Abstract
Context
The validity of urinary correction standards has not been established for most analytes.
Methods
We compared urinary creatinine and specific gravity as dilution correction standards for cotinine in a community-based study of smokers.
Results
Models of blood cotinine regressed against CR or SG (measured by total soluble solids) significantly improved the fit compared to a model without a dilution measure (P<0.01). There were no differences in model fit between CR- and SG-corrected values. Both CR and SG were significant predictors of urinary cotinine regressed against cigarettes smoked per day (P<0.01).
Conclusion
CR and SG are valid and interchangeable correction standards.
Keywords: creatinine, specific gravity, cotinine, biomarkers, total solids
INTRODUCTION
Biological exposure to industrial chemicals and xenobiotics is often measured in spot urine samples. Cotinine, the major metabolite of nicotine, is one such exposure that is frequently measured to study cancer risk and nicotine dependence. The concentration of urinary cotinine is usually expressed per units of creatinine to correct for urinary dilution since creatinine metabolism and excretion are believed to be in steady state (Chadha, Garg & Alon 2001; Boeniger, Lowry & Rosenberg 1993). However, variation in creatinine excretion by muscle mass, age, sex, race and acute protein intake can complicate comparisons between groups (James et al. 1998; Lew & Bosch 1991; Barr et al. 2005).
Differences in urinary dilution can be corrected for by measuring urinary creatinine (CR) or specific gravity (SG), which is the ratio of the relative density of urine to water and a measure of total soluble solids. A convenient and valid way to estimate SG is by the use of a handheld refractometer, which measures the angle of refraction between air and an aqueous solution. The refractive index can be converted to total soluble solids (Ross & Neely 1983). The most abundant molecules in urine are urea, electrolytes, CR and other metabolic waste products, but SG might also be affected by medical conditions that increase urinary glucose and protein amounts, which are normally at low levels or absent in urine. Like CR, SG decreases with age due to loss of muscle mass (Moriguchi et al. 2005). The effects of age, sex, body mass and other factors may differ between CR and SG (Suwazono et al. 2005).
Tobacco smoke exposure is often assessed using biomarkers including the major nicotine metabolite, cotinine. For studies requiring the quantitative assessment of exposure, blood is recognized as the fluid of choice for the measurement of cotinine. However, measurement of cotinine in urine is quite common because it is more easily obtained, particularly in population studies. Studies of urinary cotinine usually correct for dilution using CR concentrations. Urine specific gravity has not been used as a correction factor although it has been proposed that both CR and SG are equally effective measures (Haddow et al 1994). Yet the validity of both urinary CR and SG as correction standards for cotinine concentrations remains uncertain (Tricker, 2006). The Pearson correlation between blood cotinine and urinary cotinine was reported to either increase (Thompson et al. 1990) or decrease (Jatlow, McKee & O’Malley 2003) after correction for urinary CR. Further, the differential variability between urinary CR and SG by host-specific factors further complicates the choice of a correction factor (Alessio et al. 1985; Berlin et al. 1985).
In this study, urinary CR and SG were evaluated using regression techniques as correction factors for measuring urine cotinine concentrations, and in validating urinary cotinine as a biomarker of blood cotinine concentrations. We also determined the relative contribution in explaining variability in urinary cotinine when assessing exposure to cigarette smoke.
METHODS
Study Population
We recruited participants in a study of cigarette smoke biomarkers from 1995–2004. The study design and the biological collection and processing protocols were described previously (Richie Jr. et al. 1997). In brief, all subjects were non-Hispanic black and white adult smokers who lived in Yonkers, Mt. Vernon or their surrounding communities in New York. Subjects were screened for a history of major metabolic disorders. Persons with diabetes and other conditions were excluded. The subjects smoked at least five cigarettes per day for one or more years and did not use other tobacco products. All subjects fasted overnight and provided a urine and blood specimen during a morning interview. Samples were immediately put on ice and stored at −80° C. Trained interviewers administered a structured questionnaire that contained items on cigarette smoking history including cigarette brands, cigarettes smoked per day (cpd), age at smoking onset, and total years of smoking. The questionnaire also included medical history to verify the initial exclusion criteria. All subjects signed a consent form that was approved by the Institutional Review Board of the Institute for Cancer Prevention.
