Abstract
Irritable bowel syndrome (IBS) is a functional gastrointestinal (GI) disorder characterized by chronic abdominal pain associated with alterations in bowel function. Given the heterogeneity of the symptoms, multiple pathophysiologic factors are suspected to play a role. We classified women with IBS into four subgroups based on distinct symptom profiles. In-depth shotgun proteomic analysis was carried out to profile the urinary proteomes to identify possible proteins associated with these subgroups. First void urine samples with urine creatinine level ≥ 100 mg/dL were used after excluding samples that tested positive for blood. Urine from ten subjects representing each symptom subgroup was pooled for proteomic analysis. The urine proteome was analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS) using a data-independent method known as Precursor Acquisition Independent From Ion Count (PAcIFIC) that allowed extended detectable dynamic range. Differences in protein quantities were determined by peptide spectral counting followed by validation of select proteins with ELISA or a targeted single reaction monitoring (LC-SRM/MS) approach. Four IBS symptom subgroups were selected: 1) Constipation, 2) Diarrhea + Low Pain, 3) Diarrhea + High Pain, and 4) High Pain + High Pychological Distress. A fifth group consisted of Healthy Control subjects. From comparisons of quantitative spectral counting data among the symptom subgroups and controls, a total of 18 proteins that showed quantitative differences in relative abundance and possible physiological relevance to IBS were selected for further investigation. Three of the 18 proteins were chosen for validation by either ELISA or SRM. An elevated expression of gelsolin (GSN) was associated with the high pain groups. Trefoil Factor 3 (TFF3) levels were higher in IBS groups compared to controls. In this study the IBS patients subclassified by predominant symptoms showed differences in urine proteome levels. Proteins showing distinctive changes are involved in homeostasis of intestinal function and inflammatory response. These findings warrant future studies with larger, independent cohorts to enable more extensive assessment and validation of urinary protein markers as a diagnostic tool in adult with IBS.
Keywords: biomarker, irritable bowel syndrome, mass spectrometry, proteomics, urine, women
INTRODUCTION
Irritable bowel syndrome (IBS) in adults is among the most common and costly functional gastrointestinal (GI) disorders in the US.1 Diagnosis of IBS is based on the presence of abdominal pain or discomfort that is relieved by bowel movement and/or associated with bowel pattern changes, i.e., diarrhea and/or constipation. IBS is considered a functional disorder in that there is no confirmed organic cause or definitive diagnostic test. Organic diseases with symptom profiles somewhat similar to IBS include inflammatory bowel disease (IBD), celiac disease, lactose intolerance, colorectal cancer, and ovarian cancer. These conditions are typically considered before a diagnosis of IBS is made.2 In the US the overall prevalence of IBS may be as high as 17% with approximately 2 times more women affected as compared to men.1,3 Higher rates of psychological distress rates including anxiety, depression, and stress as well as reduced health-related quality of life are found in patients with IBS when compared to the general population.4–6
Mechanistic studies of IBS pathophysiology have focused on altered visceral pain sensitivity, GI motility disturbances, potential alterations in the gut microbiome, altered immune function, small intestinal bacterial overgrowth, diet (e.g., lactose malabsorption), and stress.7–11 To date there is no one clear etiologic mechanism that accounts for symptoms in all adults with IBS, and many of these studies find evidence of a particular pathology in some but not all patients with IBS. It may well be that the population of patients with a diagnosis of IBS is heterogeneous, with different patients having different etiologies. Such heterogeneity of etiology is consistent with the variations in symptom profile with which IBS patients present. For example, patients differ in severity of abdominal pain, and in presence and severity of diarrhea, constipation, bloating, and psychological distress. Such heterogeneity makes study of biomarkers more challenging than it is for a disease with a single, clear etiology. A specific biomarker may be relevant to one subset of patients with IBS but not to others, so a study that compares a heterogeneous group of IBS patients to healthy controls will have greatly reduced power. The most useful type of biomarker might be one that distinguishes IBS patients with one specific etiology from IBS patients with a different etiology. The identification and confirmation of such a biomarker would contribute to the scientific understanding of IBS, and could be very useful in guiding treatment decisions if treatments specific to etiology were available.
In the field of clinical biology, mass spectrometry (MS) based proteomics has played a leading role in biomarker discovery efforts since the early 1990’s. Quantitative proteomics, used to measure changes in protein abundance that may reflect pathophysiological alterations, has further facilitated biomarker discovery in various human diseases including cancer, interstitial cystitis, and obstructive sleep apnea.12–14 As such, proteomics screening has been applied to various biological specimens including blood, urine, saliva, and tissue for the purpose of biomarker discovery.14 Analyzing urine samples has become increasingly popular in recent years due to the non-invasive nature of sample collection and reduced complexity of the proteome over blood. To date, urine proteomic approaches have been examined for their potential to serve as biomarkers for cancer, renal failure, and inflammation.15,16 However, biomarker discovery of functional disorders with a range of clinical presentations remains challenging. IBS is one such condition for which the utility of using urine proteomics remains unexplored.
The goal of this study was to discover potential biomarker(s) related to presence of IBS or associated with a symptom profile within IBS patients. The strategy used to achieve this goal was to first, classify the IBS patients into symptom profile subtypes based on GI symptoms (bowel pattern, abdominal pain) and psychological distress. Second, use quantitative proteome analysis on pooled urine samples to discover putative biomarker(s) between IBS and healthy subjects as well as across distinctive IBS symptom subgroups. Third, use individual samples to validate putative biomarkers by ELISA.
EXPERIMENTAL SECTION
Urine Sample Collection and Processing
Urine samples were previously collected as part of two studies from adult women with IBS and without IBS. Participants were recruited from the community, screened over the phone, assessed for eligibility then either enrolled in a mechanism study (the association of sleep quality with GI symptoms),17 or a randomized controlled trial using cognitive-behavioral therapy.18 For the purposes of those studies, women provided a first voided urine specimen at multiple, predetermined time points. For the current urinary proteome study, samples with urine creatinine level ≥ 100 mg/dL and hemoglobin (presence of RBCs) ≤ 0.03mg/dL were selected. The proteomic analysis of stored samples was approved by the University Human Subjects Review Committees.
IBS Symptom Subgroup Classification
The goal for defining subgroups was to create relatively pure and distinct groups that differed from each other in symptom profile. This approach was used to maximize the chance of identifying proteins that differed among clinically distinct groups, which would then require further validation. Defining the groups was not an end in itself but a means towards the goal of biomarker discovery. These subgroup definitions are not being proposed as categories that would be useful in clinical practice.
Subgroup definitions were based on both a prospective 28-day diary and recall-questionnaire data. GI (diarrhea, constipation, abdominal pain, stool consistency) and psychological (anxiety, depression) symptoms were considered to look for logical groupings of women with IBS. Women used a diary to record symptom severity every day, on a 0 (not present) to 4 (severe) scale as previously described.19,20 The women also recorded the consistency of each stool on the daily diary. The Brief Symptom Inventory(BSI) and the Rome II questionnaire were used to measure retrospective psychological and GI symptoms, respectively.21,22 Urine samples from women under age 47 were used for this study. Ninety-four women with IBS from the previous study18 had urine samples and diary data available.
A ‘pure’ Constipation subgroup was defined as those whose bowel pattern was constipation-predominant by both the daily diary and the retrospective questionnaire. Constipation-predominant based on the 28-day diary was defined as either (a) hard stools at least 40% of days and loose stools < 40% of days, or (b) very hard stools at least 20% of days and very loose stools < 20% of days. Constipation-predominant according to the Rome II retrospective questionnaire, which asks about the last three months, was defined as either (a) hard stools ‘often’ to ‘almost always’ and loose stools ‘not at all’ to ‘occasionally’, or (b) when abdominal pain or discomfort began, the subject had harder than usual stools ‘often’ to ‘almost always’ and looser than usual stools ‘not at all’ to ‘occasionally’. A ‘pure’ Diarrhea subgroup was defined analogously. The diarrhea subgroup was further divided into two subgroups based on abdominal pain severity: Diarrhea High Pain if abdominal pain was moderate to severe on at least 40% of days, or Diarrhea Low Pain if not. The Constipation subgroup was not large enough to allow subdivision into high and low pain groups. Using only subjects who did not meet the criteria to be in one of the first three subgroups, a fourth subgroup, High Pain High Psych (psychological distress), was defined. This subgroup required moderate to severe abdominal pain on at least 40% of days, and also high psychological distress defined as a Global Severity Index (GSI) of at least 0.5. About half the subjects did not meet the criteria for any of these four ‘pure’ groups.
