Abstract
The p24α, -β, -γ, and -δ proteins are major multimeric constituents of cycling endoplasmic reticulum-Golgi transport vesicles and are thought to be involved in protein transport through the early secretory pathway. In this study, we targeted transgene overexpression of p24δ2 specifically to the Xenopus intermediate pituitary melanotrope cell that is involved in background adaptation of the animal and produces high levels of its major secretory cargo proopiomelanocortin (POMC). The transgene product effectively displaced the endogenous p24 proteins, resulting in a melanotrope cell p24 system that consisted predominantly of the transgene p24δ2 protein. Despite the severely distorted p24 machinery, the subcellular structures as well as the level of POMC synthesis were normal in these cells. However, the number and pigment content of skin melanophores were reduced, impairing the ability of the transgenic animal to fully adapt to a black background. This physiological effect was likely caused by the affected profile of POMC-derived peptides observed in the transgenic melanotrope cells. Together, our results suggest that in the early secretory pathway an intact p24 system is essential for efficient secretory cargo transport or for supplying cargo carriers with the correct protein machinery to allow proper secretory protein processing.
INTRODUCTION
Transport of cargo proteins through the early secretory pathway involves cargo selection, transport vesicle formation, quality control to recycle misfolded cargo, and cycling of the COPI- and COPII-coated vesicles between the endoplasmic reticulum (ER) and Golgi (Barlowe, 2000). One of the major constituents of the transport vesicles is the p24 family of type I transmembrane proteins that can be classified into four main subfamilies, designated p24α, -β, -γ, and -δ (Schimmöller et al., 1995; Stamnes et al., 1995; Sohn et al., 1996; Nickel et al., 1997; Dominguez et al., 1998). The p24 proteins share a number of structural characteristics, such as a relatively large lumenal putative cargo-binding domain, a coiled-coil region thought to be involved in the formation of multimeric p24 complexes, a transmembrane region, and a short cytoplasmic tail containing COPI- and COPII-binding motifs that are used for p24 traveling from the ER to the Golgi and back (for review, see Kaiser, 2000). In yeast and mammalian cells, p24 proteins form functional heterotetrameric complexes containing one representative of each subfamily, whereby the composition of the complex may differ in various cell types (Dominguez et al., 1998; Füllekrug et al., 1999; Marzioch et al., 1999; Ciufo and Boyd, 2000; Emery et al., 2000; Belden and Barlowe, 2001). Furthermore, the stability of the p24 members seems to be compromised when cells are deficient in the expression of a single p24 protein (Marzioch et al., 1999; Denzel et al., 2000). Recent evidence suggests a complex and dynamic p24 system of mostly monomers and homo-/heterodimers and that the degree of oligomerization constantly alters and largely depends on the subcellular localizations of the p24 subfamily members (Jenne et al., 2002).
The p24 proteins have been suggested to play a key role in cargo-selective protein transport at the ER/Golgi interface (Kaiser, 2000). For the elusive mechanism of action of p24, a number of functional models have been proposed, including a role as cargo receptor, membrane organizer, or regulator of vesicle budding, as well as in the ER quality control system, or excluding ER resident proteins from the vesicular lumen (Schimmöller et al., 1995; Elrod Erickson and Kaiser, 1996; Rojo et al., 1997; Bremser et al., 1999; Lavoie et al., 1999; Wen and Greenwald, 1999; Denzel et al., 2000; Kaiser, 2000; Muñiz et al., 2000; Springer et al., 2000; Belden and Barlowe, 2001). Defining the importance of a functional p24 system for proper cell physiology has however turned out to be difficult. For instance, deletion of all p24 proteins resulted in viable yeast (Marzioch et al., 1999; Springer et al., 2000), whereas genetic ablation of a single p24 family member caused early lethality in mice (Denzel et al., 2000). To investigate the significance of the p24 system in a highly specialized secretory cell, we decided to use a physiological model (background adaptation of the South-African clawed frog, Xenopus laevis) with a well-defined secretory cell (the intermediate pituitary melanotrope cell) and its single major soluble cargo protein proopiomelanocortin (POMC) (Roubos, 1997). In the trans-Golgi network/immature secretory granules of Xenopus melanotrope cells, endoproteolytic cleavage of POMC results in a number of bioactive peptides, including α-melanophore-stimulating hormone (α-MSH). This hormone mediates adaptation of the animal to a black background by causing dispersion of melanin pigment granules (melanosomes) in skin melanophores. On a black back-ground, the melanotrope cell is dedicated to produce vast amounts of POMC such that this prohormone represents ∼80% of all newly synthesized melanotrope proteins. On a white background, POMC mRNA levels are decreased ∼30-fold (Holthuis et al., 1995a) and α-MSH secretion from the melanotropes into the bloodstream is inhibited by neurons of hypothalamic origin that directly innervate the cells (Jenks et al., 1993; Tuinhof et al., 1994), leading to melanosome aggregation and, consequently, pallor of the skin. Placing Xenopus on a black or a white background therefore allows physiological manipulation of the biosynthetic and secretory activities of the melanotrope cell. Using a differential screening approach, we have identified a number of proteins coexpressed with POMC and thus differentially expressed in the melanotrope cells of black- and white-adapted Xenopus, including the POMC cleavage enzyme prohormone convertase PC2 and a member of the p24 family, namely, Xp24δ2 (Holthuis et al., 1995b). Subsequent extensive cDNA library screening resulted in the identification of all members of the p24 family that are expressed in the Xenopus melanotrope cell (Xp24α3, -β1, -γ2,3, and -δ1,2) (Rötter et al., 2002). Of these, Xp24α3, -β1, -γ3, and -δ2 constitute the major representatives and are highly up-regulated with POMC in the melanotropes during black background adaptation (at least 20-fold), whereas the two low-abundant ones (Xp24γ2 and -δ1) are not or only slightly induced (Kuiper et al., 2001; Rötter et al., 2002). The coordinate and induced expression of a selective set of Xenopus p24 proteins (Xp24α3, -β1, -γ3, and -δ2) in the melanotrope cell suggests that these p24 members are somehow involved in POMC biosynthesis. To explore the importance of p24 in the Xenopus melanotrope cell, we combined the unique properties of this cell with the technique of stable Xenopus transgenesis by using a Xenopus POMC gene promoter fragment to target transgene expression specifically to the melanotrope cell, leaving the integrity of the regulation by the hypothalamic neurons intact. For transgenic overexpression, we selected one of the Xenopus melanotrope p24 proteins coexpressed with POMC, namely, the Xp24δ2 protein, and fused it to the N terminus of the green fluorescent protein (GFP). Here, we report the effect of this transgenic manipulation of the endogenous p24 system on the functioning of the Xenopus melanotrope cells.
MATERIALS AND METHODS
Animals
X. laevis were reared in the Central Animal Facility of the University of Nijmegen (Nijmegen, The Netherlands). For the transgenesis experiments, female X. laevis were obtained directly from South Africa. For background adaptation, the animals were kept in either white or black containers under constant illumination for at least 3 wk. All animal experiments were carried out in accordance with the European Communities Council Directive 86/609/EEC for animal welfare, and permit TRC 99/15072 to generate and house transgenic Xenopus.