Analysis of biomarkers
Enzyme-Linked Immunosorbent Assay (ELISA) OraSure Technologies Inc., Bethlehem, PA) was performed to quantify levels of urinary and plasma cotinine. Urinary and serum creatinine levels were determined using a Vitros Ektachem 500 clinical chemistry analyzer. Serum creatinine measurements were determined for a subset of 312 subjects. The Cockcroft-Gault formula (CrCL (mL/min) = {[(140 − age) × weight]/72 × Cr} × adj. where Cr =serum creatinine and adj. = 1.0 for men, 0.85 for women) was calculated to estimate creatinine clearance (Gault et al. 1992). A handheld Mettler Toledo Refracto 30 refractometer measured the urinary refractive index (RI). The refractometer is calibrated to the standard temperature that is used for urine specific gravity tests (e.g. 20 °C. The RI value can be converted to units of total soluble solids (mg/ml).
Statistical analyses
Statistical analyses were conducted using SAS software version 9.1.3 (SAS Institute, Cary, NC). Significance levels were set at P<0.05. All tests were two-sided. The Student’s t-test was conducted to compare smoking and other demographic data between between men and women. Pearson correlation coefficients were calculated to determine the strength of the relationship between the two urinary adjustment factors and covariates. We calculated the partial correlations between urinary cotinine and a set of covariates including age, sex, race, BMI, cigarettes per day and creatinine clearance. The same set of covariates were correlated with total soluble solids.
We used two approaches to modeling this data to address two different questions. To determine the validity of the dilution standards for cotinine correction, we modeled blood cotinine against urinary cotinine, the dilution standards, and a set of covariates that have been either previously been reported to correlate with CR or were observed in the current data. The covariates included continuous terms for age, education, cigarettes per day and body surface area [(m2) = 0.024265 × height (cm)0.3964 × weight(kg)0.5378] (Haycock, 1978). Classification variables were constructed for sex and race.
The form of this model was:
where yi = untransformed blood cotinine regressed against urinary cotinine, a correction factor, and the above covariates. This model determines the absolute and relative validity of the correction factors in measuring biological exposure to (blood) cotinine. The results are shown in Table 2.
Table 2.
Pearson correlation of urinary creatinine and total soluble solids with age, sex, race, cigarettes per day, body mass index and creatinine clearance
| Age | Sex | Race | Cigarettes/day | BMI | Creatinine clearance | |
|---|---|---|---|---|---|---|
| Urinary creatinine | −0.03 (P=0.49) | −0.18 (P<0.01) | 0.15 (P<0.01) | −0.01 (P=0.80) | 0.10 (P<0.05) | 0.19 (P<0.01) |
| Total soluble solids | −0.04 (P=0.39) | −0.13 (P=0.01) | 0.02 (P=0.61) | 0.086 (P=0.07) | 0.149 (P<0.01) | 0.19 (P<0.01) |
Since most urinary biomarkers cannot be feasibly measured against a gold standard, a second approach did not evaluate the validity of the exposure assessment but the suitability of using either CR or SG in correcting for dilution. Traditionally, cotinine and other urinary biomarkers have been studied as a ratio to CR in correlation or regression analysis. However, dependent biological variables standardized by a denominator have unusual statistical properties which have decreased precision and sensitivity when compared to models where the denominator is treated as an independent variable (Anderson & Lydic 1997).
The form of this model was:
where yi = transformed urinary blood cotinine.
The distribution of urinary cotinine was skewed and a Cox-Box transformation indicated that the cubed root transformation of urinary cotinine improved normality more than a log or other transformations. The parameterization of the model accounted for the differences in the units in dilution standards by standardizing CR and total soluble solids by subtracting their means and then dividing by their standard deviations. The initial base model regressed urinary cotinine against the main effect variable; the number of cigarettes smoked per day. A squared term for cigarettes was found to improve the fit. Models were then fitted that included either CR or total soluble solids to determine if a term for either measurement improved the fit. Since the level of the dilution standards may vary by host factors, interaction terms were fitted between these factors and the dilution standards. Interaction terms might be significant for some but not for other models. To account for different number of predictor variables between models in explaining biomarker variation, we calculated and compared the adjusted R2 value. The best dilution standard was deemed to be the one that significantly increased the adjusted R2. The results are shown in Table 3.
