Abstract
Germ cell tumors (GCTs) most often arise in the gonads but some develop extragonadally. The aim of this study was to examine sex- and race-specific trends in incidence and survival of gonadal (GGCTs) and extragonadal GCTs (EGCTs) in the US from 1973 to 2007. We also examined the topographic distribution of EGCTs by race and sex.
We estimated age-specific and age-standardized incidence rates and 5-year relative survival rates (RSR) of GCTs using the Surveillance, Epidemiology, and End Results (SEER) Program (SEER 9 registries). GCTs and their topographic sites were identified using ICD-O morphology and topography codes.
Of 21,170 GCTs among males, 5.7% were extragonadal (whites 5.5%; blacks 16.3%). Of 2,093 GCTs among females, 39.3% were extragonadal (whites, 36.9%; blacks 51.0%). The incidence of GGCT was much higher among white (56.3/1,000,000) than black males (10.0/1,000,000) while there was no difference in incidence between white and black females (3.2/1,000,000). The rates of EGCT among men and women of both races were similar (range:1.9 – 3.4/1,000,000). The most frequent extragonadal sites were mediastinum among males and placenta among females. The 5-year RSR of testicular GCT was higher among whites (97%) than blacks (90%), as was the 5-year RSR of ovarian GCT (whites, 92%; blacks 85%). In general, the 5-year RSRs of EGCTs were lower than the 5-year RSRs of GGCTs.
The different incidence trends of GGCTs and EGCTs and distinct age-specific incidence patterns by anatomic site of EGCTs suggests that GGCTs and EGCTs may have different etiologies.
Keywords: testicular neoplasms, ovarian neoplasms, incidence, time trends, germ cell tumors, extragonadal germ cell tumors
Introduction
Germ cell tumors (GCTs) most frequently arise in the gonads, but some develop extragonadally (Schmoll 2002) (Ebi et al. 2003) (Bokemeyer et al. 2002) (Bokemeyer et al. 2003). Extragonadal germ cell tumors (EGCTs) have similar morphology as gonadal germ cell tumors (GGCT) and most often occur in the midline of the body, e.g. anterior mediastinum, retroperitoneum, pineal gland etc. Gain of isochromosome 12p is an important chromosomal marker of both GGCTs and EGCTs (Chaganti et al. 1994) (Schneider et al. 2006) (Cossu-Rocca et al. 2006) (Poulos et al. 2006).
A widely accepted hypothesis suggests an embryonic genesis of GCTs (Schmoll 2002) (Hainsworth & Greco 1992) (Oosterhuis et al. 2007). Primordial germ cells (PGCs) originate from the proximal epiblast and migrate along the midline of the body through the hindgut to the genital ridge. Once at the genital ridge, PGCs are referred to as gonocytes. Depending on the sex-chromosomal constitution and corresponding microenvironment in the gonadal ridge, gonocytes differentiate into either oocytes or pre-spermatogonia (Oosterhuis & Looijenga 2005). A disturbed migration of PGCs results in misplacement at different sites in the body’s midline. EGCTs are believed to develop after malignant transformation of these residual PGCs. Different stages of development of the precursor cells and microenvironmental conditions may determine the final histology of the tumors at different sites. This hypothesis might explain the occurrence of GCTs at various sites especially in the sagittal midline of the brain, mediastinum, and retroperitoneum (Fossa et al. 2003). Another hypothesis suggests that metastases of GGCTs in the retroperitoneal space and the posterior mediastinum of adolescent and young adult males are misdiagnosed as primary EGCT because the primary GGCT regressed (“burned out”) (Hainsworth & Greco 1992). Histologic studies of the testes of EGCT patients have revealed that some have testicular scars, i.e., fibrous tissue and microlithiasis that may reflect burned-out testicular GCTs. This misdiagnosis may be especially relevant for retroperitoneal EGCT, given the relatively high risk of metastasis to this area (Bokemeyer et al. 2002) (Bokemeyer et al. 2003) (Fossa et al. 2003) (Daugaard et al. 1987).
Recently, Oosterhuis and Looijenga proposed the classification of GCTs into five types based on the maturation stage and imprinting status of the originating germ cell (Oosterhuis & Looijenga 2005). All Type II GCTs are thought to originate through reprogramming of neoplastic primordial germ cells/gonocytes. In the testis, nonseminomatous components originate from reprogrammed intratubular germ cell neoplasia unclassified (ITGCNU, also known as testicular carcinoma in situ) lesions or seminoma cells (Mostofi et al. 1987); while in the ovary, GCTs originate from dysgerminoma cells and in the dysplastic gonad from the neoplastic primordial germ cells of gonadoblastoma. The precursor lesions in the anterior mediastinum and midline of the brain have not yet been identified. However, the fact that the tumors in these sites may be composed of germinoma (counterpart of testicular seminoma and ovarian dysgerminoma) or of germinoma combined with nongerminomatous components, is in keeping with the hypothesis that these tumors are the result of reprogramming of a neoplastic PCG. The type I tumors, not characterized by isochromosome 12p, lack a seminomatous/dysgerminomatous/germinomatous component. This suggests they originate from primitive germ cells that are immediately reprogrammed to pluripotency, without prior neoplastic proliferation of the PCGs.
Descriptive epidemiologic features such as age patterns, incidence trends, and survival could improve our understanding of EGCTs and may provide clues to differences in EGCT etiology. The aim of this study was to compare epidemiologic features including incidence trends and survival of GGCTs and EGCTs among U.S. males and females from 1973 through 2007.
Material and Methods
We extracted incidence rates, by sex and race, of gonadal and extragonadal GCTs from the Surveillance, Epidemiology, and End Results (SEER) Program original 9 registries for the years 1973–2007 (National Cancer Institutes 2009). Before incidence estimation, we restricted the cases to primary malignant GCTs among white or black persons. Other racial/ethnic groups were not included due to small numbers.
