Abstract
Although the renal proximal tubular epithelial cells are targeted in a variety of inflammatory diseases of the kidney, the signaling mechanism by which tumor necrosis factor (TNF)-α exerts its effects in these cells remains unclear. Here, we report that TNF-α elicits antiapoptotic effects in opossum kidney cells and that this response is mediated via actin redistribution through a novel signaling mechanism. More specifically, we show that TNF-α prevents apoptosis by inhibiting the activity of caspase-3 and this effect depends on actin polymerization state and nuclear factor-κB activity. We also demonstrate that the signaling cascade triggered by TNF-α is governed by the phosphatidylinositol-3 kinase, Cdc42/Rac1, and phospholipase (PLC)-γ1. In this signaling cascade, Cdc42 was found to be selectively essential for PLC-γ1 activation, whereas phosphatidylinositol-3,4,5-triphosphate alone is not sufficient to activate the phospholipase. Moreover, PLC-γ1 was found to associate in vivo with the small GTPase(s). Interestingly, PLC-γ1 was observed to associate with constitutively active (CA) Cdc42V12, but not with CA Rac1V12, whereas no interaction was detected with Cdc42(T17N). The inactive Cdc42(T17N) and the PLC-γ1 inhibitor U73122 prevented actin redistribution and depolymerization, confirming that both signaling molecules are responsible for the reorganization of actin. Additionally, the actin filament stabilizer phallacidin potently blocked the nuclear translocation of nuclear factor-κB and its binding activity, resulting in abrogation of the TNF-α-induced inhibition of caspase-3. To conclude, our findings suggest that actin may play a pivotal role in the response of opossum kidney cells to TNF-α and implicate Cdc42 in directly regulating PLC-γ1 activity.
INTRODUCTION
Tumor necrosis factor (TNF)-α is a potent proinflammatory cytokine that induces necrosis or apoptosis (Natoli et al., 1998). In addition, TNF-α elicits antiapoptotic cell signals, leading to suppression of apoptosis, which is mostly dependent on nuclear factor-κB (NF-κB), and to inflammatory response (Barnes and Karin, 1997; Chang and Yang, 2000; Idriss and Naismith, 2000; Baud and Karin, 2001; Bergmann et al., 2001; Vancurova et al., 2001). It has also been proposed that TNF-α activates cell survival signaling cascades that result in the inhibition of apoptosis independently of NF-κB (Pastorino et al., 1999; Madge and Pober, 2000). TNF-α is produced by activated macrophages and monocytes, in response to inflammation, injury, or infection, as well as by heart and kidney in response to ischemia and reperfusion (Meldrum and Donnahoo, 1999; Baud and Karin, 2001). After its production, TNF-α acts locally or in distal sites, inducing its own expression and NF-κB activation, which in turn suppress apoptosis and induce the expression of other genes promoting inflammation (Donnahoo et al., 1999; Baud and Karin, 2001).
Because TNF-α is one of the most pleiotropic proinflammatory cytokines, the elucidation of signaling mechanisms that lead to apoptotic or antiapoptotic cellular responses could facilitate the control of these biological activities in TNF-α target cells. Although TNF-α has been reported to induce the activation of key signaling molecules and to reorganize actin cytoskeleton in various cell systems (Wojciak-Stothard et al., 1998; Kim et al., 1999; Koukouritaki et al., 1999; Puls et al., 1999; Chen et al., 2000), the signaling mechanism by which TNF-α exerts its effects in proximal tubule cells remains unclear. It is well known that the renal proximal tubular epithelial cells are targeted in a variety of immune and inflammatory diseases of the kidney (Baud and Ardaillou, 1993; Healy et al., 1999). The role of inflammatory cytokines in the pathogenesis of renal diseases has also been established (Ortiz et al., 1994; Rabb, 1994; Bruijn and de Heer, 1995). Therefore, the identification of the signaling mechanisms that regulate the response of these cells to TNF-α may have major implications in the control of renal inflammatory diseases.
