Abstract
Bilateral cochlear implant users have poor sensitivity to interaural time differences (ITDs) of high-rate pulse trains, which precludes use of these stimuli to convey fine-structure ITD cues. However, previous reports of single-neuron recordings in cats demonstrated good ITD sensitivity to 1000 pulses-per-second (pps) pulses when the pulses were sinusoidally amplitude modulated. The ability of modulation to restore ITD sensitivity to high-rate pulses in humans was tested by measuring ITD thresholds for three conditions: ITD encoded in the modulated carrier pulses alone, in the envelope alone, and in the whole waveform. Five of six subjects were not sensitive to ITD in the 1000-pps carrier, even with modulation. One subject's 1000-pps carrier ITD sensitivity did significantly improve due to modulation. Sensitivity to ITD encoded in the envelope was also measured as a function of modulation frequency, including at frequencies from 4 to 16 Hz where much of the speech envelope's energy and information resides. Sensitivity was best at the modulation frequency of 100 Hz and degraded rapidly outside of a narrow range. These results provide little evidence to support encoding ITD in the carrier of current bilateral processors, and suggest envelope ITD sensitivity is poor for an important segment of the speech modulation spectrum.
INTRODUCTION
The use of two spatially-separated ears confers many advantages to normal-hearing (NH) listeners, including improved localization of sounds (Middlebrooks and Green, 1991; Blauert, 1997) and better understanding of speech in noisy environments (e.g., Bronkhorst and Plomp, 1988). Interaural time differences (ITDs) are created by the azimuth-dependent delay for sound arriving at the two ears, and are important cues for sound localization (Rayleigh, 1907; Wightman and Kistler, 1992; Macpherson and Middlebrooks, 2002) as well as understanding speech in the presence of noise (Zurek, 1993) or in the presence of competing speech (Carhart et al., 1967). ITDs are present in both the slowly varying amplitude envelope of a signal and in its more rapidly varying carrier, or fine structure. Early studies indicated that ITD cues were only used for low-frequency sounds (Rayleigh, 1907), and psychophysical studies confirmed that sensitivity to the fine-structure ITDs of pure tones is limited to frequencies of up to about 1400 Hz (Klumpp and Eady, 1956). Later studies showed that listeners can also lateralize on the basis of envelope ITDs of high-frequency sounds (Henning, 1974; Bernstein and Trahiotis, 1994).
Cochlear implant (CI) patients are increasingly choosing bilateral implantation, and numerous studies have demonstrated that these patients benefit from improved speech reception in noise (van Hoesel and Tyler, 2003; Schleich et al., 2004; Litovsky et al., 2006; Loizou et al., 2009) and improved localization (van Hoesel and Tyler, 2003; Nopp et al., 2004; Litovsky et al., 2009). However, the amount of benefit received by bilateral cochlear implant (BiCI) users is less than that achieved by NH listeners, and the benefits seem to be well accounted for by the level differences at the two ears for both localization (Grantham et al., 2007; Seeber and Fastl, 2008) and for speech reception in noise (e.g., Loizou et al., 2009; for a review see van Hoesel, 2011). Improving the ITD-based binaural benefit therefore seems an important step toward narrowing the gap in the overall benefit between BiCI and NH listeners.
In the speech coding strategies used with most modern CI processors, the input signal is divided into frequency bands by a bank of bandpass filters. The envelopes of the filter outputs are then extracted and used to amplitude modulate a set of interleaved pulse trains. As a natural result of these speech coding strategies, the envelope ITD of the input signal is represented in the envelopes of the stimulation signal. The fine structure of the signal is discarded completely and the interaural delay of the carrier pulses is almost always unrelated to the incoming sound signal.1
Even if fine-structure ITD was encoded in the carrier pulse trains of bilateral processors, benefit might be limited because BiCI patients have poor sensitivity to the ITD of un-modulated pulse trains at rates typical of modern CIs (van Hoesel and Tyler, 2003; Laback et al., 2007; van Hoesel, 2007; Poon et al., 2009; van Hoesel et al., 2009). The findings of these studies are largely consistent. The best ITD sensitivity is found with pulse trains of 50 to 100 pulses-per-second (pps), where good listeners have detection thresholds to ITDs of 100 to 200 μs, although variability among subjects is large. As pulse rates increase, ITD sensitivity drops off rapidly. For example, van Hoesel et al. (2009) measured thresholds for lateralization in six BiCI subjects pre-screened for their relatively good ITD sensitivity. For un-modulated pulse trains, with rates ranging from 100 to 1000 pps, the best ITD sensitivity was at 100 pps where the mean threshold for apical electrode pairs was about 165 μs. ITD sensitivity degraded as the pulse rate increased: The mean threshold of the six BiCI subjects increased to approximately 450 μs at 600 pps, and at 1000 pps many thresholds were above 1 ms.
Since the carrier pulse rates used in most contemporary commercial speech processors range from 800 to several thousand pps, the poor ITD sensitivity at these rates seems to preclude successfully coding relevant ITDs in the carriers. However, data from animal auditory-physiology experiments suggest amplitude modulation may improve the ITD sensitivity to high-rate pulse trains. In a pair of companion studies, Smith and Delgutte (2007, 2008) made single-neuron recordings in the inferior colliculus (IC) of bilaterally implanted cats while stimulating with pulse trains of varying interaural delay. When stimulating with constant-amplitude pulse trains of 40 pps, they recorded from units that responded to almost every pulse and were sharply tuned to ITD. As pulse rates were raised, the post-onset response fell, nearly ceasing at rates above 300 pps and resulting in an increase in neural ITD-thresholds for increasing pulse rates resembling that seen with human psychophysics. However, the authors also found that an ongoing response could be restored to a high-rate (1000-pps) pulse train by 100% amplitude modulation with a 40-Hz sinusoid, resulting in neural ITD thresholds improving to levels comparable to those for the 40-pps un-modulated pulses. This sensitivity was to carrier ITD of the 1000-pps pulse trains, not the envelopes, as it was observed even when the envelope ITD was fixed at zero. A model that successfully described the IC neurons' behavior included an interaural coincidence detector followed by a slow, inhibitory feedback with a time constant of several milliseconds. This model described how un-modulated 1000-pps pulse trains produced a buildup of inhibition which blocked the ongoing response, but the addition of modulation provided low amplitude periods during which the inhibition could dissipate, enabling an ongoing response to subsequent pulses. An alternative model predicts the observed improvement without invoking inhibition (Colburn et al., 2009), but the basic mechanism remains the same: Low amplitude intervals of a modulator restore an ongoing response.
Several psychophysical studies with human BiCI listeners have shown that ITD sensitivity to modulated high-rate pulse trains is much improved compared to un-modulated trains at the same high rates (van Hoesel and Tyler, 2003; Majdak et al., 2006; van Hoesel, 2007; van Hoesel et al., 2009). A reasonable assumption has been that this improved ITD sensitivity is due to the envelope itself, given that sensitivity to the un-modulated pulse trains is mostly nonexistent. However the physiology results also raise the possibility that at rates near 1000 pps, the modulated carrier itself is also contributing to the improved ITD sensitivity. The current study seeks to examine this possibility directly. Experiment 1 tested the hypothesis that sinusoidal amplitude modulation (SAM) improves carrier ITD sensitivity to high-rate pulse trains in human BiCI subjects. Psychophysical measures of ITD threshold were made using parameters similar to those from the animal physiological experiments, using waveforms with various combinations of envelope and carrier ITD. The results were analyzed for evidence of carrier ITD sensitivity. If amplitude modulation, which occurs naturally in CI processing, can improve carrier ITD sensitivity in human BiCI users, then modifying bilateral processors so that their interaural carrier delay agrees with the input signal's ITD may improve performance. If modulation does not improve carrier ITD sensitivity, encoding the signal's ITD in the carriers would not be indicated.
As previously mentioned, the envelope of natural sounds also carries ITD information, and most CI speech processors present this envelope ITD in their output signals (Laback et al., 2004). BiCI listeners are sensitive to this envelope ITD when acoustic click-trains are presented to their speech processors (Laback et al., 2004; van Hoesel, 2004; Senn et al., 2005), often showing thresholds between 100 and 300 μs. When the input signal is a speech token, however, ITD sensitivity in the same subjects can be very poor, with thresholds near 2 ms (Laback et al., 2004). Several factors may impact the sensitivity to the speech token. One may be the relatively low frequency of the speech envelope. For continuous speech, most of the energy in the envelope is at modulation frequencies below 30 Hz, with the range of 1 to 7 Hz being the most prominent (Elliott and Theunissen, 2009). Speech intelligibility is also mostly dependent on low modulation frequencies. Excellent speech intelligibility is maintained when envelopes are low-pass filtered to frequencies as low as 16 Hz (Drullman et al., 1994), and methods that analyze the modulation spectrum to predict speech reception in noise perform well even when restricting the analysis to frequencies below 30 Hz (Houtgast and Steeneken, 1985).
BiCI listeners have shown sensitivity to ITD presented in the envelope of high-rate pulse trains when stimuli are presented directly to a single electrode pair. However, to date, these studies have only measured ITD sensitivity for segments of the modulation spectrum above that of the speech envelope. For sinusoidal modulation, the lowest modulation frequencies studied are 50 Hz (van Hoesel and Tyler, 2003) or 100 Hz (van Hoesel, 2007; van Hoesel et al., 2009). Other studies measured ITD sensitivity for trapezoidal modulation using fixed frequencies of 12 Hz (Majdak et al., 2006) and 27 Hz (Laback et al., 2011), but the slopes of the trapezoid corresponded to higher sinusoidal frequencies. Little is known about ITD sensitivity for sinusoidal modulation frequencies below 25 Hz, even though this is the range most important for speech. In Experiment 2a we measured ITD sensitivity for sinusoidal modulation at frequencies (fm) between 4 and 500 Hz. These measurements, especially for fm between 4 and 16 Hz, are important when considering whether speech envelopes are able to deliver ITD information useful for binaural benefit. The results of Experiment 2a found a substantial dependence of ITD sensitivity on SAM fm. Experiment 2b measured additional ITD thresholds using modified versions of the SAM waveform. The motivation was to investigate which characteristic of the SAM waveform correlated with the observed ITD sensitivity.
Finally, Experiment 2c examined the effect of carrier-rate on envelope ITD sensitivity. It has been argued that a pulse rate of 5 times the modulation frequency is required to adequately represent the envelope to CI listeners (Wilson, 1997), and modeling results indicate this ratio may need to be even higher to prevent the carrier from interfering in envelope ITD detection (Colburn et al., 2009). In tension with the evidence recommending higher pulse rates is the physiological evidence showing that envelope ITD sensitivity becomes poorer at higher carrier rates in the cat IC (Smith and Delgutte, 2008). Experiment 2c sought to determine if carrier-rate contributes to the rate-limit for envelope ITD sensitivity at higher modulation frequencies when the ratio of fc /fm drops to five or less. This was assessed by measuring envelope ITD thresholds using a 4640-pps carrier at the modulation frequencies of 50, 100, 200, and 500 Hz and comparing within-subject results to 1000-pps thresholds.