The identification of these four subgroups were also supported by earlier work in which we described how IBS bowel pattern subgroups (diarrhea, constipation) differed on pain/discomfort severity, heart rate variability23 and stress hormone level (cortisol, catecholamine) especially during sleep.24
The fifth group consisted of 24 healthy control women from our early study17 who, during the telephone screening, denied any history of GI disorders and any of the GI symptoms described in the Rome II criteria. Subjects were excluded if they had any known cardiac arrhythmias or were taking medications that could interfere with sleep, such as beta blockers, antihistamines, benzodiazepines, or antidepressants (e.g., tricyclic antidepressants and selective serotonin reuptake inhibitors), or oral contraceptives. We also manually reviewed their daily diaries to ensure that the healthy control group did not report daily moderate to severe GI symptoms.
Urine sample Processing for Mass Spectrometry Analysis
Ten individual subjects were selected from each distinct symptom subgroup and from the healthy control group. Pooled samples were created using 3 mL of urine from each individual for a total of 30mL per subgroup. Urine was pooled to allow for deep proteome sequencing and due to a limited volume of urine available for individual samples as well as resource constraints. One protease inhibitor cocktail tablet (Complete™, Roche Diagnostics Corporation, Indianapolis, IN) was added per 30 mL urine to avoid proteolysis immediately after urine was thawed. The urine was centrifuged at 2000 × g for 10 min at 4°C to remove cells and debris. The supernatant was collected and processed for protein concentration and purification by 3kDa molecular weight cutoff ultrafiltration (Centriprep YM-3, Millipore, Billerica, MA) followed by trichloroacetic acid (TCA) precipitation. TCA was added to urine at 10% final concentration (w/v, made fresh), vortexed for 15 sec, and placed on ice for a minimum of 20 min. The samples were centrifuged at 14,000 × g, 4°C for 15 min and the supernatant was removed. The pellet was washed with cold acetone and then centrifuged at 14,000 × g, 4°C for 10 min. The acetone was removed from the loose pellet and air-dried thoroughly. Protein concentration was estimated by BCA protein assay (ThermoFisher Scientific Inc., Rockford, IL). Protein, 300 μg each per subgroup, was reduced with Tris(2-carboxyethyl)phosphine, alkylated with iodoacetamide, and digested with sequencing-grade trypsin (Promega, Madison, WI). Peptides then were desalted on a MacroSpin C18 column (The Nest Group, Inc., Southborough, MA) according to the manufacturer’s instructions.
Mass Spectrometry Analysis
Peptide digestion products were analyzed by electrospray ionization on a linear ion trap Velos mass spectrometer (Thermo Scientific Corp., San Jose, CA). Nanoflow HPLC was performed using a Waters NanoAquity HPLC system (Waters Corporation, Milford, MA). Peptides were trapped on a 100 μm i.d. × 20 mm long precolumn in-house packed with 200 Å (5 μm) Magic C18 particles (C18AQ; Michrom Bioresources Inc., Auburn, CA). Subsequent peptide separation was on an in-house constructed 75 μm i.d. × 180 mm long analytical column pulled using a Sutter Instruments P-2000 CO2 laser puller (Sutter Instrument Company, Novato, CA) and packed with 100 Å (5 μm) C18AQ particle. For each liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, an estimated amount of 1 μg of peptides (0.1 μg/μL) were loaded on the precolumn at 4 μL/min in water/acetonitrile (95/5) with 0.1% (v/v) formic acid. Peptides were eluted using an acetonitrile gradient flowing at 250 nL/min using mobile phase gradient of 5–35% acetonitrile over 60 min. with a total gradient time of 95 min. Ion source conditions were optimized using the tuning and calibration solution recommended by the instrument provider.
To maximize protein identification without protein fractionation, a modified version of the data-independent Precursor Acquisition Independent From Ion Count (PAcIFIC) method as originally published was employed.25 Briefly, this data-independent acquisition (DIA) method acquires tandem mass spectra at every m/z value (i.e., at each m/z “channel”) without regard for whether a precursor ion is observed or not. The acquired tandem mass spectra then are matched to peptide sequence in a database using the center of the precursor isolation window as the parent ion mass in each tandem mass spectrum file. Modifications to the published PAcIFIC analysis included increasing the mass range covered per method file from 15 to 37.5 m/z units for a total of 27 LC-MS/MS runs per sample to cover 400–1400 m/z. Consecutive scans remained 1.5 units apart with an isolation width of 2.5 m/z as previously described. For tandem MS (MS/MS) in the linear ion trap, ion population was set to 3 × 104 through use of automatic gain control, and collision energy set to 35%. For each cycle, 25 data-independent MS/MS scans were acquired per designated m/z range. Data were acquired using Xcalibur, version 2.0 (Thermo Scientific). Each experiment was done in duplicate.
Database Search and Data Processing
Acquired tandem mass spectra were searched for sequence matches against the International Protein Index (IPI) human protein database (version 3.68) using SEQUEST. The following modifications were set as search parameters: peptide mass tolerance at 3.75 Da, trypsin digestion cleavage after K or R (except when followed by P), one allowed missed cleavage site, carboxymethylated cysteines (static modification), and oxidized methionines (variable modification/differential search option). PeptideProphet and ProteinProphet, which compute a probability likelihood of each identification being correct, were used for statistical analysis of search results.13 PeptideProphet probability ≥ 0.8 and ProteinProphet probability ≥ 0.85 were used for positive identification at an error rate of less than 1%. Only the proteins identified by more than one unique peptide sequence were included in the study. Differences in relative expression of proteins were calculated using peptide spectral counting algorithm.26 The final protein spectral count was the accumulation of spectral counts from all PAcIFIC LC-MS/MS runs. Data were analyzed using one experiment first then cross referenced with a replicate experiment for consistency for computed fold changes among the different subject groups.
ELISA
To confirm the MS findings, the individual samples (n=50) that had been used in the 5 pools were analyzed for urinary neutrophil gelatinase-associated lipocalin (NGAL), gelsolin (GSN), and trefoil factor 3 (TFF3) using a commercially available ELISA kits which incorporated specific performance metrics. The NGAL assay kit (BioPorto diagnostics, Gentofte, Denmark) has a linear detection range between 25 and 600 pg/ml and a sensitivity of 8 pg/ml. The GSN assay kit (Kamiya Biomedical Company, Seattle, WA) has a linear detection range between 2 and 200 ng/mL, with a sensitivity of 2 ng/mL; the inter and intra-assay coefficients of variability based on kit controls for this assay is 14.8 and 11.7%, respectively. The ELISA assay for TFF3 (BioPorto diagnostics, Karasek, Czech Republic) has a linear detection range between 10 and 2000pg/ml, with a sensitivity of 7 pg/ml, and the inter and intra-assay variance using kit controls are 12.2 and 10.5%, respectively. TFF3 ELISA was also run on urine samples from individual subjects from the second cohort (n=68). ELISA was done in duplicate for each protein.
Selected Reaction Monitoring (SRM) Analysis for Gelsolin (GSN)
SRM method development and data analysis for GSN was performed using the Skyline software (v.0.5)27 for the targeted peptide, TGAQELLR, at m/z 444.25. Isotopically labeled peptide standard was purchased from University of Victoria Genome BC Proteomics Centre (Victoria, BC, Canada). An Eksigent NanoLC-1D plus HPLC (Eksigent Technologies, Dublin, CA) was used for the injection of desalted pooled urine digest samples onto reversed-phase capillary columns (75 μm × 15 cm) packed in-house using Magic C18AQ (5μm,100Å). Separations were performed using a flow rate of 250 nl/min with a 60-min linear gradient. Thermo Scientific TSQ Vantage mass spectrometer was used for all LC-SRM/MS analyses. SRM acquisition method was constructed using collision energy (CE) voltages at 16, with a target scan time of 0.035 s. The doubly charged precursor ions were monitored in Q1 with a resolution of 0.7 full width at half-maximum (FWHM), and seven singly charge y-ions (y1–y7) for endogenous and isotope labeled peptide was monitored in Q3 with 0.7 FWHM. Product ion ratios and chromatographic retention time were used to confirm the peptide identity within the complex mixtures. Three technical replicates were acquired per sample.
