Abstract
We have previously shown that plectin is recruited into hemidesmosomes through association of its actin-binding domain (ABD) with the first pair of fibronectin type III (FNIII) repeats and a small part of the connecting segment (residues 1328–1355) of the integrin β4 subunit. Here, we show that two proline residues (P1330 and P1333) in this region of the connecting segment are critical for supporting β4-mediated recruitment of plectin. Additional binding sites for the plakin domain of plectin on β4 were identified in biochemical and yeast two-hybrid assays. These sites are located at the end of the connecting segment (residues 1383–1436) and in the region containing the fourth FNIII repeat and the C-tail (residues 1570–1752). However, in cells, these additional binding sites cannot induce the assembly of hemidesmosomes without the interaction of the plectin-ABD with β4. Because the additional plectin binding sites overlap with sequences that mediate an intramolecular association of the β4 cytoplasmic domain, we propose that they are not accessible for binding and need to become exposed as the result of the binding of the plectin-ABD to β4. Furthermore, these additional binding sites might be necessary to position the β4 cytoplasmic domain for an optimal interaction with other hemidesmosomal components, thereby increasing the efficiency of hemidesmosome assembly.
INTRODUCTION
Hemidesmosomes (HDs) are multiprotein complexes that facilitate firm adhesion of stratified and complex epithelia to the basement membrane. They provide the linkage between the intracellular keratin intermediate filaments to the laminin constituents of the extracellular matrix (Borradori and Sonnenberg, 1999). HDs consist of at least five components, three of which are transmembrane proteins: the integrin α6β4, which serves as a receptor for the extracellular matrix component laminin-5 (Stepp et al., 1990; Sonnenberg et al., 1991; Niessen et al., 1994); the collagenous bullous pemphigoid antigen BP180 (Giudice et al., 1992); and the tetraspanin CD151 (Sterk et al., 2000). The other two components, the bullous pemphigoid antigen BP230 (Stanley et al., 1981) and plectin (Hieda et al., 1992; Gache et al., 1996), are cytoplasmic proteins that belong to the plakin family of proteins, which also include desmoplakin, envoplakin, and periplakin. These proteins are critically involved in the organization of the cytoskeleton (Ruhrberg and Watt, 1997; Leung et al., 2001).
The domains in plakins have considerable sequence homology. Their N terminus consists of a plakin domain containing a number of subdomains with high α-helical content, designated NN, Z, Y, X, W, and V, whereas the central coiled-coil rod domain is composed of heptad repeats involved in the dimerization of the plakin (Green et al., 1992). Their C-terminal end contains one or more homologous repeat sequences, referred to as plectin repeats. In plectin, a calponin-type actin-binding domain (ABD) precedes the plakin domain (McLean et al., 1996). BP230 lacks such an ABD, but variants that do contain an N-terminal ABD can be produced from the same BPAG1 gene by the use of alternative transcription start sites (BPAG1n1 and BPAG1n2) (Brown et al., 1995). Other splice variants (BPAG1-a and BPAG1-b) differ in their carboxy terminus (Leung et al., 2001) and share features of both the spectrin and plakin protein families and therefore are referred to as spectraplakins (Roper et al., 2002). Although the C-terminal end of plakins has binding properties for intermediate filaments, the N-terminal plakin domain harbors specific sequences that target the proteins to distinct membrane sites, such as desmosomes and HDs (Rezniczek et al., 1998; Geerts et al., 1999; Hopkinson and Jones, 2000; Koster et al., 2003).
Although ablation of BP230 or plectin in mice compromises the mechanical stability of the epidermal sheet, its effect on the ultrastructure of HDs is relatively weak (Guo et al., 1995, Andrä et al., 1997). In either case, HDs are formed but the attachment of intermediate filaments to the hemidesmosomal plaque is reduced or absent. The epidermal phenotype of the plectin null mutant mice resembles that of patients suffering from epidermolysis bullosa simplex, a hereditary skin blistering disease that is associated with muscular dystrophy (MD-EBS). In these patients, a wide spectrum of mutations has been identified in the plectin gene, which is presumed to be responsible for this disease (McLean et al., 1996; Smith et al., 1996; Uitto et al., 1996).
The α6β4 integrin plays an important role in the maintenance of skin integrity as indicated by the study of human disease and null mutations in mice (Vidal et al., 1995; Dowling et al., 1996; Georges-Labouesse et al., 1996; van der Neut et al., 1996; Ruzzi et al., 1997). Its loss causes a distinct form of junctional epidermolysis bullosa associated with pyloric atresia (PA-JEB), characterized by fragility and extensive blistering of the skin. In affected patients HDs are rudimentary or absent. Extensive blistering of the skin and loss of HDs are also phenomena associated with the absence of laminin-5, supporting the conclusion that this molecule is the principle ligand recognized by α6β4 in the epidermis. In contrast to the severe defects seen when α6β4 expression is lost, the symptoms associated with the absence of BP180 are relatively benign. Furthermore, only some minor abnormalities in the structure and number of HDs have been reported (Jonkman et al., 1995; McGrath et al., 1995).
The large cytoplasmic domain of the integrin β4 subunit is essential for the formation of HDs (Murgia et al., 1998; Nievers et al., 1998). It is >1000 amino acids long and contains two pairs of fibronectin type III (FNIII) repeats that are separated by a connecting segment (CS) (Borradori and Sonnenberg, 1999). The first pair of FNIII repeats and the first 35 N-terminal residues of the CS of the β4 integrin are required for the recruitment of plectin into HDs (Niessen et al., 1997a,b). The third FNIII repeat mediates binding to BP180 (Borradori et al., 1997; Schaapveld et al., 1998), whereas the third and fourth repeat have been implicated in the binding to BP230 (Hopkinson and Jones, 2000; Koster et al., 2003).
Previous studies have indicated an important role of the ABD of plectin in the recruitment of this protein into HDs (Geerts et al., 1999). However, additional binding sites for β4 on plectin have been described, but their exact function is not clear (Rezniczek et al., 1998). In this study, we confirm the presence of one or more β4 binding sites in the plakin domain of plectin, and present evidence that a nonsense mutation in β4, responsible for a lethal form of junctional epidermolysis and through which the last C-terminal 38 amino acids of the β4 cytoplasmic domain are deleted, prevents interaction of the plakin domain of plectin with β4. Based on findings with PA-JEB keratinocytes stably expressing a β4 mutant lacking one or more plectin-binding sites, a model is presented for the interaction of plectin with β4. In this model, the binding of the plectin-ABD to the first pair of FNIII repeats induces a conformational change in the β4 cytoplasmic domain through which the additional plectin binding sites in the carboxy-terminal half of the molecule, become exposed. Furthermore, we suggest that this induced conformation, stabilized by binding of plectin to β4, is most suitable for its interaction with other HD components. Finally, we identified two proline residues in the region 1328–1355 in the CS of β4 that are critical for the recruitment of plectin into HDs.
MATERIALS AND METHODS
Cell Lines and Antisera
The β4-deficient PA-JEB and plectin-deficient MD-EBS keratinocyte cell lines have been described previously (Schaapveld et al., 1998; Geerts et al., 1999). The cells were grown in keratinocyte serum-free medium (SFM) (Invitrogen, Carlsbad, CA) supplemented with bovine pituitary extract, 5 ng/ml epidermal growth factor, 100 U/ml penicillin, and 100 U/ml streptomycin. The African monkey kidney cell line COS-7 was maintained in DMEM containing 10% (vol/vol) fetal calf serum.
The mouse monoclonal antibodies (mAbs) 121 against plectin/HD1 (Hieda et al., 1992) and 233 against BP180 (Nishizawa et al., 1993) were generously provided by Dr. K. Owaribe (University of Nagoya, Nagoya, Japan). The human mAbs 5E and 10D against BP230 (Hashimoto et al., 1993) were a kind gift of Dr. T. Hashimoto (Kurume Univeristy, Kurume, Fukuoka, Japan). Mouse mAb 7A8 against plectin/HD1 was purchased from Sigma-Aldrich (St. Louis, MO). Purified rabbit polyclonal antibody D16 against the ABD of plectin/HD1 has been described previously (Geerts et al., 1999). Alexa Fluor 568 phalloidin (Molecular. Probes, Eugene, OR) was used to stain F-Actin. The mouse mAb 12CA5 against the hemagglutinin (HA)-epitope (YPYDVPDYA) and the rabbit polyclonal antibodies against the extracellular domain of β4 (sc-9090), against the extracellular domain of the interleukin 2 receptor (IL2R) (sc-665), and against plectin (C20 and sc-7572) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Secondary antibodies were obtained from Rockland (Gilbertsville, PA) (fluorescein isothiocyanate-conjugated goat anti-mouse IgG), Molecular Probes (anti-rabbit IgG, Alexa 488-conjugated goat anti-human IgG), and Amersham Biosciences (Piscataway, NJ) (horseradish peroxidase-coupled sheep anti-mouse and donkey anti-rabbit IgG).
