Abstract
High nitrogen (N) supply frequently results in a decreased photosynthetic N-use efficiency (PNUE), which indicates a less efficient use of accumulated Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco). Chloroplasts are the location of Rubisco and the endpoint of CO2 diffusion, and they play a vital important role in photosynthesis. However, the effects of chloroplast development on photosynthesis are poorly explored. In the present study, rice seedlings (Oryza sativa L., cv. ‘Shanyou 63’, and ‘Yangdao 6’) were grown hydroponically with three different N levels, morphological characteristics, photosynthetic variables and chloroplast size were measured. In Shanyou 63, a negative relationship between chloroplast size and PNUE was observed across three different N levels. Here, plants with larger chloroplasts had a decreased ratio of mesophyll conductance (gm) to Rubisco content (gm/Rubisco) and a lower Rubisco specific activity. In Yangdao 6, there was no change in chloroplast size and no decline in PNUE or gm/Rubisco ratio under high N supply. It is suggested that large chloroplasts under high N supply is correlated with the decreased Rubisco specific activity and PNUE.
Introduction
The high grain yields of most crops are dependent upon the supply of nitrogen (N) from fertilizers. The increasing cost and high energy requirement of such fertilizer, together with the adverse environmental effects of N pollution have stimulated much research activity that aiming towards enhancing the efficiency of its use. An important variable is the intrinsic N-use efficiency (NUE) in plants. A key component of NUE is the photosynthetic N-use efficiency (PNUE), defined as net photosynthetic rate (A) per unit leaf N content. Approximately 75% of N is allocated to chloroplasts [1], [2] and about 27% of this is in Ribulose–1,5–bisphosphate carboxylase/oxygenase (Rubisco) [3], [4], which carries out the primary fixation of CO2 in the Benson-Calvin cycle. Thus, Rubisco plays a pivotal role in PNUE as a major repository of N and an enzyme that limits photosynthetic rate under various conditions.
Due to the low concentration of CO2 in the atmosphere and the low affinity for CO2, the catalytic effectiveness of Rubisco is poor under ambient conditions [4]–[7]. Rubisco may operate significantly below its potential catalytic capacity in C3 plants, suggesting that under high N supply or in high N content leaves, there is excess Rubisco protein serving only as a N storage and not contributing to photosynthesis [8]–[12], especially under limiting light. The lower relative Rubisco activity in high N content leaves may thus contribute to a decreased PNUE in such leaves.
In full sunlight, photosynthesis in C3 plants is mainly limited by Rubisco activity [11], [13], [14]. Rubisco activity is related to CO2 concentration in chloroplasts [15], and therefore it has been suggested that the decreased Rubisco activity in high N content leaves is due to an insufficient supply of CO2 [16]. In the diffusion pathway from atmosphere to chloroplasts, CO2 diffuses across a boundary layer above the leaf surface, and then through the stomata into the substomatal cavity. In the substomatal cavity, CO2 dissolves in the water-filled pores of the cell wall and then diffuses through the cell wall, the plasma membrane, the cytosol, and the chloroplast envelope to enter the chloroplast. The rate of CO2 diffusion from the intercellular spaces to the carboxylation sites in chloroplasts is referred to as the mesophyll conductance, gm. It has been demonstrated that gm markedly limits chloroplast CO2 concentration relative to intercellular CO2 concentration (Ci) [17]–[20].
It is thought that chloroplast size would probably affect gm [21]. The conductance in the liquid phase in mesophyll cells is the dominant component of gm [17], [22], especially the conductance through the inner chloroplast envelope membrane, which constitutes about one half of total internal resistance [23]. Thus, gm depends upon the conductance per unit of chloroplast surface area and the surface area of chloroplasts facing the intercellular air spaces [22]. Larger chloroplasts are usually correlated with higher N content [24] and would potentially increase gm [25]. A larger chloroplast would also store more leaf N and Rubisco. However, it is not clear whether the extent of the increase in gm is sufficient to provide enough CO2 for activating the increased amount of Rubisco, and thus whether an imbalance between the increases in gm and in Rubisco content contributes to the decrease in PNUE observed in high N leaves.
Few studies have specifically investigated the relationship between chloroplast ultrastructure and PNUE that aiming at testing whether larger chloroplasts are related to lowered Rubisco activity and PNUE. We have studied the responses of two rice varieties that respond differently to N supply and provide evidence that links changes in chloroplast size with a deficiency in gm that can explain reduced PNUE and Rubisco activity. Hence, we propose a novel explanation for decreased PNUE under high N supply, and suggest an approach to plant breeding to increase N productivity.
Results
Growth response to N supply
The response of both Shanyou 63 and Yangdao 6 to the N supply was as predicted (Table 1). Increases in plant biomass were observed at high N in both cases. There was a decrease in root mass ratio (RMR, = root biomass /whole plant biomass), and an increase in leaf mass ratio (LMR, = leaf biomass /whole plant biomass) in both varieties with increasing N supply. Leaf sheath and culm mass ratio (SCMR, = leaf sheath and culm biomass/whole plant biomass) was unresponsive to N supply, except for a decrease under high N supply in Yangdao 6. SLW was also unresponsive to N supply, indicating no alterations in leaf thickness.
Table 1. Growth variables of rice seedlings grown at different N supplies.
| Variables | Shanyou 63 | Yangdao 6 | ||||
| Low-N | Int-N | High-N | Low-N | Int-N | High-N | |
| Plant dry mass | 2.92±0.73b | 4.27±0.60a | 4.89±0.49a | 1.80±0.18c | 2.21±0.45b | 3.21±.082a |
| (g plant−1) | ||||||
| RMR | 0.27±0.02a | 0.19±0.01b | 0.12±0.02c | 0.27±0.02a | 0.22±0.03b | 0.15±0.01c |
| (g plant−1) | ||||||
| SCMR | 0.48±0.02a | 0.51±0.02a | 0.48±0.04a | 0.47±0.01a | 0.47±0.04a | 0.40±0.03b |
| (g plant−1) | ||||||
| LMR | 0.26±0.01c | 0.30±0.02b | 0.41±0.02a | 0.26±0.01c | 0.31±0.02b | 0.45±0.02a |
| (g plant−1) | ||||||
| leaf area | 256±53c | 444±80b | 668±44a | 162±13c | 235±42b | 494±116a |
| (cm2 plant−1) | ||||||
| SLW | 28.98±2.73a | 29.23±1.15a | 29.87±2.02a | 29.19±1.46a | 29.19±2.06a | 29.29±1.97a |
| (g m−2) | ||||||
Rice plants (cv. Shanyou 63 and Yangdao 6) were supplied with N at three different levels (low: 20 mg L−1 N, intermediate: 40 mg L−1 N, and high: 100 mg L−1 N). Data are means ± SD of 5 individual plants. Variables were determined 40 days after the start of treatment.
Notes: Significant differences (P<5%) between N supplies or varieties were indicated by different lowercase letters or different uppercase letters, respectively. RMR, SCMR and LMR represent root mass ratio, leaf sheath and culm mass ratio and leaf mass ratio, respectively. They were calculated as the ratio of separate dry mass to whole plant dry mass. SLW represents specific leaf weight, and was calculated as the ratio of leaf fresh weight to leaf area.
