Abstract
The recent advances in designing Hsp70-based anti-cancer vaccines and the ability of the chaperone to penetrate inside a living cell prompted us to develop a non-invasive method for the treatment of surface tumors. We designed hydrogel-containing gel-forming substances and human recombinant Hsp70 and applied them on the surface of a 7-day-old B16F10 melanoma tumor. According to the results of histochemistry, Hsp70 diffused through skin layer inside the B16 tumor, and this transport was proved by biochemical data. The application of Hsp70 gel reduced the rate of tumor growth by 64 % and prolonged the life of animals by 46 %. Increased survival was correlated with the enhancement of B16-specific cytotoxicity and up-regulation of gamma–interferon production. Taken together, the data confirm the anti-tumor effect of pure recombinant Hsp70 delivered intratumorally and demonstrate the relevance of a novel non-invasive technology of Hsp70-based therapy.
Keywords: Chaperone Hsp70, Melanoma, Tumor, Gamma–interferon, CTL
Introduction
Heat shock proteins (Hsps), particularly Hsp70, are known to harness the immune response to malignancies and infections (Srivastava 2002). The immunostimulatory function of Hsp70 was first discovered when the chaperone purified from the cancerous tissue and employed as a vaccine induced the sensitivity to the autologous tumor (Udono and Srivatsava 1993). Further studies demonstrated that tumor-derived Hsp70 can deliver cancer-associated peptides into antigen-presenting cells (APCs) for the subsequent presentation of tumor antigens in complex with MHC class I antigens; the presentation triggers the formation of subpopulations of tumor-specific CD8-positive cells (Calderwood and Ciocca 2008). The activation of the specific response by Hsp70 was found to relate to its ability to bind a variety of receptors on APCs, including Lox-1, CD40, CD90, and SREC-1 scavenger receptors (Murshid et al. 2011). The immunomodulatory effects were firmly attributed to Hsp70 releasing from cancer cells and encapsulated in exosomes or occurring in a soluble form, both found to trigger innate and adaptive anti-tumor immunity (Dressel et al. 2003; Gastpar et al. 2005). The mechanism of NK cell activation by Hsp70 was studied thoroughly, and according to the data obtained by Gastpar and coauthors, the intracellular chaperone induced by a certain stressor and appearing on the surface of a cancer cell exposes its TKD peptide that becomes a target for NK cells (Gastpar et al. 2004). The fact that membrane Hsp70-positive tumor cells are subjected to granzyme B-mediated and perforin-dependent apoptosis was laid in the basis of a novel anti-cancer treatment with human recombinant granzyme B (Gehrmann et al. 2012).
Generally, in order to elicit its immunomodulatory activity, Hsp70 should occur at a cell surface or in a free or exosome-bound form in the extracellular medium. These data prompted researchers to develop anti-cancer vaccine constructs. In one of them, Hsp70-secreting murine ovarian cancer cells were employed that, when injected in mice, caused strong anti-cancer effect concomitantly with the elicitation of CD4+, CD8+ T, and NK cell activity (Chang et al. 2007). Promising data have been obtained in studies in which the complex of Hsp70 with tumor peptides derived from B16 mouse melanoma was administered in a therapeutic modality; this vaccine was found to delay tumor growth and increase life span of animals by 10–12 days (Geng et al. 2006). The data of Ito and coauthors show that the intratumoral delivery of pure Hsp70 concomitantly with heated magnetic particles can efficiently destroy B16 mouse melanoma tumor (Ito et al. 2004). More recently, the application of vaccine based on Hsp70 isolated from autologous tumor cells of two lines resulted in 25–35-day delay of animal death; importantly, pure Hsp70 from liver or from another tumor gave no effect (Kumar et al. 2009). Finally, we found that the injection of rats with pure Hsp70 inhibited the growth of rhabdomyosarcoma RA-2 tumor that was accompanied with the potent specific immune response (Guzhova et al. 2008 Kumar et al. 2009).
Positive results obtained with the use of pure Hsp70 impelled us to develop a less invasive technology of chaperone delivery to surface cancers exemplified by melanoma. Given that Hsp70 can penetrate inside cells or tissues (Guzhova et al. 2001; Ekimova et al. 2010), we suggested that the protein may cross the skin layers and migrate to a malignant tissue. To verify this suggestion, the gel-containing Hsp70 was assembled, and the transport of the chaperone was analyzed with the aid of histochemistry. We found that Hsp70 migrated through the mouse skin directly to the B16 melanoma, and this action was correlated with a delay in tumor growth. Furthermore, the anti-cancer effect of Hsp70 gel was accompanied with an increase of gamma–interferon production and specific activity of cytotoxic lymphocytes.