RESULTS
IBS Symptom Subgroups
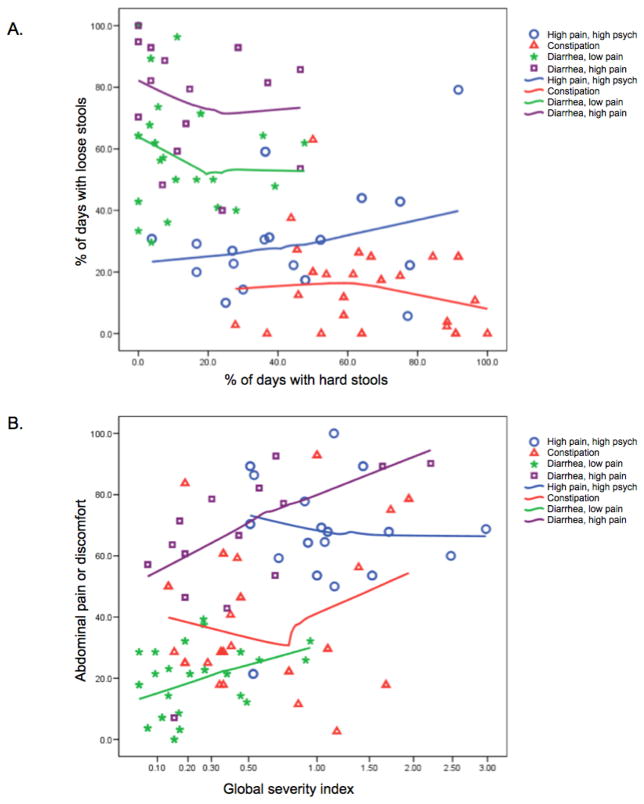
Based on the clustering of symptoms we selected four symptom groups: 1) Constipation, 2) Diarrhea + High Pain, 3) Diarrhea + Low Pain, and 4) High Pain + High Psych (Figure 1.A and 1.B). Of the 118 women included for the study, the numbers of subjects classified into the four IBS subgroups were 12 high pain high psych, 12 diarrhea high pain, 19 diarrhea low pain, and 19 constipation. Thirty-two women with IBS did not fit into any of these categories because their symptoms were not severe enough or were mixed or inconsistent (e.g., a subject with both diarrhea and constipation). There were 24 women in the healthy control group. Ten women were selected from each of these five groups, and the urine samples combined into five pooled samples for use in the MS. The supplemental Table shows the symptom profiles and numbers of subjects of each group. Samples from the remaining 68 subjects were reserved for further validation using ELISA.
Figure 1. IBS symptom subgroup classification.
A) Comparison of IBS subtype defined by retrospective questionnaire to percent of days with hard and loose stools from daily diary. B) Comparison of abdominal pain to the ‘Global Severity Index’, which is a measurement of psychological distress. The cluster analyses separate IBS into distinctive symptom phenotypes: 1) constipation, 2) diarrhea + high pain, 3) diarrhea + low pain, and 4) high pain + high psychological distress.
IBS Urinary Proteomes
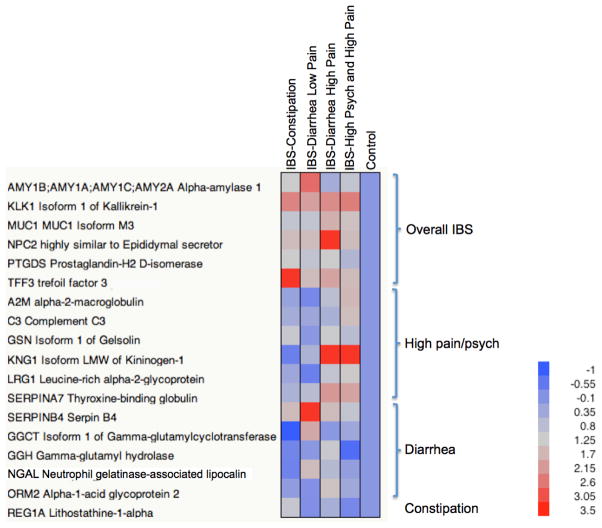
More than 750 proteins were identified by MS from each IBS subgroup and healthy controls. The selection of the initial candidate proteins was based on: the current pathophysiologic theories surrounding the cause(s) of IBS, good agreement between the two MS datasets, as well as proteins that showed the greatest expression differences relative to the controls. A total of 18 were selected for further investigation (Table 1). The correlation between the two datasets was greater than 0.5 for all but five proteins. However, we included these five proteins in the analysis because of their putative implication in IBS pathophysiology. Most of the proteins selected were annotated by Gene Ontology (GO) classification as either extracellular or membrane proteins, both of which are characteristic of the secretary urinary proteome.28 Their relative expression levels, normalized to the control group, are plotted as a heat map in Figure 2 where proteins were classified into IBS subgroups based on their phenotypic expression characteristics.
Table 1. Differentially expressed proteins identified from IBS subgroups.
The International Protein Index (IPI), protein description, Gene Ontology (GO) classifications, expression ratio (IBS/control, log2), and correlation between the two MS datasets are tabulated.
| IPI | Description | Biological Process |
Molecular Function |
Cellular Compon ent |
IBS Constipa tion/ Control |
IBS Diarr hea Low Pain/ Contr ol |
IBS Diarr hea High Pain/ Contr ol |
IBS High Pain High Psych/ Control |
Correla tion |
|---|---|---|---|---|---|---|---|---|---|
| IPI00478003 | A2M α-2-macroglobulin | protein homooligomerization | interleukin-8 binding | platelet alpha granule lumen | 0.22 | −0.26 | 0.87 | 1.66 | 0.94 |
| IPI00300786 | AMY1B;AMY1A;AMY1C;AMY2 A α-amylase 1 | carbohydrate metabolic process | alpha-amylase activity | extracellular | 1.22 | 2.74 | 0.42 | 1 | −0.43 |
| IPI00783987 | C3 Complement C3 | G-protein coupled receptor protein signaling pathway | endopeptidase inhibitor activity | extracellular | 0.42 | 0.29 | 0.53 | 1.53 | 0.91 |
| IPI00031564 | GGCT Isoform 1 of γ-glutamylcyclotransferase | N/A | N/A | N/A | −1 | 1.91 | 0 | 0.32 | 0.74 |
| IPI00023728 | GGH γ-glutamyl hydrolase | glutamine metabolic process | exopeptidase activity | lysosome | −0.32 | 0 | 1.14 | −0.74 | 0.26 |
| IPI00026314 | GSN Isoform 1 of Gelsolin | barbed-end actin filament capping | actin binding | actin cytoskeleton | 1.09 | 0 | 1.17 | 0.81 | 0.94 |
| IPI00304808 | KLK1 Isoform 1 of Kallikrein-1 | proteolysis | peptidase activity | N/A | 2.45 | 2.07 | 2.34 | 2.52 | 0.29 |
| IPI00215894 | KNG1 Isoform LMW of Kininogen-1 | elevation of cytosolic calcium ion concentration | cysteine protease inhibitor activity | extracellular | −0.42 | 0.58 | 3.31 | 3.31 | 0.22 |
| IPI00299547 | NGAL Neutrophil gelatinase-associated lipocalin | transport | transporter activity | soluble fraction | −0.36 | 1.58 | 0.74 | 0.15 | 0.75 |
| IPI00022417 | LRG1 Leucine-rich α-2-glycoprotein | N/A | protein binding | membrane | 0.32 | −0.42 | 1 | 1.32 | 0.67 |
| IPI00385172 | MUC1 Mucin 1 Isoform M3 | N/A | hormone activity | integral to plasma membrane | 1 | 1 | 1.81 | 1.46 | 0.77 |
| IPI00301579 | NPC2 highly similar to Epididymal secretor | cholesterol homeostasis | cholesterol binding | lysosome | 1.58 | 1.58 | 3.31 | 1.58 | 0.81 |
| IPI00020091 | ORM2 α-1-acid glycoprotein 2 | acute-phase response | binding | extracellular | 0.10 | −0.11 | 1.44 | 0.36 | 0.83 |
| IPI00013179 | PTGDS Prostaglandin- H2 D-isomerase | prostaglandin biosynthetic process | prostaglandin-D synthase activity | rough endoplasmic reticulum | 1.25 | 0.97 | 1.25 | 0.65 | 0.52 |
| IPI00009027 | REG1A Lithostathine-1- α | positive regulation of cell proliferation | sugar binding | N/A | 1.06 | −0.13 | 0.50 | −0.26 | 0.32 |
| IPI00292946 | SERPINA7 Thyroxine-binding globulin | N/A | serine-type endopeptidase inhibitor activity | extracellular | 0.58 | 0.81 | 2.17 | 2 | 0.99 |
| IPI00010303 | SERPINB4 Serpin B4 | regulation of proteolysis | serine-type endopeptidase inhibitor activity | cytoplasm | 1.58 | 3.31 | 1.58 | 1 | 0.99 |
| IPI00018909 | TFF3 trefoil factor 3 | defense response | N/A | extracellular | 3.31 | 1.58 | 2 | 1.58 | 0.63 |
Figure 2. Expression of putative protein biomarkers representing different IBS symptom subgroups.