EXPERIMENT 1: ITD ENCODED IN THE FINE STRUCTURE OF SAM PULSE TRAINS
Methods
Subjects
Six subjects participated in Experiment 1. All lost hearing post-lingually. Relevant binaural information and the most recent single-syllable word score are shown in Table Table I.. There were no performance-based inclusion criteria. All subjects were implanted bilaterally with Advanced Bionics CII or 90 K implants. Subject C340 received both implants simultaneously during a single surgery. The other five received their second implant after at least 1 yr of monaural implant use. All subjects had at least 9 months of binaural CI experience at the beginning of data collection. Testing was conducted at a series of monthly sessions determined by the subjects' schedules. For a few of the subjects (C109 and C128), the sessions stretched over a period greater than 1 yr. When this occurred, conditions were repeated to check consistency and verify the absence of longitudinal changes.
Table I.
Subject information. Single syllable word scores are CNC or NU6.
| Age at onset of profound hearing loss (years) | Age at onset of CI use (years) | Binaural deprivation (years) | Bilateral CI experience (years) | Binaural single-syllable word score | ITD threshold Unmodulated, 100-pps train | |||
|---|---|---|---|---|---|---|---|---|
| Subject | L | R | L | R | ||||
| C94 | 54 | 49 | 63 | 54 | 14 | 0.8 | 80% | 67 μs |
| C109 | 44 | 44 | 50 | 48 | 6 | 6 | 86% | 107 μs |
| C128 | 25 | 25 | 39 | 36 | 14 | 5 | 82% | 74 μs |
| C278 | 34 | 34 | 44 | 46 | 12 | 3 | 84% | 764 μs |
| C299 | 43 | 40 | 48 | 45 | 8 | 1 | 68% | 106 μs |
| C340 | 63 | 63 | 65 | 65 | 2 | 2.5 | 86% | 197 μs |
Stimuli
Custom interface hardware [Clarion Research Interface 2 (CRI2), Advanced Bionics] was combined with custom software to control the implanted stimulators directly; clinical speech processors were not used. The CRI2 synchronizes stimuli between the two implants to within less than 1 μs, with an ITD resolution of 13.5 μs. During testing, signals were calculated on a personal computer and transmitted to the implant via the CRI2. SAM current waveforms were generated by multiplying a carrier pulse-train by a modulator of the form
| (1) |
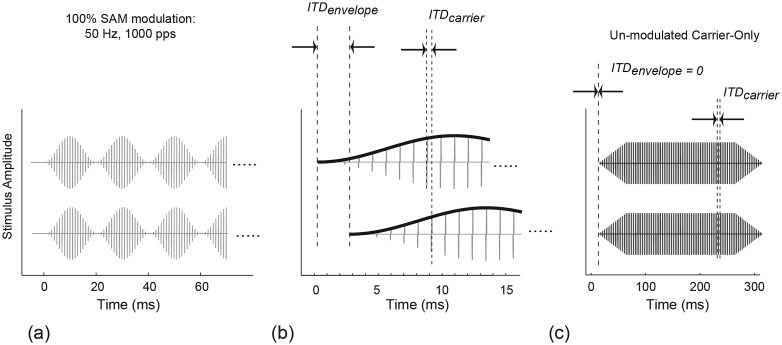
where fm is the modulation frequency and IC is the current amplitude determined for each electrode of a binaural pair (see Sec. 2A3). Carrier pulses-trains were made up of 27-μs-per-phase biphasic pulses at a carrier frequency (pulse rate) fc. In Experiment 1, carrier frequency fc was 1000 pps and modulation frequency fm was 50 Hz. These carrier and envelope rates were chosen to approximate the parameters used by Smith and Delgutte (2008) in the cat physiological experiment. The 1000-pps carrier rate is also typical of those used in commercial CIS processors. Modulation depth was 100% and all stimuli were presented on a linear current scale, i.e., without compression or logarithmic mapping. The stimulus duration was 300 ms and stimuli always contained an integer number of modulation periods. As shown in Fig. 1a, the modulator of Eq. 1 results in a gradual, non-abrupt onset. No other ramp or windowing was applied, so the onset and offset are determined solely by the shape of the modulator, which is dependent on fm.
Figure 1.
Schematic illustrations of the stimuli used in Experiment 1. (a) Raised cosine, 100% modulation as generated per Eq. 1. (b) Encoding interaural time delay. In Carrier-Only coding, the envelopes in the two ears are identical and the biphasic carrier-pulses are delayed (ITDenvelope = 0). In Envelope-Only coding, the pulses are coincident in time and only their amplitude modulation is delayed (ITDcarrier = 0). Whole-Wave encoding delays the entire lagging waveform. It is the only condition in which the envelope and the carrier ITD agree, provided the ITD does not exceed one-half the carrier period. (c) Subjects who showed sensitivity to the modulated Carrier-Only waveform were also tested with the un-modulated Carrier-Only condition. In this condition envelopes have constant amplitude, except for 50-ms onset and offset ramps. The envelope ITD = 0; ITD is encoded only in the carrier pulses.
Figure 1b illustrates interaural delays of the envelope (ITDenvelope) and of the carrier (ITDcarrier) for a SAM waveform. Experiment 1 measured sensitivity to three ITD coding conditions: Carrier-Only, Envelope-Only, and Whole-Wave. In the Carrier-Only condition, ITDenvelope is fixed at zero and the ITD is only encoded in the carrier pulses. This is one condition to which Smith and Delgutte (2008) found sensitivity to the carrier ITD. It provides a conservative measurement of carrier ITD sensitivity because there is no possibility of contribution by the envelope ITD. In fact, sensitivity to the carrier must overcome that of the zero-ITD envelope in order to be detected. Carrier-Only encoding may also present ambiguous ITD information when the delay approaches one-half the pulse period. For our 1000-pps carrier, ITDs between 500 and 1000 μs could appear as sub-500 μs ITDs leading in the opposite ear. To avoid presenting these ambiguous cues, the Carrier-Only tests limited the ITD magnitude to 450 μs (but see results for a further analysis of this issue). In the Envelope-Only condition, only the envelope is delayed. ITDcarrier is always fixed at zero, that is, the interaural carrier pulses are always coincident in time. The Whole-Wave condition delays the entire SAM waveform so that both the envelope and the carrier are delayed. Whole-Wave tests did not limit the ITD at 450 μs. When the ITD is less than 500 μs, the Whole-Wave envelope and carrier ITD are in agreement; however, for ITDs between 500 and 1000 μs, Whole-Wave envelope and carrier ITD may conflict, again because of the 1000-μs period of the carrier. For 5 of 6 subjects, Whole-Wave sensitivity was good enough that tests never presented an ITD greater than 500 μs. For subject C278, ITDs greater than 500 μs were presented.
Finally, because the hypothesis being tested is that modulation improves the ITD sensitivity to high-rate carrier pulses, thresholds were measured to un-modulated 1000-pps trains as a control condition against which to compare the modulated results. Figure 1c shows the un-modulated Carrier-Only control condition. All pulses were at constant amplitude except for linear, 50-ms onset and offset ramps meant to degrade any onset ITD cue, and the ramped-envelope ITD was fixed at zero. Only subjects who showed some sensitivity to modulated high-rate pulse trains were tested with the un-modulated signal.
Electrode pair and stimulation level
Stimuli were presented to a single bilateral electrode pair chosen as the most ITD-sensitive of all tested for the subject. The experimenter first chose one basal and one apical processor-matched pair. Processor-matched pairs are those that in daily use receive stimulation from the same frequency analysis-channel of their respective bilateral speech processors. ITD thresholds were then measured for the apical and basal pairs using a stimulus which produces the lowest ITD threshold in most subjects: 300-ms, un-modulated pulse-train of 50 or 100 pps. Processor matched pairs are often, but not always, the most sensitive for ITD (Long et al., 2003; Poon et al., 2009), therefore additional ITD thresholds were measured for candidate pairs comprising electrodes with slight interaural mismatches (up to four electrodes) relative to the processor matched locations. The electrode pair with the best ITD sensitivity of all pairs tested was selected as the subject's test pair. For most subjects, the pair with the best sensitivity was comprised of electrodes at the same position along the electrode array and corresponded to a processor-matched pair; however, mismatches of up to one electrode did occur. For three of the six subjects, the bilateral electrode pair with best ITD sensitivity was at the apical end of the electrode array; for three subjects this pair was near the base.
For each electrode pair a pair of current amplitudes (IC_left, IC_right) was determined using the following three step procedure. (1) The 50-Hz, 1000-pps SAM waveform was applied to each ear alone (monaurally) and the amplitudes adjusted until the subject judged the loudness comfortable in each ear and equal across ears. (2) Stimuli were then played simultaneously (binaurally) and amplitudes adjusted until the loudness was “most-comfortable,” the level the subject judged comfortable for listening during long periods of testing. (3) The subject reported the location of the sound image and adjustments were made to the amplitude in either ear until the image was centered in the horizontal dimension. Fusion was not used as a criterion for determining current amplitudes but subjects verified informally that all sounds were fused before the centering procedure began. Once determined, current amplitudes were fixed and used for all modulated conditions of Experiment 1. For the un-modulated condition, the current amplitude was initially set equal to the root-mean-square (rms) level of the subject's modulated stimuli and then adjusted if necessary to produce a centered auditory image.
Psychophysical procedure
ITD sensitivity was measured using a lateralization task to determine the psychophysical threshold for detection of an interaural time delay. Thresholds were measured by a 2-down/1-up adaptive procedure which converges to a 70.7% correct level (Levitt, 1971). A single trial consisted of two 300-ms sounds presented in sequence and separated by a 300-ms silence. The interaural delay of the first stimulus was always zero, and the interaural delay of the second stimulus was the magnitude of the current ITD test value. The ITD of the second stimulus was produced by delaying either the left or right ear signal, with the delayed side chosen at random with equal probability. The subjects were instructed to indicate whether they heard the second sound move to the left or right of the first. Subjects entered their responses by a keyboard and received correct-answer feedback after every trial. The instructions encouraged the subject to compare the second stimulus to the first stimulus; however, there is evidence that subjects learn to ignore the first reference trial and treat this task as a single-interval detection task (Hartmann and Raked, 1989). In this case reported thresholds correspond to a d′ value of 0.54. Threshold values were adjusted accordingly when compared with studies specifying sensitivity at other levels of d′.
