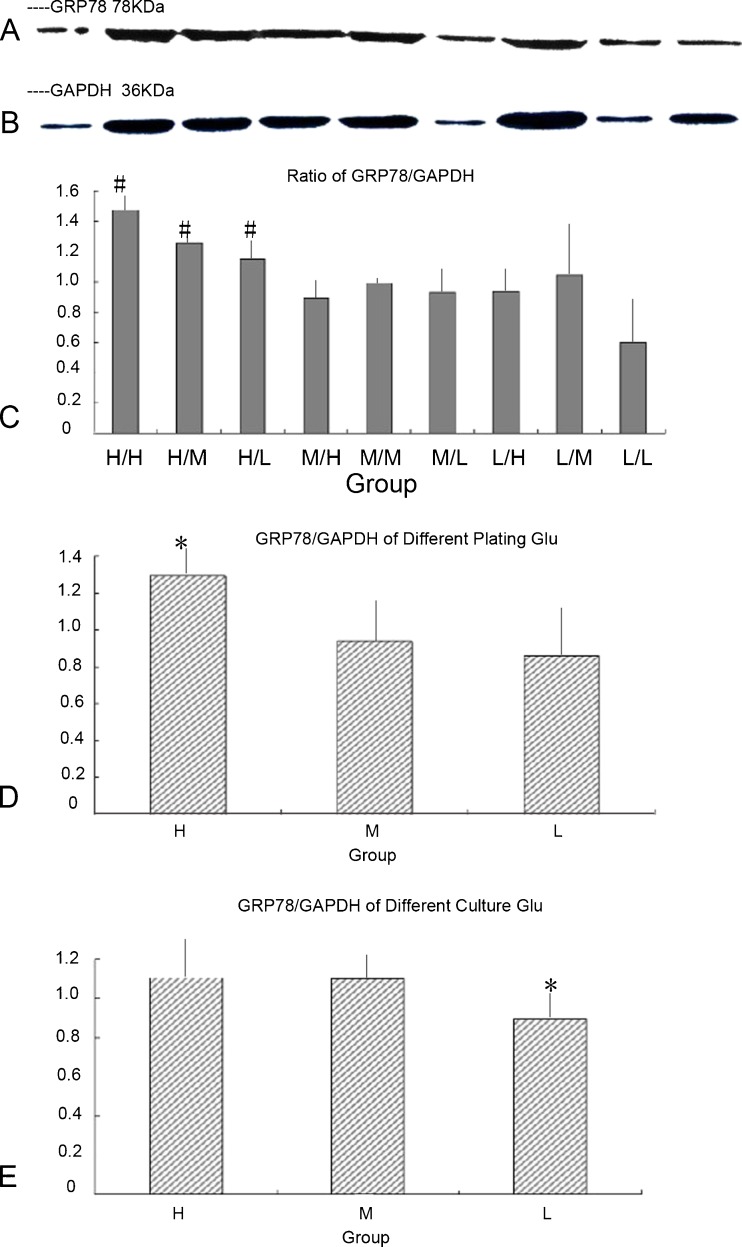
Analyses of GRP78 expression at different concentrations of glucose by Western blotting
Expression of GRP78 was higher in high glucose groups (H/H, H/M, and H/L). The relative expression of GRP78 was lower in low glucose groups (H/L, M/L, and L/L). It seemed that high-glucose DMEM during adherence had an appreciable effect on GRP78 expression, which could influence ERS in cardiomyocytes (Fig. 5).
Fig. 5.
