Abstract
Acute cold restraint stress (ACRS) has been reported to suppress host defenses against Listeria monocytogenes, and this suppression was mediated by beta1-adrenoceptors (β1-ARs). Although ACRS appears to inhibit mainly early innate immune defenses, interference with leukocyte chemotaxis and the involvement of β1-AR (or β2-AR) signaling had not been assessed. Thus, the link between sympathetic nerve stimulation, release of neurotransmitters, and changes in blood leukocyte profiles, including oxidative changes, following ACRS was evaluated. The numbers of leukocyte subsets in the blood were differentially affected by β1-ARs and β2-ARs following ACRS; CD3+ (CD4 and CD8) T-cells were shown to be decreased following ACRS, and the T cell lymphopenia was mediated mainly through a β2-AR mechanism, while the decrease in CD19+ B-cells was influenced through both β1- and β2-ARs, as assessed by pharmacological and genetic manipulations. In contrast to the ACRS-induced loss of circulating lymphocytes, the number of circulating neutrophils was increased (i.e., neutrophilia), and this neutrophilia was mediated through β1-ARs. The increase in circulating neutrophils was not due to an increase in serum chemokines promoting neutrophil emigration from the bone marrow; rather it was due to neutrophil release from the bone marrow through activation of a β1-AR pathway. There was no loss of glutathione in any of the leukocyte subsets suggesting that there was minimal oxidative stress; however, there was early production of nitric oxide and generation of some protein radicals. Premature egress of neutrophils from bone marrow is suggested to be due to norepinephrine induction of nitric oxide, which affects the early release of neutrophils from bone marrow and lessens host defenses.
Keywords: Nitric oxide, Norepinephrine, Sympathetic nervous system, Neutrophils, Cold restraint stress
Introduction
Stress is well known to increase susceptibility to bacterial infections (Biondi and Zannino 1997; Avitsur et al. 2006; Freestone et al. 2008). Although the mechanisms underlying this process are incompletely defined, there is compelling evidence that stress alters early innate immune responses to bacterial infections through stress-induced effects on beta-adrenoceptors (β-ARs), especially β1-ARs (Cao et al. 2002, 2003a; Emeny et al. 2007).
Previous studies have shown that acute cold restraint stress (ACRS) significantly inhibits host resistance to Listeria monocytogenes (LM) in BALB/c mice (Cao et al. 2002; Cao and Lawrence 2002; Cao et al. 2003a, b; Emeny et al. 2007). With the use of severe combined immunodeficiency (SCID) mice on the same BALB/c background, it was shown that one or more components of the innate immune system (i.e., neutrophils, macrophages, NK cells, dendritic cells) were more than capable of detecting and controlling early LM proliferation and thus providing partial protection against LM infection (Bhardwaj et al. 1998; Cao et al. 2003b). However, these SCID mice suffer from more chronic (i.e., weeks) LM infections, because they lack adaptive immune cells (i.e., CD8+), which are required to provide sterilizing immunity (Bhardwaj et al. 1998).
SCID is a genetic disorder, which is characterized by the inability of the adaptive immune system to mount, coordinate and sustain an appropriate antigen-specific immune response, which is due to absent T and B lymphocytes. These SCID mice showed greater early host resistance to a low dose LM challenge than that of control mice, not only after ACRS, but also without any previous stress (Cao et al. 2003b). This suggests that the adaptive immune system is not required in order to mount a robust and effective immune response to a non-lethal low dose of LM during the early phases of infection; however, it is required to completely eliminate higher doses of bacteria and prevent low dose chronic infections. Thus, the hypothesis is that the decreased host resistance seen at day 3 of LM infection in ACRS-treated mice was likely associated with a stress-induced alteration of an aspect of the innate immune response. Studies with β-AR knockout mice, β-AR antagonists, and adoptive transfer studies have concluded that decreased host resistance to LM following ACRS involves the sympathetic nervous system (SNS) and that it is mediated by β1-AR (Cao et al. 2002; Cao and Lawrence 2002; Cao et al. 2003a; Emeny et al. 2007). Since neutrophils are one of the most important early innate immune cells in defense against LM infection and are mobilized upon stressful events (Brenner et al. 1998), a main focus was on whether ACRS can modulate neutrophil trafficking.
Neutrophil release from the bone marrow (BM) is a highly regulated homeostatic process in order to maintain a readily available pool of neutrophils for responses to a microbial pathogen (bacteria, fungi, etc.) while minimizing damage to host tissue (Eash et al. 2009). It is essential that neutrophil numbers in the blood be tightly regulated because persistent neutropenia is associated with immunodeficiency (Rezaei et al. 2009), whereas excessive neutrophil infiltration and activation contributes to tissue damage in certain inflammatory disorders, such as rheumatoid arthritis (Eash et al. 2009). Neutrophil homeostasis is maintained through a balance of production, release from the BM, and clearance from circulation (Christopher and Link 2007). The BM plays a major role in the regulation of neutrophil release under two circumstances: homeostatic release of neutrophils that have reached maturity and accelerated release of mature cells in order to mediate an acute inflammatory response (Suratt et al. 2004). This study was designed to determine if ACRS affects release of neutrophils from the BM and/or causes additional redistribution of leukocytes. ACRS induced early BM neutrophil mobilization into peripheral circulation through a β1-AR based mechanism, whereas blood lymphocytes were depleted from the blood after ACRS, which was influenced by β1-AR and/or β2-AR. The early mobilization of BM neutrophils and loss of circulating lymphocytes are suggested to play a role in the ACRS-induced increased susceptibility to bacterial infections, in part, due to a process referred to as neutrophil exhaustion (Navarini et al. 2009).
Materials and methods
Animals
Male BALB/cAnNTac (BALB/c) mice (Taconic, Germantown NY) were purchased at 5–7 weeks and were housed in a pathogen-free environment with food and water ad libitum. The β1-AR deficient mice were generated by eight generations of backcrossing of the FVB/N-β1-AR−/− mice (provided by Dr. B. Kobika, Stanford University, Palo Alto, CA) to the BALB/c mice. All mice were maintained in the AAALAC-approved Animal Facility of Wadsworth Center on a 12-h light/dark (7 am to 7 pm) cycle and were allowed to acclimate for at least 1 week before they were used at the ages of 8–10 weeks.
Acute cold restraint stress
All ACRS experiments were conducted as previously described (Cao et al. 2002, 2003b). Mice were individually restrained in a well-ventilated plastic 60-ml syringe (Sherwood Medical Company, St. Louis, MO) at 4 °C for 1 h. Mice can move forward and backward in the syringe but cannot turn head to tail. The ACRS was always performed between 10 am and noon in order to minimize normal physiological changes associated with circadian rhythm. The control mice were left in their original cages undisturbed during the same time period. All animal procedures were approved by the IACUC of Wadsworth Center (Protocol# 06–278).
Beta-adrenoceptor blockers
All beta-blockers were purchased from Sigma (St. Louis, MO). All drugs were dissolved in sterile phosphate buffered saline and injected intraperitoneally (IP) based on body weight (BW). Atenolol (β1-AR antagonist) was given at 20 mg/kg BW and ICI-118,551 (β2-AR antagonist) was given at 15 mg/kg BW. All drug or vehicle (placebo) injections were given immediately prior to ACRS. Doses were chosen based on a previous study (Cao et al. 2003a).
Chemokine, antibodies, and reagents
Recombinant mouse SDF-1α (CXCL12) was purchased from R&D (Minneapolis, MN); PerCP-anti-mouse CD 45, allophycocyanin (APC)-anti-mouse CD3, phycoerythrin (PE)-anti-mouse CD19, PE:FITC anti-mouse CD4, FITC anti-mouse CD8a and FITC anti-mouse CD49b/Pan-NK (DX5) were purchased from Pharmingen (San Diego, CA). Rabbit anti-DMPO nitrone adduct and HPR-goat anti-rabbit IgG was purchased from Abcam (Cambridge, MA).
Phenylmethyl-sulfonyl fluoride (PMSF), sodium orthovanadate; benzamidine; N,N,N′,N′-tetramethylethylenediamine, and polyoxyethylenesorbitan monolaurate-Tween® 20 were purchased from Sigma. 2-Mercaptoethanol (2ME), acrylamide, sodium dodecyl sulfate (SDS), bis N,N′-methylene-bis-acrylamide, and Precision Plus Protein Unstained Standards were purchased from Bio-Rad (Hercules, CA). Dimethylsulfoxide (DMSO), BCA Protein Assay Kit, and SuperSignal® West Pico Chemiluminescent Substrate were from Pierce (Rockford, IL). Triton X-100 was from Boehringer Mannheim (Mannheim, Germany).
Beta-receptor staining
After exposure to ACRS, the mice were sacrificed, and blood was collected in pre-chilled EDTA-2 Na tubes; 200 μl of the EDTA blood was dispensed into 5-ml Polystyrene tubes and diluted with 2 ml Pharmlyse to lyse the erythrocytes (RBC). The blood lacking RBC was further diluted with an additional 2 ml PBS/0.1 % NaN3 and then washed 2× with PBS/0.1 % NaN3. Cells were resuspended in 100 μl PBS/0.1 % NaN3 containing 1 μg Fc Block/106 cells and incubated on ice for 5 min. Cell surface-specific antibodies (0.5 μg) were added to each tube and incubated on ice for an additional 30 min in the presence of Fc block. Cells were washed 2× with PBS/0.1 % NaN3, and 100 μl of fixation buffer (4 % paraformaldehyde) was added to each tube and incubated for 20 min on ice. Cells were washed 2× with permeabilization buffer. Anti-β-AR antibodies or isotype controls were added to the appropriate tubes in 100 μl of permeabilization buffer (BD Biosciences, San Jose, CA) and incubated for 30–60 min on ice. Cells were washed and incubated with FITC goat anti-rabbit F(ab)2 antibody for 30 min. Cells were washed and analyzed by flow cytometry (BD FACSCalibur).