Log2 expression ratio found in Table 1 is used to generate the plot. Proteins are grouped by different IBS symptom subgroups.
Putative overall IBS biomarkers
The following six proteins, α-amylase 1 (AMY1), kallikrein-1 (KLK1), mucin 1 (MUC1), epididymal secretor (NPC2), prostaglandin-H2 D-isomerase (PTGDS), and trefoil factor 3 (TFF3), were found to be over expressed in most of the IBS subgroups.
Amylase genes belong to a multigene family and the most of characterization has been done for pancreatic and salivary amylases.29 Alpha-amylase is a glycoprotein of 57 kDa and is the only glycosidase in human pancreatic fluid.30 The expression of this protein was elevated in most of the IBS symptom subgroups with slightly higher expression in the diarrhea with low pain group.
Kallikreins are a subgroup of serine proteases having diverse physiological functions as well as being potential disease markers in colon cancer and diabetes mellitus.31,32 KLK1 is one of the fifteen kallikrein subfamily members and it releases bradykinin from low molecular weight kininogen.33 In our study, KLK1 is overexpressed in all of the IBS symptom subgroups.
MUC1, isoform M3, is a member of the mucin family and encodes a membrane bound, glycosylated phosphoprotein. The protein serves a protective function by binding to pathogens and also functions in a cell signaling capacity.34 Expression of MUC1 was increased in IBS symptom subgroups overall with slightly greater expression in the diarrhea with high pain group.
NPC2, known as Niemann-Pick disease type C2, is an epididymal secretory protein.35 Expression of NPC2 was elevated in the most of the IBS symptom subgroups, especially in the diarrhea with high pain group.
PTGDS is known by various synonyms including glutathione-independent PGD synthase, lipocalin-type prostaglandin-D synthase (L-PGDS), and prostaglandin-D2 synthase. It is a member of the lipocalin superfamily.36 Its expression was upregulated in all IBS symptom subgroups.
TFF protects and repairs the GI mucosa by maintaining intestinal epithelial cell integrity and restoring normal intestinal permeability.37 An elevated level of TFF3 was found in most of the IBS subgroups.
Putative IBS-high pain and psychological distress biomarkers
Expression of the six proteins, α-2-macroglobulin (A2M), complement C3, gelsolin (GSN), kininogen-1 (low molecular weight) (KNG1), leucine-rich α-2-glycoprotein (LRG1), and thyroxine-binding globulin (SERPINA7) were elevated among the IBS high pain and high psych subgroup when compared with samples from the healthy control group.
A2M showed more upregulation in high pain groups including the diarrhea with high pain group. Complement system protein, C3 expression was elevated in the high pain groups, similar to the expression pattern observed for A2M. A2M is an inhibitor of multiple proteinases and function as antiprotease. It may act as a carrier protein because it also binds to numerous growth factors and cytokines.38 The complement system is a key component of innate immunity, acting to protect the host from microorganisms such as bacteria. Defects in C3 are the cause of complement component 3 deficiency, a rare defect of the complement classical pathway.39
GSN is a Ca(2+)-regulated actin filament severing, capping, and nucleating protein.40 It is an ubiquitous, multifunctional regulator of cell structure, mitochondrial membrane stability, motility and metabolism as well as apoptosis.38 GSN was upregulated among all the high pain groups including the diarrhea with high pain and constipation groups.
Expression of KNG1 was elevated in all pain groups in our study. The KNG1 gene uses alternative splicing to generate two different proteins, high molecular weight kininogen (HMWK) and low molecular weight kininogen (LMWK).39 LMWK is involved in the release of bradykinin.33,41
The leucine-rich repeat family proteins, including LRG1, are involved in protein-protein interaction, signal transduction, and cell adhesion and development.42 LRG1 was overexpressed in the three IBS subgroups with high pain.
SERPINA7 is responsible for transporting thyroid hormones in blood. It belongs to the serpin family in genomics, but the protein has no inhibitory function like many other members of the serpin family.43 The protein demonstrated the greater expression among all the IBS subgroups and especially in the high pain groups.
Putative IBS-diarrhea or -constipation biomarkers
Five proteins including SERPIN B4 (SERPINB4), γ-glutamylcyclotransferase (GGCT), γ-glutamyl hydrolase (GGH), neutrophil gelatinase-associated lipocalin (NGAL), and α-1-acid glycoprotein 2 (ORM2) showed elevated expression among the diarrhea groups whereas expression of lithostathine-1-α (REG1A) was elevated in the constipation patient group.
SERPINB4 is a serpin peptidase inhibitor and directly inhibits human granzyme, GrM proteolytic activity.44 Elevation of SERPINB4 was noted in the diarrhea with low pain group but it was also overexpressed in the high pain groups.
GGCT induces release of cytochrome c from mitochondria with resultant induction of apoptosis.45 GGH is an intracellular lysosomal glycoprotein that hydrolyzes folyl- and antifolyl-polyglutamates. It has been suggested that GGH may be a novel putative urinary biomarker of clinical outcome after chemotherapy.15
NGAL is a protein that is associated with neutrophils and is part of the innate immune system. It plays a role in inhibiting bacterial growth through its binding to iron bacterial siderophores in the intestinal lumen.46 Neutrophils and epithelial cells release NGAL as part of an antiinflammatory response.
ORM2 is a key acute phase plasma protein and is classified as an acute phase reactant. The specific function of this protein has not yet been determined, however, it may be involved in aspects of immunosuppression. Increased urinary orosomucoid has been observed in type 2 diabetes.47
REG1A is type I subclass member of the Reg gene family and also known as regenerating islet-derived 1-α. The protein has been implicated in ulcerative colitis and gastric cancer.48,49 REG1A showed slightly more expression in the constipation group.
Validation
The proteomics results were validated in the 50 individual samples by ELISA using commercially available kits on selected proteins that represented different IBS subgroups. Three proteins, NGAL, TTF3, and GSN were selected given they fit with current thinking on the pathophysiology of IBS and that commercial kits or antibodies were available.
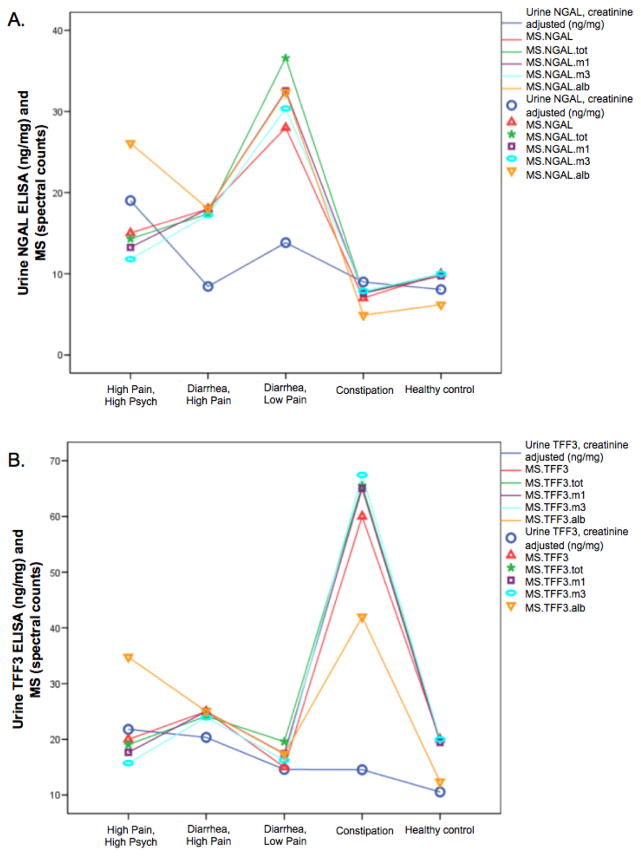
Figure 3.A shows the mean ELISA results for NGAL in comparison to the MS results with differing options for normalization. Analysis of variance showed significant differences in mean NGAL across the 5 groups (P=.008). The ELISA pattern across groups is similar to MS, with constipation and healthy controls having low NGAL and diarrhea low pain having high NGAL. However, ELISA results showed the high pain high psych group having very high NGAL, while MS results do not, with the exception of the normalization by albumin. Combining all IBS groups tended to show mean NGAL higher in IBS than in healthy controls (P=.13).