Before each test, the subject was presented with multiple example trials of both left-ear leading and right-ear leading ITD. These trials familiarized the subjects with the condition being tested and were also used to determine a starting ITD magnitude for the adaptive task at which subjects could hear the second stimulus lateralized. For the Carrier-Only condition, subjects rarely reported hearing the second stimulus lateralized; in these cases a starting ITD of 400 μs was used. The adaptive procedure used a logarithmic step size which adjusted the ITD magnitude up or down by 2 dB at each reversal (Saberi, 1995). Each test consisted of 14 reversals and a threshold was calculated as the geometric mean of the last 8 reversals (a geometric mean is appropriate because of the logarithmic step size). For Experiment 1, conditions were tested in pseudo-random order. At least 4 and as many as 13 test runs were completed for each condition and the arithmetic mean of all runs were reported.
All subjects performed a minimum practice period of at least 2 h of ITD testing before any results were included as data. Subjects C128, C340, and C109 have participated in regular testing for 2 to 8 yrs and completed at least 20 h of ITD testing before this experiment.
Results
Carrier-Only ITD sensitivity
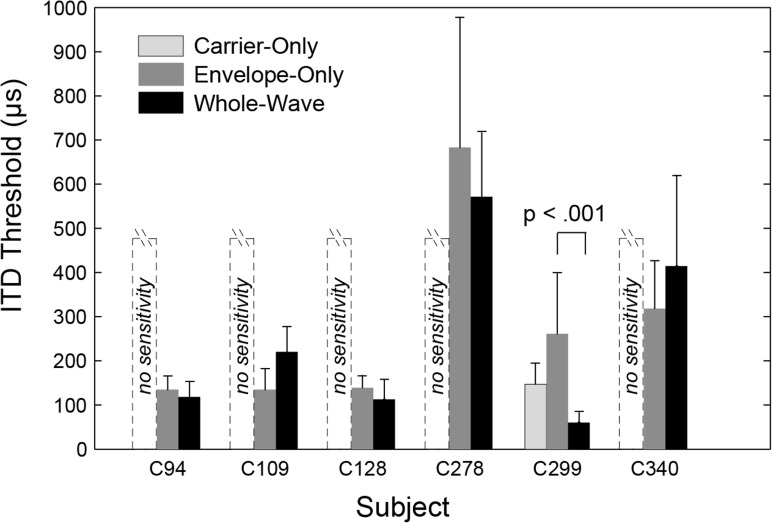
Figure 2 shows ITD thresholds for six subjects in three conditions. For the Carrier-Only condition, five of the six subjects were not able to complete the adaptive task, as depicted in Fig. 2 by an unfilled dashed bar labeled “no sensitivity.” This result was recorded if the adaptive procedure continued to track upward without reaching an ITD value at which the subject could lateralize at the 71% threshold level. When this occurred, the ITD test value eventually clipped at the 450-μs limit imposed for the Carrier-Only condition. The test was typically allowed to run until completion to allow the subject an opportunity to detect a lateralization cue using the provided feedback. Even for a condition with no sensitivity, subjects occasionally produced correct responses by chance. These caused the adaptive procedure's test ITD value to bounce between the 450-μs maximum and one or two 2-dB step-sizes below. Although the test eventually completed, the result was not recorded as evidence of sensitivity when several instances of clipping occurred. Therefore a result of “no sensitivity” for the Carrier-Only condition more precisely means that no threshold below about 400 μs could be measured.
Figure 2.
Individual subject ITD thresholds for three methods of ITD encoding. Dashed, unfilled bars labeled “no sensitivity” signify that a subject was not able to complete any adaptive test for Carrier-Only encoding, which is the case for all subjects except C299. The height of these unfilled bars serves as a reminder that given the ITD magnitude constraint for the Carrier-Only condition, only ITD thresholds below 450 μs were detectable. Error bars are standard deviations of multiple test runs. C299 alone showed a significant difference (after post hoc correction) between thresholds for the Envelope-Only and Whole-Wave conditions.
One subject, C299, was able to complete the adaptive threshold measurement with ITD encoded only in the carrier. Each of C299's multiple Carrier-Only adaptive tracks reliably converged to an ITD value below 400 μs, resulting in a mean Carrier-Only threshold of 133 μs. This is comparable with C299's mean threshold for un-modulated, low rate (100-pps) pulse trains, which is 106 μs (see Table Table I.).
The adaptive procedure assumes monotonic performance as a function of ITD, which may not be the case as the ITD tested approaches one-half the pulse period. For example, Majdak et al. (2006) measured carrier ITD sensitivity as the ITD was varied throughout the entire pulse period. For conditions when their subjects could lateralize using the pulse-train ITD, the percentage of correct lateralizations increased with ITD until reaching a maximum at one-quarter of the pulse period and then declined as the ITD approached one-half the period, where the ear receiving the leading pulse becomes ambiguous. If our subjects were similarly sensitive to the carrier ITD, their performance would vary non-monotonically, thus violating one of the assumptions for use of an adaptive method and possibly obscuring ITD sensitivity. As an alternative means of searching for carrier ITD sensitivity, lateralization performance was plotted in the form of psychometric functions. These functions were then examined for patterns that would indicate sensitivity to carrier ITD not revealed by the adaptive tests.
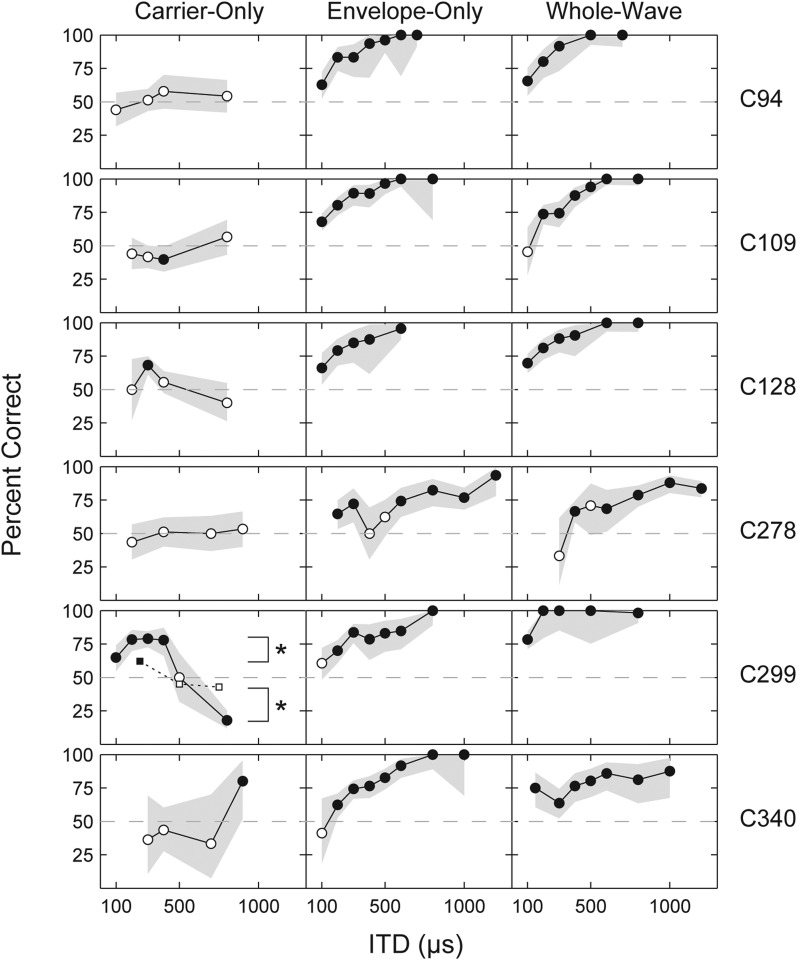
In Fig. 3, percent correct for lateralization is shown as a function of ITD magnitude. Each column corresponds to one ITD encoding condition and each row contains the data for one subject. Data in Fig. 3 were obtained by pooling the trials from the multiple adaptive test runs completed by the subjects. Trials were grouped on the basis of ITD magnitude into 100-μs wide bins centered on integer multiples of 100 μs and percent correct was calculated for the pooled trials. In order to increase the number of trials at under-sampled ITDs, or at ITDs of interest but not visited by the adaptive procedure, subjects performed additional blocks of trials at constant ITD magnitudes. The results were added to the pooled trials from the adaptive runs. Gray shaded areas surrounding the psychometric functions indicate the 95% confidence intervals, whose widths are strongly influenced by the number of trials at each ITD value. Filled symbols represent ITD values at which the percent correct was significantly different (p < 0.05) than the chance performance level of 50%. Open symbols represent performance not significantly different from chance. Confidence intervals and significant differences for the psychometric function were computed from maximum likelihood estimates using a binomial distribution.
Figure 3.
Psychometric functions for six subjects measured using each of three ITD encoding methods. Horizontal dashed lines in each panel indicate chance performance (50% correct). Filled circles represent ITD values where percent correct is significantly different than chance performance (p < 0.05). Performance at open circles is not significantly different than chance. Gray shaded regions are 95% confidence intervals. For C299 in the Carrier-Only condition, square symbols connected by a dotted line show performance when the stimulus was an un-modulated 1000-pps pulse train. In this same panel, asterisks indicate a significant improvement for modulated Carrier-Only versus un-modulated Carrier-Only conditions.
The Carrier-Only psychometric functions show differing patterns of sensitivity among users. The curves can be divided into three main sub-types. The first describes subjects with no Carrier-Only ITD sensitivity. In this group are subjects C94, C278, and C340. Curves for these subjects are mainly flat at approximately 50% correct (chance level) with no significant ability to lateralize at any ITD. (Subject C340 did show a single, dramatic jump in percent correct at 900 μs; however, as can be seen by the very wide confidence intervals for this subject's curve, the number of trials for this ITD is low, and the subject became unavailable before additional blocks could be measured.)
A single subject produced a psychometric function that clearly indicates sensitivity to the carrier. C299's function is roughly periodic with the period of the carrier pulses: Sensitivity grows to a level significantly above chance as ITD increases; then decreases to chance level as ITD reaches one-half the pulse period and the leading ear becomes ambiguous; and finally drops significantly below chance at ITD magnitudes greater than [1/2] the pulse period, because at these delays the nearest interaural pairs indicate a leading ear opposite that of the actual test ITD. Subject C299's periodic psychometric function is therefore consistent with his adaptive results showing significant sensitivity to the 1000-pps carrier.