Materials and methods
Hsp70 and gel composition
Human recombinant Hsp70 was purified from Escherichia coli cells transformed with pMSHsp70 plasmid and detoxified with the use of polymyxin B–agarose gel (Sigma, USA) as described elsewhere (Guzhova et al. 2011). The concentration of bacterial lipopolysaccharide in the final preparation was lower than 0.1 MU/ml, according to LAL test (E-toxate, Sigma, St. Louis, USA); this value is much lower than one that can have any endotoxic effect. In order to test whether Hsp70 can penetrate subcutaneously, it was conjugated with Alexa555 dye (Invitrogen, Carlsbad, CA, USA) or with NHS–biotin (Sigma, USA), according to the manufacturer’s protocol. The hydrogel was composed of carbopol (1 %), glycerol (1 %), and dimethylsulfoxide (10 %). Labeled or non-labeled Hsp70 was introduced into gel to obtain the final concentration of 0,7 mg/ml.
The safety of Hsp70 stored in gel composition was verified using chaperone immunoenzyme assay and sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis. Briefly, the gel stored for 30 days at 4 °C was diluted in a solution containing 20 mM Tris–HCl, pH 7.5, 5 mM MgCl2, vigorously vortexed, and supernatant after centrifugation at 3,000g was employed for the preparation; the samples were applied onto bottom of 96-well plate previously covered with reduced carboxymethylated lactalbumin (Lazarev et al. 2011). After reaction with the denatured protein, excess of Hsp70 was washed out, and RAF-6 polyclonal antibody was added to wells followed by anti-rabbit antibody conjugated with peroxidase. The sensitivity of the assay was found to be no less than 5 ng/ml of substrate-bound Hsp70.
Cells and animals
Mouse melanoma B16F10 cells were kindly provided by Dr. L. Sistonen (Biocenter, Turku, Finland). The cells were grown in DMEM media containing 10 % fetal bovine serum. Female C57BL/6 mice were purchased from Biomedical Technology Research Center (Moscow region, Russia). The animals were subcutaneously injected with 1 × 106 B16 cells. The application of Hsp70-containing gel or control gel (vehicle) was performed onto preliminarily shaven areas of skin surrounding a 7-day-old B16 tumor; the application was repeated every 3 days until the end of experiments (groups of animals, n = 10: Hsp70 gel and vehicle gel). Additionally, we analyzed the viability of two groups of animals injected intratumorally with 15 μg Hsp70 or phosphate-buffered saline using the same time schedule (groups, n = 10: Hsp70 injection and vehicle injection). The survival curves were estimated according to the method of Kaplan and Meier and compared using generalized Wilcoxon’s test. Tumor growth rate was estimated by weighing tumors taken from control and treated animals (n = 16) on day 18 after B16 cell engrafting. All animal experiments were in accordance with institutional guidelines for the welfare of animals.
Hsp70 penetration inside tumor
Two approaches were used to explore the route of Hsp70 applied with the hydrogel on the surface of B16 tumor. In the first part, 3 h after the application of the gel-containing Hsp70 Alexa555, the tumor was excised, fixed, and embedded into a tissue–tek compound. Cryosections were mounted in DAKO fluorescent mounting media (Dako North America Inc., Carpinteria, USA) and investigated with the use of Axiovert 200 M (Karl Zeiss, Germany). The same sections of cancerous tissue were studied with the aid of confocal microscopy using Leica TCS SP2 microscope (Leica, Germany). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI).
Hsp70 intratumoral transport was also verified by analyzing samples of tumor tissue after the application of Hsp70 bio-gel. Extracts containing proteins isolated from the tissue samples were incubated with aliquots of ATP–agarose gel (A2767 Sigma, USA); eluates from agarose gels were applied onto nitrocellulose membrane which was stained with NeutrAvidin-peroxidase (Pierce, Rockford, USA).
Gamma–interferon mesurement and CTL assays
Splenocytes were isolated from control and treated with Hsp70 gel animals (n = 3 per group) on days 14 and 20 after grafting of B16 cells using standard protocol. The level of gamma–interferon (γIFN) in the medium of splenocytes incubated with B16 cells (104 per well) for 48 h, treated with Concanavalin A (reference control) and in pure medium (spontaneous induction) was measured with the aid of mouse γIFN ELISA kit (BD Biosciences, San Jose, CA, USA). All values were equalized by reducing to the level of the cytokine in reference control samples; the latter were close in all groups of animals. The values of spontaneously released γIFN for all groups were lower than 10 pg/ml and were subtracted from the experimental data.