Antibodies
The rabbit polyclonal antibodies against portions of the lumenal and C-terminal regions of Xp24δ2 (anti-1262N and anti-1262C, respectively), against part of the lumenal region of Xp24δ1 (anti-RH6), and against a region in the lumenal part of Xp24α3 have been described previously (Kuiper et al., 2001; Rötter et al., 2002). A polyclonal antibody to human p24β1 (p24A) was kindly provided by Dr. I. Schulz (University of the Saarland, Homburg, Germany; Blum et al., 1999), to human p24γ3 by Dr. T. Nilsson (European Molecular Biology Laboratory, Heidelberg; Germany; Dominguez et al., 1998), against recombinant mature human PC2 by Dr. W.J.M. Van de Ven (University of Leuven, Belgium; Van Horssen et al., 1998), to GFP by Dr. J. Fransen (Cuppen et al., 1999), and against Xenopus POMC (ST62, recognizing only the precursor form) by Dr. S. Tanaka (Shizuoka University, Hamamatsu, Japan; Berghs et al., 1997).
Generation of Xenopus Transgenic for Xp24δ2-GFP
A linear 2166-base pair SalI/NarI DNA fragment encoding the Xenopus p24δ2 protein with the enhanced GFP protein fused in frame to its C terminus (Xp24δ2-GFP fusion protein) and cloned behind a 529-base pair Xenopus POMC gene A promoter (pPOMC; Jansen et al., 2002) fragment (construct pPOMC-Xp24δ2-GFP) was used for stable Xenopus transgenesis (Kroll and Amaya, 1996; Sparrow et al., 2000). A number of injection rounds resulted in animals transgenic for Xp24δ2-GFP and expressing the fusion protein at various levels (animals 115 and 125 with moderate and 124 with high expression levels). The number of integration sites and integrated copies of the transgene were determined by Southern blot analysis of genomic DNA isolated from transgenic livers (Ausubel et al., 2001) revealing four, one, and three sites of integration, and ∼25, 2, and ∼20 integrated copies of the transgene in animals 115, 125, and 124, respectively. To generate F1 offspring, the testes of male transgenic Xenopus frogs were isolated and for in vitro fertilization pieces of testis were incubated with eggs harvested from wild-type Xenopus females.
Microscopy
For ultrastructural analysis, electron microscopy was performed as described previously (de Rijk et al., 1990). Ultrastructural (immuno)localization studies were performed on neurointermediate lobes (NILs) of wild-type Xenopus and #124 and #224 transgenic animals expressing Xp24δ2-GFP at high levels. Entire lobes were fixed for 1 h at room temperature in 2% paraformaldehyde + 0.01% glutaraldehyde in PHEM buffer (50 mM MgCl2, 70 mM KCl, 10 mM EGTA, 20 mM HEPES, 60 mM PIPES, pH 6.8). Fixed tissue was stored in 1% paraformaldehyde in 0.1 M phosphate buffer until use. Ultrathin cryosectioning was performed as described previously (Fransen et al., 1985; Schweizer et al., 1988). Sections were incubated with an antiserum against enhanced green fluorescent protein at a 1:100 dilution followed by protein A complexed with 10-nm gold (Fransen et al., 1985). Electron microscopy experiments using the anti-Xp24δ antibodies were not successful. Electron microscopy was performed using a JEOL 1010 electron microscope operating at 80 kV. For confocal microscopy, brains with the pituitaries attached were dissected and fixed in 4% paraformaldehyde in phosphate-buffered saline. After cryoprotection in 10% sucrose-phosphate-buffered saline, sagittal 20-μm cryosections were mounted on poly-l-lysine-coated slides, dried for 2 h at 45°C, and studied with an MRC 1024 confocal laser scanning microscope (Bio-Rad, Hercules, CA). To examine direct fluorescence as a result of GFP fusion protein expression, cryosections were directly viewed under a Leica DM RA fluorescent microscope and photographs were taken with a Cohu high-performance charge-coupled device camera using the Leica Q Fluoro software. Immunohistochemistry for POMC and α-MSH was performed as described previously (Jansen et al., 2002). For light microscopy analysis of the webs and melanophores of wild-type and transgenic animals, webs were cut out, mounted on slides, and coverslipped. Digital images were obtained using a Leica MZFLIII microscope mounted with a DC200 digital color camera. Equal integration intervals and magnifications were used to capture images with Leica DC viewer software.
Western Blot Analysis
Western blot analysis was performed as described previously (Kuiper et al., 2000). For quantification, detection was performed using a BioChemi imaging system, and signals were analyzed using the Labworks 4.0 program (UVP BioImaging systems, Cambridge, United Kingdom).
Pulse and Pulse-Chase Analysis
For metabolic labeling, NILs from wild-type and transgenic Xenopus were preincubated for 30 min, pulse labeled in the presence of 5 mCi/ml Tran35S-label (ICN Radiochemicals) and chased with 0.5 mM l-methionine for the indicated time periods, and homogenized as described previously (Braks and Martens, 1994). Parts of the lysates and incubation media were analyzed directly on SDS-PAGE, while the remainder was used for immunoprecipitation, western blot and/or high-performance liquid chromatography (HPLC) analysis.
Immunoprecipitation Analysis
For immunoprecipitation analysis, NIL lysates were diluted with lysis buffer to 1 ml, and supplemented with SDS (final concentration of 0.075%) and the respective antibodies. Precipitation was performed overnight at 4°C while rotating the samples. Immune complexes were precipitated with protein A-Sepharose (Amersham Biosciences, Piscataway, NJ) and resolved by SDS-PAGE. Radiolabeled proteins were detected using autoradiography at -70°C or a PhosphoImager (Personal FX; Bio-Rad).
HPLC Analysis
For the separation of the small newly synthesized end products of POMC processing, radiolabeled NIL lysates were subjected to HPLC analysis as described previously (Martens et al., 1982a).
RESULTS
Generation of Xenopus Transgenic for Xp24δ2-GFP
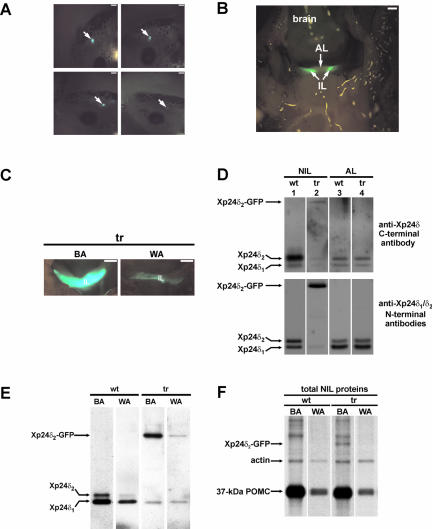
To generate Xenopus transgenic for the Xenopus p24δ2 protein with GFP fused to its C terminus (Xp24δ2-GFP), we first made a DNA construct (pPOMC-Xp24δ2-GFP) containing a 529-base pair Xenopus POMC gene promoter fragment in front of the sequence encoding the fusion protein. The GFP-moiety was fused to the C terminus of the Xp24δ2-protein to avoid interference with a possible binding of cargo to the N-terminal loop domain of Xp24δ2. The pPOMC-Xp24δ2-GFP DNA was mixed with Xenopus sperm nuclei and the mixture was microinjected into unfertilized Xenopus eggs. The different levels of expression of the fusion protein among the various transgenic animals could be readily and directly established by visual inspection of the living embryos under a fluorescence microscope (Figure 1A). Lifting the brain of the transgenic animal showed that the expression of the Xp24δ2-GFP fusion protein was restricted to cells located in the intermediate lobe of the pituitary, and no fluorescence was observed in the anterior lobe of the pituitary or in any other brain structures (Figure 1B). An immunocytochemical analysis revealed that the fusion protein was coexpressed in the melanotrope cells with POMC and α-MSH (data not shown). Adaptation of the transgenic animals to a black or a white background resulted in high and low levels of fluorescence in the intermediate pituitary, respectively (Figure 1C), suggesting that the level of Xp24δ2-GFP transgene expression was dependent on the color of the background of the animal and coregulated with POMC expression. Thus, the 529-base pairs Xenopus POMC gene promoter fragment was sufficient to drive melanotrope cell-specific expression of the transgene and give different levels of transgene expression depending on background color.