Table 3.
Beta coefficients for models of blood cotinine
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| Adjusted R2 Model | 0.40 | 0.45 | 0.44 |
| Age | 1.78 (P=0.29) | 0.045 (P<0.05) | 1.45 (P=0.08) |
| Sex | −43.4 (P<0.05) | 1.36 (P=0.09) | −49.6 (P<0.01) |
| Race | 60.1 (P<0.01) | 60.1 (P<0.01) | 66.6 (P<0.01) |
| Years of education Years | −0.16 (P=0.66) | −0.12 (P=0.97) | −1.4 (P=0.69) |
| Cigarettes per day | 11.9 (P<0.01) | 0.335 (P<0.01) | 9.67 (P<0.01) |
| Cigarettes per day2 | −0.18 (P<0.01) | 0.004 (P<0.01) | −0.15 (P<0.01) |
| Body surface area | −133.8 (P<0.01) | −0.168 (P<0.01) | −95.1 (P<0.05) |
| Urinary cotinine (μg) | 0.034 (P<0.01) | 0.043 (P<0.01) | 0.04 (P<0.01) |
| Urinary total soluble solids (mg/mL) | 1.849 (P<0.01) | ||
| Urinary creatinine (mg/mL) | −56.2 (P<0.01) |
RESULTS
The current analysis is based on 431 smokers who had urinary cotinine determinations, CR determinations and total soluble solid measurements. Table 1 shows the subject characteristics including race, sex, age, education and number of cigarettes smoked daily. As observed previously, blacks tended to smoke significantly fewer cigarettes than whites. Table 1 also shows biomarker measurements by race and sex. Black women had significantly higher mean values of urinary cotinine, urinary CR, and creatinine clearance than white women respectively. Mean levels of total soluble solids was also higher in black women. There were no significant differences in these measures between black and white men.
Table 1.
Subject characteristics and biomarker concentrations of 431 cigarette smokers, 1994–2004
| All subject | Black men | Black Women | P-value | White men | White women | P-value | |
|---|---|---|---|---|---|---|---|
| N=431 | N= 105 | N=98 | N=110 | N=118 | |||
| Age | 34.6 ± 9.9 | 35.8 ± 9.2 | 35.4 ± 8.0 | 0.73 | 33.1 ± 10.0 | 34.2 ± 11.6 | 0.46 |
| Years of education | 13.6 ± 2.4 | 12.8 ± 2.3 | 13.6 ± 2.4 | 0.03 | 13.8 ± 2.5 | 14.0 ± 2.1 | 0.61 |
| Age started smoking | 16.6 ± 4.1 | 17.2 ± 4.3 | 16.9 ± 4.6 | 0.66 | 15.9 ± 3.7 | 16.4 ± 3.8 | 0.34 |
| Cigarettes/day | 18.3 ± 10.5 | 14.7 ± 8.2 | 13.4 ± 7.1 | 0.25 | 23.5 ± 12.6 | 20.8 ± 9.4 | 0.07 |
| Weight (lbs) | 164.8 ± 34.1 | 183.4 ± 30.5 | 156.4 ± 26.0 | <0.01 | 180.7 ± 30.9 | 140.1 ± 28.1 | <0.01 |
| Body mass index | 25.2 ± 4.0 | 26.1 ± 3.5 | 26.0 ± 3.8 | 0.87 | 25.7 ± 4.0 | 23.5 ± 4.0 | <0.01 |
| Blood cotinine (ng/mL) | 346.2 ± 250.4 | 382.3 ± 267.3 | 356.3 ± 256.0 | 0.45 | 344.7 ± 253.3 | 310.0 ± 225.4 | 0.23 |
| Urinary cotinine (ng/mL) | 3396 ± 0.3081 | 3762 ± 3060 | 3812 ± 3639 | 0.90 | 3265 ± 2989 | 2846 ± 2587 | 0.21 |
| Urinary creatinine (mg/mL) | 1.07 ± 0.73 | 1.29 ± 0.81 | 1.08 ± 0.81 | 0.01 | 1.13 ± 0.67 | 0.82 ± 0.57 | <0.01 |
| Creatinine clearance (mL/min) | 102.3 ± 0.26.1 | 109.3 ± 26.6 | 88.9 ± 22.8 | <0.01 | 112.6 ± 20.3 | 88.9 ± 22.8 | <0.01 |
| Urinary total solids (mg/mL) | 36.6 ± 19.3 | 38.4 ± 19.6 | 35.7 ± 18.0 | 0.36 | 39.7 ± 19.9 | 32.8 ± 19.2 | <0.01 |
The correlation between urinary CR and total soluble solids was 0.70 (P<0.01; Figure 1). The partial Pearson correlations of urinary CR and total soluble solids with regression covariates are shown in Table 2. In general these correlations were moderate. These covariates were nevertheless included in the subsequent multiple linear models even if this sacrificed some degree of freedoms. As expected, creatinine clearance was positively associated with urinary CR.
