Abstract
The p38 mitogen-activated protein kinase PMK-1 of Caenorhabditis elegans has been associated with heavy metal, oxidative and pathogen stress. Pmk-1 is part of an operon comprising three p38 homologues, with pmk-1 expression suggested to be regulated by the operon promoter. There are contradictory reports about the cellular localization of PMK-1. We were interested to study principles of pmk-1 expression and to analyze the role of PMK-1 under heat stress. Using a translational GFP reporter, we found pmk-1 expression to be driven by a promoter in front of pmk-1. PMK-1 was detected in intestinal cells and neurons, with a cytoplasmic localization at moderate temperature. Increasing temperature above 32 °C, however, induced a nuclear translocation of PMK-1 as well as PMK-1 accumulation near to apical membranes. Testing survival rates revealed 34–35 °C as critical temperature range, where short-term survival severely decreased. Mutants of the PMK-1 pathway (pmk-1Δ, sek-1Δ, mek-1Δ) as well as a mutant of JNK pathway (jnk-1Δ) showed significantly lower survival rates than wild-type or mutants of other pathways (kgb-1Δ, daf-2Δ). Rescue and overexpression experiments verified the negative effects of pmk-1Δ on heat tolerance. Studying gene expression by RNA-seq and semi-quantitative reverse transcriptase polymerase chain reaction revealed positive effects of the PMK-1 pathway on the expression of genes for chaperones, protein biosynthesis, protein degradation, and other functional categories. Thus, the PMK-1 pathway is involved in the heat stress responses of C. elegans, possibly by a PMK-1-mediated activation of the transcription factor SKN-1 and/or an indirect or direct PMK-1-dependent activation (hyperphosphorylation) of heat-shock factor 1.
Keywords: DAF-2, HSF-1, HSP, SKN-1, TOR
Introduction
Heat is one of the most common stressors for organisms. The deleterious effects for cells include protein aggregation and denaturation, DNA and RNA damage, changes in the fluidity and permeability of membranes, or the formation of reactive oxygen species (ROS) (reviewed in Richter et al. 2010). Organisms meet this challenge by activating a conserved stress response, the heat-shock response. Chaperones are expressed to protect and stabilize proteins and to repair protein damage (GuhaThakurta et al. 2002). Chaperone expression is mediated by the heat-shock factor (HSF-1), which trimerizes upon stress and positively regulates the expression of heat-shock proteins (HSPs) (Morimoto 1998). HSPs need cofactors such as J-proteins, which stimulate the ATPase activity of HSP70 (Laufen et al. 1999) and bind unfolded proteins to prevent potentially toxic aggregations (Hageman et al. 2010). The heat-shock response also comprises the expression of membrane-modifying proteins to reduce membrane fluidity (Welker et al. 2010) or the synthesis of antioxidant enzymes (e.g., superoxide dismutase (SOD)) to detoxify ROS (Hsu et al. 2003). Moreover, the proteolytic system increases its activity to maintain cellular proteostasis (Parag et al. 1987). To control all these processes, appropriate signal processing is necessary.
In Caenorhabditis elegans, several signaling cascades are involved in the response to abiotic stressors. The insulin-like (DAF-2) signaling pathway, for instance, mediates the stress-induced translocation of the transcription factor DAF-16 to the nucleus, which drives the expression of several stress proteins leading to an increased stress resistance and elevated lifespan (Kenyon et al. 1993; Lithgow et al. 1994; Murphy et al. 2003). Moreover, mitogen-activated protein kinases (MAPKs) such as the p38, JNK (and JNK-like KGB), or ERK MAPKs are conserved signaling proteins, which fulfill various functions (Sakaguchi et al. 2004). MAPK pathways are composed of MAPK kinase kinases (MAP3Ks), MAPK kinases (MAP2Ks), and MAPKs. PMK-1, which is a C. elegans p38 MAPK homologue, and the PMK-1 pathway were first described by Berman et al. (2001). They showed that pmk-1 is part of an operon comprising three homologues of mammalian p38 MAPK. They suggested pmk-1 expression to be transcriptionally regulated by an operon promoter upstream of pmk-3. This leads to the question why the gene for the most functional protein lies downstream of two less important p38 MAPK homologues. A further question is how PMK-1 signal transduction can be performed (e.g., activation of the downstream target SKN-1; see below), when PMK-1 is localized either in cell nuclei (Berman et al. 2001) or in the cytoplasm (Bolz et al. 2010). Inactive SKN-1, for instance, is constitutively localized in the cytoplasm and shows nuclear translocation only upon its activation by PMK-1. An important role of PMK-1 for neuronal development (Sagasti et al. 2001) and several cellular stress responses has been well established. PMK-1 mediates the response to oxidative stress via the Nrf2-like transcription factor SKN-1 (Inoue et al. 2005) and regulates germline apoptosis in response to heavy metals such as cadmium (Wang et al. 2008). PMK-1 also participates in stress responses to biotic stressors. Pathogen stress due to different bacteria such as Psuedomonas aeruginosa or Yersinia pestis induces the expression of immunity genes (Kim et al. 2002; Troemel et al. 2006; Bolz et al. 2010), which is mediated by the bZIP transcription factor ATF-7 in a PMK-1-dependent manner (Shivers et al. 2010).
We were interested to study principles of pmk-1 expression and to analyze the role of the PMK-1 pathway under heat stress. We studied PMK-1 expression using a GFP reporter construct and found pmk-1 expression to be driven by an internal promoter in front of pmk-1. We verified the cytoplasmic localization of PMK-1::GFP at moderate temperature but detected PMK-1 nuclear translocations under heat stress. Survival assays revealed a higher mortality of mutants of the PMK-1 pathway under heat stress. Studying the heat-shock response of C. elegans by RNA-seq and semi-quantitative reverse transcriptase polymerase chain reaction (sqRT-PCR) revealed positive effects of the PMK-1 pathway on chaperone expression, protein biosynthesis, or proteasomal activity. The data indicate a possible connection between nuclear PMK-1 and HSF-1 activation by hyperphosphorylation of its serine residues.
Materials and methods
Wild-type and mutant strains
C. elegans N2 (Bristol variety), the deletion mutants (Δ) KU25 pmk-1 (km25) IV, KB3 kgb-1 (um3) IV, VC8 jnk-1 (gk7) IV, KU4 sek-1 (km4) X, FK171 mek-1 (ks54) X, and CB1370 daf-2 (e1370) III were obtained from the Caenorhabditis Genetics Center (http://www.cbs.umn.edu/CGC/). Worms were maintained at 20 °C on NGM with Escherichia coli OP50 as food source. According to German law, experiments carried out on the invertebrate C. elegans do not have to be announced or approved.
Transgenic strains
The pmk-1::gfp plasmid was constructed by fusion of the GFP coding sequence to 2.652 kbp upstream of the pmk-1 translational start codon (ATG) and the genomic pmk-1 locus excluding its stop codon. pmk-1 promoter and pmk-1 were amplified from N2 genomic DNA by polymerase chain reaction (PCR) (Phusion® DNA-polymerase; Finnzymes, Vantaa, Finland; primer forward: 5′-CCTCTAGAAACTTGAAGATCGTTAGAATGC-3′, primer reverse: 5′-TACCCGGGCGATTCCATTTTCTCCTCA-3′). The PCR product was checked by sequencing, and the associated XBA-I/SMA-1 fragment was inserted into pPD95.79 (Andrew Fire, Stanford, USA). Furthermore, a pmk-1 rescue strain was constructed by amplifying the same genomic region as in case of pmk-1::gfp by PCR, with the reverse primer including now the pmk-1 stop codon and both primers carrying no restriction sites (Phusion® DNA-polymerase; primer forward: 5′-CTTGAAGATCGTTAGAATGC-3′, primer reverse: 5′-CTACGATTCCATTTTCTCCT-3′). The PCR product was cloned into pJET1.2/blunt Cloning Vector (Fermentas, St. Leon-Rot, Germany) and checked by sequencing. The reporter strain pmk-1::gfp and the pmk-1 rescue strain were generated by injecting the corresponding plasmid at a concentration of 50 ng μL−1 along with the plasmid pRF4 (100 ng μL−1; Mello et al. 1991), which carried the co-injection marker rol-6(su1006) (Kramer et al. 1990), into either WT (pmk-1::gfp) or pmk-1Δ (pmk-1 rescue).
RNA interference
For RNA interference (gene knockdown), double-stranded RNA was applied by feeding the E. coli HT115 strains provided by Source BioScience LifeSciences (Nottingham, UK): sek-1-RNAi [R03G5.2], skn-1-RNAi [T19E7.2], and ctrl-RNAi (L4440, control, empty vector). The pmk-1-RNAi-strain [B0218.3] was generated by cloning a 626-bp fragment of the pmk-1 genomic sequence (primer forward: 5′-ACCGGTATACTTCATCCGACTCCACG-3′; primer reverse: 5′-GTCGACCTCTGGAGCTCTGTACCATC-3′) into the L4440 vector and transforming the E. coli strain HT115. Identity of bacteria was checked by PCR. Bacteria were grown at 37 °C for 18 h in 2×YT + Amp medium. NGM plates (1 mmol L−1 isopropyl β-d-1-thiogalactopyranoside (IPTG); 100 μg mL−1 ampicillin) were seeded with 750 μL bacterial suspension (HT115) and allowed to grow for 24 h at 20 °C to induce dsRNA-expression. Synchronized L1 larvae were seeded to these plates, grown until young-adult stage, and then submitted to heat stress conditions on NGM plates inoculated with the respective bacterial strain.