Figure 1.
Xp24δ2-GFP transgene expression is specific to Xenopus intermediate pituitary and dependent on background color. (A) Pituitary-specific fluorescence in transgenic Xenopus embryos. Shown are living stage 45 embryos, whereby the arrows indicate the locations of the pituitaries with various levels of transgene expression. Fluorescent pituitaries expressing the transgene fusion product could be detected from stage 25 onwards. Bar, 0.4 mm. (B) Fluorescence is specific to the intermediate pituitary of transgenic Xenopus. Ventrocaudal view on the brain that was lifted to reveal the bright fluorescence caused by the Xp24δ2-GFP fusion protein and observed in the intermediate lobe (IL), but not in the AL, of the pituitary of a black-adapted transgenic frog of 6 mo. Bar, 0.5 mm. (C) Fluorescence in the intermediate lobe of black-(BA) and white- (WA) adapted transgenic (tr) Xenopus. Ventrocaudal view with the anterior part of the pituitary removed. Bar, 0.5 mm. (D) Western blot analysis of p24δ protein expression in the NIL and AL of black-adapted wild-type (wt) and transgenic (tr) Xenopus. (E) Western blot analysis of p24δ protein expression in the NIL of black- and white-adapted wild-type and transgenic Xenopus using the p24δ1/-δ2 antibody mix. (F) Newly synthesized proteins produced in NILs of BA and WA wild-type and transgenic Xenopus. NILs were pulse labeled for 1 h, part of the total cell lysates was analyzed directly on SDS-PAGE, and radiolabeled proteins were visualized by fluorography.
Steady-State p24 Protein Levels in the Pituitary of Xenopus Transgenic for Xp24δ2-GFP
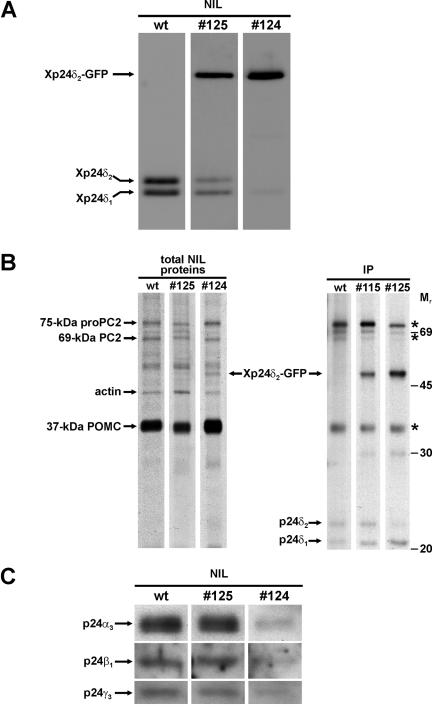
From the pituitary (consisting of the pars nervosa and the anterior and intermediate lobes), the anterior part can be dissected but the pars nervosa (biosynthetically not active nerve terminals of hypothalamic origin) is intimately associated with the intermediate pituitary (the neuroendocrine melanotrope cells). For our studies, we therefore used the anterior lobe (AL) and NIL (pars nervosa plus intermediate lobe) of the pituitary. Western blot analysis of p24 steady-state protein levels was performed on lysates of NILs and ALs of wild-type and transgenic Xenopus by using specific anti-p24 antibodies. We first used the anti-Xp24δ antibody 1262C directed against the C-terminal region of Xp24δ2 that recognizes endogenous Xp24δ1 and -δ2 with comparable affinities (Kuiper et al., 2000). With this antibody, we detected ∼8 and ∼3 times more of the ∼24-kDa Xp24δ2 protein than ∼23-kDa Xp24δ1 in the wild-type NIL and AL, respectively (Figure 1D, top). However, the C-terminally directed antibody 1262C hardly recognized the Xp24δ2-GFP fusion protein (Figure 1D, compare lanes 2 of top and bottom panels), presumably because of the fusion of GFP to the C terminus of Xp24δ2. For the simultaneous detection of the transgene and endogenous Xp24δ products, we therefore used in all subsequent experiments a mixture of anti-Xp24δ1 and anti-Xp24δ2 antibodies (RH6 and 1262N, respectively), each directed against a portion of the respective N-terminal region and specifically recognizing the corresponding Xp24δ protein. This antibody mix showed in the wild-type NIL about equal amounts of the endogenous Xp24δ1 and Xp24δ2 proteins (Figure 1D, bottom, lane 1). In the transgenic NIL, the antibodies revealed an additional product of ∼52 kDa, presumably corresponding to the transgene Xp24δ2-GFP fusion protein (∼24 kDa for Xp24δ2 and ∼28 kDa for GFP) (Figure 1D, bottom, lane 2). The fusion protein was found only in the NIL and not AL (Figure 1D, bottom), again indicating that the expression of the transgene product is melanotrope cell specific. In the transgenic cells, the fusion protein was about ∼15-fold higher in black than in white animals (Figure 1E), in line with the data obtained by direct fluorescence analysis (Figure 1C). Furthermore, metabolic labeling of wild-type and transgenic NILs revealed an approximately ninefold higher level of newly synthesized Xp24δ2-GFP fusion protein in black- than in white-adapted #124 transgenic animals, similar to the ∼10-fold difference in radiolabeled POMC precursor levels (Figure 1F). Having established that transgene expression is coregulated with POMC and specific for the melanotrope cells, we then wondered what the effect of the overexpression of the Xp24δ2-GFP fusion protein would be on the levels of the endogenous p24 proteins. For this and subsequent analyses, two male transgenic Xenopus that differed in Xp24δ2-GFP expression levels (animals 124 and 125) were selected and used to generate F1 offspring by in vitro fertilization. Western blot analysis revealed that the expression of the transgene product was approximately fourfold higher in #124 than in #125 transgenic melanotrope cells (Figure 2A). The overexpresssion of the Xp24δ2-GFP fusion protein resulted in reduced levels of the endogenous Xp24δ1 and -δ2 proteins in #125 cells (∼40 and ∼54% reduction, respectively), whereas in #124 cells the two endogenous Xp24δ proteins were even nearly completely displaced (∼87 and ∼95% reduction of Xp24δ1 and -δ2, respectively) (Figure 2A). These findings indicate that high levels of the transgene product cause low levels of the endogenous Xp24δ proteins. To examine whether the degree of competition between the exogenous and endogenous Xp24δ proteins was correlated with the level of newly synthesized Xp24δ2-GFP produced in the #124 and #125 transgenic NILs, we performed metabolic cell-labeling experiments. Direct SDS-PAGE analysis of newly synthesized NIL proteins revealed an ∼52-kDa radio-labeled product in #124 transgenic but not in #125 transgenic or wild-type cells (Figure 2B, left). The ∼52-kDa product comigrated with a radiolabeled protein immunoprecipitated with the anti-δ1/δ2 antibody mix from newly synthesized proteins produced by transgenic NILs (Figure 2B), indicating that it represents the newly synthesized Xp24δ2-GFP fusion protein. The #125 melanotrope cells produced approximately fivefold and the #124 cells at least 15-fold more newly synthesized transgene Xp24δ2-GFP product than newly synthesized endogenous Xp24δ1 protein. The lower level of immunoprecipitated newly synthesized endogenous Xp24δ2 in the #125 cells was likely due to the high amount of competing radiolabeled transgene δ2 fusion product, because in cells from the independent line 115 with less transgene expression, the amount of immunoprecipitated endogenous Xp24δ2 was not affected (Figure 2B, right). Therefore, the biosynthesis of the endogenous Xp24δ1 and -δ2 proteins does not seem to be affected by the transgene expression. Together, the above-mentioned findings indicate that in the #124 melanotrope cells the high level of Xp24δ2-GFP protein biosynthesis resulted in lower amounts of the endogenous Xp24δ1 and -δ2 proteins than in the #125 cells and, thus, that the level of transgene expression is correlated with the degree of displacement of the endogenous Xp24δ proteins by the exogenous fusion product.