DNA Constructs
The construction of expression vectors encoding chimeric proteins containing the extracellular and transmembrane domain of the IL2R and (parts of) the intracellular domain of the major β4 integrin variant β4A, pCMV-IL2R/β4, and pCMV-IL2R/β4R1281W have been described previously (Nievers et al., 1998). The vectors pCMV-IL2R/β4Δ1383–1437, pCMV-IL2R/β4Δ1383–1437, R1281W and pCMV-IL2R/β41–1670, pCMV-IL2R/β41–1670, R1281W were generated using standard cloning techniques. The retroviral expression vectors pLZRS-β41–1670, -β41–1670, R1281W, -β4Δ1383–1437, -β4Δ1383–1437, R1281W, -β41–1355, -β4P1330A, P1333A, and -β4P1610A, P1613A were obtained by cloning β4 cDNA fragments derived by site-directed mutagenesis into the retroviral vector LZRS-IRES-zeo (Sterk et al., 2000). cDNAs encoding full-length plectin as well as truncated polypeptides were subcloned into the multicloning sites of eukaryotic expression vector pcDNA-HANII (Invitrogen), which allows for expression of a recombinant protein that is tagged with HA at the N terminus. All nucleotide and amino acid positions are numbered with the ATG initiation codon at position one (accession no. U53204 for plectin and X51841 for β4). Plasmid inserts were generated by restriction enzyme digestion or polymerase chain reaction (PCR) by using the proofreading Pwo DNA polymerase (Roche Diagnostics, Indianapolis, IN) and gene-specific sense and antisense primers containing restriction site tags. Numbers in superscript correspond to the amino acid residues of subclones.
DNA Transfections and Immunofluorescence Microscopy
PA-JEB or MD-EBS cells were grown on glass coverslips to 40% confluence in 12-well tissue culture plates (Falcon; BD Biosciences, Lincoln Park, NJ). Transient transfections were performed with 0.2–0.8 μg of cDNA by using Lipofectin, according to the manufacturer's instructions (Invitrogen). Transfection mixtures were replaced by SFM medium after 5 h and incubated in this medium for 24 h. Subsequently, the SFM medium was replaced by Ham's F-12/DMEM (1:3) for an additional 16 h after which the cells were processed for immunofluorescence miscroscopy as described previously (Schaapveld et al., 1998; Geerts et al., 1999). Coverslips were mounted onto glass slides in Mowiol mounting medium (Calbiochem, San Diego, CA) containing 2.5% 1,4-diazabicyclo[2–2-2]octane (Sigma-Aldrich) and viewed under a Leica confocal scanning laser microscope.
Reverse Transcription (RT)-PCR
RNA from PA-JEB/β4 and MD-EBS cells was isolated using RNA-Bee (Tel-Test, Friendswood, TX), and cDNA was made using Superscript reverse transcriptase (Invitrogen). The cDNA was used for PCR with primers specific for the exon boundaries flanking the rod domain of plectin.
Coimmunoprecipitation
COS-7 cells were grown to 70% confluence in 10-cm culture dishes (Falcon) and transiently transfected with 7.5 μg of cDNA by using DEAE-dextran (Schaapveld et al., 1998). Cells were incubated with transfection medium for 3 h, which was then replaced by DMEM medium. After 24 h, this medium was replaced by DMEM containing 5 mM sodium butyrate. After a 24-h incubation, the cells were washed twice with phosphate-buffered saline and lysed in MPER lysis buffer (Pierce Chemical, Rockford, IL), containing a cocktail of protease inhibitors (Sigma-Aldrich) on ice for 15 min. Lysates were centrifuged at 13,000 × g for 10 min, incubated for 16 h at 4°C with 100 μl of 12CA5 mouse mAb (anti-HA), and finally for 30 min at 4°C with 30 μl of a 50% slurry of GammaBind G Sepharose (Amersham Biosciences). Beads were washed three times with lysis buffer and incubated at 95°C for 5 min in Laemmli sample buffer. Protein samples were loaded on a 4–20% gradient Tris-glycine gel (Invitrogen) and transferred to polyvinylidene difluoride membranes, which were subsequently decorated with polyclonal antibodies against IL2R or HA. Proteins were detected using the ECL Dura kit (Pierce Chemical).
Yeast Two-Hybrid Interaction Assay
Yeast two-hybrid interactions assays were performed as described by Geerts et al. (1999). cDNA fragments encoding different regions of plectin were ligated to the DNA binding domain of the pAS2.1 vector and the coding sequences of β4 to the activation domain of pACT2 (BD Biosciences Clontech, Palo Alto, CA). All nucleotide and amino acid positions are numbered with the ATG initiation codon at position one. cDNA fragments were generated by restriction enzyme digestion or PCR by using the proofreading Pwo DNA polymerase (Roche Diagnostics) and gene-specific sense and antisense primers containing restriction site tags. Numbers in superscript correspond to the amino acid residues of subclones encoded within the GAL4 (AD) or -(BD) fusion proteins.
β-Galactosidase Assay
For the quantitative analysis of β-galactosidase activity, five yeast colonies were combined and grown to an OD660 of ∼1.0 in selective medium lacking leu and trp. β-Galactosidase activity was determined at 37°C by using the yeast β-galactosidase assay kit (75768; Pierce Chemical) with O-nitrophenyl β-d-galactopyranoside as substrate. The A405 was measured in an ELISA reader, and the time at which the reaction reached a value of 0.2 was taken to calculate the β-galactosidase activity by using the equation 1000 × A405/(cell volume [milliliters] × time of reaction [minutes] × OD660). Samples that did not reach this value within 4 h were left overnight and measured the next morning. The final values are the results from three independent determinations measured in triplicate.
RESULTS
Identification of the Critical Residues in the Region 1328–1355 of the CS of β4 That Are Involved in the Recruitment of Plectin into HDs
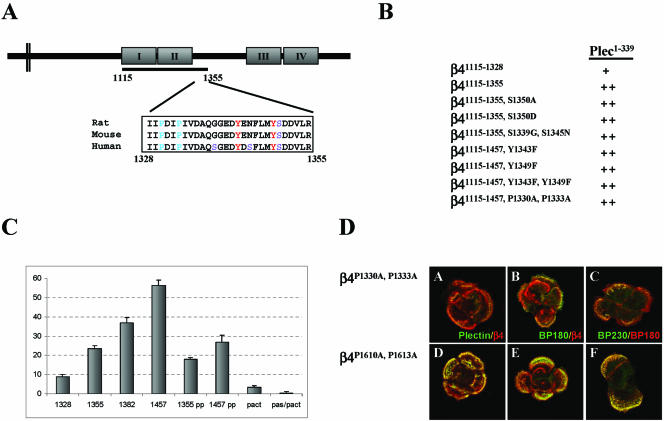
In a previous study using β4-deficient PA-JEB keratinocytes, we have shown that transient expression of a β4 mutant truncated at position 1355 (β41–1355), but not one that was truncated at position 1328 (β41–1328), resulted in the recruitment of plectin into hemidesmosome-like structures (Schaapveld et al., 1998). Similar results were obtained with cells that stably express truncated β4 subunits (our unpublished data). The recruitment of plectin by β41–1355 is facilitated by a direct interaction of the mutant β4 protein with the ABD of plectin, because a fragment containing the sequences 1115–1355 of β4 (β41115–1355) interacted with the plectin-ABD in yeast two-hybrid and biochemical assays (Geerts et al., 1999; Litjens et al., 2003). The same fragment truncated at amino acid 1328 (β41115–1328) also interacted with the plectin-ABD, but with lower affinity than β41115–1355, as assessed by quantitative β-galactosidase activity assays (Figure 1, B and C).
Figure 1.
Identification of critical residues in the connecting segment of β4 that affect the binding activity of the plectin-ABD for β4. (A) Schematic representation of the cytoplasmic domain of β4. The underlined region (1115–1355) indicates the minimal region to obtain recruitment of plectin in cell transfection experiments. The boxed sequence shows the homology between human, rat, and mouse for the region of the connecting segment that is required for recruitment of plectin and depicts the locations of serine (S), tyrosine (Y), and proline (P) residues. (B) Yeast two-hybrid analysis of the interaction of various β4 mutants with the ABD of plectin (plec1–339). Transformation mixtures were spread on SC-LT and SC-LTHA plates and grown for 5 d at 30°C. Plating efficiency on selective SC-LTHA is expressed as a percentage of plating efficiency on nonselective SC-LT plates from the same transformation. ++, >80%; +, 30–50%. (C) Quantitative β-galactosidase assay showing the strength of interactions between the various β4 constructs and plec1–339 in yeast. The values indicated are arbitrary values and representative of multiple assays. pACT, represents a negative control in which pAS-plec1–339 was cotransfected with an empty pACT vector. (D) Distribution of plectin and other HD components in PA-JEB cells stably expressing different β4 mutants. PA-JEB cells stably expressing β4P1330A, P1333A (A–C) or β4P1610A, P1613A (D–F) were stained for β4 (red; A, B, D, and E), plectin (green; A and D), BP180 (green in B and E and red in C and F) and BP230 (green; C and F). Colocalization is yellow. Note that β4P1610A, P1613A but not β4P1330A, P1333A is colocalized with plectin and that also the colocalization of β4P1330A, P1333A with BP180 and BP230 is dramatically reduced compared with that of β4P1610A, P1613A.
