Abstract
We identified increased expression and redistribution of the intracellular protein 60-kDa human heat shock protein (hHSP60) (HSPD1) to the cell surface in human endothelial cells subjected to classical atherosclerosis risk factors and subsequent immunologic cross-reactivity against this highly conserved molecule, as key events occurring early in the process of atherosclerosis. The present study aimed at investigating the role of infectious pathogens as stress factors for vascular endothelial cells and, as such, contributors to early atherosclerotic lesion formation. Using primary donor-matched arterial and venous human endothelial cells, we show that infection with Chlamydia pneumoniae leads to marked upregulation and surface expression of hHSP60 and adhesion molecules. Moreover, we provide evidence for an increased susceptibility of arterial endothelial cells for redistribution of hHSP60 to the cellular membrane in response to C. pneumoniae infection as compared to autologous venous endothelial cells. We also show that oxidative stress has a central role to play in endothelial cell activation in response to chlamydial infection. These data provide evidence for a role of C. pneumoniae as a potent primary endothelial stressor for arterial endothelial cells leading to enrichment of hHSP60 on the cellular membrane and, as such, a potential initiator of atherosclerosis.
Keywords: Inflammation, Heat shock protein 60, Autoimmunity, Chlamydia, Endothelial, Atherosclerosis
Introduction
There is increasing evidence from clinical and experimental studies for a causative role of infection and inflammation in initiation and progression of atherosclerosis (Ross 1999; Hansson 2001; Libby 2002; Wick et al. 2004). Our research group formulated the “Autoimmune Concept of Atherosclerosis,” experimental and clinical evidence for which was provided by data gathered from our and other laboratories over the past several years (Wick et al. 2001, 2004; Grundtman et al. 2011). At the heart of this concept lies the finding that stressed endothelial cells become the target of pre-existing innate and adaptive cellular and humoral immunity against eukaryotic cross-reactive HSP60 epitopes due to earlier infections or vaccinations, as well as bona fide autoimmunity against biochemically altered autologous HSP60 (Xu et al. 1993a; b; 1994; 2000; Grundtman and Wick 2011). At a molecular level, we and others have shown that hHSP60 is expressed as a first response, serving as an immunological danger signal in endothelial cells subjected to classical risk factors for atherosclerosis including cigarette smoke, oxidative stress, and oxidized LDL, rendering the cells a target of pre-existing cross-reactive autoimmunity (Xu et al. 1994; Amberger et al. 1997; Shi and Tokunaga 2004; Henderson et al. 2008; Kreutmayer et al. 2011). Clinically, a significant correlation between antibacterial HSP60 antibody levels and prevalent carotid atherosclerosis was observed, and the presence of human and chlamydial antibodies to HSP60 was identified as an independent risk factor for coronary atherosclerosis (Xu et al. 1993a; b; 1999). Severely aggravated lesion formation and enrichment of HSP60-reactive T cells within atherosclerotic lesions in rabbits and mice by immunization with recombinant HSP65 further indicate the role of HSP60 as a putative antigen, initiating and maintaining vascular inflammation (Xu et al. 1992; George et al. 1999). According to our concept of an autoimmune basis of atherosclerosis, the lifelong burden of being subjected to infectious agents, such as Chlamydia pneumoniae, would go hand-in-hand with an increased risk of developing immunologic cross-reactivity against autologous HSP60 (Mayr et al. 1999a). In a previous study, it was shown that chlamydial HSP60 (cHSP60) antibodies cross-react with hHSP60 counterparts and mediate cytotoxicity to stressed endothelial cells. Moreover, there was a strong correlation between cross-reactive anti-HSP60 and anti-C. pneumoniae antibody titers (Mayr et al. 1999b). In terms of epidemiology, population-based studies show that most individuals get infected with C. pneumoniae before the age of 20, and specific antibodies against C. pneumoniae are found in more than 70 % of people aged 50 and above (Grayston 2000). Based on increased serum antibody titers against this agent in patients with coronary heart disease, Saikku et al. (1988) suggested a possible relationship between C. pneumoniae infection and atherosclerosis. This finding was confirmed by subsequent population-based studies that found a strong correlation between the risk for coronary heart disease and detection of C. pneumoniae in coronary arterial tissue (Jackson et al. 1997; Yamashita et al. 1998). Remarkably, while more than 50 % of coronary plaque lesions harbor C. pneumoniae, the pathogen has rarely been detected in healthy vascular tissue (Shi and Tokunaga 2004). Experimentally, it has been shown that the major pro-inflammatory transcription factor NF-κB is upregulated in endothelial cells subsequent to C. pneumoniae infection, leading to increased secretion of interleukin (IL)-1, IL-8, and monocyte chemotactic protein 1 (MCP-1) (Hogdahl et al. 2008).
In summary, these lines of evidence support the notion that chlamydial infection is a potent contributor to vascular inflammation. However, classical experimental protocols used centrifugation (Dechend et al. 2003) of the endothelial cells together with the C. pneumoniae infective particles (elementary bodies) to enable productive infection of the target cells. As these treatments themselves represent stressful mechanical conditions that might induce a stress reaction in endothelial cells, they are unsuitable protocols for studying C. pneumoniae-induced stressor effects (Krüll et al. 1999; Shi and Tokunaga 2004). In order to have a noninvasive experimental setting avoiding an additional centrifugation step, we used the CV6 strain of C. pneumoniae that shows a special tropism for endothelial cells. Moreover, in the present study, we employed human umbilical vein endothelial cells (HUVECs) as well as primary adult arterial and venous endothelial cells (AECs and VECs), from the same donors. Thus, we were able to delineate the susceptibility of AECs and VECs to a C. pneumoniae-induced pro-inflammatory phenotype while avoiding a confounding effect inherent to the genetic variability of primary cells derived from different donors. In particular, we set out to investigate the threshold for surface expression of hHSP60 in primary AECs as compared to autologous VECs in response to C. pneumoniae infection. Here, we identify chlamydial infection as an inducer of hHSP60 expression in primary endothelial cells more potent than any of the classical endothelial stress factors. Moreover, we provide evidence for a particularly increased susceptibility of AECs for surface expression of hHSP60 as compared to autologous VECs.
Materials and methods
Culture of C. pneumoniae
The cardiovascular C. pneumoniae strain CV-6, a coronary artery isolate, was grown on HEp-2 cell monolayers as described (Gieffers et al. 2001). Absence of mycoplasma was shown by PCR (Stratagene, Santa Clara, CA, USA).