Microscopy
An Axiovert 100 (Zeiss, Germany), equipped with a Canon EOS 350D camera, was used for fluorescence microscopy. For microscopy, worms were anesthetized with 10 mM levamisole in M9 buffer. Image processing was carried out using Adobe Photoshop© (Adobe Systems Incorporated, San Jose, CA, USA) or ImageJ (v. 1.44; http://imagej.nih.gov/ij/).
Survival assays
All survival assays were carried out with synchronized young-adult worms derived from hypochlorite treatment (Stiernagle 2006). For heat-stress incubations, ten worms were transferred to NGM plates inoculated with the corresponding bacterial food source (OP50 or RNAi-strains), and the plates were put, within sealed plastic bags, into a thermostat-controlled (±0.1 °C) water bath set to the indicated temperature. Survival after incubation was tested by applying gentle touch stimuli with a worm pick, and worms that did not respond were scored as dead. For testing survival over time, dead worms were determined after each hour of heat incubation whereas, for endpoint analyses, dead worms were scored after 5 h of heat incubation. The data of three independent experiments with 50 worms each were pooled for Kaplan–Meier analysis. For endpoint analyses, mean values resulted from single determinations of the percentage of survivors out of ten incubated worms on 15 or 20 different experimental plates.
PMK-1 translocation assay
Animals of the reporter strain pmk-1::gfp were incubated for 5 h at 20 °C, 32 °C, 33 °C, and 34 °C. All worms with discernible intestinal cell nuclei were scored as animals showing nuclear translocation. Mean values resulted from single determinations of the percentage of worms showing PMK-1 nuclear translocation out of five incubated worms on six different experimental plates.
RNA-Seq
Approximately 500 synchronized young-adult worms (wild-type and pmk-1Δ) were incubated on 90 mm NGM plates for 5 h at 34 °C in a thermostat-controlled water bath (per strain, two plates with animals from independent experiments). After incubation, animals were washed off the plates with sterile water. After washing them two times with 1 ml sterile water to get rid of feeding bacteria and adding RNAiso-G (Segenetic, Borken, Germany), they were frozen in liquid nitrogen. After thermal disruption of worms (liquid nitrogen, 35 °C; three repetitions), chloroform extraction on ice (10 min), and centrifugation (12,000×g, 4 °C, 15 min), DNA was digested and RNA purified with a RNase-free DNase set and RNeasy® mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. In addition, several provisions were made to work under RNase-free conditions. Quality control was carried out with an Agilent Bioanalyzer® (Agilent Technologies, Böblingen, Germany). After adding the samples to RNAstableTM matrix (Biomatrica, San Diego, CA, USA) and a subsequent vacuum centrifugation for drying (2 h), samples were sent to the Beijing Genomics Institute (BGI). RNA-Seq analysis was carried out, after a final Agilent Bioanalyzer® quality check after shipment, by BGI using Illumina HiSeq2000 technology. Briefly, mRNA was purified using oligo-(dT)-magnetic beads, and first- and second-strand cDNA-synthesis was carried out using random hexamer primers. Samples were ligated with sequencing adaptors and sequenced with Illumina HiSeq2000 with a minimum 10 Megareads per sample and a sequencing quality of more than 98 % clean reads. Sequences were mapped to Wormbase release WS223, and differential gene expression was calculated with the RPKM method (reads per kilobase per million reads) out of the number of reads for one gene, the transcript length, and the overall number of reads in the sample (Mortazavi et al. 2008). P values of differentially expressed genes (DEGs) were determined referring to Audic and Claverie (1997), and the false discovery rate (FDR) was used to determine the threshold of P for DEGs. We took an FDR < 0.001 as threshold for differentially expressed genes. Raw data are available from GEO (NCBI) under accession number GSE41205.
Semi-quantitative RT-PCR
Approximately 500 synchronized young-adult worms of wildtype, pmk-1Δ, sek-1Δ or mek-1Δ, were incubated on 90 mm NGM plates for 15 or 60 min at 35 °C (heat-shock (HS)) in a thermostat-controlled water bath (see above). After HS, they were incubated at 20 °C. At different times of incubation, worms were washed off from three to four NGM plates with sterile water and cleaned several times with sterile water to exclude bacteria. RNA was isolated using RNA-Iso-G (Segenetic, Borken, Germany). After reverse transcription of 1 μg total RNA per sample using oligo(dT)18 primers (First Strand cDNA Synthesis Kit; Fermentas, St. Leon-Rot, Germany), 1 μl cDNA was used as template for semi-quantitative reverse transcriptase polymerase chain reaction (RT-PCR). Using cdc-42 as housekeeping gene (Hoogewijs et al. 2008) (primers: 5′-ATGCAGACGATCAAGTGCG-3′, 5′-TTCAGTCCCTTCTGCGTCA-3′; reaction—30 s at 94 °C, 45 s at 53 °C, 35 s at 72 °C; 28 cycles), the relative expression levels of hsp-1 (primers: 5′-ACGACTCGCAGCGTCAAGCC-3′, 5′-CGCGTGGTGCTGGTGGGATT-3′; reaction—30 s at 94 °C, 45 s at 60 °C, 60 s at 72 °C; 23 cycles), hsp-70 (primers: 5′-ACTCATGTGTCGGTATTTAT-3′, 5′-ACGGGCTTTCCTTGTTTT-3′; reaction—30 s at 94 °C, 45 s at 50.5 °C, 28 s at 72 °C; 32 cycles), hsp-70 (primers: 5′-ACTTTACCACTATTTCCGTCCAGC-3′, 5′-CCTTGAACCGCTTCTTTCTTTG-3′; reaction—30 s at 94 °C, 45 s at 64 °C, 26 s at 72 °C; 26 cycles), and hsp-16.2 (primers: 5′-ACTTTACCACTATTTCCGTCCAGC-3′, 5′- CCTTGAACCGCTTCTTTCTTTG-3′; reaction—30 s at 94 °C, 40 s at 60 °C, 40 s at 72 °C; 31 cycles) were determined. Quantification and analysis of band intensities were made using ImageJ 1.44.
DEG functional classification
For KOG (eukaryotic orthologues groups) classification, WormMart (Wormbase release WS220bugFix) was used to assign COG codes of functional categories (http://www.ncbi.nlm.nih.gov/COG/grace/fiew.cgi) to C. elegans genes. In this way, 830 from 1581 upregulated DEGs and 1,609 from 2,229 downregulated DEGs received a COG code. For gene-GO (Gene Ontology) term enrichment analysis, the functional annotation chart generated by the Database for Annotation, Visualization, and Integrated Discovery (DAVID 6.7, http://david.abcc.ncifcrf.gov/) was used to determine selected stress-related DEGs (Huang et al. 2009a, b).
Statistics
Data are given as means ± standard deviation (SD) or means ± standard error of the mean (SEM) with n indicating the number of analyzed plates. Lifespan was determined with the Kaplan–Meier estimator, and statistical significances were checked using the logrank test. The t tests were applied to test for differences between control and/or test conditions (survival experiments), and two-way analysis of variance (ANOVA) with a subsequent multiple-comparison procedure (Student–Newman–Keuls method) was used to test for differences in hsp mRNA levels over time. In case of gene-GO term enrichment analysis, Fisher’s exact test and EASE Score (modified Fisher’s exact test) were used (P value ≤ 0.05; DAVID 6.7). Chi-square analyses were used to test for the enrichment of DEGs within a gene family or group (Fig. 7, Table 2). SigmaPlot 11.0 (Systat Software, Erkrath, Germany) was used for graph preparations and other statistical analyses.
Fig. 7.
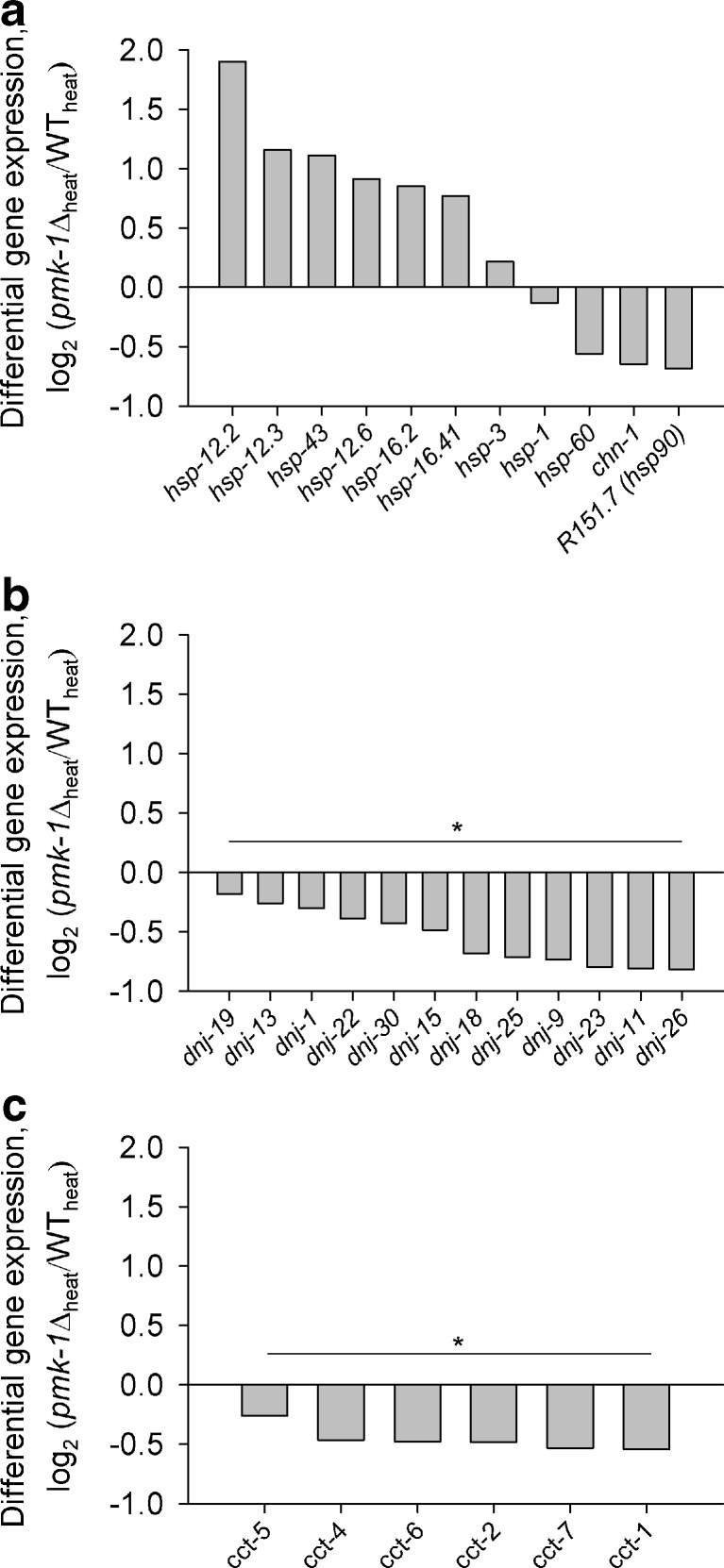
Differentially expressed genes (DEGs) for chaperones. Log2-fold changes of DEGs (pmk-1Δheat/WTheat) coding for a heat-shock proteins, b DnaJ proteins, and c members of the chaperonin-containing TCP-1 family. Asterisks and bars indicate significant enrichment of DEGs within a gene family (p < 0.001)
Table 2.