Close examination of the sequence of amino acids 1328–1355 of human β4 and comparing it with that of the mouse and the rat revealed some interesting features (Figure 1A). First, two conserved tyrosine residues (positions 1343 and 1349) and three serine residues (positions 1339, 1345, and 1350) are present in this stretch of amino acids, and they may regulate the interaction between β4 and plectin. The conserved serine residue (1350) was replaced by alanine or aspartate to prevent or mimic phosphorylation, respectively. The nonconserved serine residues (1339 and 1345) were replaced by the equivalent amino acids present in mouse and rat, and the tyrosine residues were replaced by phenylalanine. None of these mutations had an effect on the binding of β4 to plectin in a yeast two-hybrid assay (Figure 2B). In line with this, the substitution of the two tyrosine residues by phenylalanine in full-length β4, had no effect on the ability of the β4 subunits to induce the formation of HD-like structures, containing plectin, BP180, and BP230. The HD-like structures formed by β4Y1343F or β4Y1349F were indistinguishable from those formed by wild-type β4 (our unpublished data). Second, the β4 region 1328–1355 contains two proline residues at positions 1330 and 1333 that fit the consensus sequence PXXP for binding SH3 domains (Alexandropoulos et al., 1995). Substitution of these proline residues by alanine slightly reduced the binding activity of the β41115–1355 fragment with the plectin-ABD in yeast compared with the wild-type fragment (Figure 1, B and C). However, when tested in the context of a larger β4 fragment (1115–1457), containing the complete CS, the effect of the double point mutation was more pronounced. Interestingly, this larger fragment also bound more strongly to the plectin-ABD, whereas a slightly shorter β4 fragment (1115–1382) showed a binding activity intermediate between that with β41115–1355 and β41115–1457.
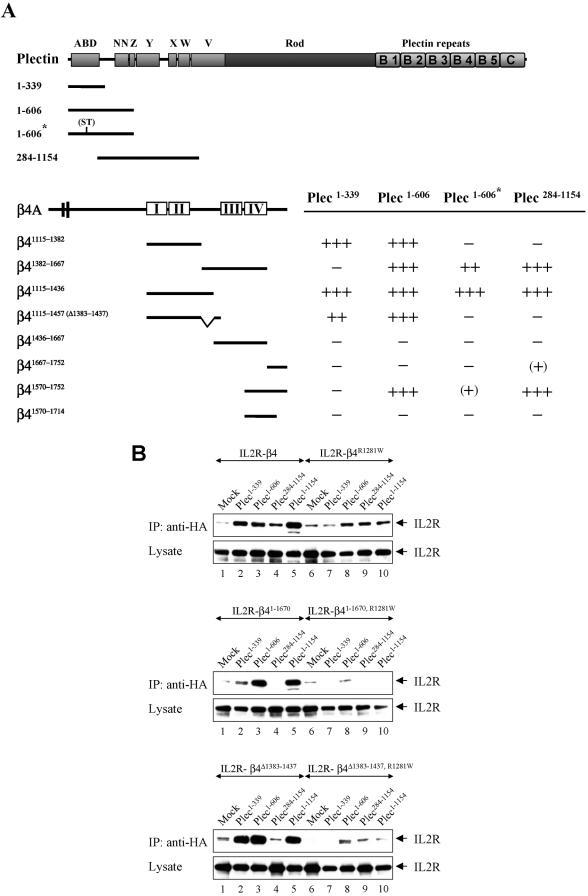
Figure 2.
Yeast two-hybrid (A) and biochemical (B) analysis of the interactions between the cytoplasmic domain of β4 and the N terminus of plectin. (A) Binding of various regions of β4 to different N-terminal fragments of plectin. Interactions were scored +++, when the number of colonies on selective SC-LTHA plates was greater than 90% of that on nonselective SC-LT plates at 5 d of growth; ++, when it was 70–90%; (+), when no or only small colonies were present at 5 d, but fully developed colonies (60–80%) were present at 10 d of growth; and -, when there were no colonies detected after 10 d of growth. The asterisk (*) indicates a double point mutation (ND>ST) in the ABD of plectin that abrogates binding of this domain to β4. (B) Different combinations of the indicated IL2R chimeric constructs and HA-tagged plectin mutants were transiently transfected into COS-7 cells and subjected to immunoprecipitation with anti-HA antibodies. Shown are immunoblot analyses of the precipitates (top) and total cell lysates (bottom), probed with anti-IL2R. Cells transfected with the various IL2R chimeric constructs and an empty vector (mock) serve as a negative control for the HA-tagged proteins that were coprecipitated with the different IL2R chimeras.
When the prolines were substituted by alanine in β41–1355 (our unpublished data) or full-length β4 (Figure 1D, A–C), and the cDNA constructs were stably introduced into PA-JEB cells, the recruitment of plectin to β4 subunits was severely compromised. In addition, the cells were no longer able to efficiently induce HD-like structures containing BP180 and BP230. In contrast, a β4 mutant carrying two proline substitutions located between the third and fourth FNIII repeat (β4P1610A, P1613A) behaved as wild-type β4 (Schaapveld et al., 1998; Sterk et al., 2000) and was colocalized with plectin, BP180, and BP230 (Figure 1D, D–F).
In conclusion, these results show that sequences in the N-terminal part of the β4 CS enforce the binding activity of the first pair of FNIII repeats with the plectin-ABD and that the two proline residues (1330 and 1333) in this region are essential for an efficient recruitment of plectin into HDs. The plectin binding site comprising the first pair of FNIII repeats and the N-terminal region of the CS will be referred to as plectin binding site-1 (PBS-1) in the remaining part of this study.
Identification of Additional Binding Sites in the CS and C-Tail of β4 That Mediate Binding to the Plakin Domain of Plectin
Although the results with the β4 mutant truncated at position 1355 indicate that the binding of the plectin-ABD to the integrin β4 subunit is sufficient for recruiting plectin into HD-like structures, they do not exclude the presence of additional binding sites for β4 on plectin that might stabilize and/or facilitate this interaction. In fact, evidence for the presence of such sites has been provided by Rezniczek et al. (1998), who showed that a plectin fragment, containing amino acids 548-1128, binds to β4 in blot overlay assays. Although at that time we were unable to confirm these results in yeast two-hybrid interaction assays, by using a similar fragment comprising most of the sequences of the plakin domain (amino acids 284-1154), we have always been intrigued by our finding that the ABD of plectin, when expressed in plectin-deficient MD-EBS keratinocytes, is only poorly localized into HD-like structures. We therefore have reevaluated the binding of plectin to β4, by using a newly isolated plectin fragment lacking the ABD. As shown in Figure 2A, this new fragment (plec284–1154) strongly interacted with β41115–1436 and β41382–1667, but not with β41115–1382 and β41436–1667. Because β41115–1436 and β41382–1667 share the stretch of amino acids 1382–1436 in the CS, we assume that this region is responsible for most, if not all, of the plectin-binding activity of these two β4 fragments. Indeed, a β4 fragment, which lacks the region 1383–1437 in the CS (β41115–1457 (Δ1383–1437)), did not interact with plec284–1154. This fragment only binds to those fragments that contain the ABD of plectin, plec1–339, or plec1–606. Additionally, the plec284–1154 fragment interacted with a C-terminal fragment of β4, containing the fourth FNIII repeat and the C terminus (β41570–1752). Because plec284–1154 did not interact with β41436–1667 and only weakly with β41667–1752, which share the fourth FNIII repeat and the extreme C-tail, respectively, with β41570–1752, it is likely that both these regions are required for strong binding.
The binding site on plectin for the CS of β4 could be allocated to a stretch of 267 amino acids (339–606), because a construct containing the first 606 amino acids of plectin (plec1–606), but not one that is truncated at position 339 (plec1–339) bound to β41382–1667. Both fragments also bound to β41115–1436, i.e., plec1–339 by virtue of its ABD and plec1–606 because it also contains the binding site for the CS of β4. The dispensability of the ABD for binding of plec1–606 to β41115–1436 was further analyzed by introducing two point mutations, D149S/D150T, in the ABD. We have previously shown that these mutations abrogate the binding of plec1–339 to a fragment of β4 containing the first pair of FNIII repeats and the complete CS (β41115–1457) (Litjens et al., 2003). In agreement with this finding, these mutations also eliminated the binding of plec1–606 to β41115–1382 or β41115–1457 (Δ1383–1437). We could, however, detect binding of the mutant plec1–606 fragment to β41115–1436. Together, these findings show the presence of a separate binding site on plec1–606 for the CS of β4. Unfortunately, binding of a fragment corresponding to the region 284–606 of plectin to β4 could not be demonstrated because of strong autotransactivation of this fragment.