Figure 3. NGAL and TFF3 expression in IBS symptom subgroups by MS and ELISA.
A) NGAL expression. Blue circle and line is the creatinine adjusted urinary NGAL expression level determined by the ELISA. All the others show the MS data with different normalizations. MS.NGAL - no normalization; NGAL.tot - normalized by the sum of all MS spectral counts for all proteins; NGAL.m1 - normalized by the sum of all proteins except uromodulin (the highest MS spectral counts); NGAL.m3 - normalized by the sum of all proteins except uromodulin, AMBP, albumin (the highest three); NGAL.alb - normalized by the spectral count for albumin. B) TFF3 expression.
TFF3 ELISA assay results are shown in Figure 3.B. ANOVA showed no significant differences across the 5 groups (P=.193) but IBS versus healthy controls showed increased TFF3 in IBS as observed in the MS (P=.067). The pattern across groups of ELISA results is similar to the MS results, with the exception of the constipation group. The spectral count from MS is high for the pooled constipation sample but is low for the mean concentration by ELISA. This discrepancy could be related to the fact that approximately 30% of TFF3 ELISA results were above the highest standard on the normal curve, meaning that we know the values are high but do not know how high. A single sample in the constipation group, which has extremely high TFF3 expression could cause the MS results from the pooled sample to be very high. This points out the caution of using pooled samples, namely the results could be influenced by one aberrant individual.
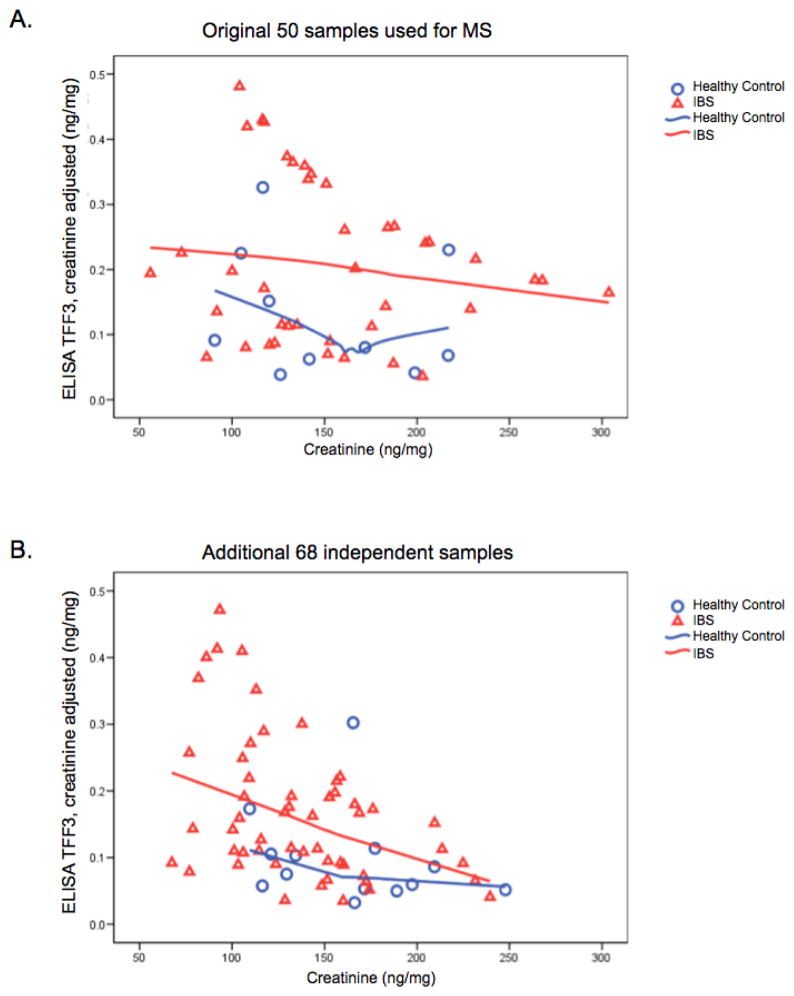
In order to further validate the IBS versus healthy control difference seen in the 50 original samples, ELISA TFF3 was run on samples from the additional 68 women who were not used in the first 50. Those results confirm the original results. TFF3 was elevated in IBS patients compared to healthy controls (P=.027). Figures 4.A and 4.B show the TFF3 results for the original 50 subjects and the additional 68 independent subjects.
Figure 4. TFF3 expression in IBS and healthy control women.
A) TFF3 expression in the 50 women used in the MS. B) TFF3 expression in the 68 independent women with and without IBS.
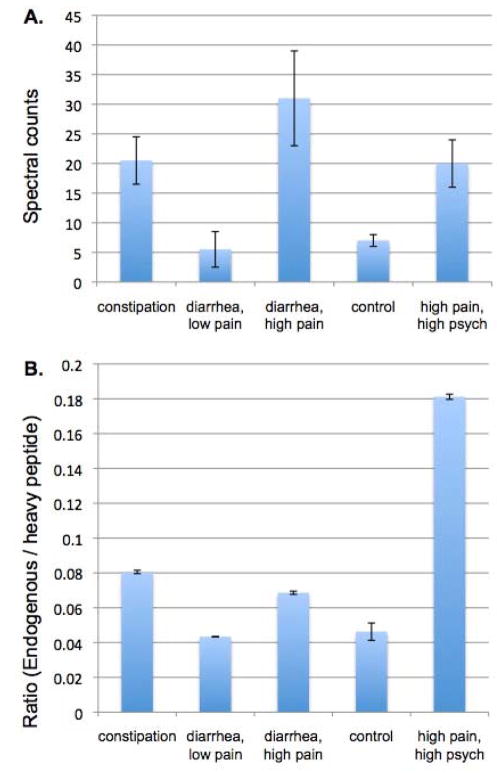
Differentially expressed GSN levels were initially validated by Western blot analysis applied to the pooled samples, where it confirmed the elevated expression in IBS subgroups with high pain.20 The commercially available ELISA for GSN was designed for use with serum or plasma, and its sensitivity was inadequate for urine samples – all samples were below the detection level. Hence, validation of GSN in the pooled samples was further carried out by SRM approach using a heavy labeled peptide by monitoring seven transition y-ions in pooled IBS samples. There was good agreement between spectral counts and the SRM approach (Figure 5).
Figure 5. Gelsolin (GSN) expression in IBS symptom subgroups by spectral counts and targeted SRM.
A) GSN expression by spectral counts from two experimental datasets. B) GSN expression measure by SRM approach. Expression is represented by endogenous peptide/heavy labeled peptide ratio for SRM.
DISCUSSION
Our study showed that differences in the urine proteome exist among symptomatically distinct groups of patients with IBS. Urinary proteomics, like that for all biofluids, is challenging because of large inter- and intra-individual variations.50 Such large variation often makes it challenging to interpret the proteomics data to determine differences between disease and normal conditions. An important advantage of our study design was the stringent processes (e.g., diaries) and clinical criteria we applied to define the IBS groups. Another advantage was the well-defined procedures for collection (first morning void) and screening (absence of blood) of urine samples. These steps increased the likelihood of identifying protein markers that would discriminate between IBS and controls as well as among IBS subgroups. We also employed an innovative shotgun protein sequencing approach, PAcIFIC, that provided deep sequencing of the urinary proteome circumventing the use of a protein enrichment process in order to increase detection of less abundant proteins.25,51,52 Pooled samples were used in the discovery phase study to help overcome resource constraints and yet allow us to analyze a large set of samples. The pros and cons of sample pooling in relation to biological variances has been discussed in the past.53,54 A disadvantage of pooling is that if one or two individuals in a pool had extremely elevated levels of a protein, it could lead to shifting of the overall mean expression of that protein in the pool. This is one reason that observed differences between pooled samples need to be validated using individual samples.