Photosynthetic variables
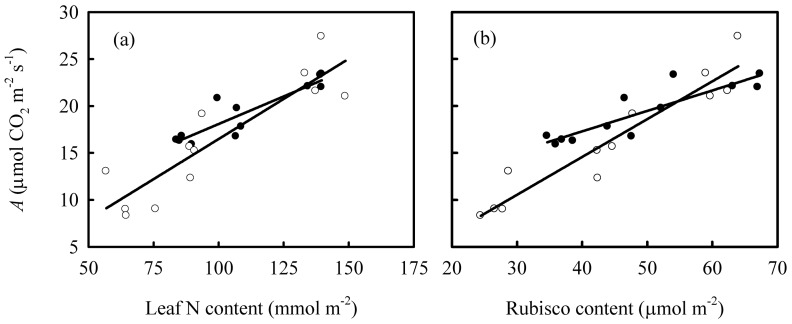
In both rice cultivars, A, N, NO3 − and relative Rubisco content were higher under high N supply compared with low N supply (Table 2). However, the responses of these varieties differed markedly when other variables were measured. Most importantly, PNUE (calculated as A/N) decreased with increasing N supply in Shanyou 63, but did not change significantly in Yangdao 6. The same trends (decrease in Shanyou 63 and no change in Yangdao 6) were observed in A/Rubisco. This phenomenon can also be observed from the relationships between A and leaf N content, and relative Rubisco content (Fig. 1). A/N and A/Rubisco were much lower in high N or Rubisco content leaves in Shanyou 63, with no significant decrease in Yangdao 6 (Fig. 1). Similarly, with increasing N supply, both the initial and maximum Rubisco activities were lower in Shanyou 63, but there were no significant differences in Yangdao 6.
Table 2. Effects of N supply level on leaf photosynthesis in rice seedlings.
| Variables | Shanyou 63 | Yangdao 6 | ||||
| Low-N | Int.-N | High-N | Low-N | Int.-N | High-N | |
| A (µmol CO2 m−2 s−1) | 16.32±0.09b | 18.76±2.88ab | 22.68±0.86a | 10.33±2.32c | 15.56±2.80b | 23.36±2.89a |
| gs (mol CO2 m−2 s−1) | 0.16±0.03a | 0.19±0.04a | 0.18±0.03a | 0.10±0.04b | 0.13±0.02b | 0.21±0.03a |
| gm (Harley) | 0.18±0.05b | 0.21±0.07ab | 0.25±0.03a | 0.11±0.03c | 0.18±0.02b | 0.26±0.05a |
| (mol CO2 m−2 s−1) | ||||||
| gm (Ethier) | 0.21±0.05b | 0.24±0.02ab | 0.28±0.04a | 0.13±0.01c | 0.19±0.03b | 0.28±0.08a |
| (mol CO2 m−2 s−1) | ||||||
| Г* (µmol CO2 mol−1) | 38.29±0.24b | 40.80±1.05ab | 42.26±1.89a | 38.53±0.68b | 39.91±1.34ab | 42.58±1.23a |
| Rd (µmol CO2 m−2 s−1) | 1.00±0.05a | 0.67±0.026b | 0.61±0.06b | 1.37±0.06a | 1.23±0.17a | 0.83±0.09b |
| N (mmol m−2) | 87.14±0.93c | 106.43±4.14b | 143.57±0.50a | 61.43±4.29c | 92.14±2.86b | 139.29±8.57a |
| NO3 − (mmol m−2) | 0.20±0.02b | 0.27±0.07b | 0.60±0.06a | 0.20±0.06b | 0.30±0.07b | 0.42±0.04a |
| Relative Rubisco content | 35.89±2.32c | 46.79±2.32b | 61.79±4.29a | 26.61±2.86c | 40.89±2.68b | 60.71±4.11a |
| (µmol m−2) | ||||||
| Chloroplast length | 4.20±0.59b | 4.69±0.62b | 5.55±0.82a | 4.51±0.61a | 4.72±0.63a | 4.87±0.67a |
| (µm) | ||||||
| Chloroplast thickness | 1.84±0.33c | 2.34±0.34b | 3.03±0.49a | 2.40±0.44a | 2.30±0.45a | 2.33±0.46a |
| (µm) | ||||||
| Initial Rubisco activity | 1.05±0.12a | 0.87±0.04b | 0.67±0.10c | 0.69±0.11a | 0.77±0.21a | 0.83±0.18a |
| (mol mol−1 Rubisco s−1) | ||||||
| Max Rubisco activity | 1.41±0.13a | 1.29±0.13ab | 1.07±0.23b | 1.31±0.36a | 1.38±0.25a | 1.17±0.06a |
| (mol mol−1 Rubisco s−1) | ||||||
| A/Rubisco | 0.45±0.03a | 0.40±0.04ab | 0.36±0.05b | 0.36±0.06a | 0.35±0.05a | 0.38±0.04a |
| (mol CO2 mol−1 Rubisco s−1) | ||||||
| PNUE (A/N) | 190±9a | 178±23ab | 164±5b | 154±51a | 171±27a | 168±24a |
| (µmol CO2 mol−1 N s−1) | ||||||
| Chloroplast volume | 7.44 | 13.44 | 26.67 | 13.59 | 13.07 | 13.84 |
| (Vchl, µm3) | ||||||
| Chloroplast surface area | 18.43 | 27.33 | 43.16 | 27.54 | 26.82 | 27.87 |
| (Schl, µm2) | ||||||
| gm/Rubisco (Harley) | 5.02 | 4.49 | 4.05 | 4.13 | 4.4 | 4.28 |
| (mol CO2 mmol−1 Rubisco s−1) | ||||||
| gm/Rubisco (Ethier) | 5.85 | 5.13 | 4.53 | 4.89 | 4.65 | 4.61 |
| (mol CO2 mmol−1 Rubisco s−1) | ||||||
Rice plants (cv. Shanyou 63 and Yangdao 6) were supplied with N at three different levels (low: 20 mg L−1 N, intermediate: 40 mg L−1 N, and high: 100 mg L−1 N). Data are means ± SD of more than 20 individual chloroplasts for their length and thickness, and 5 individual plants for other variables.
Notes: Significant differences (P<5%) between N supplies or varieties were indicated by different lowercase letters or different uppercase letters, respectively. A, gs, gm, N, Rubisco and PNUE represent leaf photosynthetic rate, stomatal conductance to CO2, mesophyll conductance to CO2, leaf nitrogen content, leaf Rubisco content, photosynthetic N-use efficiency, respectively.
Figure 1. The relationships between leaf photosynthetic rate (A) and (a) leaf N content, and (b) Rubisco content and in Shanyou 63 (closed cycles) and Yangdao 6 (open cycles).