To determine whether amplitude modulation played a role in producing Carrier-Only sensitivity, C299 was tested with an un-modulated 1000-pps pulse train [Fig. 1c] using blocks of trials with constant ITD at 250, 500, and 750 μs. Results are shown by the square symbols and dotted line in C299's Carrier-Only plot. At 250 μs the percent correct for the modulated Carrier-Only signals are significantly greater than for the un-modulated Carrier-Only trials (p < 0.05, indicated by asterisks in the C299 Carrier-Only plot). At 750 μs, modulated scores are significantly less than the un-modulated carrier, indicating better sensitivity to the reversed ITD cue. There is some sensitivity to the ITD of un-modulated pulses at 250 μs (62%, p < 0.05 different than chance) but not at 750 μs. The significantly greater sensitivity shown with the modulated carrier indicates that, for this subject, amplitude modulation is responsible for improving ITD sensitivity to high-rate pulses.
The psychometric functions of C109 and C128 fall into a third sub-type. Each shows lateralization significantly different than chance for a single ITD value, and the significance was confirmed by repeat testing with a block of 80 to 100 additional trials. C128's percent correct at 300 μs is 62%. C109's percent correct at 400 μs is 40%, i.e., below chance. While the data for these two subjects do indicate some carrier ITD sensitivity, it is isolated, not always consistent with the delivered cue, and insufficient to reach a modest threshold criterion for completion of an adaptive task.
Envelope-Only and Whole-Wave ITD sensitivity
Figure 2 shows that all subjects demonstrated some degree of sensitivity to ITD in the Envelope-Only and Whole-Wave conditions. The arithmetic group-mean for the Envelope-Only threshold was 277 μs (range 135 to 683 μs) and similar to the Whole-Wave group mean of 250 μs (range 60 to 571 μs). The largest individual difference between the Envelope-Only and Whole-Wave conditions is for subject C299, whose Envelope-Only ITD threshold of 250 μs is more than 4 times larger than his Whole-Wave ITD threshold of 61 μs.
The individual ITD test results were combined into an unbalanced, two-way analysis of variance (ANOVA) with subject and condition as factors. Because the within-subject variances were correlated with subject means, a log transformation was first applied to the test results to better satisfy the ANOVA requirement for homogeneous variances. Both main effects and interaction effects were analyzed. When subject interaction effects were taken into account, there was a significant difference between the Envelope-Only and the Whole-Wave condition (F[1,76] = 5.23, p < 0.025). A post hoc analysis using Tukey's honestly significant difference (HSD) criterion showed that C299 had a highly significant difference for Envelope-Only versus Whole-Wave ITD sensitivity (p < 0.001). No other subject showed a significant difference between the conditions. Accordingly, when C299 was removed from the group and the ANOVA re-computed, there was no significant effect of condition for the remainder of the group (F[1,65] = 0.23, p < 0.63).
Finally, the Whole-Wave and Envelope-Only psychometric functions plotted in Fig. 3 show a generally monotonic increase in performance with ITD magnitude and demonstrate broad agreement with the adaptive threshold results.
Discussion
Modulation did not improve high-rate pulse ITD sensitivity in most subjects
Experiment 1 showed that for five out of six subjects, amplitude-modulation did not produce sensitivity to the ITD of a high-rate carrier. None of these five subjects were able to complete the adaptive task using ITDs below 450 μs in the Carrier-Only condition. These subjects also performed no better in the Whole-Wave condition as compared to the Envelope-Only condition, which is further evidence that they did not benefit from the encoding of ITD in the carrier. The psychometric functions of these subjects generally confirmed the adaptive results: Lateralization of the 1000-pps pulse train in the Carrier-Only condition was generally at chance level. Two of these five subjects did show a small but significant change in lateralization at a single ITD magnitude, but the performance did not reach the 71% criterion for sensitivity.
The results from one of the six subjects differed distinctly from those of the others. C299 consistently completed the adaptive task for the Carrier-Only condition, displaying thresholds for modulated 1000-pps carriers nearly equal to those measured with un-modulated 100-pps pulses, and his sensitivity was confirmed by an analysis of his psychometric function. Further evidence that C299 benefitted from ITD encoded in the carrier was the dramatic improvement in ITD sensitivity from the Envelope-Only to the Whole-Wave condition, with threshold dropping by more than fourfold, from 250 to 61 μs. This large difference may be understood by recalling that the Envelope-Only condition does still present a carrier ITD cue; however, it is fixed at 0 μs. For a subject with carrier ITD sensitivity, this replaces an informative ITD cue with one likely to interfere with lateralization using the envelope ITD.
When the ITD was encoded only in the carrier pulses of un-modulated pulse trains, C299 showed little sensitivity to this signal. Since the modulated pulse trains produced ITD sensitivity that was significantly greater than the un-modulated condition, for this single subject it appears that amplitude modulation significantly improved ITD sensitivity to the high-rate carrier.
Several psychophysical studies with human BiCI listeners have shown that ITD sensitivity to modulated high-rate pulse trains is much improved compared to un-modulated trains at the same high rates (van Hoesel and Tyler, 2003; Majdak et al., 2006; van Hoesel, 2007; van Hoesel et al., 2009). The question asked in the current experiment is whether, for pulses near 1000 pps, this improved ITD sensitivity is due to the envelope, to improved carrier ITD sensitivity in the presence of modulation, or to a combination of the two. By explicitly isolating the carrier and envelope ITD sensitivity, this study demonstrated that for five of the six subjects at pulse rates of 1000 pps, SAM-ITD sensitivity was due to the envelope alone. For a single subject, sensitivity to the carrier ITD existed even when the envelope ITD was zero. The effect of the envelope was important, however, as modulation significantly improved the carrier ITD sensitivity. For this subject, ITD sensitivity was best when the carrier and envelope both encoded ITD, indicating the information was combined, and the subject's thresholds for the Whole-Wave condition fell to levels below those for 100-pps un-modulated pulse trains.
It would be of interest to understand why subject C299 alone demonstrated a dramatic improvement in carrier ITD sensitivity. Previous studies have reported exceptional BiCI subjects with ongoing ITD-sensitivity to pulse rates of 800 or 1000 pps (Laback et al., 2007; van Hoesel et al., 2009). These same subjects also had exceptional ITD sensitivity to pulse rates of 100 pps, where thresholds were often below 50 μs. C299, however, shows good but not exceptional ITD sensitivity at lower un-modulated (100 pps) or modulated (100 Hz) rates, with thresholds usually at the median of the six subjects studied here (Table Table I. and Fig. 4). It seems that C299 is exceptional in acquiring 1000-pps ITD-sensitivity as a result of modulation, and is not simply a subject displaying an outstanding overall ITD-sensitivity.
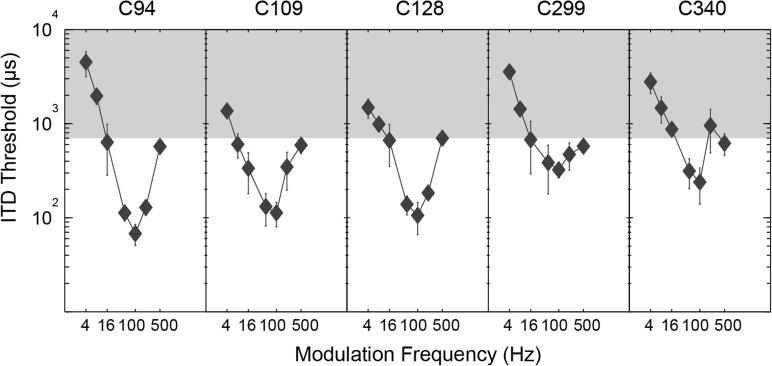
Figure 4.
ITD threshold plotted as a function of modulation frequency for five subjects. Error bars are standard deviations for multiple runs. Shaded regions indicate ITDs greater than naturally experienced by humans.
Comparison with other human BiCI studies
No previous psychophysical studies directly address whether modulation improves the carrier ITD sensitivity for pulses near 1000 pps, but previous results do provide relevant evidence. van Hoesel and Tyler (2003) measured thresholds to ITD encoded in the whole waveform using an 800-pps carrier SAM modulated at 50 Hz. The 194-μs mean threshold for 3 subjects is somewhat higher than the mean of 136 μs measured for three subjects using a 6000-pps carrier modulated at 100 Hz (van Hoesel, 2007), but compares closely with the mean of 184 μs for 6 subjects also using carriers of 6000 pps (van Hoesel et al., 2009). (For these comparisons thresholds were scaled to equal levels of d′. It is acknowledged that comparisons of absolute thresholds across studies can be confounded by subject or methodological differences.) Since ITD sensitivity at rates near 6000 pps is presumed to be poor and to not contribute to BiCI lateralization, the similar thresholds in human BiCI users for 800 and 6000-pps carriers indicate the 800-pps carrier did not contribute to ITD sensitivity. Conversely, van Hoesel and Tyler (2003) also measured Envelope-Only ITD threshold with 1 of their subjects using the 800-pps carrier. The subject's Envelope-Only ITD threshold was more than twice their Whole-Wave threshold (290 vs 120 μs). The poorer Envelope-Only threshold indicates sensitivity to the ITD of the 800-pps carrier. Since the subject showed no sensitivity to ITD in an un-modulated, 800-pps pulse train, the improved Whole-Wave performance is also consistent with modulation improving sensitivity to ITD encoded in the carrier. In another study, lateralization was measured with pulse trains at rates between 100 and 800 pps modulated by a 12.5-Hz trapezoid (Majdak et al., 2006). Whole-Wave and Envelope-Only ITD sensitivity were measured and compared within the same four subjects. With 800-pps carriers, 2 of 4 subjects' lateralization with the Whole-Wave ITD condition were significantly better than with the Envelope-Only condition. Since sensitivity to the 800-pps carrier was not compared to an un-modulated condition, the possibility exists that the two subjects showing sensitivity at 800 pps were simply demonstrating an existing ITD sensitivity to high-rate pulses, irrespective of modulation. The existing data, like our results, is not consistent across subjects in terms of the impact of amplitude modulation on sensitivity to carrier ITD. Generally, modulation does not improve carrier ITD sensitivity, but like the one subject in the present study, exceptional individuals do show a substantial improvement when ITD is encoded in the carrier.
Comparison with physiological results
For five of the six subjects, sensitivity to the carrier ITD of high-rate pulse trains was not improved by amplitude modulation. This finding differs with the single neuron data from the cat IC. A number of differences between the two experiments may explain the discrepancy. In the cat physiological experiments, neurons often only displayed ITD sensitivity for a narrow range of stimulus level, and each unit was tested at the level producing optimal ITD tuning. In the human subjects, the stimulation level could not be optimized for ITD tuning on a fiber-by-fiber basis and may result in only a fraction of the neurons operating at the optimal level to encode ITD. More generally, even when individual neurons show sharp ITD tuning, at a later stage of auditory processing their responses must be combined (or pooled) to form the percept of lateral position. This process is not precisely understood in normal or electrical hearing. It may be that some aspect of the population ITD coding is abnormal in both the acutely deafened cats and in the human subjects, but its impact only becomes apparent in behavioral measures such as the human psychophysics.