FACS analysis
Blood (600–800 μl) was collected in EDTA (K3) tubes (BD Vacutainer®, Franklin Lakes, NJ) from a cardiac puncture; 50 μl was mixed with 20 μl of a fluorescently labeled antibody cocktail against various cell surface markers in a BD Trucount™ Tube (BD Bioscience) and allowed to incubate at room temperature (RT) for 20 min. After the incubation period, 450 μl of BD FACSTM Lysing Solution (BD Bioscience) was added to each tube, and the samples were analyzed with a Becton Dickinson FACSCaliber flow cytometer.
Cell preparation
Mononuclear cells were isolated from mouse whole blood, spleens or bone marrow (BM) cells using a mouse Ficoll technique previously described (Davidson and Parish 1975). Briefly, cell suspensions were layered on top of 1 ml mouse Ficoll and centrifuged at 1,000×g (2,500 rpm) for 20 min at room temperature. The mononuclear cells were harvested and washed twice, once with PBS at room temperature and then once with mouse complete RPMI 1640 containing 10 % HI FBS, glutamine, Pen/Strep, NEAA, NaHCO3, gentamicin, sodium pyruvate at 4 °C. Cells were then counted and diluted to the appropriate density.
FACSARIA Sort
FACS Sorting of Leukocyte subsets was performed using a FACSARIA™ (Becton Dickinson, San Jose, CA). Single cell leukocyte populations were generated from mouse spleen or BM, as previously described. Erythrocytes were removed by hypotonic lysis and the remaining cells were counted using a Coulter Counter. Leukocytes (15×106) were incubated in antibody binding buffer (PBS, 0.5 % BSA plus 0.5 μg FC Block per 106 cells) for 10 min at RT. Antibody Cocktails were added based on the leukocyte subset to be sorted. CD4 cells (CD4/CD45/CD3), CD8 (CD8/CD45/CD3), CD19 (CD19/CD45/CD3), neutrophils (GR-1/CD45/CD11b), monocytes (F4/80/CD45/CD11b).
DNA isolation
A mouse tail (0.5–1 cm) was digested in 400 μl lysis buffer (10 mM Tris, 25 mM EDTA, 75 mM NaCl and 1 % SDS in Sterile MilliQ H2O at pH 8.0) containing 400 μg/ml freshly added Proteinase K (16 μl of 10 mg/ml Proteinase K Stock in 384 μl lysis buffer) and incubated at 50 °C overnight. An equal volume of phenol:chloroform (1:1, v/v) was added to each tube, vortexed and then spun at RT in an Eppendorf centrifuge at 13,800×g (13,000 rpm) for 10 min. The top 350-μl aqueous layer was transferred to a new Eppendorf tube and an equal volume (350 μl) of chloroform was added, vortexed and centrifuged as described above. The top 300-μl aqueous layer was removed and added to a new Eppendorf tube containing 750 μl of ice-cold 100 % ethanol containing 60 μl of 10 M ammonium acetate and placed in the −80 °C freezer for 15 min. After 15 min, the samples were centrifuged at 13,800×g at 4 °C for 10 min and the supernatant was aspirated being careful not to aspirate the DNA pellet. The DNA pellet was washed twice with 200 μl of ice-cold 70 % ethanol using the 4 °C centrifuge. The supernatant was aspirated off and the remaining ethanol was allowed to evaporate. The DNA pellet was resuspended in 100 μl of sterile water and dissolved in a 37 °C water bath for 2 h. DNA amount and purity were determined by measuring absorbance at 260 nm and 280 nm of a 1:100 dilution of sample in sterile water (i.e., 10 μl sample + 990 μl H2O) using water as the reference buffer. Ideally the ratio of Abs 260/280 should be within 1.8–2.0. DNA was calculated as ng/μl = Abs260 × 50 × 100 dil. Samples were labeled and stored at −20 °C until use. For isolated leukocyte subsets, the same protocol was used except all volumes were reduced by half.
PCR
Total DNA was isolated from the various purified leukocyte subsets. Primer sequences were as described by Swanson et al. (2001) for mouse β1-AR and β3-AR and Taconic Biotechnology for β2-AR. The sequences were as follows: β1-AR (FWD, 5′-AAACTCTGGTAGCGAAAGGGGAC-3′ and REV, 5′-TCTGCTCATCGTGGTGGGTAAC-3′); β 2-AR (FWD, 5′-ACTTCCTTAGGGATGAGGTTGTCC-3′ and REV, 5′-TTGCCTATCCAGATGCACTGGTAC-3′); and β3-AR (FWD, 5′-CGAAGAGCATCACAAGGAGGG-3′ and REV, 5′-CGAAACTGGTTGCGGAACTGTGT-3′). The primers were purchased from Integrated DNA Technologies, Inc. (www.idtdna.com). PCR amplification was performed using a Genius thermocycler (Techne, Inc.) initially for 3 min at 95 °C; followed by 35 optimized annealing temperature cycles (30 s at 94 °C; 30 s at 61 °C; 45 s at 72 °C), followed by 5 min at 72 °C and then held at 10 °C for up to 18 h until use.
DNA gel preparation
A 2 % agarose gel was prepared by dissolving 0.6 g Seakem® GTG® agarose in 30 ml of 0.5× TBE buffer for 2 min in a microwave. After the agarose had cooled slightly, 5 μl of ethidium bromide (10 mg/ml) was added and the agarose was poured into the mold. While the gel solidified, 2 μl of 6× DNA dye was added to each PCR sample tube and mixed. Next, 0.5× TBE buffer was used to submerge the entire gel and 4 μl of DNA Ladder or sample was added to each well. The gel was run at 140 V/2 A for 30 min in a Horizon™ 58 Horizontal Gel Electrophoresis System (Life Technologies, Inc., Gaithersburg, MD) and imaged using a FUJIFILM Luminescent Image Analyzer LAS-3000.
RT-PCR analysis
RNA isolation and analysis of expression was assayed as previously described (Swanson et al. 2001).
Oxidative stress (GSH and nitric oxide production)
Measurement of nitrite production
Serum was collected for nitrite determination as previously described (Tian et al. 1995). Briefly, Griess reagent (100 μl) (a 1:1 mixture of 1 % p-aminobenzenesulfonamide in 5 % H3PO4 and 0.1 % naphthylethylenediamine dihydrochloride in H2O) were added (v/v) to diluted serum and standard (NaNO2) in 96-well plates. Plates were incubated at room temp for 10 min and absorbance was measured at 550 nm in a plate reader and concentration was determined from the standard curve.
Intracellular GSH-NEM assay
GSH levels were measured with leukocyte subsets from control and ACRS-treated mice using forward and side light scatter as well as the general leukocyte marker CD45 to gate out debris and contaminating erythrocytes. Lymphocytes, monocytes and granulocytes were gated and analyzed for intracellular glutathione levels with a monoclonal antibody to GSH-NEM (GeoMean Intensity); 250 μl of paraformaldehyde fixed cells were aliquoted to tubes containing 250 μl of Cytofix/Cytoperm™ for 15 min on ice. One milliliter of BD Perm/Wash™ (±10 mM NEM) was added to the appropriate tubes and incubated for 20 min on ice. Cells were washed 2× with 1 ml BD Perm/Wash™ and resuspended in 100 μl BD Perm/Wash™ containing 10 μg of 8.1GSH-NEM (Alexa 647) and 1 μg of Per-CP anti-mouse CD45 and incubated for 30 min on ice in the dark. Cells were washed twice with 1 ml BD Perm/Wash™ and resuspended in 500 μl PBS for flow cytometric analysis of intracellular GSH levels.
Transwell chemotaxis assay
All cell concentrations were adjusted to approximately 10×106 cells/ml. First, 100 μl of cell suspension (1×106 cells) was added to the top of a Transwell containing 5-μm pores and incubated for 1 h at 37 °C. Then, 600 μl of pre-warmed chemotaxis medium (±100 ng/ml SDF-1α or 5 % serum from control or ACRS mice) was added to the lower compartment. After 3 h, the cells, which had migrated to the lower chamber, were harvested, stained and analyzed by flow cytometry, as previously described. Tru-count tubes were used to determine absolute counts.
For Matrigel Transwell studies, the same protocol as above was used, except the Transwell membrane had been pre-coated with 100 μl of 1 mg/ml of Matrigel and incubated at 37 °C for 1 h prior to adding cells. Matrigel is a liquid at 4 °C and gels at room temperature and above to form a thin gel like barrier on top of the Transwell pores, which prevented random migration through the 5-μm pores.
Statistical analysis
Differences in mean levels of leukocyte subsets, glutathione, and oxidative burst between stress treated or control conditions within each strain were determined using the t-test. Error bars represent the standard error of the mean (SEM). Comparisons between subsets under each experimental condition were analyzed using one-way ANOVA and Tukey’s multiple comparison post test unless otherwise described. All statistical analyses were performed with the software package SigmaStat (Jandeel Scientific, San Rafael, CA). Differences were considered significant if p < 0.05.