The lines represent the following regression equations: (a) y = 0.12x+6.33 R2 = 0.82 P<0.01 for Shanyou 63; y = 0.17x−0.60 R2 = 0.79 P<0.01 for Yangdao 6; (b) y = 0.22x+8.59R2 = 0.78 P<0.01 for Shanyou 63; y = 0.40x−1.53 R2 = 0.91 P<0.01.
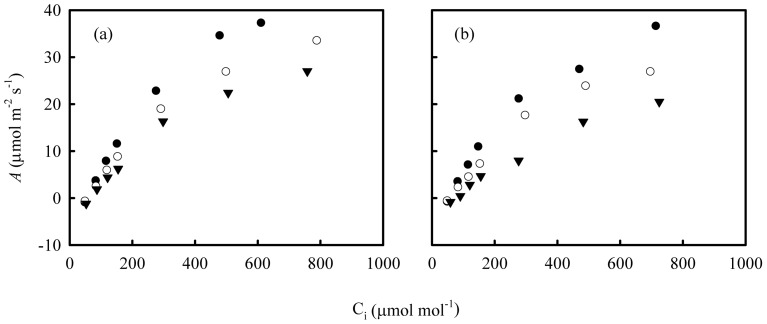
In Shanyou 63, stomatal conductance (gs) was independent of N supply, while in Yangdao 6 it increased at high N supply (Table 2). The values of gm were higher in high N in both varieties. The ratio gm/Rubisco declined markedly with increasing N supply in Shanyou 63 but remained constant in Yangdao 6. A/Ci response curves showed that photosynthesis was more responsive to N supply in Yangdao 6 than in Shanyou 63 (Fig. 2).
Figure 2. A/Ci response curves of newly expanded leaves in Shanyou 63 (a) and Yangdao 6 (b).
The symbols of solid cycles, open cycles and solid triangles represent high, intermediate and low N supply, respectively.
Chloroplast development
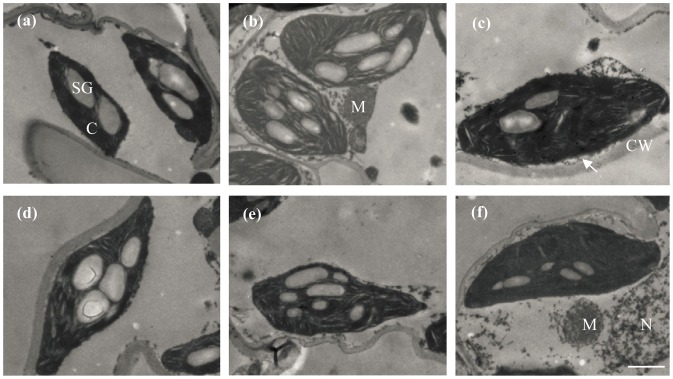
Chloroplast size also increased with increasing N supply but only in Shanyou 63, with no significant difference observed in Yangdao 6 (Table 2; Fig. 3). Chloroplast length (Lchl) was less sensitive than chloroplast thickness (Dchl) to N supply: Lchl increased by 32% compared to a 65% increase in Dchl. Single chloroplast volume (Vchl) and single chloroplast surface area (Schl) increased with increasing N supply in Shanyou 63, but Schl/Vchl decreased in high N supply. There were no significant differences in Vchl, Schl and Schl/Vchl among N supply levels in Yangdao 6 (Table 2). Compared with high N supply, chloroplasts in low N supply showed an accumulation of starch granules in both cultivars (Fig. 3).
Figure 3. Electron micrographs of chloroplasts in newly expanded leaves of Shanyou 63 (a, low-N supply; b, intermediate.-N supply; c, high-N supply) and Yangdao 6 (d, low-N supply; e, intermediate.-N supply; f, high-N supply).
Bar = 1 µm. C, chloroplasts; M, mitochondrion; N, nucleus; SG, starch granules; CW, cell wall; arrows point to plasma membrane.
Discussion
The effect of nitrogen supply on chloroplast development
As much as 75% of leaf N is invested to chloroplasts to synthesis photosynthetic apparatus, including thylakoid membranes and photosynthetic enzymes. Thereby, chloroplast development, such as chloroplast division, chloroplast grana and stroma lamellae stacking, is highly dependent on nitrogen supply. Sufficient N will significantly enlarge chloroplast size, increase chloroplast number, and enhance grana aggregation [26]–[29]. In the present study, chloroplasts were enlarged under high N supply in Shanyou 63, with chloroplast thickness more responsive than chloroplast length (Table 2 and Fig. 3). In contrast, chloroplast size, both chloroplast length and thickness, was insensitive to N supply in Yangdao 6 (Table 2 and Fig. 3).
Under full sunlight, photosynthetic assimilates should be translocated quickly out of chloroplasts to sites with high carbon sink activity to avoid starch granules formation, which will in turn inhibit leaf photosynthesis [28], [30]. It is reported that there are more and larger starch granules under high N supply for their high leaf photosynthetic capacity [27]; however, there are also numerous studies showed less and smaller starch granules under high N supply [26], [28], [30]. In the present study, starch granules were much larger under low N than under high N supply (Fig. 3). The reason is probably that high N supply can stimulate the translocation of assimilates from chloroplasts to sites with high carbon sink activity [26].
The relationship between Rubisco content and total chloroplast volume
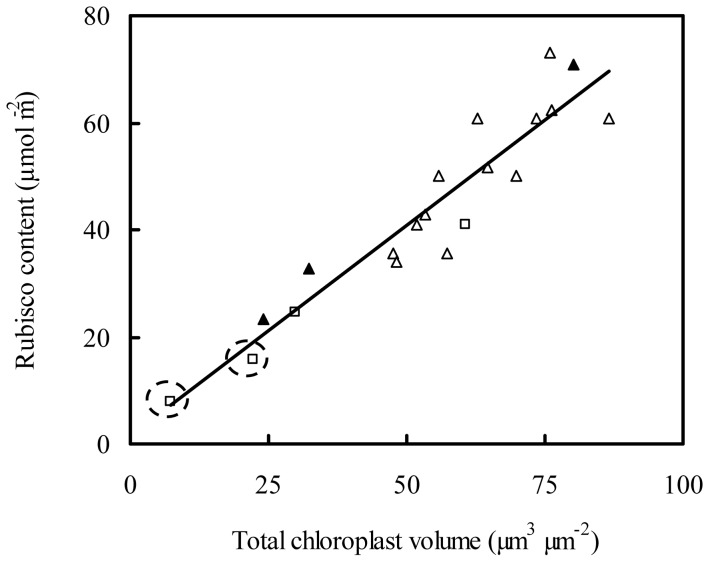
Although the level of Rubisco is sometimes excessive for photosynthesis [12], [19], [31], the increase in leaf N content at high N supply is generally accompanied by a higher Rubisco content. In the present study, this was observed in both the rice varieties, Shanyou 63 and Yangdao 6. A higher amount of Rubisco potentially be associated with either a larger total chloroplast volume (VT–chl) or a higher Rubisco concentration in chloroplasts (Rubchl). Analysis of data from a number of different plant species reveals a linear relationship between Rubisco content and VT–chl expressed on a leaf area basis (Fig. 4). This points to a constant value of Rubchl, at approximately 44 mg cm−3 (the slope of the regression equation in Fig. 4). Thus, the higher Rubisco content in high N leaves should be associated with a larger VT–chl, this hypothesis is also speculated by Evans et al. [32]. The increase in VT–chl could arise from an increase in either Vchl or chloroplast number per leaf area (nchl).
