Abstract
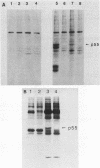
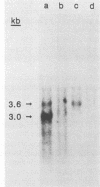
Normal human c-fgr cDNA clones were constructed by using normal peripheral blood mononuclear cell mRNA as a template. Nucleotide sequence analysis of two such clones revealed a 1,587-base-pair-long open reading frame which predicted the primary amino acid sequence of the c-fgr translational product. Homology of this protein with the v-fgr translational product stretched from codons 128 to 516, where 32 differences among 388 codons were observed. Sequence similarity with human c-src, c-yes, and fyn translational products began at amino acid position 76 of the predicted c-fgr protein and extended nearly to its C-terminus. In contrast, the stretch of 75 amino acids at the N-terminus demonstrated a greatly reduced degree of relatedness to these same proteins. To verify the deduced amino acid sequence, antibodies were prepared against peptides representing amino- and carboxy-terminal regions of the predicted c-fgr translational product. Both antibodies specifically recognized a 55-kilodalton protein expressed in COS-1 cells transfected with a c-fgr cDNA expression plasmid. Moreover, the same protein was immunoprecipitated from an Epstein-Barr virus-infected Burkitt's lymphoma cell line which expressed c-fgr mRNA but not in its uninfected fgr mRNA-negative counterpart. These findings identified the 55-kilodalton protein as the product of the human fgr protooncogene.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Tronick S. R. The role of oncogenes in human neoplasia. Important Adv Oncol. 1985:3–15. [PubMed] [Google Scholar]
- Ben-Neriah Y., Bernards A., Paskind M., Daley G. Q., Baltimore D. Alternative 5' exons in c-abl mRNA. Cell. 1986 Feb 28;44(4):577–586. doi: 10.1016/0092-8674(86)90267-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Cheah M. S., Ley T. J., Tronick S. R., Robbins K. C. fgr proto-oncogene mRNA induced in B lymphocytes by Epstein-Barr virus infection. Nature. 1986 Jan 16;319(6050):238–240. doi: 10.1038/319238a0. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Gould K. L., Cartwright C. A., Hunter T. Tyr527 is phosphorylated in pp60c-src: implications for regulation. Science. 1986 Mar 21;231(4744):1431–1434. doi: 10.1126/science.2420005. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., King C. S. Dephosphorylation or antibody binding to the carboxy terminus stimulates pp60c-src. Mol Cell Biol. 1986 Dec;6(12):4467–4477. doi: 10.1128/mcb.6.12.4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downward J., Yarden Y., Mayes E., Scrace G., Totty N., Stockwell P., Ullrich A., Schlessinger J., Waterfield M. D. Close similarity of epidermal growth factor receptor and v-erb-B oncogene protein sequences. Nature. 1984 Feb 9;307(5951):521–527. doi: 10.1038/307521a0. [DOI] [PubMed] [Google Scholar]
- Giese N. A., Robbins K. C., Aaronson S. A. The role of individual cysteine residues in the structure and function of the v-sis gene product. Science. 1987 Jun 5;236(4806):1315–1318. doi: 10.1126/science.3035718. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Inoue K., Ikawa S., Semba K., Sukegawa J., Yamamoto T., Toyoshima K. Isolation and sequencing of cDNA clones homologous to the v-fgr oncogene from a human B lymphocyte cell line, IM-9. Oncogene. 1987;1(3):301–304. [PubMed] [Google Scholar]
- Kawakami T., Pennington C. Y., Robbins K. C. Isolation and oncogenic potential of a novel human src-like gene. Mol Cell Biol. 1986 Dec;6(12):4195–4201. doi: 10.1128/mcb.6.12.4195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marth J. D., Peet R., Krebs E. G., Perlmutter R. M. A lymphocyte-specific protein-tyrosine kinase gene is rearranged and overexpressed in the murine T cell lymphoma LSTRA. Cell. 1985 Dec;43(2 Pt 1):393–404. doi: 10.1016/0092-8674(85)90169-2. [DOI] [PubMed] [Google Scholar]