Results
Effect of ACRS on the presence of leukocyte subsets in the blood
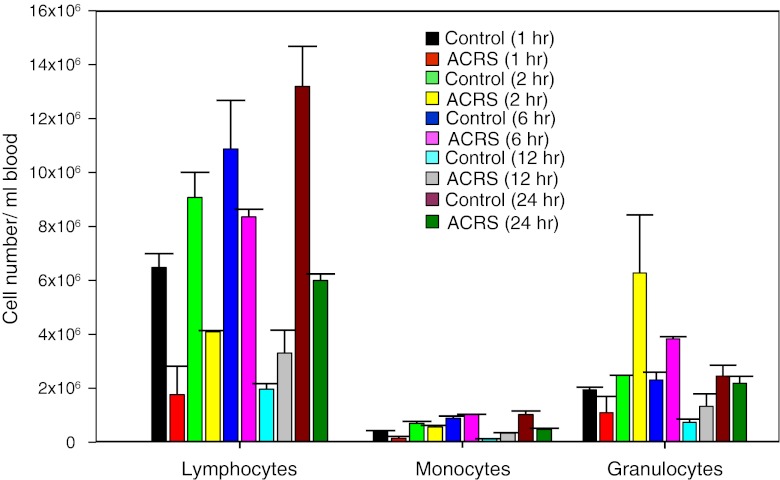
ACRS was performed between 10 am and 12 noon to minimize any potential circadian effects on the initial leukocyte populations. Mice were given ACRS for 1 h and then either sacrificed immediately or allowed to recover in their cages for varying amounts of time (Fig. 1). ACRS reduced the number of lymphocytes and monocytes in the circulation immediately following ACRS. Although there was a slight decline in the number of neutrophils immediately following ACRS, at 2 h following the initiation of ACRS, the number of neutrophils in the blood significantly increased. This lymphocytopenia/neutrophilia effect was most evident between 2 and 6 h following initiation of ACRS. The ACRS effects were less evident at 12 and 24 h due mainly to changes in overall leukocyte subsets of the control mice, which may be due to their normal diurnal leukocyte variations (Dhabhar et al. 1994). The 12 h time point corresponded to 10:00–11:00 pm, which would be when the mice are normally becoming active (Lucin et al. 2007). Since 2 h was the earliest time point that showed the major effect of ACRS (i.e., lymphocytopenia/neutrophilia), all later experiments assayed the mice at 2 h following the initiation of ACRS.
Fig. 1.
Effects of ACRS on presence of leukocytes in the blood. The absolute number of lymphocytes, monocytes and granulocytes were enumerated from 1 to 24 h following ACRS treatment. ACRS (1 h) mice and control mice were sacrificed immediately (1 h time point) or allowed to recover in their cages for varying amounts of time prior to bleeding. At early times (1 and 2 h), ACRS suppressed the numbers of lymphocytes and elevated the numbers of neutrophils
The altered distribution of leukocytes seen with ACRS is differentially mediated through β1-AR and β2-AR
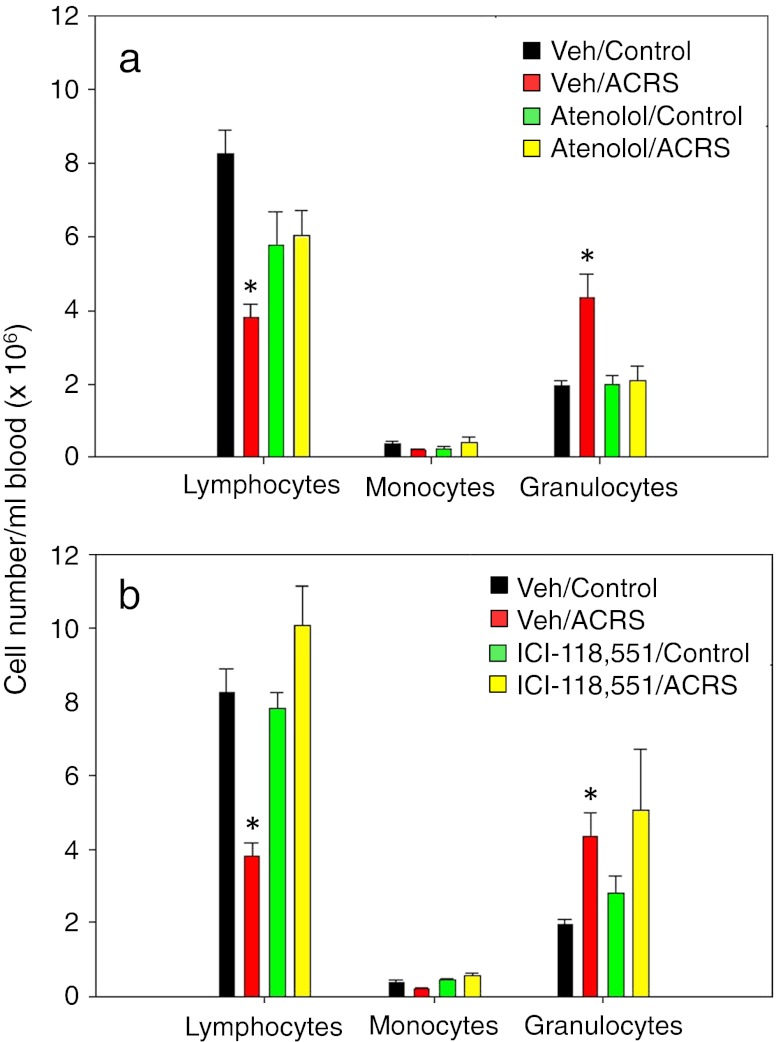
With use of the nonselective β-antagonist propanolol, it was previously suggested that leukocyte trafficking is mediated through a β-adrenergic signaling mechanism (Benschop et al. 1996a, b; Engler et al. 2004). Therefore, to determine which subtype of β-AR was involved, the selective β1-AR antagonist atenolol and the β2-AR antagonist ICI 118,551 were utilized. Male BALB/c mice were weighed and randomly put into one of the following treatment groups: vehicle (i.e., phosphate buffered saline), atenolol (20 mg/kg BW) or ICI 118,551 (15 mg/kg BW). Dosages utilized were based on a previous report (Cao et al. 2003a). Each treatment group was divided (non-stressed control group and ACRS group) as previously described. Atenolol suppressed the ACRS-induced neutrophilia (Fig. 2a); atenolol also partially prevented the ACRS-induced lymphocytopenia effect. The inability of lymphocytes to reach control levels appears to be due to the atenolol treatment itself, in that mice treated with atenolol only had the same circulating levels of lymphocytes as the ACRS-treated mice that received atenolol. ICI-118,551 prevented ACRS-induced lymphocyopenia (Fig. 2b), but it had no effect on preventing the ACRS-induced neutrophilia. Therefore, blood leukocyte redistribution following ACRS seems to be mediated by β1-AR and β2-AR, which differentially regulate blood leukocyte distribution following stress.
Fig. 2.
β-AR antagonist effects on peripheral blood leukocyte numbers after ACRS. BALB/c mice treated IP with atenolol (a) or ICI 118,551 (b) 20 min prior to ACRS. At 2 h following the initiation of ACRS, blood was assayed for presence of leukocyte subsets by flow cytometry. Statistical analysis: one-way analysis of variance was used with either Tukey or Dunn’s post hoc test. The p (<0.05) value comparisons are as follows: (*) versus vehicle control group
The altered distribution of lymphocytes in blood observed after ACRS-induced lymphocytopenia is differentially mediated through β1-AR and β2-AR
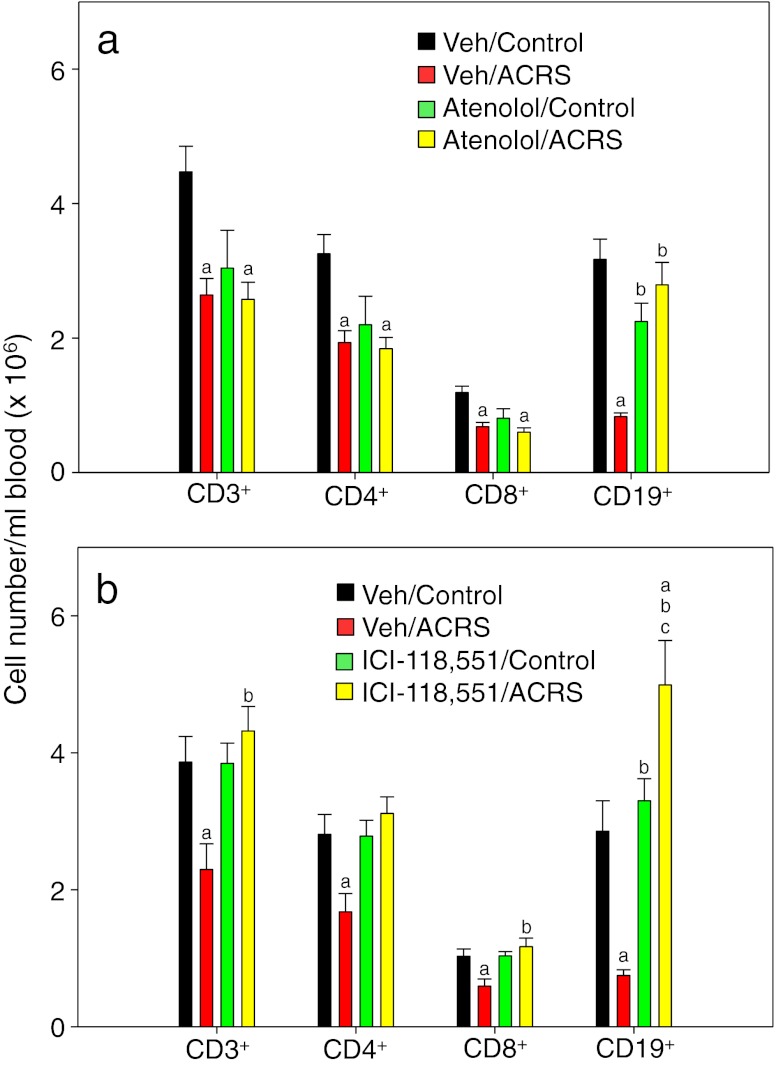
Atenolol and ICI-118,551 were further evaluated for their effects on the individual lymphocyte subsets. Atenolol-induced lymphocytopenia was mainly due to its effect on the CD3 (CD4 and CD8) T-cells and not on the CD19 B-cells (Fig. 3a). Atenolol did not prevent the depletion of CD4 or CD8 T-cells from the blood by ACRS. In fact, the atenolol injection itself substantially reduced the number of circulating T cells due to an unknown mechanism. In contrast to T-cells, atenolol prevented the loss of B-cells after ACRS. These individual lymphocyte subset results explain the results shown (Fig. 2a), with regard to atenolol’s inability to bring overall lymphocyte counts back to control mouse levels since T-cells outnumber B-cells. ICI-118,551 prevented the ACRS-induced decrease in all lymphocyte subsets (CD4, CD8, and CD19 lymphocytes; Fig. 3b), and interestingly, blocking β2-ARs caused an increased number of circulating CD19 B-cells after ACRS even above that of untreated controls.