Figure 4. The relationship between Rubisco content and total chloroplast volume per leaf area.
The line represents the regression equation: y = 0.79x+1.36, R2 = 0.88, P<0.01. Data sources: Nicotiana tabacum □ [21]; Chenopodium album ▴ [48]; Aucuba japonica Thunb. △ [24]. The two data points in dotted cycles are from transgenic tobacco with a reduced Rubisco content.
In Yangdao 6, there was no difference in chloroplast size (Table 2 and Fig. 3), despite the higher Rubisco content in leaves with higher leaf N. In this case, it is suggested that the number of chloroplasts should increase. In contrast, in Shanyou 63, the thickness and the length of chloroplasts were larger in the high N leaves compared to those with low N supply, and the higher Rubisco content at high N supply in Shanyou 63 was hence associated with a larger chloroplast volume. Wider and longer chloroplasts not only have a larger Schl, but also a larger Vchl and a decreased Schl/Vchl compared to smaller ones.
The effect of chloroplast size on mesophyll conductance, gm
The total surface area of chloroplasts facing the intercellular space (Sc) is the key determinant of gm, rather than the total chloroplast surface area per se. Sc is given by Sc = α×Schl×nchl, where α is the ratio of chloroplast surface area facing the intercellular space to total chloroplast surface area. Sc is therefore also higher when there are larger chloroplasts, the higher Sc with larger chloroplasts would potentially increase gm. The increase in Sc is again not proportional to the increase in total chloroplast volume, VT–chl ( = Vchl×nchl) and therefore the ratio Sc/VT–chl (which can be given as (α×Schl×nchl)/(Vchl×nchl), and further as α×Schl/Vchl) also decreases in large chloroplasts (Table 2). In fact, α is also likely to decrease when chloroplasts are thicker, so amplifying the decrease in this ratio. Hence, it can be concluded that the increase in chloroplast size on Shanyou 63 would be associated with an increase in gm, while it would not be proportional to the increase in total chloroplast volume and Rubisco content (Table 2). In contrast, in Yangdao 6, where chloroplast size did not change, the increase in gm at high leaf N would be attributable to an increase in chloroplast number, with no change in the Sc/VT–chl ( = α×Schl/Vchl) and gm/Rubisco ratio (Table 2).
Recent studies demonstrated that photorespiration can efficiently affect the precision of gm estimation [33], [34]. But it is now still difficult for the methods of both simultaneous measurement of gas exchange and chlorophyll fluorescence, and A/Ci reponse curve-fitting method to exclude photorespiration’s effect. It should be clear that gm in the present study was estimated without eliminating photorespiration effects. In the present study, two independent methods were conducted to improve the accuracy for gm estimation. It should be proposed that, further efforts should be done to improve accuracy for gm estimation in the method of simultaneous measurement of gas exchange and chlorophyll fluorescence to continue its convenience in gm estimation.
It is illustrated that more than 95% of mesophyll cell periphery in rice plants is covered by chloroplasts in well-grown and N sufficient leaves [35]. So, when mesophyll surface is mainly covered by chloroplasts under high N supply, gm and photosynthesis would probably be insensitive to chloroplast development if Cliq is similar. This phenomenon was observed in the present study, where photosynthesis and gm were similar between the two cultivars under high N supply while chloroplast size was substantially larger in Shanyou 63 (Table 2). When less mesophyll surface is covered by chloroplasts under low N supply, small chloroplasts would be benefit for gm and photosynthesis because they can more efficiently cover mesophyll cell surface and enhance gm (Table 2).
Would chloroplast size affect PNUE at high N?
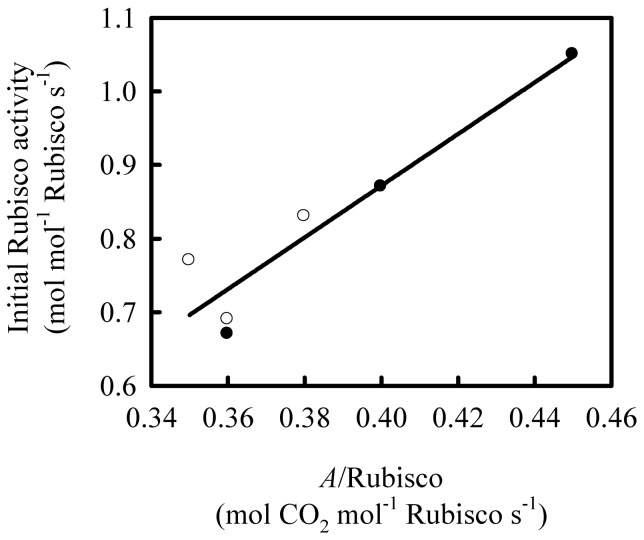
Rubisco activity can be measured under both in vivo and in vitro conditions. Because of different synthesis conditions, in vitro Rubisco activity can not always reveal its in vivo activity especially under drought stress [36]. Nevertheless, there are numerous studies showed the positive relationship between them [31], [37], [38]. The correlation was also checked in the present study (Fig. 5), and the positive relationship revealed that the slowed-down Rubisco turnover rate with increasing N supply in Shanyou 63 was probably the reason for the decreased A/Rubisco and PNUE.
Figure 5. The relationship between initial Rubisco activity and the ratio of leaf photosynthetic rate (A) to Rubisco content on Shanyou 63 (solid cycles) and Yangdao 6 (open cycles).
The line represents linear regression: y = 3.51x−0.53 R2 = 0.88 P<0.01.