GRP78 expression at different concentrations of glucose by Western blotting. a GRP78 expression by Western blotting (left to right H/H, H/M, H/L, M/H, M/M, M/L, L/H, L/M, and L/L). b GAPDH expression by Western blotting. c Relative expression of GRP78 at different concentrations of glucose. In the high-glucose groups (H/H, H/M, H/L), the relative expression of GRP78 was higher. #p < 0.05 compared with M/H, M/M, M/L, L/H, L/M, and L/L groups. d When merged with the same glucose group, the relative expression of GRP78 was higher in the high-glucose group. *p < 0.01 compared with the other groups. e When merged with the same culture glucose group, the relative expression of GRP78 was lower in the low-glucose culture group. *p < 0.01 compared with the other groups
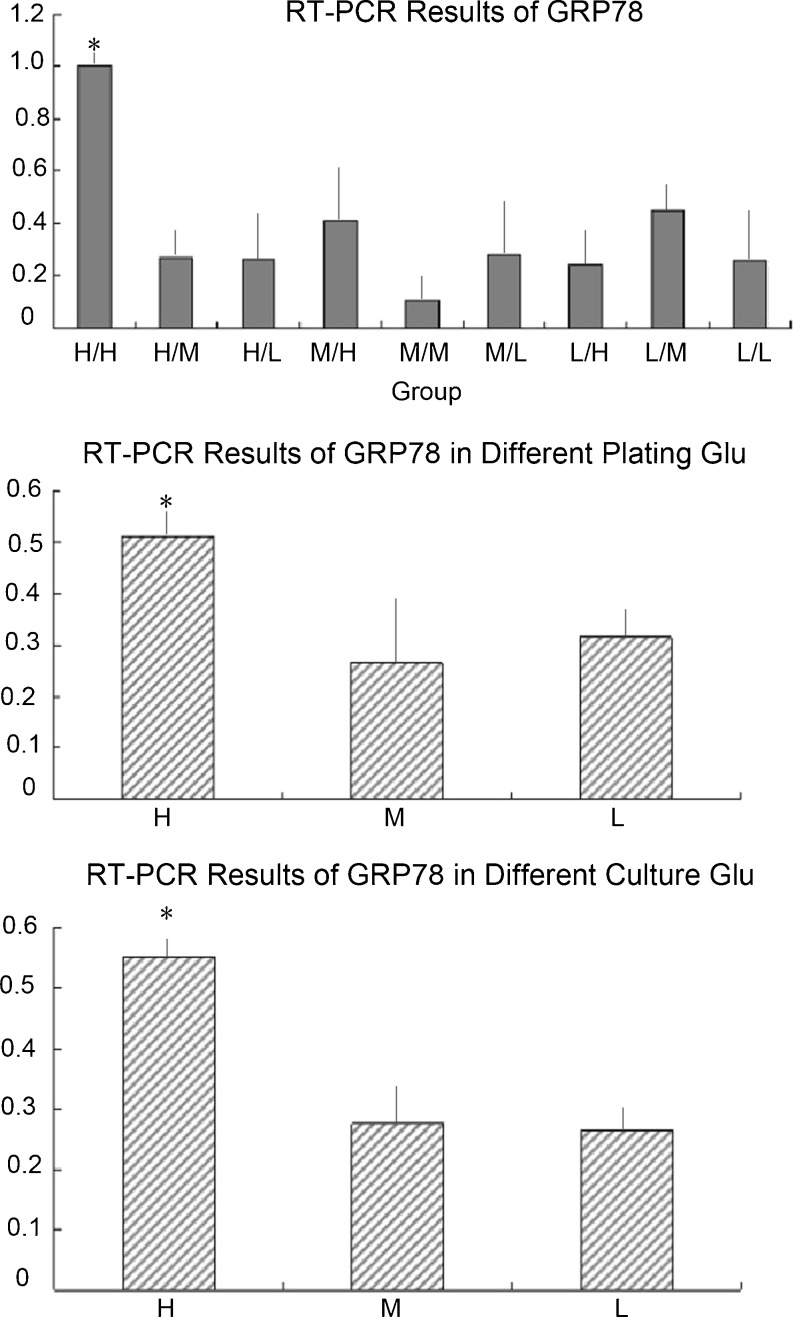
Analyses of GRP78 expression at different concentrations of glucose by reverse transcription PCR
The relative level of GRP78 was higher in high glucose groups (H/H, H/M, and H/L) than in the other groups. It also was higher in high glucose cultures (H/H, M/H, and L/H). It seemed that high glucose DMEM during adherence and culture had a considerable effect on GRP78 expression, which could influence ERS in cardiomyocytes (Fig. 6).
Fig. 6.
GRP78 expression at different concentrations of glucose by reverse transcription PCR. a Relative level of GRP78 at different glucose concentrations. In the H/H group, the relative level of GRP78 was higher. *p < 0.01 compared with the other groups. b When merged with the same glucose group, the relative level of GRP78 expression was higher in high glucose groups (H/H, H/M, and H/L). *p < 0.01 compared with the other groups. c When merged with the same glucose culture group, the relative level of GRP78 was higher in high glucose culture groups (H/L, M/L, and L/L).*p < 0.01 compared with the other groups
Analyses of different concentrations of GRP78 and the time-course of TM action in medium-concentration glucose plate/culture DMEM
We finally established a positive control of ERS in cardiomyocyte plate/culture in medium-concentration glucose DMEM. GRP78 expression was higher in TM groups compared with the M/M group (p < 0.01). This finding suggested that medium-concentration glucose DMEM could be used for the study on ERS in rat hearts (Fig. 7).
Fig. 7.