- Naharro G., Robbins K. C., Reddy E. P. Gene product of v-fgr onc: hybrid protein containing a portion of actin and a tyrosine-specific protein kinase. Science. 1984 Jan 6;223(4631):63–66. doi: 10.1126/science.6318314. [DOI] [PubMed] [Google Scholar]
- Nishizawa M., Semba K., Yoshida M. C., Yamamoto T., Sasaki M., Toyoshima K. Structure, expression, and chromosomal location of the human c-fgr gene. Mol Cell Biol. 1986 Feb;6(2):511–517. doi: 10.1128/mcb.6.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. A cDNA cloning vector that permits expression of cDNA inserts in mammalian cells. Mol Cell Biol. 1983 Feb;3(2):280–289. doi: 10.1128/mcb.3.2.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellman D., Garber E. A., Cross F. R., Hanafusa H. Fine structural mapping of a critical NH2-terminal region of p60src. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1623–1627. doi: 10.1073/pnas.82.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasheed S., Barbacid M., Aaronson S., Gardner M. B. Origin and biological properties of a new feline sarcoma virus. Virology. 1982 Feb;117(1):238–244. doi: 10.1016/0042-6822(82)90522-0. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Reddy E. P., Aaronson S. A. In vivo identification of the transforming gene product of simian sarcoma virus. Science. 1982 Dec 10;218(4577):1131–1133. doi: 10.1126/science.6293053. [DOI] [PubMed] [Google Scholar]
- Semba K., Yamanashi Y., Nishizawa M., Sukegawa J., Yoshida M., Sasaki M., Yamamoto T., Toyoshima K. Location of the c-yes gene on the human chromosome and its expression in various tissues. Science. 1985 Mar 1;227(4690):1038–1040. doi: 10.1126/science.2983418. [DOI] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. Automated preparation of DNA sequences for publication. Nucleic Acids Res. 1986 Jan 10;14(1):65–73. doi: 10.1093/nar/14.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Rettenmier C. W., Sacca R., Roussel M. F., Look A. T., Stanley E. R. The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell. 1985 Jul;41(3):665–676. doi: 10.1016/s0092-8674(85)80047-7. [DOI] [PubMed] [Google Scholar]
- Sukegawa J., Semba K., Yamanashi Y., Nishizawa M., Miyajima N., Yamamoto T., Toyoshima K. Characterization of cDNA clones for the human c-yes gene. Mol Cell Biol. 1987 Jan;7(1):41–47. doi: 10.1128/mcb.7.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tanaka A., Gibbs C. P., Arthur R. R., Anderson S. K., Kung H. J., Fujita D. J. DNA sequence encoding the amino-terminal region of the human c-src protein: implications of sequence divergence among src-type kinase oncogenes. Mol Cell Biol. 1987 May;7(5):1978–1983. doi: 10.1128/mcb.7.5.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tronick S. R., Popescu N. C., Cheah M. S., Swan D. C., Amsbaugh S. C., Lengel C. R., DiPaolo J. A., Robbins K. C. Isolation and chromosomal localization of the human fgr protooncogene, a distinct member of the tyrosine kinase gene family. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6595–6599. doi: 10.1073/pnas.82.19.6595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voronova A. F., Sefton B. M. Expression of a new tyrosine protein kinase is stimulated by retrovirus promoter insertion. Nature. 1986 Feb 20;319(6055):682–685. doi: 10.1038/319682a0. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y., Fukushige S., Semba K., Sukegawa J., Miyajima N., Matsubara K., Yamamoto T., Toyoshima K. The yes-related cellular gene lyn encodes a possible tyrosine kinase similar to p56lck. Mol Cell Biol. 1987 Jan;7(1):237–243. doi: 10.1128/mcb.7.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]