Figure 2.
Xp24δ2-GFP protein levels in transgenic Xenopus intermediate pituitary determine the degree of displacement of the endogenous p24 proteins. (A) Western blot analysis of Xp24δ protein expression in the NIL of wild-type (wt) and transgenic (#125 and #124) Xenopus by using an anti-Xp24δ1/-δ2 antibody mix. (B) Newly synthesized proteins produced in NILs of wild-type and transgenic Xenopus. NILs were pulse labeled for 1 h, and parts of the total cell lysates were analyzed directly on SDS-PAGE (left) or immunoprecipitated using an anti-Xp24δ1/-δ2 antibody mix followed by resolving the immunoprecipitates on SDS-PAGE (right). Radiolabeled proteins were visualized by fluorography. Asterisks indicate POMC- and PC2-related proteins binding nonspecifically to the antibodies. (C) Western blot analysis of Xp24α3, -β1, and -γ3 protein expression in the NIL of wild-type and transgenic Xenopus.
We next examined what the consequences of the expression of the Xp24δ2-GFP protein were on the steady-state expression levels of the major endogenous Xenopus melanotrope p24 members other than the Xp24δ proteins. Overexpression of the fusion protein in #124 transgenic melanotrope cells led to a more than fivefold reduction in the amounts of the endogenous Xp24α3, -β1, and -γ3 proteins, whereas these levels were essentially unchanged in the #125 cells (Figure 2C). The level of expression of the transgene product therefore seems to determine the degree of displacement not only of the endogenous Xp24δ proteins but also of the other endogenous p24 members. Together, we conclude that in the #124 transgenic melanotrope cells the exogenous Xp24δ2-GFP fusion protein caused a drastic reduction in the amounts of the endogenous p24 members, resulting in a p24 system predominantly consisting of the transgene product.
Microscopy Analyses of Xenopus Melanotrope Cells Transgenic for Xp24δ2-GFP
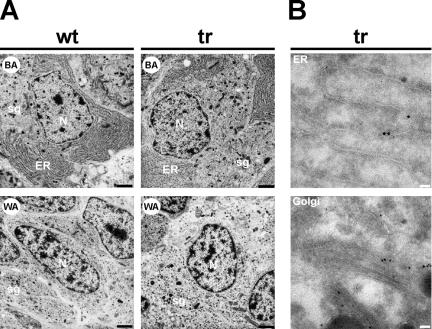
In transfected mammalian cells in culture, overexpression of p24δ1 (p23) or p24β1 (p24) caused the induction of an expansion of smooth ER membranes (Rojo et al., 1997; Blum et al., 1999). We therefore wondered what in the #124 transgenic Xenopus melanotrope cells the effect of the overexpression of the Xp24δ2-GFP protein would be on the morphology of subcellular structures. Electron microscopy analyses were performed on intermediate pituitaries of both black- and white-adapted wild-type and #124 transgenic animals. Despite the severely affected p24 system in the #124 transgenic melanotrope cells, at the ultrastructural level no gross morphological differences were observed between the wild-type and transgenic cells (Figure 3A). As expected, the melanotrope cells of black-adapted animals showed extensive ER structures as these cells are highly active in synthesizing large amounts of POMC. The melanotropes of white-adapted animals showed virtually no ER structures but many storage granules, reflecting their biosynthetic and secretory inactivity (Figure 3A). We can thus conclude that the structural changes occurring in the melanotrope cells during background adaptation of the animal are similar in the wild-type and #124 transgenic cells, and consistent with previous electron microscopy studies on wild-type Xenopus melanotrope cells (Weatherhead et al., 1971; de Rijk et al., 1990). The Xp24δ2-GFP fusion protein was found to be capable of reaching the Golgi, because confocal microscopy on the transgenic melanotrope cells revealed that both ER- and Golgi regions displayed fluorescence (our unpublished data), and immunoelectron microscopy confirmed that the Xp24δ2-GFP fusion protein was localized to structures that resemble the ER and the Golgi (Figure 3B). These results are in line with previous findings showing that endogenous Xp24δ2 localizes to the ER and the Golgi in wild-type Xenopus melanotrope cells (Kuiper et al., 2001) and that p24 proteins shuttle between the ER and the Golgi (Sohn et al., 1996; Barlowe, 1998; Dominguez et al., 1998). Together, these observations suggest that the overexpression of the transgene product was to such an extent that in the transgenic cells the early secretory pathway was not destroyed and that the transgene product was localized to the proper secretory pathway subcompartments.
Figure 3.
Electron microscopy on transgenic Xenopus intermediate pituitary cells. (A) Electron micrographs of melanotrope cells of wild-type (wt) and #124 transgenic (tr) Xenopus adapted to a black (BA) or white (WA) background. N, nucleus; ER, endoplasmic reticulum; sg, secretory/storage granule. Bar, 2 μm. (B) Pituitary glands from transgenic (tr) frogs (F1 #224, expressing high levels of Xp24δ2-GFP) were subjected to immunoelectron microscopical analysis. For immunodetection, the anti-GFP antibody was used in combination with protein-A-gold to visualize the Xp24δ2-GFP fusion protein. Immunoreactivity was found in structures that resemble the ER and the Golgi. Bars, 0.1 μm.
Background Adaptation of Xenopus Transgenic for Xp24δ2-GFP
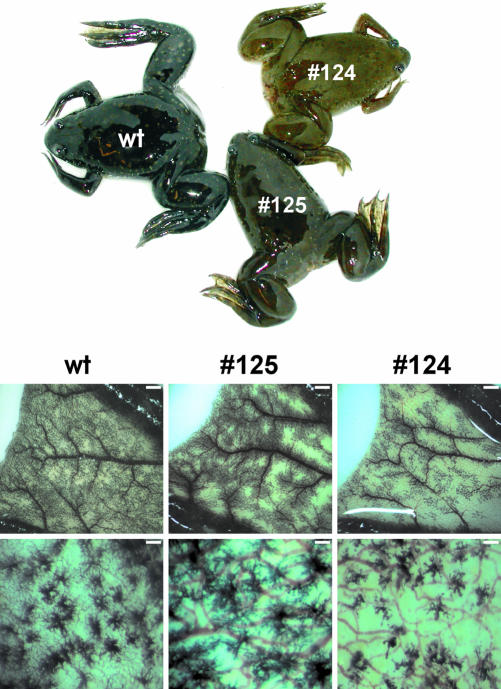
In Xenopus, the intermediate pituitary melanotrope cells to which we specifically targeted transgene expression are involved in the process of background adaptation (Jenks et al., 1977). This fact, together with the disrupted p24 machinery in the #124 Xenopus transgenic melanotrope cells, prompted us to examine the physiological consequence of this situation for background adaptation of the transgenic animal. After their metamorphosis, animals were placed on a black background for four months and thus the melanotrope cells were biosynthetically very active during a relatively long time period. As expected, wild-type Xenopus were black and contained many completely dispersed pigment-filled granules in the dermal melanophores of their webs. After the long adaptation to a black background, the skin color of the #124 transgenic animal was lighter than those of wild-type and #125 animals. On closer inspection of the webs, only in the vicinity of blood vessels were pigment-containing web melanophores observed, and the number and sizes of melanophores were clearly reduced in the #124 transgenic animal (approximately five- and ∼threefold reduction, respectively) (Figure 4). These results indicate that the transgenic manipulation of the p24 system exclusively in the Xenopus melanotrope cells led to a physiological effect regarding morphological changes in skin melanophores.
