Abstract
Although distinguishing features of masked hypertension in diabetics are well known, the significance of antihypertensive treatment on clinical practice decisions has not been fully explored. We analyzed 9691 subjects from the population-based 11-country International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcomes. Prevalence of masked hypertension in untreated normotensive participants was higher (P<0.0001) among 229 diabetics (29.3%, n=67) than among 5486 nondiabetics (18.8%, n=1031). Over a median of 11.0 years of follow-up, the adjusted risk for a composite cardiovascular end point in untreated diabetic-masked hypertensives tended to be higher than in normotensives (hazard rate [HR], 1.96; 95% confidence interval [CI], 0.97–3.97; P=0.059), similar to untreated stage 1 hypertensives (HR, 1.07; CI, 0.58–1.98; P=0.82), but less than stage 2 hypertensives (HR, 0.53; CI, 0.29–0.99; P=0.048). In contrast, cardiovascular risk was not significantly different in antihypertensive-treated diabetic-masked hypertensives, as compared with the normotensive comparator group (HR, 1.13; CI, 0.54–2.35; P=0.75), stage 1 hypertensives (HR, 0.91; CI, 0.49–1.69; P=0.76), and stage 2 hypertensives (HR, 0.65; CI, 0.35–1.20; P=0.17). In the untreated diabetic-masked hypertensive population, mean conventional systolic/diastolic blood pressure was 129.2±8.0/76.0±7.3 mm Hg, and mean daytime systolic/diastolic blood pressure 141.5±9.1/83.7±6.5 mm Hg. In conclusion, masked hypertension occurred in 29% of untreated diabetics, had comparable cardiovascular risk as stage 1 hypertension, and would require considerable reduction in conventional blood pressure to reach daytime ambulatory treatment goal. Importantly, many hypertensive diabetics when receiving antihypertensive therapy can present with normalized conventional and elevated ambulatory blood pressure that mimics masked hypertension.
Keywords: ambulatory blood pressure, conventional blood pressure, diabetes mellitus, masked hypertension, population study
Diabetes mellitus and hypertension are interrelated disorders, each powerfully predisposing to the development of the other and to the future occurrence of cardiovascular disease.1, 2 Although the distinguishing features of masked hypertension (MH)3-6 are well known, the significance of the presence or absence of antihypertensive treatment on clinical practice decisions that involve MH have been poorly understood. We do know that there is a higher prevalence of MH in treated than in nontreated hypertensive subjects,7 but the mechanism by which antihypertensive treatment is associated with a higher prevalence of MH is not known.
The current International Database on Ambulatory Blood Pressure Monitoring in Relation to Cardiovascular Outcomes (IDACO) study includes a large number of subjects with diabetes mellitus, many of whom have MH—both on and off antihypertensive treatment. These individuals were recruited in communities from 11 countries using standard protocols for conventional blood pressure (CBP) and ambulatory blood pressure (ABP) monitoring, and with a median follow-up of 11 years for cardiovascular events.
We specifically asked the following 2 questions. First, how do the cardiovascular risks in antihypertensive treated versus nontreated diabetics with MH compare with their normotensive comparator groups, stage 1 hypertensives (systolic blood pressure [SBP] 140–159 mm Hg and diastolic blood pressure [DBP] 90–99 mm Hg), and stage 2 hypertensives (SBP ≥160 mm Hg and DBP ≥100 mm Hg), and how do these risk comparisons differ between diabetics and nondiabetics? Second, what are the antihypertensive treatment implications for masked hypertensive diabetics versus those subjects without diabetes mellitus?
Methods
Study Population
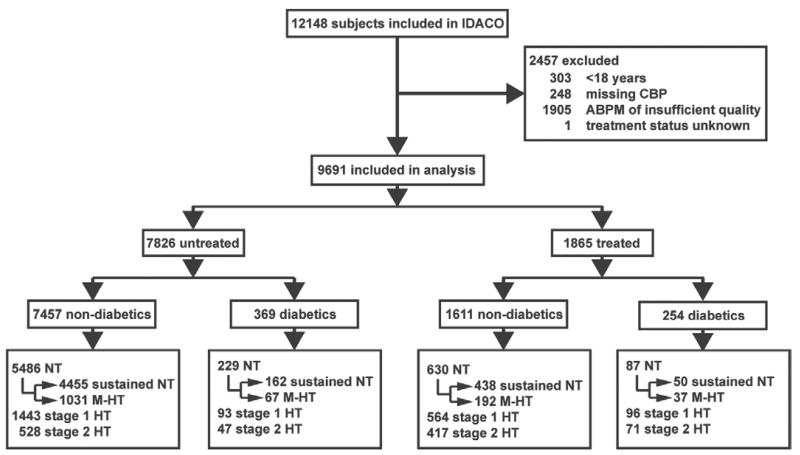
At the time of writing this report, the IDACO database8 included 11 randomly recruited population cohorts9-17 and 12 148 participants (for details, see the Expanded Methods in the online-only Data Supplement). We excluded 2457 participants, because they were younger than 18 years (n=303); because their CBP was not on the database (n=248); because they had <10 daytime or 5 nighttime BP readings (n=1905); or because their treatment status at baseline was unknown (n=1). Thus, the total number of subjects included in the present analysis totaled 9691, including 2142 residents from Copenhagen, Denmark9; 1317 inhabitants from Ohasama, Japan10; 1392 subjects from Noorderkempen, Belgium11; 1096 older men from Uppsala, Sweden12; 1438 subjects from Montevideo, Uruguay13; 349 villagers from the JingNing county, China14; 244 subjects from Novosibirsk, the Russian Federation15; 165 from Pilsen, Czech Republic16; 930 from Dublin, Ireland17; 310 from Padua, Italy16; and 308 from Kraków, Poland (Figure 1).16
Figure 1.
Flow chart of the study population. ABPM indicates ambulatory blood pressure recording; CBP, conventional blood pressure; NT, normotension (CBP <140/90 mm Hg); sustained NT (CBP <140/90 mm Hg and daytime ambulatory blood pressure [dABP] <135/85 mm Hg); M-HT, masked hypertension (CBP <140/90 mm Hg and dABP ≥135/85 mm Hg); stage 1 HT, stage-1 hypertension (CBP 140–159/90–99 mm Hg); and stage 2 HT, stage-2 hypertension (CBP ≥160/100 mm Hg). An ABPM was considered of insufficient quality if the number of daytime readings was <10 or the number of nighttime readings <5.