In the present study, we have used the opossum kidney (OP) cells that express functional characteristics of normal proximal tubular epithelial cells, to analyze whether TNF-α exerts an apoptotic or antiapoptotic cell response in these cells. We also examined whether TNF-α induces actin cytoskeleton alterations and their possible role to cell response. Finally, we attempted to identify the signaling mechanism triggered by TNF-α that leads to actin redistribution and to modulation of NF-κB and caspase-3 activity. The results presented here demonstrate that TNF-α elicits antiapoptotic effects in OK cells by inhibiting caspase-3 activity and that the pathway that transmits the signal is through the phosphatidylinositol-3 kinase (PI-3 kinase)→Cdc42→phospholipase (PLC)-γ1→actin redistribution→NF-κB nuclear translocation cascade. In addition, these results provide novel mechanistic insights: we demonstrate the essential role of Cdc42 for PLC-γ1 activation as well as the in vivo interaction between the GTP-Cdc42 and PLC-γ1, which could be implicated in the activation of the phospholipase. We also present evidence that the polymerization state of actin is required for NF-κB translocation to the nucleus, which determines the cellular response of OK cells to TNF-α.
MATERIALS AND METHODS
Materials
TNF-α was obtained from R&D Systems (Minneapolis, MN). Rhodamine-phalloidin and Slow Fade antifade kit were from Molecular Probes (Eugene, OR). The NF-κB binding specific oligonucleotide, polyclonal antibodies for p21-activated kinase (PAK)1 (rabbit), Cdc42 (rabbit), PLC-γ1 (rabbit), and NF-κB (p65) (goat) as well as monoclonal anti-phosphotyrosine (PY20) antibody were from Santa Cruz Biotechnology (Santa Cruz, CA). The digoxigenin-labeling kit and the positively charged nylon membranes were from Roche Diagnostics (Basel, Switzerland). Rabbit polyclonal anti-PI-3 kinase(p85) antibody, monoclonal anti-Rac, and Cdc42/Rac activation assay kit (including glutathione S-transferase [GST]-fusion of the PAK1 p21-binding domain [PBD, amino acids 67-150] bound to glutathione-agarose and lysis/wash buffer) were purchased from Upstate Biotechnology (Lake Placid, NY). Phospho-specific (Thr308) Akt and anti-Akt antibodies were from New England Biolabs (Beverly, MA). The APOPercentage apoptosis assay was from Biocolor (Newtownabbey, northern Ireland, United Kingdom). The caspase-3 activation assay kit was obtained from BD Biosciences Clontech (Palo Alto, CA) or from Calbiochem-Novabiochem (Darmstadt, Germany). U73122 and Clostridium difficile toxin B were from Calbiochem-Novabiochem. Phosphatidylinositol-4,5-bisphosphate (from bovine brain), phosphatidyl[2-3H]inositol 4,5-bisphosphate, phosphatidylethanolamine (from bovine brain), wortmannin, and phallacidin were obtained from Sigma-Aldrich (St. Louis, MO). Silica gel 60 sheets were from Merck (Poole, Dorset, United Kingdom). ECL Western blotting kit, protein A-Sepharose beads, and [γ-32P]ATP were from Amersham Biosciences (Piscataway, NJ). All other chemicals were obtained from usual commercial sources at the highest grade available.
Cell Culture and Transfections
OK cells were from the American Type Culture Collection (Manassas, VA) and were studied between passages 40 and 50. The culture of cells and the transfection procedure, by using the calcium-phosphate DNA coprecipitation protocol, were performed as described previously (Papakonstanti and Stournaras, 2002). Initially, cells were cultured for 48 h with complete medium. Then, the medium was changed to serum free medium 24 h before the actual experiments. TNF-α was used at a concentration of 10 ng/ml. When indicated, cells were preincubated with the PI-3 kinase inhibitor wortmannin (100 nM, 30 min), the actin stabilizing agent phallacidin (1 μM, 1 h), the inhibitor of PLC-γ1 U73122 (10 μM, 30 min), the inhibitor of Rho GTPases toxin B (50 ng/ml, 2 h), or the NF-κB inhibitor PDTC (pyrolidine dithiocarbonate) (20 μM, 1 h) and then cells were stimulated with TNF-α for the indicated times. All experiments described below were performed in confluent cells.