Cell culture
HUVECs were isolated from umbilical cords obtained with informed consent from the Department of Gynecology and Obstetrics, Innsbruck Medical University. AECs and VECs were isolated from the iliac arteries and veins of transplant donors obtained from the Clinic of Visceral, Transplant and Thorax Surgery, Innsbruck Medical University. The isolation and use of human tissues for these experiments has been approved by the Ethics Committee of Innsbruck Medical University (resolution numbers UN2979 and AM2670c). Cells were isolated by enzymatic detachment, using collagenase as described elsewhere (Amberger et al. 1997), and subsequently cultured in 0.2 % gelatin-coated (Sigma-Aldrich, St. Louis, MO, USA) polystyrene flasks (Becton Dickinson, Meylan Cedex, France) in Endothelial Cell Basal Medium-2 (EBM-2 containing EGM-2 SingleQuots supplements; Lonza, Basel, Switzerland) including 2 % fetal bovine serum and growth factors (Lonza) in a humidified atmosphere of 5 % CO2. The medium was replaced by RPMI without phenol red and antibiotics, supplemented with 10 % FCS and 5 % l-glutamine (Lonza) prior to the addition of the infectious chlamydial particles, to avoid diminished infectivity due to antibiotic compounds and because phenol red is an antioxidant (Lewinska et al. 2007) which may preclude assays on the oxidative state of cells. Chemical reagents were purchased from Merck (Darmstadt, Germany) unless stated otherwise and were of analytical grade quality.
Infection of endothelial cells with C. pneumoniae
Endothelial cells were cultivated to a maximum of six passages. To allow for an optimal comparison between AECs and VECs, cells from one donor in one enclosed experimental setting were used. Endothelial cells were infected at 80 % confluence at a low dose of 1.5 inclusion forming units per cell.
Real-time PCR (RT-PCR)
RT-PCR was performed using the LightCycler FastStart DNA Master SYBR Green kit (Roche) on the LightCycler 1.0 system (Roche). Synthesis of the first strand of cDNA from total RNA (500 ng) was achieved using First Strand cDNA Synthesis Kit (Roche) according to the manufacturer’s recommendations. The cDNA was diluted tenfold before equal amounts were added to duplicate (or triplicate) RT-PCR reactions. To ensure the highest possible accuracy, a master mix containing all reagents except for the primers was created; the primers for the gene of interest or a housekeeping gene was added directly to the glass capillary. Primer pairs are given in Table 1. Each reaction proceeded for 40 amplification cycles, followed by a melting curve analysis to ensure the specificity of each reaction, controlled by the supplied LightCycler software (version 3.5, Roche). Crossing point and melting curve analyses for each reaction were also performed using the LightCycler software.
Table 1.
List of the RT-PCR primer pairs
| Gene title | Forward | Reverse |
|---|---|---|
| HSPD1 | CCACTGCTACTGTACTGGCAC | AGCTAACATCACACCTCTCCT |
| EGR-1 | AAGCAAACCAATGGTGATCC | TGCCACATGTGAGAGTACGG |
| ICAM-1 | CTGCAGACAGTGACCATC | GTCCAGTTTCCCGGACAA |
| VCAM-1 | GGTTTCTCTGTATAGTACTGGCATG | TCATCAGACTCCTGTGCAACTTT |
| E-selectin | GAGGAATGCCTGTGTGAGCA | CCAAAGGAATCTCCAGTTTTCAGT |
| MCP-1 | CAAGCAGAAGTGGGTTCAGGAT | AGTGAGTGTTCAAGTCTTCGGAGTT |
| IL-6 | TTCTCCACAAGCGCCTTCGGTCCA | AGGGCTGAGATGCCGTCGAGGATGTA |
| IL-8 | TCTCTTGGCAGCCTTCCTGATTTC | GTGTGGTCCACTCTCAATCACTCT |
| TF | CCGAACAGTTAACCGGAAGA | TCAGTGGGGAGTTCTCCTTC |
| SOD1 | AGGGCATCATCAATTTCGA | TCCAGAAAACACGGTGGG |
| TRX-1 | GGCTTGATCATTTTGCAAGG | GTCAGACTCCAGCAGCCAAG |
| COX-1 | GAACATGGACCACCACATCC | TTTCATGCCAAACCTCTTGC |
| COX-2 | GCAGGGTTGCTGGTGGTAG | ATTTCATCTGCCTGCTCTGG |
| NOX-2 | CAAGATGCGTGGAAACTACCTAAGAT | TCCCTGCTCCCACTAACATCA |
| NOX-4 | CTGCTGACGTTGCATGTTTC | TTCTGAGAGCTGGTTCGGTT |
| SDHA | TGGGAACAAGAGGGCATCTG | CCACCACTGCATCAAATTCATG |
HSPD1 gene coding for hHsp60, EGR-1 early growth response protein 1, ICAM-1 intracellular adhesion molecule 1, VCAM-1 vascular cell adhesion molecule 1, MCP-1 monocyte chemotactic protein 1, IL interleukin, TF tissue factor, SOD1 superoxide dismutase 1 (Cu-Zn), TRX-1 thioredoxin 1, COX cyclooxygenase, NOX nicotinamide adenine dinucleotide phosphate oxidase, SDHA subunit A of the succinate dehydrogenase complex
Western blot analysis
Total protein extracts of C. pneumoniae-infected HUVECs and controls were obtained using standard NP-40 cell lysis buffer (50 mM Tris/HCl, pH 8, 150 mM NaCl, 1 % NP-40). The protein content was determined with Bradford reagent (Bio-Rad, Hercules, CA, USA) following the manufacturer’s protocol. Equal amounts of protein in loading buffer (200 mM Tris/HCl, pH 6.8, 400 mM DTT, 8 % SDS, 0.4 % bromophenol blue, and 50 % glycerol) were loaded onto 12 % Tris/Glycine SDS-PAGels, purchased from Lonza, and run at 25 mA for 1 h. Proteins were blotted to a nitrocellulose membrane (Schleicher & Schuell, Dassel, Germany), and hHSP60 was stained using a mouse anti-human HSP60 antibody (clone II-13, prepared from hybridoma cells in our lab) (Singh and Gupta 1992). As a loading control, mouse anti-human α-tubulin antibody (from Santa Cruz Biotechnology, Santa Cruz, CA, USA; catalogue number: sc-23948) was used. Polyclonal rabbit anti-mouse immunoglobulins, covalently bound to horseradish peroxidase (P0161, Dako, Glostrup, Denmark) as secondary antibody, allowed for detection of the signal with the ECL method (Pierce, Rockford, IL, USA).