Comparison of DAF-16 target genes from previous studies with DEGs (pmk-1Δheat/WTheat) from this study
| DAF-16 target genes | DEGs (RNA-seq) showing gene identity or identical regulation with respect to a previous study | Gene identity or identity in regulation |
|---|---|---|
| 497a | 138* | 27.8 % |
| Upregulated, 254 | Upregulated, 76 (downregulated, 2) | 29.9 % |
| Downregulated, 243 | Downregulated, 27 (upregulated, 33) | 11.1 % |
| 953b | 183* | 19.2 % |
| Upregulated, 558 | Upregulated, 94 (downregulated, 11) | 16.8 % |
| Downregulated, 395 | Downregulated, 17 (upregulated, 61) | 4.3 % |
| 791c | 307* | 38.8 % |
| Upregulated, 791 | Upregulated, 307 | 38.8 % |
Results
Survival under heat stress
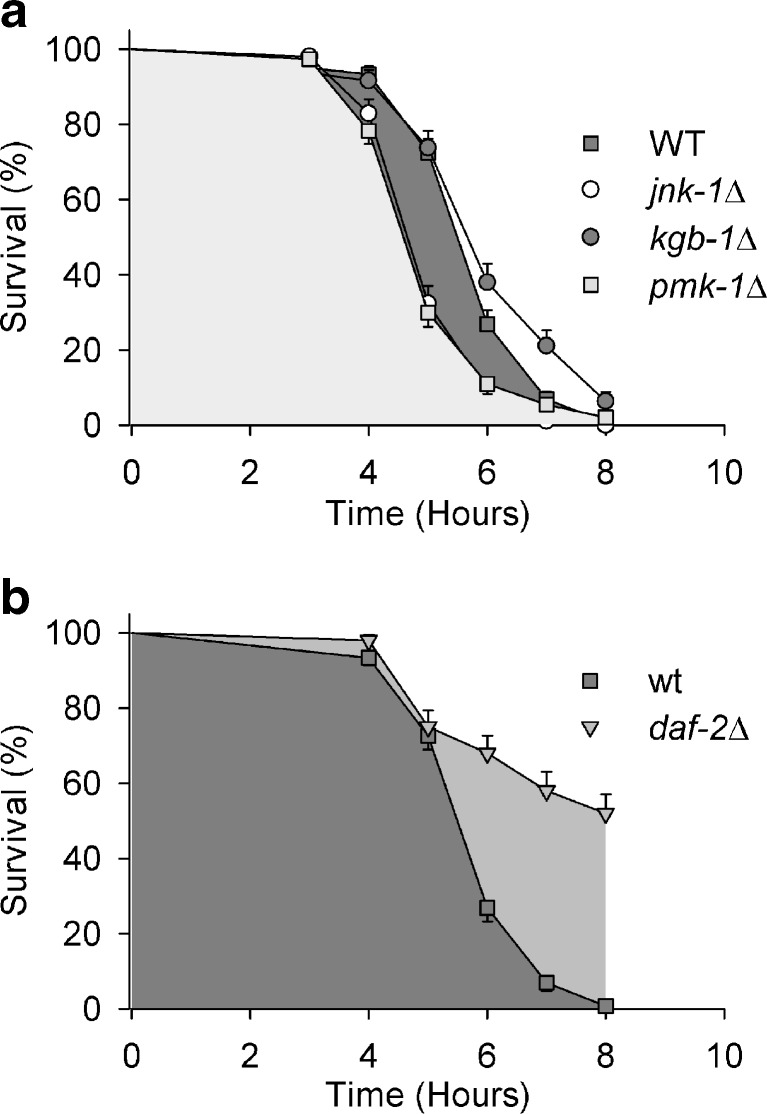
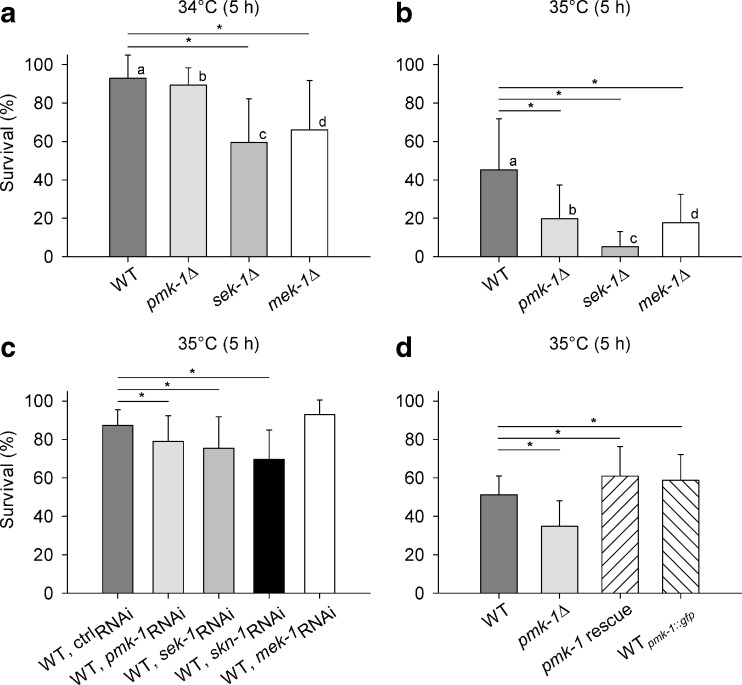
Heat tolerance was tested in wild-type (WT), MAPK deletion mutants (pmk-1Δ, jnk-1Δ, kgb-1Δ) and in a mutant of the insulin-like receptor DAF-2 (daf-2Δ) by scoring every hour for survival at 35 °C over a period of 8 h. The KGB-1 (kinase, GLH-binding 1) pathway seems not to be involved in heat tolerance, since there was only a minor difference in survival between WT and kgb-1Δ (Fig. 1a). The significant reductions in survival of pmk-1Δ and jnk-1Δ in comparison to WT, however, show a yet not described role of the PMK-1 pathway for the heat tolerance of C. elegans, aside from the already known contribution of the JNK-1 pathway to this physiological property (Wolf et al. 2008). The daf-2 mutant (Fig. 1b) exhibited its well-known resistance to heat (Lithgow et al. 1994). To assess the critical temperature, WT, pmk-1Δ, and deletion mutants of the two MAP2Ks upstream of PMK-1 (sek-1Δ, mek-1Δ) were exposed for 5 h to either 34 °C or 35 °C. The survival rate decreased significantly between 34 °C and 35 °C (Fig. 2a, b) showing this temperature range to be close to the critical (transition) temperature between survival and mortality. Heat stress affected sek-1Δ and partly mek-1Δ even more negatively than pmk-1Δ. RNA interference (RNAi) against pmk-1, sek-1, and mek-1 also showed that PMK-1 and SEK-1 are involved in heat tolerance mechanisms (Fig. 2c), even when pmk-1- or sek-1-RNAi reduced survival not as much as pmk-1 or sek-1 mutation. The latter effect may have been due to a less effective downregulation of gene expression by RNAi and/or the application of different feeding bacteria (HT115 vs. OP50), with the different survival rates of WT (Fig. 2b, c) favoring the second alternative. Knockdown (RNAi) of the downstream transcription factor SKN-1 of PMK-1 (WT, skn-1RNAi) also reduced the heat tolerance of C. elegans. In a further experimental series, the heat tolerance of a pmk-1 rescue strain as well as that of pmk-1::gfp, which carries additional extrachromosomal copies of the pmk-1 gene, was tested together with the tolerance of WT and pmk-1Δ to heat (Fig. 2d). Both strains, pmk-1 rescue and pmk-1::gfp, showed a better survival than pmk-1Δ and even WT.