BiCI physiological studies also differ from human behavioral data in that anesthetized cats show a lower rate-limit for ITD sensitivity to un-modulated pulses [10 to 200 pps (Smith and Delgutte, 2007)] as compared to awake human subjects [250 to 600 pps (van Hoesel, 2007)]. A preliminary study (Chung et al., 2012) has shown that anesthesia itself may be responsible for this difference in rate limit, as awake rabbits exhibit rate limits that are substantially higher than anesthetized cats or rabbits and more similar to those seen with human psychophysics. Since the modulator's ability to restore responses to the high-rate carrier is presumed to depend on an interaction between the modulator frequency and the carrier rate (Smith and Delgutte, 2008; Colburn et al., 2009), a different combination of these variables may be necessary to produce carrier sensitivity in awake, BiCI subjects than was found to be effective in anesthetized cats.
Finally, acutely deafened cats were used in the physiology study whereas it has been shown that congenitally deafened cats have poorer ITD tuning as compared to their acutely deafened counterparts (Hancock et al., 2010b). While the relatively late onset of hearing impairment in our human subjects suggests the development of normal binaural hearing, their 2 to 14 yrs of binaural deprivation may have resulted in deterioration of their binaural circuits as compared to the acutely deafened animals. This hypothesis is weakened by (1) more recent data which show that ITD tuning from long-term deafened cats is closer to that of acutely deafened cats than it is to that of congenitally deafened cats (Hancock et al., 2010a) and (2) results showing no indication that the length of binaural deprivation degrades ITD sensitivity provided it is preceded by a period of normal binaural development (Litovsky et al., 2010). While the effect of binaural deprivation cannot be ruled out, it appears unlikely to explain the difference between results from our human subjects and the physiological results measured in cat.
EXPERIMENT 2: FREQUENCY DEPENDENCE OF ENVELOPE ITD SENSITIVITY
In Experiment 1, most subjects showed no sensitivity to ITD encoded in a modulated, high-rate carrier, but all subjects showed sensitivity to the ITD encoded in the envelope. Experiment 2 measured the envelope sensitivity for SAM waveforms whose modulation frequencies spanned the modulation spectrum of speech. Several aspects of envelope ITD sensitivity were examined. After preliminary pilot studies, three experiments (Experiments 2a, 2b, and 2c) were designed. Threshold measurements for all three experiments were then performed concurrently with runs intermingled in pseudo-random order. At least three threshold measurements were made for each condition reported. Five of the research subjects from Experiment 1 participated in Experiment 2a. Of these five, four also completed Experiments 2b and 2c. Electrode pair selection and the adaptive threshold procedure were identical to those used in Experiment 1.
Experiment 2a: Envelope ITD sensitivity as a function of SAM modulation frequency
Stimuli
Envelope-Only ITD sensitivity was measured at the sinusoidal modulation frequencies of 4, 8, 16, 50, 100, 200, and 500 Hz using a 1000-pps carrier. Waveforms were again computed using the modulator of Eq. 1 without amplitude compression or ramping. Envelope-Only ITD coding was used to most nearly replicate the condition currently experienced by today's BiCI listeners, wherein pulse carriers are not informative with respect to ITD. For the fm frequencies of 4, 8, and 16 Hz the stimulus duration was reduced from 300 to 250 ms to present only an integer number of modulator periods and thereby prevent abrupt offset transients. When the current amplitudes from Experiment 1 were used at different values of fm, subjects reported the stimuli to be slightly softer (at low fm) or louder (at high fm) than most comfortable. Therefore stimulus currents were readjusted. The procedure was the same as that used for Experiment 1 (Sec. 2A3) except it was repeated at each fm and iteratively adjusted until the subjects settled on a single pair of amplitudes that produced the closest match to most-comfortable at all modulation frequencies. Once chosen, this single pair of current amplitudes was used for all Experiment 2a modulation frequencies so that all were tested at the same peak and rms levels. The most comfortable loudness was typically reported to occur at the fm frequency of 100 Hz; however, the difference at other values of fm was modest and even at the extreme values of 4 and 500 Hz subjects reported the stimulus to be comfortable.
Results
Figure 4 shows ITD sensitivity as a function of modulation frequency for five subjects. For every subject, the best ITD threshold occurred at an fm of 100 Hz, where the group mean was 154 μs. Individual 100-Hz thresholds ranged from 68 to 245 μs, a nearly fourfold difference. Large differences in absolute sensitivity across subjects, such as those seen here, are common in CI psychophysics. In contrast, the pattern of ITD sensitivity for each subject is remarkably similar, showing a V (or bandpass) shape with threshold rising as fm varies above or below the 100-Hz best frequency. The shaded region in Fig. 4 indicates values of ITD that are outside the range human listeners experience naturally. For modulation frequencies below 50 Hz, SAM ITD sensitivity becomes extremely poor, with many thresholds outside the range listeners would normally experience and therefore not useful in providing binaural benefit.
A repeated-measures ANOVA showed a highly significant effect of fm on ITD sensitivity (F[6,24] = 15.0, p < 0.0001) and post hoc analysis (Tukey's HSD, p < 0.05) showed a significant decrease in ITD threshold for each fm frequency from 4 to 50 Hz, no significant difference between 50, 100, and 200 Hz, and a significant increase in ITD threshold between 100 and 500 Hz.
Experiment 2b: Envelope ITD sensitivity: Effect of waveform shape
Stimuli
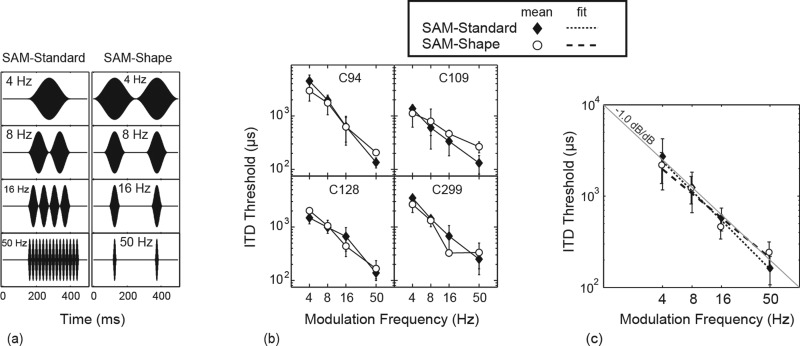
As the modulation frequency drops from 50 to 4 Hz, several waveform parameters co-vary. Among these is the shape of each modulation cycle, which changes as a function of fm such that at lower fm there is a decrease in onset and offset slope. Also, because the stimulus duration used in Experiment 2a was roughly constant, lower values of fm result in a reduction in the number of the cycles presented. In both acoustic hearing (Hafter and Dye, 1983) and CI hearing (van Hoesel, 2008), there is evidence that listeners integrate multiple cycles of a stimulus to improve detection. Optimal integration predicts that the threshold is reduced by , where n is the number of independent presentations (or “looks”). Although for many conditions integration is found to be less than optimal (McFadden and Moffitt, 1977), it is possible that the smaller number of cycles contributes to the poorer ITD-sensitivity seen at low modulation frequencies. In Experiment 2b, an additional set of stimuli were designed to try to isolate the effect of waveform shape on ITD sensitivity at fm frequencies between 4 and 50 Hz.
In Fig. 5a, the panels of the left column show the fixed duration SAM waveform (previously used in Experiment 2a) at the modulation frequencies 4, 8, 16, and 50 Hz. This will be referred to as the SAM-Standard stimulus. For SAM-Standard stimuli the shape and number of modulation cycles all vary as a function of fm. The right panels of Fig. 5a show the SAM-Shape stimuli, in which the shape of the modulation cycles varies with fm (as in the SAM-Standard condition), but in which the number of cycles is fixed at 2 and the time between cycle peaks is fixed at 250 ms.2 For the SAM-Shape condition, the modulator of Eq. 1 was again used to generate waveforms; however, periods of zero stimulation were inserted between modulation cycles such that the modulation peaks were always 250 ms apart. No additional ramping was used. The SAM-Shape waveforms of Experiment 2b used the same binaural amplitude level used in Experiment 2a. This produced equal peak current levels but a reduced rms level, and subjects reported the loudness level to drop for the SAM-Shape conditions with fm of 8, 16, and 50 Hz.
Figure 5.
(a) Schematic illustrations of the stimuli used in Experiment 2b. For the SAM-Standard waveform (left panels), the modulator shape and the number and repetition rate of modulator cycles vary with modulation frequency. For the SAM-Shape waveform (right panels), the modulator shape varies as for the SAM-Standard waveform, but the number and repetition rate of cycles are held constant. (b) ITD thresholds for four subjects as a function of fm for the two conditions. (c) Group means and linear regression fits of data in (b). Also plotted for reference is a thin gray line at −1.0 dB/dB slope. Correlation statistics for the fitted lines and comparisons with the reference slope are shown in Table Table II..
Results
Figure 5b displays the individual ITD thresholds, as a function of fm, for the four subjects who completed Experiment 2b. The SAM-Standard thresholds are those previously described in Experiment 2a. Again, while absolute thresholds for individual subjects vary across a broad range, the trend of the effect due to fm shows a marked similarity across all subjects. For the SAM-Shape stimulus, ITD sensitivity degrades in a pattern very similar to that for the SAM-Standard condition. A repeated-measures ANOVA with frequency and stimulus type as fixed factors showed no significant difference between the SAM-Standard and SAM-Shape conditions (F[1,9] = 1.26, p = 0.34). There was a significant effect of modulation frequency (F[3,9] = 14.22, p < 0.001) and no interaction between modulation frequency and stimulus condition (F[3,9] = 1.48, p = 0.283). The SAM-Shape condition attempted to isolate modulator cycle shape as the waveform feature which varied at different values of fm. The strong similarity between the SAM-Shape and SAM-Standard condition suggests that the same feature may also determine SAM-Standard ITD-sensitivity, at least for fm frequencies between 4 and 50 Hz.
Having observed a significant effect of modulation frequency on ITD sensitivity for each stimulus condition, the form of the dependence on fm was analyzed. Linear-regression models fit to the data from each condition are plotted in Fig. 5c along with mean across-subject thresholds as a function of fm. Data are plotted on a log-log scale with the span of the horizontal and vertical axis each set to 20 dB. For reference, a gray line running diagonally across the plot illustrates the slope of –1.0 dB/dB. A line fit to the data with slope –1.0 dB/dB would describe an inverse linear relationship between threshold and modulation frequency.