Figure 1.
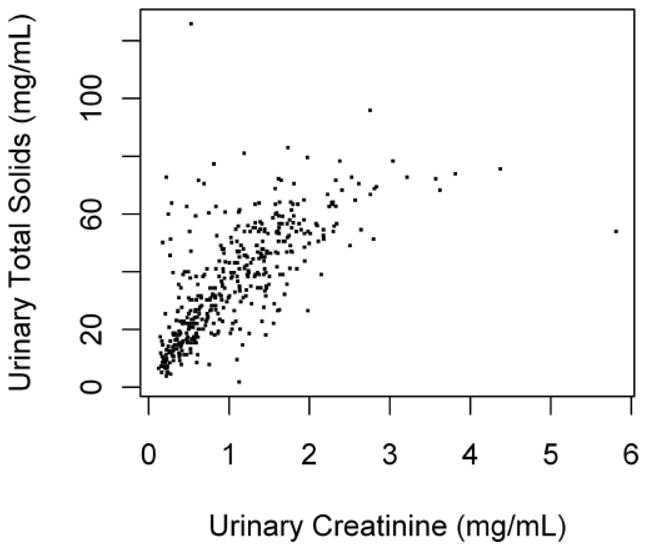
Legend. Scatter plot of urinary creatinine vs. total solids
To determine the validity of CR and SG dilution standards for the correction of urinary cotinine values in predicting blood cotinine levels, regression models were fitted with either CR or SG as independent variables (Table 3). As expected, cigarettes per day, body surface area, race and gender were significant predictors of blood cotinine levels. In addition, both urinary CR and total soluble solids were significant predictors in these models and the adjusted R2 was about the same in the models that included CR or SG.
We then examined the relative contribution of CR and SG in explaining variability in urinary cotinine. The initial model of transformed urinary cotinine included age, sex, race, BMI, age of smoking onset, years of education, the number of cigarettes per day (cpd) and its squared term (Table 4, Model 1). The linear and quadratic effects of daily smoking cigarettes were significant in all models. Age, age of smoking onset, and BMI were also significant demographic covariates that predicted urinary cotinine. Subsequent models included these covariates and either urinary CR or urinary total soluble solids. Both total soluble solids and urinary CR were found to improve the fit of the model that did not include any dilution standard (e.g. Table 4, Models 2 and 3 vs. Model 1). The R2 for the model that included CR was slightly higher than the R2 for the model that included a term for total soluble solids (>0.35 vs. 0.33).
Table 4.