We used ICD-O-3 (International Classification of Disease for Oncology) topography and morphology codes to classify the tumors (2002). Topograpy code C62 identified testicular tumors while code C56 identified ovarian tumors. All other topographic sites were considered extragonadal. Among males, morphology codes 9060-9062, 9064 identified seminomas, while codes 9065-9102 identified nonseminomas. Among females, morphology codes 9060-9064 identified dysgerminomas, the histologic equivalent of seminomas, while other GCTs were identified by histologic type: embryonal carcinoma (9070), yolk sac tumor (9071), teratoma (9080-9084), mixed germ cell tumor (9085), and choriocarcinoma (9100-9101). For simplicity we collectively refer to this grouping as non-dysgerminomas (Nogales et al. 2003).
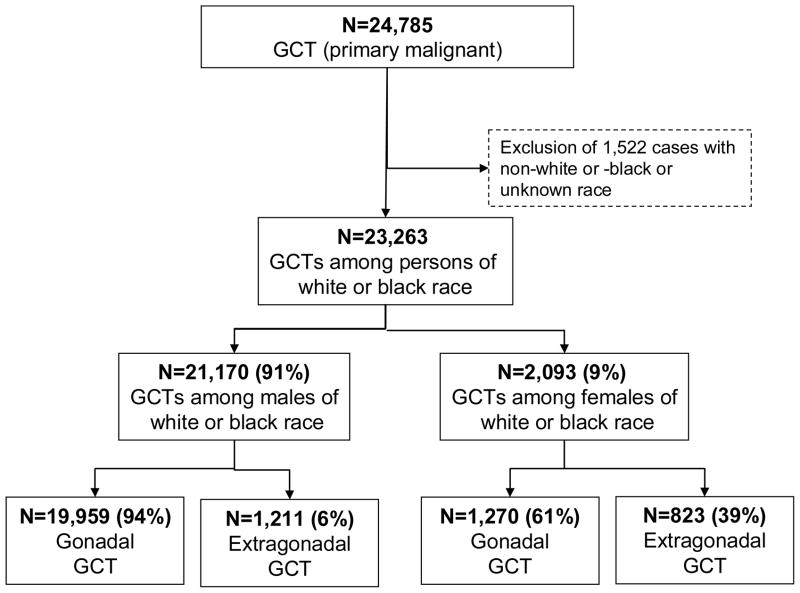
We analysed the incidence of EGCTs by sex and race using two digit ICD-O topography codes (C00, C01, C02, …, C80). Herein, we report topography-specific incidence rates by sex and race if the absolute number of cases for the incidence rates were 16 or more for the overall registration period 1973–2007. In addition, certain extragonadal sites mentioned in prior studies were analyzed in detail, including pineal gland (C75.3), pituitary gland (C75.1), brain (C71.0–71.9), thymus (C37.9), mediastinum (C38.1–3), retroperitoneum (C48.0), pelvis (C49.5, 76.3), placenta (C58), and uterus (C54–55). Details of the inclusion/exclusion criteria and the corresponding counts are presented in Figure 1.
Figure 1.
Gonadal and extragonadal germ cell tumors among whites and blacks, SEER-9, 1973–2007
Statistical methods
We calculated crude, age-specific and age-standardized incidence rates of GCTs stratified by sex, race, and histologic group. To study the incidence among children, we calculated age-specific rates for age groups 0–14 years. Incidence estimates based on less than 16 cases were not reported.
We used a median/average smoothing process to model age-specific incidence rates (1-year age groups) to increase clarity of the underlying pattern. We first calculated the median incidence for every three contiguous 1-year age groups and then calculated weighted means (0.25 for age group x−1, 0.50 for age group x, and 0.25 for age group x+1) (Selvin 2001).
We estimated 5-year relative survival rates (RSR) by dividing observed survival rates by the expected survival rates of persons of the same age and sex. The expected survival rates were obtained from population life tables of the SEER program. We chose 1990–2007 as the years of diagnoses and follow-up period. We excluded cases identified solely by death certificate or autopsy. Relative survival estimates are presented only for sites that had at least 60 registered cases in at least one of the strata of sex and race.
Results
Between 1973 and 2007, 23,263 GCTs were diagnosed among white and black persons in the SEER-9 registries (Figure 1). Among white and black men, 5.5% and 16.3% of all GCTs were EGCTs, respectively, while among white and black women, these proportions were 36.9% and 51.0%, respectively.
The overall incidence of GGCT was, as anticipated, much higher among white males (56.3/1 million) than black males (10.0/1 million) (Table 1). In contrast there was no difference in incidence of GGCT between white and black females (3.2/1 million). The rates of EGCT among men and women of both races were on par with the rate of GGCTs among women, ranging from 1.8 – 3.4/1 million (Table 1). Among white males, the incidence of seminoma was higher than that of nonseminoma. In contrast, among white females, the incidence of dysgerminoma was lower than that of non-dysgerminoma (Table 2). The findings by histology were similar among black persons (results not shown).
Table 1.