Not surprisingly, some of the proteins that showed distinctive differences in IBS samples were the ones involved in homeostasis of intestinal function. MUC1 and TFF3 are mucosal epithelial membrane-bound molecules synthesized by the goblet cells.55,56 The functional integrity of the intestine plays an important role in the first line defense system against chemical injury or unwanted microbes. The intestinal mucus layer and membrane-bound molecules such as TFF3 are the major components to protect/repair the mucosal epithelium. Thus, any change among these molecular components would affect delicate intestinal environment and result in increased permeability that might cause inflammation as well as injury to the intestinal mucosal cells, which has been described in some patients with IBS.57 Expression of TFF3 was higher in individuals with IBS as compared to healthy controls. Mice studies of gut inflammation have noted increased TFF3 mRNA levels during colitis induction, an indication that TFF3 genes are upregulated early in inflammation and that their mRNA levels could be used as early markers of inflammation.58 The use of a non-invasive marker such as urine TFF3 may also serve as an early indicator of altered intestinal defenses. However, TFF3 is also expressed in urinary tract epithelia and excreted in urine.59 Thus, to elucidate the role of TFF3 in IBS additional studies are warranted.
Other proteins including L-PGDS and NGAL also have been suggested as markers reflecting inflammatory activity.60 Dextran sulfate sodium (DSS)-induced colitis in mice showed L-PGDS expressing cells play pro-inflammatory roles in colitis.61 The same study showed that the increased level of L-PGDS is linked to severity of ulcerative colitis in humans. Serum NGAL levels are elevated in patients with Crohn’s disease and ulcerative colitis.62 Measuring metalloproteinase (MMP-9) together with NGAL in urine of children and young adults with IBD, Manfredi et al. found elevations relative to age and sex-matched control samples.63 They found that the sensitivity of MMP/NGAL in identifying patients with IBD was greater than erythrocyte sedimentation rate levels and c-reactive protein. In our MS results NGAL showed elevated expression in both diarrhea groups (high and low pain). ELISA confirmed the subgroup differences especially for the diarrhea with low pain group. In a single study, NGAL levels were found to be higher in fecal dialysate samples from patients with Crohn’s disease relative to healthy controls suggesting that fecal proteomic approaches also may be useful to examine NGAL as a putative biomarker.64
A targeted SRM approach used for GSN in pooled samples confirmed the increased expression among the IBS groups with high pain. One of the hypotheses about the function of circulating GSN is that it scavenges actin released from cells at the site of injury. Levels of plasma GSN decrease during acute injury and inflammation.65,66 The administration of recombinant plasma GSN in mice and rats improves outcomes following sepsis or burn injuries.65 An insufficiency of GSN in mice has also been shown to cause increased permeability of the vascular pulmonary barrier, suggesting that GSN is important in regulating membrane permeability.67
The study of the utility of urine proteomics as biomarkers for IBS subgroups is in its infancy. The ideal biomarker would be one that either separates IBS subgroups from each other or separates IBS patients from healthy individuals as well as from patients with other diseases such as IBD. Ideally, it would be collected non-invasively from a readily accessible body fluid, have excellent specificity and sensitivity, and be measurable over time to monitor and assess treatment outcomes or disease trajectory. Whether urine protein measures will meet these criteria remains to be tested. Other potential biomarkers for subgroups of IBS including scintography for transit alterations and immune or permeability markers for post infectious IBS-diarrhea also hold promise.68,69
In summary, urine samples from 40 women demonstrating one of four clinical subtypes of IBS revealed selective alterations in specific components of the urinary proteome as assessed by MS when compared with samples from healthy control subjects. Physiological roles have been partially elucidated for some of the proteins identified in this study, however, their function in IBS needs to be investigated further. Although group differences among IBS subgroups have been found in our shotgun proteomics, going beyond the discovery phase to validation studies was challenging. Especially, it should be pointed out that the bowel and pain subgroup patterns noted in pooled urine samples by MS analysis were not always found when the individual urine samples from the pools were assayed with ELISA. In many cases the commercially available ELISA sensitivity is out of the detection level needed for urine samples. For one protein, TFF3, for which quantitative assessments were possible for the whole cohort (40 IBS + 10 healthy) and an independent IBS cohort of 68 individuals, the results suggest it holds promise for identifying IBS and worth further investigation.
Our findings further suggest that future studies with a larger, independent cohort should be considered to enable more extensive assessment and validation of urinary protein markers as a diagnostic tool in adults with IBS.
Supplementary Material
Acknowledgments
Financial support was obtained through grants from NINR, NIH (R01 NR004142 and P30 NR04001).
ABBREVIATIONS
- ELISA
Enzyme-linked immunosorbent assay
- SRM
Single Reaction Monitoring
Footnotes
AUTHOR CONTRIBUTIONS
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript. *YAG, CK: These authors contributed equally.
Supporting Information.
Supporting information 1 shows the symptom profiles of each group and numbers of subjects within each of the groups.
Supporting information 2 includes detail for the proteins listed in the Table 1. Database source, protein, sum of spectral counts, ProteinProphet score, and sequence coverage are tabulated for the duplicated experiments for the five study groups.
This material is available free of charge via the Internet at http://pubs.acs.org,
References
- 1.Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104(Suppl 1):S1–35. doi: 10.1038/ajg.2008.122. [DOI] [PubMed] [Google Scholar]
- 2.Yoon SL, Grundmann O, Koepp L, Farrell L. Management of irritable bowel syndrome (IBS) in adults: conventional and complementary/alternative approaches. Altern Med Rev. 2011;16(2):134–51. [PubMed] [Google Scholar]
- 3.Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis. 2009;41(11):772–80. doi: 10.1016/j.dld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Jerndal P, Ringstrom G, Agerforz P, Karpefors M, Akkermans LM, Bayati A, Simren M. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22(6):646–e179. doi: 10.1111/j.1365-2982.2010.01493.x. [DOI] [PubMed] [Google Scholar]
- 5.DiBonaventura M, Sun SX, Bolge SC, Wagner JS, Mody R. Health-related quality of life, work productivity and health care resource use associated with constipation predominant irritable bowel syndrome. Curr Med Res Opin. 2011;27(11):2213–22. doi: 10.1185/03007995.2011.623157. [DOI] [PubMed] [Google Scholar]
- 6.Suarez-Hitz KA, Otto B, Bidlingmaier M, Schwizer W, Fried M, Ehlert U. Altered psychobiological responsiveness in women with irritable bowel syndrome. Psychosom Med. 2012;74(2):221–31. doi: 10.1097/PSY.0b013e318244fb82. [DOI] [PubMed] [Google Scholar]
- 7.Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5(4):261–8. doi: 10.1177/1756283X11436241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Camilleri M, Katzka DA. Irritable bowel syndrome: methods, mechanisms, and pathophysiology. Genetic epidemiology and pharmacogenetics in irritable bowel syndrome. Am J Physiol Gastrointest Liver Physiol. 2012;302(10):G1075–84. doi: 10.1152/ajpgi.00537.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martinez C, Vicario M, Ramos L, Lobo B, Mosquera JL, Alonso C, Sanchez A, Guilarte M, Antolin M, de Torres I, Gonzalez-Castro AM, Pigrau M, Saperas E, Azpiroz F, Santos J. The jejunum of diarrhea-predominant irritable bowel syndrome shows molecular alterations in the tight junction signaling pathway that are associated with mucosal pathobiology and clinical manifestations. Am J Gastroenterol. 2012;107(5):736–46. doi: 10.1038/ajg.2011.472. [DOI] [PubMed] [Google Scholar]