Fig. 1.
Survival over time under heat stress. Kaplan–Meier survival analysis of a WT and MAPK mutants (pmk-1Δ, jnk-1Δ, kgb-1Δ), or b WT and a daf-2 mutant at 35 °C (means ± SEM, n = 3 test groups with 50 worms each). In comparison to WT, pmk-1Δ and jnk-1Δ showed a significant decrease, and daf-2Δ a significant increase in survival under heat stress (p < 0.05)
Fig. 2.
Survival under heat stress. Survival rates of WT, different mutants of the p38 (PMK-1) signaling pathway of C. elegans (pmk-1Δ, sek-1Δ, mek-1Δ), RNAi-treated WT (ctrl-RNAi, pmk-1-RNAi, sek-1-RNAi, skn-1-RNAi, mek-1-RNAi) as well as a pmk-1 rescue and a pmk-1::gfp strain after 5 h at a 34 °C or b–d 35 °C (means ± SD, n = 20 [a, b] or 15 [c, d] test groups with ten worms each). Asterisks and bars indicate significant differences to WT; small letters denote significant differences between 34 °C and 35 °C within strains (p < 0.05)
PMK-1 expression and localization
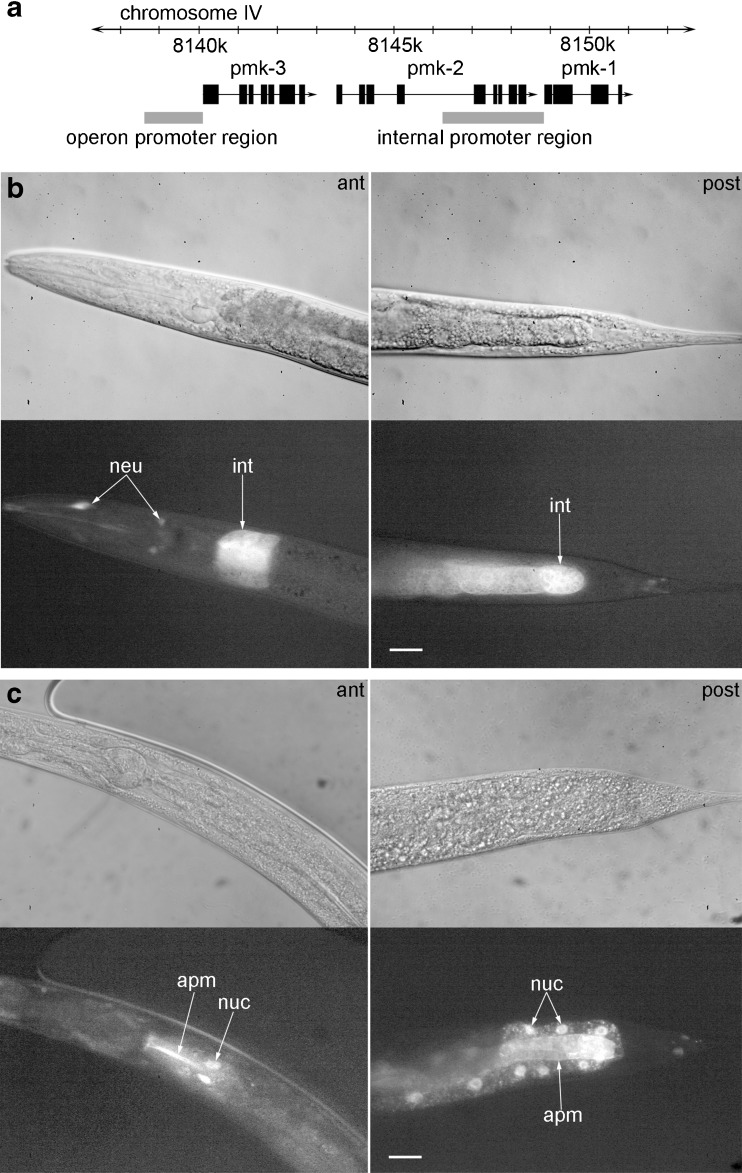
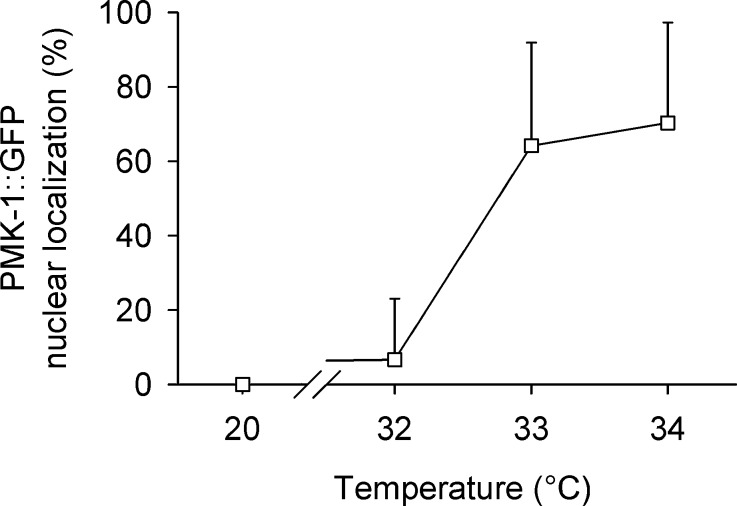
To study pmk-1 expression in light of the operon structure of the three p38 homologues of C. elegans (pmk-3, pmk-2, and pmk-1), which have been reported to be controlled by an operon promoter upstream of pmk-3, we generated a pmk-1::gfp carrying transgenic strain, with 2,652 bp upstream of the pmk-1 translational start codon as promoter sequence (Fig. 3a), and the pmk-1 genomic sequence fused to the sequence for GFP. Alignment with ClustalW2 of this promoter sequence and the sequence of the operon promoter revealed high sequence identity (pairwise score, 66 %). The transgenic strain exhibited pmk-1::gfp expression in the cytoplasm of anterior and posterior intestinal cells as well as in some neurons of the head region (Fig. 3b), which proves the presence of an internal promoter for pmk-1. Applying heat stress (35 °C, 5 h) to the transgenic strain caused PMK-1::GFP to translocate into the cell nuclei of anterior and posterior intestinal cells as well as to accumulate near to their apical membranes, which indicates both a nuclear and cytoplasmic function of PMK-1 (Fig. 3c). Testing the nuclear translocation of PMK-1::GFP revealed an increase in nuclear localization between 32 °C and 34 °C (Fig. 4), which indicates a role of nuclear PMK-1 for the heat stress response of C. elegans.
Fig. 3.
PMK-1 expression and nuclear translocation. a Schematic overview of the sequence regions, which carry the operon (Berman et al. 2001) or internal pmk-1 promoter (this study). Localization of pmk-1::gfp expression under b control conditions (20 °C) or c after heat stress (35 °C, 5 h). At 20 °C, GFP fluorescence was detected in the cytoplasm of anterior and posterior intestinal cells as well as a few neurons of the head region. Upon heat stress, PMK-1::GFP translocated into intestinal cell nuclei and accumulated, in addition, near to the apical membrane of intestinal cells. Corresponding DIC (top) and fluorescence (bottom) images are shown (magnification, 400×; scale bar, 20 μm; ant: anterior, post: posterior, int: intestine, neu: neurons, apm: apical membrane)
Fig. 4.
PMK-1 nuclear translocation. Percentage of worms showing nuclear localization of PMK-1::GFP after 5 h under different temperature conditions (20 °C, 32 °C, 33 °C, 34 °C; means ± SD, n = 6 test groups with five worms each)
HSP expression
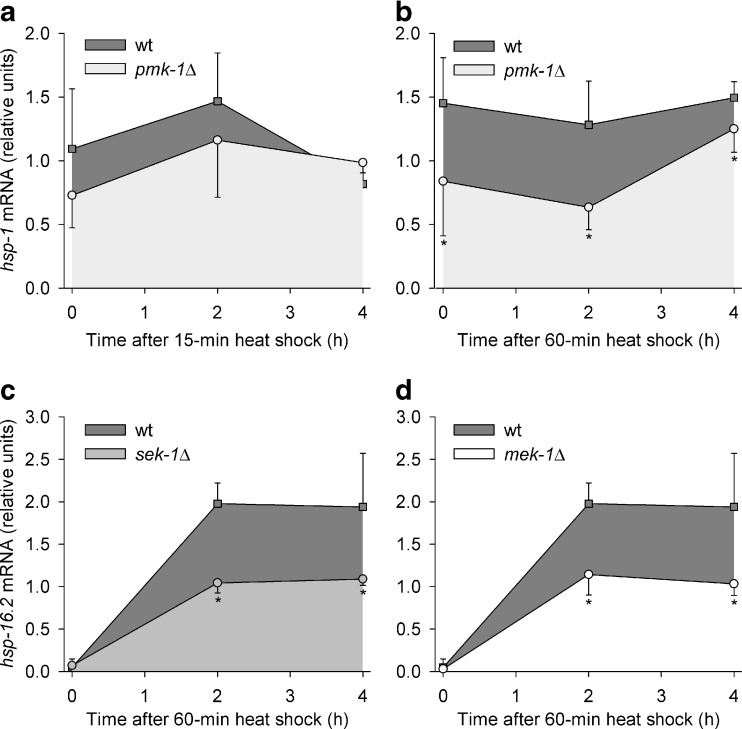
To further elucidate the heat stress response of C. elegans, we studied the expression of genes for HSPs as an obvious heat tolerance mechanism. The expression of one constitutive HSP70 (hsp-1; F26D10.3), two inducible HSP70s (C12C8.1 and F44E5.5), and one small heat-shock protein (hsp-16.2; Y46H3A.3) was monitored in WT, pmk-1Δ, sek-1Δ, and mek-1Δ over a period of 4 h after a heat shock (HS; 35 °C) of 15 min and/or 60 min duration. The hsp-1 mRNA level showed a tendency to lower values in pmk-1Δ than in WT after a heat shock of 15 min and significantly lower values after a heat shock of 60 min duration (Fig. 5a, b; Table 1). The hsp-16.2 mRNA level was lower in sek-1Δ and mek-1Δ (but not in pmk-1Δ, Table 1) than in WT after a HS of 60-min duration (Fig. 5c, d; Table 1). The inducible hsp70 mRNAs were either lower expressed in sek-1Δ and mek-1Δ (but not in pmk-1Δ) than in WT after a HS of 60-min duration (gene F44E5.5) or were not affected by mutations in the PMK-1 pathway (gene C12C8.1) (Table 1).