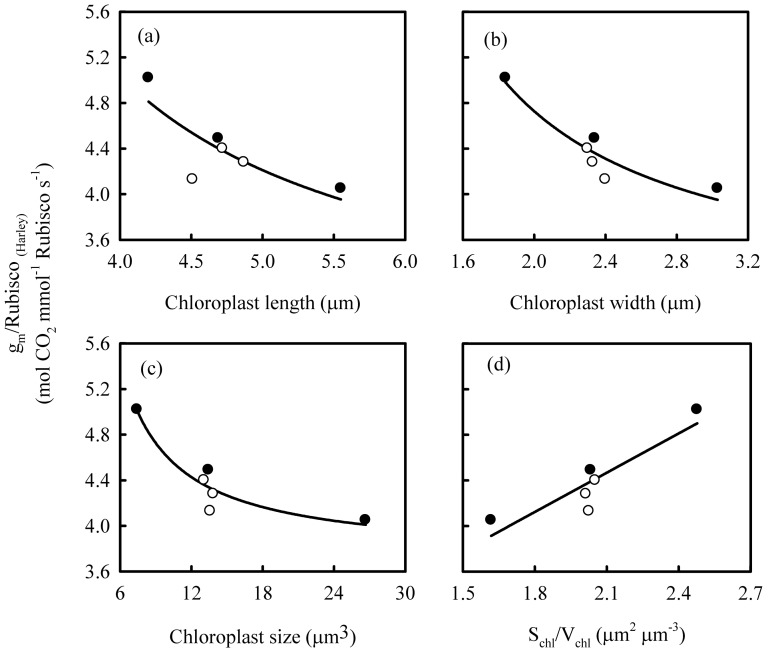
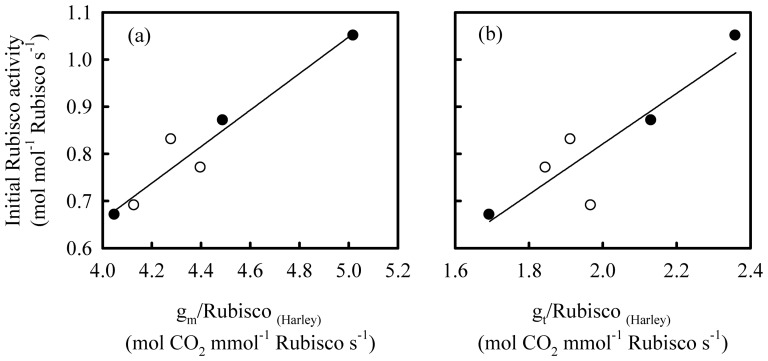
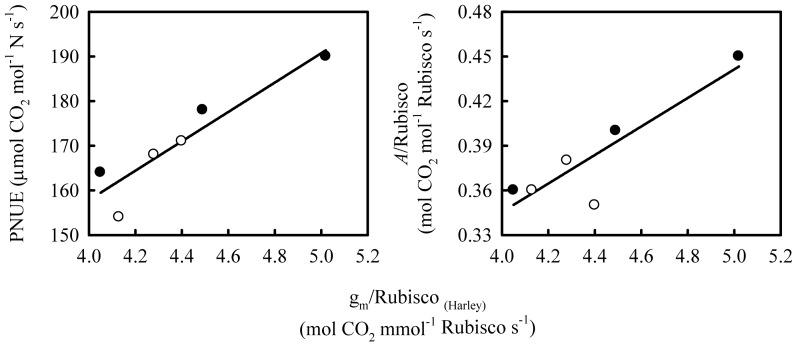
In large chloroplasts, the ratio of Sc to Rubisco content ( = Rubchl×VT–chl) is lower, and hence gm per unit Rubisco content also lower, compared to the values in smaller chloroplasts (Fig. 6). Because of the correlation between gm and total conductance (gt), responses of gt/Rubisco to chloroplast size were similar with those of gm/Rubisco (Data not shown). The lower gm/Rubisco and gt/Rubisco ratio would result in an insufficient supply of CO2 in chloroplasts and a consequent decrease in Rubisco activity (Fig. 7). A decrease in gm/Rubisco and a reduction in Rubisco activity at high leaf N were observed in Shanyou 63 but not in Yangdao 6 (Table 2). As a consequence, in the former variety, there was a decreased A/Rubisco and PNUE, whereas these variables were unchanged in the latter variety. Therefore, the lower gm/Rubisco ratio induced by chloroplast enlargement under high N supply in Shanyou 63 at least partially explains the lowered Rubisco efficiency and decline in PNUE (Fig. 8). However, in Yangdao 6, the constant gm/Rubisco would ensure a sufficient supply of CO2, and thus Rubisco efficiency and PNUE would not decrease under high N supply. It is suggested that whether PNUE decreases under high N would, at least partially, depends on the mechanism by which the Rubisco content increases i.e. whether through increasing Vchl or by increasing nchl. Thus, we hypothesize that a high N–dependent increase in chloroplast size would cause a decrease in gm per unit Rubisco that results in a fall in PNUE, an effect that does not occur if the response to high N is an increase in chloroplast number.
Figure 6. The relationships between chloroplast size and the ratio of mesophyll conductance (gm) to Rubisco content on Shanyou 63 (solid cycles) and Yangdao 6 (open cycles).
Chloroplast surface area (Schl) and volume (Vchl) were calculated from the Cesaro formula. The lines represent the following regressions: (a) y = 2.54x/(x−1.98) R2 = 0.63 P > 0.05; (b) y = 2.99x/(x−0.73) R2 = 0.88 P<0.01; (c) y = 3.72x/(x−1.91) R2 = 0.89 P<0.01; (d) y = 1.15x+2.06 R2 = 0.82 P<0.05.
Figure 7. The relationships between initial Rubisco activity and (a) the ratio of mesophyll conductance (gm) to Rubisco content, and (b) the ratio of total conductance (gt) to Rubisco content on Shanyou 63 (solid cycles) and Yangdao 6 (open cycles).
The lines represent the following regressions: (a) y = 0.39x−0.89 R2 = 0.93 P<0.01; (b) y = 0.54x−0.25 R2 = 0.80 P<0.05.
Figure 8. The relationships between the ratio of mesophyll conductance (gm) to Rubisco content and (a) photosynthetic N-use efficiency (PNUE) and (b) the ratio of leaf photosynthetic rate (A) and Rubisco content on Shanyou 63 (solid cycles) and Yangdao 6 (open cycles).
The lines represent the following regressions: (a) y = 32.89x+26.28 R2 = 0.86 P<0.01; (b) y = 0.096x−0.038 R2 = 0.80 P<0.05.
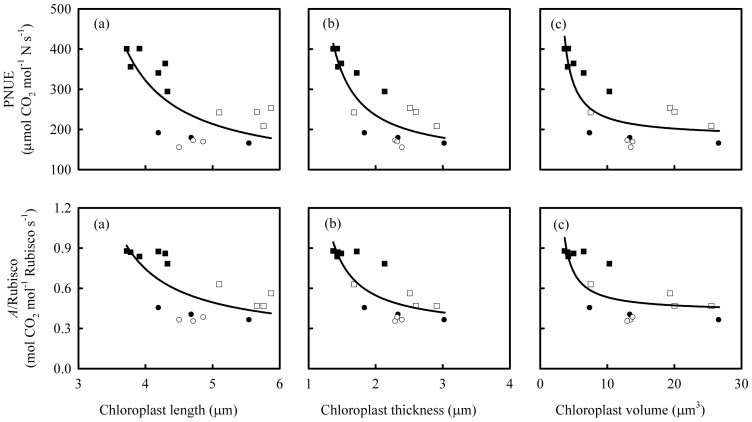
This hypothesis was tested for its general applicability in rice plants grown under different conditions (Fig. 9). The results showed that PNUE and A/Rubisco were negatively related to chloroplast size. The relationships were again stronger with chloroplast thickness than chloroplast length. With similar Rubisco content, leaves with smaller chloroplasts have a higher CO2 assimilation rate. Thus, the lack of balance between gm and Rubisco content as chloroplasts increase in size is a tenable explanation of observed decreases in Rubisco turnover rate and the lowered PNUE.
Figure 9. The relationships between chloroplast size and photosynthetic N-use efficiency (PNUE), and the ratio of leaf photosynthetic rate (A) to Rubisco.