Plasmids
pCMV expression plasmid encoding the double mutant IκBα (S32A/S36A), which is not phosphorylated (Whiteside et al., 1995), was kindly provided by Dr. A. Zantema (Laboratory for Molecular Carcinogenesis, Sylvius Laboratories, Leiden University, The Netherlands). Wild-type IKK-2 and IKK-2(S177E, S181E) were kindly provided by Dr. A. Rao (The Center for Blood Research and, Department of Pathology, Harvard Medical School, Boston, MA) and Dr. F. Mercurio (Celgene Research, San Diego, California). pCMV expression plasmids encoding the Cdc42(T17N) or Cdc42V12 and pEXV expression plasmids encoding the Rac1(T17N) or Rac1V12 were kindly provided by Dr. A. Moustakas (Ludwing Institute for Cancer Research, Uppsala, Sweden).
Expression and Purification of Recombinant Proteins
The pCMV or pEXV plasmids were transformed into Escherichia coli DH10B for protein expression. Recombinant proteins from 250 ml of culture were purified by the high purity plasmid purification system (Invitrogen, Carlsbad, CA) according to the manufacture's instructions.
Immunoprecipitation, Kinase Assays, and Immunoblotting Analysis
Cells were washed three times with ice-cold phosphate-buffered saline (PBS) and suspended in cold lysis buffer containing 1% Nonidet P-40, 50 mM Tris, pH 7.5, and 150 mM NaCl supplemented with protease and phosphatase inhibitors. Cleared lysates were preadsorbed with protein A-Sepharose for 1 h at 4°C, centrifuged, and the supernatants (equal amounts of protein) were subjected to immunoprecipitation by using the indicated antibodies and the A-Sepharose beads.
PAK1 kinase assay was performed according to the procedure of Knaus et al. (1995), by using autophosphorylation to assess activity, and the lipid kinase activity of PI-3 kinase was measured by the method of Auger et al. (1989) with minor modifications as described previously (Papakonstanti and Stournaras, 2002).
For immunoblot analysis, the cell lysates or the immunoprecipitates were suspended in Laemmli's sample buffer and separated by SDS-PAGE. Proteins were transferred onto nitrocellulose membrane, and the incubation with antibodies was performed as described previously (Papakonstanti and Stournaras, 2002). Blots were developed using the ECL system and the band intensities were quantitated by PC-based image analysis (Image Analysis, St. Catherines, ONT, Canada).
PLC-γ1 Activity Assay
PLC-γ1 activity was measured according to Arkinstall et al., (1995) with minor modifications. To prepare substrate, 1 μCi of phosphatidyl[2-3H]inositol 4,5-bisphosphate ([3H]PIP2; 10 Ci/mmol) was mixed with 110 μl of phosphatidylethanolamine (10 mg/ml) and dried under nitrogen stream. Then, 120 μl of 2 μg/μl PIP2 dissolved in 50 mM HEPES with 100 mM KCl and 10 mM deoxycholate (pH 7.0) was added, and phospholipids were resuspended in 1 ml of substrate solution (20 mM HEPES, 100 mM NaCl, pH 7.2) by using a sonicator. To measure PLC-γ1 activity, 25 μl of substrate was mixed with 25 μl of buffer B (50 mM Tris, 50 mM maleic acid, 40 mM LiCl, 20 mM MgCl2, pH 7.0) and 25 μl of 2.2 mM CaCl2 calculated to give 5 × 10-5 M free Ca2+ (based on 0.5 mM EGTA). We have decided to use this free Ca2+ concentration because we observed maximum enzymatic activity under these conditions. Reactions were started by addition of immunoprecipitated PLC-γ1 (from equal amounts of protein) suspended in 25 μl of 5 mM Tris, pH 7.4, containing 2 mM EGTA. After incubation at 37°C for 10 min with agitation, reactions were stopped by the addition of methanol:chloroform:HCl (200:100:1), chloroform, and 0.25 M HCl. After mixing vigorously and centrifuging to separate the phases, 1 ml of the upper aqueous phase was counted by liquid scintillation.