Like the two fragments that share the region 1382–1436 of the CS, the C-terminal fragment of β4 (β41570–1752) that interacts with plec284–1154, also strongly binds to plec1–606. However, unexpectedly, binding of this β4 fragment is almost abolished by the above-mentioned double point mutation in the ABD that abrogates the binding of plec1–606 to β41115–1382. Of note is that the ABD itself does not bind β41570–1752. This suggests that the ABD is part of a large binding surface that also includes the region 284-1154 of the plakin domain of plectin. For binding in yeast, the stretch of amino acids 284–606 must be connected to either the N-terminal ABD or to the region after it, up to position 1154. The importance of sequences in the C-tail of β4 for proper functioning of α6β4 in HDs is underscored by the fact that homozygosity for a nonsense mutation in the β4 gene for this region (Q1714X) leads to a lethal form of junctional epidermolysis bullosa (Nakano et al., 2001). As a result of this mutation, the C-terminal β4 fragment (β41570–1714) is unable to bind to the plectin fragments plec1–606 and plec284–1154.
Together, these results show that there are two additional plectin binding sites on β4, one at the end of the CS (PBS-2, residues 1382–1436) and another in a fragment including the fourth FNIII repeat and the C-tail (PBS-3, residues 1570–1752) that mediate interaction with the plakin domain of plectin.
Coimmunoprecipitations Reveal the Importance of PBS-2 and PBS-3 in Binding to Plectin
To confirm the results obtained by the yeast two-hybrid assay, different HA-tagged plectin constructs were tested for association with the cytoplasmic domain of β4 (expressed as a chimeric construct with the extracellular and transmembrane domain of the IL2R) in COS-7 cells. On lysis of the cells and immunoprecipitation of the plectin constructs with anti-HA antibodies, associated IL2R/β4 was detected by immunoblotting.
As shown in Figure 2B, β4 was shown to be associated with all plectin constructs that contain the ABD, i.e., plec1–339, plec1–606, and plec1–1154. Furthermore, the IL2R/β4 chimera was coprecipitated with plec284–1154, which interacts with PBS-2 and PBS-3 on β4. This association, however, seemed somewhat weaker than that with plec1–339, which may point to a lower affinity. A β4 subunit in which the binding site for the plectin-ABD is mutated (β4R1281W) bound to plec1–606, plec284–1154, and plec1–1154. No association was detected with plec1–339 that lacks sequences capable of interacting with PBS-2 and PBS-3 on β4. Deletion of PBS-2 (β4Δ1383–1437) or truncation of β4 at position 1670, by which PBS-3 is removed (β41–1670), eliminated the binding to plec284–1154, whereas binding to plec1–339, plec1–606, or plec1–1154 was unaffected. When in the β4Δ1383–1437 and β41–1670 constructs R1281 had also been replaced, binding to these latter plectin constructs was either greatly reduced (β4Δ1383–1437, R1281W) or even abrogated (β41–1670, R1281W).
Together, these results confirm that plectin binds to β4 by three sites, PBS1–3. Furthermore, the results indicate that the affinity of β4 for PBS-2 and PBS-3 is weaker than that for PBS-1.
MD-EBS Keratinocytes Express a Rod-less Variant of Plectin
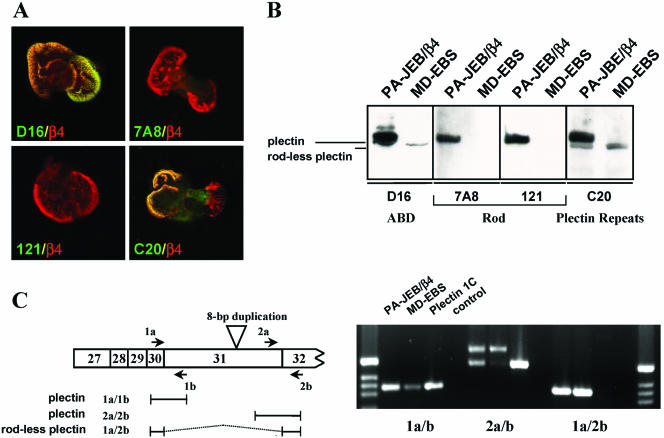
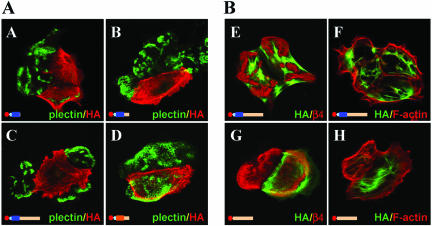
In further studies on the binding of β4 to plectin, we used MD-EBS keratinocytes (Geerts et al., 1999). These cells have been established from an EBS patient homozygous for an 8-base pair duplication in exon 31 (Smith et al., 1996), which encodes the entire rod domain of plectin (McLean et al., 1996). As a consequence, the cells did not react with the antiplectin mAbs 121 and 7A8 that recognize epitopes on the central rod domain of plectin (Foisner et al., 1994; Okumura et al., 1999). Unexpectedly, in further analyses, it became clear that the MD-EBS cells expressed a plectin variant that reacted with antibodies against the N-terminal plectin-ABD and the C-terminal plectin repeats and that was colocalized with β4 in HD-like structures (Figure 3A). Because a plectin variant lacking the central rod domain has been previously described in a variety of tissues (Elliott et al., 1997), we wondered whether the plectin molecule expressed in the MD-EBS cells is identical to this variant. Immunoblotting of cell lysates from PA-JEB/β4 cells with antibodies against the plectin-ABD and the C-terminus showed the presence of two closely spaced bands of ∼300 kDa, of which only the slower band reacted with the antibodies 121 and 7A8 (Figure 3B). By contrast, MD-EBS cells only contained the faster migrating band with which the antibodies against the ABD and the C-terminus of plectin gave positive reaction, whereas the mAbs 121 and 7A8 did not. Using RT-PCR with a primer just downstream of the rod domain, we were able to show the presence of a mRNA that does not contain the sequences encoding the rod (Figure 4C). This variant could be found in both PA-JEB/β4 keratinocytes as well as in MD-EBS cells. The RT-PCR also indicated that in MD-EBS cells, mRNA for the full-length plectin containing the rod domain was present. However, judging from the PCR, it was greatly reduced in quantity compared with the PA-JEB sample.
Figure 3.
MD-EBS cells express a rod-less variant of plectin. (A) Immunofluorescence of MD-EBS cells stained for β4 (red) and different domains of plectin (green). The rod domain of plectin is recognized by the mAbs 121 and 7A8, and the N-terminal ABD and C-terminal plectin repeats by the polyclonal antibodies D16 and C20, respectively. (B) Analysis by Western blotting of MD-EBS and PA-JEB/β4 cells by using different anti-plectin antibodies. Rod-less plectin, detected with D16 or C20, migrates just below wild-type (full-length) plectin. (C) PCR with specific primers to detect the presence of transcripts for rod-less plectin, on cDNA synthesized from mRNA of PA-JEB/β4 and MD-EBS cells. As a positive control, a cDNA construct of full-length plectin 1C was used.
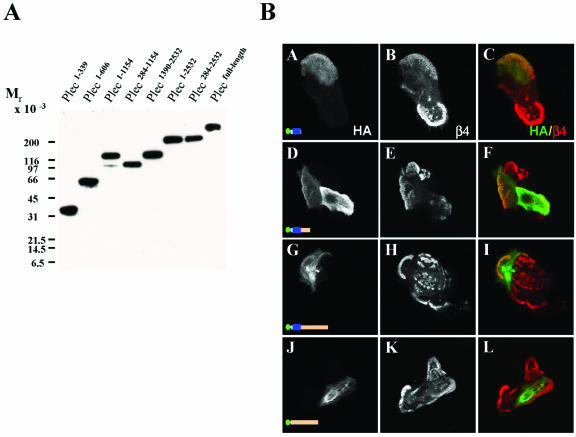
Figure 4.
Expression and distribution of different fragments of plectin in transfected COS-7 cells and MD-EBS keratinocytes. (A) Lysates of COS-7 cells, transfected with the indicated HA-tagged plectin constructs were analyzed by immunoblotting by using anti-HA antibodies. (B) MD-EBS keratinocytes were transiently transfected with cDNAs encoding HA-tagged plec1–339 (A–C), plec1–606 (D–F), plec1–1154 (G–I), or plec284–1154 (J–L). Cells were fixed and double immunolabeled for the different HA-tagged plectin mutants (green; A, D, G, and J) and β4 (red; B, E, H, and K). Merged images are shown in C, F, I, and L. Colocalized staining is yellow.
We conclude from these data that MD-EBS keratinocytes do not contain full-length plectin but that they express the plectin variant lacking the central rod domain. Furthermore, it is apparent from the results that dimerization of plectin via its central rod domain is not essential for its localization in HDs.