The lines represent the following regression equations: (a) y = 91.04x/(x–2.87), R2 = 0.53, P<0.01; (b) y = 121.76x/(x–0.96), R2 = 0.75, P<0.01; (c) y = 180.56x/(x–2.12), R2 = 0.72, P<0.01; (d) y = 0.21x/(x–2.86), R2 = 0.53, P<0.01; (e) y = 0.29x/(x–0.95), R2 = 0.69, P<0.01; (f) y = 0.42x/(x–2.06), R2 = 0.63, P<0.01. Data sources: data of solid squares were collected from Wuyujing 3 (Oryza sativa L. ssp. japonica) with different N supplies; data of open squares were from Shanyou 63 with different N forms and water supply [42]; data of solid and open cycles were from Shanyou 63 and Yangdao 6 with different N supplies.
Materials and Methods
Plant material and growth conditions
After germination on moist filter paper, rice seeds (Oryza sativa L., ssp. indica hybrid, cv. ‘Shanyou 63’, and ssp. indica inbred, cv. ‘Yangdao 6’) were transferred to 2.0 mM CaSO4 for germination at 25±5°C. After 3 days, the rice seedlings were transferred to 6–L rectangular containers (30×20×10 cm) and ¼-strength nutrient solution (for composition, see below). Three days later, the seedlings were transferred to a ½-strength nutrient solution, and after 5 days, the seedlings were supplied with full–strength nutrient solution for 1 week. Seedlings were supplied with nutrient solution containing three different N levels: low N (20 mg L−1), intermediate N (40 mg L−1), and high N (100 mg L−1). The N sources were equimolar amounts of (NH4)2SO4 and Ca(NO3)2. In addition, the macronutrients in the solution were as follows (mg L−1): 10 P as KH2PO4, 40 K as K2SO4 and KH2PO4, and 40 Mg as MgSO4. The micronutrients were (mg L−1): 2.0 Fe as Fe–EDTA, 0.5 Mn as MnCl2·4H2O, 0.05 Mo as (NH4)6Mo7O24·4H2O, 0.2 B as H3BO3, 0.01 Zn as ZnSO4·7H2O, 0.01 Cu as CuSO4·5H2O, and 0.0028 Si as Na2SiO3·9H2O. To compensate for the lower Ca, additional CaCl2 was added to the low N and intermediate N treatments. A nitrification inhibitor (dicyandiamide) was added to each nutrient solution to prevent the oxidation of ammonium. Nutrient solutions were changed every 2 days, and the pH was adjusted to 5.50±0.05 every day with HCl or NaOH. All treatments were planted in 5 individual containers and were placed in a completely randomised design.
Plants were grown in a greenhouse at 25/18°C day/night temperature. Light was supplied by SON–T AGRO 400W bulbs, with the light intensity maintained at a minimum of 1000 µmol photons m−2 s−1 (PAR) at the leaf level and a 14 h photoperiod.
Gas exchange and fluorescence measurements
Forty days after the start of treatment, photosynthesis and chlorophyll fluorescence were simultaneously measured on light–adapted leaves using a Li–Cor 6400 infrared gas analyzer. Leaf temperature during the measurement was maintained at 30.5±1.1°C, with a photosynthetic photon flux density (PPFD) of 1500 µmol photons m−2 s−1. The CO2 concentration in the cuvette was adjusted to the ambient CO2 concentration (424.3±1.9 µmol mol−1), and the relative humidity was maintained at 50%. After equilibration to a steady state, the fluorescence was measured (Fs) and a 0.8 s saturating pulse of light (approx. 8000 µmol m−2 s−1) was applied to measure the maximum fluorescence (Fm’). Gas exchange variables were also recorded simultaneously. The efficiency of photosystem II (ΦPSII) was calculated as ΦPSII = 1−Fs/Fm’.
Total electron transport rate (JT) was calculated as JT = ΦPSII×PPFD×αleaf×β, where αleaf and β were leaf absorption and the proportion of quanta absorbed by photosystem II, respectively. The product αleaf×β was determined from the slope of relationship between ΦPSII and the quantum efficiency of CO2 uptake (ΦCO2), obtained by varying light intensity under non–photorespiratory conditions at less than 2% O2 [39]. The variable JT method [17] was used to calculate gm using the equation gm = A/{Ci−Γ*[JT+8(A+Rd)]/[JT−4(A+Rd)]}, where A is the rate of leaf photosynthetic CO2 uptake per unit leaf area, Г* is CO2 compensation point and Rd is the rate of dark respiration. Г* and Rd were measured following Laisk’s method [40], as described by Brooks and Farquhar [41] with minor modifications. The process was described in detail in our previous study [42]. gt was calculated as gt = gs×gm/(gs+gm) [43].
After the above gas exchange measurement, A/Ci response curves were conducted on the same leaves. Leaf temperature, PPFD, and relative humidity during measurements were maintained as mentioned above. Prior to measurements, leaves were placed in the cuvette at a PPFD of 1500 µmol photons m−2 s−1; CO2 concentration in the cuvette was maintained at 400 µmol CO2mol−1 with a CO2 mixer. Ten minutes later, CO2 concentration in the cuvette was controlled across a series of 1000, 800, 600, 400, 200, 150, 100, and 50 µmol CO2 mol−1. After equilibration to a steady state, data were recorded automatically. gm was then calculated based on gas exchange measurements themselves according to the method in Ethier and Livingston [44], which is modified from that in Farquhar et al. [13].
Relative Rubisco content and activity measurements
The Rubisco content of newly expanded leaves was determined according to the method of Makino et al. [45], [46]. Briefly, samples of newly expanded leaves were immersed in liquid N, and then stored at −70°C. For analysis, 0.5 g were ground in a solution containing 50 mM Tris–HCl (pH 8.0), 5 mM β–mercaptoethanol, and 12.5% glycerol (v/v), and then centrifuged at 1500 g for 15 min at 4°C. The supernatants were mixed with a solution containing 2% (w/v) SDS, 4% (v/v) β–mercaptoethanol and 10% (v/v) glycerol, boiled in a water bath for 5 min before SDS–PAGE using a 4% (w/v) stacking gel, and a 12.5% (w/v) separating gel. After electrophoresis, the gels were stained with 0.25% Commassie Blue for 12 h, and destained. Gel slices containing the large subunits and small subunits of Rubisco were transferred to a 10-mL cuvette containing 2 ml of formamide and incubated at 50°C in a water bath for 8 h. The absorbance of the wash solution was measured at 595 nm. Protein concentrations were determined using bovine serum albumin as a standard.