To estimate the cytotoxic activity of lymphocytes, splenocytes were isolated from control and treated mice (n = 3) on day 14 after tumor injection and added to B16 cells (104 cells per well) at 50:1 and 100:1 ratios for 6 h. The cytolytic activity of splenocytes was measured as LDH activity in a culture medium with the help of CytoTox 96 non-radioactive cytotoxicity assay (Promega Inc., Madison, USA).
Statistical analysis
The survival curves were estimated according to the Kaplan–Meier method, and the curves were compared using the generalized Wilcoxon’s test. Observations are generally reported as mean ± SE. One- or two-tailed unpaired Student’s t tests were used to evaluate the differences between the control and treatment groups; differences were considered to be statistically significant when p < 0.05.
Results and discussion
The aims of this study were to elaborate a novel technology for the non-invasive intratumoral delivery of Hsp70 and to test it in the model of B16F10 mouse melanoma. Since our earlier data showed that the protein may easily penetrate inside living cells, we considered several forms of Hsp70 application and chose hydrogel-like substance. This matter was composed from ordinarily used in cosmetics and pharmacology gel-forming substance, carbopol, to which glycerol and DMSO were added. We assumed that these additives can protect Hsp70 from denaturation and/or proteolysis. To prove the safety of Hsp70, we extracted the protein from the gel by diluting the latter in aqueous solution and subjected it to a chaperone immunoenzyme assay (Lazarev et al. 2011) and to SDS–polyacrylamide gel electrophoresis; the data showed that the protein stored in gel for 1 month at 4 °C retained its substrate-binding (chaperonic) activity and molecular structure as proved by the only 70 kD band on the polyacrylamide gel (Pankratova, Margulis, Guzhova, unpublished observation). The reduction of total amount of Hsp70 embedded in gel by 8–10 % can relate to losses due to incomplete dissociation of the gel structure. Generally, these data prove that the hydrogel composition is a good candidate for Hsp70 storage.
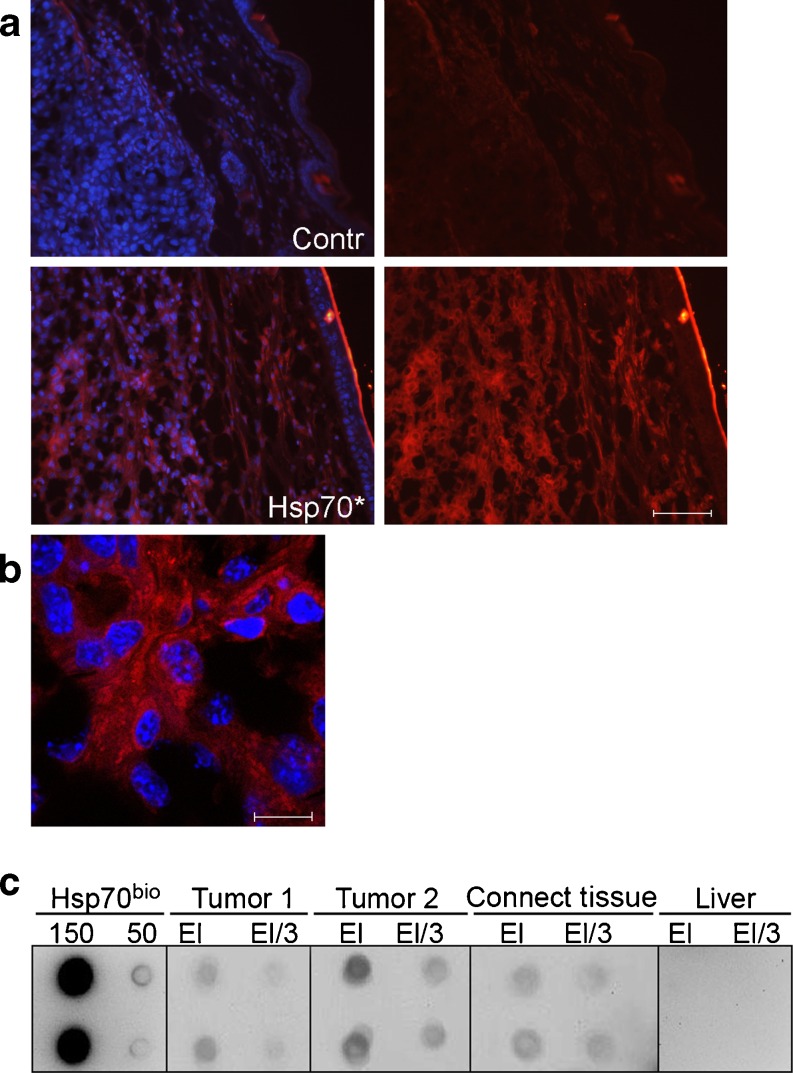
Suggesting that Hsp70 can migrate from the gel to tumor, we employed microscopy to localize the chaperone labeled with Alexa555 (red) in mouse tissues after its application as part of a gel composition. The labeling procedure was performed instead of immunostaining in order to exclude possible recognition of self-chaperone with an anti-Hsp70 antibody. It was found that 3 h after gel application, Hsp70 was distributed throughout the whole volume of tumor and connective tissue; this distribution extended to skin layer seen as a sharp red line in the right upper part of the images, Fig. 1a, lower panel. We found a much weaker red self-fluorescence on the slices obtained from the same tissue of control mice untreated with Hsp70 gel that is probably related to hair follicles or other dense granular structures, Fig. 1a, upper panel. To analyze the route of Hsp70 Alexa555 in cancerous tissue in more details, we applied confocal microscopy using the same tissue sections as in above study. The enhanced resolution allows to observe Hsp70 Alexa555 distribution throughout extracellular matrix and its possible penetration inside the tumor cells where the protein appears to locate near the nuclei (blue staining), Fig. 1b. The intracellular transport of Hsp70 is a well-established phenomenon unless the mechanisms underlying it are not yet clear (Murshid et al. 2011).