Figure 4.
Wild-type and transgenic Xenopus adapted to a black background. Wild-type (wt) and #125 and #124 transgenic animals were placed on a black background for 4 mo. Shown below are the pigment-containing dermal melanophores in the webs. Bars, 1 mm (top) and 250 μm (bottom).
Steady-State Protein levels of POMC and PC2 in Xenopus Melanotrope Cells Transgenic for Xp24δ2-GFP
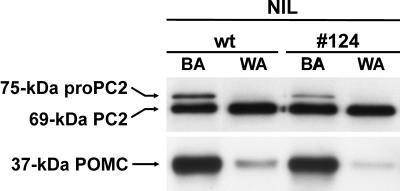
Because the process of background adaptation is mediated by α-MSH, a cleavage product of POMC, we next examined by Western blot analysis whether the altered p24 system had affected the steady-state level of the 37-kDa POMC precursor in the transgenic melanotrope cells. No differences in POMC levels were observed between wild-type and #124 transgenic NILs of black-adapted animals (Figure 5). On white-background adaptation of the transgenic animals, the amount of the POMC protein decreased to similar levels as observed in wild-type melanotrope cells of white animals (at least 10-fold reduction; Figure 5). Likewise, in the #124 transgenic cells of black-adapted animals, the steady-state amounts of both the proenzyme and mature forms of the POMC cleavage enzyme PC2 (75-kDa proPC2 and 69-kDa PC2, respectively) were not affected when compared with those in the wild-type situation. Furthermore, in both the inactive wild-type and transgenic cells of white animals, the expression of the proPC2 protein was greatly reduced (at least 15-fold), whereas the level of mature PC2 remained essentially the same as in black-adapted animals (Figure 5). These results indicate that the steady-state POMC and proPC2 protein levels, and the changes in these levels induced by the process of background adaptation were not affected by the introduction of the transgene into the melanotrope cells.
Figure 5.
POMC and PC2 protein levels are similar in the intermediate pituitary of wild-type and transgenic Xenopus and dependent on background color. Western blot analysis of NIL proteins of wild-type and #124 transgenic Xenopus adapted to a black (BA) or white (WA) background.
Biosynthesis and Processing of Newly Synthesized POMC and proPC2 in Xenopus Melanotrope Cells Transgenic for Xp24δ2-GFP
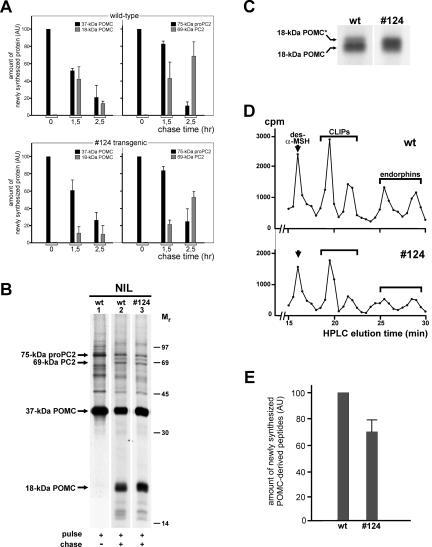
We next studied the dynamics of protein synthesis by performing in vitro pulse- and pulse-chase analyses of newly synthesized proteins produced in wild-type and transgenic NILs. Because besides the melanotrope cells, the Xenopus NIL consists of nerve terminals of hypothalamic origin that are biosynthetically not active (the pars nervosa), the radio-labeled proteins are synthesized by the melanotropes. During the 10-min pulse incubation of wild-type NILs, the 37-kDa POMC precursor protein was clearly the major newly synthesized protein (Figure 6, A and B, lane 1). During the subsequent 1.5-h and 2.5-h chase incubations, 37-kDa POMC was gradually processed to an 18-kDa cleavage product (Figure 6A, top left and B, lane 2). This product represents the N-terminal portion of 37-kDa POMC, is generated by the first endoproteolytic cleavage step during POMC processing and contains the only N-linked glycosylation site present in the POMC molecule (Martens, 1986). The amount of the 18-kDa POMC protein was lower for the 2.5-h than for the 1.5-h time point, because during the chase period this newly synthesized product is processed further (to γ-MSH; Martens et al., 1982b) (Figure 6A, top left). During the 10-min pulse, the POMC cleavage enzyme PC2 was synthesized as a 75-kDa proenzyme form that in the course of the subsequent 1.5-h and 2.5-h chase incubations was processed to a 69-kDa mature form of PC2 that represents the end product of proPC2 processing (Figure 6A, top right, and B, lanes 1 and 2). Within the time frame of these pulse-chase experiments, virtually no newly synthesized 18-kDa POMC and mature PC2 was released into the incubation medium (<10% of the cellular content). In the #124 transgenic melanotrope cells, similar amounts of 37-kDa POMC were synthesized during the 10-min pulse incubation as in wild-type cells. However, the amounts of 18-kDa POMC that were produced in the transgenic cells after 1.5 h and 2.5 h of chase were less than those synthesized in the wild-type cells (Figure 6A). Moreover, reloading of the samples on a higher-percentage polyacrylamide gel revealed that a substantial portion of the newly synthesized 18-kDa product produced in the #124 transgenic cells migrated slower than the majority of 18-kDa POMC synthesized in the wild-type cells (Figure 6C). We refer to this slower-migrating product as 18-kDa POMC* and the normal product as 18-kDa POMC without an asterisk. The nature of the difference between the two 18-kDa POMC products is presently unknown. During the 10-min pulse, the amount of newly synthesized 75-kDa proPC2 produced in the transgenic melanotrope cells was similar to that synthesized by the wild-type cells. In contrast, as for the reduced rate of 37-kDa POMC processing, during the 1.5-h and 2.5-h chase periods the rate of conversion of newly synthesized proPC2 into mature PC2 was lower in the transgenic than in the wild-type cells (Figure 6A).
Figure 6.
Biosynthesis and processing of newly synthesized POMC and proPC2 in wild-type and transgenic Xenopus intermediate pituitary cells. (A) NILs of black-adapted wild-type (wt) and #124 transgenic animals were pulse labeled for 10 min or pulse labeled for 10 min and chased for 1.5 or 2.5 h. Newly synthesized proteins were extracted from the lobes, resolved by SDS-PAGE directly before (for POMC analysis) or after immunoprecipitation (for PC2 analysis), and visualized by fluorography. The amounts of newly synthesized 37-kDa POMC, 18-kDa POMC cleavage product, 75-kDa proPC2, and 69-kDa mature PC2 were quantified by densitometric scanning and are presented in arbitrary units (AUs), relative to the amount of 37-kDa POMC or of 75-kDa proPC2 produced during the pulse. Shown are the means ± SEM (n = 3, except n = 5 for the 2.5-h chase values). (B) Newly synthesized proteins produced by NILs of black-adapted wild-type (wt) and #124 transgenic animals during a 10-min pulse (lane 1) or during a 10-min pulse/2.5-h chase (lanes 2 and 3) were extracted from the lobes, resolved by SDS-PAGE on 12,5% gels, and visualized by fluorography. (C) Samples corresponding to B, lanes 2 and 3, were reloaded for the separation of newly synthesized 18-kDa POMC and 18-kDa POMC* by SDS-PAGE on 15% gels. (D) Samples corresponding to B, lanes 2 and 3, were subjected to HPLC analysis to separate the newly synthesized POMC-derived peptides des-N-α-acetyl-α-MSH (des-α-MSH), CLIPs, and endorphins. (E) The amounts of the five peptides (des-α-MSH, two CLIPs, and two endorphins) produced in the #124 transgenic cells were calculated on the basis of the HPLC profiles and are presented in AUs, relative to the corresponding peaks in the wild-type profile, showing a 30 ± 11% reduction in the #124 peptide amount (n = 4).
