Abstract
Epithelial cells and fibroblasts both express heat shock transcription factors, HSF1 and HSF4, yet they respond to heat shock differentially. For example, while HSP70 is induced in both cell types, the small heat shock protein, αB-crystallin gene (CRYAB) that contains a canonical heat shock promoter, is only induced in fibroblasts. A canonical heat shock promoter contains three or more inverted repeats of the pentanucleotide 5′-nGAAn-3′ that make the heat shock element. It is known that, in vitro, promoter architecture (the order and spacing of these repeats) impacts the interaction of various heat shock transcription factors (HSFs) with the heat shock promoter, but in vivo relevance of these binding preferences so far as the expression is concerned is poorly understood. In this report, we first establish cell-type-dependent differential expression of CRYAB in four established cell lines and then working with adult human retinal pigment epithelial cells and NIH3T3 fibroblasts and employing chromatin immunoprecipitation, attempt to relate expression to promoter occupancy by HSF1 and HSF4. We show that HSF4 occupies only CRYAB and not HSP70 promoter in epithelial cells, while HSF1 occupies only HSP70 promoter in both cell types, and cryab promoter, only in heat shocked fibroblasts; HSF4, on the other hand, is never seen on these two promoters in NIH3T3 fibroblasts. This comparative analysis with CRYAB and HSP70 demonstrates that differential heat shock response is controlled by cell-type-dependent access of HSFs (HSF1 and HSF4) to specific promoters, independent of the promoter architecture.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-012-0386-7) contains supplementary material, which is available to authorized users.
Keywords: Heat shock promoter, HSF1, HSF4, Cell-type specific, HSP70, αB-crystallin
Introduction
Although the dogma of the heat shock response (Morimoto 1993) is considered universal, there is a large lacuna in our understanding of its regulation in various cell types and tissues (Bienz 1984; Morimoto and Fodor 1984; Murray et al. 2004). In eukaryotes, heat shock response manifests in the differential activation of heat shock genes. This is exemplified by the differential expression of cryab, a small heat shock protein gene, which is expressed in multiple tissues in a developmentally controlled fashion as well as in a large number of neurodegenerations (Andley 2007; Bhat 2003; Horwitz 2000). Cryab contains a canonical heat shock promoter that has been shown to be activated by heat shock (Klemenz et al. 1991) and osmotic stress (Dasgupta et al. 1992). It is constitutively expressed in adult human retinal pigment epithelial (ARPE19) cells (Gangalum et al. 2011). As part of our investigations on the transcriptional regulation of this gene, we noted that when ARPE19 cells are heat shocked, αB-crystallin (αB) is not induced, an observation that goes against previously reported induction of cryab in the mouse fibroblast cell line, NIH3T3 (Klemenz et al. 1991). It is also known that cryab is not induced in ocular lenses subjected to heat shock under conditions where heat shock protein hsp70 is induced (Collier and Schlesinger 1986; de Jong et al. 1986). Similar findings have been reported for the malignant human epithelial cell line HEp2, which when exposed to a heat shock shows HSP70 but not CRYAB induction (Laramie et al. 2008). We have previously reported that αB is a protein of kidney epithelial cell lines and not kidney fibroblasts (Nagineni and Bhat 1989).
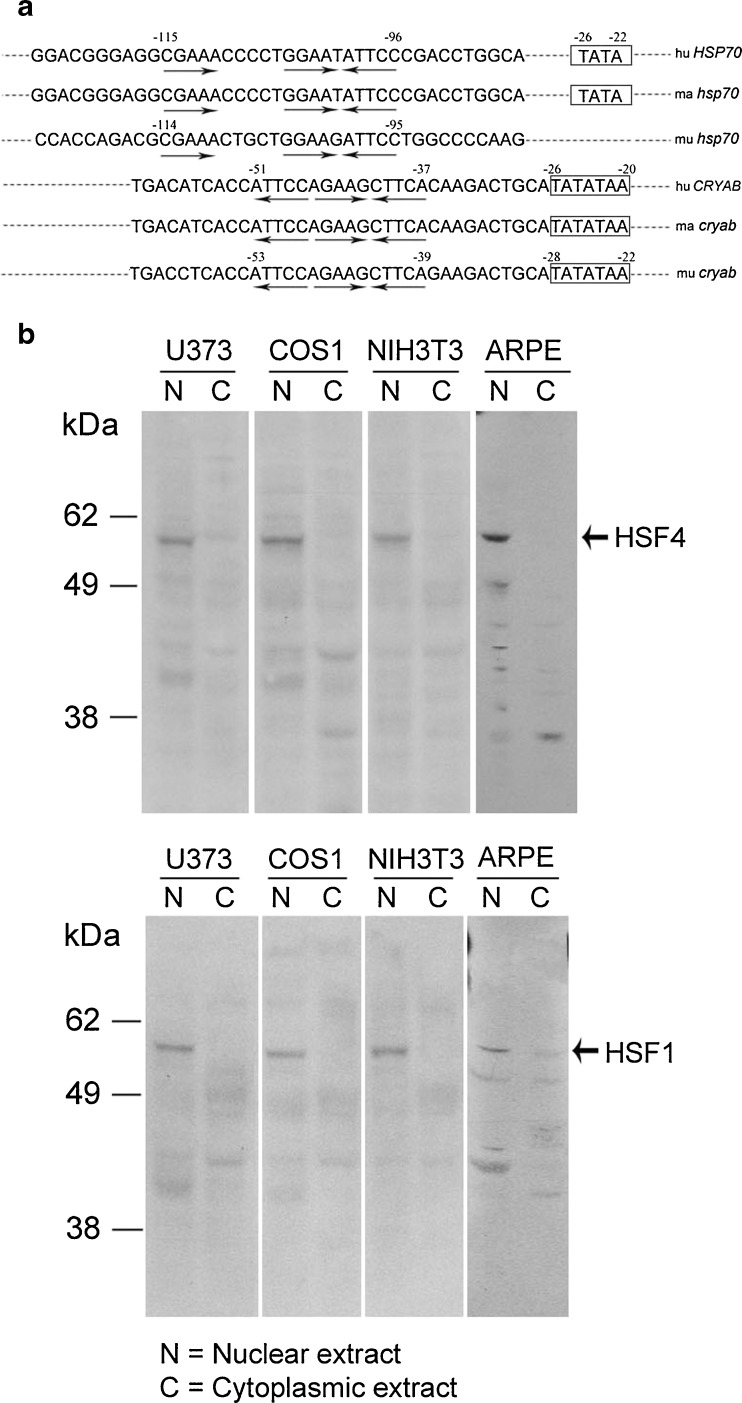
The molecular basis of how two canonical promoters (HSP70 and CRYAB) respond to heat shock differentially within the same cell remains to be understood. In vitro studies have suggested that promoter architecture has an influence on heat shock transcription factor (HSF)/heat shock element (HSE) interactions (Yamamoto et al. 2009). HSP70 and CRYAB promoters present excellent examples of canonical heat shock promoters with variations in their promoter architecture; they contain slightly different versions of the arrangement of the 5′-nGAAn-3′ motifs in their respective HSEs (Fig. 1 a). The promoter of HSP70 contains “discontinuous” 5′-nGAAn-3′ motifs, while the CRYAB promoter has a “continuous,” uninterrupted arrangement of 5′-nGAAn-3′ motifs in its HSE (Fig. 1a). In vitro, HSF1 and HSF4 have been shown to interact differentially with “continuous” and “discontinuous” 5′-nGAAn-3′ motif-containing HSEs (Yamamoto et al. 2009). Thus, the efficiency of binding of a specific HSF with a specific version of the canonical HSE may determine the activation of a particular heat shock gene. Alternatively, the selectivity of HSF/HSE interaction may be developmentally predisposed (cell-type dependent) and independent of the promoter architecture.