Fig. 1.
Migration of Hsp70 from gel into B16F10 tumor and subcutaneous tissue. a Morphological tracing of Hsp70 transport. Hsp70 was labeled with Alexa555 dye (Invitrogen, USA) according to instructions of the manufacturer and introduced in carbopol–glycerol–DMSO gel (700 μg of Hsp70 Alexa555 per milliliter of gel); 70 μl of gel was applied onto 7-day-old B16 tumor, and the sections of the tissue were prepared for the light microscopy. In the left part of Fig. 1, the pattern of staining of cell nuclei with DAPI is presented. Scale bar, 50 μm. b Microscopic analysis of Hsp70 transport into cancerous tissue. The sections were prepared as in A and studied with the aid of Leica TCS SP2 confocal microscope. Scale bar, 5 μm. c Biochemical proof of Hsp70 penetration inside B16 tumor. B16 tumor and adjacent tissue treated with gel-containing biotinylated Hsp70 were cut to obtain pieces with the approximate volume of 10–15 μl each. The proteins contained in each piece were extracted and subjected to the reaction with ATP–agarose gel; after washing, the proteins captured by the gel were applied on nitrocellulose, and spots were stained with neutravidin and biotinilated peroxidase solution. The dots are presented in pairs in the following order (from left to right): pure Hsp70 bio, 150 and 50 nanograms per dot; B16 tumor from animal no. 1, eluate 1:1(EL) and 1:3 (El/3); tumor from animal no. 2, eluate 1:1(EL) and 1:3(El/3); eluate obtained after processing of connective tissue adjacent to the tumor no. 2, eluate 1:1(EL) and 1:3(El/3); and eluate obtained after processing of liver of the animal no. 2, eluate 1:1(EL) and 1:3(El/3)
To prove the subcutaneous migration of Hsp70, we biotinylated the protein, introduced the preparation into gel, and applied it on the surface of a growing tumor. Three hours after the application of Hsp70 bio-containing gel, we obtained extracts from the pieces of tumor and adjacent tissue. These extracts were incubated with ATP–agarose known to bind Hsp70; after washing, the proteins bound to the gel were eluted with the SDS-containing buffer and applied onto nitrocellulose membrane. Hsp70 bio in dots was analyzed with the aid of staining with NeutrAvidin–peroxidase. The data of staining show that the protein reached tumor and adjacent connective tissue within 3 h, Fig. 1c. Interestingly, there were no traces of Hsp70 bio in the liver, the organ that was earlier shown to absorb most of the radioactively labeled protein when it was injected intravenously (Nishikawa et al. 2008). This fact may reflect a tumor-targeted transportation of the chaperone especially at the beginning of its penetration inside the organism, and this targeting is supported by our observations of Hsp70-I131 appearance in B16 tumor after intravenous injection (Shevtsov, Pozdnyakov, Margulis, unpublished observations). Indeed, the courageous suggestion that Hsp70 targets specifically to cancerous tissue must be established using molecular approaches.