Age-standardized incidence rates (cases per 1 million, US 2000 population standard), gonadal and extragonadal germ cell tumors among white and black males and females in the US (SEER-9, 1973–2007)
| Overall | By Calendar Period
|
||||
|---|---|---|---|---|---|
| 1973–1984 | 1985–1996 | 1997–2007 | |||
| White Males | Population size | 328,840,919 | 102,224,234 | 112,934,860 | 113,681,825 |
| Gonadal germ cell tumors | |||||
| Count | 19,517 | 4688 | 7130 | 7699 | |
| ASR | 56.3 | 44.0 | 57.0 | 65.6 | |
| SE | 0.4 | 0.7 | 0.7 | 0.7 | |
| Extragonadal germ cell tumors | |||||
| Count | 1125 | 318 | 423 | 384 | |
| ASR | 3.3 | 3.0 | 3.5 | 3.3 | |
| SE | 0.1 | 0.2 | 0.2 | 0.2 | |
| Black Males | Population size | 44,167,482 | 11,496,501 | 15,095,037 | 17,575,944 |
| Gonadal germ cell tumors | |||||
| Count | 442 | 92 | 135 | 215 | |
| ASR | 10.0 | 8.3 | 8.5 | 11.9 | |
| SE | 0.5 | 1.0 | 0.8 | 0.8 | |
| Extragonadal germ cell tumors | |||||
| Count | 86 | 15 | 36 | 35 | |
| ASR | 1.8 | 2.3 | 1.8 | ||
| SE | 0.2 | 0.4 | 0.3 | ||
| White Females | Population size | 337,913,118 | 106,268,059 | 116,539,442 | 115,105,617 |
| Gonadal germ cell tumors | |||||
| Count | 1095 | 387 | 387 | 321 | |
| ASR | 3.2 | 3.3 | 3.3 | 2.9 | |
| SE | 0.1 | 2.1 | 0.2 | 0.2 | |
| Extragonadal germ cell tumors | |||||
| Count | 641 | 239 | 217 | 185 | |
| ASR | 1.9 | 2.1 | 1.8 | 1.7 | |
| SE | 0.1 | 0.1 | 0.1 | 0.1 | |
| Black Females | Population size | 48,568,799 | 12,576,823 | 16,672,993 | 19,318,983 |
| Gonadal germ cell tumors | |||||
| Count | 175 | 51 | 65 | 59 | |
| ASR | 3.2 | 3.4 | 3.4 | 2.9 | |
| SE | 0.2 | 0.5 | 0.4 | 0.4 | |
| Extragonadal germ cell tumors | |||||
| Count | 182 | 58 | 56 | 68 | |
| ASR | 3.4 | 4.0 | 2.9 | 3.3 | |
| SE | 0.3 | 0.6 | 0.4 | 0.4 | |
ASR: age-standardized rate; SE: standard error of ASR
Table 2.
Age-standardized incidence rates (cases per 1 million) of extragonadal germ cell tumors by primary site, sex and histologic group (whites only); SEER-9, 1973–2007
| Topography (ICD-O) | White Males
|
White Females
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seminoma | Nonseminoma | Dysgerminoma | Non-Dysgerminoma | |||||||||
| N | ASR | SE | N | ASR | SE | N | ASR | SE | N | ASR | SE | |
|
|
||||||||||||
| Gonadal germ cell tumors | ||||||||||||
| Testis (C62.0–C62.9) | 10,994 | 32.7 | 0.33 | 8,398 | 23.1 | 0.25 | ||||||
| Ovary (C56) | 393 | 1.1 | 0.06 | 701 | 2.0 | 0.08 | ||||||
| Primary sites of extragonadal germ cell tumors | ||||||||||||
| Thymus (C37.9) | 9 | 9 | 1 | 1 | ||||||||
| Mediastinum (C38.1–C38.3) | 194 | 0.6 | 0.04 | 212 | 0.6 | 0.04 | 12 | 26 | 0.1 | 0.02 | ||
| Retroperitoneum (C48.0) | 73 | 0.2 | 0.03 | 81 | 0.2 | 0.03 | 1 | 12 | ||||
| Pelvis (C49.5, C76.3) | 4 | 35 | 0.1 | 0.02 | 0 | 60 | 0.2 | 0.02 | ||||
| Uterus (C54–C55) | 1 | 45 | 0.1 | 0.02 | ||||||||
| Female genital tract (C57) | 3 | 20 | 0.1 | 0.01 | ||||||||
| Placenta (C58) | 0 | 312 | 0.9 | 0.05 | ||||||||
| Brain, NOS (C71.0–C71.9) | 59 | 0.2 | 0.02 | 24 | 0.1 | 0.01 | 26 | 0.1 | 0.02 | 15 | ||
| Pineal gland (C75.3) | 127 | 0.4 | 0.03 | 48 | 0.1 | 0.02 | 7 | 3 | ||||
| Pituitary gland (C75.1) | 17 | 0.0 | 0.01 | 3 | 11 | 5 | ||||||
| Malignant neoplasm of other and ill-defined sites (C76excl. C76.3) | 2 | 6 | 0 | 2 | ||||||||
| Other sites* | 16 | 0.0 | 0.01 | 44 | 0.1 | 0.02 | 6 | 46 | 0.1 | 0.02 | ||
| Unknown primary site (C80.9) | 57 | 0.2 | 0.02 | 105 | 0.3 | 0.03 | 6 | 21 | 0.1 | 0.01 | ||
| All extragonadal germ cell tumors | 558 | 1.6 | 0.07 | 567 | 1.6 | 0.07 | 74 | 0.2 | 0.03 | 567 | 1.6 | 0.07 |
all other sites had less than 16 registered cases in each sex-race stratum of the registration period 1973–2007
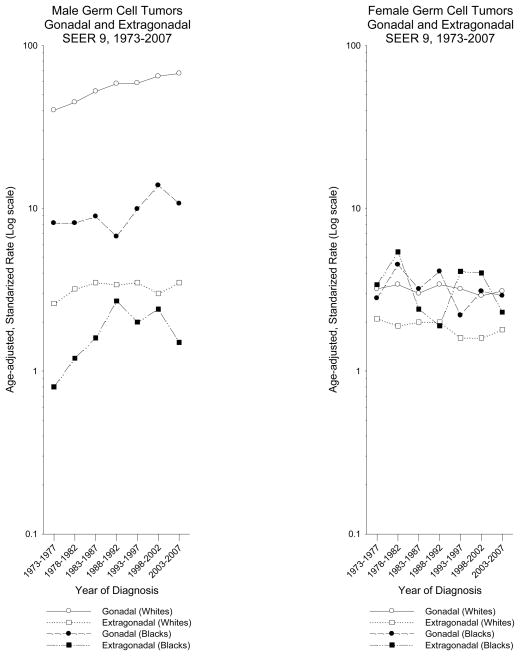
Trends in incidence of GCTs by race and sex are provided in Figure 2. The incidence of GGCTs among white males continuously increased from 1973 through 2007 (average annual percent change (APC) in incidence = 1.7%, 95% confidence interval (95%CI): 1.4% to 1.9%). In contrast, the incidence of EGCTs among white males remained fairly constant over the entire time period (APC = 0.6%, 95%CI: −0.2% to 1.4%). Among black males, the incidence of GGCTs increased (APC = 1.5%, 95%CI: 0.3% to 2.7%), with the largest increase between 1988 and 2002. The incidence of EGCTs among black males increased from 1973 until 1992, then plateaued before declining in the latest time period; however, these rates are based on small numbers making calculation of the difficult. Among white and black females, the incidence rates for GGCT and EGCTs had similar trends with small decreases over time (APCs: GGCT white = −0.2%, 95%CI: −0.9% to 0.4%, GGCT black = −1.2%, 95%CI: −3.1% to 0.6%, EGCT white = −0.7%, 95%CI: −1.4% to 0.2%, EGCT black = −0.8%, 95%CI: −2.4% to 0.8%). The fluctuation of rates among black females was large, likely due to small numbers.