Fig. 1.
a Heat shock promoter sequences of HSP70 and cryab genes in various species corresponding to the cell lines used in this study. The HSP70 genes show discontinuous arrangement of inverted 5′-nGAAn-3′ motifs (arrows) in the heat shock element (upper three sequences) (Morgan et al. 1987; Wu et al. 1986), while cryab promoters (bottom three sequences) show a continuous arrangement of these motifs. The numbering is given 5′ upstream of the transcription start site (TSS) (+1) were known. Rat sequence, not shown here, is similar to the human sequence except for one base at −51, which is G in the rat (Somasundaram and Bhat 2000). hu Homo sapiens (human) (Dubin et al. 1990); ma Macaca mulatta (monkey); mu Mus musculus (mouse) (Gopal-Srivastava et al. 1996). Macaca mulatta sequences are not numbered for lack of information about TSS; ma cryab (accession number XM-002799762.1 ) and ma hsp70 (accession number AC148662.1) sequences were obtained from NCBI databases. b Immunoblots showing presence of HSF4 (upper panel) and HSF1 (lower panel) in various cell lines. Antibodies used and the immunoblotting have been previously described (Somasundaram and Bhat 2004). Note that both HSF4 as well as HSF1 are predominantly detected in the nucleus. N nucleus, C cytoplasm
In view of these possibilities, we sought an experimental paradigm that would address both the cell type specificity as well as the possible role of promoter architecture in the heat shock response of a cell. We choose (a) to investigate representative cell lines of known epithelial and fibroblastic lineage to establish cell-type specific expression patterns of HSP70 and αB in response to a heat shock and (b) relate that expression pattern to promoter interactions of the two transcription factors, HSF1 and HSF4, which are known to regulate heat shock genes in response to heat shock and developmental cues, respectively (Akerfelt et al. 2007; Somasundaram and Bhat 2004).
Materials and methods
Construction of recombinant molecules
Rat αB-crystallin complementary DNA (cDNA; Bhat et al. 1991) was cloned into the NotI site (underlined) of pTurbo GFP-pRL vector (Axxora LLC., San Diego, CA, USA) by PCR using primers: forward (F), 5′-AATAAAGCGGCCGCGAGACATAGCCATCCACCACCCCT-3′; reverse (R), 5′-AATAAAGCGGCCGCCTACTTCTTAGGGGCTGCAGTGA-3′), 3′ of the turboGFP (GFP) (the underlined indicates NotI sites). This recombinant clone was further modified to validate the appropriate reading frames (oligonucleotides used for these modifications: sense, 5′-ATGCAGATGCCGGTGAAGAAA AGCGGCCGCGAGACATAGCC-3′ and antisense 5′-GGCTATGTCTCGCGGCCGCTTTTCTTCACCGGCATCTGCAT-3′). These manipulations were done using the Quick Change site-directed mutagenesis kit (Agilent, Santa Clara, CA, USA).
Next, rat cryab promoter region (−896/+44) (Srinivasan and Bhat 1994) was subcloned upstream of the hybrid GFPαB coding sequence into EcoRI–BamHI sites (underlined) of above modified construct (F, 5′-ATCTAAGAATTCACACCACCCAAAATAGTGCAGAGC-3′ and R, 5′-ATCTAAGGATCCGATGGCTAGATGAGTGTAGAGTCG- 3′). All constructions were verified by sequencing.
Transfection and generation of stable cell lines expressing hybrid GFPαB
All cell lines were purchased from ATCC (Manassas, VA, USA). ARPE19 cell line was cultured in DMEM/F12 medium supplemented with 10 % fetal bovine serum (FBS) and sodium bicarbonate. Human glioblastoma-astrocytoma (U373 MG) cell line was maintained in MEM medium supplemented with 10 % FBS. Monkey kidney fibroblast (COS1) cell line was cultured in DMEM medium containing 10 % FBS. Mouse embryo fibroblast (NIH3T3) cell line was grown in DMEM supplemented with 10 % fetal calf serum. The cultures were maintained, humidified in 5 % CO2 at 50–80 % confluence, at 37 °C. All culture media contained 100 U/ml penicillin and 0.1 mg/ml streptomycin (Invitrogen, Carlsbad). Cells were transfected using Lipofectamin 2000 (Invitrogen) followed by selection with 1 mg/ml G418 (Invitrogen).
The copy number of GFPαB plasmids integrated into these cell lines was examined by quantitative real-time PCR of genomic DNAs from transfected cells using a GFPαB plasmid DNA as the standard. The copy number of GFPαB copies per cell line varies from 17 to 24/cell (ARPE = 22, NIH3T3 = 20, COS1 = 24, and U373 = 17).