Fig. 3.
Peripheral blood leukocytes of BALB/c mice that were injected IP with vehicle (PBS), atenolol (20 mg/kg BW), or ICI 118,551 (15 mg/kg BW) 20 min prior to initiation of ACRS. At 2 h following the initiation of ACRS, a whole blood sample was taken for analysis by flow cytometry. Statistical analysis: one-way analysis of variance with Tukey post hoc test. The p (<0.05) value comparisons are as follows: a versus vehicle control group; b versus vehicle ACRS group; c versus treatment (atenolol or ICI-118,551) control group
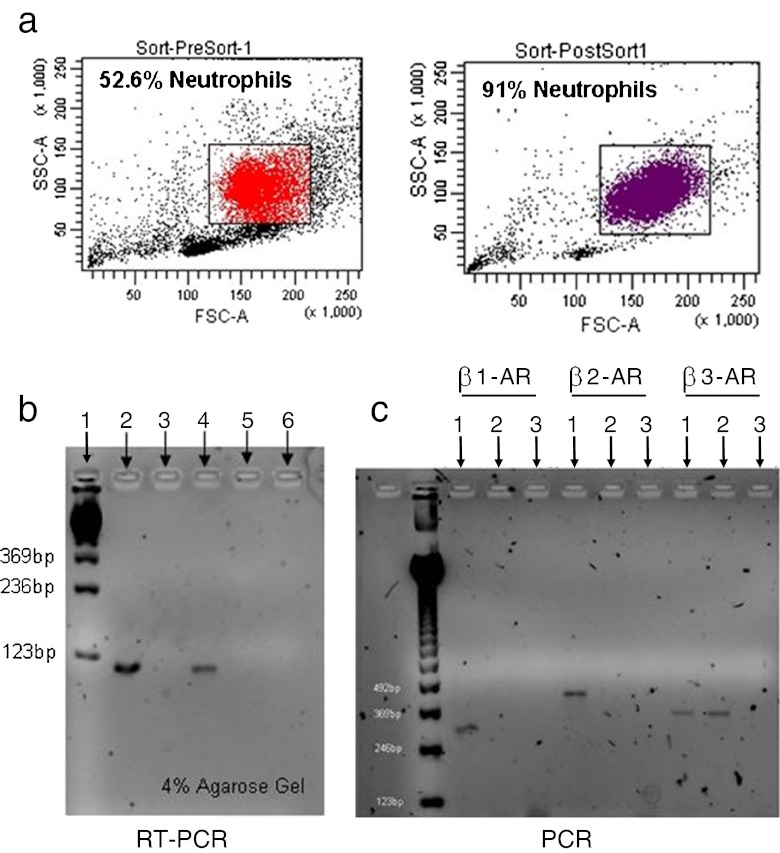
Mouse neutrophils express β1-AR mRNA
Since mouse neutrophil trafficking was shown to be altered through β1-AR, in that atenolol prevented the increase after ACRS, the expression of β1-AR by neutrophils was assessed since it has been reported that human neutrophils express β2-ARs but not β1-ARs (de Coupade et al. 2004). Mouse neutrophils were isolated from mouse femurs and purified by sorting with FACSAria (FACSORT). BM neutrophil preparations were enriched from 53 % to 91 % (Fig. 4a). The purified neutrophils were then used to determine mRNA β1-AR expression by RT-PCR analysis. Neutrophils isolated from BALB/c mice express β1-AR mRNA (Fig. 4b). In order to ensure that the β1-AR and β2-AR deficiency was appropriately detected, PCR was performed on mice lacking β1-AR and β2-AR (β1β2KO) (Fig. 4c).
Fig. 4.
Bone marrow neutrophils were isolated and sorted based on GR-1+/CD11b+ staining by BD FACSAria sorting, which enriched the neutrophil population from 53 % to 91 % (a). This concentrated neutrophil population was used to determine β1-AR mRNA expression (b) in reference to BALB/c (lanes 2 & 4) and β1β2KO (lanes 3 & 5) neutrophil (lanes 2 & 3) and spleen (lanes 4 & 5) preparations. Lane 1 contained nucleotide bp control markers and lane 6 was a negative control. PCR was performed on the same mice to ensure they expressed the proper knock-out genes (c); lanes 1, 2 and 3 represent BALB/c cells, β1β2KO cells, and negative control, respectively
Mouse leukocyte expression of beta-ARs
Since our RT-PCR neutrophil population was not 100 % pure, to rule out any potential contamination from other cell types and to confirm that neutrophil β1-AR mRNA expression translates into expression of the actual receptor, β1-AR protein expression by leukocyte subsets was evaluated (Fig. 5). All leukocyte subsets expressed β1-ARs, confirming the β1-AR mRNA data of neutrophils.
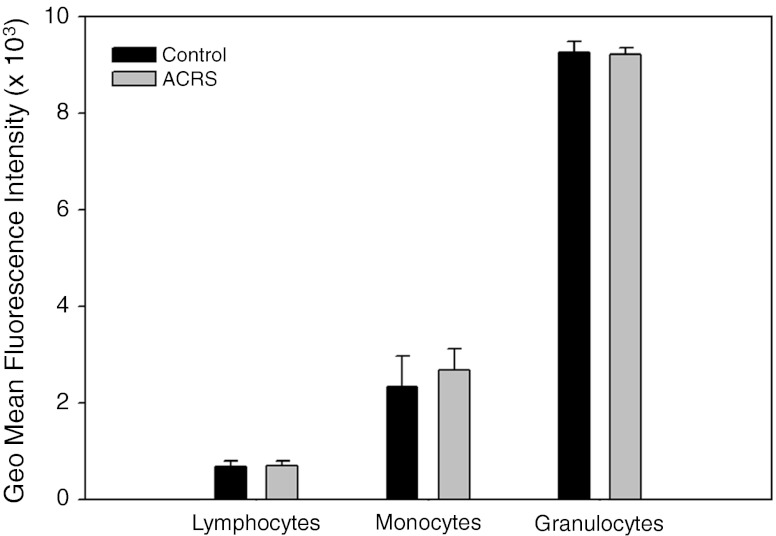
Fig. 5.
Expression of β1-AR by peripheral blood leukocytes. After RBC lysis, cells were washed, fixed, and permeabilized. Based on flow cytometric separation of blood leukocytes with light scatter and antigens (see Materials and methods), the permeabilized subpopulations were assayed for the amount of β1-AR (Geomean of Fluroescence Intensity) with rabbit IgG or rabbit polyclonal antibodies to the intracellular cytoplasmic domain of β1-AR followed by FITC-goat anti-rabbit IgG
ACRS does induce oxidative stress and nitric oxide production although it did not affect the glutathione levels of the leukocyte subsets
It was previously reported that ACRS lowers the expression of surface thiols on leukocytes, which suggests oxidative stress (Emeny and Lawrence 2006). ACRS also enhances early expression of perforin, which affects cell membranes and cell viability (Emeny et al. 2007). Similar to the expression of perforin, ACRS induced early production of nitric oxide, which was measured as nitrite (Fig. 6), and generated protein radicals (data not shown), as assayed with the nitrone spin trap 5,5-dimethyl-1-pyrroline N-oxide (DMPO), which forms a conjugate with cysteine, tyrosine and tryptophan radicals (Ramirez et al. 2003). Although there are signs of ACRS-induced oxidative stress, at 2–6 h following the initiation of ACRS, there was no difference in the amount of GSH in any leukocytes subsets between the control and ACRS-treated mice (Fig. 7). However, there were differences in the quantity of GSH in lymphocytes, monocytes and neutropils with the greatest amount of GSH in neutrophils.