Table Table II. displays statistics of the linear models. Slopes of the lines fit to the SAM-Standard and the SAM-Shape data are close in magnitude and not significantly different from –1.0 dB/dB, and the amount of variance accounted for by the linear model is large, in both cases being greater than 85%. Both the SAM-Standard and the SAM-Shape stimuli are well characterized by an inverse linear dependence of threshold on waveform shape, independent of whether the number of cycles and repetition rate remained fixed or varied.
Table II.
Summary of statistics for linear regression fits to data of experiment 2b. The p values in the right column are for the hypothesis that the fitted slopes are different than –1.0 (two sided t-test; df: 14). A slope of −1.0 dB/dB describes an inverse linear relationship between threshold and modulation frequency.
| Condition | R2 | Slope of fit (dB/dB) | p value for slope ≠ –1.0 |
|---|---|---|---|
| SAM-Standard | 0.87 | –1.1 | <0.522 |
| SAM-Shape | 0.86 | –0.9 | <0.245 |
Experiment 2c: Envelope ITD sensitivity: Effect of higher carrier rate
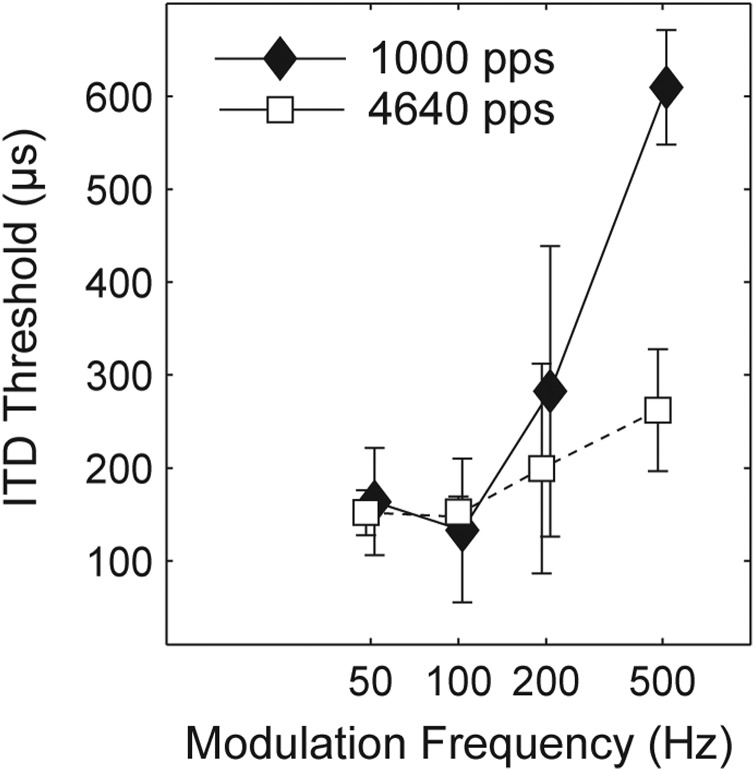
Stimuli
Thresholds were measured at 50, 100, 200, and 500-Hz fm using the higher carrier rate of 4640 pps. At this higher pulse rate the stimuli sounded considerably louder and were rated by the subjects as too loud for comfortable testing. Therefore, current levels were adjusted downward to produce centered images at a comfortable loudness level to match the same sensation level used at 1000 pps. van Hoesel (2007) found ITD sensitivity to be poorer when measured at 60% of dynamic range (DR) as opposed to when measured at 80% of DR. In the experiment conducted here, adjusting currents to produce comfortable loudness approximates conditions of equal % of DR. This also corresponds to the fitting of a clinical speech processor, where current levels are adjusted at different carrier rates in order to maintain comfortable listening levels. For Experiment 2c, once a comfortable current level was determined for the 4640-pps carrier rate, the same level was used for all values of fm.
Results
Figure 6 plots mean thresholds for four subjects at the 4640-pps carrier rate along with the mean 1000-pps data previously described in Experiment 2a. For the higher sampling rate, the increase in thresholds above 100 Hz is reduced, indicating less degradation of sensitivity as the modulation rate increases. A repeated-measures ANOVA with fm and fc as fixed factors showed a significant effect of carrier rate (F[1,9] = 12.49, p < 0.04). It can be seen in Fig. 6 that much of the difference in rate occurs at fm = 500 Hz. Post hoc comparisons at each modulation frequency confirm that the effect of carrier rate is only significant at 500 Hz (Tukey's HSD, p < 0.05). The poor mean threshold at fm = 500 Hz for the 1000-pps sampling rate is not surprising given that the envelope is poorly represented at this rate. In summary, a higher carrier rate appears to support better ITD sensitivity at higher modulation frequencies. For the carrier rates we tested, the effect is only significant when the modulation frequency encoding the ITD approaches half the pulse rate, and ITD sensitivity is not significantly degraded as long as the carrier rate is at least 5 times the modulation frequency of the envelope carrying the ITD. In contrast to the physiological results (Smith and Delgutte, 2008) there is no evidence that high carrier rates degrade envelope ITD sensitivity.
Figure 6.
Mean ITD threshold (four subjects) as a function of modulation frequency, with carrier rate as a parameter. Thresholds between carrier rates only differ significantly for the modulation frequency 500 Hz. (Symbols are plotted with a small horizontal offset to enhance their visibility.)
Discussion
In accord with previous studies (van Hoesel, 2007; van Hoesel et al., 2009), Experiment 2 (Figs. 46) showed a decrease in BiCI envelope ITD sensitivity as the modulation frequency increased above 100 Hz. In these earlier studies, however, 100 Hz was the lowest modulation frequency tested. In the current study, ITD thresholds were also measured for modulation frequencies down to 4 Hz. When fm dropped below 50 Hz, ITD sensitivity decreased substantially. The result was a V-shaped curve of ITD threshold versus fm, with very poor ITD sensitivity for the modulation frequencies where most of the energy in the envelope of speech occurs.
Limits of ITD sensitivity at lower values of fm
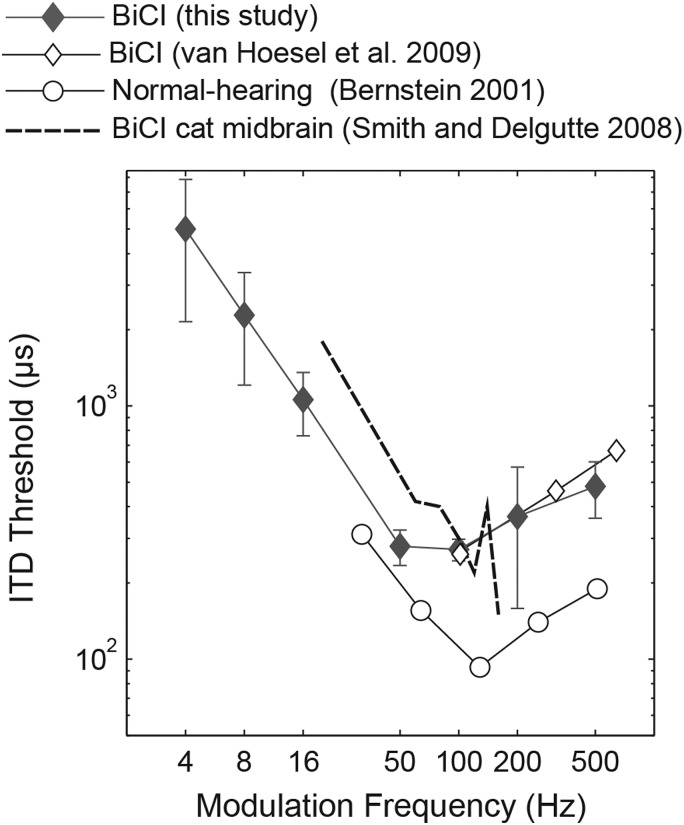
Measures of envelope ITD threshold with NH listeners are relatively uncommon for fm below 50 Hz. Bernstein (2001) reported ITD thresholds for SAM modulation of a 4000-Hz carrier with fm as low as 32 Hz. We have re-plotted these data in Fig. 7 along with mean thresholds from our BiCI subjects from Experiment 2. (Here our thresholds are scaled upward to compare with a detection level of d′ = 1 used in the NH study.) NH subjects had a best SAM ITD for the modulation frequency of 128 Hz, much like the BiCI subjects' best fm of 100 Hz, although the NH mean threshold at 128 Hz is 90 μs, 3 times lower than the BiCI group mean of 312 μs. For the NH group, thresholds decreased at a rate of –0.9 dB/dB over the range of fm from 32 to 128 Hz, which compares closely with a rate of –1.1 dB/dB for BiCI subjects over the range of fm from 4 to 50 Hz. The similar drop in envelope ITD sensitivity for low modulation frequencies suggests it is not unique to CI users and may be caused by similar mechanisms in NH listeners.
Figure 7.
Comparison of SAM envelope ITD sensitivity. Human BiCI thresholds from this study using a carrier of 1000 pps for fm of 4 to 16 Hz and 5000-pps for fm of 50 to 500 Hz (carriers chosen for comparison with previous studies). Additional human BiCI thresholds are from Fig. 1 in van Hoesel et al. (2009) using a 6000-pps carrier. NH human psychophysics are from Fig. 9 in Bernstein (2001) using a 4-kHz tone carrier. BiCI single-neuron data from cat IC is from Fig. 5(b) in Smith and Delgutte (2008) using a 1000-pps carrier. All thresholds are specified at a sensitivity of d′ = 1.
Smith and Delgutte (2008) measured neural ITD just-noticeable-differences (JNDs) from bilaterally implanted cats when ITD was encoded only in the SAM envelope of a 1000-pps pulse train. Their average results across neurons are also plotted in Fig. 7. Envelope JNDs were calculated from the rate-versus-ITD functions of neurons in the cat mid-brain. The functions were mostly Gaussian shaped, and the width and onset-time of the functions decreased as 1/fm, resembling the change in shape of the stimuli. As with the human psychophysical data, the ITD JND was approximately a constant fraction of the modulation period. The neural JND is computed from the change in mean firing rate normalized by the variance of the neural response. A straight-forward explanation of the improved ITD sensitivity for the neural data, and for the human psychophysics, is that the higher modulation frequencies simply cause a criterion firing rate to occur at a shorter interaural delay, resulting in a lower ITD threshold.