Rubisco activity was measured according to Jin et al. [7] with minor modification. Briefly, about 0.1 g of newly expanded leaves were ground with 2 ml of a solution containing 50 mM Tris–HCl (pH 7.5), 10 mM β–mercaptoethanol, 12.5% (v/v) glycerol, 1 mM EDTA–Na2, 10 mM MgCl2, and 1% (m/v) PVP–40. After centrifugation at 15,000 g for 1 min at 4°C, the activity in the supernatants were assayed. The initial Rubisco specific activity was measured at 30°C by adding 100 µL of the supernatant to 900 µL of assay solution containing 56 mM HEPES–NaOH (pH 7.5), 1 mM EDTA–Na2, 20 mM MgCl2, 3 mM DTT, 11 mM NaHCO3, 6 mM ATP, 6 mM creatine phosphate, 0.2 mM NADH, 11 units of phosphocreatine kinase, 11 units of glyceraldehyde–3–phosphate dehydrogenase, 11 units of phosphoglycerate kinase, 11 mM Tris–HCl (pH 7.5), and 0.7 mM RuBP. The absorbance at 340 nm was recorded at 3 s intervals for 30 s. To measure total Rubisco specific activity, 100 µL of the supernatant were added to 200 µL of activation medium containing 1 mM EDTA–Na2, 50 mM MgCl2, 15 mM NaHCO3, and 50 mM Tris–HCl (pH 7.5), and incubated at 30°C for 10 min. Following the addition 700 µL of assay solution, the activity was determined by recording the absorbance at 340 nm at 3 s intervals for 30 s.
Chloroplast ultrastructure
Leaf pieces of approximately 1–2 mm2 were cut from the middle of newly expanded leaves using two razor blades, then they were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4, and post–fixed with 2% osmium tetroxide. Specimens were dehydrated in a graded acetone series and embedded in Epon 812. Leaf sections, 70 nm thick, were cut with a Power Tome–XL ultramicrotome, stained with 2% uranyl acetate, and examined under an H–7650 transmission. Chloroplast length and thickness were calculated from at least 20–30 chloroplasts. Chloroplasts were assumed to be ellipsoids of revolution, which is a shape that is generated by rotating an ellipse around one of its axes. According to the Cesaro formula [47], Schl was calculated as Schl = 4×π×(a×b2)2/3, where a = Lchl/2, b = Dchl/2. Vchl was calculated from the Cesaro formula, Vchl = (4/3)×π×a×b2.
Measurement of biomass, leaf nitrate and N content
Nitrate from newly expanded leaves was extracted in boiling water for 30 min and reacted with salicylic acid, and the color was determined at a wavelength of 410 nm. 45 days after treatments were started when all measurements had been completed, plants were harvested and separated into root, leaf sheath and culm, and leaf fractions. Leaf area and leaf fresh weight were determined and specific leaf weight (SLW) was calculated as the ratio of leaf fresh weight to leaf area. All samples were oven-dried at 105°C first for 30 min, and then at 70°C to constant weight. The dried leaves were digested with H2SO4–H2O2 at 260–270°C, and the total leaf N concentration was determined using a digital colorimeter (AutoAnalyzer 3; Bran+Luebbe).
Statistics
To test the differences between varieties and N supplies, data were analyzed using two-way analysis of variance (ANOVA) and the least significant difference (LSD) test with the SAS 9.0 statistical software package. Different lowercase letters were used to indicate significant differences (P<5%) among N supplies, and different uppercase letters were used between varieties.
Acknowledgments
We wish to thank Professor Peter Horton FRS (University of Sheffield, UK) for assistance in preparation of the manuscript.
Funding Statement
This work was supported by the National Basic Research Program of China (2013CB127403) and the National Natural Science Foundation of China (NSFC-31272236). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Evans JR, Terashima I (1987) Effects of nitrogen nutrition on electron transport components and photosynthesis in spinach. Aust J Plant Physiol 14: 59–68. [Google Scholar]
- 2. Poorter H, Evans JR (1998) Photosynthetic nitrogen–use efficiency of species that differ inherently in specific leaf area. Oecologia 116: 26–37. [DOI] [PubMed] [Google Scholar]
- 3. Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C3 plants. Oecologia 78: 9–19. [DOI] [PubMed] [Google Scholar]
- 4. Makino A, Shimada T, Takumi S, Kaneko K, Matsuoka M, et al. (1997) Does decrease in Ribulose–1,5–bisphosphate carboxylase by antisense RbcS lead to a higher N–use efficiency of photosynthesis under conditions of saturating CO2 and light in rice plants? Plant Physiol 114: 483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jensen RG (2000) Activation of Rubisco regulates photosynthesis at high temperature and CO2 . Proc Natl Acad Sci USA 97: 12937–12938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spreitzer RJ, Salvucci ME (2002) Rubisco: structure, regulatory interactions, and possibilities for a better enzyme. Ann Rev Plant Biol 53: 449–479. [DOI] [PubMed] [Google Scholar]
- 7. Jin SH, Hong J, Li XQ, Jiang DA (2006) Antisense inhibition of Rubisco activase increases Rubisco content and alters the proportion of Rubisco activase in stroma and thylakoids in chloroplasts of rice leaves. Ann Bot 97: 739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Stitt M, Schulze D (1994) Does Rubisco control the rat eof photosynthesis and plant growth? An exercise in molecular ecophysiology. Plant Cell Environ 17: 465–487. [Google Scholar]
- 9. Cheng LL, Fuchigami LH (2000) Rubisco activation state decreases with increasing nitrogen content in apple leaves. J Exp Bot 51: 1687–1694. [DOI] [PubMed] [Google Scholar]
- 10. Murchie EH, Hubbart S, Chen Y, Peng S, Horton P (2002) Acclimation of rice photosythesis to irradiance under field conditions. Plant Physiol 139: 1999–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Manter DK, Kerrigan J (2004) A/Ci curve analysis across a range of woody plant species: influence of regression analysis parameters and mesophyll conductance. J Exp Bot 55: 2581–2588. [DOI] [PubMed] [Google Scholar]
- 12. Warren CR, Dreyer E, Adams MA (2003) Photosynthesis–Rubisco relationships in foliage of Pinus sylvestris in response to nitrogen supply and the proposed role of Rubisco and amino acids as nitrogen stores. Trees 17: 359–366. [Google Scholar]
- 13. Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149: 78–90. [DOI] [PubMed] [Google Scholar]
- 14. Sage RF (1990) A model describing the regulation of ribulose–1,5–bisphosphate carboxylase, electron transport, and triose phosphate use in response to light intensity and CO2 in C3 plants. Plant Physiol 94: 1728–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Flexas J, Ribas–Carbó M, Bota J, Galmés J, Henkle M, et al. (2006) Decreased Rubisco activity during water stress is not induced by decreased relative water content but related to conditions of low stomatal conductance and chloroplast CO2 concentration. New Phytol 172: 73–82. [DOI] [PubMed] [Google Scholar]
- 16. Warren CR (2004) The photosynthetic limitation posed by internal conductance to CO2 movement is increased by nutrient supply. J Exp Bot 55: 2313–2321. [DOI] [PubMed] [Google Scholar]