Heat shock and immunoblotting
All the stably transfected cell lines were cultured without antibiotics and G418 for 24 h before heat shock. The culture dishes with cells were sealed with Parafilm (Pechiney, Chicago) and incubated in a 43 °C water bath for 1 hr. After heat shock, the cells were transferred immediately to the humidified CO2 incubator at 37 °C, and collected at various time points (0, 4, 8, 12, 16, and 24 h) for isolation of total protein and RNA.
Cell lysates were prepared in the T-PER reagent (Pierce, Rockford, IL, USA) containing protease inhibitor cocktail (Sigma-Aldrich Co. LLC), electrophoresed (30 μg /sample) and immunoblotted. The immunoreactive bands were quantified using Odyssey Dual wavelength IR system (LiCOR Biosciences, Lincoln, NE, USA). Primary antibodies, mouse monoclonal anti-actin, rabbit polyclonal anti-HSP70 (Santa Cruz Biotechnologies) and rabbit anti-αB (Gangalum et al. 2004) were used with secondary antibodies tagged with IR-dyes 680 (anti-rabbit) and 800 (anti-mouse) (LiCOR Biosciences). Anti-HSF1 and anti-HSF4 were used as described (Somasundaram and Bhat 2004). Anti-αB detects the endogenous αB (∼20 kDa) as well as the hybrid GFPαB (∼47 kDa) (see Figs. 2a, b and 4a, b).
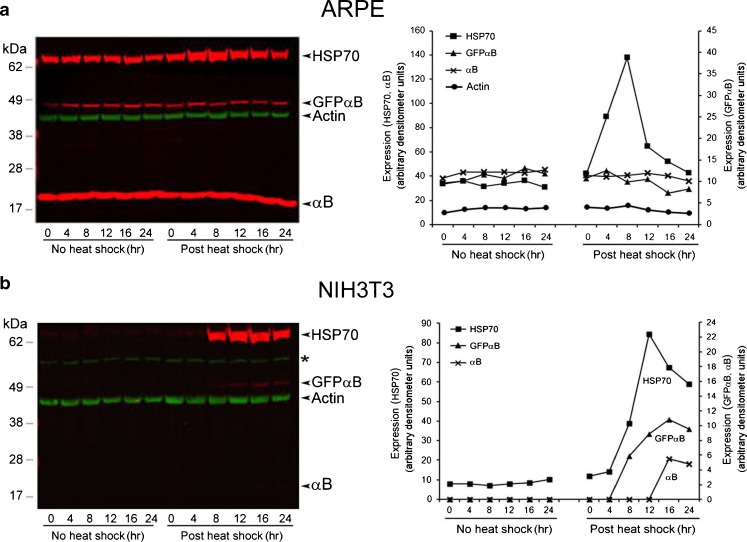
Fig. 2.
Differential expression of Hsp70 and αB in ARPE-GFPαB and NIH3T3-GFPαB cells. a Expression of Hsp70 and αB was followed post-heat shock by immunoblotting. The left panel shows the immunoblots, and the right panel shows plots of densitometry quantitation of immune reactions (see “Materials and methods”). Bands with similar intensities were plotted together using either the left or the right y-axis (arbitrary densitometer units). The green bands in the immunoblots show actin as an internal control for loading (only shown in the densitometry plots in a). In ARPE-GFPαB cells, only endogenous HSP70 is increased. b The endogenous HSP70, endogenous αB, and recombinant GFPαB are induced in NIH3T3-GFPαB cells. Protein standards (kDa) are shown on the left, and the identity of each reactive band is shown on the right of each immunoblot. Asterisk shown in immunoblot (b) indicates nonspecific band reacting with anti-actin. These experiments were repeated three times. Shown above are the data obtained from a typical experiment
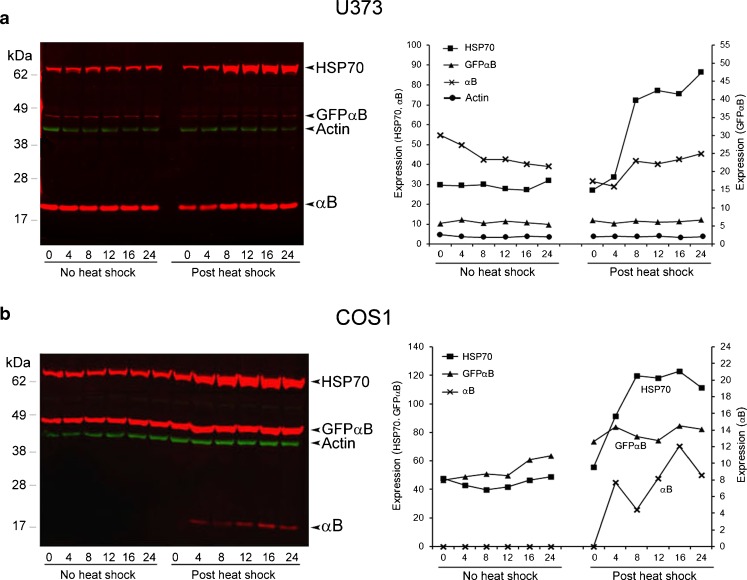
Fig. 4.
