Abstract
Parkinson's disease (PD) is characterized by typical extrapyramidal motor features and increasingly recognized non-motor symptoms such as working memory (WM) deficits. Using functional magnetic resonance imaging (fMRI), we investigated differences in neuronal activation during a motor WM task in 23 non-demented PD patients and 23 age- and gender-matched healthy controls. Participants had to memorize and retype variably long visuo-spatial stimulus sequences after short or long delays (immediate or delayed serial recall). PD patients showed deficient WM performance compared to controls, which was accompanied by reduced encoding-related activation in WM-related regions. Mirroring slower motor initiation and execution, reduced activation in motor structures such as the basal ganglia and superior parietal cortex was detected for both immediate and delayed recall. Increased activation in limbic, parietal and cerebellar regions was found during delayed recall only. Increased load-related activation for delayed recall was found in the posterior midline and the cerebellum. Overall, our results demonstrate that impairment of WM in PD is primarily associated with a widespread reduction of task-relevant activation, whereas additional parietal, limbic and cerebellar regions become more activated relative to matched controls. While the reduced WM-related activity mirrors the deficient WM performance, the additional recruitment may point to either dysfunctional compensatory strategies or detrimental crosstalk from “default-mode” regions, contributing to the observed impairment.
Introduction
Parkinson's disease (PD) has traditionally been recognized as a motor disorder, characterized by bradykinesia, tremor, rigidity and postural instability. Recent research, however, revealed a more complex picture of a multicentric neurodegeneration [1], [2], where non-motor symptoms such as neuro-psychiatric, autonomic, sensory, and sleep disturbances have a profound impact on patients' morbidity and quality of life [3]. Some non-motor features such as the REM-sleep behavior disorder (RBD), depression or hyposmia may even precede the motor symptoms by many years [4]. Cognitive impairment is one of the most common non-motor symptoms in PD. It has already been observed in initial disease stages and tends to worsen over time, developing into dementia in between up to 90% of PD cases [5], [6]. Even non-demented or de-novo PD patients may have deficits in executive functions such as planning, concept formation, rule use, and working memory (WM) [7], [8] similar to patients with frontal lobe lesions [9]. WM impairment, however, has been argued to be one of the most relevant cognitive deficits [10], [11]. In line with the role of dopamine in WM [12], [13], several studies suggested a link between fronto-striatal dopamine deficiency and cognitive impairment in PD [14], [15]. Given that WM is not a mental capacity [16]–[20], however, it is not surprising that WM impairments in PD are not uniform. There is evidence that visuo-spatial WM is predominantly affected even in medicated PD patients [15]–[17], [19]–[23] with the most specific impairment seen in the transformation of spatial WM information into action, i.e., “memory–motor transformations” [24]–[26] with increased load or retention time leading to further performance deterioration [25], [27].
Physiologically, motor sequence reproduction involves: (1) an internal representation of the sequence, (2) WM processes to maintain this representation, and (3) the transformation of acquired representations into sequences of motor commands. While there is a large body of work [24], [26], [28]–[34] on the neuronal correlates of motor sequence learning and more abstract/sensory WM processes (such as the n-back or Sternberg task) in PD, the neurobiological underpinnings of impaired memory–motor transformations are less well understood. In this context, it is interesting to note that during sequence-learning PD patients seem to recruit additional brain regions, which was interpreted as compensation for functionally impaired pathways in order to maintain a normal level of performance [28], [35], [36]. Whether this also holds true in the context of memory–motor transformations, in which pronounced deficits seem prevalent in PD, however, remains open. The current study thus investigated the neural basis underlying motor WM in PD using functional magnetic resonance imaging (fMRI). To probe memory–motor transformations, we implemented a sequence reproduction task in which a visuo-spatial sequence was followed either by a short or long retention interval and finally a cued manual reproduction [37]. The specific aims were to investigate (i) whether memory–motor transformations and hence motor WM performance is impaired in non-demented PD patients, (ii) whether PD patients show hyperactivation similar to those interpreted as compensatory networks in sequence learning and (iii) how these behavioral and neuronal effects are modulated by recall delay and WM load.
Methods
Participants
23 PD patients (mean age: 67.2±6.2 (SD), male: 14) and 23 age- and gender-matched healthy control (HC) subjects (mean age: 65±4.41 (SD), male: 13) were included into this study (Table 1). All patients fulfilled the standard UK Brain Bank criteria for PD [38]. The following inclusion criteria were employed: (a) no past history of psychiatric or neurological illness including dementia and mild cognitive impairment; (b) a score of at least 26 (out of 30) on the Mini Mental Status Examination (MMSE) (c) no prior exposure to neuroleptic or antidepressant agents (d) no history of substance abuse; (e) no past medical history of severe hypertension, cardiovascular disease, autoimmune disease, or diabetes mellitus; and f) no contraindications to MRI. Additionally, we collected data of the Montreal Cognitive Assessment Test (MOCA) [39] of 12 patients (mean [SD] 26.75±1.22) and of the Parkinson Neuropsychometric Dementia Assessment (PANDA) [40] for 18 patients (mean [SD] 25.37±4.25), also revealing no signs of dementia. Importantly, none of the patients presented with impairment in activities of daily living as assessed by a detailed anamnesis. Patients were not asked to withdraw their medication; therefore, all examinations were performed in the “on-state” (levodopa equivalent daily dose (LEDD) mean: 426.15±417.45 (SD) mg).
Table 1. Demographic and clinical data.
| PD | Controls | |
| N/Gender (male) | 23/14 | 23/13 |
| Age (years) | 67.2±6.2 | 65±4.4 |
| Education (years) | 13±3 | 14.9±3.9 |
| Disease duration (years) | 4.7±4.2 | n.a. |
| UPDRS-III | 23.9±16.1 | n.a. |
| Hoehn & Yahr | 1.5±0.9 | n.a. |
| PDQ-39 | 19.6±12.2 | n.a. |
| LEDD (mg) | 426.15±417.45 | n.a. |
| MMSE | 28.6±1.2 | 29.0±1.1 |
| Digit Span Forward (raw score) | 9±2.2 | 10.7±1.8 |
| Digit Span Backward (raw score) | 6.2±2.7 | 6.9±1.8 |
| Digit Span (standard score) | 10.6±3.2 | 12.2±2.2 |
| TMT-A (s) | 39.8±25.6 | 26.1±9 |
| TMT-B (s) | 88.9±53.7 | 56±20.9 |
Abbr.: PD, Parkinson's Disease; HC, Healthy Controls; SD, Standard Deviation; UPRDS, Unified Parkinson's Disease Rating Scale; PDQ, Parkinson's Disease Questionnaire; LEDD, Levodopa Equivalent Daily Dose; MMSE, Mini-Mental State Examination; TMT-A/B, Trail Making Test versions A and B; s, seconds; %, percent.
Before MRI scanning, all subjects underwent a clinical examination including the Unified Parkinson's Disease Rating Scale (UPDRS) [41], Hoehn and Yahr staging [42], Parkinson's Disease Questionnaire (PDQ-39) for quality of life [43], the Structured Clinical Interview for DSM-IV (SCID) to confirm absence of psychiatric comorbidity [44] and a neuropsychological test battery. The latter included the forward and backward digit span subtest of the Wechsler Memory Scale (WMS/WAIS) [45], the Trail Making Test versions A and B (TMT-A and TMT-B) [46] [47] as well as a 10 s finger-tapping test (performed three times on each side and averaged to reflect basic motor speed) and a pointing test (horizontal pointing with the index finger between two spots 30 cm apart; average time of three trials per side). All subjects were classified as right-handed by the Edinburgh inventory [48].
Ethics statement
Written informed consent was obtained from all participants prior to examination. The study had been approved by the local ethics committee of the RWTH Aachen University Hospital.
MR Imaging
Motor working-memory task
In the motor WM task performed in the scanner, subjects had to memorize and retype (on a response key pad) a visually presented spatial sequence. At the start of each event, a visual cue (the German word “Achtung”) was displayed for 500 ms, indicating the beginning of the next trial. The cue was followed by the target stimuli consisting of red dots displayed in a sequential order on a two-dimensional schematic drawing of a hand. Each trial probed either the left or right hand and involved the indication of four (stimulus duration: 2.9 s) or five (stimulus duration: 3.5 s) randomly chosen locations corresponding the sequence to be memorized. Following a delay interval of either 500 or 7000 ms a go-cue (green circle, presented for 500 ms), instructed the participants to reproduce the sequence manually by typing the corresponding fingers on the keypad. Each of the ensuing eight different conditions (left or right hand, memory load of four or five items, delay of 500 or 7000 ms) was presented six times each. The ensuing 48 events followed in a randomized order and were separated from each other by a jittered delay between 4500 and 6500 ms. Stimuli were presented with MR-compatible goggles using Presentation® software (Neurobehavioral Systems, Inc.), and responses were collected using MRI-compatible keypads (LUMItouch, Photon Control Inc.). All subjects were familiarized with the task before scanning.
MRI Acquisition and preprocessing
MRI was carried out on a Siemens 3T Trio Tim scanner (Siemens Medical Solutions, Erlangen, Germany) using a gradient echo-planar imaging (EPI) sequence (TR = 2200 ms, TE = 30 ms, flip angle = 90°, matrix = 64×64 voxels, slice thickness 3 mm, field of view = 1200×1200 mm2). Additionally, high-resolution T1-weighted whole-brain images were acquired using an MPRAGE sequence (TR = 1900 ms, TE = 2.5 ms, matrix size = 256×256, 176 sagittal slices, voxel size = 1×1×1 mm3, field of view = 250×250 mm2).