BP Measurement
Methods used for CBP and ABP measurements are described in detail in the Expanded Methods. CBP was the average of 2 consecutive readings obtained either at the person’s home,11, 13-16 or at an examination center.10, 12, 17, 18 Portable monitors were programmed to obtain ABP readings at 30-minute intervals throughout the whole day,10, 17 or at intervals ranging from 1518 to 3012 minutes during daytime and from 3018 to 6012 minutes at night.
We categorized the CBP according to the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC 7)19 guidelines. Normotension was a level <140 mm Hg systolic and <90 mm Hg diastolic. Stage 1 hypertension encompassed 140 to 159 mm Hg systolic or 90 to 99 mm Hg diastolic. CBP of at least 160 mm Hg systolic or 100 mm Hg diastolic was classified as stage 2 hypertension. Ambulatory hypertension was a daytime ABP of 135 mm Hg systolic or 85 mm Hg diastolic or more.20 Sustained normotension was normotension on both CBP and ABP measurement. Masked hypertension was ambulatory hypertension in participants with a normal CBP. Patients on antihypertensive drug treatment were classified according to their treated BP. The term normotension in treated subjects refers to successfully treated hypertensive patients; that is, hypertensive subjects whose BP, both CBP and ABP, are controlled on antihypertensive drug therapy.
Other Measurements
We used the questionnaires originally administered in each cohort to obtain information on each participant’s medical history and smoking and drinking habits. Diabetes mellitus was the use of antidiabetic drugs,9-16 a fasting blood glucose concentration of at least 7.0 mmol/L,9-16 a random blood glucose concentration of at least 11.1 mmol/L,10, 11, 14-16 a self-reported diagnosis,11, 13-17 or diabetes mellitus documented in practice or hospital records.13 Glomerular filtration rate was estimated using the Modification of Diet in Renal Disease study equation.21
Ascertainment of Events
The composite cardiovascular end point included fatal and nonfatal stroke, transient ischemic attacks, death from ischemic heart disease, sudden death, nonfatal myocardial infarction, angina pectoris, coronary revascularization, fatal and nonfatal heart failure, and fatal and nonfatal peripheral arterial disease. A restricted definition of the composite cardiovascular end point not including transient ischemic attacks, angina pectoris, and nonfatal peripheral arterial disease was used for sensitivity analyses. In all outcome analyses, we only considered the first event within each category.
Statistical Analysis
For database management and statistical analysis, we used SAS software, version 9.3 (SAS Institute, Cary, NC). For comparison of means and proportions, we applied the large-sample z-test and the χ2 statistic, respectively. The risk association with MH was assessed using Cox regression analysis, stratified for cohort, and adjusted for sex, age, body mass index, smoking and drinking, serum cholesterol, and history of cardiovascular complications. We compared hazard ratios between groups by testing the significance of the appropriate interaction term. Statistical significance was an α-level of <0.05 on 2-sided tests.
Results
Baseline Characteristics
As shown in the flow chart, 9691 participants were included in the analysis (Figure 1). Of these, 4584 (47.3%) were women and 1865 (19.2%) used antihypertensive drug treatment. Mean (±SD) age was 52.5±15.8 years. At enrolment, 2738 (28.4%) participants were smokers and 4746 (52.3%) reported intake of alcohol. In the entire study population, CBP averaged (±SD) 130.2±20.3 mm Hg systolic and 79.4±11.5 mm Hg diastolic. The daytime ABP were 129.9±15.0 mm Hg and 78.8±9.1 mm Hg, respectively.
A total of 623 (6.4%) participants had diabetes mellitus distributed as follows over the various cohorts: 73 (3.4%) in Copenhagen, 232 (17.6%) in Ohasama, 38 (2.7%) in Noorderkempen, 121 (11.0%) in Uppsala, 88 (6.1%) in Montevideo, 0 (0.0%) in JingNing, 6 (2.5%) in Novosibirsk, 8 (4.8%) in Pilsen, 32 (3.4%) in Dublin, 11 (3.6%) in Padua, and 14 (4.6%) in Krakow.
On CBP measurement 6432 (66.4%) participants were normotensive, and 2196 (22.7%) and 1063 (11.0%) had stage 1 or stage 2 hypertension. Of the 6432 subjects with conventional normotension, 1327 (20.6%) had MH. The characteristics of the untreated and treated study participants by BP status and the presence or absence of diabetes mellitus are shown in Table 1 and Table S1 in the online-only Data Supplement.
Table 1.
Baseline Characteristics of the 6432 Conventional Normotensive Subjects Broken Down by Treatment Status, Diabetic Status, and Ambulatory Blood Pressure Category
| Characteristic | Untreated |
Treated |
||||||
|---|---|---|---|---|---|---|---|---|
| Nondiabetics |
Diabetics |
Nondiabetics |
Diabetics |
|||||
| Sustained NT, n=4455 |
Masked HT, n=1031 |
Sustained NT, n=162 |
Masked HT, n=67 |
Sustained NT #, n=438 |
Masked HT, n=192 |
Sustained NT #, n=50 |
Masked HT, n=37 |
|
| Number with characteristic (%) | ||||||||