Heat shock induces αB in COS1 cells (fibroblasts) and not in U373MG cells (epithelial). (a, b) U373-GFPαB and COS1-GFPαB cells when exposed to a heat shock (43 °C for 1 h) show expression pattern similar to those seen in Fig. 2a and b. The left panel shows the immunoblots, and the right panel shows plots of densitometry quantitation of immune reactions. Bands with similar intensities were plotted together using either the left or the right y-axis. The green bands in the immunoblots show actin as an internal control for loading (only shown in the densitometry plots in a). Only HSP70 is induced in U373MG cells (a). In comparison all the three proteins, HSP70, endogenous αB, and the hybrid GFPαB, are induced in COS1 cells (b), corroborating the data obtained with ARPE-GFPαB and NIH3T3-GFPαB cells (Fig. 2a, b). These experiments were repeated three times. Shown above are the data obtained from a typical experiment
Reverse transcription qPCR
Total RNA was extracted with PureLink RNA mini kit (Invitrogen) and treated with DNAse I (Amplification grade, Invitrogen) to remove the DNA contamination. One microgram of this DNA-free RNA was reverse transcribed in a 20-μl reaction with Superscript II RT (Invitrogen) according to manufacturer’s instruction. One microliter of this cDNA was used in a 10-μl real-time quantitative PCR reaction in triplicate with SYBR Green Master Mix (Roche) employing the Light Cycler 480 (Roche) with thermal cycling conditions as follows: denature at 95 °C 5 min, followed by 45 cycles of 15 s at 95 °C, 20 s at 56 °C, and 30 s at 72 °C. At the end, the reaction tubes were incubated at 37 °C for 10 min. PCR reactions were normalized with reference to an internal control, Actin, which was determined to be the most consistent within an arbitrary range of two cycles (see Supplemental data Fig. 1).To calculate the relative change of expression, the 2- ΔΔCT method (Livak and Schmittgen 2001) was used. Primers for discriminating between recombinant GFPαB expression and endogenous αB transcripts were designed such that there was no cross-interference (see Fig. 3). All primers sequences are listed in the supplemental data.
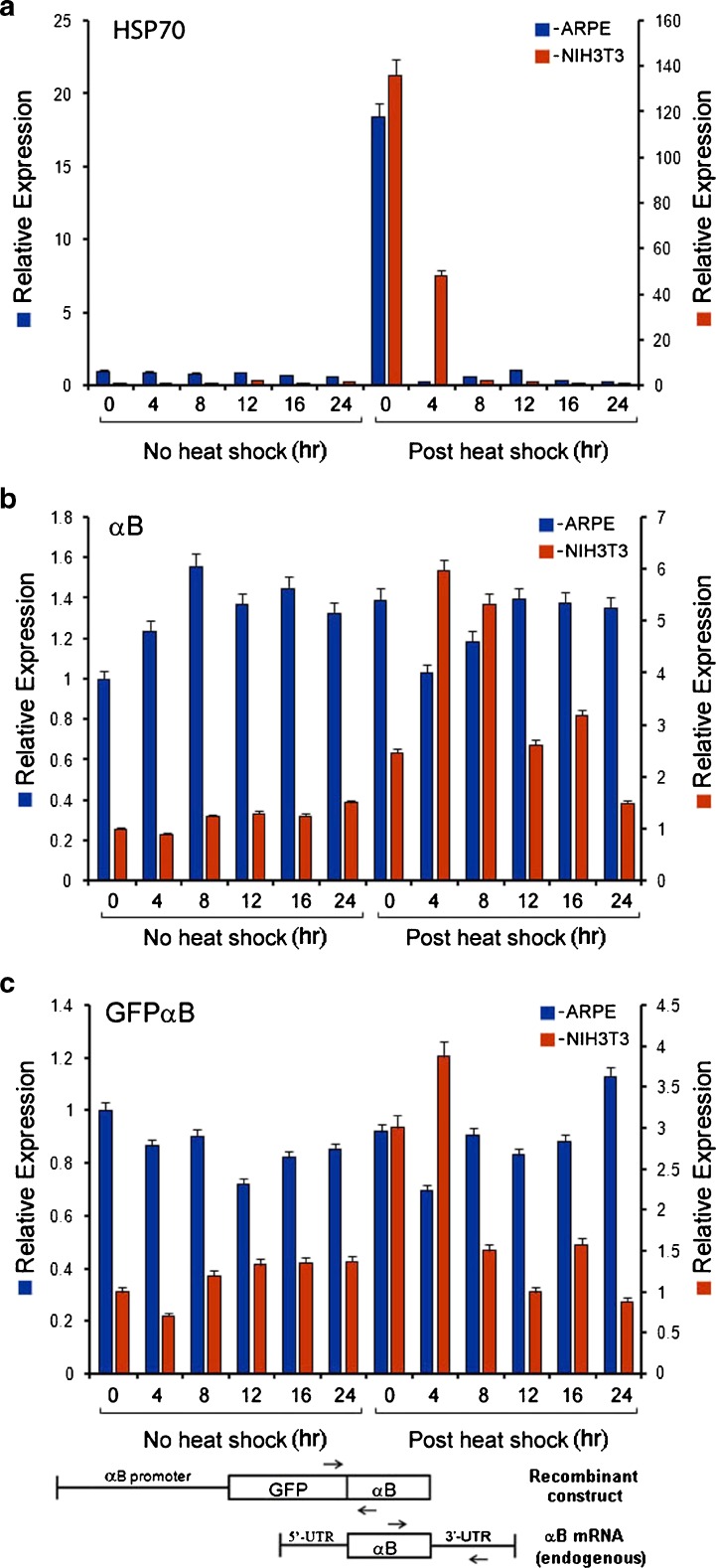
Fig. 3.
Differential activation of CRYAB heat shock promoter in ARPE-GFPαB and NIH3T3-GFPαB cells. RT-qPCR data show increased levels of transcripts for endogenous HSP70 in both the cell types (a, blue and red bars); however, endogenous αB (b) and hybrid GFPαB (c) transcript levels are seen elevated only in NIH3T3-GFPαB cells only (b and c, red bars, postheat shock). The data shown are the average of triplicate determinations ± SE for each time point. These experiments were repeated three times. Note that while both αB as well as GFPαB transcripts are present in ARPE-GFPαB cells, there is no discernable change in their levels upon heat shock (b and c, respectively, blue bars). These transcript levels follow the pattern of protein levels seen in Fig. 2a and b. For GFPαB transcripts, two primers, one from GFP and the other from αB coding sequences were used. For endogenous αB transcripts, one of the primers used was from the 3′ untranslated sequence of the αB mRNA, which is lacking in the permanently transfected GFPαB recombinant gene construction. This allows discrimination between the recombinant αB and endogenous sequences. The primer locations are schematically depicted at the bottom
Chromatin immunoprecipitation
Chromatin immunoprecipitation (ChIP) was performed with native ARPE and NIH3T3 cells using ChIP-IT® Express kit (Active Motif) with minor modifications. Antibodies used in ChIP assay were protein G-purified rabbit polyclonal HSF1 or HSF4 antibody or normal serum (Sigma Genosys). The reverse cross-linked DNA fragments were purified by PureLink PCR purification kit (Invitrogen) before final PCR amplification. All amplicons cover the key HSE motifs in respective promoters (Fig 5). See supplemental data for list of primers used.