To allow for magnetic-field saturation, image acquisition was preceded by three dummy images which were discarded prior to data analysis. Images were analyzed using SPM8 (www.fil.ion.ucl.ac.uk/spm). The EPI images were corrected for head movement by affine registration using a two-pass procedure. This included an initial realignment of all images to the first image and a subsequent realignment to the mean of the realigned images. After realignment, the mean EPI image of each participant was spatially normalized to the MNI (Montreal Neurological Institute) reference space using the unified segmentation approach [49]. The resulting parameters that define the deformation field necessary to move the participant's data into the space of the MNI tissue probability maps were then combined with the deformation field transforming between the latter and the MNI single subject template. The ensuing deformation was subsequently applied to the individual EPI volumes that were thereby transformed into the MNI single subject space and resampled at 1.5×1.5×1.5 mm3 voxel size. Finally, these normalized images were spatially smoothed with a Gaussian kernel of 8-mm full width at half-maximum.
Data analysis
Behavioral data analysis
Task accuracy and response times were analyzed using the SPSS software package (SPSS v17.0, Chicago, Illinois, USA). The rate of correct reproductions, initial reaction time (i.e. the time interval between go-signal and first button press), and mean interresponse time (i.e. the time interval between the first and last button press divided by the number of items in the sequence minus one [as there are, e.g., three intervals between four responses]) was calculated for each subject and compared between conditions and groups. The effect of the between-subject factor group, and the within-subject factors delay (immediate or delayed) and memory load (4 or 5 items) on each performance measure was examined by a 2×2×2 mixed design analyses of variance (ANOVA). P-values below 0.05 were considered significant. For significant factors or interactions, pair-wise comparisons were computed with the Bonferroni correction for multiple comparisons.
Functional MRI data
Imaging data were analyzed using the general linear model as implemented in SPM8. In particular, we used six condition regressors reflecting encoding, immediate (direct) and delayed recall (retrieval) for the left and right hand, respectively. In addition, a parametric modulator for each regressor was introduced to capture load-related differences in local activation. In contrasts to the alternative procedure of modelling low and high load trials separately, this approach has the advantage that it allows for a more robust estimation of the main effects (based on more trials) without losing sensitivity to differences between both low- and high-load trials. Given the relatively modest performance rates in each group, we did not restrict our analysis to correct trials but rather included all those trials in which subjects pressed the required number of buttons, independently of whether the sequence was correct or not. This ensured that subjects tried to perform the task while at the same time providing a sufficient number of the estimation of neuronal responses. Each of the ensuing regressors was modelled by convolving a canonical hemodynamic response form with a boxcar reference vector reflecting the onset and duration of the respective events. That is, for the encoding, the width of the boxcar function reflected the time from the appearance of the stimulus to the end of the last item being displayed. For (immediate and delayed) recall, it corresponded from the onset of the go-cue to the last response. In addition, residual motion artefacts were modelled by including the six-parameters (three translational and three rotational) [50] estimated in the realignment preprocessing as regressors of no nuisance regressors into the model. Low-frequency signal drifts were removed by employing a highpass filter with a cut-off period of 128 seconds. After correction of the time series for dependent observations according to an autoregressive first-order correlation structure, parameter estimates of the HRF regressors were calculated for each voxel using weighted least squares to provide maximum-likelihood estimators based on the temporal autocorrelation of the data [51]. The individual first-level contrasts for each condition and its parametric modulation by load (all relative to the implicit baseline) were then fed into a second-level random-effects ANOVA. In this group analysis, mean parameter estimates were computed within in each group (controls, patients) for the three conditions (encoding, immediate recall and delayed recall) as well as their modulation by item load. The two different delays that were implemented to different delay periods represented direct and delayed retrieval. The only reason why “direct retrieval” was performed with a delay of 500 ms is to avoid attentional blink phenomena/surprise by the immediately appearing go. On the other hand the manipulation of WM load was set up to reflect easy and difficult items (low and high WM load). For that however, the available levels were rather limited as sequence length of three items or less resulted in ceiling effects in the control population (almost perfect reproduction), whereas item sequences of six or more items led to floor effects in the patient group (many patients performing at less than ten percent success). It is important to emphasize that the different magnitude ratios have no direct bearing on our analysis rather we compared no/short delay versus long delay and easy versus difficult memory load in a categorical fashion. We allowed for violations of sphericity by modeling nonindependence across images from the same subject and allowing unequal variances between conditions and subjects as implemented in SPM8.
Differences between conditions or groups were then tested by applying appropriate linear contrasts to the ANOVA parameter estimates. All effects were investigated as main effects across both respond hands, as this study was neither aimed nor well suited (given the relatively low number of trials) to study lateralization effects. Rather, left/right trials were randomized and counter-balanced only to avoid a potential confound of stimulus- or response-side. Conjoint main effects were tested by means of a conjunction analysis using the minimum statistics approach [52]. The resulting SPM(T) maps were then thresholded at P<0.05 conducting a family-wise error (FWE) correction on the cluster-level (cluster forming threshold at voxel level P<0.001; [53]). For investigation of load-related effects, a slightly more liberal cluster-level threshold of p<0.001 (uncorrected) was employed.
Voxel-based morphometry (VBM)
As structural brain changes may principally confound functional MRI data, we performed voxel-based morphometry (VBM) [54] to control for gray matter differences between patients and controls in the fMRI data analysis. T1-weighted images of all subjects were processed and analysed with SPM8 and the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm). Briefly, T1-weighted images were spatially normalized by high-dimensional warping with a standard template and segmented into gray matter (GM), white matter and cerebrospinal fluid. To correct for individual brain sizes and allow comparing the absolute amount of tissue volume [55], voxel values were multiplied (“modulated”) by the non-linear component of the Jacobian determinant derived from the spatial normalization. Finally, modulated GM images were smoothed with a Gaussian kernel of 8-mm FWHM. Using a general linear model, voxel-wise gray matter differences between patients and controls were examined using independent-sample t-tests and by including age as a nuisance covariate. For the statistical analysis, we employed a family-wise error (FWE) corrected threshold (on cluster level) of p<0.05.
Anatomical allocation
All results were anatomically labeled by reference to probabilistic cytoarchitectonic maps of the human brain using the SPM Anatomy Toolbox [56], [57]. Using a Maximum Probability Map (MPM), activations were assigned to the most probable histological area at their respective locations. Details on these cytoarchitectonic regions can be found in the following publications reporting on the cerebellum [58], thalamus [59], premotor cortex (PMC, BA 6; [60]), primary motor cortex (M1, BA 4a, BA 4p) [61], primary somatosensory cortex (BA 3a, BA 3b) [62], [63]), parietal operculum (OP4) [64], insula (lg2) [65], Broca's region (BA 45) [66], inferior, superior parietal cortex and superior parietal lobule (IPC, SPC and SPL; PGp; 7P; 7PC) [67]–[69], intraparietal sulcus (IPS; hlP1; hlP3) [70], visual cortex (BA 17; BA 18 [71]; hOC3 (V3); hOC4 (V4) [72]; hOC5 (V5/MT+)) [73] and hippocampus (Hipp (EC)) [74]. Brain regions not yet histologically mapped were macroanatomically labeled by reference to the WFU Pickatlas (version 2.4) [75].
Results
Clinical and neuropsychological data
Results of the clinical and neuropsychological examination are summarized in Table 1. There was no significant difference between both groups with respect to age (p = 0.38), gender (p = 0.59), years of education (p = 0.06) or MMSE score (p = 0.15). PD patients demonstrated significant deficits in nearly all neuropsychological tests as indicated by two-sample t-tests. In particular, they performed worse in forward digit span subtest of the WMS (t (44) = −2.77, p = 0.008); TMT-A (t (44) = 2.415, p<0.02) and TMT-B (t (44) = 2.73, p<0.009). Increase in completion time between the TMT-B and TMT-A, which may be interpreted as a marker for executive control, was also significantly elevated (worse) in PD patients (t (44) = 2.54, p<0.015). As expected, patients were also significantly slowed in the pointing and finger-tapping examinations. The only neuropsychological test not reaching statistical significance was the backward digit span subtest of the WMS (p<0.2) in which the patients recalled on average one item less than the controls but both groups showed a pronounced inter-individual variability. The WMS age-appropriate standard scores that have been converted from the sum of the raw scores of both, the digit span forward and backward tests, however demonstrated significantly more decline in PD patients than in controls (t (44) = −2.035, p<0.048).
Behavioral data
Multiple mixed design ANOVAs confirmed that performance accuracy (i.e. correct sequence reproductions) was significantly lower in PD patients than in HC across all conditions [F(1, 41) = 11.329; p<0.002]. Also, higher memory load [F(1, 44) = 68.481; p<0.001] and delayed response initiation [F(1, 44) = 13.496; p<0.001] caused additional decline in performance accuracy in both groups. Neither factor, however, showed a significant interaction with “group”, indicating that patients and controls perform worse with longer sequences or delays. Likewise, there was no significant load×delay interaction. Furthermore, PD patients used more time to respond as indicated by significantly higher mean interresponse time in PD compared to HC [F(1, 44) = 4.219; p = 0.046]. Likewise, higher memory load but not delay periods caused longer interresponse time intervals in both groups [F(1, 44) = 63.481; p<0.001]. There was no significant interaction between these factors or with group. Finally, initial reaction time was prolonged by delayed response initiation compared to immediate responses [F(1, 44) = 18.161; p<0.001] but not significantly different between low- and high-load conditions. Please see also Table S1.