Fig. 5.
HSP expression over time. Changes in a, bhsp-1 or c, dhsp-16.2 transcript levels after heat shocks (35 °C) of a 15 min or b–d 60 min duration in WT, pmk-1Δ, sek-1Δ, and mek-1Δ (means ± SD; per point in time, n = 3–4 test groups with approximately 500 worms each). Asterisks indicate points in time, when significant differences between WT and mutants existed (p < 0.05)
Table 1.
Relative mRNA levels of constitutive hsp70 (hsp-1), two inducible hsp70 (C12C8.1, F44E5.5), and the small hsp16.2 (hsp-16.2) in WT, pmk-1Δ, sek-1Δ, and mek-1Δ at different points in time (0, 2, and 4 h) after a 15- or 60-min heat shock (HS; 35 °C) (means ± SD, n = 3–4 plates per point in time)
| Gene | Time after HS (h) | WT | pmk-1Δ | sek-1Δ | mek-1Δ | ||
|---|---|---|---|---|---|---|---|
| 15-min HS | 60-min HS | 15-min HS | 60-min HS | 60-min HS | 60-min HS | ||
| hsp-1 (F26D10.3) | 0 | 1.09 ± 0.47 | 1.45 ± 0.36 | 0.73 ± 0.25 | 0.84 ± 0.43 | 1.42 ± 0.86 | 1.6 ± 0.13 |
| 2 | 1.47 ± 0.38 | 1.28 ± 0.34 | 1.16 ± 0.45 | 0.63 ± 0.18 | 0.98 ± 0.18 | 1.18 ± 0.43 | |
| 4 | 0.82 ± 0.16 | 1.49 ± 0.13 | 0.99 ± 0.08 | 1.25 ± 0.18 | 0.89 ± 0.31 | 1.49 ± 0.44 | |
| hsp70 (C12C8.1) | 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| 2 | 0.24 ± 0.08 | 0.28 ± 0.05 | 0.18 ± 0.08 | 0.63 ± 0.28 | 0.22 ± 0.06 | 0.22 ± 0.09 | |
| 4 | 0.02 ± 0.04 | 0.44 ± 0.29 | 0.04 ± 0.02 | 0.53 ± 0.23 | 0.05 ± 0.04 | 0.26 ± 0.18 | |
| hsp70 (F44E5.5) | 0 | 0 ± 0 | 0.03 ± 0.05 | 0 ± 0 | 0.02 ± 0.03 | 0 ± 0 | 0 ± 0 |
| 2 | 0.79 ± 0.17 | 1.5 ± 0.29 | 0.71 ± 0.17 | 1.39 ± 0.49 | 0.21 ± 0.20 | 0.33 ± 0.14 | |
| 4 | 0.23 ± 0.16 | 1.32 ± 0.98 | 0.35 ± 0.12 | 1.59 ± 0.46 | 0.05 ± 0.01 | 0.37 ± 0.20 | |
| hsp-16.2 (Y46H3A.3) | 0 | 0.05 ± 0.10 | 0.03 ± 0.05 | 0.03 ± 0.05 | 0.07 ± 0.01 | ||
| 2 | 1.98 ± 0.25 | 1.67 ± 0.37 | 1.14 ± 0.24 | 1.04 ± 0.12 | |||
| 4 | 1.94 ± 0.63 | 2.42 ± 0.64 | 1.03 ± 0.14 | 1.09 ± 0.07 | |||
Bold typing indicates significantly lower expression in comparison to WT under identical heat stress conditions (p < 0.05)
RNA-seq
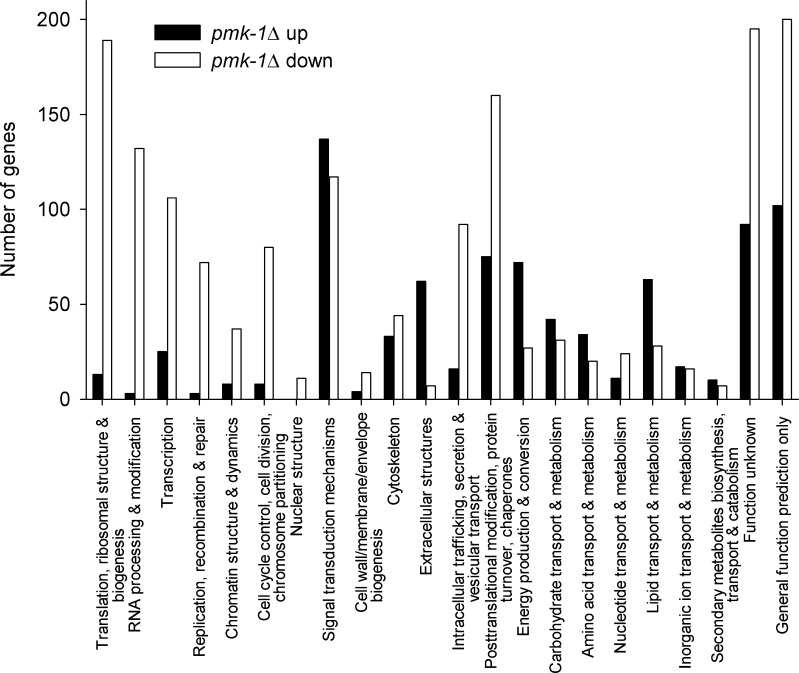
To get a comprehensive view of gene expression under heat stress in WT and pmk-1Δ, we carried out RNA-seq analyses. To avoid higher shares of dead worms in the mRNA preparation, we incubated WT and pmk-1Δ for 5 h at 34 °C, at which condition survival was not much impeded (Fig. 2a) but at which a nuclear translocation of PMK-1 was detected (Fig. 4). When applying a threshold for the FDR of below 0.001, 3,810 DEGs were detected, of which 1,581 genes were upregulated and 2,229 genes downregulated in pmk-1Δ. The functional classification of DEGs according to KOG categories revealed in pmk-1Δ an upregulation of 830 genes, which were mainly related to signal transduction mechanisms, extracellular structures, and metabolism, whereas the 1,609 classified downregulated genes were especially found in the categories translation, RNA processing, transcription, replication and DNA repair, chromatin structure, cell cycle control, intracellular trafficking, as well as protein turnover and chaperones (Fig. 6). Screening the DEGs for hsp expression revealed hsp-1 mRNA slightly downregulated (log2 ratio, −0.13) and hsp-16.2 mRNA upregulated (log2 ratio, 0.85) in the pmk-1 mutant in comparison to WT (Fig. 6a). The mRNA level for the two inducible HSP70s (F44E5.5, C12C8.1) was not different in WT and pmk-1Δ. However, when comparing these data with the results from sqRT-PCR (Fig. 5, Table 1), the different experimental conditions have to be considered (34 °C for 5 h versus 35 °C for 15 or 60 min, followed by a 4-h measuring period at 20 °C). Nevertheless, there were negative effects of pmk-1 mutation on the expression of hsp-1, hsp-60, chn-1, a putative hsp-90 (R151.7), as well as genes (dnj, cct) for several members of the DnaJ domain protein and chaperonin-containing TCP-1 families (Fig. 7; Table 3). Comparing the target genes of the transcription factor DAF-16 of the DAF-2 pathway (Murphy et al. 2003; McElwee et al. 2003; Iser et al. 2011) with our data showed a significant contribution of DAF-16 target genes to the DEGs for pmk-1Δheat/WTheat (Table 2). Performing a gene-GO term enrichment analysis for selected functional categories, which were suggested to cover stress-related genes (Kültz 2003, 2005), revealed for pmk-1Δ a significant enrichment (P < 0.05) of downregulated DEGs in the GO terms cell cycle, DNA repair/chromatin stability, molecular chaperones, protein degradation, and translation/protein biosynthesis. DEGs upregulated in pmk-1Δ were more frequently found in the GO category aging, which contains many DAF-16 target genes, and smaller numbers of DEGs were assigned to the categories response to heat or oxidative stress (mainly DAF-16-dependent small heat-shock proteins and several genes related to glutathione metabolism) (Table 4).
Fig. 6.
Number of up- or downregulated DEGs (pmk-1Δheat/WTheat) within functional categories (KOG)
Table 3.