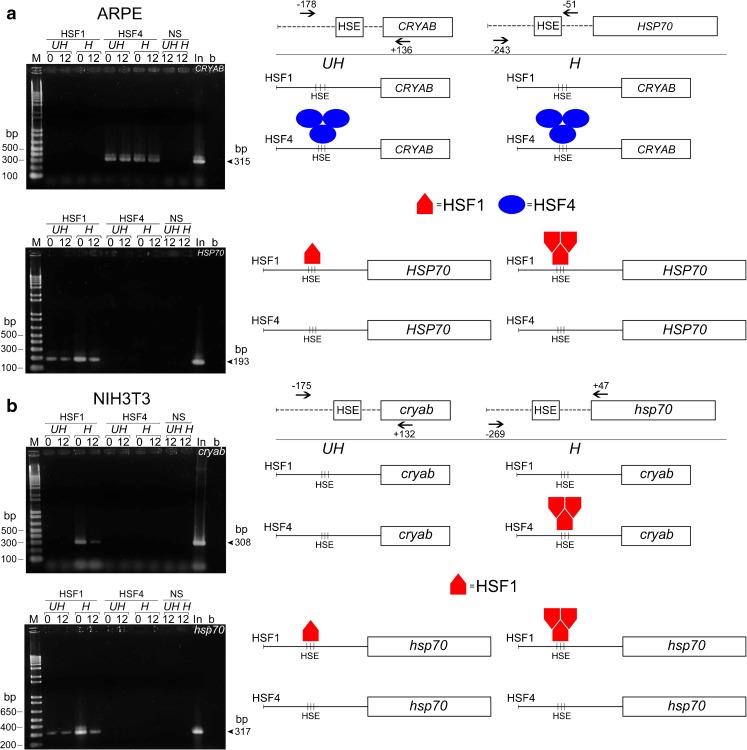
Fig. 5.
Occupancy of HSP70 and cryab promoters by HSF1 and HSF4 is cell-type specific. Native ARPE cells and NIH3T3 cells were processed for ChIP assay with HSF1 and HSF4-specific antibodies. The left panels show agarose gel electrophoresis of the PCR products. The right panels show schematic representation of the data obtained. a Note that in ARPE cells there is no HSF1 on the cryab promoter either in control (unheated, UH) or heat shocked (H) cells, either at 0 h or at 12 h postheat shock; HSF4 is seen only on the cryab promoter both in heated and unheated cells ( blue ovals). There is no detectable HSF4 on the HSP70 promoter; HSF1 is only seen on the HSP70 promoter in unheated cells, and its enhanced binding is seen in heat-shocked ARPE cells (red pentagons). b In NIH3T3 cells HSF1 is seen on the cryab promoter only in heated cells (red pentagons). HSF1 is also present on the hsp70 promoter in both the heated as well as unheated cells (red pentagon), but it is enhanced in heat shocked cells (red pentagons) just as in ARPE cells in a. Note that there is no HSF4 on either the cryab promoter or the HSP70 promoter in NIH3T3 cells under any condition. The first lane of each gel shows the DNA markers (M, bp base pairs), the last lane is the H2O control. H heat shocked, UH not heat shocked, In input DNA before immunoprecipitation, NS normal serum. The sizes of amplicons are indicated on the right side of the agarose gels with an arrow pointing to the PCR product. The location of the primers for cryab and HSP70 promoters in ARPE and NIH3T3 cells are schematically depicted. This experiment was repeated twice
Results and discussion
Transcription from the heat shock promoter is activated upon interaction of a trimeric HSF with the HSE (Voellmy 2004; Wu 1995). There are three known mammalian HSFs, namely, HSF1, HSF2, and HSF4. HSF3 is a chicken HSF, although a mouse homologue has recently been reported (Fujimoto et al. 2010). HSFs are a family of closely related transcription factors, which share appreciable sequence homologies, in particular in their DNA binding domains. HSF1 has been considered to be the master regulator of the heat shock response, while HSF2 and HSF4 have been shown to regulate the heat shock promoter developmentally (Akerfelt et al. 2007; Somasundaram and Bhat 2004). The HSF4, in comparison to HSF1, is not inducible and is constitutively bound to the DNA.
We first examined four cell lines ARPE19 and U373MG (epithelial) and NIH3T3 and COS1 (fibroblasts) for the expression of Hsp70 and αB. Importantly, all these cell lines express HSF1 as well as HSF4 (Fig. 1b).
Heat shock does not induce αB in epithelial cells
We generated ARPE-GFPαB and NIH3T3-GFPαB cells permanently transfected with recombinant hybrid GFPαB sequences driven by the rat cryab promoter (see “Materials and methods”). This manipulation allows us (a) comparison with previous studies (Klemenz et al. 1991) that employed transfected recombinant promoter-reporter constructs and (b) provides information about the endogenous promoter activity at the same time. The number of copies of the transfected plasmids in all the four cell lines was comparable (see “Materials and methods”), and more importantly, the expression from the transfected plasmids mirrored the expression of the endogenous promoter (see below). These two cell lines were exposed to a heat shock (43 °C for 1 h), and the expression of HSP70 and αB were examined, immediately before and at various time points after heat shock.