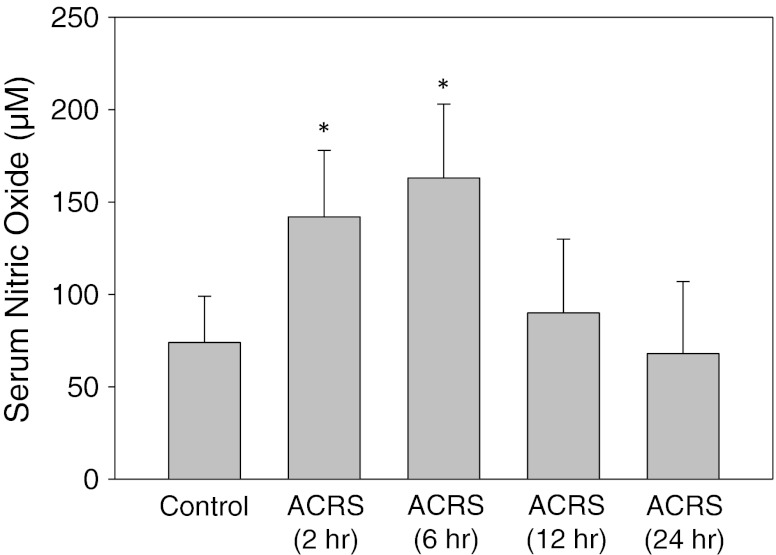
Fig. 6.
ACRS induction of nitric oxide. Serum was deproteinized and the level of nitric oxide was assayed by conversion to nitrite as described in Methods. The sera were measured from 2 to 24 h after ACRS initiation; at 2 and 6 h, there were significant (*) increases in nitric oxide
Fig. 7.
Leukocyte subsets from whole blood were measured at 2 h following the initiation of ACRS for GSH Levels. RBCs were removed (i.e., hypotonic lysis of RBCs) from the whole blood sample and washed thoroughly. The leukocytes were fixed with 2 % paraformaldehyde and permeabilized using Cytofix/Cytoperm. Permeabilized cells were incubated with 10 mM NEM for 20 min, washed and then analyzed by Flow cytometry using a fluorescently labeled 8.1 GSH-NEM antibody. ACRS induced no significant change in any glutathione levels
The neutrophilia following ACRS is due to release (activation/mobilization) of neutrophils from the bone marrow
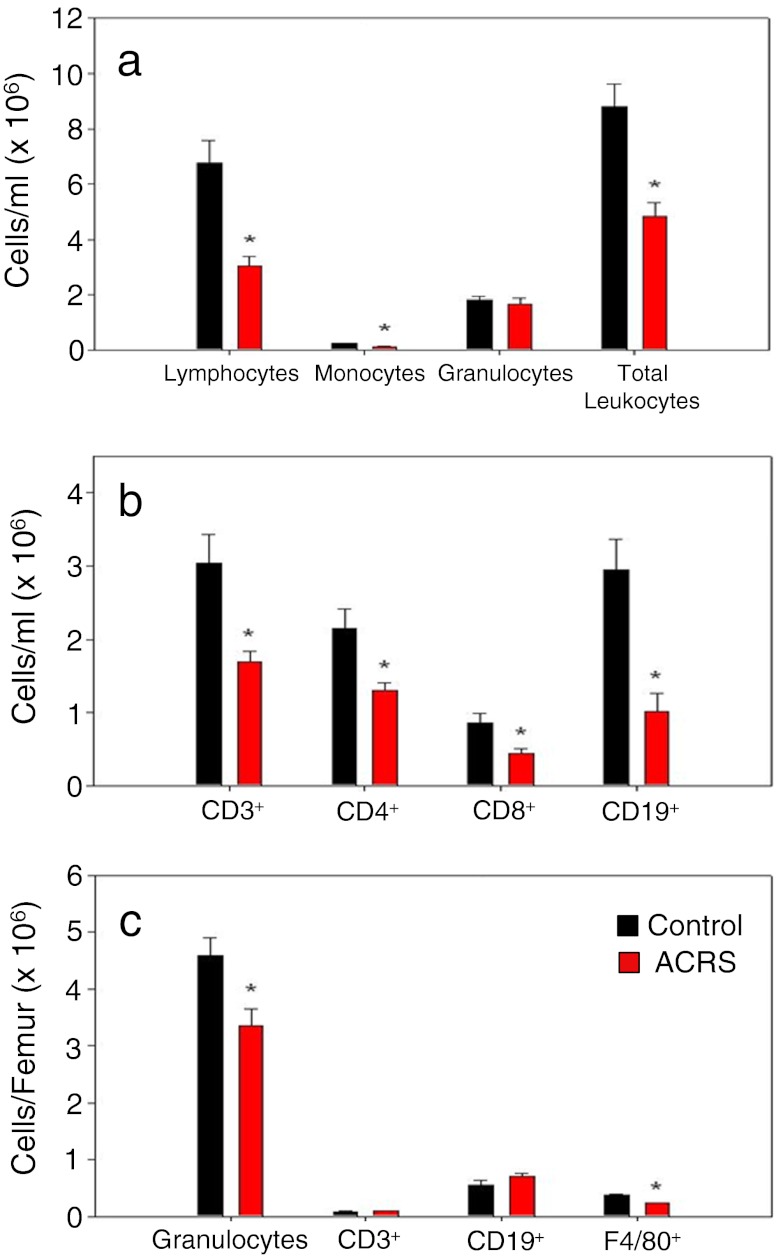
Control and ACRS BALB/c mice were evaluated for their leukocyte profiles 24 h following ACRS to determine if the increase in circulating neutrophils seen during the first 6 h following the initiation of ACRS came from the BM, thus causing BM neutrophil depletion (Fig. 8). At 24 h, in the whole blood, all lymphocyte subsets (i.e., CD4, CD8 and CD19) were still below their normal circulating levels. These results are comparable to the circulating lymphocyte levels seen at 2 h following initiation of ACRS. However, by 24 h, the granulocyte population had reverted back to its normal circulating levels as seen in the control mice and had reduced the absolute numbers of granulocytes in the BM. Therefore, ACRS initiates mobilization of neutrophils from the BM, which depletes the BM of its normal neutrophil reservoir.
Fig. 8.
BALB/c mice were split into two groups: control or ACRS. Mice in the ACRS group were subjected to ACRS for 1 h at 4 °C. Whole blood and bone marrow was harvested 24 h after the initiation of stress to evaluate leukocyte profiles; *p < 0.05
ACRS-induced neutrophilia was not mediated by an increase in circulating chemotactic factors specific to neutrophils
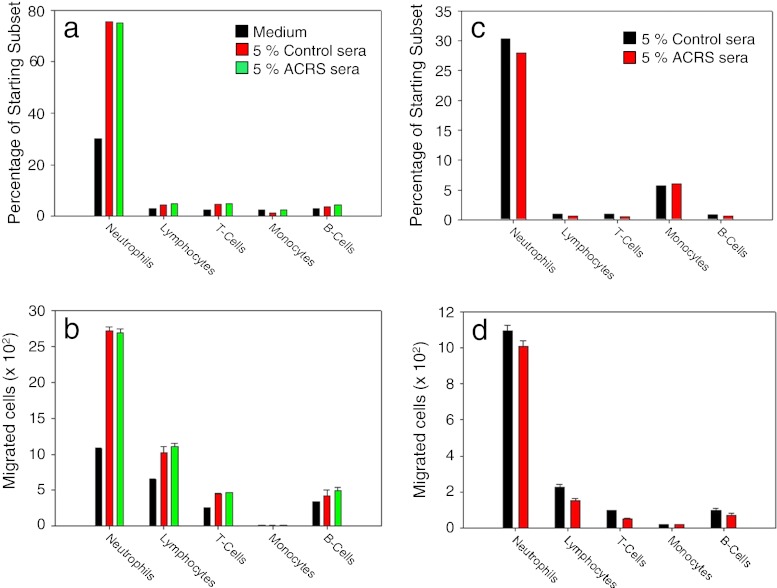
To determine if ACRS elevated the levels of chemotactic factors in serum, which could lead to the increase in circulating neutrophils following ACRS, sera from BALB/c control mice and BALB/c mice immediately following ACRS were collected. The control and ACRS sera were pooled and evaluated for their ability to attract different leukocyte subsets. Medium containing 5 % ACRS serum did not induce enhanced chemotaxis of any leukocyte subset from a normal BALB/c whole blood sample (Fig. 9a,b). Due to the naturally high chemokinetic potential of neutrophils to actively migrate through the 5-μm Transwell pores in the absence of a stimulus, the same experiment was repeated using a Transwell pre-treated with 100 μl of 1 mg/ml of Matrigel. The Matrigel layer reduced the overall absolute numbers of leukocytes migrating to the bottom well, as well as, their overall percentage from the starting population. However, as in the previous experiment, there was no difference between the ACRS and control sera with regard to chemotactic potential on any leukocyte subset (Fig. 9c,d).
Fig. 9.