Transcription factor binding-motifs of chaperone-coding DEGs (pmk-1Δheat/WTheat) showing sequence, public name and protein family, direction of regulation, and the number of motifs, 1 kbp upstream of the translational start codon (SKN-1 motif, [TA][TA]T[GA]TCAT (Boellmann et al. 2004); HSE motif, TTC[CT][AC]GAA (GuhaThakurta et al. 2002); HSAS motif (heat-shock-associated site), GGGT[CT][TA][CT] (GuhaThakurta et al. 2002); DBE motif (Daf-16 binding-element), T[GA]TTTAC (Murphy 2006); DAE motif (Daf-16-associated element), CTTATCA (Murphy 2006))
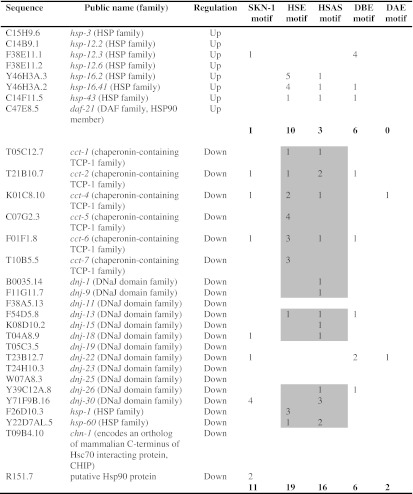
Bold figures are total numbers of motifs. Gray areas indicate detected HSE and HSAS motifs in case of downregulated cct, dnj, and hsp genes
Table 4.
Gene-GO term enrichment analysis for selected stress-related DEGs (pmk-1Δheat/WTheat) from this study
| GO term | Number of genes | Fisher’s exact P valuea | EASE scoreb |
|---|---|---|---|
| Downregulated genes in pmk-1Δ | |||
| Cell cycle | |||
| GO:0051726 regulation of cell cycle | 38 | <0.0001 | <0.0001 |
| DNA repair/chromatin stability | |||
| GO:0006281 DNA repair | 38 | <0.0001 | <0.0001 |
| Molecular chaperones | |||
| GO:0051082 unfolded protein binding | 16 | 0.0004 | 0.0013 |
| GO:0031072 heat-shock protein binding | 14 | 0.0022 | 0.0065 |
| Protein degradation | |||
| GO:0000502 proteasome complex | 29 | 0.0001 | 0.0001 |
| GO:0051603 proteolysis involved in cellular protein catabolic process | 64 | 0.0001 | 0.0001 |
| Translation/protein biosynthesis | |||
| GO:0003735 structural constituent of ribosome | 80 | <0.0001 | <0.0001 |
| GO:0042254 ribosome biogenesis | 29 | <0.0001 | <0.0001 |
| GO:0006414 translational elongation | 9 | 0.0001 | 0.0006 |
| GO:0006413 translational initiation | 12 | 0.0001 | 0.0005 |
| Upregulated genes in pmk-1Δ | |||
| “Daf-16 targets” | |||
| GO:0007568 aging | 42 | <0.0001 | <0.0001 |
| Stress response | |||
| GO:0009408 response to heat | 7 | 0.0064 | 0.0240 |
| GO:0006979 response to oxidative stress | 7 | 0.0290 | 0.0790 |
aFisher’s exact P value (determined by DAVID 6.7)
bEASE Score: modified Fisher’s exact P value (determined by DAVID 6.7)
Discussion
This study has revealed a significant contribution of the PMK-1 pathway (SEK-1 and MEK-1, PMK-1, SKN-1) to the heat tolerance of C. elegans (Figs. 1 and 2). Experiments with a pmk-1 rescue strain verified the negative effect of pmk-1 mutation on the heat tolerance of C. elegans (Fig. 2d). The higher survival rates of heat-stressed pmk-1 rescue (construct in pmk-1Δ) or pmk-1::gfp (construct in WT) than heat-stressed WT was likely due to pmk-1 overexpression, since transgenic animals usually carry a surplus of injected constructs and genes (Evans 2006). Thus, there are three signaling pathways, the DAF-2 (Henderson and Johnson 2001), JNK-1(Wolf et al. 2008), and PMK-1 pathways, which are involved in the heat-stress response of C. elegans (Fig. 1). The lower survival rate of sek-1Δ (or WT, sek-1RNAi) than mek-1Δ (or WT, mek-1RNAi) under heat stress (Fig. 2b, c) supports previous reports on a dominance of SEK-1 over MEK-1 for the activation of PMK-1 (Tanaka-Hino et al. 2002; Kim et al. 2002). Nevertheless, MEK-1 also contributed to the activation of this MAPK, because heat tolerance was negatively affected by both pmk-1Δ and mek-1Δ (Fig. 2b; cf. Mizuno et al. 2004). Since SEK-1 also promotes DAF-16 nuclear translocation (Kondo et al. 2005) and DAF-16-dependent gene expression (Lin et al. 2001; Henderson and Johnson 2001), a lack of expressed DAF-16-dependent stress genes (e.g., hsp-16.2) in sek-1Δ (Fig. 5c) may have contributed to the very low survival rate of this mutant under heat stress (Fig. 2a, b). Maybe, the lower survival rate of mek-1Δ than pmk-1Δ at 34 °C (Fig. 2a) is also linked to DAF-2 signaling. Since skn-1-RNAi also lowered the heat tolerance of C. elegans (Fig. 2c), the negative effects of pmk-1Δ on heat tolerance could be (at least partly) due to an absent activation of the transcription factor SKN-1, which is phosphorylated by PMK-1 and other kinases under oxidative stress (Inoue et al. 2005; An et al. 2005).
In addition to this mechanism, PMK-1 seems to be directly involved in heat-stress responses, because heat caused the yet not reported nuclear translocation of a p38 MAPK (PMK-1::GFP) in C. elegans (Figs. 3c and 4). Only in case of an external control of pmk-1 expression (intestine-specific vha-6 promoter) have cytoplasmic and nuclear localizations of PMK-1 been hitherto shown (Bolz et al. 2010). In mammals, however, a nuclear translocation of p38 MAPK has already been reported (Raingeaud et al. 1995; Wood et al. 2009). Moreover, the observed expression of pmk-1::gfp (Fig. 3b), which evidently carried an internal promoter but not the operon promoter (Fig. 3a), represents another example of operon gene control in C. elegans by both operon and internal promoters (Huang et al. 2007), as a previous study has already reported pmk gene control by the operon promoter (Berman et al. 2001). Aside from the nuclear translocation of PMK-1 in intestinal cells, there was also an accumulation of PMK-1::GFP near to the apical membranes of intestinal cells, suggesting an additional site-specific, but yet unknown cytoplasmic function of PMK-1. Concerning nuclear PMK-1 functions, there was, in a small range of increasing temperatures, a marked decrease in survival rate of WT and several mutants (34–35 °C; Fig. 2a, b) and an increase in PMK-1 nuclear translocation in the transgenic strain (32–34 °C; Fig. 4), which, however, showed a better survival rate than WT at 35 °C due to pmk-1 overexpression (Fig. 2d). Thus, the (not detectable) nuclear translocation of PMK-1 in WT may be shifted toward a lower temperature range in comparison to the transgenic strain, but, nevertheless, a link exists between PMK-1 nuclear translocation, heat stress, and heat-stress responses.
Studying the expression of genes for heat-shock proteins after heat stress (15-min or 60-min HS, 35 °C) by sqRT-PCR revealed downregulated mRNA levels in case of constitutive hsp70 (hsp-1) for pmk-1Δ as well as inducible hsp70 (F44E5.5) and hsp-16.2 for sek-1Δ and mek-1Δ (Fig. 5, Table 1). RNA-seq analyses of DEGs under heat stress (5 h, 34°) revealed a downregulated expression of chaperone genes such as hsp-1, hsp-60, chn-1, hsp-90 (R151.7), 12 DNaJ domain (dnj) genes, and six chaperonin-containing TCP-1 (cct) genes in pmk-1Δ in comparison to WT (Fig. 7, Table 3). DnaJ proteins are cofactors of HSP70, which stimulate the ATPase cycle of HSP70 and promote the binding and delivery of HSP70 client proteins (reviewed in Richter et al. 2010). They were also suggested to prevent an aggregation of partially denatured or misfolded proteins (Hageman et al. 2010). Chaperonin-containing TCP-1 proteins are thought to be involved in the correct folding of the cytoskeleton components actin and tubulin (reviewed in Frydman 2001) and also to fulfill chaperone function for other cytoplasmic proteins (Kubota 2002).
In consequence, there is a connection between the PMK-1 pathway and the expression of genes for chaperones (heat-shock proteins), with the downregulated expression of these genes in pmk-1Δ (or sek-1Δ and mek-1Δ) likely being a major reason for the reduced survival of the mutants of the PMK-1 pathway near to the thermal limit of C. elegans (around 34–35 °C). The mechanisms by which PMK-1 affects chaperone expression may include cytoplasmic or nuclear interactions between PMK-1 and the transcription factors SKN-1 (An et al. 2005; Inoue et al. 2005) or ATF-7 (Shivers et al. 2010) as well as an independent nuclear function of PMK-1 after translocation. SKN-1 is localized in its inactive state in the cytoplasm and translocates into the nucleus after its phosphorylation by cytoplasmic PMK-1. ATF-7 is located in the nucleus, functions as transcriptional repressor, and is switched to an activator of transcription (deactivation of its repressive action) after its phosphorylation by nuclear pmk-1 (Shivers et al. 2010). In addition, nuclear PMK-1 may further promote SKN-1-mediated gene expression by preventing the return of non-phosphorylated SKN-1 to the cytoplasm. Anyway, the increasing nuclear localization of PMK-1 with increasing temperature (Fig. 4) supports the idea of an important nuclear function of PMK-1.