We determined the time needed for Hsp70 transition to tumor with the use of the biotinylated chaperone. The results of this study show that Hsp70 can be detected already 1 h after the application though its amount is low; 3 h appear to be the point of maximal protein accumulation in tumor, and during the next 7–8 h, its amount steadily declines (data not shown). This reduction made us to use in further experiments the schedule of multiple applications of the protein with the gel.
The concentration of Hsp70 in cancerous tissue from two animals was rather high, and according to our calculations, it amounted to 5 μg/ml of tumor, a level comparable to that used by Ito and coauthors when treating B16 melanoma by intratumoral injection of pure chaperone (Ito et al. 2004).
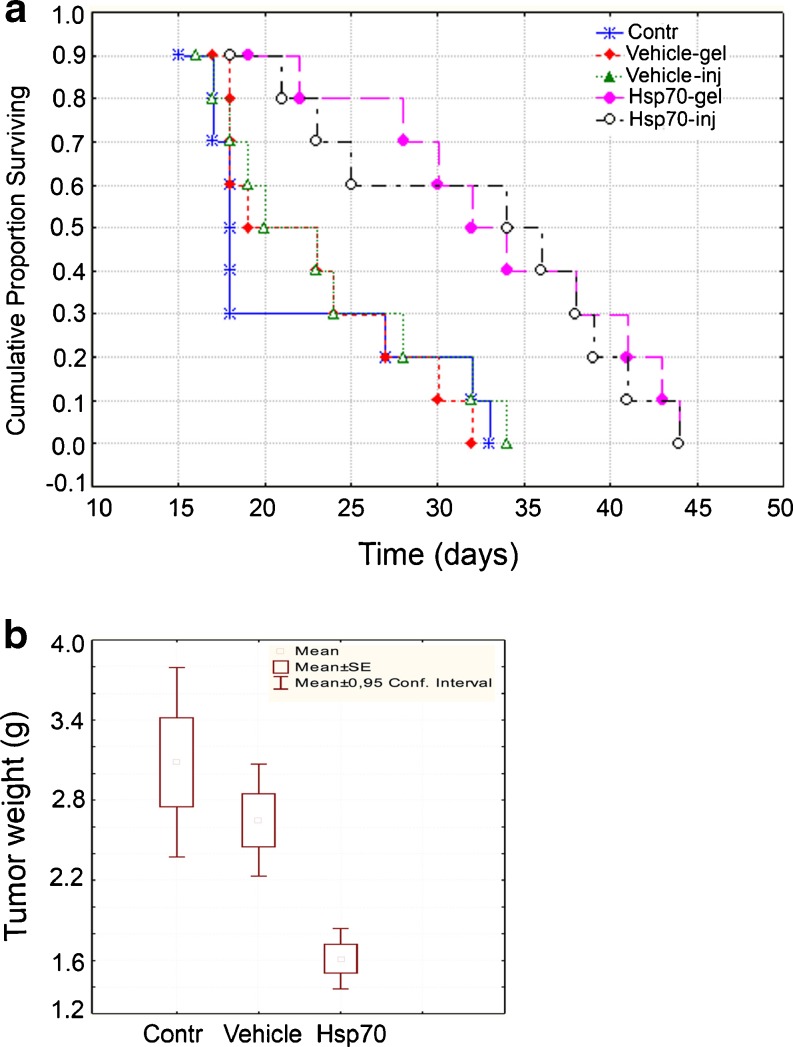
For the experiments aimed to study the anti-cancer effects of Hsp70 gel, we applied it in a volume of 70 μl (50 μg of Hsp70) on the skin area over a tumor starting from the seventh day after B16 cell injection when the tumor was first observable. The application was performed with 3-day intervals till the termination of the study. In the same experiment, the viability of two groups of animals, intratumorally injected with Hsp70 or with phosphate-buffered saline, was analyzed. The data demonstrate that the delivery of Hsp70 injected or applied as the gel component increase the survival rate by almost 50 %, and this result is statistically significant (p < 0.01), Fig. 2a. Importantly, both methods of delivery gave similar data on prolonging the survival of treated with Hsp70 animals by 15 days. Similar estimates of survival prolongation of B16 tumor-bearing mice were reported by two groups (Ito et al. 2004; Geng et al. 2006), and this concordance proves the pharmacological relevance of fully non-invasive technology of Hsp70 application onto tumor as compared with injection of the chaperone in tumor.