After conversion of 37-kDa POMC into the 18-kDa N-terminal POMC cleavage product, the remaining C-terminal half of the POMC molecule was processed further to a number of peptides that were analyzed by HPLC, namely, des-N-α-acetyl-α-MSH (the nonacetylated form of α-MSH), two corticotrophin-like intermediate lobe peptides (CLIPs), and two endorphins (Figure 6D). In the Xenopus melanotrope cells, des-N-α-acetyl-α-MSH is the major form of α-MSH and its acetylation occurs just before release, thereby making the acetylated form (α-MSH) the released (and more bioactive) product (Martens et al., 1981). HPLC analysis revealed that, after a 10-min pulse/2.5-h chase and relative to the peptides produced in wild-type melanotrope cells, the amounts of the small POMC cleavage products (des-N-α-acetyl-αMSH, CLIPs, and endorphins) were reduced in the #124 cells (Figure 6E). Thus, besides the production of a lower amount of 18-kDa POMC and the additional form of the 18-kDa POMC cleavage product (18-kDa POMC*), reduced amounts of the POMC-derived peptides were synthesized in the #124 transgenic cells.
DISCUSSION
The type I transmembrane p24 proteins are abundantly present in ER- and Golgi-derived transport vesicles and are therefore thought to play an important role in some aspect of cargo-selective transport through the early secretory pathway. The complex and dynamic behavior of this protein family has hampered functional analyses. In this study, we used the X. laevis intermediate pituitary melanotrope cell with one major secretory cargo protein (the prohormone POMC) and melanotrope cell-specific transgene expression of a GFP-tagged Xenopus p24 family member as a model to explore the importance of a functional p24 complex for a highly specialized secretory cell. Of the four abundant melanotrope p24 members upregulated with POMC (Xp24α3,-β1, -γ3, and -δ2) and thus likely somehow involved in the biosynthesis of the prohormone, Xp24δ2 was chosen for transgenic expression. The microscopy analyses revealed that the GFP-tag did not prevent the Xp24δ2-GFP fusion protein from reaching the Golgi. The two selected, independent transgenic lines 125 and 124 displayed moderate and high expression levels of the Xp24δ2-GFP fusion protein, respectively. From the Western blot and biosynthetic studies on the Xenopus p24δ proteins, we conclude that the level of newly synthesized Xp24δ2-GFP produced in the transgenic cells determined the degree of displacement of the endogenous Xp24δ2 and -δ1 proteins by the fusion protein. Thus, high levels of newly synthesized fusion protein, as produced in the #124 transgenic melanotrope cells, caused the near-absence of endogenous Xp24δ1 and -δ2. Due to the lower level of transgene expression in the #125 cells, substantial amounts of the endogenous Xp24δ proteins were still present, albeit at lower steady-state levels than in the wild-type cells. In the #124 cells, the high level of Xp24δ2-GFP effectively displaced not only the endogenous Xp24δ proteins, but also the normally abundant Xp24α3, -β1, and -γ3 family members such that the resulting p24 system consisted mainly of the transgene product. It therefore seems that the number of ER/Golgi subcompartments that can harbor p24 proteins is limited and that the relative amounts of the various newly synthesized p24 family members expressed in a cell determine the final composition of the p24 machinery in the early secretory pathway (by a “displacement effect”). In transiently transfected cells in culture, overexpression of a single p24 member resulted in aberrant ER structures (Rojo et al., 2000). Because our ultrastructural analysis did not reveal gross morphological changes, the level of transgene expression may have been relatively less than the amount of exogenous p24 produced in the transfected cells and thus to an extent that did not destroy the early secretory pathway in the transgenic Xenopus melanotrope cells.
Of special interest was that the number and sizes of the melanophores in the skin of the #124 transgenic animals were clearly reduced and as a result, these animals were not able to fully adapt to a black background. Because in Xenopus the intermediate pituitary melanotrope cells regulate skin melanophores, the phenotype of the #124 animal urged us to investigate in detail the functioning of the transgenic melanotropes. From the Western blot analyses of POMC and the POMC cleavage enzyme PC2, it seemed that the steady-state levels of these proteins were similar in the wild-type and transgenic melanotrope cells. We then examined the dynamics of protein biosynthesis and for this study we focused on the major newly synthesized secretory cargo protein POMC, its well-defined processing products and PC2. The results of the in vitro metabolic cell labeling studies suggested that in the #124 transgenic melanotrope cells the distortion of the endogenous p24 complex did not affect the level of POMC and proPC2 biosynthesis. However, relative to the wild-type melanotrope cells, the transgenic cells produced lower amounts of newly synthesized 18-kDa POMC, of the newly synthesized peptides derived from POMC (des-N-α-acetyl-αMSH, CLIPs, and endorphins) and of newly synthesized mature PC2. This effect may have been caused by a lower rate of transport of newly synthesized POMC and proPC2 through the secretory pathway, resulting in a lower rate of precursor protein processing and the observed reduced amounts of the newly synthesized precursor-derived peptides produced within the time frame of the pulse-chase experiments. Alternatively, the distorted p24 system may have exerted a more direct effect on the POMC processing event itself, e.g., because it failed to provide the proper processing conditions in the various secretory pathway subcompartments. In considering such a role in processing, a recently proposed model for ER- to-Golgi cargo transport is of special interest (Mironov et al., 2003). According to this model, which was based on the results of high-resolution morphological studies, secretory proteins would exit the ER by bulk flow in large transport carriers emerging from specialized ER exit sites, and this process would not involve budding and fusion of COPII-coated vesicles. In adjacent COPII-coated exit sites, a specific set of “machinery proteins” would be recruited and subsequently incorporated into the outgoing secretory cargo-containing carrier, e.g., for providing the correct lumenal environment in the carrier (Mironov et al., 2003). Because of their well-established ability to bind COPII (Fiedler et al., 1996; Nickel et al., 1997; Dominguez et al., 1998), p24 proteins may be involved in the COPII-dependent targeting of the machinery proteins to the secretory cargo transport carriers. In view of the results from our transgenic studies, the proteins recruited by p24/COPII could include components of the biosynthetic machinery that are needed for proper prohormone processing. Thus, in the #124 transgenic Xenopus melanotrope cells with the severely distorted p24 system, the set of machinery proteins incorporated into the outgoing POMC-containing carriers may be incomplete and these cells would therefore lack a fully functional POMC processing system.
The observation that the #124 pigment-containing skin cells were found only in the vicinity of blood vessels suggests that these transgenic animals have a shortage of the factor(s) responsible for the signaling to these cells. The reduced size and pigment content of the melanophores may be attributed to the lower amount of intermediate pituitary α-MSH, the POMC-derived peptide with a well-established role in background adaptation of amphibians by causing both the dispersion and synthesis of melanin in dermal melanophores (Hadley et al., 1981). An intriguing explanation for the lower number of skin melanophores in the #124 animal concerns the 18-kDa POMC cleavage product. The N-terminal 52 amino acids of the mammalian counterpart of Xenopus 18-kDa POMC (16-kDa POMC, also named pro-γ-MSH), resulting from a postsecretional cleavage of 16-kDa POMC by a serine protease localized on the target cell membrane, has been found to act as a growth factor (Bicknell et al., 2001). Hence, a deficit in normal melanotrope 18-kDa POMC may have resulted in insufficient mitogenic activity to produce normal quantities of skin melanophores in the #124 transgenic animal.