Beta coefficients for models of urinary cotinine
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| R2 Model | 0.180 | 0.362 | 0.332 |
| Age | 0.029 (P=0.19) | 0.043 (P<0.05) | 0.046 (P<0.05) |
| Sex | −0.222 (P=0.59) | 0.511 (P=0.17) | 0.187 (P=0.62) |
| Race | 2.029 (P=0.47) | 2.25 (P<0.01) | 2.622 (P<0.01) |
| Years of education | −0.208 (P=0.47) | −0.053 (P=0.51) | −0.106 (P=0.20) |
| Age smoking onset | −0.098 (P=0.06) | −0.085 (P=0.07) | −0.091 (P=0.05) |
| Cigarettes per day | 0.433 (P<0.01) | 0.397 (P<0.01) | 0.333 (P<0.01) |
| Cigarettes per day2 | −0.004 (P<0.01) | 0.005 (P<0.01) | −0.004 (P<0.01) |
| BMI | −0.109 (P<0.05) | −0.135 (P<0.01) | −0.168 (P<0.01) |
| Urinary total soluble solids (mg/mL) | 1.851 (P<0.01) | ||
| Total soluble solids* race | N.S. | ||
| Urinary creatinine (mg/mL) | 3.401 (P<0.01) | ||
| Urinary creatinine * race | −0.882(P<0.05) |
Beta estimates for model 3 are based on a final model that excluded all nonsignificant interaction terms (e.g. race*education, race*cigarettes per day, race*total solids).
Since urinary CR, total soluble solids, cigarettes per day and education vary by race, further models were fitted that included interaction terms for CR and race, total soluble solids and race and cpd and race. The interaction term of urinary CR and race was significant (p-value=0.019) which increased the adjusted R2 from 0.35 with CR alone to 0.36. The interaction effect of total soluble solids and race was not significant (p-value=0.92). In the above models, the interaction terms for race and cpd, as well as race and education years were not significant. These nonsignificant terms were excluded from the final models (Models 2 and 3).
DISCUSSION
The current study shows that urinary CR and SG are equivalent correction factors for cotinine. Both measures were significant predictors of blood cotinine, validating their use as a dilution standard for assessing exposure to cotinine. The increased precision of the model is modest compared to a model without the dilution factor, but does validate their effect and need in accounting for differences in urinary dilution. The effect of CR on urinary cotinine levels did differ significantly by race, indicating that in a multiracial population SG performs better than CR if effect modification of CR by race is not accounted for.
Our results also show that multivariate control for dilution factors as independent variables and as effect modifiers may be a better method to compare biomarker concentrations between individuals than when modeling the marker as a fixed ratio. This might be particularly true in a diverse population where we showed significant interactive effects between race and urinary CR. This modeling approach also allows us to directly compare the ability of the two dilution standards in explaining the variation in urinary cotinine levels. Creatinine and SG were both significant predictors of urinary cotinine, and the improvement in fit of the model compared to the initial model was about the same. These findings indicate that both methods may be interchangeable in explaining the variation in urinary cotinine levels. The levels of cotinine are correlated with levels of other tobacco smoke metabolites in urine, and the dilution standards may be predictive of other tobacco smoke constituents as well. Because urinary CR and SG were highly correlated, these correction measures are probably interchangeable when accounting for dilution in studies of other urinary compounds. Ideally, it would be desirable to confirm this by examining their relationships with a battery of urinary biomarkers. Few studies have directly compared these normalization methods in exposure models. The effects of CR and SG on occupational exposure to 1,6-hexamethylene was recently determined in automotive painters. Both were significant predictors (Gaines et al. 2010). The correction of urinary estrogen concentrations was also similar between CR and SG (Miller et al. 2004). Although all subjects in the current study were current cigarette smokers, which may limit the generalizability of the findings, creatinine clearance is similar in smokers and nonsmokers (Halimi et al. 2000).
The optimal conditions for using CR as a dilution measure have been studied extensively. For example, the validity of the spot sample is generally considered high only when collected at certain times of the day (Heavner et al. 2006) or in persons who are not malnourished (Nermell et al. 2008). The variability in CR remains a source of concern, particularly when comparing cotinine or other urinary markers in diverse populations. The low correlation between CR and urine SG in some data indicate that the two correction methods are not interchangeable (Alessio et al. 1985). However the correlation between CR and SG was high in other studies (Trevisan et al. 1994; Jones et al. 1998; Carrieri, Trevisan & Bartolucci 2001) and in our data which included individuals without reported metabolic disorders.