Figure 2.
Incidence time trends of gonadal and extragonadal germ cell tumors among males and females, US SEER-9, 1973–2007 (Cases per 100,000)
As shown in Table 3, the most frequent extragonadal sites were mediastinum, pineal gland, retroperitoneum, and brain among males and placenta, pelvis, uterus, and brain among females. Although there was a tendency toward higher rates of EGCTs among white than black females, rates of placental GCTs were higher among black females (white: 0.9 per 1 million; black: 1.7 per 1 million).
Table 3.
Age-standardized incidence rates (cases per 1 million) of gonadal and extragonadal germ cell tumors by primary site, sex and race; SEER-9, 1973–2007
| Topography (ICD-O) | Males
|
Females
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | White | Black | |||||||||
| N | ASR | SE | N | ASR | SE | N | ASR | SE | N | ASR | SE | |
|
|
||||||||||||
| Gonadal germ cell tumors | ||||||||||||
| Testis (C62.0–C62.9) | 19,517 | 56.3 | 0.41 | 442 | 10.0 | 0.49 | ||||||
| Ovary (C56) | 1,095 | 3.2 | 0.10 | 175 | 1.9 | 0.1 | ||||||
| Primary sites of extragonadal germ cell tumors | ||||||||||||
| Thymus (C37.9) | 18 | 0.1 | 0.01 | 0 | 2 | 0 | ||||||
| Mediastinum (C38.1–C38.3) | 406 | 1.2 | 0.06 | 25 | 0.5 | 0.11 | 38 | 0.1 | 0.02 | 3 | ||
| Retroperitoneum (C48.0) | 154 | 0.5 | 0.04 | 7 | 13 | 6 | ||||||
| Pelvis (C49.5, C76.3) | 39 | 0.1 | 0.02 | 4 | 60 | 0.2 | 0.02 | 18 | 0.3 | 0.17 | ||
| Uterus (C54–C55) | 46 | 0.1 | 0.02 | 23 | 0.3 | 0.09 | ||||||
| Female genital tract (C57) | 23 | 0.1 | 0.01 | 2 | ||||||||
| Placenta (C58) | 312 | 0.9 | 0.05 | 92 | 1.7 | 0.17 | ||||||
| Brain, NOS (C71.0–C71.9) | 83 | 0.2 | 0.03 | 8 | 41 | 0.1 | 0.02 | 11 | ||||
| Pineal gland (C75.3) | 175 | 0.5 | 0.04 | 23 | 0.4 | 0.09 | 10 | 0 | ||||
| Pituitary gland (C75.1) | 20 | 0.1 | 0.01 | 0 | 16 | 0.0 | 0.01 | 1 | ||||
| Malignant neoplasm of other and ill-defined sites (C76excl. C76.3) | 8 | 1 | 2 | 0 | ||||||||
| Other sites* | 60 | 0.2 | 0.02 | 9 | 52 | 0.2 | 0.02 | 19 | 0.4 | 0.09 | ||
| Unknown primary site (C80.9) | 162 | 0.5 | 0.04 | 9 | 27 | 0.1 | 0.02 | 7 | ||||
| All extragonadal germ cell tumors | 1,125 | 3.3 | 0.10 | 86 | 1.8 | 0.20 | 641 | 1.9 | 0.07 | 182 | 3.4 | 0.25 |
all other sites had less than 16 registered cases in each sex-race stratum of the registration period 1973–2007; ASR: age-standardized rate; SE: standard error of ASR
Histology-specific analyses among white persons revealed that EGCTs of the brain, pineal gland and pituitary gland were predominantly seminomas/dysgerminomas (67%, 74%, 78%, respectively). In contrast, EGCTs of the pelvis were predominately nonseminomas/nondysgerminomas (96%) (Table 3). The frequencies of EGCTs by site were similar among black persons, although based on small numbers (results not shown).