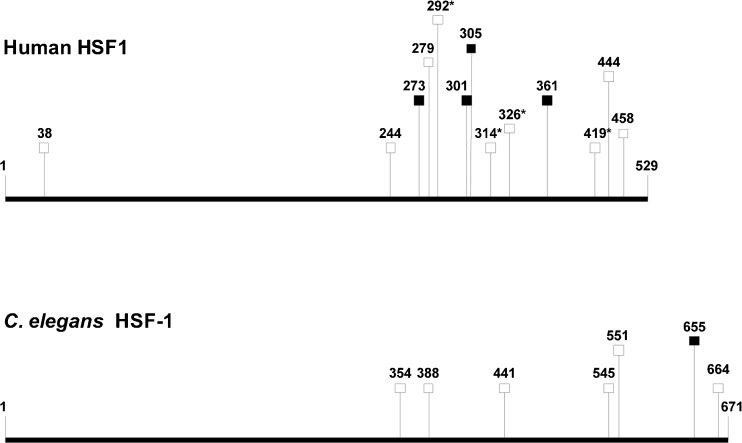
To further elucidate the mechanisms by which PMK-1 affects heat tolerance, we checked for specific binding motifs for the transcription factors SKN-1, HSF-1, and DAF-16 (binding motifs for ATF-7 are yet not known) in promoters (1 kbp upstream of translational start codons) of the up- or downregulated chaperone genes (Table 3). Surprisingly, we only found 11 SKN-1 and eight DAF-16 (DBE, DAE) motifs in promoters of the 22 downregulated chaperone genes (upregulated chaperone genes will be discussed later), but 35 HSF-1 (HSE, HSAS) motifs, particularly in front of the cct genes. The data (Table 3) suggest firstly alternative (i.e., gene control of a chaperone family by different transcription factors), but also redundant (i.e., gene control of a chaperone by different transcription factors) activation modes for chaperone gene expression and secondly, possible indirect or direct effects of (nuclear) PMK-1 on HSF-1 function. Stress activation of mammalian HSF1 requires its hyperphosphorylation at serine residues (12 serine residues for heat-activated human HSF1; Guettouche et al. 2005). MAPKs phosphorylate serine residues, which are immediately followed by a proline residue (Ser-Pro) (Sheridan et al. 2008). Four of the hyperphosphorylated serine residues of HSF1 belong to this sequence type (Fig. 8), including Ser326 that contributed significantly to HSF1 activation (Guettouche et al. 2005). MAPKs show some additional preference for sequences, which include a proline or another aliphatic residue at the −2 position (Pro-X-Ser-Pro, with X indicating any residue) (Sheridan et al. 2008), but serine residues in this sequence type were not hyperphosphorylated (Fig. 8). The comparison of human and worm heat-shock factors revealed quite a high number of Ser-Pro sequences in both proteins (human HSF1, 9 Ser-Pro pairs; C. elegans HSF-1, 6 Ser-Pro pairs) as well as four Pro-X-Ser-Pro sequences in HSF1 and only one in C. elegans HSF-1 (Fig. 8). Thus, it is possible that hyperphosphorylation of serine residues (Ser-Pro) in HSF-1 by the MAPK PMK-1 contributed to the elevated expression of chaperone genes in C. elegans wild-type (e.g., cct genes). Concerning the upregulated chaperone genes (Table 3), we detected a few DAF-16 target genes (Murphy et al. 2003) (hsp-12.3, hsp-12.6, hsp-16.2, hsp-16.41). Another well-known target gene of DAF-16, sod-3, was also upregulated in pmk-1Δ (log2-fold change, 1.58; data not shown). The upregulated expression of the small chaperones indicates an elevated nuclear translocation of DAF-16, the transcription factor of the DAF-2 pathway, in pmk-1Δ. This fits to the rather high identity of DAF-16 target genes and DEGs from pmk-1Δheat/WTheat (maximally 38.8 %), with these DEGs mostly upregulated in pmk-1Δ (Table 2). The compensatory increase in DAF-16-regulated gene expression in pmk-1Δ may be due to the missing phosphorylation target (PMK-1) of SEK-1, with the consequence of a redirection of SEK-1 kinase activity towards DAF-2 signaling resulting in the promotion of DAF-16 nuclear translocation (see above). However, the markedly higher heat tolerance of daf-2Δ than pmk-1Δ (Fig. 1) shows that such a mechanism may improve the heat tolerance of pmk-1Δ but does not suffice to compensate for the loss of normal hsp, dnj, and cct expression levels (Fig. 7) resulting in a lower heat tolerance of pmk-1Δ in comparison to WT.
Fig. 8.
Schematic overview of human HSF1 and C. elegans HSF-1 amino acid sequences. Squares mark putative minimal (open symbols; motif: Ser-Pro) or extended (filled symbols; motif: Pro-X-Ser-Pro) MAPK phosphorylation sites (see text for details). Asterisks indicate phosphorylation sites detected by Guettouche et al. (2005)
A functional (KOG) classification of DEGs (Fig. 6) as well as a gene-GO term enrichment analysis (Table 4) also revealed a significant enrichment of downregulated DEGs in the categories translation/protein biosynthesis and proteasomal degradation aside from downregulated DEGs in other categories related to DNA or RNA function, cell cycle control, intracellular trafficking, or chaperones. This downregulation must originate from strain (pmk-1Δ) properties and not from heat stress, even if heat stress has promoted the effect. Accordingly, PMK-1 seems to exert positive influence on the expression of genes for protein biosynthesis and proteasomal subunits. Actually, there is a recent report (Cully et al. 2010) showing in both mammals and Drosophila that p38 is a positive regulator of the target of rapamycin (TOR) complex 1 (TORC1), which regulates protein biosynthesis and growth and promotes, in addition, the necessary gene expressions. In addition to a possible TORC1 activation, DAF-16 recruitment to the nucleus is evidently higher in pmk-1Δ and lower in WT (see above). Since nuclear DAF-16 inhibits the expression of daf-15, which codes for C. elegans raptor, an important protein of the TOR complex (Jia et al. 2004), DAF-2 signaling could also contribute to the reduced expression of genes for protein biosynthesis in pmk-1Δ. The degradation of damaged or aggregated proteins is mediated by 26S proteasomes, and the high share of downregulated proteasomal genes in pmk-1Δ indicates a connection between these genes and PMK-1, which may be based on an SKN-1-dependent control of proteasomal gene expression (Kahn et al. 2008). Anyway, the downregulation of proteasomal genes additionally impedes the heat tolerance of pmk-1Δ by a reduced degradation of damaged proteins.
Acknowledgments
We thank Frank Nunes, Daniel Runde, and Ulrike Gigengack for supporting material and measurements, the Caenorhabditis Genetics Center for providing C. elegans strains, and the Deutsche Forschungsgemeinschaft for financial support (Pa 308/13-1).
References
- An JH, Vranas K, Lucke M, Inoue H, Hisamoto N, Matsumoto K, Blackwell TK. Regulation of the Caenorhabditis elegans oxidative stress defense protein SKN-1 by glycogen synthase kinase-3. Proc Natl Acad Sci U S A. 2005;102(45):16275–16280. doi: 10.1073/pnas.0508105102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audic S, Claverie JM. The significance of digital gene expression profiles. Genome Res. 1997;7(10):986–995. doi: 10.1101/gr.7.10.986. [DOI] [PubMed] [Google Scholar]
- Berman K, McKay J, Avery L, Cobb M. Isolation and characterization of pmk-(1-3): three p38 homologs in Caenorhabditis elegans. Mol Cell Biol Res Commun. 2001;4(6):337–344. doi: 10.1006/mcbr.2001.0300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boellmann F, Guettouche T, Guo Y, Fenna M, Mnayer L, Voellmy R. DAXX interacts with heat shock factor 1 during stress activation and enhances its transcriptional activity. Proc Natl Acad Sci U S A. 2004;101(12):4100–4105. doi: 10.1073/pnas.0304768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolz DD, Tenor JL, Aballay A. A conserved PMK-1/p38 MAPK is required in Caenorhabditis elegans tissue-specific immune response to Yersinia pestis infection. J Biol Chem. 2010;285(14):10832–10840. doi: 10.1074/jbc.M109.091629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully M, Genevet A, Warne P, Treins C, Liu T, Bastien J, Baum B, Tapon N, Leevers SJ, Downward J. A role for p38 stress-activated protein kinase in regulation of cell growth via TORC1. Mol Cell Biol. 2010;30(2):481–495. doi: 10.1128/MCB.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans TC (2006) Transformation and microinjection. WormBook: 1–11. doi:10.1895/wormbook.1.108.1
- Frydman J. Folding of newly translated proteins in vivo: the role of molecular chaperones. Annu Rev Biochem. 2001;70:603–647. doi: 10.1146/annurev.biochem.70.1.603. [DOI] [PubMed] [Google Scholar]
- Guettouche T, Boellmann F, Lane WS, Voellmy R. Analysis of phosphorylation of human heat shock factor 1 in cells experiencing a stress. BMC Biochem. 2005;6:4. doi: 10.1186/1471-2091-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GuhaThakurta D, Palomar L, Stormo GD, Tedesco P, Johnson TE, Walker DW, Lithgow G, Kim S, Link CD. Identification of a novel cis-regulatory element involved in the heat shock response in Caenorhabditis elegans using microarray gene expression and computational methods. Genome Res. 2002;12(5):701–712. doi: 10.1101/gr.228902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hageman J, Rujano MA, van Waarde MAWH, Kakkar V, Dirks RP, Govorukhina N, Oosterveld-Hut HMJ, Lubsen NH, Kampinga HH. A DNAJB chaperone subfamily with HDAC-dependent activities suppresses toxic protein aggregation. Mol Cell. 2010;37(3):355–369. doi: 10.1016/j.molcel.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11(24):1975–1980. doi: 10.1016/S0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR (2008) Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in Caenorhabditis elegans. BMC Mol Biol 9:9. doi:10.1186/1471-2199-9-9 [DOI] [PMC free article] [PubMed]