Reference values for blood CR vary somewhat between different studies and laboratories. We defined the cutpoints as: (0.8–1.4 mg/dl for men; 0.6–1.1 mg/dl for women). About 53% of women and 74% of men in our study had clinically normal CR levels. Using a cutpoint of ≥1.5 mg/dl as an indicator of abnormal values, about 1% of nonhypertensive, nondiabetic adults under 50 had elevated levels in the National Health and Interview Survey (NHANES) (Clase, Garg & Bryce 2002). Similarly, about 1.5% of our subjects had elevated levels. In NHANES data, about 39% of subjects had low creatinine clearance levels defined as > 80 ml/min using the Cockroft-Gault formula. About 22% of our subjects had low clearance levels. Renal insufficiency is fairly common in US adults but there are many factors that influence the measurement of creatinine clearance. These include the timing of sample collection, strenuous exercise, stress, menstrual cycle, dehydration, the analytic method and others Further, the Cockroft-Gault formula was based on studies conducted in hospitalized whites and may not estimate well creatinine clearance in blacks (Coresh et al. 1998). However, blood CR and creatinine clearance was similar for blacks and whites in our data.
CONCLUSIONS
In summary, our study showed that both urinary CR and specific gravity in individuals without metabolic disorders are similar and effective dilution standards.
Acknowledgments
This work was supported by research grants PO1 CA68384 and K07 CA104231 from the National Cancer Institute.
Footnotes
The authors have no financial disclaimers.
References
- Alessio L, Berlin A, Dell’Orto A, et al. Reliability of urinary creatinine as a parameter used to adjust values of urinary biological indicators. Int Arch Occup Environ Health. 1985;55:99–106. doi: 10.1007/BF00378371. [DOI] [PubMed] [Google Scholar]
- Anderson D, Lydic R. Ratio data and the quantification of drug effects. Biobehavioral Reviews. 1997;1:55–57. [Google Scholar]
- Barr DB, Wilder LC, Caudill SP, et al. Urinary creatinine concentrations in the U.S. population: implications for urinary biologic monitoring measurements. Environ Health Perspect. 2005;113:192–200. doi: 10.1289/ehp.7337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin A, Alessio L, Sesana G, et al. Problems concerning the usefulness of adjustment of urinary cadmium for creatinine and specific gravity. Int Arch Occup Environ Health. 1985;55:107–11. doi: 10.1007/BF00378372. [DOI] [PubMed] [Google Scholar]
- Boeniger MF, Lowry LK, Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–27. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Carrieri M, Trevisan A, Bartolucci GB. Adjustment to concentration-dilution of spot urine samples: correlation between specific gravity and creatinine. Int Arch Occup Environ Health. 2001;74:63–67. doi: 10.1007/s004200000190. [DOI] [PubMed] [Google Scholar]
- Chadha V, Garg U, Alon US. Measurement of urinary concentration: a critical appraisal of methodologies. Pediatr Nephrol. 2001;16:374–82. doi: 10.1007/s004670000551. [DOI] [PubMed] [Google Scholar]
- Clase CM, Garg AX, Bryce BA. Prevalence of low glomerular filtration rate in nondiabetic Americans: Third National Health and Nutrition Examination Survey (NHANES III) J Am Soc Nephrol. 2002;13:1338–39. doi: 10.1097/01.asn.0000013291.78621.26. [DOI] [PubMed] [Google Scholar]
- Coresh J, Toto RD, Kirk KA, et al. Creatinine clearance as a measure of GFR in screenees for the African-American Study of Kidney Disease and Hypertension pilot study. Am J Kidney Dis. 1998;32:32–42. doi: 10.1053/ajkd.1998.v32.pm9669421. [DOI] [PubMed] [Google Scholar]
- Gaines LG, Fent KW, Flack SL, et al. Effect of creatinine and specific gravity normalization on urinary biomarker 1,6-hexamethylene diamine. J Environ Monit. 2010;12:591–99. doi: 10.1039/b921073c. [DOI] [PubMed] [Google Scholar]