Immunofluorescence staining of cells and confocal microscopy
For immunofluorescence analysis, HUVECs, AECs, and VECs were grown in RPMI medium (Lonza) on 18 × 18 mm glass coverslips (Nunc, Rochester, NY, USA) coated with 10 % gelatine (Sigma-Aldrich, St. Louis, MO, USA). Cells were washed with phosphate-buffered saline (PBS), pH 7.2, fixed, and permeabilized with 99.5 % acetone at −20 °C for 2.5 min for intracellular staining. Subsequently, cells were allowed to dry for 30 min at room temperature (RT), followed by blocking with 0.1 % bovine serum albumin (Sigma-Aldrich) in PBS for 30 min at RT. For surface staining, cells were kept for all steps at 4 °C and fixed with 4 % paraformaldehyde after incubation with the antibodies. Identical blocking solution and reagents were used for both staining protocols. Staining of eukaryotic HSP60 was performed with either the mouse anti-human HSP60 antibody (clone II-13) or rabbit anti-human HSP60 antibody (sc-13966, Santa Cruz Biotechnology). Staining of chlamydial HSP60, human intracellular adhesion molecule 1 (ICAM-1), and human vascular cell adhesion molecule 1 (VCAM-1) was performed using mouse anti-chlamydial HSP60 antibody (Stressgen, Enzo Life Sciences, Plymouth Meeting, PA, USA), mouse anti-human ICAM-1, and mouse anti-human VCAM-1 antibodies (both e-Bioscience, San Diego, CA, USA). The matching isotype control antibodies were from Dako. After four washing steps with PBS, staining was visualized using either goat anti-mouse IgG Alexa 488- or Alexa 568- and goat anti-rabbit Alexa 568-labeled secondary antibodies (Invitrogen, Carlsbad, CA, USA). Cells were then washed again, nuclei were stained with Hoechst 33342 (Sigma-Aldrich) according to the manufacturer’s protocol, and the coverslips were mounted with Fluoromount G on to the specimen holder (Nunc, Rochester, NY, USA). Time-resolved 3D stacks were acquired with an SP5 confocal microscope (Leica Microsystems, Wetzlar, Germany) equipped with a fast resonant scanner. We used an HCX PL APO lambda blue ×63 1.4 NA oil immersion objective. Imaging was performed with a 476-nm laser line for EGFP, a 561-nm laser line for AlexaFluor568 and mCherry excitation, and 633 nm for AlexaFluor647. Fluorescence emission was detected from 493 to 555 nm (EGFP), 566 to 742 nm (AlexaFluor568, mCherry), and 638 to 750 nm (AlexaFluor647). Images were acquired using the LAS AF acquisition software Version 2.1.
OxyBlot analysis of oxidative protein modification
To assess the formation of protein carbonyl groups as a measure of oxidative protein modification, the OxyBlot protein oxidation detection kit (Millipore, S7150) was used according to the manufacturer’s detailed protocol. For OxyBlot analysis, 107 cells were washed twice with cold PBS and lysed (150 mM NaCl, 0.1 % Triton X-100, 30 mM Tris, 1 mM PMSF, 10 % glycerol, peptide inhibitors) on ice for 15 min. The cellular debris was removed by centrifugation (10,000×g, 4 °C, 15 min). The supernatant was incubated with 2 μg of the respective antibodies and 40 μl of protein A/protein G agarose mix (Oncogene Research Products, Darmstadt, Germany) on a shaker for 2 h at 4 °C. Bound proteins were collected by centrifugation. The pellet was washed four times in lysis buffer, and subsequently suspended in “carbonyl-moiety to dinitrophenylhydrazone (DNP)-derivative converting solution.” After washing in Tris-buffered saline, the pellets were suspended for electrophoretic separation in Western blot loading buffer. The separated DNP-containing proteins were detected in Western blots (with DNP-specific antibodies) as described (Bernhard et al. 2005).
Statistics
Data are presented as mean ± SD. Statistical analyses were performed using computer software (SPSS 17.0). Variable levels between different treatment groups were compared using ANOVA.
Results
Infection with C. pneumoniae leads to upregulation of hHSP60 and its relocalization to the endothelial cell surface
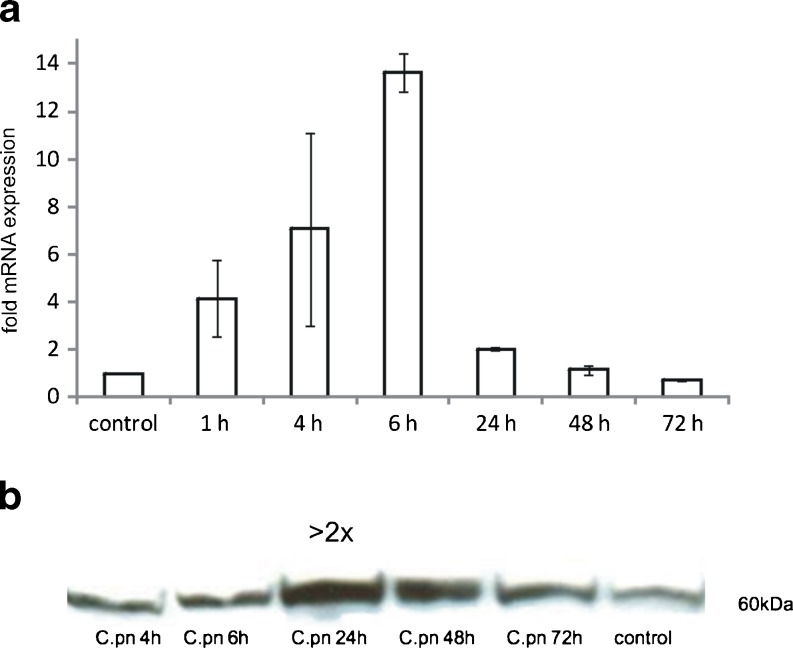
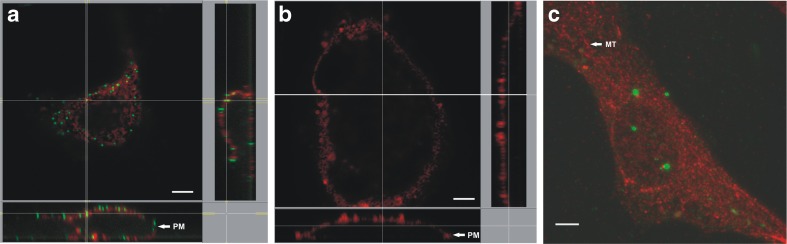
Based on our previous finding of increased hHSP60 expression in vascular endothelial cells in the presence of a diverse range of pro-atherogenic stimuli, we examined the effect of chlamydial infection on expression of hHSP60 in endothelial cells. As shown in Fig. 1a, chlamydial infection of HUVECs led to a significantly increased expression of hHSP60 mRNA with a maximum of expression at 6 h. As determined by Western blot analysis, hHSP60 levels were markedly increased upon infection with C. pneumoniae (Fig. 1b) with a peak of expression at 24 h (more than twofold upregulation). RT-PCR and Western blot analyses showed increased hHsp60 mRNA and protein expression, respectively, in C. pneumoniae-infected HUVECs, but this approach did not allow any conclusions about the subcellular localization of the HSP60 molecules. Therefore, confocal microscopy of primary adult endothelial cells was performed (Fig. 2), clearly showing in AECs (Fig. 2a) redistribution of hHSP60 from the mitochondria to the plasma membrane together with adhesion molecules. Interestingly, neither hHSP60 surface expression nor hHSP60 presence was visible in the autologous VECs (Fig. 2b). As shown in Fig. 2c, surface expression of hHSP60 was accompanied by pronounced accumulation of intracellular chlamydial particles (cHSP60 staining) demonstrating successful infection of endothelial cells in our experimental setting.