BALB/c mice were split into two groups: control or ACRS. Mice in the ACRS group were subjected to ACRS for 1 h at 4 °C. Whole blood was immediately collected and allowed to clot for 30 min. The serum from the control and ACRS was removed and pooled for future analysis. All chemotaxis experiments used a chemotaxis media containing 5 % control or ACRS serum in a transwell chemotaxis system without (a, b) or with transwells pre-coated with 100 μl of 1 mg/ml Matrigel (c, d). There were no differences between control and ACRS sera
Bone marrow neutrophil mobilization/activation is prevented in β1-AR−/− mice
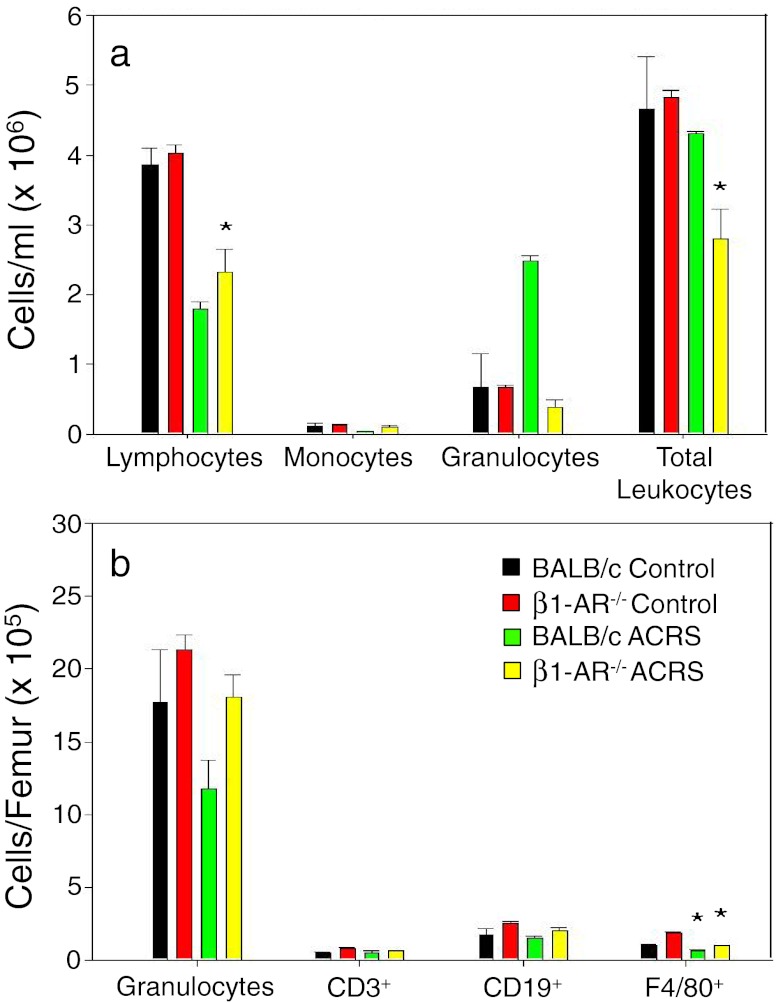
The following experiment was conducted to determine if β1-AR−/− mice would display a similar whole blood and BM leukocyte profile 2 h following the initiation of ACRS as compared to our initial studies using the β1-AR antagonist atenolol in BALB/c mice. Lymphocytes numbers in whole blood were decreased in both the BALB/c and β1-AR−/− mice due to a decrease of CD3+ and CD19+ cells in the BALB/c mice and only CD3+ cells in the β1-AR−/− mice. In contrast, neutrophil presence in the blood was increased with BALB/c mice but not with β1-AR−/− mice (Fig. 10a). These results are consistent with those with atenolol. Again, neutrophil mobilization was associated with BM neutrophil depletion (Fig. 10b).
Fig. 10.
BALB/c and β1-AR−/− mice were split into two groups: control or ACRS. Mice in the ACRS group were subjected to ACRS for 1 h at 4 °C; 2 h after the initiation of ACRS, whole blood and bone marrow were harvested to determine leukocyte profiles by flow cytometry; *p < 0.05
Responsiveness of BM neutrophils from BALB/c and B1KO mice sensitive to SDF-1α with or without ACRS
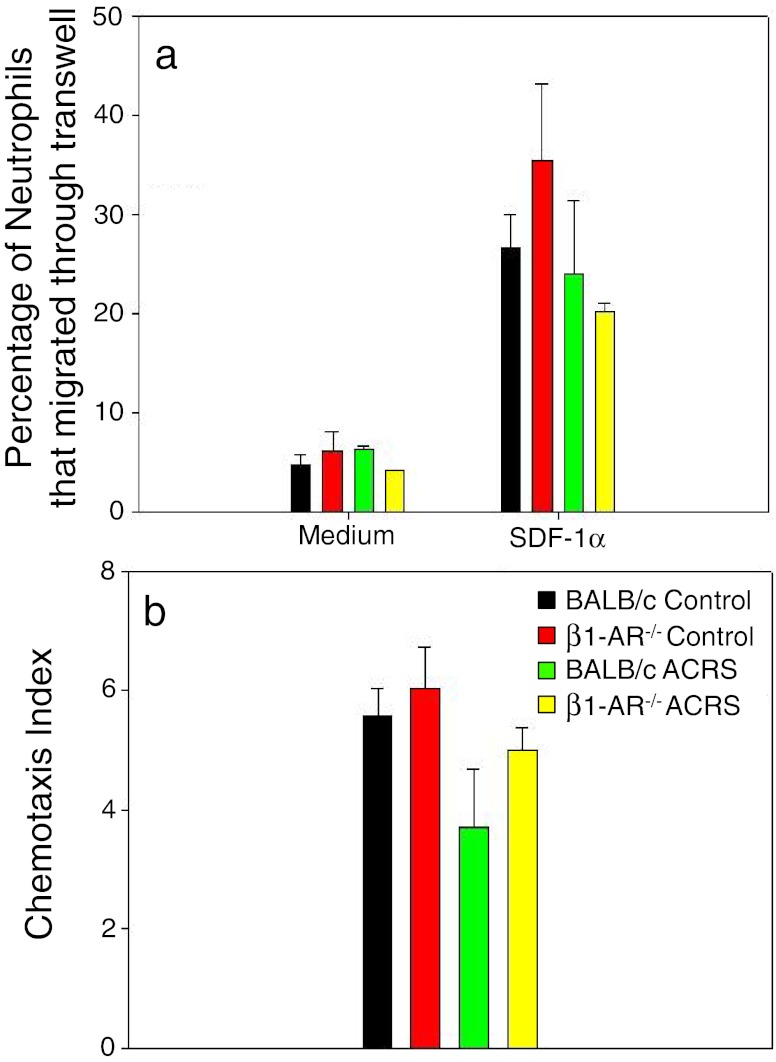
It has been suggested that the SDF-1α/CXCR4 chemokine axis is what retains neutrophils in the BM. To determine if stress or the absence of B1-AR makes neutrophils less sensitive to SDF-1α, either through desensitization or surface down regulation of CXCR4, leukocyte subsets harvested from the BM were evaluated for sensitivity to the chemokine SDF-1α, using a modified Matrigel Transwell chemotaxis system. ACRS seems to make neutrophils somewhat less responsive to SDF-1α, but there were no significant differences (Fig. 11).
Fig. 11.
BALB/c or β1-AR−/− mice were split into two groups: control or ACRS. Mice in the ACRS group were subjected to ACRS for 1 h at 4 °C. At 2 h following the initiation of ACRS, bone marrow was harvested and used to determine neutrophil chemotaxis to SDF-1α (100 ng/ml) using the modified Matrigel Transwell chemotaxis assay. There were no significant effects of ACRS or absence of β1-AR on chemotaxis
Discussion
Stress has long been associated with alterations in leukocyte whole blood profiles and increased susceptibility to infection and disease. Our ACRS/LM model is consistent with that finding, in that mice exposed to ACRS have an increased LM bacterial burden in their livers and spleens compared to control mice (Cao et al. 2002; Cao and Lawrence 2002; Cao et al. 2003a, b), and as shown herein, ACRS has an immediate and relatively short-term (≤24 h) affect on the peripheral blood leukocyte profile. This characteristic stress-induced response of lymphopenia and neutrophilia seen in whole blood is a common finding (Dhabhar et al. 1994, 1996), and it has been shown to be linked with elevated norephinephrine (NE) levels (Gruber-Olipitz et al. 2004). The early (2–6 h) leukocyte trafficking changes after ACRS are posited to be responsible for ACRS interference with host defenses against LM. Previously, no changes in cytokines or T cell activities could account for ACRS-induced loss of host defenses.
ACRS kinetically altered the leukocyte profiles in whole blood. Between 2 and 6 h following the initiation of ACRS, altered levels of lymphocytes and neutrophils were observed in the blood of the ACRS treated mice. The early effects of ACRS on the peripheral blood leukocyte profile occurred at the same time as the elevated expression of NO. Although NO production is usually considered a positive factor in defenses against LM, when it is produced may be critical in the development of efficient immunity against intracellular pathogens. Induction of NO production has been connected to release of hematopoietic progenitors from their BM niche (Récalde et al. 2012). Thus, early production of NO, which is influenced by SNS release of NE, may be associated with inopportune modulation of immune system defenses. The later (12–24 h) observed changes of leukocyte numbers in the blood likely correspond to circadian rhythm influences, in that the later times correspond to about 10 pm, which is the time when mice are normally becoming more active due to their nocturnal tendencies and corticosterone levels peak (Lucin et al. 2007). The 12-h time point may be due to normal diurnal fluctuations of leukocyte profiles (Dhabhar et al. 1994). ACRS-induced interference with host defenses previously had been shown to relate to NE release and not the elevated level of corticosterone (Cao et al. 2002).