Influence of the Presence of β4 Binding Sites in the Plakin Domain on the Localization of Plectin into HDs
To determine the contribution of the additional β4 binding sites in the plakin domain of plectin on its localization into HD-like structures, we prepared a series of HA-tagged deletion mutants of plectin and expressed the truncated polypeptides in MD-EBS cells by transient transfection. The sizes of the mutant proteins were verified by immunoblotting the lysates of transiently transfected COS-7 cells with antibodies against the HA-tag (Figure 4A).
We first tested the distribution of the plectin-ABD plec1–339, and consistent with previous observations, this fragment was found to be largely colocalized with filamentous actin (our unpublished data) and only rarely together with α6β4 in HD-like structures (Figure 4B, A–C). Extension of the plectin-ABD at the C-terminus so that it contains sequences that can interact with PBS-2 and PBS-3 of β4 (plec1–606 and plec1–1154) did not obviously improve its localization in HD-like structures (Figure 4B, D–I). Plec284–1154, which interacts with PBS-2 and PBS-3 but not with PBS-1, was not colocalized with β4, but produced a filamentous staining pattern (Figure 4B, J–L). To ensure that the results were not unique for MD-EBS cells, the different plectin fragments were also transiently expressed in PA-JEB/β4 cells. The results were similar, except that in PA-JEB/β4 cells the HDs contained hardly any plec1–339, plec1–606, or plec1–1154, and these fragments frequently seemed to have a dominant negative effect on the localization of endogenous plectin in HDs (Figure 5, A–C). No such effect was seen with a plectin fragment (plec1–606) in which the ABD of plectin had been replaced by that of dystrophin (Figure 5D). Intriguingly, the plec1–1154 and plec284–1154 fragments often looked like dense, distorted filament structures: they were not colocalized with either β4 or with F-actin (Figure 5, E–H).
Figure 5.
Effects of transiently expressed fragments of plectin on the localization of endogenous plectin into HDs and their localization relative to that of β4 and F-actin in PA-JEB/β4 keratinocytes. (A) PA-JEB/β4 keratinocytes were transiently transfected with cDNAs encoding HA-tagged plec1–339 (A), plec1–606 (B), plec1–1154 (C), or plec-1C1–65/dystrophin-ABD11–337/plec339–606 chimera (D) and stained with mAb 121 against the plectin rod domain to detect endogenous plectin (green) and mAb anti-HA to detect the expressed HA-tagged proteins (red). Note that the plectin fragments containing the ABD domain of plectin, but not the one containing the ABD of dystrophin, exert a dominant-negative effect on the hemidesmosomal localization of endogenous plectin. In B, PA-JEB/β4 cells were transfected with plec1–1154 (E and F) and plec284–1154 (G and H). The expressed proteins are in green and the integrin β4 subunit (E and G) and F-actin (F and H) are in red. Colocalization is yellow.
These data suggest that the presence of the additional β4 binding sites in the plakin domain, are not sufficient to recruit plectin into HDs and furthermore that the additional β4 binding sites do not noticeably contribute to the localization of the ABD in HDs.
Extension of a Plectin Fragment to Include the Rod Domain Allows Recruitment into HDs, Even in the Absence of the ABD in MD-EBS and PA-JEB/β4 Cells
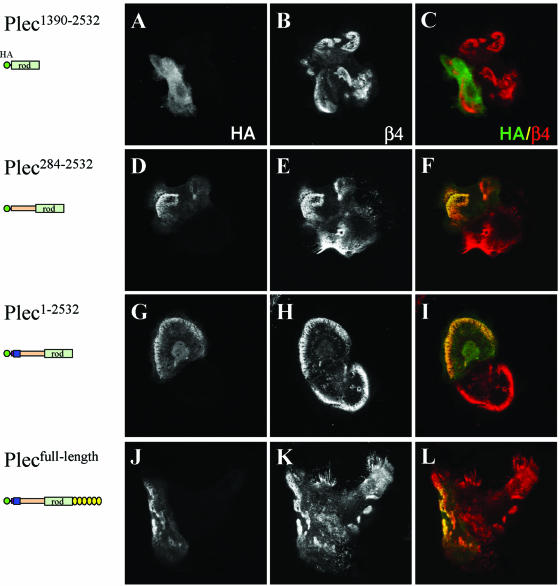
The rod domain of plectin is considered to be involved in homodimerization of the plectin molecule (Wiche, 1998) and thus, through its ability to dimerize the β4 binding sites, could contribute to an efficient localization of it in HDs. To test this possibility, we have expressed various plectin fragments containing the central rod domain in MD-EBS keratinocytes. The rod domain by itself (plec1390–2532) is not localized in HD-like structures (Figure 6, A–C). However, different N-terminal plectin constructs that contain either two of the three β4 binding sites (those that are located on the plakin domain, plec284–2532), or all three (the ABD and plakin domain plec1–2532), together with the rod domain did become colocalized with β4 in HD-like structures (Figure 6, D–I). The number of transfected cells in which the two mutant plectin proteins were colocalized in HD-like structures was small (∼5–10%) but clearly larger than that of cells that were transfected with either of the corresponding plectin constructs lacking the rod domain. No further improvement was obtained when a full-length plectin molecule was used (Figure 6, J and K), suggesting that most, if not all, of the important information for the localization of plectin in HDs is contained within the N-terminal end of this molecule. Similar results were obtained when PA-JEB/β4 cells were used (our unpublished data).
Figure 6.
N-terminal plectin fragments containing the rod domain are localized in HD-like structures. MD-EBS keratinocytes were transiently transfected with HA-tagged plec1390–2532 (A–C), plec284–2532 (D–F), plec1–2532 (G–I), or full-length plectin (J–L). Cells were stained for HA-tagged proteins (A, D, G, and J) and β4 (B, E, H, and K). Merged images are shown in C, F, I, and L. Colocalization is yellow.
Together, these results show that the β4 binding sites in the plakin domain, although not able to mediate recruitment of this domain as a monomer, can do so when they are dimerized by the rod domain.
Roles of PBS-2 and PBS-3 in the Recruitment of Components into HDs
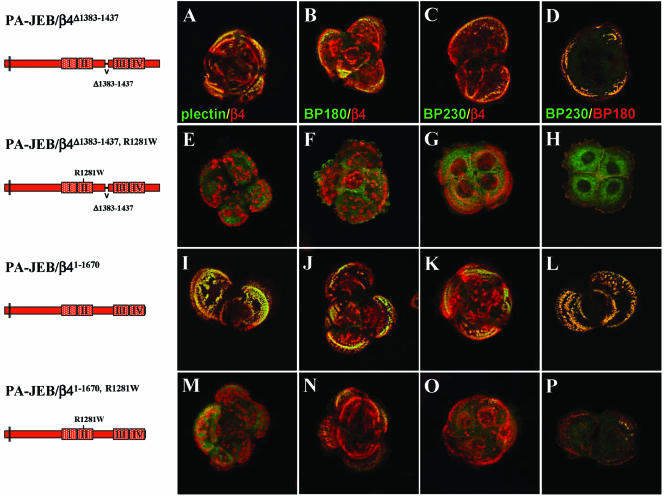
Despite the fact that β4R1281W contains binding sites for BP180 and BP230, it is unable to efficiently recruit these molecules into HDs (Geerts et al., 1999; Koster et al., 2001). One possible explanation is that the β4 cytoplasmic domain is folded and that the binding sites for BP180 and BP230 in the carboxy-terminal half of the molecule are not accessible (Koster et al., 2003). Similarly, it may explain why β4R1281W is unable to recruit plectin in spite of the fact that PBS-2 and PBS-3 are intact in this mutant β4 subunit. Indeed, we have previously shown that sequences in the CS of β4 can interact with sequences in its C-tail, and thus that the β4 cytoplasmic domain can adapt a folded conformation by intramolecular interaction. Interestingly, the sites involved in β4 self-association overlap with those that mediate binding to plectin. Therefore, their deletion inevitably will also destroy binding of plectin to PBS-2 and PBS-3. To examine whether the deletion of PBS-2 or PBS-3 in β4 can relieve an autoinhibitory effect of the folding of the β4 cytoplasmic domain on the binding to plectin, BP180, and BP230, different β4 constructs have been generated in which either PBS-2 or PBS-3 had been deleted. First, we tested the effects of deleting these sites in wild-type β4 on the ability of these mutants to recruit plectin. Consistent with the observation that a β4 subunit truncated at position 1355 recruits plectin, the stable expression of β4Δ1383–1437, which lacks PBS-2, or β41–1667, which lacks PBS-3, resulted in the incorporation of plectin into HDs (Figure 7, A and I). However, in contrast to the β41–1355 mutant, β41–1667, and to a lesser extent β4Δ1383–1437, did recruit BP180 and BP230 into HDs (Figure 7, B–D and J–L). When R1281 was also replaced by tryptophan in these constructs, not only the recruitment of plectin was prevented but also that of BP180 and BP230 was severely compromised. In fact, the amount of BP180 and BP230 in HDs of PA-JEB cells that stably express β4R1281W, β4Δ1383–1437, R1281W, or β41–1667, R1281W was similar. Thus, the prevention of intramolecular folding of the β4 cytoplasmic domain does not result in a more efficient localization of plectin, BP180 or BP230 into HDs.