Functional MRI Data
Condition-related effects were tested as main effects across all participants, i.e. both groups, and are shown in the supplementary material (Figures S1, S2, S3, S4, S5, and S6). A detailed assessment of task-related effects (against implicit baseline), differences between condition (encoding, direct and delayed recall) and load-related (higher activation in the five compared to the four item condition as reflected by the parametric modulator) is outside the scope of this work. Although we are not able to eliminate a potential limitation of the current study, which might be a possible confounding effect of motor execution during the task, we would nevertheless like to note, that all effects resonate well with known networks for working memory and memory–motor transformations (e.g. [22], [37], [76]–[79]), confirming the effectiveness of our experimental setup and the appropriateness of the imaging and analysis approach.
Encoding
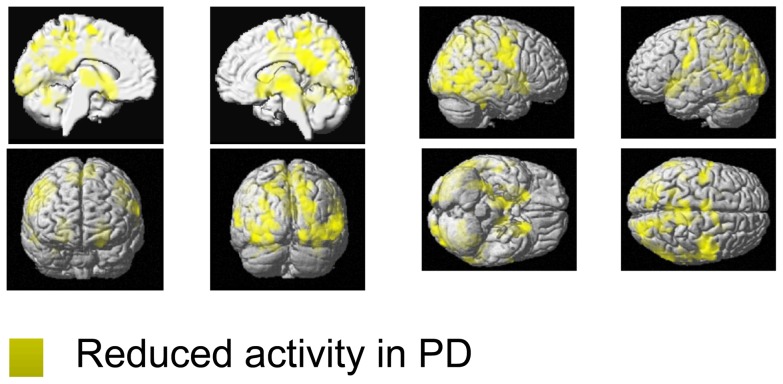
FMRI results are summarized in Tables 2, 3, and 4 as well as visualized in Figures 1, 2, 3, and 4. Relative to controls, PD patients showed reduced encoding-related activity in a large, bilateral network (Table 2A, Figure 1). In particular, reduced activation in patients was most pronounced in the bilateral putamen, extending to the bilateral thalamus and temporo-occipital cortex. Furthermore, the bilateral temporal gyrus, bilateral superior parietal cortex, bilateral dorsal and ventral occipital cortex including left posterior fusiform gyrus and left cerebellar lobule VI were less activated in patients. Further reductions were observed in the bilateral pre- and primary motor cortex, bilateral inferior frontal gyrus, right precuneus, medial superior parietal cortex, bilateral SMA as well as the right inferior parietal cortex. For additional information including cluster size, stereotaxic location and histological allocation confer Table 2A. We found no region that showed significantly higher activation in PD patients relative to controls during encoding (Table 3A).
Table 2. Reduced working memory related functional MRI results in PD compared to controls.
| Macroanatomical location | Cytoarchitectonic location | MNI coordinates of local maxima | z-score | kE | ||
| x | y | z | ||||
| A) Reduced activation in PD compared to controls during encoding | ||||||
| Left Putamen | −24 | 15 | −9 | 6.82 | 17869 | |
| Right Putamen | 21 | 17 | −11 | 5.65 | ||
| Right thalamus | 12 | −15 | −3 | 6.66 | ||
| Right occipital cortex | hOC5 | 54 | −66 | 3 | 6.65 | |
| Right superior parietal occipital cortex | 17 | −65 | 47 | 6.1 | ||
| Right dorsal occipital cortex | 29 | −87 | 26 | 5.71 | ||
| Right ventral occipital cortex | FG1 | 35 | −69 | −12 | 5.57 | |
| Right inferior temporal cortex | 33 | −50 | −20 | 5.51 | ||
| Right lateral occipital cortex | 60 | −51 | 0 | 4.9 | ||
| Right superior temporal gyrus | 62 | −54 | 11 | 4.62 | ||
| Left thalamus | −12 | −14 | 2 | 4.78 | ||
| Left dorsal occipital cortex | −24 | −89 | 9 | 6.41 | 5089 | |
| Left ventral occipital cortex | FG1 | −39 | −86 | −12 | 4.9 | |
| Left inferior temporal cortex | −41 | −60 | −14 | 5.21 | ||
| Left cerebellum Lobule VI | Lobule VI | −17 | −65 | −27 | 4.48 | |
| Left occipital cortex | hOC5 | −45 | −72 | 0 | 4.17 | |
| Right precentral gyurs | Area 6 | 38 | −8 | 45 | 6.08 | 4087 |
| Right motorcortex | Area 4p | 42 | −11 | 38 | 6.04 | |
| Right inferior precentral gyrus | Area 4p | 54 | −3 | 27 | 5.25 | |
| Left middle occipital gyrus | −30 | −69 | 26 | 5.95 | 876 | |
| Left superior parietal occipital cortex | −15 | −78 | 42 | 4.4 | 1038 | |
| Precuneus | 5 | −54 | 17 | 5.9 | 6715 | |
| Posterior cingulate cortex | 6 | −39 | 26 | 5.14 | ||
| Retrosplenial cortex | 12 | −62 | 23 | 4.79 | ||
| Right paracentral gyrus | Area 3a/Area 4p | 14 | −33 | 59 | 4.49 | |
| Right paracentral gyrus | Area 3a/Area 4p | −9 | −35 | 72 | 4.33 | |
| Left superior parietal lobule | Area 7PC | −24 | −51 | 48 | 5.7 | 423 |
| Left Motorcortex | Area 4p | −42 | −14 | 36 | 5.18 | 1709 |
| Left inferior frontal gyrus | Area 3a/Area 4p | −45 | −9 | 30 | 4.99 | |
| Left superior temporal gyrus | −62 | −54 | 6 | 5.01 | 1149 | |
| Left parieto-occipital junction | −44 | −38 | 26 | 4.18 | ||
| SMA | Area 6 | −5 | −9 | 65 | 4.91 | 1118 |
| SMA | Area 6 | 8 | 3 | 59 | 4.7 | |
| Right inferior parietal cortex | Area PFcm | 62 | −29 | 15 | 4.64 | 579 |
| Right inferior parietal cortex | Area PFcm | 51 | −38 | 21 | 4.49 | |
| Right middle temporal gyrus | 56 | −17 | −11 | 4.5 | 355 | |
| B) Reduced activation in PD compared to controls during direct recall | ||||||
| Left primary motor cortex | Area 4a | −42 | −14 | 47 | 5.96 | 2930 |
| Left SMA | Area 6 | −3 | −8 | 62 | 5.12 | |
| Left dorsal precentral gyrus | Area 6 | −39 | −6 | 53 | 5.42 | |
| Left superior parietal lobule | Area 7PC | −30 | −53 | 57 | 5.68 | 1365 |
| Left intraparietal sulcus | Areas hIP1–3 | −30 | −42 | 42 | 5.26 | |
| Right superior parietal lobule | Area 7P | 14 | −78 | 54 | 5.84 | 807 |
| Left Putamen | −30 | −11 | 3 | 4.8 | 676 | |
| Right dorsal precentral gyrus | 35 | −3 | 51 | 5.33 | 397 | |
| C) Reduced activation in PD compared to controls during delayed recall | ||||||
| Left Putamen | −32 | 3 | −8 | 5.12 | 646 | |
| SMA | Area 6 | −3 | −8 | 59 | 5.31 | 590 |
| SMA | Area 6 | 11 | 0 | 56 | 3.82 | |
| Left superior parietal lobule | Area 7PC | −32 | −50 | 56 | 5.3 | 547 |
| Left primary motor cortex | Area 4 | −39 | −15 | 51 | 5.28 | 493 |
| D) Reduced load effects in PD compared to controls | ||||||
| Encode | ||||||
| Right Medial Orbitofrontal cortex | 2 | 41 | −20 | 4.87 | 582 | |
| Left anterior inferior temporal sulcus | −39 | −5 | −29 | 4.52 | 301 | |
| Direct recall | ||||||
| No significant effect | ||||||
| Delayed recall | ||||||
| Left anterior Insula | −30 | 27 | 3 | 4.87 | 391 | |
Abbr.: kE: cluster size; x, y, z: MNI co-ordinates; PD, Parkinson's disease, HC healthy controls.
Table 3. Increased working memory related activation in PD.
| Condition | Macroanatomical location | Cytoarch. location | MNI coordinates of local maxima | Z-score | kE | ||
| x | y | z | |||||
| A) Increased activation in PD compared to controls during encoding, recall and delayed recall | |||||||
| Encode | no significant effects | ||||||
| Direct recall | no significant effects | ||||||
| Delayed recall | Left posterior parahippocampal gyrus | −17 | −51 | 6 | 4.49 | 1586 | |
| Right posterior parahippocampal gyrus | 20 | −42 | −3 | 4.21 | |||
| Retrosplenial cortex | 3 | −38 | 9 | 4.16 | |||
| Right cerebellum | Lobule VIIa | 24 | −83 | −23 | 4.17 | 1194 | |
| Left cerebellum | Lobule VIIa | −27 | −72 | −23 | 3.66 | 921 | |
| Right inferior frontal gyrus | Area 45 | 51 | 26 | 21 | 4.84 | 566 | |
| Right superior parietal occipital cortex | 9 | −84 | 48 | 4.3 | 480 | ||
| Right posterior middle frontal gyrus | 36 | 12 | 50 | 4.49 | 427 | ||
| Left medial superior parietal lobule | Area 7A | −3 | −62 | 66 | 4.97 | 362 | |
| B) Increased load-effects in PD compared to controls | |||||||
| Encode | no significant effects | ||||||
| Direct recall | no significant effects | ||||||
| Delayed recall | Right cerebellum | Lobule I–IV | 11 | −36 | −21 | 4.31 | 366 |
| Right posterior cingulate | Area 7A | 5 | −41 | 35 | 4.68 | 362 | |
Abrr.: kE: cluster size; x, y, z: MNI co-ordinates; PD, Parkinson's disease, HC healthy controls.