- 10.Spiller R, Lam C. An Update on Post-infectious Irritable Bowel Syndrome: Role of Genetics, Immune Activation, Serotonin and Altered Microbiome. J Neurogastroenterol Motil. 2012;18(3):258–68. doi: 10.5056/jnm.2012.18.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stasi C, Rosselli M, Bellini M, Laffi G, Milani S. Altered neuro-endocrine-immune pathways in the irritable bowel syndrome: the top-down and the bottom-up model. J Gastroenterol. 2012 doi: 10.1007/s00535-012-0627-7. [DOI] [PubMed] [Google Scholar]
- 12.Gozal D, Jortani S, Snow AB, Kheirandish-Gozal L, Bhattacharjee R, Kim J, Capdevila OS. Two-dimensional differential in-gel electrophoresis proteomic approaches reveal urine candidate biomarkers in pediatric obstructive sleep apnea. Am J Respir Crit Care Med. 2009;180(12):1253–61. doi: 10.1164/rccm.200905-0765OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goo YA, Tsai YS, Liu AY, Goodlett DR, Yang CC. Urinary proteomics evaluation in interstitial cystitis/painful bladder syndrome: a pilot study. Int Braz J Urol. 2010;36(4):464–78. doi: 10.1590/s1677-55382010000400010. discussion 478–9. [DOI] [PubMed] [Google Scholar]
- 14.Goo YA, Goodlett DR. Advances in proteomic prostate cancer biomarker discovery. J Proteomics. 2010;73(10):1839–50. doi: 10.1016/j.jprot.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 15.Pollard C, Nitz M, Baras A, Williams P, Moskaluk C, Theodorescu D. Genoproteomic mining of urothelial cancer suggests {gamma}-glutamyl hydrolase and diazepam-binding inhibitor as putative urinary markers of outcome after chemotherapy. Am J Pathol. 2009;175(5):1824–30. doi: 10.2353/ajpath.2009.090155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matheson A, Willcox MD, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev. 2010;26(3):150–71. doi: 10.1002/dmrr.1068. [DOI] [PubMed] [Google Scholar]
- 17.Heitkemper M, Jarrett M, Burr R, Cain KC, Landis C, Lentz M, Poppe A. Subjective and objective sleep indices in women with irritable bowel syndrome. Neurogastroenterol Motil. 2005;17(4):523–30. doi: 10.1111/j.1365-2982.2005.00700.x. [DOI] [PubMed] [Google Scholar]
- 18.Jarrett ME, Cain KC, Burr RL, Hertig VL, Rosen SN, Heitkemper MM. Comprehensive self-management for irritable bowel syndrome: randomized trial of in-person vs. combined in-person and telephone sessions. Am J Gastroenterol. 2009;104(12):3004–14. doi: 10.1038/ajg.2009.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cain KC, Jarrett ME, Burr RL, Rosen S, Hertig VL, Heitkemper MM. Gender differences in gastrointestinal, psychological, and somatic symptoms in irritable bowel syndrome. Dig Dis Sci. 2009;54(7):1542–9. doi: 10.1007/s10620-008-0516-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Voss J, Goo YA, Cain K, Woods N, Jarrett M, Smith L, Shulman R, Heitkemper M. Searching for the noninvasive biomarker holy grail: are urine proteomics the answer? Biol Res Nurs. 2011;13(3):235–42. doi: 10.1177/1099800411402056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Derogatis L. BSI: Brief Symptom Inventory; Administration, Scoring and Procedures Manual. 4. Minneapolis: National Computer Systems; 1993. [Google Scholar]
- 22.Drossman D, Corazziari E, Talley N, et al., editors. Rome II: The functional gastrointestinal disorders: Diagnosis, Pathophysiology, and Treatment: a Multinational Consensus. 2. McLean, Degnon Associates; 2000. [Google Scholar]
- 23.Jarrett ME, Burr RL, Cain KC, Rothermel JD, Landis CA, Heitkemper MM. Autonomic nervous system function during sleep among women with irritable bowel syndrome. Dig Dis Sci. 2008;53(3):694–703. doi: 10.1007/s10620-007-9943-9. [DOI] [PubMed] [Google Scholar]
- 24.Burr RL, Jarrett ME, Cain KC, Jun SE, Heitkemper MM. Catecholamine and cortisol levels during sleep in women with irritable bowel syndrome. Neurogastroenterol Motil. 2009;21(11):1148–e97. doi: 10.1111/j.1365-2982.2009.01351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Panchaud A, Scherl A, Shaffer SA, von Haller PD, Kulasekara HD, Miller SI, Goodlett DR. Precursor acquisition independent from ion count: how to dive deeper into the proteomics ocean. Anal Chem. 2009;81(15):6481–8. doi: 10.1021/ac900888s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu H, Sadygov RG, Yates JR. 3rd, A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal Chem. 2004;76(14):4193–201. doi: 10.1021/ac0498563. [DOI] [PubMed] [Google Scholar]
- 27.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, Kern R, Tabb DL, Liebler DC, MacCoss MJ. Skyline: an open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics. 2010;26(7):966–8. doi: 10.1093/bioinformatics/btq054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, Harris MA, Hill DP, Issel-Tarver L, Kasarskis A, Lewis S, Matese JC, Richardson JE, Ringwald M, Rubin GM, Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25(1):25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gumucio DL, Wiebauer K, Caldwell RM, Samuelson LC, Meisler MH. Concerted evolution of human amylase genes. Mol Cell Biol. 1988;8(3):1197–205. doi: 10.1128/mcb.8.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Whitcomb DC, Lowe ME. Human pancreatic digestive enzymes. Dig Dis Sci. 2007;52(1):1–17. doi: 10.1007/s10620-006-9589-z. [DOI] [PubMed] [Google Scholar]
- 31.Kim JT, Song EY, Chung KS, Kang MA, Kim JW, Kim SJ, Yeom YI, Kim JH, Kim KH, Lee HG. Up-regulation and clinical significance of serine protease kallikrein 6 in colon cancer. Cancer. 2011;117(12):2608–19. doi: 10.1002/cncr.25841. [DOI] [PubMed] [Google Scholar]
- 32.Miranda GM, Magalhaes CA, Bosco AA, Reis JS, Ribeiro-Oliveira A, Jr, Nogueira AI, Leite RB, Miranda PA, Figueiredo AF. Increased tissue kallikrein amidase activity in urine of patients with type 1 diabetes under insulin therapy in those with gestational diabetes mellitus not under insulin therapy. Biochem Biophys Res Commun. 2011;406(1):141–5. doi: 10.1016/j.bbrc.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 33.Tang SC, Leung JC, Lai KN. The kallikrein-kinin system. Contrib Nephrol. 2011;170:145–55. doi: 10.1159/000325650. [DOI] [PubMed] [Google Scholar]
- 34.Camilleri M. Probiotics and irritable bowel syndrome: rationale, putative mechanisms, and evidence of clinical efficacy. J Clin Gastroenterol. 2006;40(3):264–9. doi: 10.1097/00004836-200603000-00020. [DOI] [PubMed] [Google Scholar]
- 35.Rosenbaum AI, Maxfield FR. Niemann-Pick type C disease: molecular mechanisms and potential therapeutic approaches. J Neurochem. 2011;116(5):789–95. doi: 10.1111/j.1471-4159.2010.06976.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nagata A, Suzuki Y, Igarashi M, Eguchi N, Toh H, Urade Y, Hayaishi O. Human brain prostaglandin D synthase has been evolutionarily differentiated from lipophilic-ligand carrier proteins. Proc Natl Acad Sci U S A. 1991;88(9):4020–4. doi: 10.1073/pnas.88.9.4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu LF, Teng X, Guo J, Sun M. Protective Effect of Intestinal Trefoil Factor on Injury of Intestinal Epithelial Tight Junction Induced by Platelet Activating Factor. Inflammation. 2011 doi: 10.1007/s10753-011-9320-x. [DOI] [PubMed] [Google Scholar]
- 38.Ho AS, Cheng CC, Lee SC, Liu ML, Lee JY, Wang WM, Wang CC. Novel biomarkers predict liver fibrosis in hepatitis C patients: alpha 2 macroglobulin, vitamin D binding protein and apolipoprotein AI. J Biomed Sci. 2010;17:58. doi: 10.1186/1423-0127-17-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singer L, Whitehead WT, Akama H, Katz Y, Fishelson Z, Wetsel RA. Inherited human complement C3 deficiency. An amino acid substitution in the beta-chain (ASP549 to ASN) impairs C3 secretion. J Biol Chem. 1994;269(45):28494–9. [PubMed] [Google Scholar]