Fig. 1.
qRT-PCR analysis of human HSP60 (hHSP60) expression in HUVECs infected with C. pneumoniae. Control: succinate dehydrogenase (SDHA). Mean ± SD, n = 3. Relative change in expression of the target gene mRNA is shown in relation to untreated samples with SDHA serving as an internal control (a). Western blot analysis of hHSP60 expression in HUVECs infected with C. pneumoniae for the given time points. Shown is one representative experiment out of three (b)
Fig. 2.
Confocal microscopy. Human HSP60 (hHsp60) stained at the cell surface of nonpermeabilized cells (a, b). Orthogonal view of a z-stack of donor matched primary endothelial cells (48 h post-C. pneumoniae infection). ICAM (red), hHSP60 (green). Bar = 5 μm. AECs (a) or VECs (b). Chlamydial HSP60 (cHSP60) in C. pneumoniae-infected HUVECs, permeabilized for intracellular staining. Maximum intensity projection of HUVEC (z-stack, 48 h post-C. pneumoniae infection). cHSP60 (green), hHSP60 (red). Bar = 5 μm (c)
Infection with C. pneumoniae leads to increased expression of chemokines and adhesion molecules in endothelial cells
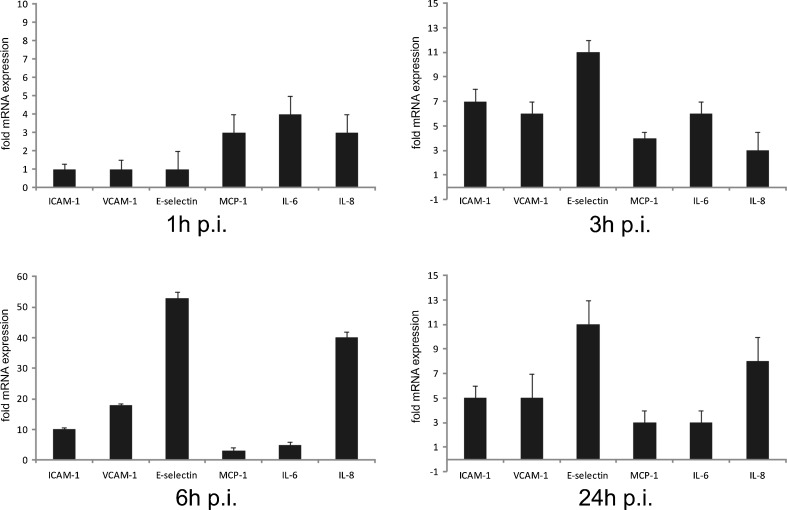
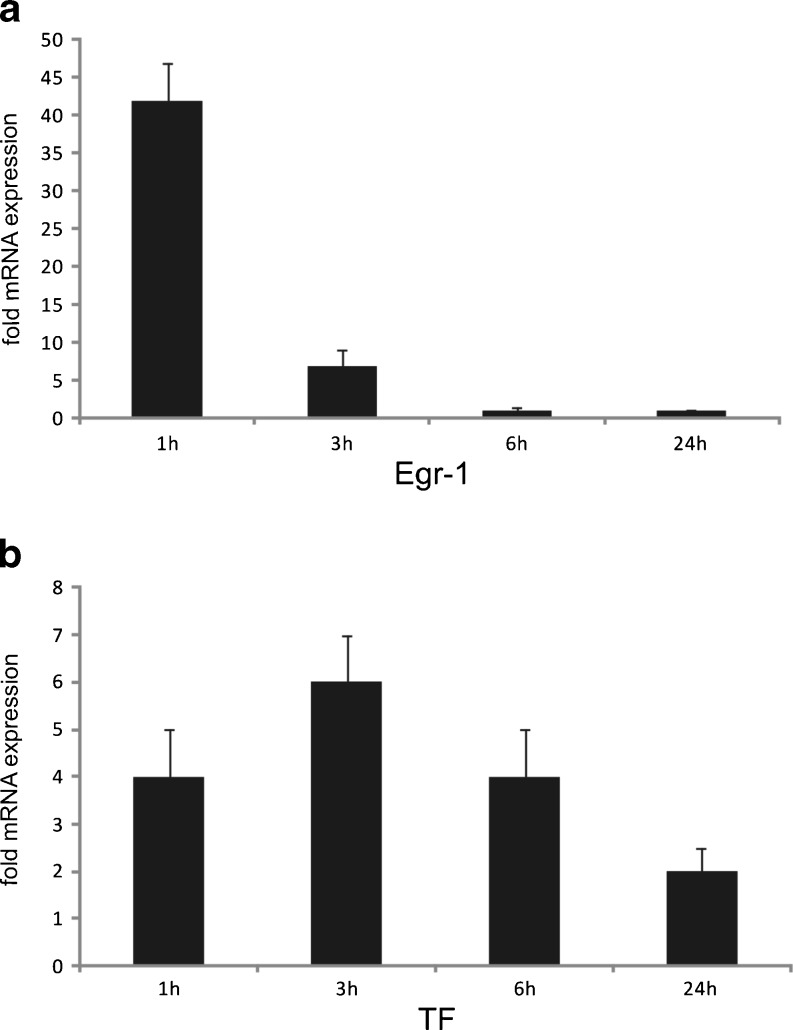
Early atherosclerotic lesions are characterized by the presence of activated T cells and monocyte-derived macrophages. Importantly, lymphocyte and monocyte trafficking is highly dependent on chemoattractant chemokines produced at sites of ongoing inflammation. As outlined in Fig. 3, analysis by RT-PCR revealed pronounced upregulation of MCP-1 and IL-8 in endothelial cells, which are known to be the most potent chemokines for macrophage migration. Moreover, expression of adhesion molecules ICAM-1, VCAM-1, and E-selectin was increased starting at 3 h, with a peak of expression reached at 6 h post-infection. An increased expression of all the investigated adhesion molecules was sustained for up to 24 h post-infection (Fig. 3). Additionally, we found the transcription factor early growth response gene-1 (Egr-1), a key mediator of inflammation and gene expression after vascular injury, to be markedly upregulated at early time points of infection (Fig. 4a).
Fig. 3.
RT-PCR analysis of adhesion molecules and pro-inflammatory cytokines in HUVECs. Cells were subjected to C. pneumoniae infection for the indicated time periods. Mean ± SD, n = 3. Relative change in expression of the target gene mRNA is shown in relation to untreated samples with SDHA serving as an internal control
Fig. 4.
RT-PCR analysis of the early growth response gene-1 (Egr-1) in C. pneumoniae-infected HUVECs. Cells were subjected to C. pneumoniae infection for the indicated time periods. Short but pronounced upregulation of Egr-1 mRNA occurs as an early event at 1 h after infection. Mean ± SD, n = 3 (a). RT-PCR analysis of human tissue factor (TF) in HUVECS infected with C. pneumoniae. Mean ± SD, n = 3 (b). Relative change in expression of the target gene mRNA is shown in relation to untreated samples with SDHA serving as an internal control
Infection with C. pneumoniae leads to a pro-coagulative state in endothelial cells
Besides its role in the gradual process of atherosclerotic lesion formation, chlamydial infection has been suggested to play a role in acute vascular events such as acute coronary syndrome. In the present study, infection with C. pneumoniae led to upregulation of tissue factor (TF) (Fig. 4b). Increased expression of TF would facilitate onset of the external pathway of blood coagulation and, thus, enhance the risk of acute vascular thrombosis.