Table 4. Condition by group interaction.
| Macroanamtomical location | Cytoarchitectonic location | MNI coordinates of local maxima | z-score | kE | |||
| x | y | z | |||||
| A) Reduced activation in PD for direct recall | |||||||
| Right posterior superior parietal lobule | 7P | 14 | −78 | 54 | 5.94 | 804 | |
| Left posterior superior frontal gyrus | −38 | −3 | 51 | 4.74 | 669 | ||
| B) Increased activation in delayed recall in PD | |||||||
| Right cerebellum | Lobule VIIa Crus I | 24 | −83 | −23 | 4.21 | 1102 | |
Figure 1. Functional working-memory related correlates in PD and controls during the encoding phase.
Regions showing significantly lower activity (yellow) in PD relative to healthy controls during the encoding phase of the motor WM task. All significant effects are displayed on the MNI single subject template and the color bar represents T-values.
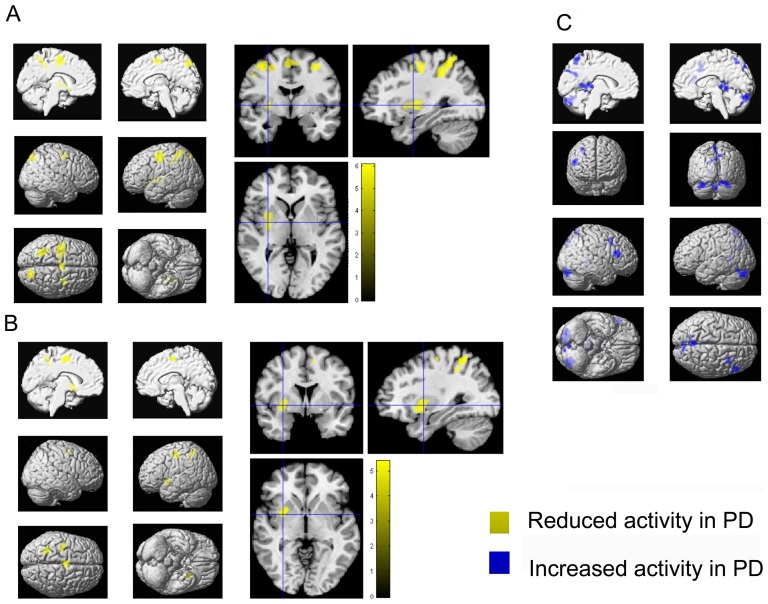
Figure 2. Functional working-memory related correlates in PD and controls during direct and delayed recall.
A–C) Regions showing significantly lower activity (yellow) in PD relative to healthy controls during A) direct recall and B) delayed recall. C) Regions showing significantly higher activity (blue) in PD relative to healthy controls during delayed recall. All significant effects are displayed on the MNI single subject template and the color bar represents T-values.
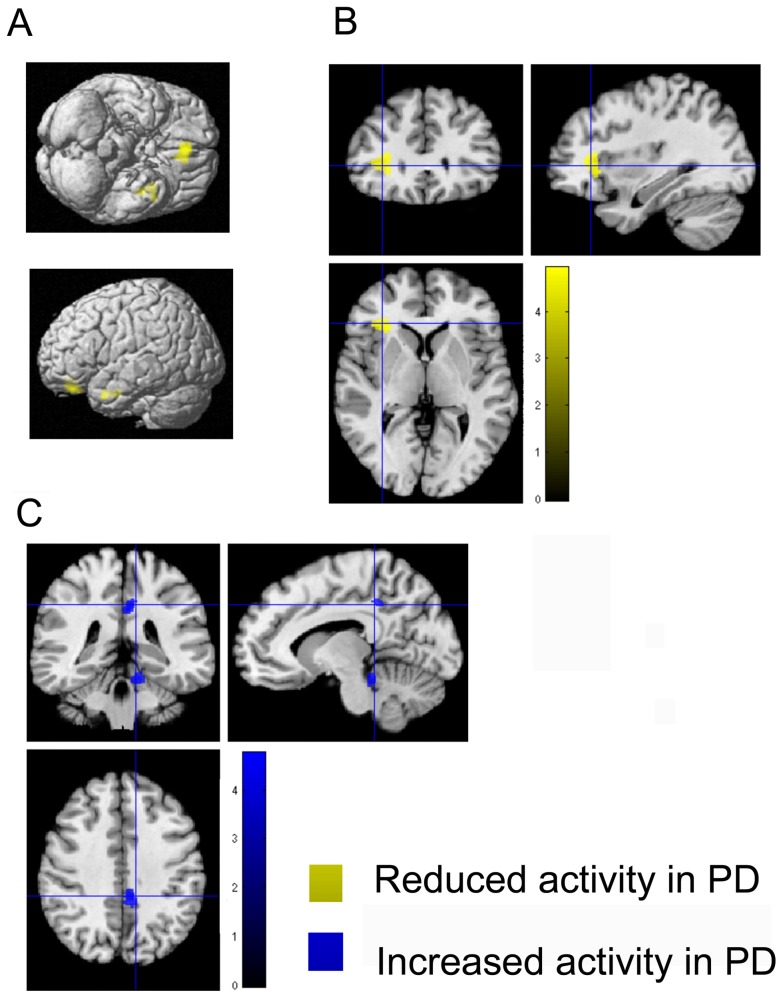
Figure 3. Functional working-memory related correlates in PD and controls during load-related modulation.
A–B) Regions showing significantly lower load-related modulation in PD during the encoding phase A) and delayed recall B). C) Regions showing significantly higher load-related modulation in PD relative to healthy controls during delayed recall. All significant effects are displayed on the MNI single subject template and the color bar represents T-values.
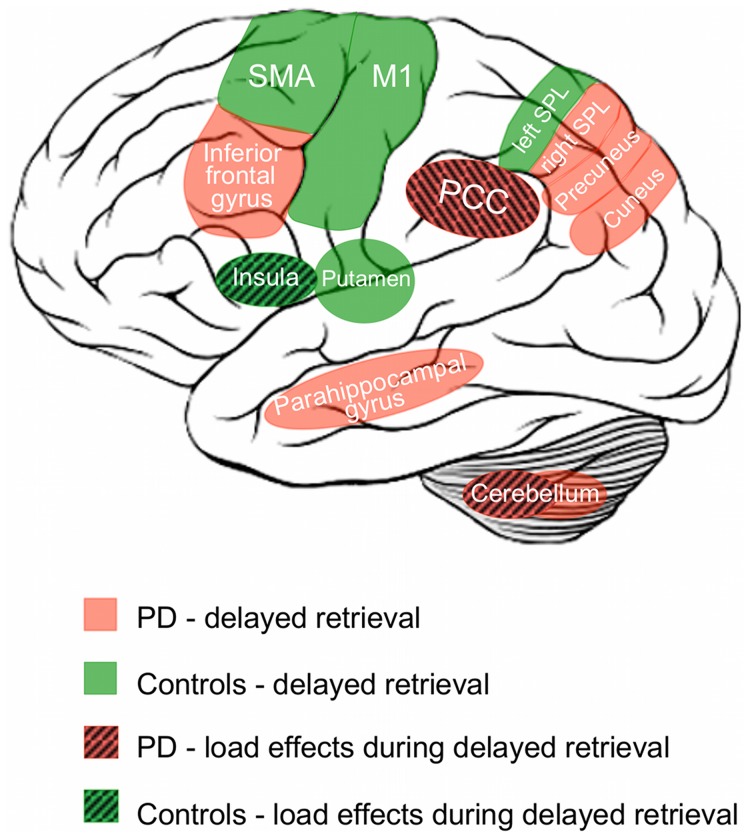
Figure 4. Schematic overview of working-memory related activation patterns in PD and controls.
Schematic summary of brain regions showing task- or load-related differences during the delayed recall condition representing memory - motor transformation.
Direct and delayed recall
During immediate recall, when subjects had to retype the memorized sequences after a delay of only 500 ms, PD patients showed reduced activation relative to controls in the left precentral gyrus, left SMA, bilateral dorsal precentral gyrus, bilateral superior parietal lobule, left intraparietal sulcus and middle and posterior parts of the left putamen (Table 2B, Figure 2A). In turn, no brain area showed significantly increased activation in PD relative to controls (Table 3A).