Heat shock does not change the endogenous αB levels in ARPE-GFPαB cells, which constitutively express this gene (Fig. 2a). In comparison, NIH3T3-GFPαB cells, which do not make αB constitutively, show induction of this protein (Fig. 2b, NIH3T3). In both these cells lines, however, Hsp70 is induced (Fig. 2a, b, Hsp70 panels). Heat shock does not induce either the endogenous αB or the hybrid GFPαB in ARPE-GFPαB cells, but the induction of HSP70 is obvious (Fig. 2a). In comparison, in NIH3T3-GFPαB, all the three, Hsp70, GFPαB, and αB, are induced (Fig. 2b), albeit at various levels. Although αB signal is weaker, it is significant considering that there is no detectable αB in the control cells (Fig. 2b). This observation of the induction of endogenous αB (activation of the cryab promoter) is also supported by the increased expression of the transfected recombinant hybrid GFPαB, which is driven by the rat cryab promoter (Fig. 2b) confirming previously reported induction of αB in NIH3T3 cells (Klemenz et al. 1991). These expression patterns (presence of the protein) in ARPE19 and NIH3T3 cells were further corroborated by estimation of corresponding RNA transcript levels by quantitative real-time PCR (RT-qPCR) (Fig. 3). Our experience shows that it is difficult to find an absolute control gene that does not change (Kulkarni et al. 2011). We, however, chose actin on the basis of its relatively limited variation in both the heated as well as unheated cultures within our experimental parameters (see Supplemental data Fig 1).
Elevated levels of Hsp70 transcripts are seen both in ARPE-GFPαB and NIH3T3-GFPαB cells (Fig. 3a). This induction is seen immediately after the heat shock both in ARPE-GFPαB as well as in NIH3T3-GFPαB cells (Fig. 3a, 0 time point, immediately postheat shock). Comparison of the transcript induction profile of HSP70 with αB shows stark differences between the ARPE-GFPαB and NIH3T3-GFPαB cells (Fig. 3b). In ARPE-GFPαB cells, αB transcript levels at various time points without the heat shock and postheat shock (Fig. 3b, blue bars) do not show any significant differences; however, significant change (increase) in αB transcripts levels is noticeable in heat shocked NIH3T3-GFPαB cells (Fig. 3b, reds bars, postheat shock). Interestingly this increase in CRYAB promoter activity is also confirmed by increased levels of transcripts from the transfected recombinant GFPαB, driven by rat cryab promoter, in NIH3T3-GFPαB cells (Fig. 3c, 0 and 4 h time points, red bars, postheat shock); in comparison, there is no significant change seen in GFPαB transcripts in ARPE-GFPαB cells (Fig. 3c, blue bars), indicating cell-type specific activation of the cryab promoter (in NIH3T3-GFPαB cells).
αB is only induced in fibroblasts upon heat shock
In order to establish the veracity of the above observations (Figs. 2 and 3), on the differential expression of αB and Hsp70 in epithelial cells and fibroblasts, we investigated two additional established cell lines, human glioblastoma U373MG (epithelial origin) and monkey kidney cell line COS1 (fibroblast origin). These cells were stably transfected with hybrid GFPαB driven by the rat cryab promoter as above. Heat shock does not increase expression of αB or recombinant GFPαB in U373-GFPαB cells (Fig. 4a), but both are induced in COS1-GFPαB cells (Fig. 4b). Note however, as above, the obvious induction of Hsp70 in both the cell lines (Fig. 4a, b).
Although only four cells lines were studied, the data presented above clearly points to differential response of epithelial cell lines, ARPE and U373 cells (Figs. 2a and 4a) and fibroblasts, NIH3T3 (Fig. 2b), and COS1 cells (Fig. 4b) with respect to the expression of αB. It follows, therefore, that many cell types that do not constitutively express αB may do so upon exposure to heat shock. An important corollary to these observations is that only some cell types will respond to heat shock with induced expression of αB. These observations are in harmony with our earlier observations on the presence of αB in kidney epithelial cell lines and not in kidney fibroblasts (Nagineni and Bhat 1989). Additionally, analyses of previously published microarray data (Murray et al. 2004) from the Botstein laboratory indicates that upon heat shock, CRYAB is expressed noticeably only in a specific cell line in culture (Supplemental data Fig. 2).
The above data (Figs. 2, 3, and 4) also indicate that the endogenous promoter and the transfected recombinant promoter respond equally to the molecular environment within the respective cells, suggesting that heterologous heat shock promoters used to target inducible expression of reporter genes (Guo et al. 2008) will work only in those cells or tissues in which endogenous heat shock promoter is inducible by heat shock. It should also be noted that the transfected recombinant constructions used in this study contain an almost complete promoter of the rat cryab gene (Srinivasan and Bhat 1994).
HSF4 occupies CRYAB and not HSP70 promoter in ARPE19 cells
Presence of multiple HSFs in a cell raises the possibility of different HSFs activating the same promoter. For instance, here, in the paradigm of epithelial cells and fibroblasts, HSF1 and HSF4 could activate CRYAB and HSP70 depending on the promoter architecture and/or simply by their access to one of the two or both promoters. The HSF:HSE interactions have previously been studied with gel-shift assays (Somasundaram and Bhat 2000; Wu 1995). This has led to in vitro binding studies on the characteristics of the HSEs and their relationship to binding efficiency of the HSF in question (Yamamoto et al. 2009). For example, in vitro, HSF4 binds robustly to hsp70 HSE than cryab HSE (Somasundaram and Bhat 2004). Recent in vitro binding studies suggest that human HSF4 has higher binding affinity for promoters containing gaps between 5′-nGAAn-3′ motifs, while HSF1 has higher affinity for continuous HSEs containing no gaps between 5′-nGAAn-3′ motifs (Yamamoto et al. 2009). HSP70 promoter represents a “discontinuous” heat shock promoter with a gap between the first two 5′-nGAAn-3′ motifs, and cryab represents an example of a “continuous” heat shock promoter with no gaps between 5′-nGAAn-3′ motifs (see Fig. 1).
We investigated whether observed expression patterns of CRYAB and HSP70 in the epithelial cells and fibroblasts, detailed above, could be related to the specific promoter occupancy by HSF1 and/or HSF4. We used ChIP to examine the presence of HSF1 and HSF4 on the cryab and HSP70 promoters before and after heat shock in native untransfected ARPE19 and NIH3T3 cells.