C. pneumoniae-infected endothelial cells show evidence of increased oxidative stress
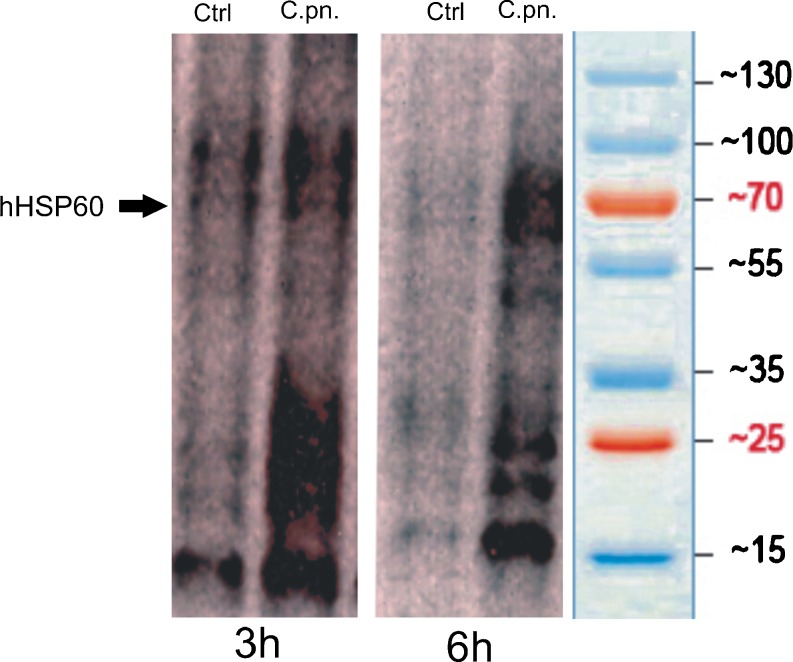
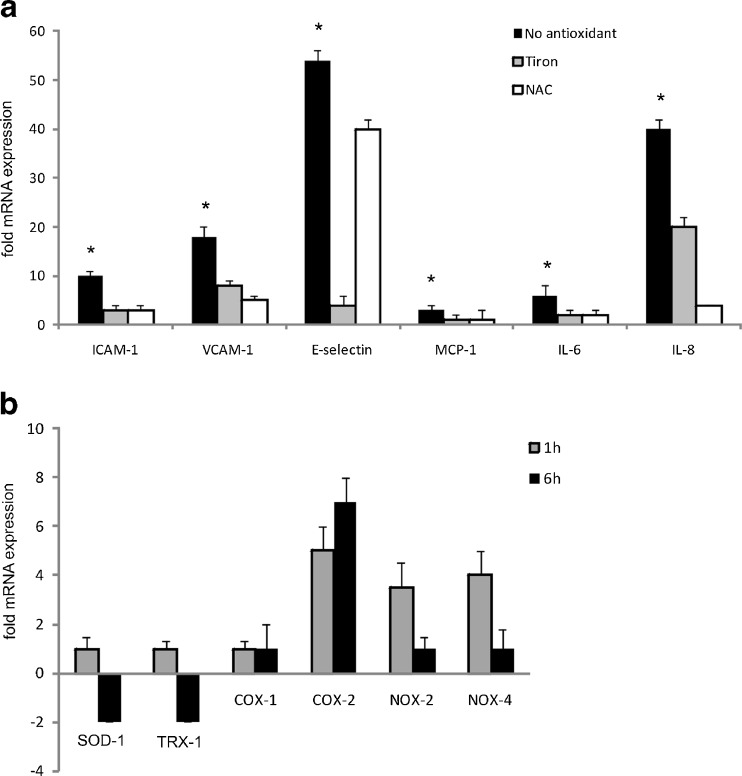
The cellular redox state plays a key role in several cellular fate decisions ranging from regulation of genes and redox-sensitive pathways to triggering cell death mechanisms. To get a deeper insight into the molecular mechanisms involved in the pro-atherosclerotic activities of C. pneumoniae, we examined if the endothelial redox balance was affected by C. pneumoniae infection by measuring the levels of protein carbonyl formation with the OxyBlot-based technique. C. pneumoniae-infected cells showed increased levels of protein carbonyl moieties as compared to noninfected cells (Fig. 5). To determine the pathophysiological significance, if any, of this finding, we treated cells with various antioxidants before infection with C. pneumonia. Figure 6a shows that the presence of both the broad spectrum antioxidant N-acetylcysteine and the superoxide anion scavenger tiron (4,5-dihydroxy-1,3-benzene disulfonic acid) significantly attenuated the inflammatory response of endothelial cells upon C. pneumoniae infection. Taken together, these results strongly suggest that redox-sensitive signaling pathways mediate the upregulation of genes relevant for vascular inflammation.
Fig. 5.
Endothelial cells infected with C. pneumoniae show evidence of increased oxidative stress. OxyBlot analysis of proteins from uninfected control (Ctrl) and C. pneumoniae-infected (C.pn.) HUVECs (3 and 6 h after infection). Representative images of three independent experiments are shown
Fig. 6.
RT-PCR analysis of adhesion molecules and pro-inflammatory cytokines in C. pneumoniae-infected HUVECs in the presence of antioxidants. Cells were pre-incubated either with the superoxide radical scavenger tiron or N-acetylcysteine (NAC) for 30 min and infected with C. pneumoniae for 6 h. Mean ± SD, n = 3. (*P < 0.05) (a). RT-PCR analysis of ROS-producing enzymes in HUVEC subjected to C. pneumoniae infection. HUVECs infected with C. pneumoniae show transient upregulation of the NADPH oxidase isoforms NOX-2 and NOX-4 at 1 h, while upregulation of COX-2 persists at 6 h, when downregulation of TRX-1 as well as SOD-1 is detected. Mean ± SD, n = 3 (b). Relative change in expression of the target gene mRNA is shown in relation to untreated samples with SDHA serving as an internal control
C. pneumoniae infection causes downregulation of thioredoxin and superoxide dismutase and increased expression of NADPH oxidase
Based on the finding of an increased ROS production in C. pneumoniae-infected cells, we investigated whether chlamydial infection has an impact on the antioxidative defense systems of endothelial cells. We found the expression of thioredoxin-1 (TRX-1), a major antioxidative defense system in endothelial cells, and superoxide dismutase-1 (SOD-1) to be strongly downregulated. Concomitantly, the prototypical endothelial NADPH oxidase-4 (NOX-4) and NADPH oxidase-2 (NOX-2), the two major sources of ROS in the vasculature (Lassègue and Griendling 2010), were upregulated, pointing towards a redox imbalance within C. pneumoniae-infected cells. Furthermore, our experiments showed increased expression of the ROS-producing enzyme cyclooxygenase-2 (COX-2) but not of COX-1 upon chlamydial infection pointing towards an additional role of COX-2 in the increased cellular ROS level associated with C. pneumoniae infection (Fig. 6b).