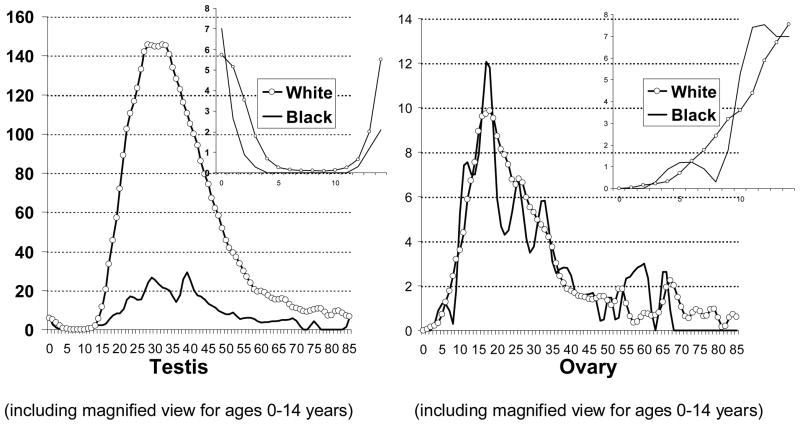
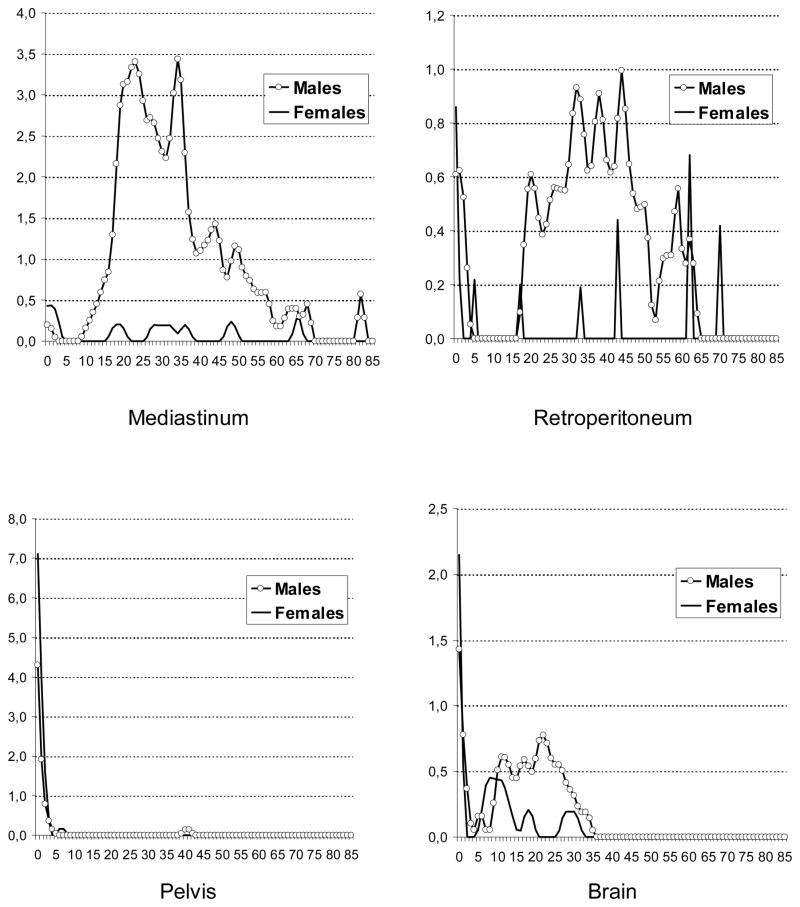
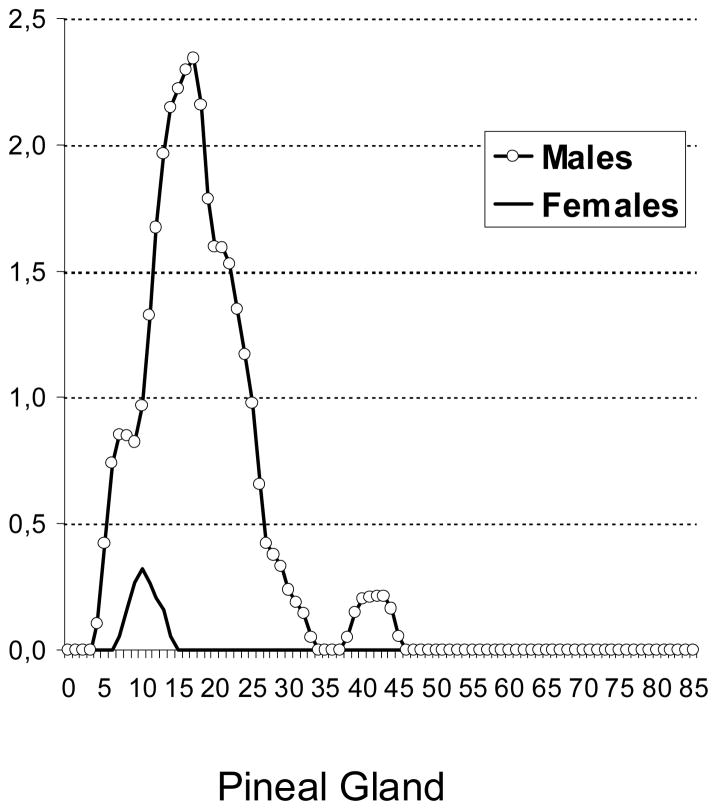
The age-specific incidence patterns of testicular GCTs had an early peak at ages 0–1 years and a steep increase starting at puberty. In contrast, ovarian GCTs had a steep increase starting at age 5 years (Figure 3). Among EGCTs, brain tumors had an early peak (0–1 year) and a second peak starting at puberty while pineal tumors had a steep increase starting at age 5 years. Mediastinal and retroperitoneal GCTs showed steep increases starting at puberty, while pelvic GCTs had only one age peak, at 0–1 years (Figure 4).
Figure 3.
Age-specific incidence rates of gonadal germ cell tumors among by race, US SEER-9, 1973–2007(Cases per 1 million)
Figure 4.
Sex- and age-specific incidence rates of extragonadal germ cell tumors among whites, US SEER-9, 1973–2007 (Cases per 1 million)
The 5-year RSRs of testicular GCTs were higher among white (97%) than black males (90%) (Table 4) Stratification by histologic group revealed that seminoma RSRs among white (98%) and black males (96%) were very similar (results not shown). The RSRs of nonseminomas, however, were considerably lower among black (78%) than white males (94%) (results not shown). Among females, the 5-year RSR of ovarian GCTs was higher among white (92%) than black females (85%). Among white females, the RSR was higher for dysgerminoma (96%) than non-dysgerminoma (90%) (results not shown). There were too few cases among black females to stratify by histology.