ChIP assays clearly demonstrate that, in ARPE19 cells, HSF4 is bound to CRYAB promoter before and after heat shock (at 0 h, immediately after heat shock and at 12 h postheat shock; Fig. 5a, CRYAB panel, blue ovals). Importantly, HSF1 is not seen on this promoter under either of the two conditions (Fig. 5a, CRYAB panel), but enhanced presence of HSF1 is seen on the HSP70 promoter in ARPE19 cells exposed to heat shock (Fig. 5a, HSP70 panel, red pentagons). Interestingly, in these cells, no HSF4 is detectable on HSP70 promoter, either before or after heat shock (Fig. 5a, HSP70 panel). Considering that both HSP70 as well as CRYAB promoters are active in ARPE19 cells and that both HSF4 as well as HSF1 are available, these data suggest compartmentalization of the two HSF-related activities, even under heat shock conditions as indicated by the absence of HSF1 on CRYAB promoter and its presence on the HSP70 promoter. Based on these data, we conclude that, in human ARPE19 cells, the access of HSF1 and HSF4 to HSP70 and CRYAB promoters is selectively controlled and determined by the cell type.
HSF1 binding to cryab promoter is detected only after heat shock in NIH3T3 cells
The ChIP assay reveals a different picture in the fibroblasts (NIH3T3 cells) exposed to the heat shock. No HSF4 binding is seen either on cryab or hsp70 promoters either before or after heat shock (Fig. 5b, cryab and hsp70 panels), but HSF1 binding to cryab promoter is seen only after heat shock, while HSF1 binding to hsp70 promoters is enhanced postheat shock (Fig. 5b, cryab and hsp70 panels, H, 0 lanes). None or little HSF1 binding is seen on the cryab promoter before heat shock (Fig. 5b, cryab panel, UH, 0 and 12 lanes); in comparison, unchanged basal HSF1 binding is seen on the Hsp70 promoter (Fig. 5b, hsp70 panel, UH, 0 and 12 lanes). The HSF1 binding returns to normal level (as in no heat sock) at the 12-h time point (Fig. 5a, HSP70 panel, H, 0 and 12 lanes and Fig. 5b, hsp70 panel, H, 0 and 12 lanes). Notably, there is no detectable HSF1 on the CRYAB promoter in unheated ARPE19 cells or NIH3T3 cells; it appears on the cryab promoter only in heat shocked NIH3T3 cells (Fig. 5b, cryab panel, red pentagons). It is apparent from the above data that, under heat shock, the unoccupied promoter of cryab in NIH3T3 cells becomes accessible to HSF1; in ARPE19, however, where the promoter is already occupied by HSF4, HSF1 does not gain access to this promoter. In comparison, in the same cells, HSF1 does gain access to the hsp70 promoter, indicating that cell-type dictates promoter occupancy of HSFs.
HSF1 and HSF4 interactions with heat shock promoters are dependent on cell type
Interestingly, the data presented in Fig. 5 shows that in vitro binding patterns reported previously showing HSF4 preference for the “discontinuous” and HSF1 preference for the “continuous” promoters (Yamamoto et al. 2009) are not followed in vivo. Our data (Fig. 5) clearly shows that HSF access to either of the two promoters is controlled innately by the cell-type. In ARPE cells, HSF1 is seen on the HSP70 promoter (which contains discontinuous 5′-nGAAn-3′ motifs), while HSF4 is bound to the CRYAB promoter (with continuous 5′-nGAAn-3′ motifs). HSF1 does not gain access to CRYAB promoter in ARPE19 cells upon heat shock (Fig. 5a) but gains access to the cryab promoter in heat shocked cells in NIH3T3 (Fig. 5b, red pentagons). While these data clearly indicate that in vitro HSF binding affinities (Yamamoto et al. 2009) may not reflect functional in vivo patterns, it also emphasizes that it is the developmental state/origin of the cell rather than the HSE architecture that determines HSF/HSE interaction and, therefore, the promoter activity.
The data presented in this report suggest that the stress response in eukaryotes does not override developmental predisposition that dictates specific access of HSFs to specific heat shock promoters, possibly controlled by epigenetic modifications (Bernstein et al. 2007). It is interesting to note that while Hsp70 promoter binds HSF4 in vitro (Somasundaram and Bhat 2004), we do not find it on this promoter in these in vivo studies.
The specific HSF/HSE interactions such as those described here ensure that various heat shock proteins are not only synthesized during heat shock or a stress response but before and after the stress episode as required by various cellular physiologies. It is noteworthy that ChIP analyses (Fig. 5) did not reveal any cross-talk (Fujimoto et al. 2008) between HSF4 and HSF1 so far as their physical presence on the HSP70 and CRYAB promoters is concerned. Assuming that both HSF1 and HSF4 are expressed in the same cell, it is tempting to speculate that the presence of one HSF (e.g., HSF4 on the CRYAB promoter in ARPE cells) may preclude binding of the other HSF (e.g., HSF1) to this promoter. Whether this is true must await single cell studies on the expression of various HSFs and their mechanistic relationships with respect to the activation of heat shock promoters in phenotypically homogeneous populations of cells.
Electronic supplementary material
(DOCX 269 kb)
Acknowledgments
We want to thank Janice Canaria for technical assistance. This work was supported by NIH-NEI grants to SPB.