Figure 7.
Distribution of HD components in PA-JEB keratinocytes expressing different β4 mutants. PA-JEB cells, stably expressing β4Δ1383–1437 (A–D), β4Δ1383–1437, R1281W (E–H), β41–1670 (I–L), or β41–1670, R1281W (M–P), were double immunostained for plectin (green) and β4 (red) (A, E, I, and M), for BP180 (green) and β4 (red) (B, F, J, and N), for BP230 (green) and β4 (red) (C, G, K, and O), or for BP230 (green) and BP180 (red) (D, H, L, and P). Colocalization is yellow.
DISCUSSION
The binding of α6β4 to plectin is a critical step in the formation of HDs in cultured keratinocytes. When this binding cannot occur, the recruitment of BP180 and BP230 into HDs is severely compromised. In previous studies, we have shown that the ABD of plectin interacts with the first pair of FNIII repeats and a small fragment of the CS (PBS-1, residues 1115–1355) of β4 (Geerts et al., 1999; Koster et al., 2001; Litjens et al., 2003). In this study, we have further evaluated the binding of plectin to the cytoplasmic domain of β4. In line with observations made by Rezniczek et al. (1998), we show that, in addition to the ABD, the plakin domain of plectin also interacts with the integrin β4 subunit. In this interaction, the CS (PBS-2, residues 1382–1436) and the FNIII-4/C-tail (PBS-3, residues 1570–1752) of β4 are involved. Importantly, these additional binding sites are not required for the recruitment of plectin into HDs. We suggest that their function might be to stabilize the binding of the plectin-ABD to β4 and/or to support a conformation of the β4 cytoplasmic domain that is optimal for its interaction with other HD components. In addition, we identified two proline residues in the CS of β4 that play a critical role in plectin binding and thus in the formation of HDs.
Binding of a Single Plectin Molecule to the Integrin β4 Subunit in Cells
Because the β4 cytoplasmic domain has two separate binding regions for the plakin domain of plectin, it is possible that a single β4 subunit can bind two plectin molecules (Figure 8). Moreover, the ABD not only mediates binding to PBS-1 but also seems to be involved in the binding of plectin to PBS-3. A role of the plectin-ABD in the binding to PBS-3 of β4 is suggested because a double point mutation in the plectin-ABD that previously has been shown to abrogate the binding of this domain to PBS-1 on β4 (Litjens et al., 2003) reduces the binding activity of plec1–606 for β41570–1752. Arginine 1281, which is located in the loop region that connects two β strands (EC') in the second FNIII repeat, is critical for binding of the ABD to PBS-1 (Koster et al., 2001). In the fourth FNIII repeat, arginine is present at an identical position (R1630) and like R1281, this residue, might also be involved in binding to the plectin-ABD. However, the binding of plec1–606 to PBS-3 (β41570–1752) was not affected when R1630 was replaced by tryptophan (our unpublished data). Thus, there is no evidence that this amino acid is critical for binding of the plectin-ABD to the PBS-3 of β4. Furthermore, it is unlikely that PBS-3 is a site to which a second plectin molecule can bind, because plectin is not recruited into HDs of retrovirally transduced PA-7EB cells when PBS-1 alone, or in combination with PBS-2, has been deleted from β4 (Figure 7).
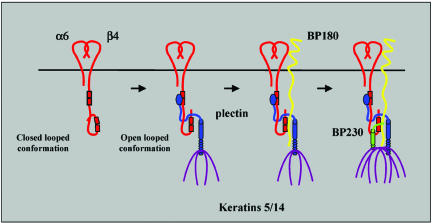
Figure 8.
Model for the assembly of hemidesmosomes in cultured keratinocytes. There may be two conformational states of the β4 subunit: an inactive one in which the β4 cytoplasmic domain is folded backwards into a closed loop by intramolecular association, and, when plectin is bound to β4, an active one (an open-looped conformation). For rendering the β4 cytoplasmic domain active, binding of the ABD of plectin to the first pair of FNIII repeats might be required to allow subsequent binding of the plakin domain to the CS and the C-terminal tail. It is believed that this sequence of interactions supports a conformation of the cytoplasmic domain of β4 that renders it able to optimally interact with BP180 via its third FNIII repeat and with BP230 via its third and fourth FNIII repeats of β4. For the recruitment of BP180, it must interact with both β4 and plectin. The recruitment of BP230 into HDs is facilitated through interaction with both BP180 and β4.
The results suggest that there is one large binding interface on β4 that interacts with a single plectin molecule. Consistent with a model in which one β4 subunit interacts with a single plectin molecule, mutations such as R1281W and Q1714X are likely to have varying effects on the formation and/or stability of HDs in afflicted patients. The effects of R1281W will be primarily on the formation of HDs because it prevents the binding of plectin to β4 and therefore, the subsequent binding of BP180 and BP230. On the contrary, Q1714X will affect the stability of HDs. This mutation will only weaken the binding of β4 to plectin, but not abrograte it. Therefore, the consequences of it in cultured keratinocytes are less dramatic, and defects only become apparent under in vivo conditions of mechanical stress.
Binding of Plectin to PBS-2 and PBS-3 Depends on the Binding to PBS-1
The finding that β4R1281W is unable to recruit plectin into HDs suggests that in this mutant β4 subunit, PBS-2 and PBS-3 are not accessible for interaction with plectin because the β4 cytoplasmic domain is folded into a closed loop (Figure 8). A similar model was presented to explain why β4R1281W is unable to recruit BP180 and BP230, despite the fact that the binding sites for these two molecules were intact (Koster et al., 2003). Indeed, the sequences that mediate an intramolecular association of the β4 cytoplasmic domain overlap with those involved in the binding to plectin (Geerts et al., 1999; Nievers et al., 2000). However, when the intramolecular association was disrupted by also mutating PBS-2 or PBS-3, then plectin, BP180, or BP230 were still not recruited by β4R1281W into HDs. One could interpret this result as evidence that our model is not correct. However, another explanation for the fact that plectin is not recruited might be that the affinity of PBS-2 or PBS-3 by themselves is insufficient to mediate this recruitment and that it can only occur when both sites are available. Consistent with the latter notion, no binding of plec284–1154 to β41–1670, R1281W was seen in the biochemical assays and the binding to β4Δ1383–1437, R1281W was only weak. Therefore, the possibility remains that an intramolecular association of the β4 cytoplasmic domain is responsible for the inability of plectin to interact with PBS-2 and PBS-3. The mechanism by which PBS-2 and PBS-3 are exposed is not known. Perhaps, the binding of the ABD to PBS-1 induces a conformational change in the cytoplasmic domain of β4, by which the PBS-2 and PBS-3 become uncovered and available for additional interactions with plectin (Figure 8). The implication of such a model would be that without the ABD, no plectin can be recruited into HDs. The questions remains, how does plec284–2532 colocalize with β4 in HDs of EBS-MD cells? We believe that the explanation for this apparent discrepancy might lie in the fact that the EBS-MD cells are not really plectin deficient; they express a rod-less plectin variant. It is therefore feasible that the rod-less plectin variant, when bound to β4, has the same unfolding effect on its cytoplasmic domain as wild-type plectin. Clearly, more experiments are required to determine whether this model is correct.
Requirements for the Recruitment of BP180 and BP230 into HDs
The PBS-2 and PBS-3 in the β4 cytoplasmic domain are different from the sites that mediate binding to BP180 and BP230, which are located in the third FNIII repeat and in the region comprising the C-terminal part of the CS and the third and fourth FNIII repeats, respectively (Koster et al., 2003). Hence, in contrast to the effect on the binding of plectin, the deletion of PBS-2 or PBS-3 from β4R1281W should not affect the ability of the mutant β4 cytoplasmic domains (β4Δ1383–1437, R1281W and β41–1670, R1281W) to interact with BP180 and BP230. The only defect of these two mutants is the inability to engage in an intramolecular association. Nevertheless, the two double mutant β4 subunits are not able to recruit BP180 and BP230. Thus, it does not seem likely that folding of the β4 cytoplasmic domain into a closed loop is responsible for the failure of β4R1281W to recruit these molecules into HDs. Presumably, BP180 and BP230 can only be efficiently recruited into HDs, when BP180 not only interacts with β4 but also with plectin. Indeed, we have shown previously that BP180 can directly interact with plectin (Koster et al., 2003). Moreover, in keratinocytes from GABEB patients, which lack BP180, less plectin is found to be associated with β4 in HDs (Koster et al., 2003). However, it should be emphasized that our data do not exclude the possibility that a conformational change in the β4 cytoplasmic domain, induced by the binding to plectin, also contributes to a more efficient recruitment of BP180 and BP230. Specifically, in the plectin induced open looped conformation of β4, the binding sites for BP180 on these two molecules might be in optimal positions to each other for binding to occur. Once BP180 is bound, BP230 will become incorporated into HDs through its interaction with β4 and BP180 (Koster et al., 2003).