- 40.Pottiez G, Haverland N, Ciborowski P. Mass spectrometric characterization of gelsolin isoforms. Rapid Commun Mass Spectrom. 2010;24(17):2620–4. doi: 10.1002/rcm.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sotiropoulou G, Pampalakis G, Diamandis EP. Functional roles of human kallikrein-related peptidases. J Biol Chem. 2009;284(48):32989–94. doi: 10.1074/jbc.R109.027946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Zhang Y, Qiu F, Qiu Z. Proteomic identification of exosomal LRG1: a potential urinary biomarker for detecting NSCLC. Electrophoresis. 2011;32(15):1976–83. doi: 10.1002/elps.201000598. [DOI] [PubMed] [Google Scholar]
- 43.Qi X, Loiseau F, Chan WL, Yan Y, Wei Z, Milroy LG, Myers RM, Ley SV, Read RJ, Carrell RW, Zhou A. Allosteric modulation of hormone release from thyroxine and corticosteroid-binding globulins. J Biol Chem. 2011;286(18):16163–73. doi: 10.1074/jbc.M110.171082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Koning PJ, Kummer JA, de Poot SA, Quadir R, Broekhuizen R, McGettrick AF, Higgins WJ, Devreese B, Worrall DM, Bovenschen N. Intracellular serine protease inhibitor SERPINB4 inhibits granzyme M-induced cell death. PLoS One. 2011;6(8):e22645. doi: 10.1371/journal.pone.0022645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masuda Y, Maeda S, Watanabe A, Sano Y, Aiuchi T, Nakajo S, Itabe H, Nakaya K. A novel 21-kDa cytochrome c-releasing factor is generated upon treatment of human leukemia U937 cells with geranylgeraniol. Biochem Biophys Res Commun. 2006;346(2):454–60. doi: 10.1016/j.bbrc.2006.05.161. [DOI] [PubMed] [Google Scholar]
- 46.Urbschat A, Obermuller N, Haferkamp A. Biomarkers of kidney injury. Biomarkers. 2011;16(Suppl 1):S22–30. doi: 10.3109/1354750X.2011.587129. [DOI] [PubMed] [Google Scholar]
- 47.Matheson A, Willcox MD, Flanagan J, Walsh BJ. Urinary biomarkers involved in type 2 diabetes: a review. Diabetes Metab Res Rev. 2010;26(3):150–71. doi: 10.1002/dmrr.1068. [DOI] [PubMed] [Google Scholar]
- 48.Sekikawa A, Fukui H, Suzuki K, Karibe T, Fujii S, Ichikawa K, Tomita S, Imura J, Shiratori K, Chiba T, Fujimori T. Involvement of the IL-22/REG Ialpha axis in ulcerative colitis. Lab Invest. 2010;90(3):496–505. doi: 10.1038/labinvest.2009.147. [DOI] [PubMed] [Google Scholar]
- 49.Yamagishi H, Fukui H, Sekikawa A, Kono T, Fujii S, Ichikawa K, Tomita S, Imura J, Hiraishi H, Chiba T, Fujimori T. Expression profile of REG family proteins REG Ialpha and REG IV in advanced gastric cancer: comparison with mucin phenotype and prognostic markers. Mod Pathol. 2009;22(7):906–13. doi: 10.1038/modpathol.2009.41. [DOI] [PubMed] [Google Scholar]
- 50.Nagaraj N, Mann M. Quantitative analysis of the intra- and inter-individual variability of the normal urinary proteome. J Proteome Res. 2011;10(2):637–45. doi: 10.1021/pr100835s. [DOI] [PubMed] [Google Scholar]
- 51.Panchaud A, Jung S, Shaffer SA, Aitchison JD, Goodlett DR. Faster, quantitative, and accurate precursor acquisition independent from ion count. Anal Chem. 2011;83(6):2250–7. doi: 10.1021/ac103079q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hengel SM, Murray E, Langdon S, Hayward L, O’Donoghue J, Panchaud A, Hupp T, Goodlett DR. Data-independent proteomic screen identifies novel tamoxifen agonist that mediates drug resistance. J Proteome Res. 2011;10(10):4567–78. doi: 10.1021/pr2004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Diz AP, Truebano M, Skibinski DO. The consequences of sample pooling in proteomics: an empirical study. Electrophoresis. 2009;30(17):2967–75. doi: 10.1002/elps.200900210. [DOI] [PubMed] [Google Scholar]
- 54.Zhang SD, Gant TW. Effect of pooling samples on the efficiency of comparative studies using microarrays. Bioinformatics. 2005;21(24):4378–83. doi: 10.1093/bioinformatics/bti717. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12(5):319–30. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lievin-Le Moal V, Servin AL. The front line of enteric host defense against unwelcome intrusion of harmful microorganisms: mucins, antimicrobial peptides, and microbiota. Clin Microbiol Rev. 2006;19(2):315–37. doi: 10.1128/CMR.19.2.315-337.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Camilleri M, Madsen K, Spiller R, Van Meerveld BG, Verne GN. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24(6):503–12. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hoebler C, Gaudier E, De Coppet P, Rival M, Cherbut C. MUC genes are differently expressed during onset and maintenance of inflammation in dextran sodium sulfate-treated mice. Dig Dis Sci. 2006;51(2):381–9. doi: 10.1007/s10620-006-3142-y. [DOI] [PubMed] [Google Scholar]
- 59.Rinnert M, Hinz M, Buhtz P, Reiher F, Lessel W, Hoffmann W. Synthesis and localization of trefoil factor family (TFF) peptides in the human urinary tract and TFF2 excretion into the urine. Cell Tissue Res. 2010;339(3):639–47. doi: 10.1007/s00441-009-0913-8. [DOI] [PubMed] [Google Scholar]
- 60.Wallenius V, Elias E, Bergstrom GM, Zetterberg H, Behre CJ. The lipocalins retinol-binding protein-4, lipocalin-2 and lipocalin-type prostaglandin D2-synthase correlate with markers of inflammatory activity, alcohol intake and blood lipids, but not with insulin sensitivity in metabolically healthy 58-year-old Swedish men. Exp Clin Endocrinol Diabetes. 2011;119(2):75–80. doi: 10.1055/s-0030-1265212. [DOI] [PubMed] [Google Scholar]
- 61.Hokari R, Kurihara C, Nagata N, Aritake K, Okada Y, Watanabe C, Komoto S, Nakamura M, Kawaguchi A, Nagao S, Urade Y, Miura S. Increased expression of lipocalin-type-prostaglandin D synthase in ulcerative colitis and exacerbating role in murine colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300(3):G401–8. doi: 10.1152/ajpgi.00351.2010. [DOI] [PubMed] [Google Scholar]
- 62.Lawrance IC, Fiocchi C, Chakravarti S. Ulcerative colitis and Crohn’s disease: distinctive gene expression profiles and novel susceptibility candidate genes. Hum Mol Genet. 2001;10(5):445–56. doi: 10.1093/hmg/10.5.445. [DOI] [PubMed] [Google Scholar]
- 63.Manfredi MA, Zurakowski D, Rufo PA, Walker TR, Fox VL, Moses MA. Increased incidence of urinary matrix metalloproteinases as predictors of disease in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2008;14(8):1091–6. doi: 10.1002/ibd.20419. [DOI] [PubMed] [Google Scholar]
- 64.Nielsen OH, Gionchetti P, Ainsworth M, Vainer B, Campieri M, Borregaard N, Kjeldsen L. Rectal dialysate and fecal concentrations of neutrophil gelatinase-associated lipocalin, interleukin-8, and tumor necrosis factor-alpha in ulcerative colitis. Am J Gastroenterol. 1999;94(10):2923–8. doi: 10.1111/j.1572-0241.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 65.Li GH, Arora PD, Chen Y, McCulloch CA, Liu P. Multifunctional roles of gelsolin in health and diseases. Med Res Rev. 2010 doi: 10.1002/med.20231. [DOI] [PubMed] [Google Scholar]
- 66.Haverland N, Pottiez G, Wiederin J, Ciborowski P. Immunoreactivity of anti-gelsolin antibodies: implications for biomarker validation. J Transl Med. 2010;8:137. doi: 10.1186/1479-5876-8-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Becker PM, Kazi AA, Wadgaonkar R, Pearse DB, Kwiatkowski D, Garcia JG. Pulmonary vascular permeability and ischemic injury in gelsolin-deficient mice. Am J Respir Cell Mol Biol. 2003;28(4):478–84. doi: 10.1165/rcmb.2002-0024OC. [DOI] [PubMed] [Google Scholar]
- 68.Barbara G, Stanghellini V. Biomarkers in IBS: when will they replace symptoms for diagnosis and management? Gut. 2009;58(12):1571–5. doi: 10.1136/gut.2008.169672. [DOI] [PubMed] [Google Scholar]
- 69.Rao AS, Camilleri M, Eckert DJ, Busciglio I, Burton DD, Ryks M, Wong BS, Lamsam J, Singh R, Zinsmeister AR. Urine sugars for in vivo gut permeability: validation and comparisons in irritable bowel syndrome-diarrhea and controls. Am J Physiol Gastrointest Liver Physiol. 2011;301(5):G919–28. doi: 10.1152/ajpgi.00168.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.