| Male | 1941 (43.6) | 661 (64.1)§ | 71 (43.8) | 38 (56.7) | 174 (39.7) | 98 (51.0)† | 23 (46.0) | 19 (51.4) |
| History of CV events | 191 (4.3) | 53 (5.1) | 8 (4.9) | 6 (9.0) | 94 (21.5) | 41 (21.4) | 12 (24.0) | 8 (21.6) |
| Current smokers | 1368 (30.8) | 405 (39.4)§ | 28 (17.3) | 21 (31.3)* | 89 (20.4) | 54 (28.4)* | 7 (14.0) | 9 (24.3) |
| Current drinkers | 2026 (46.7) | 629 (63.8)§ | 63 (42.9) | 26 (42.6) | 145 (36.5) | 90 (50.3)† | 11 (24.4) | 16 (50.0)* |
| BMI>25 kg/m2 | 1584 (35.6) | 536 (52.0)§ | 59 (36.4) | 40 (59.7)† | 230 (52.5) | 111 (57.8) | 34 (68.0) | 27 (73.0) |
| BMI>30 kg/m2 | 306 (6.9) | 115 (11.2)§ | 12 (7.4) | 13 (19.4) | 82 (18.7) | 30 (15.6) | 9 (18.0) | 11 (29.7) |
| Mean values±SD | ||||||||
| Age, years | 45.0±15.1 | 50.6±14.2§ | 54.8±13.5 | 60.5±10.8† | 60.8±12.4 | 62.6±10.5 | 63.9±11.1 | 67.1±8.1 |
| Body mass index, kg/m2 | 24.2±3.8 | 25.6±3.8§ | 24.5±3.6 | 26.7±4.4§ | 26.1±4.9 | 26.1±4.1 | 26.6±4.6 | 27.9±4.5 |
| Blood glucose, mmol/L | 91.2±15.2 | 91.6±14.1 | 127.8±41.0 | 144.3±49.7* | 100.8±19.7 | 99.7±18.0 | 130.7±47.7 | 148.1±44.7 |
| Serum cholesterol, mmol/L | 5.4±1.1 | 5.8±1.2§ | 5.5±1.0 | 5.8±1.2 | 5.6±1.1 | 5.6±1.1 | 5.3±1.1 | 5.5±0.9 |
| Serum creatinine, μmol/L | 86.1±15.3 | 89.5±14.8§ | 86.1±15.5 | 90.2±23.8 | 93.7±32.0 | 97.2±48.2 | 89.6±21.4 | 89.2±17.5 |
| GFR, mL/min per 1.73 m2 | 80.8±37.6 | 79.8±16.1 | 77.8±16.9 | 76.2±18.8 | 70.5±17.0 | 70.6±17.9 | 72.5±22.3 | 69.4±12.2 |
| Conventional SBP, mm Hg | 116.4±11.1 | 124.9±9.2§ | 120.2±12.5 | 129.2±8.0§ | 123.6±10.0 | 127.8±8.7§ | 127.2±8.9 | 127.6±8.1 |
| Conventional DBP, mm Hg | 73.0±7.9 | 78.7±7.0§ | 72.3±8.3 | 76.0±7.3† | 74.9±8.5 | 77.4±8.1‡ | 72.6±8.7 | 76.0±10.0 |
| Daytime SBP, mm Hg | 119.8±8.2 | 138.8±8.4§ | 120.1±9.1 | 141.5±9.1§ | 121.6±8.2 | 142.2±9.8§ | 124.1±8.1 | 143.6±8.7§ |
| Daytime DBP, mm Hg | 74.0±5.8 | 84.9±6.3§ | 72.5±6.0 | 83.7±6.5§ | 73.3±6.6 | 84.8±7.3§ | 73.0±5.7 | 83.9±7.3§ |
| Nighttime SBP, mm Hg | 104.1±9.3 | 114.9±11.0 | 105.2±10.2 | 120.3±14.3§ | 108.2±11.6 | 119.5±13.3 | 114.1±13.2 | 121.0±16.2* |
| Nighttime DBP, mm Hg | 60.3±6.6 | 66.7±7.6§ | 60.6±6.6 | 68.6±7.7§ | 62.0±7.7 | 68.9±8.4§ | 63.9±8.4 | 68.1±8.8* |
BMI indicates body mass index; DBP, diastolic blood pressure; GFR, glomerular fi ltration rate; HT, hypertension; NT, normotension; SBP, systolic blood pressure; and SD, standard deviation.
Sustained NT is a conventional blood pressure <140/90 mm Hg and a daytime ambulatory blood pressure <135/85 mm Hg. Masked HT is a conventional blood pressure <140/90 mm Hg with a daytime ambulatory blood pressure ≥135/85 mm Hg. GFR was estimated using the Modifi cation of Diet in Renal Disease Study equation.21 To convert blood glucose, serum cholesterol, and serum creatinine from SI units to mg/dL, divide by 0.0555, 0.0259, and 88.4, respectively.
Treated subjects with sustained NT are hypertensive subjects, whose conventional and ambulatory blood pressures are normalized on antihypertensive therapy.
Signifi cance of the difference between sustained NT and masked HT:
P<0.05
P<0.01
P<0.001
P<0.0001.
Prevalence of Masked Hypertension in Subjects With and Without Diabetes Mellitus
The prevalence of MH in untreated participants normotensive on CBP measurement was higher (P<0.0001) among the 229 diabetics (29.3%, n=67) than among the 5486 nondiabetics (18.8%, n=1031). The sex- and age-adjusted odds ratio for untreated MH in diabetics versus nondiabetics was 1.46 (95% confidence interval [CI], 1.08–1.98; P=0.014). After further adjustment for the systolic CBP, history of cardiovascular complications, current smoking status, alcohol intake, body mass index, and total cholesterol, the odds ratio decreased to 1.35 (CI, 0.98-1.86; P=0.065). Similarly, in antihypertensive-treated subjects with normalized CBP, the prevalence of MH was higher (P=0.027) among 87 diabetics (42.5%, n=37) than among 630 nondiabetics (30.5%, n=192). The sex- and age-adjusted odds ratio in treated participants was 1.59 (CI, 1.00–2.52; P=0.051), and the fully adjusted odds ratio was 1.59 (CI, 0.98–2.58; P=0.058).
Risk Associated With Masked Hypertension and Diabetes Mellitus
In the overall study population, the median follow-up was 11.0 years (5th to 95th percentile interval, 2.5–18.1 years). During 106 087 person-years of follow-up, 1412 subjects experienced a fatal or nonfatal cardiovascular complication (14.0 per 1000 person-years). The risks associated with MH in untreated and treated nondiabetics and diabetics are illustrated in Figure 2 (adjusted for cohort, sex, and age only) and in Figure 3 (full adjustment).
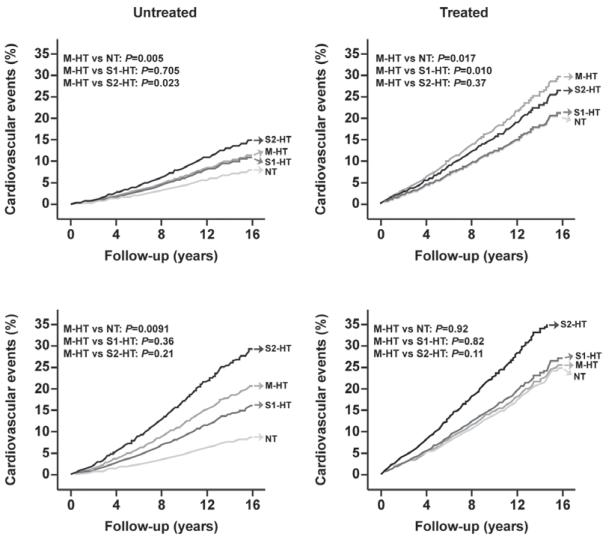
Figure 2.
Cohort-, sex-, and age-adjusted incidence of cardiovascular events in untreated (left) and treated (right) nondiabetic (upper) and diabetic (lower) subjects with normal conventional and ambulatory blood pressures (normotension [NT], masked hypertension [M-HT], stage 1 hypertension [S1-HT], and stage 2 hypertension [S2-HT]). Incidence was standardized to the distribution of cohort (see Methods section in the online-only Data Supplement), female sex (47.3%), and mean age (52.5 years) in the whole study population.