References
- Akerfelt M, Trouillet D, Mezger V, Mezger V, Sistonen L, Sistonen L. Heat shock factors at a crossroad between stress and development. Ann N Y Acad Sci. 2007;1113:15–27. doi: 10.1196/annals.1391.005. [DOI] [PubMed] [Google Scholar]
- Andley UP. Crystallins in the eye: function and pathology. Prog Retin Eye Res. 2007;26:78–98. doi: 10.1016/j.preteyeres.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Bernstein BE, Meissner A, Lander ES. The mammalian epigenome. Cell. 2007;128:669–681. doi: 10.1016/j.cell.2007.01.033. [DOI] [PubMed] [Google Scholar]
- Bhat SP. Crystallins, genes and cataract. Prog Drug Res. 2003;60:205–262. doi: 10.1007/978-3-0348-8012-1_7. [DOI] [PubMed] [Google Scholar]
- Bhat SP, Horwitz J, Srinivasan A, Ding L (1991) αB-crystallin exists as an independent protein in the heart and in the lens. Eur J Biochem 102:775–781 [DOI] [PubMed]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci USA. 1984;81:3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier NC, Schlesinger MJ. Induction of heat-shock proteins in the embryonic chicken lens. Exp Eye Res. 1986;43:103–117. doi: 10.1016/S0014-4835(86)80049-5. [DOI] [PubMed] [Google Scholar]
- Dasgupta S, Hohman TC, Carper D. Hypertonic stress induces alpha B-crystallin expression. Exp Eye Res. 1992;54:461–470. doi: 10.1016/0014-4835(92)90058-Z. [DOI] [PubMed] [Google Scholar]
- de Jong WW, Hoekman WA, Mulders JW, Bloemendal H. Heat shock response of the rat lens. J Cell Biol. 1986;102:104–111. doi: 10.1083/jcb.102.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubin RA, Ally AH, Chung S, Piatigorsky J. Human alpha B-crystallin gene and preferential promoter function in lens. Genomics. 1990;7:594–601. doi: 10.1016/0888-7543(90)90204-8. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Oshima K, Shinkawa T, Wang BB, Inouye S, Hayashida N, Takii R, Nakai A. Analysis of HSF4 binding regions reveals its necessity for gene regulation during development and heat shock response in mouse lenses. J Biol Chem. 2008;283:29961–29970. doi: 10.1074/jbc.M804629200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujimoto M, Fujimoto M, Hayashida N, Katoh T, Oshima K, Shinkawa T, Prakasam R, Prakasam R, Tan K, Tan K, Inouye S, et al. A novel mouse HSF3 has the potential to activate nonclassical heat-shock genes during heat shock. Mol Biol Cell. 2010;21:106–116. doi: 10.1091/mbc.E09-07-0639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangalum RK, Schibler MJ, Bhat SP. Small heat shock protein alphaB-crystallin is part of cell cycle-dependent Golgi reorganization. J Biol Chem. 2004;279:43374–43377. doi: 10.1074/jbc.C400371200. [DOI] [PubMed] [Google Scholar]
- Gangalum RK, Atanasov IC, Zhou ZH, Bhat SP. AlphaB-crystallin is found in detergent-resistant membrane microdomains and is secreted via exosomes from human retinal pigment epithelial cells. J Biol Chem. 2011;286:3261–3269. doi: 10.1074/jbc.M110.160135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopal-Srivastava R, Cvekl A, Piatigorsky J. Pax-6 and alphaB-crystallin/small heat shock protein gene regulation in the murine lens. Interaction with the lens-specific regions, LSR1 and LSR2. J Biol Chem. 1996;271:23029–23036. doi: 10.1074/jbc.271.38.23029. [DOI] [PubMed] [Google Scholar]
- Guo ZS, Li Q, Bartlett DL, Yang JY, Fang B. Gene transfer: the challenge of regulated gene expression. Trends Mol Med. 2008;14:410–418. doi: 10.1016/j.molmed.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Horwitz J. The function of alpha-crystallin in vision. Semin Cell Dev Biol. 2000;11:53–60. doi: 10.1006/scdb.1999.0351. [DOI] [PubMed] [Google Scholar]
- Klemenz R, Frohli E, Steiger RH, Schafer R, Aoyama A. Alpha B-crystallin is a small heat shock protein. Proc Natl Acad Sci USA. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulkarni B, Mohammed I, Hopkinson A, Dua HS. Validation of endogenous control genes for gene expression studies on human ocular surface epithelium. PLoS One. 2011;6:e22301. doi: 10.1371/journal.pone.0022301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laramie JM, Chung TP, Brownstein B, Stormo GD, Cobb JP. Transcriptional profiles of human epithelial cells in response to heat: computational evidence for novel heat shock proteins. Shock. 2008;29:623–630. doi: 10.1097/shk.0b013e318157f33c. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Morgan WD, Williams GT, Morimoto RI, Greene J, Kingston RE, Tjian R. Two transcriptional activators, CCAAT-box-binding transcription factor and heat shock transcription factor, interact with a human hsp70 gene promoter. Mol Cell Biol. 1987;7:1129–1138. doi: 10.1128/mcb.7.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimoto RI. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Morimoto R, Fodor E. Cell-specific expression of heat shock proteins in chicken reticulocytes and lymphocytes. J Cell Biol. 1984;99:1316–1323. doi: 10.1083/jcb.99.4.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JI, Whitfield ML, Trinklein ND, Myers RM, Brown PO, Botstein D. Diverse and specific gene expression responses to stresses in cultured human cells. Mol Biol Cell. 2004;15:2361–2374. doi: 10.1091/mbc.E03-11-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagineni CN, Bhat SP. Alpha B-crystallin is expressed in kidney epithelial cell lines and not in fibroblasts. FEBS Lett. 1989;249:89–94. doi: 10.1016/0014-5793(89)80022-5. [DOI] [PubMed] [Google Scholar]
- Somasundaram T, Bhat SP. Canonical heat shock element in the alpha B-crystallin gene shows tissue-specific and developmentally controlled interactions with heat shock factor. J Biol Chem. 2000;275:17154–17159. doi: 10.1074/jbc.M000304200. [DOI] [PubMed] [Google Scholar]
- Somasundaram T, Bhat SP. Developmentally dictated expression of heat shock factors: exclusive expression of HSF4 in the postnatal lens and its specific interaction with alphaB-crystallin heat shock promoter. J Biol Chem. 2004;279:44497–44503. doi: 10.1074/jbc.M405813200. [DOI] [PubMed] [Google Scholar]
- Srinivasan AN, Bhat SP. Complete structure and expression of the rat alpha B-crystallin gene. DNA Cell Biol. 1994;13:651–661. doi: 10.1089/dna.1994.13.651. [DOI] [PubMed] [Google Scholar]
- Voellmy R. On mechanisms that control heat shock transcription factor activity in metazoan cells. Cell Stress Chaperones. 2004;9:122–133. doi: 10.1379/CSC-14R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: structure and regulation. Annu Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301. [DOI] [PubMed] [Google Scholar]
- Wu BJ, Kingston RE, Morimoto RI. Human HSP70 promoter contains at least two distinct regulatory domains. Proc Natl Acad Sci USA. 1986;83:629–633. doi: 10.1073/pnas.83.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto N, Takemori Y, Sakurai M, Sugiyama K, Sakurai H. Differential recognition of heat shock elements by members of the heat shock transcription factor family. FEBS J. 2009;276:1962–1974. doi: 10.1111/j.1742-4658.2009.06923.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 269 kb)