Table 4.
5-year relative survival (RSR in %) of gonadal and extragonadal germ cell tumors by primary site, sex and race; SEER-9, 1990–2007
| Topography (ICD-O) | Males
|
Females
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| White | Black | White | Black | |||||||||
| N | RSR (%) | SE | N | RSR(%) | SE | N | RSR(%) | SE | N | RSR(%) | SE | |
|
|
||||||||||||
| Gonadal germ cell tumors | ||||||||||||
| Testis (C62.0–C62.9) | 11,468 | 97 | 0.2 | 292 | 90 | 2.1 | - | - | - | - | - | - |
| Ovary (C56)* | - | - | - | - | - | - | 545 | 92 | 1.3 | 87 | # 85 | #4.1 |
| Primary sites of extragonadal germ cell tumors | ||||||||||||
| Mediastinum (C38.1–C38.3)* | 209 | 58 | 3.6 | 15 | - | - | 14 | - | - | 1 | - | - |
| Retroperitoneum (C48.0) | 65 | 81 | 5.4 | 2 | - | - | 7 | - | - | 5 | - | - |
| Placenta (C58)* | - | - | - | - | - | - | 122 | 94 | 2.4 | 40 | 88 | 5.3 |
| Brain, NOS (C71.0–C71.9)* | 61 | 83 | 5.4 | 7 | - | - | 29 | * 65 | * 9.1 | 9 | - | - |
| Pineal gland (C75.3)* | 112 | 90 | 3.1 | 17 | - | - | 2 | - | - | 0 | - | - |
| All extragonadal germ cell tumors* | 608 | 68 | 2.1 | 57 | 71 | 6.4 | 308 | 82 | 2.3 | 103 | 78 | 4.3 |
Persons with multiple primaries were included;
The relative cumulative survival increased from a prior interval and has been adjusted;
SE: estimated standard error of the RSR
Overall, the 5-year RSRs of EGCTs were generally lower than those of GGCTs (Table 4). Among white males, the highest 5-year RSR among EGCTs was that of the pineal gland (90%), followed by the brain (83%), retroperitoneum (81%) and mediastinum (58%). Among women, placental GCTs had similar 5-year RSRs to those of gonadal GCTs. A comparison of 5-year RSRs of EGCTs of the brain among white persons found better survival among males (83%) than females (65%). Small numbers precluded comparison of other sites by sex and race.
Discussion
Our study provides evidence that incidence trends and age patterns of GGCTs and EGCTs differ. While the incidence of testicular GCT increased from 1973 through 2007, the incidence of EGCTs among men remained virtually constant. Among females, the incidence of all GCTs showed a small decrease over time.
The different time trends of GGCTs and EGCTs suggest that the etiology may differ, although it is believed that GCTs originate from primitive germ cells of which the developmental potential differs according to its stage of maturation and pattern of genomic imprinting (Oosterhuis & Looijenga 2005). The considerably lower incidence of GCTs among females than males is likely related to the lower number of germ cells in the ovaries than the testes (Moller & Evans 2003) (Giambartolomei et al. 2009). Further, in contrast to testicular germ cells, ovarian germ cells do not proliferate after puberty. Finally, sex differences in etiologic factors or mechanisms might explain observed incidence differences by sex.
In the current study, GCTs of the pelvis had a distinctive feature: they occurred, almost exclusively, among newborns or young infants and were predominantly nonseminomas/nondysgerminomas. Sacrococcygeal teratoma is one of the most common congenital tumors. Its estimated prevalence is 1/27,000 live births with a female-to-male ratio of 4:1. About 10% of congenital sacrococcygeal teratomas are malignant (Lakhoo 2010) (Swamy et al. 2008). According to a Kiel Pediatric Tumor Registry report, the majority of GCTs of the abdomen, retroperitoneum and sacrococcyx in infants are teratomas (Harms et al. 1989). Sacrococcygeal tumors develop totally or partially in front of the sacrum. Sacrococcygeal tumors that develop totally in front of the sacrum should be included with retro- and intraperitoneal tumors (Harms et al. 1989). Similar to Harms et al. (Harms et al. 1989), we observed higher incidence rates of pelvic GCTs among females than males. The peak in incidence of pelvic GCTs at early ages most likely reflects that many are congenital sacrococcygeal GCTs.
Mediastinal GCTs occurred overwhelmingly among males. Among males, the steep increases in incidence of mediastinal and retroperitoneal EGCTs at onset of puberty may reflect a hormone-related promotion of neoplastically transformed cells that arose during embryogenesis. Among white males, the age-specific incidence pattern of mediastinal GCTs was similar to the pattern of testicular GCTs. Evidence based on case series and case reports suggests that mediastinal nonseminomatous GCTs are associated with Klinefelter syndrome (McKenney et al. 2007) (Oosterhuis et al. 2007). These tumors are known to have a poor prognosis (IGCCCG 1997), which has been explained by resistance to cisplatin-based chemotherapy (Bokemeyer et al. 2002). Also similar to testicular GCT, the estimated 5-year RSR of mediastinal nonseminoma was considerably lower than that of mediastinal seminoma.