- Hsu AL, Murphy CT, Kenyon C. Regulation of aging and age-related disease by DAF-16 and heat-shock factor. Science. 2003;300(5622):1142–1145. doi: 10.1126/science.1083701. [DOI] [PubMed] [Google Scholar]
- Huang P, Pleasance ED, Maydan JS, Hunt-Newbury R, O’Neil NJ, Mah A, Baillie DL, Marra MA, Moerman DG, Jones SJ. Identification and analysis of internal promoters in Caenorhabditis elegans operons. Genome Res. 2007;17(10):1478–1485. doi: 10.1101/gr.6824707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- Huang DW, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Hisamoto N, An JH, Oliveira RP, Nishida E, Blackwell TK, Matsumoto K. The C. elegans p38 MAPK pathway regulates nuclear localization of the transcription factor SKN-1 in oxidative stress response. Genes Dev. 2005;19(19):2278–2283. doi: 10.1101/gad.1324805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iser WB, Wilson MA, Wood WH, 3rd, Becker K, Wolkow CA. Co-regulation of the DAF-16 target gene, cyp-35B1/dod-13, by HSF-1 in C. elegans dauer larvae and daf-2 insulin pathway mutants. PLoS One. 2011;6(3):e17369. doi: 10.1371/journal.pone.0017369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131(16):3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Kahn NW, Rea SL, Moyle S, Kell A, Johnson TE. Proteasomal dysfunction activates the transcription factor SKN-1 and produces a selective oxidative-stress response in Caenorhabditis elegans. Biochem J. 2008;409(1):205–213. doi: 10.1042/BJ20070521. [DOI] [PubMed] [Google Scholar]
- Kenyon C, Chang J, Gensch E, Rudner A, Tabtiang R. A C. elegans mutant that lives twice as long as wild type. Nature. 1993;366(6454):461–464. doi: 10.1038/366461a0. [DOI] [PubMed] [Google Scholar]
- Kim DH, Feinbaum R, Alloing G, Emerson FE, Garsin DA, Inoue H, Tanaka-Hino M, Hisamoto N, Matsumoto K, Tan MW, Ausubel FM. A conserved p38 MAP kinase pathway in Caenorhabditis elegans innate immunity. Science. 2002;297(5581):623–626. doi: 10.1126/science.1073759. [DOI] [PubMed] [Google Scholar]
- Kondo M, Yanase S, Ishii T, Hartman PS, Matsumoto K, Ishii N. The p38 signal transduction pathway participates in the oxidative stress-mediated translocation of DAF-16 to Caenorhabditis elegans nuclei. Mech Ageing Dev. 2005;126(6–7):642–647. doi: 10.1016/j.mad.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Kramer JM, French RP, Park EC, Johnson JJ. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H. Function and regulation of cytosolic molecular chaperone CCT. Vitam Horm. 2002;65:313–331. doi: 10.1016/S0083-6729(02)65069-1. [DOI] [PubMed] [Google Scholar]
- Kültz D. Evolution of the cellular stress proteome: from monophyletic origin to ubiquitous function. J Exp Biol. 2003;206:3119–3124. doi: 10.1242/jeb.00549. [DOI] [PubMed] [Google Scholar]
- Kültz D. Molecular and evolutional basis of the cellular stress response. Annu Rev Physiol. 2005;67:225–257. doi: 10.1146/annurev.physiol.67.040403.103635. [DOI] [PubMed] [Google Scholar]
- Laufen T, Mayer MP, Beisel C, Klostermeier D, Mogk A, Reinstein J, Bukau B. Mechanism of regulation of hsp70 chaperones by DnaJ cochaperones. Proc Natl Acad Sci U S A. 1999;96(10):5452–5457. doi: 10.1073/pnas.96.10.5452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. 2001;28(2):139–145. doi: 10.1038/88850. [DOI] [PubMed] [Google Scholar]
- Lithgow GJ, White TM, Hinerfeld DA, Johnson TE. Thermotolerance of a long-lived mutant of Caenorhabditis elegans. J Gerontol. 1994;49(6):B270–B276. doi: 10.1093/geronj/49.6.B270. [DOI] [PubMed] [Google Scholar]
- McElwee JJ, Bubb K, Thomas JH. Transcriptional outputs of the Caenorhabditis elegans forkhead protein DAF-16. Aging Cell. 2003;2(2):111–121. doi: 10.1046/j.1474-9728.2003.00043.x. [DOI] [PubMed] [Google Scholar]
- Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C. elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10(12):3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno T, Hisamoto N, Terada T, Kondo T, Adachi M, Nishida E, Kim DH, Ausubel FM, Matsumoto K. The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J. 2004;23(11):2226–2234. doi: 10.1038/sj.emboj.7600226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Regulation of the heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12(24):3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Mortazavi A, Williams BA, McCue K, Schaeffer L, Wold B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat Methods. 2008;5(7):621–628. doi: 10.1038/nmeth.1226. [DOI] [PubMed] [Google Scholar]
- Murphy CT. The search for DAF-16/FOXO transcriptional targets: approaches and discoveries. Exp Gerontol. 2006;41(10):910–921. doi: 10.1016/j.exger.2006.06.040. [DOI] [PubMed] [Google Scholar]
- Murphy CT, McCarroll SA, Bargmann CI, Fraser A, Kamath RS, Ahringer J, Li H, Kenyon C. Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277–283. doi: 10.1038/nature01789. [DOI] [PubMed] [Google Scholar]
- Parag HA, Raboy B, Kulka RG. Effect of heat shock on protein degradation in mammalian cells: involvement of the ubiquitin system. EMBO J. 1987;6(1):55–61. doi: 10.1002/j.1460-2075.1987.tb04718.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270(13):7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Richter K, Haslbeck M, Buchner J. The heat shock response: life on the verge of death. Mol Cell. 2010;40(2):253–266. doi: 10.1016/j.molcel.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Sagasti A, Hisamoto N, Hyodo J, Tanaka-Hino M, Matsumoto K, Bargmann CI. The CaMKII UNC-43 activates the MAPKKK NSY-1 to execute a lateral signaling decision required for asymmetric olfactory neuron fates. Cell. 2001;105(2):221–232. doi: 10.1016/S0092-8674(01)00313-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi A, Matsumoto K, Hisamoto N. Roles of MAP kinase cascades in Caenorhabditis elegans. J Biochem. 2004;136(1):7–11. doi: 10.1093/jb/mvh097. [DOI] [PubMed] [Google Scholar]
- Sheridan DL, Kong Y, Parker SA, Dalby KN, Turk BE. Substrate discrimination among mitogen-activated protein kinases through distinct docking sequence motifs. J Biol Chem. 2008;283(28):19511–19520. doi: 10.1074/jbc.M801074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivers RP, Pagano DJ, Kooistra T, Richardson CE, Reddy KC, Whitney JK, Kamanzi O, Matsumoto K, Hisamoto N, Kim DH. Phosphorylation of the conserved transcription factor ATF-7 by PMK-1 p38 MAPK regulates innate immunity in Caenorhabditis elegans. PLoS Genet. 2010;6(4):e1000892. doi: 10.1371/journal.pgen.1000892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle T (2006) Maintenance of C. elegans. WormBook :1–11. doi:10.1895/wormbook.1.101.1 [DOI] [PMC free article] [PubMed]
- Tanaka-Hino M, Sagasti A, Hisamoto N, Kawasaki M, Nakano S, Ninomiya-Tsuji J, Bargmann CI, Matsumoto K. SEK-1 MAPKK mediates Ca2+ signaling to determine neuronal asymmetric development in Caenorhabditis elegans. EMBO Rep. 2002;3(1):56–62. doi: 10.1093/embo-reports/kvf001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troemel ER, Chu SW, Reinke V, Lee SS, Ausubel FM, Kim DH. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006;2(11):e183. doi: 10.1371/journal.pgen.0020183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Tang M, Pei B, Xiao X, Wang J, Hang H, Wu L. Cadmium-induced germline apoptosis in Caenorhabditis elegans: the roles of HUS1, p53, and MAPK signaling pathways. Toxicol Sci. 2008;102(2):345–351. doi: 10.1093/toxsci/kfm220. [DOI] [PubMed] [Google Scholar]
- Welker S, Rudolph B, Frenzel E, Hagn F, Liebisch G, Schmitz G, Scheuring J, Kerth A, Blume A, Weinkauf S, Haslbeck M, Kessler H, Buchner J. Hsp12 is an intrinsically unstructured stress protein that folds upon membrane association and modulates membrane function. Mol Cell. 2010;39(4):507–520. doi: 10.1016/j.molcel.2010.08.001. [DOI] [PubMed] [Google Scholar]
- Wolf M, Nunes F, Henkel A, Heinick A, Paul RJ. The MAP kinase JNK-1 of Caenorhabditis elegans: location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J Cell Physiol. 2008;214(3):721–729. doi: 10.1002/jcp.21269. [DOI] [PubMed] [Google Scholar]
- Wood CD, Thornton TM, Sabio G, Davis RA, Rincon M. Nuclear localization of p38 MAPK in response to DNA damage. Int J Biol Sci. 2009;5(5):428–437. doi: 10.7150/ijbs.5.428. [DOI] [PMC free article] [PubMed] [Google Scholar]