Effects of different concentrations of GRP78 and time-course of tunicamycin action in medium-glucose culture DMEM. a GRP78 expression by Western blotting (left to right M/M, 5 μg/mL TM for 6 h, μg/mL TM for 12 h, 5 μg/mL TM for 24 h). b GAPDH expression by Western blotting. c Relative concentration of GRP78 at different times and concentrations. *p < 0.01 compared with the other groups
Discussion
Primary cultures of NRMCs enable researchers to study and understand the morphological, biochemical, and electrophysiological characteristics of the heart. This model is also a valuable tool for pharmacological and toxicological studies. Increasing numbers of studies now focus on ERS in heart disease. In particular, ERS stress has been shown to be involved in several of the processes involved in CVDs, such as ischemia–reperfusion injury, cardiomyopathy, cardiac hypertrophy, heart failure, and atherosclerosis (Simpson and Savion 1982; Duan et al. 2009). In preliminary experiments, we found that ERS might already be exhibited in the conventional protocol of the primary culture of NRMCs. We hypothesized that further research on the relationship between ERS and cardiomyocyte culture could help to improve the protocol.
Probably, the most important result of the present study was that the conventional high-glucose culture medium used during primary culture of NRMCs could induce ERS. Western blotting and gene expression data confirmed ERS in high glucose plated/cultured cells. We used plate and culture cells in high glucose medium, and it seemed to elicit the best morphological, biochemical, and electrophysiological characteristics (Mitcheson et al. 1998). Obviously, high glucose levels will affect diabetes mellitus (DM), and ERS has been shown in DM patients (Eizirik et al. 2008). In addition, several studies on cultured cardiomyocytes have explored mechanisms in subjects with DM and heart disease.
The results of the present study suggest that medium-concentration glucose culture might be the best condition for the primary culture of NRMCs. Under these conditions, cultured cardiomyocytes showed good morphological and biochemical characteristics, produced a good yield of cells, and the cells were viable. Importantly, ERS was not observed during the plate/culture process. We also compared the change in GRP78 expression in different glucose media and noted the appreciable influence of ERS in high-glucose plate/culture processes. We believe that the practical value of this new culture method is very useful, especially if studying ERS in CVDs.
Conclusion
We describe here that the use of conventional high-glucose culture media during primary culture of NRMCs induced ERS. We describe here an improved protocol for the primary culture of NRMCs, especially if studying ERS in CVDs.
Acknowledgments
This study was supported by the National Science Foundation for Young Scholars of China (grant number 30900612), the Grant of Medical Science Research Foundation of Zhejiang Province (grant number 2009B127), and from the Zhejiang Provincial Natural Science Foundation (grant number Y2090393), People’s Republic of China.
References
- Chomczynski P, Sacchin N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Ann Biochem. 1987;162:156–159. doi: 10.1016/0003-2697(87)90021-2. [DOI] [PubMed] [Google Scholar]
- Duan XH, Qi YF, Tang CS. Endoplasmic reticulum stress and cardiovascular diseases. J Geriatr Cardiol. 2009;6:49–55. [Google Scholar]
- Eizirik DL, Cardozo AK, Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for ADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/S0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- Kasten FH. Rat myocardial cells in vitro: mitosis and differentiated properties. In Vitro. 1972;8:128–150. doi: 10.1007/BF02619489. [DOI] [PubMed] [Google Scholar]
- Mitcheson JS, Hancox JC, Levi AJ. Cultured adult cardiac myocytes: future applications, culture methods, morphological and electrophysiological properties. Cardiovasc Res. 1998;39:280–300. doi: 10.1016/S0008-6363(98)00128-X. [DOI] [PubMed] [Google Scholar]
- Piper HM, Volz A, Schwartz P. (1990) Adult ventricular rat heart muscle cells. In: Piper HM (ed) Cell culture techniques in heart and vessel research. Springer, Berlin, pp 36–60
- Shen J, Hughes C, Chao C, Cai J, Bartels C, Gessner T, Subjeck J. Conduction of glucose regulated protein and doxorubicin resistance in Chinese hamster cells. Proc Nati Acad Sci. 1987;4:3278–3282. doi: 10.1073/pnas.84.10.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson P, Savion S. Differentiation of rat myocytes in single cell cultures with and without proliferating nonmyocardial cells. Cirac Res. 1982;50:101–106. doi: 10.1161/01.RES.50.1.101. [DOI] [PubMed] [Google Scholar]
- Zingarelli B, Salzman AL, Szabo C. Genetic disruption of poly (ADP-Ribose) synthetase inhibits the expression of P-selectin and intercellular adhesion molecule-1 in myocardial ischemia/reperfusion injury. Circ Res. 1998;83:85–94. doi: 10.1161/01.RES.83.1.85. [DOI] [PubMed] [Google Scholar]