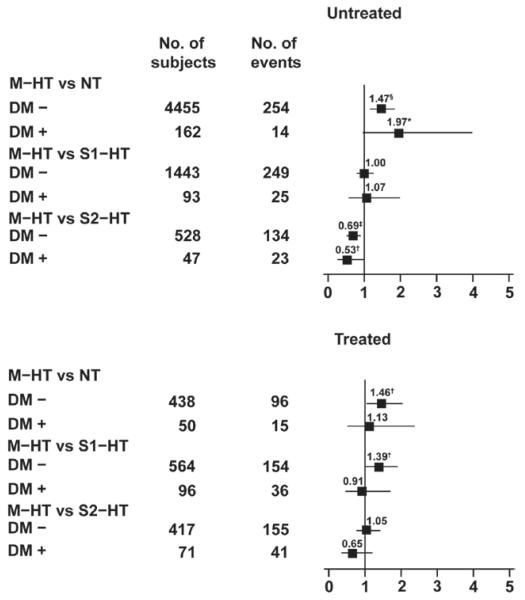
Figure 3.
Hazard ratios for the composite cardiovascular end point in untreated (left) and treated (right) conventional normotensive subjects without (DM−) and with (DM+) diabetes mellitus and with masked hypertension (M-HT; conventional blood pressure [CBP] <140/90 mm Hg and daytime ambulatory blood pressure [dABP] ≥135/85 mm Hg). The sustained normotensives (NT; CBP <140/90 mm Hg and dABP <135/85 mm Hg), stage 1 hypertensives (S1-HT; CBP 140-159/90-94 mm Hg), and stage 2 hypertensives (S2-HT; CBP ≥160/95 mm Hg) were used as reference groups. Horizontal lines denote the 95% confidence interval. All analyses were adjusted for cohort, sex, age, body mass index, smoking and drinking, history of cardiovascular disease, and total serum cholesterol. Numbers are the number of subjects (left column) and number of events (right column) in the reference groups. Significance of the hazard ratios: *0.05≤P<0.06; †P<0.05; ‡P<0.01; and §P<0.001.
The diabetic subjects not receiving antihypertensive treatment included 162 sustained normotensives, 67 masked hypertensives, 93 stage 1 hypertensives, and 47 stage 2 hypertensives; within these 4 groups, the numbers of cardiovascular events were as follows: 14 (7.2 per 1000 person-years), 18 (27.1), 25 (28.5), and 23 (67.0), respectively. With adjustment for cohort, sex, age, body mass index, smoking and drinking, history of cardiovascular disease, and total serum cholesterol, Cox proportional hazards regression in untreated diabetics showed that the cardiovascular risk in MH was similar to that in stage 1 hypertension (hazard rate [HR], 1.07; CI, 0.58–1.98; P=0.82), and tended to be higher than in sustained normotension (HR, 1.97; CI, 0.97-3.97; P=0.059) but lower than in stage 2 hypertension (HR, 0.53; CI, 0.29–0.99; P=0.048; Figure 3). In nondiabetics not receiving antihypertensive treatment these HRs were 1.00 (CI, 0.80–1.25; P=0.99), 1.47 (CI, 1.18–1.83; P=0.0006), and 0.69 (CI, 0.54–0.89; P=0.0043), respectively (Figure 3). Although the untreated diabetics were at higher risk than the untreated nondiabetics (HR, 1.73; CI, 1.36-2.20; P<0.0001), the HRs comparing the risk in the various BP categories were similar (P>0.12) in diabetics and nondiabetics.
The number of cardiovascular events in treated diabetics was 15 (28.8 per 1000 person-years) in the 50 subjects with normalized CBP and ABP, 14 (41.9) in the 37 masked hypertensives, 36 (43.9) in the 96 stage 1 hypertensives, and 41 (77.9) in the 71 stage 2 hypertensives. The adjusted cardiovascular risk was not significantly different in masked hypertensives, as compared with sustained normotensives (HR, 1.13; CI, 0.54–2.35; P=0.75), stage 1 hypertensives (HR, 0.91; CI, 0.49–1.69; P=0.76), and stage 2 hypertensives (HR, 0.65; CI, 0.35–1.20; P=0.17). In treated nondiabetics, the cardiovascular risk in MH was higher than in sustained normotension (HR, 1.46; CI, 1.06–2.02; P=0.022) and stage 1 hypertenison (HR, 1.39; CI, 1.03–1.89; P=0.032), and similar to that in stage 2 hypertension (HR, 1.05; CI, 0.77–1.42; P=0.77; Figure 3). Sensitivity analyses based on the restricted definition of the composite cardiovascular end point produced similar results (Figure S1).
ABP Versus CBP in Diabetic Subjects With Masked Hypertension
Table 2 shows the mean daytime and nighttime SBP and DBP by various categories of the CBP in the 67 subjects with untreated MH. The table also shows mean conventional and nighttime BP in various categories of the daytime ABP. In all diabetic subjects with untreated MH, the conventional, daytime, and nighttime ABP averaged 129.2±8.0/76.0±7.3 mm Hg, 141.5±9.1/83.7±6.5 mm Hg, and 120.3±14.3/68.6±7.7 mm Hg, respectively. In diabetic subjects with treated MH, these values were similar (P>0.24; ie, 127.6±8.1/76.0±10.0 mm Hg, 143.6±8.7/83.9±7.3 mm Hg, and 121.0±16.2/68.1±8.8 mm Hg, respectively). Figure S2 shows the association between the daytime and conventional SBP and DBP in untreated and treated diabetic subjects with MH. In the 67 diabetic subjects with untreated MH, the 5th to 95th percentile interval of the CBP ranged from 112 to 139 mm Hg systolic and from 65 to 88 mm Hg diastolic.
Table 2.