In their review of EGCTs, McKenney et al. (McKenney et al. 2007) recently stated that “most purely retroperitoneal GCTs in adults represent metastases from an undiscovered or occult primary in the testicle, or, rarely, ovary”. If retroperitoneal EGCTs are metastases of GGCTs, this would imply that these tumors are more aggressive than localized GGCTs and that these tumors are more frequently associated with metachronous GGCTs. Accordingly, one would expect lower survival rates for retroperitoneal GCTs than GGCTs and a greater likelihood of metachronous GGCTs among patients with retroperitoneal than mediastinal GCTs. Our findings among white males corroborate these hypotheses. Although we were not able to adjust the RSRs for stage at diagnosis, the 5-year RSR was lower for retroperitoneal GCTs than for testicular GCTs. Interestingly, if retroperitoneal EGCTs were mainly metastases from occult cancer of the testis, one might also expect an increase of the incidence of these metastases if the stage distribution at diagnosis of testicular cancer had remained constant over time. Previous analyses of the SEER data by our group, however, found that testicular cancers have been diagnosed at increasingly earlier stages over time. Thus, it is conceivable that the decrease in retroperitoneal EGCT incidence may be due to fewer later stage testicular cancers being diagnosed (McGlynn et al. 2005).
Bokemeyer et al. noted the inferior prognosis of retroperitoneal GCTs in contrast with gonadal GCTs (Bokemeyer et al. 2002). Among 635 EGCT patients, the authors observed metachronous testicular tumors among 4.2% of men with retroperitoneal GCTs and only 1.1% of men with mediastinal GCTs (Bokemeyer et al. 2002). Similarly, Fossa et al. reported that 18 of 53 (34%) men with retroperitoneal GCT and 3 of 15 (20%) men with mediastinal GCT presented with testicular carcinoma in situ (Fossa et al. 2003). Although these observations are in line with a multi-site development of GCTs, they could also indicate that some retroperitoneal GCTs are metastases from burned-out testicular GCTs.
The vast majority of GCTs of the brain and pineal gland (predominantly seminoma/dysgerminoma) among white males occur during the second and third decades of life. In contrast to pineal gland GCTs, EGCTs of the brain had an early age peak at ages 0–1 years. The incidence of EGCTs of the placenta (predominantly choriocarcinoma) was higher among black than white females and may be due to higher birth rates among black (19/1000) than white (14/1000) women (Martin et al. 2010).
Our study had several notable strengths, including a large sample size (23,263). The data were drawn from an extensive population-based collection of registries with considerable time-depth covering almost four decades. In addition, the SEER registries are known to have well-validated, comprehensive histologic and topographic data. Nevertheless, several factors may limit our results. A substantial number of EGCTs were registered as having an unknown primary site, complicating the interpretation of the topographical distribution. Even with large numbers overall, several subsite- and histology-specific analyses could not be conducted as the numbers of cases were too small to allow a meaningful analysis. In addition, genetic analyses of poorly differentiated carcinomas involving primarily midline structures report that several of these tumors are actually EGCTs (Motzer et al. 1991) thus the incidence of EGCTs may be underestimated (Hainsworth & Greco 1992). Finally, the estimation of RSR of GCTs did not account for tumor stage and treatment modalities.
In conclusion, we provide detailed incidence and survival analysis of gonadal and extragonadal GCTs among black and white persons in the US from 1973 to 2007. The different incidence trends of GGCTs and EGCTs and distinct age-specific incidence patterns by anatomic site of EGCTs suggests that GGCTs and EGCTs may have different etiologies. In general, the prognosis of EGCTs was poorer than the prognosis of GGCTs.
Acknowledgments
Funding: Dr. Stang was a recipient of a grant from the Deutsche Forschungsgemeinschaft (DFG), grant number STA 621/6-1. Dr. Rusner was a recipient of a grant from the Deutsche Forschungsgemeinschaft (DFG), grant number RU 1659/1-1. Drs. Trabert, Cook, Wentzensen and McGlynn are supported by the NCI Intramural Research Program, NIH, DHHS.
Footnotes
All authors had substantial contributions to research design, data analysis, interpretation of data, drafting and revising the paper.
Reference List
- International Classification of Diseases for Oncology. 3. Geneva: World Health Organization; 2002. [Google Scholar]
- Bokemeyer C, Hartmann JT, Fossa SD, Droz JP, Schmol HJ, Horwich A, Gerl A, Beyer J, Pont J, Kanz L, Nichols CR, Einhorn L. Extragonadal germ cell tumors: relation to testicular neoplasia and management options. APMIS. 2003;111:49–59. doi: 10.1034/j.1600-0463.2003.11101081.x. [DOI] [PubMed] [Google Scholar]
- Bokemeyer C, Nichols CR, Droz JP, Schmoll HJ, Horwich A, Gerl A, Fossa SD, Beyer J, Pont J, Kanz L, Einhorn L, Hartmann JT. Extragonadal germ cell tumors of the mediastinum and retroperitoneum: results from an international analysis. J Clin Oncol. 2002;20:1864–1873. doi: 10.1200/JCO.2002.07.062. [DOI] [PubMed] [Google Scholar]