With the use of β-AR antagonists, the results suggest CD3+ lymphocyte trafficking is mediated through β2-AR, CD19+ lymphocyte trafficking is mediated through a combination of β1- and β2-ARs, and neutrophil trafficking is affected mainly by β1-AR. However, it has been previously reported that cell egress from the BM is regulated by β2-ARs (Katayama et al. 2006). Since SCID mice, β1-AR−/− mice, mice pretreated with the β1-AR antagonist, and irradiated mice that received BM cells from β1-AR−/− mice had increased host resistance against LM infection compared to their wild type and knock-out/antagonist controls (Cao et al. 2002; Cao and Lawrence 2002; Cao et al. 2003a, b; Emeny et al. 2007), results suggest that decreased host resistance to LM following ACRS may be mediated by the early changes in the leukocyte changes in the blood. Although it appears that β1-ARs affect both host defenses and leukocyte presence in blood, human neutrophils have been reported to be devoid of β1-AR (de Coupade et al. 2004, 2007). However, mouse neutrophils are shown to express β1-ARs (Figs. 4 and 5), but this evidence still does not indicate direct NE influences on leukocytes are responsible for the trafficking changes.
The present analysis of ACRS influences on the peripheral blood leukocyte profile was undertaken to address β-AR effects on ACRS-induced modulation of host defenses. SNS release of NE has been reported to induce NO production by endothelial cells (Récalde et al. 2012) and β2-AR signaling of osteoblasts has been reported to alter the CXCL12 (SDF-1a) interaction with CXCR4, which maintains hematopoietic progenitors in their BM niche (Katayama et al. 2006). As noted, β-AR antagonists have implicated β2-ARs in the release of cells from the BM niche; however, most antagonists can affect β1-AR and β2-AR. The increased presence of neutrophils in the blood corresponded with a decreased number of neutrophils in the BM, and the release of neutrophils from BM was prevented with Atenonol and was also absent with β1-AR−/− mice. The neutrophils were not being called into circulation by an increase in circulating chemokines but rather probably released from the BM stromal environment due to SDF-1α desensitization through a β1-AR mechanism. The mechanism could be a direct effect of the SNS on neutrophils, themselves, and/or an indirect on the BM stromal niche. However, one of the proposed mechanisms involves the disruption of SDF-1α/CXCR4 interaction holding the cells in the BM.
SDF-1α (CXCL12) acts through one known receptor CXCR4 that is expressed on CD34+ progenitors, megakaryocytes, platelets, B cells, T cells, mononuclear phagocytes and neutrophils (Bleul et al. 1996, 1997; Förster et al. 1998; Möhle et al. 1998; Kowalska et al. 1999; Nagase et al. 2002). It has been reported that the SDF-1α/CXCR4 axis plays a critical role in the retention of myeloid cells within the BM during their development (Nagasawa et al. 1996; Ma et al. 1998; Martin et al. 2003). Mobilization of neutrophils into peripheral circulation occurs with G-CSF, which can disrupt the SDF-1α/CXCR4 axis within the BM environment (Suratt et al. 2004; Kim et al. 2006; Eash et al. 2009).
G-CSF is the principle cytokine responsible for regulating the proliferation and differentiation of myeloid progenitors (Basu et al. 2002) and is a potent inducer of the release of neutrophils from the BM into peripheral circulation (Demetri and Griffin 1991). G-CSF has been shown to directly down regulate CXCR4 expression on neutrophils at the cellular level (Kim et al. 2006), while others have reported that it stimulates proteases to enzymatically breakdown SDF-1α within the BM. The BM microenvironment is rich in proteolytic enzymes released by neutrophils, including metalloproteinase-9 (MMP-9), neutrophil elastase and cathepsin G (Lévesque et al. 2002), all of these factors have been shown to cleave and functionally inactivate SDF-1α (Delgado et al. 2001; McQuibban et al. 2001; Valenzuela-Fernandez et al. 2002). Both mechanisms would have the potential to release neutrophils from the BM.
As suggested, ACRS induced neutrophil exhaustion through a β1-AR mechanism is posited to be responsible for the decreased host resistance to LM infection. Neutrophils are one of the most important innate immune cells in the early defenses against LM infection. However, the innate immune system response to LM is a sequential set of steps, which allow the neutrophils to sense a bacterial infection (i.e., cytokines, chemokines, inflammatory markers) and respond in the appropriate manner and time sequence. Once the neutrophils receive their external cues and become activated, they mobilize to the area of infectious foci (i.e., hepatocytes, spleen) to clear damaged and infected cells while simultaneously killing off the LM. Afterwards, the neutrophils, which have a limited life span once leaving the BM, are cleared in order to prevent excessive damage to the host tissues and organs beyond what is required to clear the infection. This normal neutrophil response has its maximum effect around day 3 following a low dose LM infection. This is the day when the greatest number of neutrophils have left the BM (Navarini et al. 2009) in response to the infection and when that bacterial burden within the organs (i.e., liver and spleen) become stabilized before the gradual decline in bacterial burden over the next 7–10 days. Since neutrophils have a finite life span once leaving the BM, the neutrophils need to be released from the BM at the appropriate rate and time for optimization of host defenses. Additionally, released neutrophils enhance inflammatory processes, which also need to be properly timed. Elevated levels of enzymes, such as myeloperoxidase and metalloproteinases, and oxidants could interfere with T cell activation and enhance tissue accessibility of bacterial invasion.
Although LM infection, itself, is a stressor, the stress cues increase gradually over time and are correlated with the LM infection cues emanating from the infectious foci. However, it has been reported that if you give addition inflammatory or stress signals (i.e., bacterial antigens) in conjunction with a live dose of LM that it generates a scenario where a normal low dose infection of LM, which the animal would normally fight off, appears as a much higher dose in which the animal appears sicker and may actually become lethal (Navarini et al. 2009). It has been proposed that our ACRS model, through the immediate mobilization/activation of neutrophils, results in a similar scenario. By exhausting or tapping into the normal pool of mature neutrophils too early, it generates a scenario where a normal low dose infections (<104) mimics an infection seen with higher levels of LM (>104), which results in a more serious infection that could be lethal. Cold Stress and infectious challenge have been shown to evoke a sympathoadrenal response and increase NE turnover in the BM (Tang et al. 1999).
The mechanisms of early mobilization/activation of BM neutrophils leading to neutrophil exhaustion through a stress induced β1-AR mechanism within the BM is suggested to be why otherwise normally healthy individuals may succumb to what should be a relatively mild inoculum of a bacteria after stressful events. Timely modulation of NE signaling might provide a preventive treatment regime to prevent stress-induced opportunistic infections. Several groups have tried controlling infections by stimulating neutrophil production with G-CSF; however, with little to no success. One common theme among these experiments was that G-CSF was given after the individual had already been exposed to the pathogen; however, the literature has suggested that there is a point during a lethal infection when increases in the G-CSF levels can no longer help control infection (Navarini et al. 2009). The key may relate to the treatment being given at the most appropriate time. This would not conducive to controlling normal daily exposures to pathogens, but it might play an important role as a pre-treatment of individuals who are about to undergo what is known to be a stress event either psychologically or physically and would be prone to bacterial infections. A common example would be an individual in a hospital environment who is undergoing treatment or surgery. Assuming it would not cause any unwanted side effects within other organ systems, it would be interesting to know if giving individuals a β1-AR antagonist prior to a stressful event, like surgery, decreased incidence of post-operative infections within a hospital setting.
Acknowledgements
We thank the staff of the Immunology Core of Wadsworth Center for their assistance with the flow cytometry. This work was partially supported by National Science Foundation Nanobiology Technology Center Grant BSCECS9876771 and NIEHS U01 ES016014.
Footnotes
This work was submitted in partial fulfillment of PhD degree from the Department of Environmental Health Sciences, University at Albany School of Public Health.