- 17. Harley PC, Loreto F, Marco GD, Sharkey TD (1992) Theoretical considerations when estimating the mesophyll conductance to CO2 flux by analysis of the response of photosynthesis to CO2 . Plant Physiol 98: 1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Loreto F, Harley PC, Marco GD, Sharkey TD (1992) Estimation of mesophyll conductance to CO2 flux by three different methods. Plant Physiol 98: 1437–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Eichelmann H, Laisk A (1999) Ribulose–1,5–bisphosphate carboxylase/oxygenase content, assimilatory charge, and mesophyll conductance in leaves. Plant Physiol 119: 179–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bernacchi CJ, Portis AR, Nakano H, von Caemmerer S, Long SP (2002) Temperature response of mesophyll conductance. Implications for the determination of Rubisco enzyme kinetics and for limitations to photosynthesis in vivo. Plant Physiol 130: 1992–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Evans JR, von Caemmerer S, Setchell BA, Hudson GS (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21: 475–495. [Google Scholar]
- 22. Hanba YT, Shibasaka M, Hayashi Y, Hayakawa T, Kasamo K, et al. (2004) Overexpression of the barley aquaporin HvPIP2;1 increases internal CO2 conductance and CO2 assimilation in the leaves of transgenic rice plants. Plant Cell Physiol 45: 521–529. [DOI] [PubMed] [Google Scholar]
- 23. Uehlein N, Otto B, Hanson DT, Fischer M, McDowll N, et al. (2008) Function of nicotiana tabacum aquaporins as chloroplast gas pores challenges the concept of membrane CO2 permeability. Plant Cell 20: 648–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Muller O, Oguchi R, Hirose T, Werger MJA, Hikosaka K (2009) The leaf anatomy of a broad–leaved evergreen allows an increase in leaf nitrogen content in winter. Physiol. Plantarum 136: 299–309. [DOI] [PubMed] [Google Scholar]
- 25. Li Y, Gao YX, Xu XM, Shen QR, Guo SW (2009) Light–saturated photosynthetic rate in high–nitrogen rice (Oryza sativa L.) leaves is related to chloroplastic CO2 concentration. J Exp Bot 60: 2351–2360. [DOI] [PubMed] [Google Scholar]
- 26. Ariovich D, Cresswell CF (1983) The effect of nitrogen and phosphorus on starch accumulation and net photosynthesis in two variants of Panicum maximum Jacq. Plant Cell Environ 6: 657–664. [Google Scholar]
- 27. Bondada BR, Syvertsen JP (2003) Leaf chlorophyll, net gas exchange and chloroplast ultrastructure in citrus leaves of different nitrogen status. Tree Physiol 23: 553–559. [DOI] [PubMed] [Google Scholar]
- 28. Bondada BR, Syvertsen JP (2005) Concurrent changes in net CO2 assimilation and chloroplast ultrastructure in nitrogen deficient citrus leaves. Environ Exp Bot 54: 41–48. [Google Scholar]
- 29. Antal T, Mattila H, Hakala-Yatkin M, Tyystjärvi T, Tyystjärvi E (2010) Acclimation of photosynthesis to nitrogen deficiency in Phaseolus vulgaris. Planta 232: 887–898. [DOI] [PubMed] [Google Scholar]
- 30. Doncheva S, Vassileva V, Ignatov G, Pandev S (2001) Influence of nitrogen deficiency on photosynthesis and chloroplast ultrastructure of pepper plants. Agric Food Sci in Finland 10: 59–64. [Google Scholar]
- 31. Quick WP, Schurr U, Fichtner K, Schulze ED, Rodermel SR, et al. (1991) The impact of decreased Rubisco on photosynthesis, growth, allocation and storage in tobacco plants which have been transformed with antisense rbcS. Plant J 1: 51–58. [Google Scholar]
- 32. Evans JR, Kaldenhoff R, Genty B, Terashima I (2009) Resistances along the CO2 diffusion pathway inside leaves. J Exp Bot 60: 2235–2248. [DOI] [PubMed] [Google Scholar]
- 33. Tholen D, Zhu XG (2011) The mechanistic basis of internal conductance: A theoretical analysis of mesophyll cell photosynthesis and CO2 diffusion. Plant Physiol 156: 90–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tholen D, Ethier G, Genty B, Pepin S, Zhu XG (2012) Variable mesophyll conductance revisited. Theoretical background and experimental implication. Plant Cell Environ 35: 2087–2103. [DOI] [PubMed] [Google Scholar]
- 35. Sage TL, Sage RF (2009) The functional anatomy of rice leaves: Implications for refixation of photorespiratory CO2 and efforts to engineer C4 photosynthesis into rice. Plant and Cell Physiology 50: 756–772. [DOI] [PubMed] [Google Scholar]
- 36. Bota J, Medrano H, Flexas J (2004) Is photosynthesis limited by decreased Rubisco activity and RuBP content under progressive water stress? New Phytol 162: 671–681. [DOI] [PubMed] [Google Scholar]
- 37. Jiang CZ, Rodermel SR, Shibles RM (1993) Photosynthesis, Rubisco activity and amount, and their regulation by transcription in senescing soybean leaves. Plant Physiol 101: 105–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Law RD, Crafts-Brandner SJ (1999) Inhibition and acclimation of photosynthesis to heat stress is closely correlated with activation of ribulose-1,5-bisphosphate carboxylase/oxygenase. Plant Physiol 120: 173–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Valentini R, Epron D, Angelis PD, Matteducci G, Dreyer E (1995) In–situ estimation of net CO2 assimilation, photosynthetic electron flow and photorespiration in turkey oak (Q. cerris L) leaves. Diurnal cycles under different levels of water–supply. Plant Cell Environ 18: , 631–640. [Google Scholar]
- 40.Laisk AK (1977) Kinetics of photosynthesis and photorespiration in C3-plants. (In Russian) Nauka, Moscow.
- 41. Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose–1,5–bisphosphate carboxylase/oxygenase and the rate of respiration in the light. Planta 165: 397–406. [DOI] [PubMed] [Google Scholar]
- 42. Li Y, Ren BB, Yang XX, Xu GH, Shen QR, et al. (2012) Chloroplast downsizing under nitrate nutrition restrained mesophyll conductance and photosynthesis in rice (Oryza sativa L.) under drought conditions. Plant Cell Physiol 53: 892–900. [DOI] [PubMed] [Google Scholar]
- 43. Grassi G, Magnani F (2005) Stomatal, mesophyll conductance and biochemical limitations to photosynthesis as affected by drought and leaf ontogeny in ash and oak trees. Plant Cell Environ 28: 834–849. [Google Scholar]
- 44. Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27: 137–153. [Google Scholar]
- 45. Makino A, Mae T, Chira K (1985) Photosynthesis and rubulose–1,5–bisphosphate carboxylase/oxygenase in rice leaves from emergence through senescence. Planta 166: 414–420. [DOI] [PubMed] [Google Scholar]
- 46. Makino A, Mae T, Chira K (1986) Colorimetric measurement of protein stained with coomassie brilliant blue ron sodium dodecyl sulfate–polyacrylamide gel electrophoresis by eluting with formamide. Agric Biol Chem 50: 1911–1912. [Google Scholar]
- 47. Ivanova LA, P’yankov VI (2002) Structural adaptation of the leaf mesophyll to shading. Russian J Plant Physiol 49: 419–431. [Google Scholar]
- 48. Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light–acclimation need change in leaf anatomy? Plant Cell Environ 26: 505–512. [Google Scholar]