Cross-Classifi cation of Daytime and Nighttime Systolic and Diastolic Blood Pressures Versus Levels of Corresponding Conventional Blood Pressures in Untreated Diabetic Subjects With Masked Hypertension
| n | Daytime SBP | Nighttime SBP | |
|---|---|---|---|
| Conventional SBP | |||
| <120 | 8 | 140.0±7.2 | 118.0±17.2 |
| 120–124 | 7 | 142.1±11.2 | 118.9±14.2 |
| 125–129 | 17 | 139.6±8.5 | 116.9±11.1 |
| 130–134 | 15 | 139.4±7.0 | 116.6±11.5 |
| 135–139 | 20 | 145.1±10.4 | 127.3±16.2 |
| ALL | 67 | 141.5±9.1 | 120.3±14.3 |
| Conventional DBP | |||
| <70 | 14 | 81.5±5.1 | 65.4±6.0 |
| 70–74 | 16 | 83.2±8.4 | 67.8±7.2 |
| 75–79 | 10 | 84.2±7.8 | 70.3±10.5 |
| 80–84 | 16 | 84.9±5.4 | 69.8±6.5 |
| 85–89 | 11 | 85.2±5.5 | 70.4±8.9 |
| ALL | 67 | 83.7±6.5 | 68.6±7.7 |
| Daytime SBP | |||
| <135 | 9 | 118.9±12.4 | 127.3±7.6 |
| 135–139 | 31 | 114.3±12.4 | 128.2±7.7 |
| 140–144 | 10 | 119.8±6.5 | 129.4±7.9 |
| ≥145 | 17 | 132.3±15.1 | 131.8±8.8 |
| ALL | 67 | 120.3±14.3 | 129.2±8.0 |
| Daytime DBP | |||
| <80 | 19 | 65.6±6.4 | 73.1±7.0 |
| 80–84 | 20 | 67.0±8.5 | 78.0±8.4 |
| 85–89 | 18 | 70.6±5.6 | 76.1±6.5 |
| ≥90 | 10 | 73.9±8.7 | 77.6±6.2 |
| ALL | 67 | 68.6±7.3 | 76.0±7.3 |
DBP indicates diastolic blood pressure; and SBP, systolic blood pressure.
Discussion
There were 2 important findings in this 11-country IDACO study. First, 42.5% of the antihypertensive-treated diabetics with normalized CBP had an on-treatment daytime ABP within the hypertensive range. These presumed masked hypertensive subjects had similar cardiovascular risk as treated subjects with sustained normotension and those with uncontrolled stage 1 and stage 2 hypertension. Second, the untreated masked hypertensive diabetic population represented 29.3% of the normotensive CBP population, showed greater risk than those with sustained normotension, showed equivalent cardiovascular risk to a stage 1 diabetic population, but less risk as compared with stage 2 hypertension. Although untreated and treated diabetics were at higher risk than the untreated and treated nondiabetics, respectively, the HRs comparing the risk in the various BP categories were similar in diabetics and nondiabetics.
Cardiovascular Risk in Antihypertensive-Treated Subjects With Masked Hypertension
When Pickering first coined the term MH in 2002,22 he was referring to untreated subjects with elevated ABP in the presence of normal CBP. When dealing with a population that has received antihypertensive therapy, the normotensive comparator group may be at increased risk, as we have shown to be true when evaluating treated white-coat hypertension.23 The same relation applies to treated hypertension in general,24 and specifically to MH. Indeed, the present study showed that the antihypertensive-treated diabetics with presumed MH were at the same cardiovascular risk as the comparator group with normalized CBP and ABP, whereas untreated diabetic subjects with MH tended to have higher cardiovascular risk than their sustained normotensive comparator group.
Sustained Hypertensives Undergoing Antihypertensive Treatment may Mimic Masked Hypertension
There is abundant evidence from previous studies that antihypertensive treatment will lower ABP values by only 60% to 70% of the reduction in CBP pressures, that is, approximately a 3-mm Hg SBP reduction of CBP for a 2-mm Hg SBP reduction of ABP.25-27 The findings in the present study are consistent with this treatment effect: the prevalence of MH in the normotensive diabetic population receiving antihypertensive therapy was 42.5% and in those who were untreated was 29.3%; thus, there was an approximate ratio of 1.5 to1.0 (or 3 to 2), comparing the prevalence of treated with untreated MH in the diabetic population. Our working hypothesis is that a significant number of subjects with diabetic MH actually had sustained hypertension before beginning antihypertensive therapy; with therapy, they normalized CBP but continued to have elevated ABP values, and thus mimicked MH. Indeed, if antihypertensive treatment would have equally reduced systolic CBP and ABP, the untreated and treated diabetic MH prevalence would be equal. In summary, this is the first study, to our knowledge, to show that antihypertensive-treated diabetics can present with normalized CBP and elevated ABP that mimics MH; in reality, many of these subjects were sustained hypertensives masquerading as MH.
Patient compliance with treatment and/or adequacy of antihypertensive therapy by the physician may have a direct affect on the prevalence of MH. The presence of effective antihypertensive therapy may (1) in large part normalize both CBP and ABP, and present as optimally treated BP, so that MH is greatly reduced or totally eliminated. More commonly, insufficient antihypertensive therapy may (2) in large part normalize CBP, whereas ABP remains elevated, suggesting that a significant number of untreated sustained hypertensives were converted to treated MH; this results in a particularly high prevalence of MH. Because the prevalence of MH is higher in treated versus untreated diabetics (and nondiabetics), as noted in the current study and generally as noted in the literature,28-30 this suggests that a large number of physicians that treat hypertensive diabetics (or nondiabetics) erroneously focus primarily on normalizing CBP rather than monitoring for normalization of ABP or home BP.
Antihypertensive Treatment Goals for Diabetics With Masked Hypertension
Previous studies have shown that diabetic subjects have not only a high prevalence of MH,31, 32 but also high rates of target organ damage33, 34 and a cardiovascular risk profile similar to sustained hypertension, so that out-of-office BP monitoring35, 36 and antihypertensive therapy can be justified in subjects with these characteristics. Furthermore, not only the present study, but also a previous IDACO publication have shown that the cardiovascular risk is the summation of the risk of diabetes mellitus plus the risk of hypertension.37 In the present study, diabetic subjects with untreated MH had a mean CBP of 129.2/76.0 mm Hg (with values that ranged as low as 110/60 mm Hg) and corresponding mean daytime ABP of 141.5/83.7 mm Hg. Therefore, if the primary treatment strategy is reaching daytime ABP treatment target goal, this would inevitably lead to further reduction in CBP values.