Fig. 2.
Application of Hsp70 gel prolongs survival of B16 tumor-bearing animals and inhibits tumor growth. a Survival curves were generated using the method of Kaplan and Meier and compared using the generalized Wilcoxon’s test. b Tumor growth rate was established by weighing cancerous tissues taken from control and treated animals (n = 16) on day 18 after B16 cell engrafting. The data on the tumor mass are generally reported as mean ± SE
The efficacy of anti-tumor activity elicited by Hsp70 gel was proved by the results of the measurement of B16 tumor weight in animals of three groups. To our surprise, there was a difference between the two control groups; however, after calculations, it was found to be statistically insignificant, Fig. 2b. The data of tumor weighing demonstrated a considerable difference between both control groups (control and vehicle) and the one including the animals treated with Hsp70 gel, Fig. 2b. Almost twofold reduction of B16 tumor growth caused by intratumorally delivered pure Hsp70 or its complex with cancer antigens was reported by the abovementioned groups (Ito et al. 2004; Geng et al. 2006), and the similarity of their and our results also proves the adequacy of the technology described here.
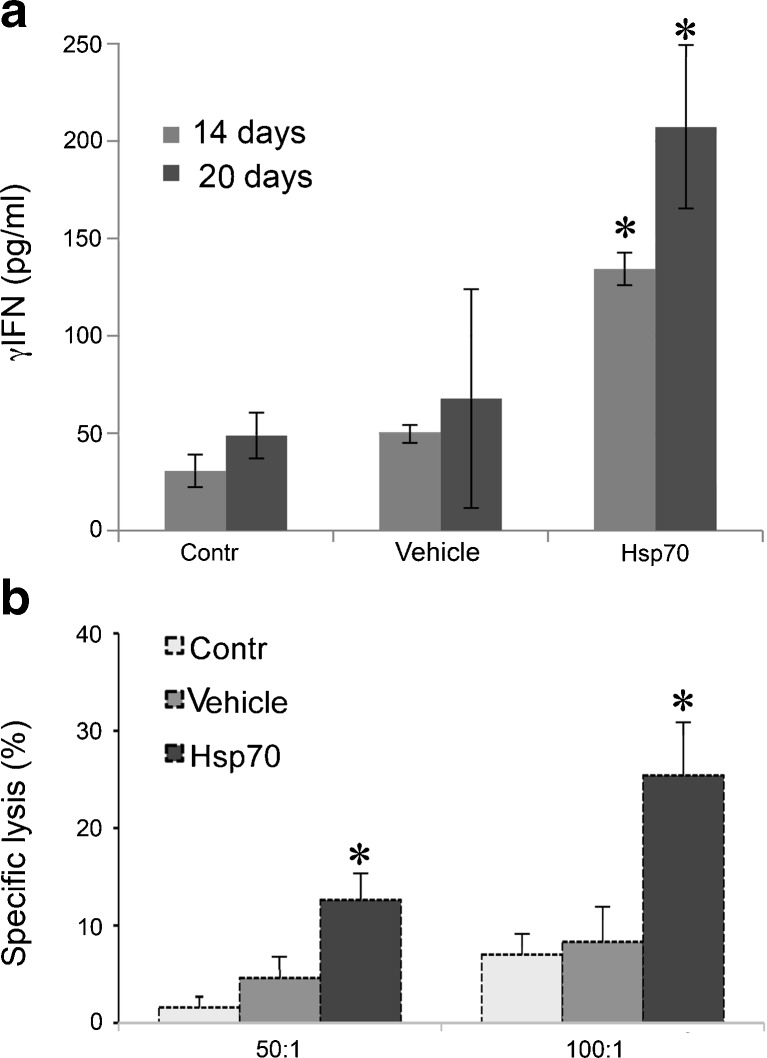
The anti-tumor effects of the molecular chaperones are always ascribed to their ability to activate innate and adaptive immunity, and it appeared reasonable to measure the two key parameters characterizing the development of the immune cell response, namely, γ–IFN production and the cytolytic activity of lymphocytes. To measure the γ–IFN level in animals belonging to three groups, we obtained splenocytes and incubated them with B16 cells. The data show that the splenocytes isolated from mice treated with Hsp70 gel responded to tumor cells already on day 14; whereas a week later, the cytokine production exceeded the values for control animals threefold, Fig. 3a. The response of splenocytes isolated from control or treated with vehicle animals was significantly lower proving the generation of Hsp70-triggered specific immune response.