Together, our results are most consistent with a role for p24 in the transport of newly synthesized secretory cargo proteins through the early stages of the secretory pathway or in the processing of secretory cargo by recruiting the proper components of the biosynthetic machinery into ER- to-Golgi cargo transport carriers. Furthermore, our transgenic approach in a physiological context has shown that distortion of the complex p24 system results in an affected profile of prohormone-derived bioactive peptides with the eventual consequence at the level of the target cell of the secretory signals.
Acknowledgments
We thank Ron Engels for animal care; Tony Coenen, Huib Croes, and Coen Van der Meij for technical assistance; Drs. Roland Kuiper and Jutta Rötter for helpful discussions; and Drs. Wiljan Hendriks and Bruce Jenks for critical reading of the manuscript. We also thank Drs. Irene Schulz, Tommy Nilsson, Wim Van deVen, Jack Fransen, and Shige Tanaka for providing antibodies. This work was supported by grant 811.38.002 from the Netherlands Organization for Scientific Research-Earth and Life Sciences.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-08-0600. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-08-0600.
References
- Ausubel, F.M., Brent, R., Kingston, R.E., Moore, D.D., Seidman, J.G., Smith, J.A., and Struhl, K. (eds.) (2001). Current Protocols in Molecular Biology, New York: John Wiley & Sons. 2.2.1-2.2.3.
- Barlowe, C. (1998). COPII and selective export from the endoplasmic reticulum. Biochim. Biophys. Acta 1404, 67-76. [DOI] [PubMed] [Google Scholar]
- Barlowe, C. (2000). Traffic COPs of the early secretory pathway. Traffic 1, 371-377. [DOI] [PubMed] [Google Scholar]
- Belden, W.J., and Barlowe, C. (2001). Deletion of yeast p24 genes activates the unfolded protein response. Mol. Biol. Cell 12, 957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghs, C.A., Tanaka, S., Van Strien, F.J., Kurabuchi, S., and Roubos, E.W. (1997). The secretory granule and pro-opiomelanocortin processing in Xenopus melanotrope cells during background adaptation. J. Histochem. Cytochem. 45, 1673-1682. [DOI] [PubMed] [Google Scholar]
- Bicknell, A.B., Lomthaisong, K., Woods, R.J., Hutchinson, E.G., Bennett, H.P., Gladwell, R.T., and Lowry, P.J. (2001). Characterization of a serine protease that cleaves pro-γ-melanotropin at the adrenal to stimulate growth. Cell 105, 903-912. [DOI] [PubMed] [Google Scholar]
- Blum, R., Pfeiffer, F., Feick, P., Nastainczyk, W., Kohler, B., Schafer, K.H., and Schulz, I. (1999). Intracellular localization and in vivo trafficking of p24A and p23. J. Cell Sci. 112, 537-548. [DOI] [PubMed] [Google Scholar]
- Braks, J.A., and Martens, G.J.M. (1994). 7B2 is a neuroendocrine chaperone that transiently interacts with prohormone convertase PC2 in the secretory pathway. Cell 78, 263-273. [DOI] [PubMed] [Google Scholar]
- Bremser, M., Nickel, W., Schweikert, M., Ravazzola, M., Amherdt, M., Hughes, C.A., Sollner, T.H., Rothman, J.E., and Wieland, F.T. (1999). Coupling of coat assembly and vesicle budding to packaging of putative cargo receptors. Cell 96, 495-506. [DOI] [PubMed] [Google Scholar]
- Ciufo, L.F., and Boyd, A. (2000). Identification of a lumenal sequence specifying the assembly of Emp24p into p24 complexes in the yeast secretory pathway. J. Biol. Chem. 275, 8382-8388. [DOI] [PubMed] [Google Scholar]
- Cuppen, E., Wijers, M., Schepens, J., Fransen, J., Wieringa, B., and Hendriks, W. (1999). A FERM domain governs apical confinement of PTP-BL in epithelial cells. J. Cell Sci. 112, 3299-3308. [DOI] [PubMed] [Google Scholar]
- de Rijk, E.P., Jenks, B.G., and Wendelaar Bonga, S.E. (1990). Morphology of the pars intermedia and the melanophore-stimulating cells in Xenopus laevis in relation to background adaptation. Gen. Comp. Endocrinol. 79, 74-82. [DOI] [PubMed] [Google Scholar]
- Denzel, A., Otto, F., Girod, A., Pepperkok, R., Watson, R., Rosewell, I., Bergeron, J.J., Solari, R.C., and Owen, M.J. (2000). The p24 family member p23 is required for early embryonic development. Curr. Biol. 10, 55-58. [DOI] [PubMed] [Google Scholar]
- Dominguez, M., Dejgaard, K., Füllekrug, J., Dahan, S., Fazel, A., Paccaud, J.P., Thomas, D.Y., Bergeron, J.J., and Nilsson, T. (1998). gp25l/emp24/p24 protein family members of the cis-Golgi network bind both Cop I and II coatomer. J. Cell Biol. 140, 751-765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elrod Erickson, M.J., and Kaiser, C.A. (1996). Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol. Biol. Cell 7, 1043-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery, G., Rojo, M., and Gruenberg, J. (2000). Coupled transport of p24 family members. J. Cell Sci. 113, 2507-2516. [DOI] [PubMed] [Google Scholar]
- Fiedler, K., Veit, M., Stamnes, M.A., and Rothman, J.E. (1996). Bimodal interaction of coatomer with the p24 family of putative cargo receptors. Science 273, 1396-1399. [DOI] [PubMed] [Google Scholar]
- Fransen, J.A., Ginsel, L.A., Hauri, H.P., Sterchi, E., and Blok, J. (1985). Immuno-electronmicroscopical localization of a microvillus membrane disaccharidase in the human small-intestinal epithelium with monoclonal antibodies. Eur. J. Cell Biol. 38, 6-15. [PubMed] [Google Scholar]
- Füllekrug, J., Suganuma, T., Tang, B.L., Hong, W., Storrie, B., and Nilsson, T. (1999). Localization and recycling of gp27 (hp24γ3): complex formation with other p24 family members. Mol. Biol. Cell 10, 1939-1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley, M.E., Heward, C.B., Hruby, V.J., Sawyer, T.K., and Yang, Y.C. (1981). Biological actions of melanocyte-stimulating hormone. CIBA Found. Symp. 81, 244-262. [DOI] [PubMed] [Google Scholar]
- Holthuis, J.C., Jansen, E.J.R., van Riel, M.C., and Martens, G.J.M. (1995a). Molecular probing of the secretory pathway in peptide hormone-producing cells. J. Cell Sci. 108, 3295-3305. [DOI] [PubMed] [Google Scholar]
- Holthuis, J.C., van Riel, M.C., and Martens, G.J.M. (1995b). Translocon-associated protein TRAP δ and a novel TRAP-like protein are coordinately expressed with pro-opiomelanocortin in Xenopus intermediate pituitary. Biochem. J. 312, 205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen, E.J.R., Holling, T.M., van Herp, F., and Martens, G.J.M. (2002). Transgene-driven protein expression specific to the intermediate pituitary melanotrope cells of Xenopus laevis. FEBS Lett. 516, 201-207. [DOI] [PubMed] [Google Scholar]
- Jenks, B.G., de Koning, H.P., Valentijn, K., and Roubos, E.W. (1993). Dual action of GABAA receptors on the secretory process of melanotrophs of Xenopus laevis. Neuroendocrinology 58, 80-85. [DOI] [PubMed] [Google Scholar]
- Jenks, B.G., Overbeeke, A.P., and McStay, B.F. (1977). Synthesis, storage and release of MSH in the pars intermedia of the pituitary gland of Xenopus laevis during background adaptation. Can. J. Zool. 55, 922-927. [Google Scholar]