Discussion
The most striking finding in our study was the observation of upregulation and surface expression of hHSP60 in arterial endothelial cells upon chlamydial infection. Of note, AECs proved to be markedly more susceptible to a chlamydia-mediated stress reaction in terms of surface expression of hHSP60 as compared to VECs from the same donors, which might be explained by the lifelong exposure of AECs to arterial blood pressure and flow conditions. This finding places C. pneumoniae among acquired primary stress factors, especially for AECs, and is in agreement with our notion of a T-cell-mediated immunopathology underlying the atherosclerotic process. Expression of hHSP60 on the surface of endothelial cells might represent a major target for pre-existing hHSP60 auto- and cross-reactive immunity (Wick 2000). The nonphysiological expression of such an intracellular antigen on the cell surface of stressed arterial endothelial cells would lead to a vicious cycle of infiltration of the vascular wall by cross-reactive T cells and destruction of stressed endothelial cells (Curry et al. 2000). A study investigating the clonality of such atherosclerotic plaque-derived T cells in cohorts of both anti-C. pneumoniae seropositive and seronegative patients found plaques of seronegative patients to harbor clones of T cells that recognize hHSP60 epitopes, while in seropositive patients, these T cells were found to cross-react with cHSP60 due to molecular mimicry of certain HSP60 epitopes (Benagiano et al. 2005). This observation is in agreement with the finding of our previous cross-sectional studies, namely, that T-cell reactivity to bacterial and human HSP60 is an independent risk factor for incipient atherosclerosis in a cohort of clinically healthy young adults (Knoflach et al. 2007; Rossmann et al. 2008; Knoflach et al. 2009). As HSP60 constitutes a highly conserved molecule, there is significant homology in the protein structure of human and bacterial HSP60 (Young and Elliott 1989). Lifelong reactivation of chlamydial infection in the vasculature (Peters et al. 2005) would lead to a chronic immune reaction against cHSP60 and would significantly increase the risk for immunological cross-reactivity of human chlamydia-specific T cells against stressed AECs. Besides recruitment of cross-reactive T cells, increased IL-8 and MCP-1 production paralleled by expression of adhesion molecules as demonstrated in the present and other studies points to increased trafficking of monocytes into the vascular wall (Krüll et al. 1999; Molestina et al. 1999; Kothe et al. 2000; Hogdahl et al. 2008). Besides being a causative agent of systemic and local inflammation, chlamydial particles appear to be potent inducers of a pro-coagulative state of the physiologically inert vasculature. Our finding of increased endothelial TF expression early after chlamydial infection suggests an increased risk for acute thrombotic events in people subjected to chlamydial infection. An in vivo relevance of this finding is suggested by Hoshida et al. (2005) who found serum levels of anti-chlamydial IgM and hHSP60 antibody levels to be significantly higher in acute coronary syndrome patients as opposed to a clinically stable control cohort.
In the present study, several major pro-atherogenic changes observed upon chlamydial infection in HUVECs were found to depend on increased production of ROS, since application of antioxidants markedly ameliorated the endothelial stress response in the presence of C. pneumonia. Transcriptional upregulation of COX-2 as well as NOX-2 and NOX-4 paralleled by downregulation of SOD-1 and TRX-1 demonstrates a shift toward a more oxidative cellular redox state. COX-2 is involved in various inflammatory processes including atherosclerosis, and its inhibition was shown to significantly ameliorate C. pneumoniae-mediated effects on vascular remodeling (Rupp et al. 2004). Thus, chlamydial infection causes a remarkable redox imbalance in the host cell, with a diminished antioxidative defense on the one hand and critical enhancement of ROS-levels with upregulated NOX-2 and NOX-4 genes on the other.
In a recent study, it has been shown that LPS induces a superoxide-dependent expression of inflammatory cytokines and prostaglandins via upregulation of NADPH oxidase in a rat model of hypertension (Zhang et al. 2010). Besides LPS-mediated signaling events, cHSP60 has been found to directly cause endothelial dysfunction by downregulation of endothelial nitric oxide synthase and associated mitochondrial dysfunction in human coronary artery endothelial cells, and application of antioxidants was shown to abrogate HSP60-mediated endothelial dysfunction (Chen et al. 2009). Our finding of an increased oxidative state is in agreement with previous studies that could demonstrate increased levels of ROS in smooth muscle cells subsequent to infection with C. pneumoniae that seemed to depend on a functional NADPH oxidase activity (Dechend et al. 2003). A contributory role of superoxide anion in this process is suggested by our finding of downregulation of SOD-1 (Fig. 6b) as an early event after C. pneumoniae infection and by the markedly reduced endothelial stress response to C. pneumoniae infection in the presence of the superoxide radical scavenger tiron (Fig. 6a). This finding suggests that antioxidant therapy might hold promise as a preventive measure against atherogenesis in the presence of risk factors such as infection with C. pneumoniae.
In summary, the finding of a markedly increased susceptibility of arterial endothelial cells as compared to venous endothelial cells from the same donor for redistribution and enrichment of hHSP60 on the cellular surface further lends support to our hypothesis of atherosclerosis being the result of immunological cross-reactivity against eukaryotic hHSP60 under stressful conditions. Our data extend the list of established stress factors for endothelial cells that all converge upon the immunological danger signal hHSP60 being exposed on the cell surface. Compared to the data of previous studies on the degree of endothelial cell stressing capacity of canonical risk factors of atherosclerosis as reflected by the abundant expression of hHSP60, C. pneumoniae emerged as the most potent endothelial cell stressor (Amberger et al. 1997; Henderson et al. 2008; Wick et al. 2008; Grundtman et al. 2011). The cross-recognition of shared epitopes between human and chlamydial HSP60 by autoreactive cellular and humoral immunity might represent an as yet unappreciated mechanism underlying the atherogenic potential of recurrent infection or reactivation of chlamydial particles in patients who fail to clear the infection. We believe that further in vivo studies are warranted to delineate the association of persistent chlamydial infection with markers of systemic inflammation in order to design novel therapeutic approaches aimed at counteracting endothelial cell activation and associated vascular autoimmune pathogenic mechanisms.
Acknowledgments
We thank Rajam Csordas-Iyer for critical reading and editorial assistance. This work was supported by the European Initiative to Fight Chlamydial Infections by Unbiased Genomics (ECIBUG; # 818496 to GW), the Austrian Research Fund (FWF grant # 14741 to GW), and the TOLERAGE Health Research Grant (HEALTH-F4-2008-202156 to GW).
Footnotes
Simone Kreutmayer and Adam Csordas contributed equally to this work.