- Chaganti RS, Rodriguez E, Mathew S. Origin of adult male mediastinal germ-cell tumours. Lancet. 1994;343:1130–1132. doi: 10.1016/s0140-6736(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Cossu-Rocca P, Zhang S, Roth LM, Eble JN, Zheng W, Karim FW, Michael H, Emerson RE, Jones TD, Hattab EM, Cheng L. Chromosome 12p abnormalities in dysgerminoma of the ovary: a FISH analysis. Mod Pathol. 2006;19:611–615. doi: 10.1038/modpathol.3800576. [DOI] [PubMed] [Google Scholar]
- Daugaard G, von der MH, Olsen J, Rorth M, Skakkebaek NE. Carcinoma-in-situ testis in patients with assumed extragonadal germ-cell tumours. Lancet. 1987;2:528–530. doi: 10.1016/s0140-6736(87)92922-9. [DOI] [PubMed] [Google Scholar]
- Ebi H, Nakata M, Tahara M, Igarashi T, Kawada K, Itoh K, Ueda R, Minami H. Extragonadal germ cell tumors in Japan. Cancer Sci. 2003;94:1107–1111. doi: 10.1111/j.1349-7006.2003.tb01408.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fossa SD, Aass N, Heilo A, Daugaard G, Skakkebaek E, Stenwig AE, Nesland JM, Looijenga LH, Oosterhuis JW. Testicular carcinoma in situ in patients with extragonadal germ-cell tumours: the clinical role of pretreatment biopsy. Ann Oncol. 2003;14:1412–1418. doi: 10.1093/annonc/mdg373. [DOI] [PubMed] [Google Scholar]
- Giambartolomei C, Mueller CM, Greene MH, Korde LA. A mini-review of familial ovarian germ cell tumors: an additional manifestation of the familial testicular germ cell tumor syndrome. Cancer Epidemiol. 2009;33:31–36. doi: 10.1016/j.canep.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainsworth JD, Greco FA. Extragonadal germ cell tumors and unrecognized germ cell tumors. Semin Oncol. 1992;19:119–127. [PubMed] [Google Scholar]
- Harms D, Schmidt D, Leuschner I. Abdominal, retroperitoneal and sacrococcygeal tumours of the newborn and the very young infant. Report from the Kiel Paediatric Tumour Registry. Eur J Pediatr. 1989;148:720–728. doi: 10.1007/BF00443094. [DOI] [PubMed] [Google Scholar]
- IGCCCG. International Germ Cell Consensus Classification: a prognostic factor-based staging system for metastatic germ cell cancers. International Germ Cell Cancer Collaborative Group. J Clin Oncol. 1997;15:594–603. doi: 10.1200/JCO.1997.15.2.594. [DOI] [PubMed] [Google Scholar]
- Lakhoo K. Neonatal teratomas. Early Hum Dev. 2010;86:643–647. doi: 10.1016/j.earlhumdev.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Mathews TJ, Kirmeyer S, Osterman MJ. Births: Final data for 2007. Natl Vital Stat Rep. 2010;58:1–85. [PubMed] [Google Scholar]
- McGlynn KA, Devesa SS, Graubard BI, Castle PE. Increasing incidence of testicular germ cell tumors among black men in the United States. J Clin Oncol. 2005;23:5757–5761. doi: 10.1200/JCO.2005.08.227. [DOI] [PubMed] [Google Scholar]
- McKenney JK, Heerema-McKenney A, Rouse RV. Extragonadal germ cell tumors: a review with emphasis on pathologic features, clinical prognostic variables, and differential diagnostic considerations. Adv Anat Pathol. 2007;14:69–92. doi: 10.1097/PAP.0b013e31803240e6. [DOI] [PubMed] [Google Scholar]
- Moller H, Evans H. Epidemiology of gonadal germ cell cancer in males and females. APMIS. 2003;111:43–46. doi: 10.1034/j.1600-0463.2003.11101071.x. [DOI] [PubMed] [Google Scholar]
- Mostofi FK, Sesterhenn IA, Davis CJ., Jr Immunopathology of germ cell tumors of the testis. Semin Diagn Pathol. 1987;4:320–341. [PubMed] [Google Scholar]
- Motzer RJ, Rodriguez E, Reuter VE, Samaniego F, Dmitrovsky E, Bajorin DF, Pfister DG, Parsa NZ, Chaganti RS, Bosl GJ. Genetic analysis as an aid in diagnosis for patients with midline carcinomas of uncertain histologies. J Natl Cancer Inst. 1991;83:341–346. doi: 10.1093/jnci/83.5.341. [DOI] [PubMed] [Google Scholar]
- National Cancer Institutes, D. S. R. P. C. S. B. Surveillance, Epidemiology, and End Results (SEER) program. 2009 ( www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER 9 Regs Limited-Use.
- Nogales F, Talerman A, Kubik-Huch RA, Tavassoli FA, Devouassoux-Shisheboran M. Germ cell tumours. In: Tavassoli FA, Devilee P, editors. Pathology and genetics of tumours of the breast and female genital organs. Lyon: International Agency for Research on Cancer (IARC) Press; 2003. pp. 163–179. [Google Scholar]
- Oosterhuis JW, Looijenga LH. Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer. 2005;5:210–222. doi: 10.1038/nrc1568. [DOI] [PubMed] [Google Scholar]
- Oosterhuis JW, Stoop H, Honecker F, Looijenga LH. Why human extragonadal germ cell tumours occur in the midline of the body: old concepts, new perspectives. Int J Androl. 2007;30:256–263. doi: 10.1111/j.1365-2605.2007.00793.x. [DOI] [PubMed] [Google Scholar]
- Poulos C, Cheng L, Zhang S, Gersell DJ, Ulbright TM. Analysis of ovarian teratomas for isochromosome 12p: evidence supporting a dual histogenetic pathway for teratomatous elements. Mod Pathol. 2006;19:766–771. doi: 10.1038/modpathol.3800596. [DOI] [PubMed] [Google Scholar]
- Schmoll HJ. Extragonadal germ cell tumors. Ann Oncol. 2002;13(Suppl 4):265–272. doi: 10.1093/annonc/mdf669. [DOI] [PubMed] [Google Scholar]
- Schneider DT, Zahn S, Sievers S, Alemazkour K, Reifenberger G, Wiestler OD, Calaminus G, Gobel U, Perlman EJ. Molecular genetic analysis of central nervous system germ cell tumors with comparative genomic hybridization. Mod Pathol. 2006;19:864–873. doi: 10.1038/modpathol.3800607. [DOI] [PubMed] [Google Scholar]
- Selvin S. Smoothing Sequential Data. In: Selvin S, editor. Epidemiologic Analysis: A Case-oriented Approach. New York: Oxford University Press; 2001. pp. 237–247. [Google Scholar]
- Swamy R, Embleton N, Hale J. Sacrococcygeal teratoma over two decades: birth prevalence, prenatal diagnosis and clinical outcomes. Prenat Diagn. 2008;28:1048–1051. doi: 10.1002/pd.2122. [DOI] [PubMed] [Google Scholar]