Tight BP control (systolic CBP <130 mm Hg and diastolic CBP <80 mm Hg) appears to be applicable for reduction in stroke events, in young diabetics, and in diabetics of short-duration. In contrast, usual BP control (systolic CBP <140 mm Hg and diastolic CBP <90 mm Hg) appears to be more applicable to reduction of ischemic heart disease events and in older and longer-duration diabetics.38-40 Importantly, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) study, the largest of the intervention studies that compared intense with usual care reduction in BP control in hypertensive diabetics, did not recruit subjects with MH specifically.41 Therefore, at the present time, there are no credible outcome studies in diabetics with MH to prove the benefit of antihypertensive therapy or to indicate how low to go with the reduction in daytime and nighttime ABP to achieve optimal reduction in cardiovascular risk. Furthermore, any significant reduction in ABP would be associated with even larger reductions in CBP values, which are already lower than JNC7 recommended guidelines.19 Thus, there is the possibility that with antihypertensive treatment in diabetic subjects with MH, one may have to balance the increased cardiovascular risk of lower diastolic CBP and ABP values with the potential benefit of further reduction in systolic CBP and ABP values.42
Strengths and Limitations
Our study must be interpreted within the context of its strengths and potential limitations. First, the CBP was measured under differing conditions in the cohorts. However, in all but 1 cohort, BP was measured in the sitting position, and in all cohorts, the average of only 2 CBP measurements was used for analysis. In addition, all of the cohorts implemented rigorous quality control programs for BP measurement. Second, ABP monitoring was not standardized in terms of device type and intervals between successive readings. However, all ABP means were weighted for the interval between successive readings. By design, this meta-analysis was based on data from individuals, rather than from aggregate data from each individual study. Third, the analysis rested on 11 population-based cohorts over 3 continents with an overrepresentation of European subjects, and might therefore not be representative for other ethnic groups, in particular blacks. Fourth, the confidence intervals around the hazard ratios comparing the risks in masked hypertensives versus normotensives and stage 1 and stage 2 hypertensives were wide, reflecting limited statistical power to accurately assess differences between these subgroups. Finally, a possible limitation of the study is the question of reproducibility of MH. However, generally, high reproducibility have been shown in adults with MH in previous studies.7, 43, 44
Perspectives
Using the 11-country IDACO population database and measuring CBP and 24-hour ABP, we noted a higher prevalence of MH in diabetics than nondiabetics; this finding was more prominent in treated versus nontreated diabetics. Of significance, cardiovascular risk in diabetics not receiving antihypertensive treatment and presenting with MH was significantly greater than in their normotensive comparator group and was equivalent to the risk in diabetics with stage 1 hypertension. In contrast, antihypertensive-treated diabetics with MH on 24-hour ABP monitoring had cardiovascular risk that was equal to treated normotensives and stage 1 and stage 2 hypertensive subjects, strongly suggesting that a significant percentage of these subjects had sustained hypertension that mimicked MH in the presence of normalized CBP and elevated ABP. Hence, the term MH should be used with caution in the presence of antihypertensive therapy. Furthermore, because antihypertensive therapy always decreases CBP more than ABP, there is the danger that reliance on CBP as target treatment goal will result in suboptimal control of BP in subjects with either sustained hypertension or MH; thus, out-of-office BP monitoring should be used to focus on home and/or ABP target goals in both diabetics and nondiabetics. Unfortunately, there are no specific treatment guidelines based on randomized controlled trials in either diabetic or nondiabetic subjects with MH or sustained hypertension masquerading as MH, so that antihypertensive treatment goals remain empirical.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first study to hypothesize that antihypertensive-treated diabetics may present with normalized conventional blood pressure (CBP) and elevated ambulatory blood pressure (ABP) that mimics masked hypertension (MH); in reality, many of these subjects may be sustained hypertensives that masquerade as MH.
What Is Relevant?
The prevalence of MH is (1) significantly higher in diabetics than non-diabetics and is (2) higher in treated than untreated diabetics; we postulate that treatment converts many sustained hypertensives into “MH” because of a greater lowering of CBP than ABP.
Summary
Diabetics with untreated MH have cardiovascular risk equal to stage 1 hypertension and require considerable reduction in CBP to reach ABP treatment goals. In the absence of randomized controlled trials, treatment goals remain empirical.
Acknowledgments
We gratefully acknowledge the expert assistance of Sandra Covens and Sonja Zuba (Studies Coordinating Center, Leuven, Belgium). The IDACO investigators are listed in Reference 8.
Sources of Funding: The European Union (grants IC15-CT98-0329-EPOGH, LSHM-CT-2006–037093 InGenious HyperCare, HEALTH-F4-2007–201550 HyperGenes, HEALTH-F7-2011–278249 EU-MASCARA, and the European Research Council Advanced Research Grant 294713 EPLORE) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0734.09) supported the Studies Coordinating Centre (Leuven, Belgium). The European Union (grants LSHM-CT-2006–037093 and HEALTH-F4-2007–201550) also supported the research groups in Shanghai, Kraków, Padova, and Novosibirsk. The Danish Heart Foundation (grant 01-2-9-9A-22914) and the Lundbeck Fonden (grant R32-A2740) supported the studies in Copenhagen. The Ohasama study received support via Grant-in-Aid for Scientific Research (22590767, 22790556, 23249036, 23390171, and 23790242) from the Ministry of Education, Culture, Sports, Science and Technology, Japan; Health Labour Sciences Research Grant (H23-Junkankitou [Seishuu]-Ippan-005) from the Ministry of Health, Labour and Welfare, Japan; Japan Arteriosclerosis Prevention Fund; and a Grant from the Central Miso Research Institute, Tokyo, Japan. The National Natural Science Foundation of China (grants 30871360 and 30871081), Beijing, China, and the Shanghai Commissions of Science and Technology (grant 07JC14047 and the “Rising Star” program 06QA14043) and Education (grant 07ZZ32 and the “Dawn” project) supported the JingNing study in China. The Comisión Sectorial de Investigación Científica de la Universidad de la República (Grant I+D GEFA-HT-UY) and the Agencia Nacional de Innovación e Investigación supported research in Uruguay.
Footnotes
Disclosures: None.
This paper was sent to Morris Brown, Consulting editor, for review by expert referees, editorial decision, and final disposition.
The online-only Data Supplement is available with this article at http://hyper.ahajournals.org/lookup/suppl/doi:10.1161/HYPERTENSIONAHA.111.00289/-/DC1.