References
- Amberger A, Maczek C, Jürgens G, Michaelis D, Schett G, Trieb K, Eberl T, Jindal S, Xu Q, Wick G. Co-expression of ICAM-1, VCAM-1, ELAM-1 and Hsp60 in human arterial and venous endothelial cells in response to cytokines and oxidized low-density lipoproteins. Cell Stress Chaperones. 1997;2:94–103. doi: 10.1379/1466-1268(1997)002<0094:CEOIVE>2.3.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benagiano M, D'Elios MM, Amedei A, Azzurri A, van der Zee R, Ciervo A, Rombolà G, Romagnani S, Cassone A, Prete D. Human 60-kDa heat shock protein is a target autoantigen of T cells derived from atherosclerotic plaques. J Immunol. 2005;174:6509–6517. doi: 10.4049/jimmunol.174.10.6509. [DOI] [PubMed] [Google Scholar]
- Bernhard D, Csordas A, Henderson B, Rossmann A, Kind M, Wick G. Cigarette smoke metal-catalyzed protein oxidation leads to vascular endothelial cell contraction by depolymerization of microtubules. FASEB J. 2005;19:1096–1107. doi: 10.1096/fj.04-3192com. [DOI] [PubMed] [Google Scholar]
- Chen C, Chai H, Wang X, Lin PH, Yao Q. Chlamydia heat shock protein 60 decreases expression of endothelial nitric oxide synthase in human and porcine coronary artery endothelial cells. Cardiovasc Res. 2009;83:768–777. doi: 10.1093/cvr/cvp150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curry AJ, Portig I, Goodall JC, Kirkpatrick PJ, Gaston JS. T lymphocyte lines isolated from atheromatous plaque contain cells capable of responding to Chlamydia antigens. Clin Exp Immunol. 2000;121:261–269. doi: 10.1046/j.1365-2249.2000.01294.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dechend R, Gieffers J, Dietz R, Joerres A, Rupp J, Luft FC, Maas M. Hydroxymethylglutaryl coenzyme A reductase inhibition reduces Chlamydia pneumoniae-induced cell interaction and activation. Circulation. 2003;108:261–265. doi: 10.1161/01.CIR.0000083367.93022.78. [DOI] [PubMed] [Google Scholar]
- George J, Shoenfeld Y, Afek A, Gilburd B, Keren P, Shaish A, Kopolovic J, Wick G, Harats D. Enhanced fatty streak formation in C57BL/6J mice by immunization with heat shock protein-65. Arterioscler Thromb Vasc Biol. 1999;19:505–510. doi: 10.1161/01.ATV.19.3.505. [DOI] [PubMed] [Google Scholar]
- Gieffers J, Füllgraf H, Jahn J, Klinger M, Dalhoff K, Katus HA, Solbach W, Maas M. Chlamydia pneumoniae infection in circulating human monocytes is refractory to antibiotic treatment. Circulation. 2001;103:351–356. doi: 10.1161/01.CIR.103.3.351. [DOI] [PubMed] [Google Scholar]
- Grayston JT. Background and current knowledge of Chlamydia pneumoniae and atherosclerosis. J Infect Dis. 2000;181(Suppl 3):S402–S410. doi: 10.1086/315596. [DOI] [PubMed] [Google Scholar]
- Grundtman C, Kreutmayer SB, Almanzar G, Wick MC, Wick G. Heat shock protein 60 and immune inflammatory responses in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:960–968. doi: 10.1161/ATVBAHA.110.217877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundtman C, Wick G. The autoimmune concept of atherosclerosis. Curr Opin Lipidol. 2011;22:327–334. doi: 10.1097/MOL.0b013e32834aa0c2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson GK. Immune mechanisms in atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:1876–1890. doi: 10.1161/hq1201.100220. [DOI] [PubMed] [Google Scholar]
- Henderson B, Csordas A, Backovic A, Kind M, Bernhard D, Wick G. Cigarette smoke is an endothelial stressor and leads to cell cycle arrest. Atherosclerosis. 2008;201:298–305. doi: 10.1016/j.atherosclerosis.2008.02.022. [DOI] [PubMed] [Google Scholar]
- Hogdahl M, Soderlund G, Kihlstrom E. Expression of chemokines and adhesion molecules in human coronary artery endothelial cells infected with Chlamydia (Chlamydophila) pneumoniae. APMIS. 2008;116:1082–1088. doi: 10.1111/j.1600-0463.2008.01145.x. [DOI] [PubMed] [Google Scholar]
- Hoshida S, Nishino M, Tanouchi J, Kishimoto T, Yamada Y. Acute Chlamydia pneumoniae infection with heat-shock-protein-60-related response in patients with acute coronary syndrome. Atherosclerosis. 2005;183:109–112. doi: 10.1016/j.atherosclerosis.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Jackson LA, Campbell LA, Kuo CC, Rodriguez DI, Lee A, Grayston JT. Isolation of Chlamydia pneumoniae from a carotid endarterectomy specimen. J Infect Dis. 1997;176:292–295. doi: 10.1086/517270. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Mayrl B, Kind M, Gaston JS, van der Zee R, Faggionato A, Mayr A, Willeit J, Wick G. T-cell reactivity against HSP60 relates to early but not advanced atherosclerosis. Atherosclerosis. 2007;195:333–338. doi: 10.1016/j.atherosclerosis.2006.09.021. [DOI] [PubMed] [Google Scholar]
- Knoflach M, Kiechl S, Penz D, Zangerle A, Schmidauer C, Rossmann A, Shingh M, Spallek R, Griesmacher A, Bernhard D, Robacher P, Buchberger W, Draxl W, Willeit J, Wick G. Cardiovascular risk factors and atherosclerosis in young women: atherosclerosis risk factors in female youngsters (ARFY study) Stroke. 2009;40:1063–1069. doi: 10.1161/STROKEAHA.108.525675. [DOI] [PubMed] [Google Scholar]
- Kothe H, Dalhoff K, Rupp J, Müller A, Kreuzer J, Maass M, Katus HA. Hydroxymethylglutaryl coenzyme A reductase inhibitors modify the inflammatory response of human macrophages and endothelial cells infected with Chlamydia pneumoniae. Circulation. 2000;101:1760–1763. doi: 10.1161/01.CIR.101.15.1760. [DOI] [PubMed] [Google Scholar]
- Kreutmayer SB, Messner B, Knoflach M, Henderson B, Niederegger H, Böck G, Van der Zee R, Wick G, Bernhard D. Dynamics of heat shock protein 60 in endothelial cells exposed to cigarette smoke extract. J Mol Cell Cardiol. 2011;51:777–780. doi: 10.1016/j.yjmcc.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krüll M, Klucken AC, Wuppermann FN, Fuhrmann O, Magerl C, Seybold J, Hippenstiel S, Hagemann JH, Jantos CA, Suttorp N. Signal transduction pathways activated in endothelial cells following infection with Chlamydia pneumoniae. J Immunol. 1999;162:4834–4841. [PubMed] [Google Scholar]