In the long delay condition (in which the subjects had to reproduce the sequence after 7000 ms) PD patients featured significantly less activation in the left putamen, superior parietal cortex and precentral gyrus as well as in bilateral SMA (Table 2C, Figure 2B). Additionally, PD patients showed increased bilateral activation (compared to controls) in the posterior parahippocampal gyrus and cerebellar lobule VIIa. Moreover, increased activation was found in the right inferior frontal gyrus, and the posterior midline including the retrosplenial cortex, while in the left hemisphere increased activation was found in the medial superior parietal cortex (Table 3A, Figure 2C). Again, additional details for all effects, including cluster size, stereotaxic location and histological allocation, are provided by the tables 2B/C and 3A. A schematic overview of working-memory related activation patterns in PD and controls is illustrated in Figure 4.
Load-related modulations
PD patients showed significantly lower load-related effects during encoding, i.e., significantly less modulation of neuronal activity when memorizing five as compared to four items in the right medial orbitofrontal cortex and the left anterior inferior temporal sulcus relative to healthy controls during encoding (Table 2D, Figure 3A). During delayed recall, PD patients showed significantly lower load-related modulation of activity in the left anterior insula (Figure 3B). In contrast, PD patients showed significantly higher load-related modulation during delayed recall in the right posterior cingulate cortex and right cerebellar lobule I–IV (Table 3B, Figure 3C). Again, details regarding details on cluster size, stereotaxic location and histological allocation are given in the tables 2D and 3B, for an overview please see Figure 4.
Condition by group interaction
Furthermore, we compute the “group×task” interaction to statistically assess, whether the factor “group” (PD vs. controls) modulates the within-group factor “task” (direct vs. delayed retrieval). Evidently, two possible interaction effects may be computed, representing the opposite direction of the “group×task” interaction. In particular, given the order of the relevant regressors as ConDirect ConDelayed PatDirect PatDelayed, these two terms are [1 −1 −1 1] and [−1 1 1 −1].
The first tests, whether the difference in the neuronal activation between controls and patients for direct retrieval is greater than the difference between the two groups for delayed retrieval (ConDirect – PatDirect)>(ConDelayed – PatDelayed). Alternatively, however, this may be interpreted as a test, where the difference in neuronal activation between patients and controls for delayed retrieval is greater than the difference between the two groups for direct retrieval (PatDelayed – ConDelayed)>(PatDirect – ConDirect). To differentiate these two alternative accounts for the (same) [1 −1 −1 1] interaction, we constrained our analysis by a conjunction with the minuend of the two alternatives, i.e., forcing the direction of the observed effect. The contrast [1 −1 −1 1] ∩ [1 0 −1 0] hence tests for regions, where patients show a specific reduction in activation during direct retrieval (ConDirect – PatDirect)>(ConDelayed – PatDelayed). Testing for this interaction at p<0.05 (cluster-level FWE correction for multiple comparisons, cf. Fig. S7a; Table 4A), yielded two significant regions in the left posterior superior frontal gyrus and right posterior superior parietal lobule (area 7P) in which activity in PD patients was specifically reduced during direct retrieval. In turn [1 −1 −1 1] ∩ [0 −1 0 1] tests for regions, where patients show a specific increase in activation during delayed retrieval (PatDelayed – ConDelayed)>(PatDirect – ConDirect). Testing for this interaction at p<0.05 (cluster-level FWW, cf. Fig. S7b; Table 4B), yielded one significant effect in the right cerebellum (lobule VIIa Crus I).
The second interaction term [−1 1 1 −1] tests, whether the difference in the neuronal activation between controls and patients for delayed retrieval is greater than the difference between the two groups for direct retrieval (ConDelayed – PatDelayed)>(ConDirect – PatDirect). Alternatively, however, this may be interpreted as a test, where the difference in neuronal activation between patients and controls for direct retrieval is greater than the difference between the two groups for delayed retrieval (PatDirect – ConDirect)>(PatDelayed – ConDelayed). Testing for this interaction yielded no significant effect, even when lowering the threshold to p<0.001 uncorrected.
Voxel-based morphometry
In our sample of PD patients and age- and sex-matched controls, no significant differences in gray-matter volume or differences in total brain volume were detected. That is, we found no evidence for significant (at p<0.05 corrected for multiple comparisons) regionally specific (given that total brain volume was included as a covariate into the analysis) atrophy in our groups of PD patients. In other words, the examined patients showed the above described neuropsychological and functional differences in spite of neither featuring clinical signs of dementia (dementia screening tests) nor significant atrophy (VBM).
Discussion
This fMRI study investigated aberrations in neuronal responses during a motor WM task in non-demented patients with PD. In spite of absence of clinical dementia and significant brain atrophy, we demonstrated that: (I) PD patients performed significantly worse on the motor WM task than closely matched healthy controls. II) There was no group by load or delay interaction on performance rates. (III) Impaired task performance was associated with reduced task-related activity in all phases but in particular during encoding. (IV) During sequence encoding PD patients showed reduced activity in a widespread network comprising the basal ganglia, motor, cingulate and parieto-occipital cortices. (V) During recall, reduced activation was found in cerebral motor networks, superior parietal structures, and the putamen. Increased activation was found in the bilateral posterior parahippocampal gyrus and the posterior cerebellum as well as in the posterior midline when recall was delayed. (VI) In PD, significantly reduced load-modulations were observed in the orbitofrontal cortex and anterior insula, while the posterior cingulate cortex and the cerebellum showed increased load-modulation in patients.
Aberrant encoding-related activity in PD
The encoding phase involves stimulus processing and the formation of transient motor representations [80]. In particular, there is solid evidence for subliminal activation of the motor system, i.e. covert action, simulation being triggered by observing an action or receiving information representing actions such as words or motor-related spatial cues as in the present experiment (for review: [81]). The observed widespread reduction of activity during encoding in PD is in line with previous studies reporting reduced activation during action simulation [82], [83] and motor programming [84] in these patients. This interpretation as implicitly triggered motor activation holds particularly for the effects in premotor cortices [85] and matches previous reports of malfunctioning mesial motor areas in PD [86]–[88], i.e., regions strongly involved in the interface between cognitive and motor processes. The dorsal lateral premotor cortex, in turn, is predominantly associated with planning and execution sensory-guided movements [89] and externally triggered movements [90]. Our study thus provides evidence for reduced stimulus-driven triggering of activation within the cortical motor system by highly associative action-related spatial stimuli. Furthermore, the reduced activation in the putamen during both encoding and subsequent recall is well in accordance with earlier fMRI studies linking this region to impaired spatial motor WM [91], [92]. The putamen was shown to actively contribute to stimulus maintenance [93] and also associated with episodic memory encoding [94]. Reduced activation in the putamen may thus reflect potentially dopamine-dependent aberrations during the maintenance of motor representations. Decreased activation in the posterior parietal lobe and in particular the precuneus finally resonates well with recent findings, that this region plays a key role in multiple higher cognitive processes [95] including attentive tracking [96], visuo-spatial [97] and motor imagery [98]–[101]. It may hence represent a hub of cognitive functioning, which is disturbed in patients with PD resulting in impaired task performance. When further considering the recently discussed association of the medial superior parietal cortex with imaginative processes and prospective cognition (but not actual task execution in many goal directed [motor] tasks, cf. [102]), it may be speculated, that insufficient imagery and simulation within or controlled by the precuneus may represent a key component of this reduced task performance in PD patients.
In summary, our results thus suggest that impaired motor WM in patients with PD may represent a composite deficit related to insufficient triggering of implicit enactment by the cortical motor system, reduced basal ganglia activation resulting in impaired transfer into short term storage and finally reduced simulation and imagery under the guidance of superior and medial parietal cortices.
Aberrant recall-related activity in PD
Delayed response initiation and prolonged interresponse times may be regarded as direct reflection of bradykinesia, a clinical hallmark of PD. Longer delay intervals furthermore decreased task performance but did not result in longer interresponse times and actually speeded up response initiation. Furthermore, there was no significant group by delay or load interaction. These results hence point to dissociation between task difficulty and motor slowing, which are both present in patients with PD but reflected in different measures derived from the employed motor WM task. The PD-related slowing is neuronally reflected by decreased activation in the (pre-) motor and (particularly superior) parietal cortex as well as the left putamen. All of these areas are directly involved in the preparation and execution of voluntary movements. Consequently, we would conjecture that their reduced activation should best be interpreted as neuronal correlates of the slowed motor response in the patients, rather than with respect to the impaired (cognitive) task performance. In other words, whereas the reduced activity during encoding may be primarily responsible for deficits in the correct encoding and hence recall of sequences, most of the effects seen during the reproduction period may be attributable to impaired motor control and difficulties in initiating and performing the sequence reproduction.
In contrast, increased activation was observed only in the context of delayed recall in several regions, including the parahippocampus. The latter findings is particularly thought-provoking given reports on PD pathology in this region [103], [104] and its involvement for spatial localization tasks [105]. Its strategic position within the medial temporal lobe makes it well suited to participate in the long-term storage [106] of currently available information [107] indicating a correspondence to the integrative functions of an episodic buffer [108] that is predictive of subsequent long-term memory [109]. In sequence learning tasks, increased parahippocampal activation [110]–[112] was found in PD subjects with better learning performance [33]. In contrast to these findings indicating a supportive role, we observed parahippocampal hyperactivity in spite of deficient task performance. This may relate to the concurrently decreased activation of cortical motor systems but potentially also to the increased activation of the posterior cingulate cortex. The latter is particularly interesting as this region is frequently associated with the default mode system of the human brain [102] and failure to deactivate it may lead to impaired task performance. While it is tempting to speculate about a dysbalance between the default mode and cortical motor network during the delayed recall of action sequences from working memory, further data seems to be first needed to dissociate motor (bradykinesia) related effects from neuronal correlates of cognitive performance and supportive from disruptive effects. We would hence only conclude that impaired task performance may result from a complex interplay of reduced (cortical and striatal motor system) and increased (parahippocampus) beneficial as well as potentially detrimental (posterior cingulate) activation.