References
- 1.Kannel WB, Wilson PW, Zhang TJ. The epidemiology of impaired glucose tolerance and hypertension. Am Heart J. 1991;121(4 Pt 2):1268–1273. doi: 10.1016/0002-8703(91)90432-h. [DOI] [PubMed] [Google Scholar]
- 2.Stamler J, Vaccaro O, Neaton JD, Wentworth D. Diabetes, other risk factors, and 12-yr cardiovascular mortality for men screened in the Multiple Risk Factor Intervention Trial. Diabetes Care. 1993;16:434–444. doi: 10.2337/diacare.16.2.434. [DOI] [PubMed] [Google Scholar]
- 3.Bobrie G, Clerson P, Ménard J, Postel-Vinay N, Chatellier G, Plouin PF. Masked hypertension: a systematic review. J Hypertens. 2008;26:1715–1725. doi: 10.1097/HJH.0b013e3282fbcedf. [DOI] [PubMed] [Google Scholar]
- 4.Hänninen MR, Niiranen TJ, Puukka PJ, Mattila AK, Jula AM. Determinants of masked hypertension in the general population: the Finn-Home study. J Hypertens. 2011;29:1880–1888. doi: 10.1097/HJH.0b013e32834a98ba. [DOI] [PubMed] [Google Scholar]
- 5.Mancia G, Bombelli M, Seravalle G, Grassi G. Diagnosis and management of patients with white-coat and masked hypertension. Nat Rev Cardiol. 2011;8:686–693. doi: 10.1038/nrcardio.2011.115. [DOI] [PubMed] [Google Scholar]
- 6.Zhou J, Liu C, Shan P, Zhou Y, Xu E, Ji Y. Prevalence and distinguishing features of masked hypertension in type 2 diabetic patients. J Diabetes Complicat. 2013;27:82–86. doi: 10.1016/j.jdiacomp.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Bobrie G, Clerson P, Cuchet A, Mahmoudi A, Postel-Vinay N, Chatellier G. Prevalence and mechanism of masked hypertension: the ol’mesures survey. Arch Mal Coeur Vaiss. 2006;99:760–763. [PubMed] [Google Scholar]
- 8.Thijs L, Hansen TW, Kikuya M, et al. IDACO Investigators The International Database of Ambulatory Blood Pressure in relation to Cardiovascular Outcome (IDACO): protocol and research perspectives. Blood Press Monit. 2007;12:255–262. doi: 10.1097/mbp.0b013e3280f813bc. [DOI] [PubMed] [Google Scholar]
- 9.Hansen TW, Jeppesen J, Rasmussen F, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and mortality: a population-based study. Hypertension. 2005;45:499–504. doi: 10.1161/01.HYP.0000160402.39597.3b. [DOI] [PubMed] [Google Scholar]
- 10.Ohkubo T, Hozawa A, Yamaguchi J, Kikuya M, Ohmori K, Michimata M, Matsubara M, Hashimoto J, Hoshi H, Araki T, Tsuji I, Satoh H, Hisamichi S, Imai Y. Prognostic significance of the nocturnal decline in blood pressure in individuals with and without high 24-h blood pressure: the Ohasama study. J Hypertens. 2002;20:2183–2189. doi: 10.1097/00004872-200211000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Staessen JA, Bieniaszewski L, O’Brien ET, Imai Y, Fagard R. An epidemiological approach to ambulatory blood pressure monitoring:the Belgian Population Study. Blood Press Monit. 1996;1:13–26. [PubMed] [Google Scholar]
- 12.Ingelsson E, Björklund-Bodegård K, Lind L, Arnlöv J, Sundström J. Diurnal blood pressure pattern and risk of congestive heart failure. JAMA. 2006;295:2859–2866. doi: 10.1001/jama.295.24.2859. [DOI] [PubMed] [Google Scholar]
- 13.Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H, Hypertension Working Group Ambulatory blood pressure: normality and comparison with other measurements. Hypertension. 1999;34(4 Pt 2):818–825. doi: 10.1161/01.hyp.34.4.818. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Wang JG, Gao P, Guo H, Nawrot T, Wang G, Qian Y, Staessen JA, Zhu D, The JingNing population study Are published characteristics of the ambulatory blood pressure generalizable to rural Chinese? Blood Press Monit. 2005;10:125–134. doi: 10.1097/00126097-200506000-00003. [DOI] [PubMed] [Google Scholar]
- 15.Kuznetsova T, Malyutina S, Pello E, Thijs L, Nikitin Y, Staessen JA. Ambulatory blood pressure of adults in Novosibirsk, Russia: interim report on a population study. Blood Press Monit. 2000;5:291–296. doi: 10.1097/00126097-200010000-00007. [DOI] [PubMed] [Google Scholar]
- 16.Kuznetsova T, Staessen JA, Kawecka-Jaszcz K, Babeanu S, Casiglia E, Filipovsky J, Nachev C, Nikitin Y, Peleskã J, O’Brien E. Quality control of the blood pressure phenotype in the European Project on Genes in Hypertension. Blood Press Monit. 2002;7:215–224. doi: 10.1097/00126097-200208000-00003. [DOI] [PubMed] [Google Scholar]
- 17.O’Brien E, Murphy J, Tyndall A, Atkins N, Mee F, McCarthy G, Staessen J, Cox J, O’Malley K. Twenty-four-hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens. 1991;9:355–360. doi: 10.1097/00004872-199104000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Hansen TW, Jeppesen J, Rasmussen S, Ibsen H, Torp-Pedersen C. Ambulatory blood pressure monitoring and risk of cardiovascular disease: a population based study. Am J Hypertens. 2006;19:243–250. doi: 10.1016/j.amjhyper.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. National Heart, Lung, and Blood Institute. National High Blood Pressure Education Program Coordinating Committee Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 20.Mancia G, De Backer G, Dominiczak A, et al. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) Eur Heart J. 2007;2007;28:1462–1536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 21.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D, Modification of Diet in Renal Disease Study Group A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 22.Pickering TG, Davidson K, Gerin W, Schwartz JE. Masked hypertension. Hypertension. 2002;40:795–796. doi: 10.1161/01.hyp.0000038733.08436.98. [DOI] [PubMed] [Google Scholar]
- 23.Franklin SS, Thijs L, Hansen TW, Li Y, Boggia J, Kikuya M, Björklund-Bodegård K, Richart T, Ohkubo T, Jeppesen J, Pedersen CT, Dolan E, Kuznetsova T, Stolarz-Skrzypek K, Tikhonoff V, Malyutina S, Casiglia E, Nikitin Y, Lind L, Sandoya E, Kawecka-Jaszcz K, Imai Y, Wang J, Ibsen H, O’Brien E, Staessen JA, International Database on Ambulatory Blood Pressure in Relation to Cardiovascular Outcome Investigators Significance of white-coat hypertension in older persons with isolated systolic hypertension: A meta-analysis using hte international database on ambulatory blood pressure monitoring in relation to cardiovascular outcomes population. Hypertension. 2012;59:564–571. doi: 10.1161/HYPERTENSIONAHA.111.180653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clement DL, De Buyzere ML, De Bacquer DA, de Leeuw PW, Duprez DA, Fagard RH, Gheeraert PJ, Missault LH, Braun JJ, Six RO, Van Der Niepen P, O’Brien E, Office versus Ambulatory Pressure Study Investigators Prognostic value of ambulatory blood-pressure recordings in patients with treated hypertension. N Engl J Med. 2003;348:2407–2415. doi: 10.1056/NEJMoa022273. [DOI] [PubMed] [Google Scholar]