Fig. 3.
Hsp70 gel application induces elevation of gamma–interferon production and cytotoxic response. a γ–IFN level in medium of splenocytes isolated from control animals, and animals treated with vehicle and with Hsp70 gel on days 14 and 20 after B16 tumor injection; b cytotoxic activity was measured using splenocytes derived on day 14 from animals as indicated in 3A and with the aid of standard protocol. Cell death was determined with the help of Cytotox-96 assay (see the text for details)
Next, we checked the response of cytotoxic lymphocytes to B16 cells possibly affected by the application of Hsp70 gel. To measure the response, we used a standard cytotoxic T lymphocyte (CTL) assay which comprised splenocytes taken on day 14 after the tumor injection from animals of three groups: the control group and the groups treated with vehicle and with Hsp70 gels. Cocultivation of effector and tumor cells resulted in cytolysis of the latter as evidenced by the enhanced LDH activity in cell medium, Fig. 3b. At both ratios between effector and target cells, the value of specific cell lysis is at least 2.5-fold higher in mice treated with Hsp70 gel than in control animals; this difference is statistically significant.
The values of immune response parameters, γ–IFN production, and CTL activity are in good agreement with data obtained using other vaccine constructs based on Hsp70 chaperone. First, the immunization of B16F10 melanoma-bearing mice with the autologous Hsp70 was shown to induce an approximately twofold increase of γ–IFN and Il2 cytokine productions (Geng et al. 2006). In another study, mice-bearing sarcoma S180 tumor was immunized with Hsp70 isolated from the tumor, and the vaccination induced a threefold and twofold elevation of γ–IFN production of the specific cytotoxic response (Guo et al. 2011). Using the same cancer cells, S180 mouse sarcoma, Kumar and coauthors proved that the injection of the autologous Hsp70 induced more than 100 % increase of cytolytic response of mice with tumors (Kumar et al. 2009). Taken together, these data confirm that Hsp70 migrating from the hydrogel is immunologically not less active than the other protein constructs administered to an organism bearing a tumor. Thus, we demonstrated a novel way to apply Hsp70 superficially, proved its adequacy as a tool for the therapy of surface cancer, mouse melanoma, and linked this anti-tumor activity to the immunostimulatory effect of Hsp70.
Acknowledgments
The authors thank Dr. Irina V. Romanova for the histological analysis and Dr. Maxim A. Shevtsov for fruitful discussions. The work was supported by grants from the Russian Foundation for Basic Research 10-04-01049, 11-08-00445 and the Program of Russian Academy of Sciences “Molecular and Cellular Biology.”
Footnotes
Sergey V. Abkin and Katerina M. Pankratova contributed equally to this work.
References
- Calderwood SK, Ciocca DR. Heat shock proteins: stress proteins with Janus-like properties in cancer. Int J Hyperthermia. 2008;24:31–39. doi: 10.1080/02656730701858305. [DOI] [PubMed] [Google Scholar]
- Chang CL, Tsai YC, He L, Wu TC, Hung CF. Cancer immunotherapy using irradiated tumor cells secreting heat shock protein 70. Cancer Res. 2007;67(20):10047–10057. doi: 10.1158/0008-5472.CAN-07-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressel R, Grzeszik C, Kreiss M, Lindemann D, Herrmann T, Walter L, Gunther E. Differential effect of acute and permanent heat shock protein 70 overexpression in tumor cells on lysability by cytotoxic T lymphocytes. Cancer Res. 2003;63(23):8212–8120. [PubMed] [Google Scholar]
- Ekimova IV, Nitsinskaya LE, Romanova IV, Pastukhov YF, Margulis BA, Guzhova IV. Exogenous protein Hsp70/Hsc70 can penetrate into brain structures and attenuate the severity of chemically-induced seizures. J Neurochem. 2010;115(4):1035–1044. doi: 10.1111/j.1471-4159.2010.06989.x. [DOI] [PubMed] [Google Scholar]