Effects of increased memory load
Increased memory load significantly reduced the accuracy of sequence recall in both groups without a particular effect on PD patients or an interaction with delay. Nevertheless, decreased load-related effects in PD were observed in the medial orbitofrontal and temporal cortices (during encoding) and in the anterior insula (during delayed recall). In turn, activation was increased in the posterior cingulate cortex. The latter set of effects may be particularly relevant, as these two regions are considered part of antagonistic “saliency”/task positive (anterior insula [113]) and “default”/task-negative (posterior cingulate [114]) networks. This argues for a dysbalance between these networks in PD that may result in increased cross-talk from resting-state networks, insufficient recruitment of task-relevant and attention-related areas and ultimately impaired task performance. Finally, it is important to point out, that most effects in the current study were observed when looking at delayed rather than immediate recall in spite of the fact that we observed no significant group×delay interaction, i.e., performance was not particularly impaired in this task. A potential explanation for this discrepancy is the per se higher difficulty of this condition (cf. lower performance across both groups) and the additional involvement of memory – motor transformations. The latter may not be necessary in the immediate recall condition, where sensory and (implicitly triggered) motor representations may still be active.
Conclusions
Here we investigated differences in task performance and neuronal correlates in a motor WM task between non-demented PD patients and healthy control subjects. We found that reduced task performance was associated with widespread attenuation of task-related activity in a bilateral WM network. Furthermore, bradykinesia seems differentiable from cognitive performance and related to hypoactivity of the striatal and cortical motor system. Moreover, we observed increased activation in limbic areas that were previously associated with beneficial (parahippocampus) and detrimental (posterior cingulate) effects in PD patients.
Supporting Information
Left side - main effect (compared to resting baseline across both groups). Right side - load related effects during encoding (main effects across both groups).
(TIF)
Left side - main effect of direct recall (compared to resting baseline across both groups). Right side - load related effects during direct recall (main effects across both groups).
(TIF)
Left side - main effect of delayed recall (compared to resting baseline across both groups). Right side - load related effects during delayed recall (main effects across both groups).
(TIF)
Left side – increased activation during encoding relative to direct recall across both groups. Right side - increased activation during encoding relative to delayed recall across both groups.
(TIF)
Left side – increased activation during direct recall relative to encoding across both groups. Right side - increased activation during delayed recall relative to encoding across both groups.
(TIF)
Left side – conjunction between direct and delayed recall across both groups. Right side - conjunction between encoding, direct and delayed recall across both groups.
(TIF)
A - Interaction (ConDirect – PatDirect)>(ConDelayed – PatDelayed): Regions in which patients showed a significant specific reduction of activity during direct retrieval as tested by the interaction (ConDirect – PatDirect)>(ConDelayed – PatDelayed) in conjunction with the respective main effect (ConDirect – PatDirect), as well as the mean parameter estimates and 90% confidence intervals for the individual conditions at the location of the local maxima. B - Interaction (PatDelayed – ConDelayed)>(PatDirect – ConDirect): Regions in which patients showed a significant specific increase of activity during delayed retrieval as tested by the interaction (PatDelayed – ConDelayed)>(PatDirect – ConDirect) in conjunction with the respective main effect (PatDelayed – ConDelayed), as well as the mean parameter estimates and 90% confidence intervals for the individual conditions at the location of the local maxima.
(TIF)
Working memory task performance accuracy in patients with Parkinson's disease (PD) and healthy controls (HC) during direct recall, delayed recall and all conditions. Hits and misses are given for the 4-sequence and 5-sequence.
(DOC)
Acknowledgments
We thank all participants for their enduring collaboration and interest in research.
Funding Statement
CR was funded by the Medical Faculty of the RWTH Aachen University Rotation Programme. SBE was funded by the Human Brain Project (R01-MH074457-01A1) and the Helmholtz Alliance on Systems Biology (Human Brain Model). SBE and KR were funded by the Excellence Initiative of the German federal and state governments. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Lim SY, Lang AE (2010) The nonmotor symptoms of Parkinson's disease–an overview. Mov Disord 25 Suppl 1: S123–130. [DOI] [PubMed] [Google Scholar]
- 2. Braak H, Del Tredici K (2008) Invited Article: Nervous system pathology in sporadic Parkinson disease. Neurology 70: 1916–1925. [DOI] [PubMed] [Google Scholar]
- 3. Thanvi BR, Munshi SK, Vijaykumar N (2003) Neuropsychiatric non-motor aspects of Parkinson's disease. Postgraduate Medical Journal 561–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Braak H, Del K, Rüb U, Vos RAID, Jansen ENH, et al. (2003) Staging of brain pathology related to sporadic Parkinson ' s disease. Neurobiology of Aging 24: 197–211. [DOI] [PubMed] [Google Scholar]
- 5. Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, et al. (2008) Dementia and survival in Parkinson disease: a 12-year population study. Neurology 70: 1017–1022. [DOI] [PubMed] [Google Scholar]
- 6. Hely Ma, Reid WGJ, Adena Ma, Halliday GM, Morris JGL (2008) The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Movement disorders : official journal of the Movement Disorder Society 23: 837–844. [DOI] [PubMed] [Google Scholar]
- 7. Taylor AE, Saint-Cyr JA, Lang AE (1986) Frontal lobe dysfunction in Parkinson's disease. The cortical focus of neostriatal outflow. Brain 109 (Pt 5) 845–883. [DOI] [PubMed] [Google Scholar]
- 8. Kehagia AA, Barker RA, Robbins TW (2010) Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson ' s disease. The Lancet Neurology 9: 1200–1213. [DOI] [PubMed] [Google Scholar]
- 9. Lewis SJG, Dove A, Robbins TW, Barker RA, Owen AM (2003) Cognitive Impairments in Early Parkinson's Disease Are Accompanied by Reductions in Activity in Frontostriatal Neural Circuitry. Neurology 23: 6351–6356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Owen AM, James M, Leigh PN, Summers BA, Marsden CD, et al. (1992) Fronto-striatal cognitive deficits at different stages of Parkinson's disease. Brain 115 (Pt 6) 1727–1751. [DOI] [PubMed] [Google Scholar]
- 11. Dubois B, Pillon B (1997) Cognitive deficits in Parkinson's disease. J Neurol 244: 2–8. [DOI] [PubMed] [Google Scholar]
- 12. Williams GV, Goldman-Rakic PS (1995) Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature 376: 572–575. [DOI] [PubMed] [Google Scholar]
- 13. Landau SM, Lal R, Neil JPO, Baker S, Jagust WJ, et al. (2009) Striatal Dopamine and Working Memory. Cerebral Cortex 445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lange KW, Robbins TW, Marsden CD, James M, Owen AM, et al. (1992) L-dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology (Berl) 107: 394–404. [DOI] [PubMed] [Google Scholar]
- 15. Fournet N, Moreaud O, Roulin JL, Naegele B, Pellat J (2000) Working memory functioning in medicated Parkinson's disease patients and the effect of withdrawal of dopaminergic medication. Neuropsychology 14: 247–253. [DOI] [PubMed] [Google Scholar]
- 16. Morris RG, Downes JJ, Sahakian BJ (1988) Planning and spatial working memory in Parkinson ' s disease. Journal of Neurology 757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bradley VA, Welch JL, Dick DJ (1989) Visuospatial working memory in Parkinson ' s disease. Memory 1228–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cummings JL (1993) Frontal-subcortical circuits and human behavior. Arch Neurol 50: 873–880. [DOI] [PubMed] [Google Scholar]
- 19. Owen AM, Doyon J, Dagher A, Sadikot A, Evans AC (1998) Abnormal basal ganglia outflow in Parkinson ' s disease identified with PET Implications for higher cortical functions. Psychology 949–965. [DOI] [PubMed] [Google Scholar]
- 20. Possin KL, Filoteo JV, Song DD, Salmon DP (2008) Spatial and Object Working Memory Deficits in Parkinson's Disease are Due to Impairment in Different Underlying Processes. Neuropsychology 22: 585–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV (1991) Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain 114 (Pt 5) 2095–2122. [DOI] [PubMed] [Google Scholar]
- 22. Owen AM, Beksinska M, James M, Leigh PN, Summers BA, et al. (1993) Visuospatial memory deficits at different stages of Parkinson's disease. Neuropsychologia 31: 627–644. [DOI] [PubMed] [Google Scholar]
- 23. Postle BR, Jonides J, Smith EE, Corkin S, Growdon JH (1997) Spatial, but Not Object, Delayed Response Is Impaired in Early Parkinson ' s Disease. Neuropsychology 11: 171–179. [DOI] [PubMed] [Google Scholar]