References
- Avitsur R, Padgett DA, Sheridan JF. Social interactions, stress, and immunity. Neurol Clin. 2006;24:483–491. doi: 10.1016/j.ncl.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Basu S, Hodgson G, Katz M, Dunn AR. Evaluation of role of G-CSF in the production, survival, and release of neutrophils from bone marrow into circulation. Blood. 2002;100:854–861. doi: 10.1182/blood.V100.3.854. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Jacobs R, Sommer B, Schürmeyer TH, Raab JR, Schmidt RE, Schedlowski M. Modulation of the immunologic response to acute stress in humans by beta-blockade or benzodiazepines. FASEB J. 1996;10:517–524. doi: 10.1096/fasebj.10.4.8647351. [DOI] [PubMed] [Google Scholar]
- Benschop RJ, Rodriguez-Feuerhahn M, Schedlowski M. Catecholamine-induced leukocytosis: early observations, current research, and future directions. Brain Behav Immun. 1996;10:77–91. doi: 10.1006/brbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- Bhardwaj V, Kanagawa O, Swanson PE, Unanue ER. Chronic Listeria infection in SCID mice: requirements for the carrier state and the dual role of T cells in transferring protection or suppression. J Immunol. 1998;160:376–384. [PubMed] [Google Scholar]
- Biondi M, Zannino LG. Psychological stress, neuroimmunomodulation, and susceptibility to infectious diseases in animals and man: a review. Psychother Psychosom. 1997;66:3–26. doi: 10.1159/000289101. [DOI] [PubMed] [Google Scholar]
- Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleul CC, Wu L, Hoxie JA, Springer TA, Mackay CR. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci U S A. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner I, Shek PN, Zamecnik J, Shephard RJ. Stress hormones and the immunological responses to heat and exercise. Int J Sports Med. 1998;19:130–143. doi: 10.1055/s-2007-971895. [DOI] [PubMed] [Google Scholar]
- Cao L, Lawrence DA. Suppression of host resistance to Listeria monocytogenes by acute cold/restraint stress: lack of direct IL-6 involvement. J Neuroimmunol. 2002;133:132–143. doi: 10.1016/S0165-5728(02)00371-5. [DOI] [PubMed] [Google Scholar]
- Cao L, Filipov NM, Lawrence DA. Sympathetic nervous system plays a major role in acute cold/restraint stress inhibition of host resistance to Listeria monocytogenes. J Neuroimmunol. 2002;125:94–102. doi: 10.1016/S0165-5728(02)00039-5. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Lawrence DA. Acute cold/restraint stress inhibits host resistance to Listeria monocytogenes via beta1-adrenergic receptors. Brain Behav Immun. 2003;17:121–133. doi: 10.1016/S0889-1591(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Cao L, Hudson CA, Lawrence DA. Immune changes during acute cold/restraint stress-induced inhibition of host resistance to Listeria. Toxicol Sci. 2003;74:325–334. doi: 10.1093/toxsci/kfg146. [DOI] [PubMed] [Google Scholar]
- Christopher MJ, Link DC. Regulation of neutrophil homeostasis. Curr Opin Hematol. 2007;14:3–8. doi: 10.1097/00062752-200701000-00003. [DOI] [PubMed] [Google Scholar]
- Davidson WF, Parish CR. A procedure for removing red cells and dead cells from lymphoid cell suspensions. J Immunol Methods. 1975;7:291–300. doi: 10.1016/0022-1759(75)90026-5. [DOI] [PubMed] [Google Scholar]
- de Coupade C, Gear RW, Dazin PF, Sroussi HY, Green PG, Levine JD. Beta 2-adrenergic receptor regulation of human neutrophil function is sexually dimorphic. Br J Pharmacol. 2004;143:1033–1041. doi: 10.1038/sj.bjp.0705972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Coupade C, Brown AS, Dazin PF, Levine JD, Green PG. Beta(2)-Adrenergic receptor-dependent sexual dimorphism for murine leukocyte migration. J Neuroimmunol. 2007;186:54–62. doi: 10.1016/j.jneuroim.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MB, Clark-Lewis I, Loetscher P, Langen H, Thelen M, Baggiolini M, Wolf M. Rapid inactivation of stromal cell-derived factor-1 by cathepsin G associated with lymphocytes. Eur J Immunol. 2001;31:699–707. doi: 10.1002/1521-4141(200103)31:3<699::AID-IMMU699>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Demetri GD, Griffin JD. Granulocyte colony-stimulating factor and its receptor. Blood. 1991;78:2791–2808. [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Diurnal and acute stress-induced changes in distribution of peripheral blood leukocyte subpopulations. Brain Behav Immun. 1994;8:66–79. doi: 10.1006/brbi.1994.1006. [DOI] [PubMed] [Google Scholar]
- Dhabhar FS, Miller AH, McEwen BS, Spencer RL. Stress-induced changes in blood leukocyte distribution. Role of adrenal steroid hormones. J Immunol. 1996;157:1638–1644. [PubMed] [Google Scholar]
- Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–4719. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeny RT, Lawrence DA, et al. Cold-restraint-induced immune and biochemical changes inhibit host resistance to Listeria. In: Ader R, et al., editors. Psychoneuroimmunology, vol. IV. Academic Press: Academic Pressx; 2006. pp. 1035–1051. [Google Scholar]
- Emeny RT, Gao D, Lawrence DA. Beta1-adrenergic receptors on immune cells impair innate defenses against Listeria. J Immunol. 2007;178:4876–4884. doi: 10.4049/jimmunol.178.8.4876. [DOI] [PubMed] [Google Scholar]
- Engler H, Dawils L, Hoves S, Kurth S, Stevenson JR, Schauenstein K, Stefanski V. Effects of social stress on blood leukocyte distribution: the role of alpha- and beta-adrenergic mechanisms. J Neuroimmunol. 2004;156:153–162. doi: 10.1016/j.jneuroim.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Förster R, Kremmer E, Schubel A, Breitfeld D, Kleinschmidt A, Nerl C, Bernhardt G, Lipp M. Intracellular and surface expression of the HIV-1 coreceptor CXCR4/fusin on various leukocyte subsets: rapid internalization and recycling upon activation. J Immunol. 1998;160:1522–1531. [PubMed] [Google Scholar]
- Freestone PP, Sandrini SM, Haigh RD, Lyte M. Microbial endocrinology: how stress influences susceptibility to infection. Trends Microbiol. 2008;16:55–64. doi: 10.1016/j.tim.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Gruber-Olipitz M, Stevenson R, Olipitz W, Wagner E, Gesslbauer B, Kungl A, Schauenstein K. Transcriptional pattern analysis of adrenergic immunoregulation in mice. Twelve hours norepinephrine treatment alters the expression of a set of genes involved in monocyte activation and leukocyte trafficking. J Neuroimmunol. 2004;155:136–142. doi: 10.1016/j.jneuroim.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS. Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell. 2006;124:407–421. doi: 10.1016/j.cell.2005.10.041. [DOI] [PubMed] [Google Scholar]
- Kim HK, De La Luz Sierra M, Williams CK, Gulino AV, Tosato G. G-CSF down-regulation of CXCR4 expression identified as a mechanism for mobilization of myeloid cells. Blood. 2006;108:812–820. doi: 10.1182/blood-2005-10-4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalska MA, Ratajczak J, Hoxie J, Brass LF, Gewirtz A, Poncz M, Ratajczak MZ. Megakaryocyte precursors, megakaryocytes and platelets express the HIV co-receptor CXCR4 on their surface: determination of response to stromal-derived factor-1 by megakaryocytes and platelets. Br J Haematol. 1999;104:220–229. doi: 10.1046/j.1365-2141.1999.01169.x. [DOI] [PubMed] [Google Scholar]
- Lévesque JP, Hendy J, Takamatsu Y, Williams B, Winkler IG, Simmons PJ. Mobilization by either cyclophosphamide or granulocyte colony-stimulating factor transforms the bone marrow into a highly proteolytic environment. Exp Hematol. 2002;30:440–449. doi: 10.1016/S0301-472X(02)00788-9. [DOI] [PubMed] [Google Scholar]
- Lucin KM, Sanders VM, Jones TB, Malarkey WB, Popovich PG. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp Neurol. 2007;207:75–84. doi: 10.1016/j.expneurol.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/S1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–43508. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- Möhle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Nagase H, Miyamasu M, Yamaguchi M, Imanishi M, Tsuno NH, Matsushima K, Yamamoto K, Morita Y, Hirai K. Cytokine-mediated regulation of CXCR4 expression in human neutrophils. J Leukoc Biol. 2002;71:711–717. [PubMed] [Google Scholar]
- Navarini AA, Lang KS, Verschoor A, Recher M, Zinkernagel AS, Nizet V, Odermatt B, Hengartner H, Zinkernagel RM. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci U S A. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez DC, Chen YR, Mason RP. Immunochemical detection of hemoglobin-derived radicals formed by reaction with hydrogen peroxide: involvement of a protein-tyrosyl radical. Free Radic Biol Med. 2003;34:830–839. doi: 10.1016/S0891-5849(02)01437-5. [DOI] [PubMed] [Google Scholar]
- Récalde A, Richart A, Guérin C, Cochain C, Zouggari Y, Yin KH, Vilar J, Drouet I, Lévy B, Varoquaux O, Silvestre JS. Sympathetic nervous system regulates bone marrow-derived cell egress through endothelial nitric oxide synthase activation: role in postischemic tissue remodeling. Arterioscler Thromb Vasc Biol. 2012;32:643–653. doi: 10.1161/ATVBAHA.111.244392. [DOI] [PubMed] [Google Scholar]
- Rezaei N, Moazzami K, Aghamohammadi A, Klein C. Neutropenia and primary immunodeficiency diseases. Int Rev Immunol. 2009;28:335–366. doi: 10.1080/08830180902995645. [DOI] [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood. 2004;104:565–571. doi: 10.1182/blood-2003-10-3638. [DOI] [PubMed] [Google Scholar]
- Swanson MA, Lee WT, Sanders VM. IFN-γ production by Th1 cells generated from naïve CD4+ T cells exposed to norepinephrine. J Immunol. 2001;166:232–240. doi: 10.4049/jimmunol.166.1.232. [DOI] [PubMed] [Google Scholar]
- Tang Y, Shankar R, Gamelli R, Jones S. Dynamic norepinephrine alterations in bone marrow: evidence of functional innervation. J Neuroimmunol. 1999;96:182–189. doi: 10.1016/S0165-5728(99)00032-6. [DOI] [PubMed] [Google Scholar]
- Tian L, Noelle RJ, Lawrence DA. Activated T cells enhance nitric oxide production by murine splenic macrophages through gp39 and LFA-1. Eur J Immunol. 1995;25:306–309. doi: 10.1002/eji.1830250152. [DOI] [PubMed] [Google Scholar]
- Valenzuela-Fernandez A, Planchenault T, Baleux F, Staropoli I, Le-Barillec K, Leduc D, Delaunay T, Lazarini F, Virelizier JL, Chignard M, Pidard D, Arenzana-Seisdedos F. Leukocyte elastase negatively regulates stromal cell-derived factor-1 (SDF-1)/CXCR4 binding and functions by amino-terminal processing of SDF-1 and CXCR4. J Biol Chem. 2002;277:15677–15689. doi: 10.1074/jbc.M111388200. [DOI] [PubMed] [Google Scholar]