- Gastpar R, Gehrmann M, Bausero MA, Asea A, Gross C, Schroeder JA, Multhoff G. Heat shock protein 70 surface-positive tumor exosomes stimulate migratory and cytolytic activity of natural killer cells. Cancer Res. 2005;65:5238–5247. doi: 10.1158/0008-5472.CAN-04-3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gastpar R, Gross C, Rossbacher L, Ellwart J, Riegger J, Multhoff G. The cell surface-localized heat shock protein 70 epitope TKD induces migration and cytolytic activity selectively in human NK-cells. J Immunol. 2004;172:972–980. doi: 10.4049/jimmunol.172.2.972. [DOI] [PubMed] [Google Scholar]
- Gehrmann M, Stangl S, Kirshner A, Foulds GA, Sievert W, Doss BT, Walch A, Pockley AG, Multhoff G. Immunotherapeutic targeting of membrane Hsp70-expressing tumors using recombinant human granzyme B. PLoS One. 2012;7(7):e41341. doi: 10.1371/journal.pone.0041341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng H, Zhang GM, Xiao H, Yuan Y, Li D, Zhang H, Qiu H, He YF, Feng ZH. HSP70 vaccine in combination with gene therapy with plasmid DNA encoding sPD-1 overcomes immune resistance and suppresses the progression of pulmonary metastatic melanoma. Int J Cancer. 2006;118:2657–2664. doi: 10.1002/ijc.21795. [DOI] [PubMed] [Google Scholar]
- Guo Q-Y, Yuan M, Peng J, Cui X-M, Song G, Sui X, Lu S-B. Antitumor activity of mixed heat shock protein/peptide vaccine and cyclophosphamide plus interleukin-12 in mice sarcoma. J Exp Clin Cancer Res. 2011;30:24. doi: 10.1186/1756-9966-30-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzhova I, Kislyakova K, Moskaliova O, Fridlanskaya I, Tytell M, Cheetham M, Margulis B. In vitro studies show that Hsp70 can be released by glia and that exogenous Hsp70 can enhance neuronal stress tolerance. Brain Res. 2001;914:66–73. doi: 10.1016/S0006-8993(01)02774-3. [DOI] [PubMed] [Google Scholar]
- Guzhova IV, Komarova EI, Pimenova AA, Bakhtin IB, Kaminskaia EV, Margulis BA. The role of extracellular chaperone Hsp70 in creating antitumor immunity in rat rhabdomyosarcoma RA-2 model. Vopr Onkol. 2008;54:611–617. [PubMed] [Google Scholar]
- Guzhova IV, Lazarev VF, Kaznacheeva AV, Ippolitova MV, Muronetz VI, Kinev AV, Margulis BA. Novel mechanism of Hsp70 chaperone-mediated prevention of polyglutamine aggregates in a cellular model of huntington disease. Hum Mol Genet. 2011;20:3953–3963. doi: 10.1093/hmg/ddr314. [DOI] [PubMed] [Google Scholar]
- Ito A, Matsuoka F, Honda H, Kobayashi T. Antitumor effects of combined therapy of recombinant heat shock protein 70 and hyperthermia using magnetic nanoparticles in an experimental subcutaneous murine melanoma. Cancer Immunol Immunother. 2004;53:26–32. doi: 10.1007/s00262-003-0416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Deepak P, Acharya A. Autologous Hsp70 immunization induces anti-tumor immunity and increases longevity and survival of tumor-bearing mice. Neoplasma. 2009;56:259–268. doi: 10.4149/neo_2009_03_259. [DOI] [PubMed] [Google Scholar]
- Lazarev VF, Onokhin KV, Antimonova OI, Polonik SG, Guzhova IV, Margulis BA. Kinetics of chaperone activity of proteins Hsp70 and Hdj1 in human leukemia U-937 cells after preconditioning with thermal shock or compound u-133. Biochemistry (Mosc) 2011;76(5):590–595. doi: 10.1134/S0006297911050099. [DOI] [PubMed] [Google Scholar]
- Murshid A, Theriault J, Gong J, Calderwood SK. Investigating receptors for extracellular heat shock proteins. Methods Mol Biol. 2011;787:289–302. doi: 10.1007/978-1-61779-295-3_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa M, Takemoto S, Takakura Y. Heat shock protein derivatives for delivery of antigens to antigen presenting cells. Int J Pharm. 2008;354(1–2):23–27. doi: 10.1016/j.ijpharm.2007.09.030. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Udono H, Srivatsava PK. Heat shock protein 70-associated peptides elicit specific cancer immunity. J Exp Med. 1993;178:1391–1396. doi: 10.1084/jem.178.4.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]