- Jenne, N., Frey, K., Brügger, B., and Wieland, F.T. (2002). Oligomeric state and stoichiometry of p24 proteins in the early secretory pathway. J. Biol. Chem. 277, 46504-46511. [DOI] [PubMed] [Google Scholar]
- Kaiser, C. (2000). Thinking about p24 proteins and how transport vesicles select their cargo. Proc. Natl. Acad. Sci. USA 97, 3783-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll, K.L., and Amaya, E. (1996). Transgenic Xenopus embryos from sperm nuclear transplantations reveal FGF signaling requirements during gastrulation. Development 122, 3173-3183. [DOI] [PubMed] [Google Scholar]
- Kuiper, R.P., Bouw, G., Janssen, K.P.C., Rötter, J., van Herp, F., and Martens, G.J.M. (2001). Localization of p24 putative cargo receptors in the early secretory pathway depends on the biosynthetic activity of the cell. Biochem. J. 360, 421-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper, R.P., Waterham, H.R., Rötter, J., Bouw, G., and Martens, G.J.M. (2000). Differential induction of two p24δ putative cargo receptors upon activation of a prohormone-producing cell. Mol. Biol. Cell 11, 131-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavoie, C., Paiement, J., Dominguez, M., Roy, L., Dahan, S., Gushue, J.N., and Bergeron, J.J. (1999). Roles for α(2)p24 and COPI in endoplasmic reticulum cargo exit site formation. J. Cell Biol. 146, 285-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, G.J.M. (1986). Expression of two proopiomelanocortin genes in the pituitary gland of Xenopus laevis: complete structures of the two preprohormones. Nucleic Acids Res. 14, 3791-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, G.J.M., Biermans, P.P., Jenks, B.G., and Van Overbeeke, A.P. (1982a). Biosynthesis of two structurally different pro-opiomelanocortins in the pars intermedia of the amphibian pituitary gland. Eur. J. Biochem. 126, 17-22. [DOI] [PubMed] [Google Scholar]
- Martens, G.J.M., Jenks, B.G., and Overbeeke, A.P. (1981). N α-acetylation is linked to α-MSH release from pars intermedia of the amphibian pituitary gland. Nature 294, 558-560. [DOI] [PubMed] [Google Scholar]
- Martens, G.J.M., Jenks, B.G., and Van Overbeeke, A.P. (1982b). Biosynthesis of a γ3-melanotropin-like peptide in the pars intermedia of the amphibian pituitary gland. Eur. J. Biochem. 126, 23-28. [DOI] [PubMed] [Google Scholar]
- Marzioch, M., Henthorn, D.C., Herrmann, J.M., Wilson, R., Thomas, D.Y., Bergeron, J.J., Solari, R.C., and Rowley, A. (1999). Erp1p and Erp2p, Partners for Emp24p and Erv25p in a Yeast p24 Complex. Mol. Biol. Cell 10, 1923-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov, A.A., et al. (2003). ER-to-Golgi carriers arise through direct en bloc protrusion and multistage maturation of specialized ER exit domains. Dev. Cell 5, 583-594. [DOI] [PubMed] [Google Scholar]
- Muñiz, M., Nuoffer, C., Hauri, H.P., and Riezman, H. (2000). The Emp24 complex recruits a specific cargo molecule into endoplasmic reticulum-derived vesicles. J. Cell Biol. 148, 925-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickel, W., Sohn, K., Bunning, C., and Wieland, F.T. (1997). p23, a major COPI-vesicle membrane protein, constitutively cycles through the early secretory pathway. Proc. Natl. Acad. Sci. USA 94, 11393-11398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojo, M., Emery, G., Marjomaki, V., McDowall, A.W., Parton, R.G., and Gruenberg, J. (2000). The transmembrane protein p23 contributes to the organization of the Golgi apparatus. J. Cell Sci. 113, 1043-1057. [DOI] [PubMed] [Google Scholar]
- Rojo, M., Pepperkok, R., Emery, G., Kellner, R., Stang, E., Parton, R.G., and Gruenberg, J. (1997). Involvement of the transmembrane protein p23 in biosynthetic protein transport. J. Cell Biol. 139, 1119-1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rötter, J., Kuiper, R.P., Bouw, G., and Martens, G.J.M. (2002). Cell-typespecific and selectively induced expression of members of the p24 family of putative cargo receptors. J. Cell Sci. 115, 1049-1058. [DOI] [PubMed] [Google Scholar]
- Roubos, E.W. (1997). Background adaptation by Xenopus laevis: a model for studying neuronal information processing in the pituitary pars intermedia. Comp. Biochem. Physiol. A. Physiol. 118, 533-550. [DOI] [PubMed] [Google Scholar]
- Schimmöller, F., Singer Krüger, B., Schröder, S., Krüger, U., Barlowe, C., and Riezman, H. (1995). The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO J. 14, 1329-1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer, A., Fransen, J.A., Bachi, T., Ginsel, L., and Hauri, H.P. (1988). Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulo-vesicular compartment at the cis-side of the Golgi apparatus. J. Cell Biol. 107, 1643-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn, K., Orci, L., Ravazzola, M., Amherdt, M., Bremser, M., Lottspeich, F., Fiedler, K., Helms, J.B., and Wieland, F.T. (1996). A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J. Cell Biol. 135, 1239-1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow, D.B., Latinkic, B., and Mohun, T.J. (2000). A simplified method of generating transgenic Xenopus. Nucleic Acids Res. 28, E12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer, S., Chen, E., Duden, R., Marzioch, M., Rowley, A., Hamamoto, S., Merchant, S., and Schekman, R. (2000). The p24 proteins are not essential for vesicular transport in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97, 4034-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes, M.A., Craighead, M.W., Hoe, M.H., Lampen, N., Geromanos, S., Tempst, P., and Rothman, J.E. (1995). An integral membrane component of coatomer-coated transport vesicles defines a family of proteins involved in budding. Proc. Natl. Acad. Sci. USA 92, 8011-8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuinhof, R., Artero, C., Fasolo, A., Franzoni, M.F., Ten Donkelaar, H.J., Wismans, P.G., and Roubos, E.W. (1994). Involvement of retinohypothalamic input, suprachiasmatic nucleus, magnocellular nucleus and locus coeruleus in control of melanotrope cells of Xenopus laevis: a retrograde and anterograde tracing study. Neuroscience 61, 411-420. [DOI] [PubMed] [Google Scholar]
- Van Horssen, A.M., Van Kuppeveld, F.J., and Martens, G.J.M. (1998). Manipulation of disulfide bonds differentially affects the intracellular transport, sorting, and processing of neuroendocrine secretory proteins. J. Neurochem. 71, 402-409. [DOI] [PubMed] [Google Scholar]
- Weatherhead, B., Thornton, V.F., and Whur, P. (1971). Effects of change of background colour on the ultrastructure of the `MSH cells' of the pars intermedia of Xenopus laevis. J. Endocrinol. 49, 25-26. [PubMed] [Google Scholar]
- Wen, C., and Greenwald, I. (1999). p24 proteins and quality control of LIN-12 and GLP-1 trafficking in Caenorhabditis elegans. J. Cell Biol. 145, 1165-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]