- 24. Helmuth LL, Mayr U, Daum I (2000) Sequence learning in Parkinson ' s disease : a comparison of spatial- attention and number-response sequences. Neuropsychologia 38: 1443–1451. [DOI] [PubMed] [Google Scholar]
- 25. Ketcham CJ, Hodgson TL, Kennard C, Stelmach GE (2003) Memory-motor transformations are impaired in Parkinson's disease. Experimental brain research 149: 30–39. [DOI] [PubMed] [Google Scholar]
- 26. Seidler RD, Tuite P, Ashe J (2007) Selective impairments in implicit learning in Parkinson's disease. Brain Res 1137: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yaguez L, Lange HW, Homberg V (2006) Differential effect of Huntington's and Parkinson's diseases in programming motor sequences of varied lengths. J Neurol 253: 186–193. [DOI] [PubMed] [Google Scholar]
- 28. Nakamura T, Ghilardi MF, Mentis M, Dhawan V, Fukuda M, et al. (2001) Functional Networks in Motor Sequence Learning : Abnormal Topographies in Parkinson ' s Disease. Human Brain Mapping 60: 42–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ghilardi M-f, Eidelberg D, Silvestri G, Ghez C (2003) The differential effect of PD and normal aging on early explicit sequence learning. Neurology 01961: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 30. Smith JG, McDowall J (2006) The implicit sequence learning deficit in patients with Parkinson's disease: a matter of impaired sequence integration? Neuropsychologia 44: 275–288. [DOI] [PubMed] [Google Scholar]
- 31. Muslimovic D, Post B, Speelman JD, Schmand B (2007) Motor procedural learning in Parkinson's disease. Brain : a journal of neurology 130: 2887–2897. [DOI] [PubMed] [Google Scholar]
- 32. Price A, Shin JC (2009) The impact of Parkinson's disease on sequence learning: perceptual pattern learning and executive function. Brain and cognition 69: 252–261. [DOI] [PubMed] [Google Scholar]
- 33. Carbon M, Reetz K, Ghilardi MF, Dhawan V, Eidelberg D (2010) Early Parkinson's disease: longitudinal changes in brain activity during sequence learning. Neurobiol Dis 37: 455–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kwak Y, Müller MLTM, Bohnen NI, Dayalu P, Seidler RD (2010) Effect of dopaminergic medications on the time course of explicit motor sequence learning in Parkinson's disease. Journal of neurophysiology 103: 942–949. [DOI] [PubMed] [Google Scholar]
- 35. Mentis MJ, Dhawan V, Nakamura T (2003) Enhancement of brain activation during trial-and-error sequence learning in early PD. Neurology [DOI] [PubMed] [Google Scholar]
- 36. Mallol R, Barrós-loscertales A, López M, Belloch V, Antònia M, et al. (2007) Compensatory cortical mechanisms in Parkinson ' s disease evidenced with fMRI during the performance of pre-learned sequential movements. 7: 1–7. [DOI] [PubMed] [Google Scholar]
- 37. Kellermann TS, Sternkopf MA, Schneider F, Habel U, Turetsky BI, et al. (2012) Modulating the processing of emotional stimuli by cognitive demand. Soc Cogn Affect Neurosci 7: 263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nasreddine ZS, Philips NA, Bedirian V, Charbonneau S, Whitehead V, et al. (2005) The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699. [DOI] [PubMed] [Google Scholar]
- 40. Kalbe E, Calabrese P, Kohn N, Hilker R, Riedel O, et al. (2008) Screening for cognitive deficits in Parkinson's disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord 14: 93–101. [DOI] [PubMed] [Google Scholar]
- 41.Fahn S, Elton R (1987) Members of the UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinsons Disease: Macmillan Health Care Information. pp. 153–164.
- 42. Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17: 427–442. [DOI] [PubMed] [Google Scholar]
- 43. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R (1995) The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res 4: 241–248. [DOI] [PubMed] [Google Scholar]
- 44.Wittchen H-U, Zaudig M, Fydrich T (1997) Strukturiertes Klinisches Interview für DSM-IV. Göttingen: Hogrefe.
- 45.Wechsler D (1987) Wechsler Memory Scale - Revised: Manual. Psychology.
- 46. Reitan RM (1985) Relationships between measures of brain functions and general intelligence. J Clin Psychol 41: 245–253. [DOI] [PubMed] [Google Scholar]
- 47. Sanchez-Cubillo I, Perianez JA, Adrover-Roig D, Rodriguez-Sanchez JM, Rios-Lago M, et al. (2009) Construct validity of the Trail Making Test: role of task-switching, working memory, inhibition/interference control, and visuomotor abilities. J Int Neuropsychol Soc 15: 438–450. [DOI] [PubMed] [Google Scholar]
- 48. Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- 49. Ashburner J, Friston KJ (2005) Unified segmentation. Neuroimage 26: 839–851. [DOI] [PubMed] [Google Scholar]
- 50. Friston KJ, Holmes AP, Poline JB, Grasby PJ, Williams SC, et al. (1995) Analysis of fMRI time-series revisited. NeuroImage 2: 45–53. [DOI] [PubMed] [Google Scholar]
- 51. Kiebel SJ, Glaser DE, Friston KJ (2003) A heuristic for the degrees of freedom of statistics based on multiple variance parameters. Neuroimage 20: 591–600. [DOI] [PubMed] [Google Scholar]
- 52. Nichols T, Brett M, Andersson J, Wager T, Poline JB (2005) Valid conjunction inference with the minimum statistic. Neuroimage 25: 653–660. [DOI] [PubMed] [Google Scholar]
- 53. Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, et al. (1996) A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp 4: 58–73. [DOI] [PubMed] [Google Scholar]
- 54. Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11: 805–821. [DOI] [PubMed] [Google Scholar]
- 55. Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, et al. (2001) A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage 14: 21–36. [DOI] [PubMed] [Google Scholar]
- 56. Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, et al. (2005) A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage 25: 1325–1335. [DOI] [PubMed] [Google Scholar]
- 57. Eickhoff SB, Paus T, Caspers S, Grosbras M-h, Evans AC, et al. (2007) Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuro Image 511–521. [DOI] [PubMed] [Google Scholar]
- 58. Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009) A probabilistic MR atlas of the human cerebellum. Neuroimage 46: 39–46. [DOI] [PubMed] [Google Scholar]
- 59. Behrens TE, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, et al. (2003) Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magn Reson Med 50: 1077–1088. [DOI] [PubMed] [Google Scholar]
- 60. Geyer S (2004) The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Adv Anat Embryol Cell Biol 174: I–VIII, 1–89. [DOI] [PubMed] [Google Scholar]
- 61. Geyer S, Ledberg A, Schleicher A, Kinomura S, Schormann T, et al. (1996) Two different areas within the primary motor cortex of man. Nature 382: 805–807. [DOI] [PubMed] [Google Scholar]
- 62. Geyer S, Schleicher A, Zilles K (1999) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Neuroimage 10: 63–83. [DOI] [PubMed] [Google Scholar]
- 63. Geyer S, Schormann T, Mohlberg H, Zilles K (2000) Areas 3a, 3b, and 1 of human primary somatosensory cortex. Part 2. Spatial normalization to standard anatomical space. Neuroimage 11: 684–696. [DOI] [PubMed] [Google Scholar]
- 64. Eickhoff SB, Schleicher A, Zilles K (2006) The Human Parietal Operculum. I. Cytoarchitectonic Mapping of Subdivisions differences. [DOI] [PubMed] [Google Scholar]
- 65.Kurth F, Eickhoff SB, Schleicher A (2010) Cytoarchitecture and Probabilistic Maps of the Human Posterior Insular Cortex. Cerebral Cortex. [DOI] [PMC free article] [PubMed]
- 66. Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, et al. (1999) Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol 412: 319–341. [DOI] [PubMed] [Google Scholar]
- 67. Caspers S, Eickhoff SB, Geyer S, Scheperjans F, Mohlberg H, et al. (2008) The human inferior parietal lobule in stereotaxic space. Brain Struct Funct 212: 481–495. [DOI] [PubMed] [Google Scholar]
- 68. Scheperjans F, Eickhoff SB, Homke L, Mohlberg H, Hermann K, et al. (2008) Probabilistic maps, morphometry, and variability of cytoarchitectonic areas in the human superior parietal cortex. Cereb Cortex 18: 2141–2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, et al. (2008) Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cereb Cortex 18: 846–867. [DOI] [PubMed] [Google Scholar]
- 70. Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, et al. (2006) Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. J Comp Neurol 495: 53–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K (2000) Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? Neuroimage 11: 66–84. [DOI] [PubMed] [Google Scholar]
- 72. Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, et al. (2007) Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Hum Brain Mapp 28: 1045–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, et al. (2007) Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cereb Cortex 17: 562–574. [DOI] [PubMed] [Google Scholar]
- 74. Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, et al. (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210: 343–352. [DOI] [PubMed] [Google Scholar]
- 75. Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19: 1233–1239. [DOI] [PubMed] [Google Scholar]
- 76. Ghilardi MF, Eidelberg D, Silvestri G, Ghez C (2003) The differential effect of PD and normal aging on early explicit sequence learning. Neurology 60: 1313–1319. [DOI] [PubMed] [Google Scholar]
- 77.Baddeley A, Hitch GJ (1974) Recent advances in learning and motivation. Working memory. New York. pp. 47–90.