Results from Experiment 2b (Fig. 5 and Table Table II.) show a strong correlation between envelope ITD thresholds and waveform shape. This is evidence that the shape of the modulation cycle determines ITD sensitivity to SAM waveforms, and we hypothesize that the onset slope is the important waveform characteristic. Recently, Laback et al. (2011) measured envelope ITD sensitivity in seven BiCI subjects using trapezoidal modulation. They varied the slope of the trapezoids with other parameters held constant, using slopes of 6%, 8%, and 12% of DR per millisecond (%DR/ms). They found no change in ITD threshold as a function of slope. As the authors pointed out, the slopes tested may have been outside the range for which slope impacts ITD sensitivity. To explore this possibility, we estimated the SAM modulation frequencies of our study that correspond to their trapezoid slopes by equalizing the time for each type of signal to rise from threshold to peak amplitude.3 The estimated equivalent fm values for the trapezoid slopes were 40 Hz (6% DR/ms), 54 Hz (8% DR/ms), and 80 Hz (12% DR/ms). The lack of an effect on ITD sensitivity in this range is consistent with our results which show insignificant differences between thresholds for 50 and 100 Hz (Sec. 3A2, Fig. 6). In the present study, a significant improvement in ITD threshold only occurred for modulation frequencies from 4 to 50 Hz. Estimates of onset slopes corresponding to this range are 0.6 to 8% DR/ms, a range that is mostly below that tested in the Laback et al. (2011) study.
For NH listeners, several studies have reported measures of envelope ITD sensitivity made while methodically varying features of the envelope shape (Bernstein and Trahiotis, 2009; Klein-Hennig et al., 2011; Laback et al., 2011). Each of these studies included measurements of ITD thresholds as the slope of the envelope was increased. While each study used different envelope shapes and different techniques for increasing the envelope slope, results were consistent in showing that NH envelope ITD sensitivity improved as the slope of the envelope was increased, although the improvement plateaued after a certain level of slope was reached. The improvement in NH envelope ITD sensitivity with increased envelope onset slope is consistent with the interpretation of Experiment 2b which infers that the slope of the modulation cycle is the important correlate for BiCI ITD thresholds.
These same studies also revealed an effect of off-time on ITD sensitivity with NH listeners, and in one study, with BiCI listeners (Laback et al., 2011). Increasing the off-time (the period of zero or below-threshold silence inserted between individual envelope cycles) produced an increase in envelope ITD sensitivity, although again only up to a certain duration, after which sensitivity plateaued. It is unlikely that the off-time parameter impacted thresholds measured with the SAM-Shape stimuli of Experiment 2b, because off-times for this condition range from 63 to 230 ms, durations considerably greater than the 10 to 20 ms at which the effect of off-times plateaued for both NH and BiCI subjects. For the Standard-SAM condition, it is possible that as modulation frequency increased, the benefit of increased envelope slope and increased number of modulation cycles was counteracted by the detrimental effect of reduced off-times. Experiments 2a and 2b were designed to investigate the ITD sensitivity for the SAM modulation spectrum that is important for speech, and were not methodical explorations of the envelope features which determine envelope ITD sensitivity. Nevertheless they demonstrate that the inverse of modulation frequency is an excellent predictor of envelope ITD sensitivity in the range of fm from 4 to 50 Hz.
Limits of ITD sensitivity at higher values of fm
Figures 46 show that the benefit of increasing modulation frequency only persisted up to a frequency of 100 Hz. Above that fm, ITD sensitivity began to deteriorate. Some of this loss of sensitivity can be attributed to insufficient sampling of the envelope by the carrier pulse train; however, this effect was only significant when the modulation frequency approached one-half the carrier rate. When the carrier rate was 4640 pps and at least 10 times greater than the maximum modulation frequency, the decline in ITD sensitivity was reduced. For the range of fm between 100 and 500 Hz, the rate of change in envelope ITD threshold as a function of modulation frequency was –0.35 dB/dB.
SUMMARY AND CONCLUSIONS
Psychophysical and physiological studies of BiCI ITD-sensitivity both show the common feature of an upper pulse-rate limit. One factor likely to contribute to this limit is the presence of adaptation or saturation in the auditory pathway acting to block sustained neural responses. Experiment 1 tested whether 100% amplitude modulation applied to 1000-pps pulses would periodically allow recovery from this adaptation, thereby producing a sustained response instead of the purely onset response seen for un-modulated high-rate pulses. The desired effect of the sustained response would be an improved ITD sensitivity to the high-rate pulses. One motivation for the study was to gain insight into the mechanisms producing the high pulse-rate limit on ITD sensitivity in BiCI listeners. A second motivation was to test whether modifying bilateral processors to encode the ITD of the incoming signal into the device's interaural carrier-delay might improve BiCI ITD sensitivity, given that the pulse trains undergo amplitude modulation as a result of CI speech processing. In general, even 100% amplitude modulation did not improve carrier ITD sensitivity at 1000 pps, with the notable exception of a single subject. These results indicate that, absent additional manipulations, patients are not likely to benefit from encoding ITD in the carriers pulse trains of their current bilateral sound processors.
While the 50-Hz SAM modulation used here was mostly unsuccessful at improving sensitivity to carrier ITD encoded in high-rate pulses, the fact that one subject did benefit is intriguing because this subject had otherwise good, but not exceptional, overall ITD sensitivity and because when carrier ITD and envelope ITD sensitivity were combined, thresholds fell below those of 100-pps pulse trains. Understanding the conditions for which modulation does convey high-rate sensitivity could be important in understanding the limitations BiCI listeners face in perceiving ITD cues. It is possible that there is interaction between carrier rate and modulation frequency, and ITD sensitivity to high-rate carriers could arise at different combinations of these two parameters than those tested here.
Results from our Experiment 2 showed that BiCI envelope ITD thresholds at modulation frequencies of 4 to 16 Hz were poor, with most thresholds greater than the maximum ITD that would naturally be experienced by human listeners. ITD thresholds measured with additional stimuli showed that it is the shape of the low fm modulators that determines sensitivity, while low modulation cycle counts or cycle rates have minimal effects. Speech contains both slow-onset, long-duration modulations at the syllable rate, as well as fast-onset modulations, most typically at consonant boundaries. The poor envelope ITD sensitivity for the former (similar to those seen in Fig. 5 for low fm) indicates that slow modulations are not likely to deliver envelope ITD information useful for a BiCI binaural benefit. For fast-onset modulations, even when these modulations are relatively few and infrequent (similar to the 50-Hz SAM-Shape stimuli in Fig. 5), ITD thresholds are low enough to indicate sensitivity potentially useful for localization or sound segregation.
ACKNOWLEDGMENTS
This work was supported by NIH Grant Nos. R01-DC005775 and R01-DC007528. Advanced Bionics provided the binaural-research-interface hardware.
Portions of this work were previously presented at the 34th Midwinter Research Meeting of the Association for Research in Otolaryngology, Baltimore, MD (2011) and at the Conference on Implantable Auditory Prostheses, Asilomar (2011).
Footnotes
In fact, the ITD between the carrier pulse trains oscillates between zero and the inter-pulse period, with the oscillation rate determined by the frequency mismatch between two processors' system clocks. For example, when the carrier pulse ITD was measured for two commercial processors identically programmed with a typical CIS implementation, it cycled between –500 and +500 μs every 4 s. If any sensitivity to the pulse-train ITD did exist, this varying cue might be even more confounding than if the ITD was incorrect but constant.
The use of the term “modulation frequency” and the variable fm are not completely accurate for the SAM-Shape stimulus because the modulator cycles-per-second is fixed by design. Noting this, use of the variable fm is retained for notational convenience.
Onset slopes for sinusoidal stimuli were estimated using a methodology similar to that used by Laback et al. (2011) for trapezoidal stimuli to facilitate comparisons to those data. DR was defined as the current range from threshold to maximum-comfortable-level measured using unmodulated 1000-pps pulse trains. The slope of the sinusoidal stimulus was computed as: Slope = %DR/Δt, where %DR is the percentage of the DR represented by the threshold to the peak current of the SAM envelope (approximately 60%DR at the most comfortable listening level) and Δt is the time between the threshold and peak levels of the SAM envelope waveform. The use of a single scalar value to estimate the onset slope of a sinusoidal envelope is, of course, an approximation since the instantaneous slope of the envelope is a function of time that varies above and below this estimate. Across all electrodes tested, thresholds for 1000 pps, un-modulated pulses ranged from 109 to 149 μAmps, SAM envelope peak currents ranged from 700 to 1300 μAmps, and thresholds were between 11% and 20% of the envelope peak current.
References
- Bernstein, L. R. (2001). “ Auditory processing of interaural timing information: new insights,” J. Neurosci. Res. 66, 1035–1046. 10.1002/jnr.10103 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (1994). “ Detection of interaural delay in high-frequency sinusoidally amplitude-modulated tones, two-tone complexes, and bands of noise,” J. Acoust. Soc. Am. 95, 3561–3567. 10.1121/1.409973 [DOI] [PubMed] [Google Scholar]
- Bernstein, L. R., and Trahiotis, C. (2009). “ How sensitivity to ongoing interaural temporal disparities is affected by manipulations of temporal features of the envelopes of high-frequency stimuli,” J. Acoust. Soc. Am. 125, 3234–3242. 10.1121/1.3101454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blauert, J. (1997). Spatial Hearing (MIT Press, Cambridge, MA: ), pp. 36–200. [Google Scholar]
- Bronkhorst, A. W., and Plomp, R. (1988). “ The effect of head-induced interaural time and level differences on speech intelligibility in noise,” J. Acoust. Soc. Am. 83, 1508–1516. 10.1121/1.395906 [DOI] [PubMed] [Google Scholar]
- Carhart, R., Tillman, T. W., and Johnson, K. R. (1967). “ Release of masking for speech through interaural time delay,” J. Acoust. Soc. Am. 42, 124–138. 10.1121/1.1910541 [DOI] [PubMed] [Google Scholar]
- Chung, Y., Hancock, K., Nam, S., and Delgutte, B. (2012). “ The upper limit of temporal coding of electric pulse trains in the inferior colliculus of awake animals wearing cochlear implants,” Assoc. Res. Otolaryngol. Abstr. 796.