Role of Proline Residues in β4 Binding to Plectin
The region 1328–1355 of β4 is crucial for the recruitment of plectin into HDs (Niessen et al., 1997b; Schaapveld et al., 1998). A β4 subunit that is truncated at position 1355, but not one that is truncated at position 1328, can mediate this recruitment. In the present study we show that two proline residues (P1330 and P1333) in this region 1328–1355 play a critical role in the recruitment of plectin into HDs. When these prolines are substituted by alanines in β41–1355 or in full-length β4, recruitment of plectin into HDs was severely compromised. It is possible that the proline residues in the CS directly contribute to the binding of the ABD to the first pair of FNIII repeats. However, because the effect of mutating these residues on the binding of β41115–1355 to the plectin-ABD in yeast is relatively weak, it seems more likely that they affect the structure of the protein. The increase in binding activity of the plectin-ABD to β4, when the CS is extended past residue 1355 indicates that this region has a positive influence on the binding of the ABD to β4. This positive effect of the CS, however, is almost completely abrogated when the two prolines are substituted by alanines. We believe that the proline residues do not directly participate in the binding to plectin, but instead keep the remaining part of the CS in such a position that the exposure of the PBS-1 is optimal. As a result of the alanine replacements, the binding of plectin is severely compromised.
N-Terminal Fragments of Plectin Are Distributed into an Aberrant Filamentous Network
In transfected keratinocytes, plec1–1154 was often distributed in a densely distorted filamentous pattern that only partly overlapped with that of F-actin. A similar pattern was observed with plec284–1154, which lacked the ABD, suggesting that the plakin domain in plec1–1154 is largely responsible for this abnormal distribution pattern. Moreover, the introduction of an analogous construct of BP230 (BP2301–887) showed the same results (our unpublished data). It seems likely that the aberrant distribution pattern of plec284–1154 is due to its truncation and the exposure of a cryptic binding site(s) for one or more filamentous proteins, because a larger plectin fragment including the rod domain was not distributed in this abnormal pattern. In fact, plec284–2532, as well as plec1–2532, was localized in HD-like structures in transfected cells. Although it is clear that the masking of such cryptic binding sites on the plakin domain might be essential for its localization into HDs, dimerization of the β4 binding sites, which could occur because of the presence of the heptad repeats in the rod domain, may have facilitated this localization. The notion that dimerization of plectin via the rod domain is not a prerequisite for its localization into HDs is illustrated by the fact that a rod-less variant that is expressed in the MD-EBS cells also becomes localized in HDs.
MD-EBS Cells Contain Rod-less Plectin That Is Recruited to HDs
Because antibodies against the rod domain of plectin did not react with MD-EBS cells, it was concluded that they do not contain plectin (Geerts et al., 1999). We now show that the MD-EBS cells express a plectin variant lacking the rod domain, which reacts with antibodies against the N- and C-terminal ends of the protein. As shown by RT-PCR, this rod-less variant is produced as a result of alternative RNA splicing by which exon 31, which encodes the entire rod domain of plectin, is omitted from the primary transcript. An identical variant has been described by Elliott et al. (1997) and is expressed in a variety of tissues.
It is of interest that many mutations in MD-EBS patients are missense mutations that are confined to exon 31 of the PLEC1 gene and cause premature termination of protein translation (Uitto et al., 1996). Such mutations are predicted to result in low steady-state levels of plectin mRNA due to nonsense-mediated mRNA decay, thereby resulting in a loss of protein expression. In these cells, differentially spliced transcripts that do not contain these mutations are produced and translated into proteins. Therefore, patients carrying missense mutations in exon 31 may not express full-length plectin, but may still express the normal rod-less variant. Because this variant is still localized in HDs, it could fulfill important functions in HD assembly and integrity. The presence of these rod-less variants may also explain why the phenotype of plectin-null mice is much more dramatic that of MD-EBS patients; they die almost immediately after birth (Andrä et al., 1997).
Acknowledgments
We thank Dr. P. James for the yeast strain PJ69-4A and Drs. K. Owaribe and T. Hashimoto for providing antibodies. We are grateful to Dr. C.P.E. Engel-friet for critical reading of the manuscript. This work was supported by grants from the Dystrophic Epidermolysis Bullosa Research Association (DEBRA Foundation, Crowthorne, United Kingdom) and the Dutch Cancer Society (NKI 99-2039).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0697. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0697.
Abbreviations used: ABD, actin-binding domain; BP, bullous pemphigoid; CS, connecting segment; FNIII, fibronectin type III repeat; HD, hemidesmosome; IL2R, interleukin 2 receptor; MD-EBS, muscular dystrophy associated with epidermolysis bullosa simplex; PA-JEB, pyloric atresia associated with junctional epidermolysis bullosa; PBS, plectin binding site.
References
- Alexandropoulos, K., Cheng, G., and Baltimore, D. (1995). Proline-rich sequences that bind to Src homology 3 domains with individual specificities. Proc. Natl. Acad. Sci. USA 92, 3110-3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrä, K., Lassmann, H., Bittner, R., Shorny, S., Fässler, R., Propst, F., and Wiche, G. (1997). Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 11, 3143-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori, L., Koch, P.J., Niessen, C.M., Erkeland, S., van Leusden, M.R., and Sonnenberg, A. (1997). The localization of bullous pemphigoid antigen 180 (BP180) in hemidesmosomes is mediated by its cytoplasmic domain and seems to be regulated by the β4 integrin subunit. J. Cell Biol. 136, 1333-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borradori, L., and Sonnenberg, A. (1999). Structure and function of hemidesmosomes: more than simple adhesion complexes. J. Investig. Dermatol. 112, 411-418. [DOI] [PubMed] [Google Scholar]
- Brown, A., Bernier, G., Mathieu, M., Rossant, J., and Kothary, R. (1995). The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1. Nat. Genet. 10, 301-306. [DOI] [PubMed] [Google Scholar]
- Dowling, J., Yu, Q.C., and Fuchs, E. (1996). β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134, 559-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott, C.E., Becker, B., Oehler, S., Castãnón, M.J., Hauptmann, R., and Wiche, G. (1997). Plectin transcript diversity: identification and tissue distribution of variants with distinct first coding exons and rodless isoforms. Genomics 42, 115-125. [DOI] [PubMed] [Google Scholar]
- Foisner, R., Feldman, B., Sander, L., Seifert, G., Artlieb, U., and Wiche, G. (1994). A panel of monoclonal antibodies to rat plectin: distinction by epitope mapping and immunoreactivity with different tissues and cell lines. Acta Histochem. 96, 421-438. [DOI] [PubMed] [Google Scholar]
- Gache, Y., Chavanas, S., Lacour, J.P., Wiche, G., Owaribe, K., Meneguzzi, G., and Ortonne, J.P. (1996). Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Investig. 97, 2289-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts, D., Fontao, L., Nievers, M.G., Schaapveld, R.Q.J., Purkis, P.E., Wheeler, G.N., Lane, E.B., Leigh, I.M., and Sonnenberg, A. (1999). Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 147, 417-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges-Labouesse, E., Messaddeq, N., Yehia, G., Cadalbert, L., Dierich, A., and Le Meur, M. (1996). Absence of integrin α6 leads to epidermolysis bullosa and neonatal death in mice. Nat. Genet. 13, 370-373. [DOI] [PubMed] [Google Scholar]
- Giudice, G.J., Emery, D.J., and Diaz, L.A. (1992). Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J. Investig. Dermatol. 99, 243-250. [DOI] [PubMed] [Google Scholar]
- Green, K.J., Virata, M.L., Elgart, G.W., Stanley, J.R., and Parry, D.A. (1992). Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: members of a new gene family involved in organization of intermediate filaments. Int. J. Biol. Macromol. 14, 145-153. [DOI] [PubMed] [Google Scholar]
- Guo, L., Degenstein, L., Dowling, J., Yu, Q.C., Wollmann, R., Perman, B., and Fuchs, E. (1995). Gene targeting of BPAG 1, abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell 81, 233-243. [DOI] [PubMed] [Google Scholar]