- 25.Mancia G, Parati G. Office compared with ambulatory blood pressure in assessing response to antihypertensive treatment: a meta-analysis. J Hypertens. 2004;22:435–445. doi: 10.1097/00004872-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 26.Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET, Treatment of Hypertension Based on Home or Office Blood Pressure (THOP) Trial Investigators Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004;291:955–964. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 27.Staessen JA, Byttebier G, Buntinx F, Celis H, O’Brien ET, Fagard R, Ambulatory Blood Pressure Monitoring and Treatment of Hypertension Investigators Antihypertensive treatment based on conventional or ambulatory blood pressure measurement. A randomized controlled trial. JAMA. 1997;278:1065–1072. [PubMed] [Google Scholar]
- 28.Andalib A, Akhtari S, Rigal R, Curnew G, Leclerc JM, Vaillancourt M, Tardif JC. Determinants of masked hypertension in hypertensive patients treated in a primary care setting. Intern Med J. 2012;42:260–266. doi: 10.1111/j.1445-5994.2010.02407.x. [DOI] [PubMed] [Google Scholar]
- 29.Charvat J, Chlumsky J, Szabo M, Zakovicova E, Zamrazil V. The association of masked hypertension in treated type 2 diabetic patients with carotid artery IMT. Diabetes Res Clin Pract. 2010;89:239–242. doi: 10.1016/j.diabres.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 30.Uzu T, Nakao K, Kume S, Araki H, Isshiki K, Araki S, Kawai H, Ugi S, Kashiwagi A, Maegawa H. High sodium intake is associated with masked hypertension in Japanese patients with type 2 diabetes and treated hypertension. Am J Hypertens. 2012;25:1170–1174. doi: 10.1038/ajh.2012.102. [DOI] [PubMed] [Google Scholar]
- 31.Leitão CB, Canani LH, Kramer CK, Boza JC, Pinotti AF, Gross JL. Masked hypertension, urinary albumin excretion rate, and echocardiographic parameters in putatively normotensive type 2 diabetic patients. Diabetes Care. 2007;30:1255–1260. doi: 10.2337/dc06-2131. [DOI] [PubMed] [Google Scholar]
- 32.Ng CM, Yiu SF, Choi KL, Choi CH, Ng YW, Tiu SC. Prevalence and significance of white-coat hypertension and masked hypertension in type 2 diabetics. Hong Kong Med J. 2008;14:437–443. [PubMed] [Google Scholar]
- 33.Liu JE, Roman MJ, Pini R, Schwartz JE, Pickering TG, Devereux RB. Cardiac and arterial target organ damage in adults with elevated ambulatory and normal office blood pressure. Ann Intern Med. 1999;131:564–572. doi: 10.7326/0003-4819-131-8-199910190-00003. [DOI] [PubMed] [Google Scholar]
- 34.Ogedegbe G, Agyemang C, Ravenell JE. Masked hypertension: evidence of the need to treat. Curr Hypertens Rep. 2010;12:349–355. doi: 10.1007/s11906-010-0140-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Brien E, Asmar R, Beilin L, Imai Y, Mancia G, Mengden T, Myers M, Padfield P, Palatini P, Parati G, Pickering T, Redon J, Staessen J, Stergiou G, Verdecchia P, European Society of Hypertension Working Group on Blood Pressure Monitoring Practice guidelines of the European Society of Hypertension for clinic, ambulatory and self blood pressure measurement. J Hypertens. 2005;23:697–701. doi: 10.1097/01.hjh.0000163132.84890.c4. [DOI] [PubMed] [Google Scholar]
- 36.Pickering TG, Houston Miller N, Ogedegbe G, Krakoff LR, Artinian NT, Goff D, A joint scientific statement from the American Heart Association, American Society of Hypertension, and Preventive Cardiovascular Nurses Association Call to action on use and reimbursement for home blood pressure monitoring. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sehestedt T, Hansen TW, Li Y, et al. Are blood pressure and diabetes additive or synergistic risk factors? Outcome in 8494 subjects randomly recruited from 10 populations. Hypertens Res. 2011;34:714–721. doi: 10.1038/hr.2011.6. [DOI] [PubMed] [Google Scholar]
- 38.Parati G, Bilo G, Ochoa JE. Benefits of tight blood pressure control in diabetic patients with hypertension: importance of early and sustained implementation of effective treatment strategies. Diabetes Care. 2011;34(Suppl 2):S297–S303. doi: 10.2337/dc11-s243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reboldi G, Gentile G, Angeli F, Ambrosio G, Mancia G, Verdecchia P. Effects of intensive blood pressure reduction on myocardial infarction and stroke in diabetes: a meta-analysis in 73,913 patients. J Hypertens. 2011;29:1253–1269. doi: 10.1097/HJH.0b013e3283469976. [DOI] [PubMed] [Google Scholar]
- 40.Reboldi G, Gentile G, Manfreda VM, Angeli F, Verdecchia P. Tight blood pressure control in diabetes: evidence-based review of treatment targets in patients with diabetes. Curr Cardiol Rep. 2012;14:89–96. doi: 10.1007/s11886-011-0236-8. [DOI] [PubMed] [Google Scholar]
- 41.Cushman WC, Evans GW, Byington RP, et al. ACCORD Study Group Effects of intensive blood-pressure control in type 2 diabetes mellitus. N Engl J Med. 2010;362:1575–1585. doi: 10.1056/NEJMoa1001286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Burnier M, Grassi G. Ambulatory blood pressure monitoring in diabetic patients: new data, new questions. J Hypertens. 2011;29:198–200. doi: 10.1097/HJH.0b013e328342d4d7. [DOI] [PubMed] [Google Scholar]
- 43.Ben-Dov IZ, Ben-Arie L, Mekler J, Bursztyn M. Reproducibility of white-coat and masked hypertension in ambulatory BP monitoring. Int J Cardiol. 2007;117:355–359. doi: 10.1016/j.ijcard.2006.04.088. [DOI] [PubMed] [Google Scholar]
- 44.Viera AJ, Hinderliter AL, Kshirsagar AV, Fine J, Dominik R. Reproducibility of masked hypertension in adults with untreated borderline office blood pressure: comparison of ambulatory and home monitoring. Am J Hypertens. 2010;23:1190–1197. doi: 10.1038/ajh.2010.158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.