- Lassègue B, Griendling KK. NADPH oxidases: functions and pathologies in the vasculature. Arterioscler Thromb Vasc Biol. 2010;30:653–661. doi: 10.1161/ATVBAHA.108.181610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinska A, Wnuk M, Slota E, Bartosz G. Total anti-oxidant capacity of cell culture media. Clin Exp Pharmacol Physiol. 2007;34:781–786. doi: 10.1111/j.1440-1681.2007.04637.x. [DOI] [PubMed] [Google Scholar]
- Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- Mayr M, Metzler B, Kiechl S, Willeit J, Schett G, Xu Q, Wick G. Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis. Circulation. 1999;99:1560–1566. doi: 10.1161/01.CIR.99.12.1560. [DOI] [PubMed] [Google Scholar]
- Mayr M, Xu Q, Wick G. Atherogenic effects of chronic infections: the role of heat shock protein 60 in autoimmunity. Isr Med Assoc J. 1999;1:272–277. [PubMed] [Google Scholar]
- Molestina RE, Miller RD, Ramirez JA, Summersgill JT. Infection of human endothelial cells with Chlamydia pneumoniae stimulates transendothelial migration of neutrophils and monocytes. Infect Immun. 1999;67:1323–1330. doi: 10.1128/iai.67.3.1323-1330.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, Hess S, Endlich K, Thalmann J, Holzberg D, Kracht M, Schaefer M, Bartling G, Klos A. Silencing or permanent activation: host-cell responses in models of persistent Chlamydia pneumoniae infection. Cell Microbiol. 2005;7:1099–1108. doi: 10.1111/j.1462-5822.2005.00534.x. [DOI] [PubMed] [Google Scholar]
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Rossmann A, Henderson B, Heidecker B, Seiler R, Fraedrich G, Singh M, Parson W, Keller M, Grubeck-Loebenstein B, Wick G. T-cells from advanced atherosclerotic lesions recognize hHSP60 and have a restricted T-cell receptor repertoire. Exp Gerontol. 2008;43:229–237. doi: 10.1016/j.exger.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Rupp J, Berger M, Reiling N, Gieffers J, Lindschau C, Haller H, Dalhoff K, Maass M. Cox-2 inhibition abrogates Chlamydia pneumoniae-induced PGE2 and MMP-1 expression. Biochem Biophys Res Commun. 2004;320:738–744. doi: 10.1016/j.bbrc.2004.05.210. [DOI] [PubMed] [Google Scholar]
- Saikku P, Leinonen M, Mattila K, Ekman MR, Nieminen MS, Mäkelä PH, Huttunen JK, Valtonen V. Serological evidence of an association of a novel Chlamydia, TWAR, with chronic coronary heart disease and acute myocardial infarction. Lancet. 1988;2:983–986. doi: 10.1016/S0140-6736(88)90741-6. [DOI] [PubMed] [Google Scholar]
- Shi Y, Tokunaga O. Chlamydia pneumoniae (C. pneumoniae) infection upregulates atherosclerosis-related gene expression in human umbilical vein endothelial cells (HUVECs) Atherosclerosis. 2004;177:245–253. doi: 10.1016/j.atherosclerosis.2004.07.014. [DOI] [PubMed] [Google Scholar]
- Singh B, Gupta RS (1992) Expression of human 60-kD heat shockprotein (HSP60 or P1) in Escherichia coli and the development and characterization of corresponding monoclonal antibodies. DNA Cell Biol 11:489–496 [DOI] [PubMed]
- Wick G, Knoflach M, Xu Q. Autoimmune and inflammatory mechanisms in atherosclerosis. Annu Rev Immunol. 2004;22:361–403. doi: 10.1146/annurev.immunol.22.012703.104644. [DOI] [PubMed] [Google Scholar]
- Wick G, Perschinka H, Millonig G. Atherosclerosis as an autoimmune disease: an update. Trends Immunol. 2001;22:665–669. doi: 10.1016/S1471-4906(01)02089-0. [DOI] [PubMed] [Google Scholar]
- Wick G. Atherosclerosis—an autoimmune disease due to an immune reaction against heat-shock protein 60. Herz. 2000;25:87–90. doi: 10.1007/PL00001957. [DOI] [PubMed] [Google Scholar]
- Wick MC, Mayerl C, Backovic A, van der Zee R, Jaschke W, Dietrich H, Wick G. In vivo imaging of the effect of LPS on arterial endothelial cells: molecular imaging of heat shock protein 60 expression. Cell Stress Chaperones. 2008;13:275–285. doi: 10.1007/s12192-008-0044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Dietrich H, Steiner HJ, Gown AM, Schoel B, Mikuz G, Kaufmann SH, Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb Vasc Biol. 1992;12:789–799. doi: 10.1161/01.ATV.12.7.789. [DOI] [PubMed] [Google Scholar]
- Xu Q, Kiechl S, Mayr M, Metzler B, Egger G, Oberhollenzer F, Willeit J, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis: clinical significance determined in a follow-up study. Circulation. 1999;100:1169–1174. doi: 10.1161/01.CIR.100.11.1169. [DOI] [PubMed] [Google Scholar]
- Xu Q, Luef G, Weimann S, Gupta RS, Wolf H, Wick G. Staining of endothelial cells and macrophages in atherosclerotic lesions with human heat-shock protein-reactive antisera. Arterioscler Thromb Vasc Biol. 1993;13:1763–1769. doi: 10.1161/01.ATV.13.12.1763. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Perschinka H, Mayr M, Egger G, Oberhollenzer F, Willeit J, Kiechl S, Wick G. Serum soluble heat shock protein 60 is elevated in subjects with atherosclerosis in a general population. Circulation. 2000;102:14–20. doi: 10.1161/01.CIR.102.1.14. [DOI] [PubMed] [Google Scholar]
- Xu Q, Schett G, Seitz CS, Hu Y, Gupta RS, Wick G. Surface staining and cytotoxic activity of heat-shock protein 60 antibody in stressed aortic endothelial cells. Circ Res. 1994;75:1078–1085. doi: 10.1161/01.RES.75.6.1078. [DOI] [PubMed] [Google Scholar]
- Xu Q, Willeit J, Marosi M, Kleindienst R, Oberhollenzer F, Kiechl S, Stulnig T, Luef G, Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993;341:255–259. doi: 10.1016/0140-6736(93)92613-X. [DOI] [PubMed] [Google Scholar]
- Yamashita K, Ouchi K, Shirai M, Gondo T, Nakazawa T, Ito H. Distribution of Chlamydia pneumoniae infection in the athersclerotic carotid artery. Stroke. 1998;29:773–778. doi: 10.1161/01.STR.29.4.773. [DOI] [PubMed] [Google Scholar]
- Young RA, Elliott TJ. Stress proteins, infection, and immune surveillance. Cell. 1989;59:5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Yu Y, Wei SG, Felder RB. Centrally administered lipopolysaccharide elicits sympathetic excitation via NAD(P)H oxidase-dependent mitogen-activated protein kinase signaling. J Hypertens. 2010;28:806–816. doi: 10.1097/HJH.0b013e3283358b6e. [DOI] [PMC free article] [PubMed] [Google Scholar]