- Colburn, H. S., Chung, Y., Zhou, Y., and Brughera, A. (2009). “ Models of brainstem responses to bilateral electrical stimulation,” J. Assoc. Res. Otolaryngol. 10, 91–110. 10.1007/s10162-008-0141-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drullman, R., Festen, J. M., and Plomp, R. (1994). “ Effect of temporal envelope smearing on speech reception,” J. Acoust. Soc. Am. 95, 1053–1064. 10.1121/1.408467 [DOI] [PubMed] [Google Scholar]
- Elliott, T. M., and Theunissen, F. E. (2009). “The modulation transfer function for speech intelligibility,” PLoS Comput. Biol. 5, e1000302. 10.1371/journal.pcbi.1000302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham, D. W., Ashmead, D. H., Ricketts, T. A., Labadie, R. F., and Haynes, D. S. (2007). “ Horizontal-plane localization of noise and speech signals by postlingually deafened adults fitted with bilateral cochlear implants,” Ear Hear. 28, 524–541. 10.1097/AUD.0b013e31806dc21a [DOI] [PubMed] [Google Scholar]
- Hafter, E. R., and Dye, R. H., Jr. (1983). “ Detection of interaural differences of time in trains of high-frequency clicks as a function of interclick interval and number,” J. Acoust. Soc. Am. 73, 644–651. 10.1121/1.388956 [DOI] [PubMed] [Google Scholar]
- Hancock, K., Noel, V., and Delgutte, B. (2010a). “ Neural coding of ITD with bilateral cochlear implants: Effects of auditory experience,” Assoc. Res. Otolaryngol. Abstr. 795.
- Hancock, K. E., Noel, V., Ryugo, D. K., and Delgutte, B. (2010b). “ Neural coding of interaural time differences with bilateral cochlear implants: effects of congenital deafness,” J. Neurosci. 30, 14068–14079. 10.1523/JNEUROSCI.3213-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann, W. M., and Raked, B. (1989). “ On the minimum audible angle—a decision theory approach,” J. Acoust. Soc. Am. 85, 2031–2041. 10.1121/1.397855 [DOI] [PubMed] [Google Scholar]
- Henning, G. B. (1974). “ Detectability of interaural delay in high-frequency complex waveforms,” J. Acoust. Soc. Am. 55, 84–90. 10.1121/1.1928135 [DOI] [PubMed] [Google Scholar]
- Houtgast, T., and Steeneken, H. J. M. (1985). “ A review of the MTF concept in room acoustics and its use for estimating speech intelligibility in auditoria,” J. Acoust. Soc. Am. 77, 1069–1077. 10.1121/1.392224 [DOI] [Google Scholar]
- Klein-Hennig, M., Dietz, M., Hohmann, V., and Ewert, S. D. (2011). “ The influence of different segments of the ongoing envelope on sensitivity to interaural time delays,” J. Acoust. Soc. Am. 129, 3856–3872. 10.1121/1.3585847 [DOI] [PubMed] [Google Scholar]
- Klumpp, R. G., and Eady, H. R. (1956). “ Some measurements of interaural time difference thresholds,” J. Acoust. Soc. Am. 28, 859–860. 10.1121/1.1908493 [DOI] [Google Scholar]
- Laback, B., Majdak, P., and Baumgartner, W. D. (2007). “ Lateralization discrimination of interaural time delays in four-pulse sequences in electric and acoustic hearing,” J. Acoust. Soc. Am. 121, 2182–2191. 10.1121/1.2642280 [DOI] [PubMed] [Google Scholar]
- Laback, B., Pok, S. M., Baumgartner, W. D., Deutsch, W. A., and Schmid, K. (2004). “ Sensitivity to interaural level and envelope time differences of two bilateral cochlear implant listeners using clinical sound processors,” Ear Hear. 25, 488–500. 10.1097/01.aud.0000145124.85517.e8 [DOI] [PubMed] [Google Scholar]
- Laback, B., Zimmermann, I., Majdak, P., Baumgartner, W. D., and Pok, S. M. (2011). “ Effects of envelope shape on interaural envelope delay sensitivity in acoustic and electric hearing,” J. Acoust. Soc. Am. 130, 1515–1529. 10.1121/1.3613704 [DOI] [PubMed] [Google Scholar]
- Levitt, H. (1971). “ Transformed up-down methods in psychoacoustics,” J. Acoust. Soc. Am. 49(2 ), 467–477. 10.1121/1.1912375 [DOI] [PubMed] [Google Scholar]
- Litovsky, R. Y., Jones, G. L., Agrawal, S., and van Hoesel, R. (2010). “ Effect of age at onset of deafness on binaural sensitivity in electric hearing in humans,” J. Acoust. Soc. Am. 127, 400–414. 10.1121/1.3257546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R. Y., Parkinson, A., and Arcaroli, J. (2009). “ Spatial hearing and speech intelligibility in bilateral cochlear implant users,” Ear Hear. 30, 419–431. 10.1097/AUD.0b013e3181a165be [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litovsky, R., Parkinson, A., Arcaroli, J., and Sammeth, C. (2006). “ Simultaneous bilateral cochlear implantation in adults: A multicenter clinical study,” Ear Hear. 27, 714–731. 10.1097/01.aud.0000246816.50820.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loizou, P. C., Hu, Y., Litovsky, R., Yu, G., Peters, R., Lake, J., and Roland, P. (2009). “ Speech recognition by bilateral cochlear implant users in a cocktail-party setting,” J. Acoust. Soc. Am. 125, 372–383. 10.1121/1.3036175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long, C. J., Eddington, D. K., Colburn, H. S., and Rabinowitz, W. M. (2003). “ Binaural sensitivity as a function of interaural electrode position with a bilateral cochlear implant user,” J. Acoust. Soc. Am. 114, 1565–1574. 10.1121/1.1603765 [DOI] [PubMed] [Google Scholar]
- Macpherson, E. A., and Middlebrooks, J. C. (2002). “ Listener weighting of cues for lateral angle: The duplex theory of sound localization revisited,” J. Acoust. Soc. Am. 111, 2219–2236. 10.1121/1.1471898 [DOI] [PubMed] [Google Scholar]
- Majdak, P., Laback, B., and Baumgartner, W. D. (2006). “ Effects of interaural time differences in fine structure and envelope on lateral discrimination in electric hearing,” J. Acoust. Soc. Am. 120, 2190–2201. 10.1121/1.2258390 [DOI] [PubMed] [Google Scholar]
- McFadden, D., and Moffitt, C. M. (1977). “ Acoustic integration for lateralization at high frequencies,” J. Acoust. Soc. Am. 61, 1604–1608. 10.1121/1.381473 [DOI] [PubMed] [Google Scholar]
- Middlebrooks, J. C., and Green, D. M. (1991). “ Sound localization by human listeners,” Annu. Rev. Psychol. 42, 135–159. 10.1146/annurev.ps.42.020191.001031 [DOI] [PubMed] [Google Scholar]
- Nopp, P., Schleich, P., and D'Haese, P. (2004). “ Sound localization in bilateral users of MED-EL COMBI 40/40+ cochlear implants,” Ear Hear. 25, 205–214. 10.1097/01.AUD.0000130793.20444.50 [DOI] [PubMed] [Google Scholar]
- Poon, B. B., Eddington, D. K., Noel, V., and Colburn, H. S. (2009). “ Sensitivity to interaural time difference with bilateral cochlear implants: Development over time and effect of interaural electrode spacing,” J. Acoust. Soc. Am. 126, 806–815. 10.1121/1.3158821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayleigh, L. (1907). “ On our perception of sound direction,” Philos. Mag. 13, 214–232. [Google Scholar]
- Saberi, K. (1995). “ Some considerations on the use of adaptive methods for estimating interaural-delay thresholds,” J. Acoust. Soc. Am. 98, 1803–1806. 10.1121/1.413379 [DOI] [PubMed] [Google Scholar]
- Schleich, P., Nopp, P., and D'Haese, P. (2004). “ Head shadow, squelch, and summation effects in bilateral users of the MED-EL COMBI 40/40+ cochlear implant,” Ear Hear. 25, 197–204. 10.1097/01.AUD.0000130792.43315.97 [DOI] [PubMed] [Google Scholar]
- Seeber, B. U., and Fastl, H. (2008). “ Localization cues with bilateral cochlear implants,” J. Acoust. Soc. Am. 123, 1030–1042. 10.1121/1.2821965 [DOI] [PubMed] [Google Scholar]
- Senn, P., Kompis, M., Vischer, M., and Haeusler, R. (2005). “ Minimum audible angle, just noticeable interaural differences and speech intelligibility with bilateral cochlear implants using clinical speech processors,” Audiol. Neuro-Otol. 10, 342–352. 10.1159/000087351 [DOI] [PubMed] [Google Scholar]
- Smith, Z. M., and Delgutte, B. (2007). “ Sensitivity to interaural time differences in the inferior colliculus with bilateral cochlear implants,” J. Neurosci. 27, 6740–6750. 10.1523/JNEUROSCI.0052-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, Z. M., and Delgutte, B. (2008). “ Sensitivity of inferior colliculus neurons to interaural time differences in the envelope versus the fine structure with bilateral cochlear implants,” J. Neurophysiol. 99, 2390–2407. 10.1152/jn.00751.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2004). “ Exploring the benefits of bilateral cochlear implants,” Audiol. Neuro-Otol. 9, 234–246. 10.1159/000078393 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2007). “ Sensitivity to binaural timing in bilateral cochlear implant users,” J. Acoust. Soc. Am. 121, 2192–2206. 10.1121/1.2537300 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2008). “ Observer weighting of level and timing cues in bilateral cochlear implant users,” J. Acoust. Soc. Am. 124, 3861–3872. 10.1121/1.2998974 [DOI] [PubMed] [Google Scholar]
- van Hoesel, R. J. (2011). “ Bilateral cochlear implants,” in Auditory Prostheses: New Horizons, edited by Zeng F.-G., Popper A. N., and Fay R. R. (Springer, New York: ), pp. 13–57. [Google Scholar]
- van Hoesel, R. J., Jones, G. L., and Litovsky, R. Y. (2009). “ Interaural time-delay sensitivity in bilateral cochlear implant users: Effects of pulse rate, modulation rate, and place of stimulation,” J. Assoc. Res. Otolaryngol. 10, 557–567. 10.1007/s10162-009-0175-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoesel, R. J., and Tyler, R. S. (2003). “ Speech perception, localization, and lateralization with bilateral cochlear implants,” J. Acoust. Soc. Am. 113, 1617–1630. 10.1121/1.1539520 [DOI] [PubMed] [Google Scholar]
- Wightman, F. L., and Kistler, D. J. (1992). “ The dominant role of low-frequency interaural time differences in sound localization,” J. Acoust. Soc. Am. 91, 1648–1661. 10.1121/1.402445 [DOI] [PubMed] [Google Scholar]
- Wilson, B. S. (1997). “ The future of cochlear implants,” Br. J. Audiol. 31, 205–225. 10.3109/03005369709076795 [DOI] [PubMed] [Google Scholar]
- Zurek, P. M. (1993). “ Binaural advantages and directional effects of speech intelligibility,” in Acoustical Factors Affecting Hearing Aid Performance, edited by Studebaker G. A. and Hochberg I. (Allyn and Bacon, Boston: ), pp. 255–276. [Google Scholar]