- Hashimoto, T., Amagai, M., Ebihara, T., Gamou, S., Shimizu, N., Tsubata, T., Hasegawa, A., Miki, K., and Nishikawa, T. (1993). Further analyses of epitopes for human monoclonal anti-basement membrane zone antibodies produced by stable human hybridoma cell lines constructed with Epstein-Barr virus transformants. J. Investig. Dermatol. 100, 310-315. [DOI] [PubMed] [Google Scholar]
- Hieda, Y., Nishizawa, Y., Uematsu, J., and Owaribe, K. (1992). Identification of a new hemidesmosomal protein, HD 1, a major, high molecular mass component of isolated hemidesmosomes. J. Cell Biol. 116, 1497-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkinson, S.B., and Jones, J.C.R. (2000). The N terminus of the transmembrane protein BP180 interacts with the N-terminal domain of BP230, thereby mediating keratin cytoskeleton anchorage to the cell surface at the site of the hemidesmosome. Mol. Biol. Cell. 11, 277-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonkman, M.F., de Jong, M.C., Heeres, K., Pas, H.H., van der Meer, J.B., Owaribe, K., Martinez de Velasco, A.M., Niessen, C.M., and Sonnenberg, A. (1995). 180-kD bullous pemphigoid antigen (BP180) is deficient in generalized atrophic benign epidermolysis bullosa. J. Clin. Investig. 95, 1345-1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster, J., Geerts, D., Favre, B., Borradori, L., and Sonnenberg, A. (2003). Analysis of the interactions between BP180, BP230, plectin and the integrin α6β4 important for hemidesmosome assembly. J. Cell Sci. 116, 387-399. [DOI] [PubMed] [Google Scholar]
- Koster, J., Kuikman, I., Kreft, M., and Sonnenberg, A. (2001). Two different mutations in the cytoplasmic domain of the integrin β4 subunit in nonlethal forms of epidermolysis bullosa prevent interaction of β4 with plectin. J. Investig. Dermatol. 117, 1405-1411. [DOI] [PubMed] [Google Scholar]
- Leung, C.L., Zheng, M., Prater, S.M., and Liem, R.K. (2001). The BPAG1 locus: Alternative splicing produces multiple isoforms with distinct cytoskeletal linker domains, including predominant isoforms in neurons and muscles. J. Cell Biol. 154, 691-697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litjens, S.H.M., Koster, J., Kuikman, I., van Wilpe, S., de Pereda, J.M., and Sonnenberg, A. (2003). Specificity of binding of the plectin actin-binding domain to β4 integrin. Mol. Biol. Cell 14, 4039-4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath, J.A., Gatalica, B., Christiano, A.M., Li, K., Owaribe, K., McMillan, J.R., Eady, R.A.J., and Uitto, J. (1995). Mutations in the 180-kD bullous pemphigoid antigen (BPAG2), a hemidesmosomal transmembrane collagen (COL17A1), in generalized atrophic benign epidermolysis bullosa. Nat. Genet. 11, 83-86. [DOI] [PubMed] [Google Scholar]
- McLean, W.H.I., et al. (1996). Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 10, 1724-1735. [DOI] [PubMed] [Google Scholar]
- Murgia, C., Blaikie, P., Kim, N., Dans, M., Petrie, H.T., and Giancotti, F.G. (1998). Cell cycle and adhesion defects in mice carrying a targeted deletion of the integrin β4 cytoplasmic domain. EMBO J. 17, 3940-3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, A., Pulkkinen, L., Murrell, D., Rico, J., Lucky, A.W., Garzon, M., Stevens, C.A., Robertson, S., Pfendner, E., and Uitto, J. (2001). Epidermolysis bullosa with congenital pyloric atresia: novel mutations in the 4 integrin gene (ITGB4) and genotype/phenotype correlations. Pediatr. Res. 49, 618-626. [DOI] [PubMed] [Google Scholar]
- Nishizawa, Y., Uematsu, J., and Owaribe, K. (1993). HD4, a 180 kDa bullous pemphigoid antigen, is a major transmembrane glycoprotein of the hemidesmosome. J. Biochem. 113, 493-501. [DOI] [PubMed] [Google Scholar]
- Niessen, C.M., Hogervorst, F., Jaspars, L.H., de Melker, A.A., Delwel, G.O., Hulsman, E.H.M., Kuikman, I., and Sonnenberg, A. (1994). The 64 integrin is a receptor for both laminin and kalinin. Exp. Cell Res. 211, 360-367. [DOI] [PubMed] [Google Scholar]
- Niessen, C.M., Hulsman, E.H.M., Rots, E.S., Sánchez-Aparicio, P., and Sonnenberg, A. (1997a). Integrin α6β4 forms a complex with the cytoskeletal protein HD1 and induces its redistribution in transfected COS-7 cells. Mol. Biol. Cell 8, 555-566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen, C.M., Hulsman, E.H.M., Oomen, L.C.J.M., Kuikman, I., and Sonnenberg, A. (1997b). A minimal region on the integrin β4 subunit that is critical to its localization in hemidesmosomes regulates the distribution of HD1/plectin in COS-7 cells. J. Cell Sci. 110, 1705-1716. [DOI] [PubMed] [Google Scholar]
- Nievers, M.G., Schaapveld, R.Q.J., Oomen, L.C.J.M., Fontao, L., Geerts, D., and Sonnenberg, A. (1998). Ligand-independent role of the β4 integrin subunit in the formation of hemidesmosomes. J. Cell Sci. 111, 1659-1672. [DOI] [PubMed] [Google Scholar]
- Nievers, M.G., Kuikman, I., Geerts, D., Leigh, I.M., and Sonnenberg, A. (2000). Formation of hemidesmosome-like structures in the absence of ligand binding by the α6β4 integrin requires binding of HD1/plectin to the cytoplasmic domain of the β4 integrin subunit. J. Cell Sci. 113, 963-973. [DOI] [PubMed] [Google Scholar]
- Okumura, M., Uematsu, J., Hirako, Y., Nishizawa, Y., Shimizu, H., Kido, N., and Owaribe, K. (1999). Identification of the hemidesmosomal 500 kDa protein (HD1) as plectin. J. Biochem. 126, 1144-1150. [DOI] [PubMed] [Google Scholar]
- Rezniczek, G.A., de Pereda, J.M., Reipert, S., and Wiche, G. (1998). Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeleton: direct interaction between the β4 subunit and plectin at multiple molecular sites. J. Cell Biol. 141, 209-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper, K., Gregory, S.L., and Brown, N.H. (2002). The `Spectraplakins': cytoskeletal giants with characteristics of both spectrin and plakin families. J. Cell Sci. 115, 4215-4225. [DOI] [PubMed] [Google Scholar]
- Ruhrberg, C., and Watt, F.M. (1997). The plakin family: versatile organizers of cytoskeletal architecture. Curr. Opin. Genet. Dev. 7, 392-397. [DOI] [PubMed] [Google Scholar]
- Ruzzi, L., Gagnoux-Palacios, L., Pinola, M., Belli, S., Meneguzzi, G., D'Alessio, M., and Zambruno, G. (1997). A homozygous mutation in the integrin α6 gene in junctional epidermolysis bullosa with pyloric atresia. J. Clin. Investig. 99, 2826-2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaapveld, R.Q.J., Borradori, L., Geerts, D., van Leusden, M.R., Kuikman, I., Nievers, M.G., Niessen, C.M., Steenbergen, R.D.M., Snijders, P.J.F., and Sonnenberg, A. (1998). Hemidesmosome formation is initiated by the β4 integrin subunit, requires complex formation of β4 and HD1/plectin, and involves a direct interaction between β4 and the bullous pemphigoid antigen 180. J. Cell Biol. 142, 271-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, F.J.D., et al. (1996). Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat. Genet. 13, 450-457. [DOI] [PubMed] [Google Scholar]
- Sonnenberg, A., Calafat, J., Janssen, H., Daams, H., van der Raaij-Helmer, L.M.H., Falcioni, R., Kennel, S.J., Aplin, J.D., Baker, J., Loizidou, M., and Garrod, D. (1991). Integrin α6β4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J. Cell Biol. 113, 907-917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley, J.R., Hawley-Nelson, P., Yuspa, S.H., Shevach, E.M., and Katz, S.I. (1981). Characterization of bullous pemphigoid antigen: a unique basement membrane protein of stratified squamous epithelia. Cell 24, 897-903.s [DOI] [PubMed] [Google Scholar]
- Stepp, M.A., Spurr-Michaud, S., Tisdale, A., Elwell, J., and Gipson, I.K. (1990). α6β4 integrin heterodimer is a component of hemidesmosomes. Proc. Natl. Acad. Sci. USA 87, 8970-8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk, L.M.Th, Geuijen, C.A.W., Oomen, L.C.J.M., Calafat, J., Janssen, H., and Sonnenberg, A. (2000). The tetraspan molecule CD151, a novel constituent of hemidesmosomes, associates with the integrin α6β4 and may regulate the spatial organization of hemidesmosomes. J. Cell Biol. 149, 969-982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto, J., Pulkkinen, L., Smith, F.J.D., and McLean, W.H.I. (1996). Plectin and human genetic disorders of the skin and muscle. The paradigm of epidermolysis bullosa with muscular dystrophy. Exp. Dermatol. 5, 237-246. [DOI] [PubMed] [Google Scholar]
- van der Neut, R., Krimpenfort, P., Calafat, J., Niessen, C.M., and Sonnenberg, A. (1996). Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat. Genet. 13, 366-369. [DOI] [PubMed] [Google Scholar]
- Vidal, F., Aberdam, D., Miquel, C., Christiano, A.M., Pulkkinen, L., Uitto, J., Ortonne, J.P., and Meneguzzi, G. (1995). Integrin β4 mutations associated with junctional epidermolysis bullosa with pyloric atresia. Nat. Genet. 10, 229-234. [DOI] [PubMed] [Google Scholar]
- Wiche, G. (1998). Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 111, 2477-2486. [DOI] [PubMed] [Google Scholar]