- 78. Cowan N (1988) Evolving conceptions of memory storage, selective attention, and their mutual constraints within the human information-processing system. Psychol Bull 104: 163–191. [DOI] [PubMed] [Google Scholar]
- 79. Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, et al. (2012) Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage 60: 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jeannerod M (1994) The representing brain: Neural correlates of motor intention and imagery. Behavioral and Brain Sciences 17: 187–202. [Google Scholar]
- 81. Jeannerod M (2001) Neural simulation of action: a unifying mechanism for motor cognition. NeuroImage 14: S103–109. [DOI] [PubMed] [Google Scholar]
- 82. Dominey P, Decety J, Broussolle E, Chazot G, Jeannerod M (1995) Motor imagery of a lateralized sequential task is asymmetrically slowed in hemi-Parkinson's patients. Neuropsychologia 33: 727–741. [DOI] [PubMed] [Google Scholar]
- 83. Thobois S, Dominey PF, Decety J, Pollak PP, Gregoire MC, et al. (2000) Motor imagery in normal subjects and in asymmetrical Parkinson's disease: a PET study. Neurology 55: 996–1002. [DOI] [PubMed] [Google Scholar]
- 84. Roland PE (1980) Quantitative assessment of cortical motor dysfunction by measurement of the regional cerebral blood flow. Scand J Rehabil Med Suppl 7: 27–41. [PubMed] [Google Scholar]
- 85. Jeannerod M (1994) [Contribution of JM Charcot to the study of motor localizations in man]. Rev Neurol (Paris) 150: 536–542. [PubMed] [Google Scholar]
- 86. Eckert T, Peschel T, Heinze HJ, Rotte M (2006) Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 253: 199–207. [DOI] [PubMed] [Google Scholar]
- 87. Rowe J, Stephan KE, Friston K, Frackowiak R, Lees A, et al. (2002) Attention to action in Parkinson's disease: impaired effective connectivity among frontal cortical regions. Brain 125: 276–289. [DOI] [PubMed] [Google Scholar]
- 88. Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, et al. (1992) Impaired mesial frontal and putamen activation in Parkinson's disease: a positron emission tomography study. Ann Neurol 32: 151–161. [DOI] [PubMed] [Google Scholar]
- 89. Berardelli A, Rothwell JC, Thompson PD, Hallett M (2001) Pathophysiology of bradykinesia in Parkinson's disease. Brain 124: 2131–2146. [DOI] [PubMed] [Google Scholar]
- 90. Grafton ST, Hazeltine E, Ivry RB (1998) Abstract and effector-specific representations of motor sequences identified with PET. J Neurosci 18: 9420–9428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Postle BR, Druzgal TJ, D'Esposito M (2003) Seeking the neural substrates of visual working memory storage. Cortex 39: 927–946. [DOI] [PubMed] [Google Scholar]
- 92. Postle BR, Esposito MD (1999) Dissociation of human caudate nucleus activity in spatial and nonspatial working memory : an event-related fMRI study. Cognitive Brain Research 107–115. [DOI] [PubMed] [Google Scholar]
- 93. Cairo TA, Liddle PF, Woodward TS, Ngan ET (2004) The influence of working memory load on phase specific patterns of cortical activity. Brain Res Cogn Brain Res 21: 377–387. [DOI] [PubMed] [Google Scholar]
- 94. Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A (2011) Cooperation between the hippocampus and the striatum during episodic encoding. J Cogn Neurosci 23: 1597–1608. [DOI] [PubMed] [Google Scholar]
- 95. Cavanna AE, Trimble MR (2006) The precuneus: a review of its functional anatomy and behavioural correlates. Brain 129: 564–583. [DOI] [PubMed] [Google Scholar]
- 96. Culham JC, Brandt SA, Cavanagh P, Kanwisher NG, Dale AM, et al. (1998) Cortical fMRI activation produced by attentive tracking of moving targets. J Neurophysiol 80: 2657–2670. [DOI] [PubMed] [Google Scholar]
- 97. Suchan B, Yaguez L, Wunderlich G, Canavan AG, Herzog H, et al. (2002) Hemispheric dissociation of visual-pattern processing and visual rotation. Behav Brain Res 136: 533–544. [DOI] [PubMed] [Google Scholar]
- 98. Stephan KM, Fink GR, Passingham RE, Silbersweig D, Ceballos-Baumann AO, et al. (1995) Functional anatomy of the mental representation of upper extremity movements in healthy subjects. J Neurophysiol 73: 373–386. [DOI] [PubMed] [Google Scholar]
- 99. Gerardin E, Sirigu A, Lehericy S, Poline JB, Gaymard B, et al. (2000) Partially overlapping neural networks for real and imagined hand movements. Cereb Cortex 10: 1093–1104. [DOI] [PubMed] [Google Scholar]
- 100. Hanakawa T, Immisch I, Toma K, Dimyan MA, Van Gelderen P, et al. (2003) Functional properties of brain areas associated with motor execution and imagery. J Neurophysiol 89: 989–1002. [DOI] [PubMed] [Google Scholar]
- 101. Malouin F, Richards CL, Jackson PL, Dumas F, Doyon J (2003) Brain activations during motor imagery of locomotor-related tasks: a PET study. Hum Brain Mapp 19: 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Schilbach L, Bzdok D, Timmermans B, Fox PT, Laird AR, et al. (2012) Introspective minds: using ALE meta-analyses to study commonalities in the neural correlates of emotional processing, social & unconstrained cognition. PLoS One 7: e30920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Carbon M, Ghilardi MF, Feigin A, Fukuda M, Silvestri G, et al. (2003) Learning networks in health and Parkinson's disease: reproducibility and treatment effects. Hum Brain Mapp 19: 197–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Grahn JA, Parkinson JA, Owen AM (2009) The role of the basal ganglia in learning and memory: neuropsychological studies. Behav Brain Res 199: 53–60. [DOI] [PubMed] [Google Scholar]
- 105. Postma A, Kessels RP, van Asselen M (2008) How the brain remembers and forgets where things are: the neurocognition of object-location memory. Neurosci Biobehav Rev 32: 1339–1345. [DOI] [PubMed] [Google Scholar]
- 106. Squire LR, Stark CE, Clark RE (2004) The medial temporal lobe. Annu Rev Neurosci 27: 279–306. [DOI] [PubMed] [Google Scholar]
- 107. Jonides J, Lewis RL, Nee DE, Lustig CA, Berman MG, et al. (2008) The mind and brain of short-term memory. Annu Rev Psychol 59: 193–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Luck D, Danion JM, Marrer C, Pham BT, Gounot D, et al. (2010) The right parahippocampal gyrus contributes to the formation and maintenance of bound information in working memory. Brain Cogn 72: 255–263. [DOI] [PubMed] [Google Scholar]
- 109. Axmacher N, Schmitz DP, Weinreich I, Elger CE, Fell J (2008) Interaction of working memory and long-term memory in the medial temporal lobe. Cereb Cortex 18: 2868–2878. [DOI] [PubMed] [Google Scholar]
- 110. Dagher A, Owen AM, Boecker H, Brooks DJ (2001) The role of the striatum and hippocampus in planning: a PET activation study in Parkinson's disease. Brain 124: 1020–1032. [DOI] [PubMed] [Google Scholar]
- 111. Moody TD, Bookheimer SY, Vanek Z, Knowlton BJ (2004) An implicit learning task activates medial temporal lobe in patients with Parkinson's disease. Behav Neurosci 118: 438–442. [DOI] [PubMed] [Google Scholar]
- 112. Beauchamp MH, Thompson DK, Howard K, Doyle LW, Egan GF, et al. (2008) Preterm infant hippocampal volumes correlate with later working memory deficits. Brain 131: 2986–2994. [DOI] [PubMed] [Google Scholar]
- 113. Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB (2010) A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct 214: 519–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Hampson M, Driesen NR, Skudlarski P, Gore JC, Constable RT (2006) Brain connectivity related to working memory performance. J Neurosci 26: 13338–13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Left side - main effect (compared to resting baseline across both groups). Right side - load related effects during encoding (main effects across both groups).
(TIF)
Left side - main effect of direct recall (compared to resting baseline across both groups). Right side - load related effects during direct recall (main effects across both groups).
(TIF)
Left side - main effect of delayed recall (compared to resting baseline across both groups). Right side - load related effects during delayed recall (main effects across both groups).
(TIF)
Left side – increased activation during encoding relative to direct recall across both groups. Right side - increased activation during encoding relative to delayed recall across both groups.
(TIF)
Left side – increased activation during direct recall relative to encoding across both groups. Right side - increased activation during delayed recall relative to encoding across both groups.
(TIF)
Left side – conjunction between direct and delayed recall across both groups. Right side - conjunction between encoding, direct and delayed recall across both groups.
(TIF)
A - Interaction (ConDirect – PatDirect)>(ConDelayed – PatDelayed): Regions in which patients showed a significant specific reduction of activity during direct retrieval as tested by the interaction (ConDirect – PatDirect)>(ConDelayed – PatDelayed) in conjunction with the respective main effect (ConDirect – PatDirect), as well as the mean parameter estimates and 90% confidence intervals for the individual conditions at the location of the local maxima. B - Interaction (PatDelayed – ConDelayed)>(PatDirect – ConDirect): Regions in which patients showed a significant specific increase of activity during delayed retrieval as tested by the interaction (PatDelayed – ConDelayed)>(PatDirect – ConDirect) in conjunction with the respective main effect (PatDelayed – ConDelayed), as well as the mean parameter estimates and 90% confidence intervals for the individual conditions at the location of the local maxima.
(TIF)
Working memory task performance accuracy in patients with Parkinson's disease (PD) and healthy controls (HC) during direct recall, delayed recall and all conditions. Hits and misses are given for the 4-sequence and 5-sequence.
(DOC)