- Gault MH, Longerich LL, Harnett JD, et al. Predicting glomerular function from adjusted serum creatinine. Nephron. 1992;62:249–56. doi: 10.1159/000187054. [DOI] [PubMed] [Google Scholar]
- Haddow JE, Knight GJ, Palomaki GE, et al. Replacing creatinine measurements with specific gravity values to adjust urine cotinine concentrations. Clin Chem. 1994;40:562–64. [PubMed] [Google Scholar]
- Halimi JM, Giraudeau B, Vol S, et al. Effects of current smoking and smoking discontinuation on renal function and proteinuria in the general population. Kidney Int. 2000;58:1285–92. doi: 10.1046/j.1523-1755.2000.00284.x. [DOI] [PubMed] [Google Scholar]
- Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: A height weight formula validated in infants, children and adults. J Pediatr. 1978;93:662–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- Heavner DL, Morgan WT, Sears SB, et al. Effect of creatinine and specific gravity normalization techniques on xenobiotic biomarkers in smokers’ spot urine samples. J Pharmaceut Biomed Anal. 2006;40:928–42. doi: 10.1016/j.jpba.2005.08.008. [DOI] [PubMed] [Google Scholar]
- James GD, Sealey JE, Alderman M, et al. A longitudinal study of urinary creatinine and creatinine clearance in normal subjects. Race, sex, and age differences. Am J Hypertens. 1998;1:124–31. doi: 10.1093/ajh/1.2.124. [DOI] [PubMed] [Google Scholar]
- Jatlow P, McKee S, O’Malley SS. Correction of urine cotinine concentrations for creatinine excretion: is it useful? Clin Chem. 2003;49:1932–34. doi: 10.1373/clinchem.2003.023374. [DOI] [PubMed] [Google Scholar]
- Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Disease. 1998;32:992–99. doi: 10.1016/s0272-6386(98)70074-5. [DOI] [PubMed] [Google Scholar]
- Lew SW, Bosch JP. Effect of diet on creatinine clearance and excretion in young and elderly healthy subjects and in patients with renal disease. J Am Soc Nephrol. 1991;2:856–65. doi: 10.1681/ASN.V24856. [DOI] [PubMed] [Google Scholar]
- Miller RC, Brindle E, Holman DJ, et al. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clin Chem. 2004;50:924–32. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- Moriguchi J, Ezaki T, Tsukahara T, et al. Decreases in urine specific gravity and urinary creatinine in elderly women. Int Arch Occup Environ Health. 2005;78:438–45. doi: 10.1007/s00420-004-0597-z. [DOI] [PubMed] [Google Scholar]
- Nermell B, Lindberg AL, Rahman M, et al. Urinary arsenic concentration adjustment factors and malnutrition. Environ Res. 2008;106 doi: 10.1016/j.envres.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Richie JP, Jr, Carmella SG, Muscat JE, et al. Differences in the urinary metabolites of the tobacco-specific lung carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone in black and white smokers. Cancer Epidemiol Biomarkers Prev. 1997;6:783–90. [PubMed] [Google Scholar]
- Ross DL, Neely AE. Textbook of Urinalysis and Body Fluids. Norwalk, CT: Appleton-Century-Crofts; 1983. [Google Scholar]
- Suwazono Y, Akesson A, Alfvén T, et al. Creatinine versus specific gravity-adjusted urinary cadmium concentrations. Biomarkers. 2005;10:117–26. doi: 10.1080/13547500500159001. [DOI] [PubMed] [Google Scholar]
- Thompson SG, Barlow RD, Wald NJ, et al. How should urinary cotinine concentrations be adjusted for urinary creatinine concentration? Clin Chim Acta. 1990;187:289–95. doi: 10.1016/0009-8981(90)90114-8. [DOI] [PubMed] [Google Scholar]
- Trevisan A, Nicoletto G, Maso S, et al. Biological monitoring of cadmium exposure: reliability of spot urine samples. Int Arch Occup Environ Health. 1994;65:373–75. doi: 10.1007/BF00383246. [DOI] [PubMed] [Google Scholar]
- Tricker AR. Biomarkers derived from nicotine and its metabolites: a review. Beitr Tabakforsch Int. 2006;22:147–75. [Google Scholar]