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear proteins were extracted from untreated or TNF-α-treated cells according to the procedure of Dignam et al. (1983) with minor modifications. Labeling of NF-κB binding-specific oligonucleotide (5′-AGT TGA GGG GAC TTT CCC AGG C-3′) was performed using a nonradioactive technique with digoxigenin-11-ddUTP under the instructions provided by the kit (Roche Diagnostics). For binding reactions, 10 μg of nuclear protein was incubated with 0.21 pmol of 3′ end-labeled probe and 5 μg poly(dI-dC) and 0.1 μg of poly l-lysine in binding buffer in a final volume of 20 μl for 15 min at room temperature. Specificity was determined using 1 μg of anti-p65 or anti-p50 antibodies, which were added to proteins 45 min before using the labeled probe and by competition with 25-fold excess of unlabeled probe. The samples were analyzed on 6% native polyacrylamide gels and then were transferred in positively charged nylon membranes, blocked in blocking buffer (1% blocking reagent, 0.1 M maleic acid, 0.15 M NaCl, pH 7.5), and finally incubated with anti-digoxigenin-Fab fragments conjugated with alkaline phosphatase. Bands were visualized by chemiluminescence using the CSPD system (Roche Diagnostics).
In NF-κB (p65) translocation studies the cytosolic or nuclear extracts were subjected to SDS-PAGE by using a 10% running gel, and immunoblot analysis was performed as described above.
Apoptosis Assay
APOPercentage apoptosis assay is based on the staining of the cells with a dye, which enters the cells after the translocation of phosphatidylserine that, during apoptosis, is moved from the plasma membrane inner leaflet to the outer leaflet where it triggers recognition and phagocytosis of the apoptotic cells (Fadok et al., 1992; Martin et al., 1995). This assay was performed as described in the instructions of the kit. The cells were washed with PBS and photographed using an inverted microscope and then the Dye Release Reagent was added and the absorbance was measured using a microplate colorimeter at 540 nm with reference at 620 nm.
Caspase-3 Assay
The activity of caspase-3 in whole cell lysates was determined using the BD Biosciences Clontech ApoAlert CPP32 or the Calbiochem colorimetric assay kit according to the manufacturers' instructions. The caspase-3 activity was determined by incubating cell lysates with the caspase-3 substrate (the peptide DEVD conjugated to the chromophore p-nitroanilide) for 2 h at 37°C. The absorbance of each sample was measured at 405 nm by using a 96-well colorimetric plate reader.
Affinity Precipitation
Affinity precipitation with GST-PBD was performed using an assay based on the method of Benard et al. (1999). Cells were lysed in Mg2+ lysis buffer (MLB), which was provided by the assay kit (UBI, Lake Placid, NY), mixed with 8 μg of GST-PBD bound to glutathione-agarose, and incubated for 1 h at 4°C. Precipitates were washed three times with MLB and suspended in Laemmli's sample buffer. Proteins were separated by 11% SDS-PAGE, transferred onto nitrocellulose membrane, and blotted with anti-Cdc42, anti-Rac1, or anti-PLC-γ1 antibody.
Immunofluorescence Microscopy
Direct fluorescence staining of microfilaments by rhodamine-phalloidin and double labeling with additional indirect staining for fluorescence of Myc-tagged dominant negative Cdc42(T17N) to detect the transfected cells, by using monoclonal anti-Myc epitope antibody and the fluorescein isothiocyanate-conjugated goat anti-mouse antibody, were performed as described previously (Are et al., 2000; Papakonstanti and Stournaras, 2002). All specimens were examined with an Olympus BH-2 microscope equipped with epifluorescence illumination. Micrographs were photographed with a 35-mm (C-35AD-4) camera and Kodak P3200 black and white films.
DNase I Inhibition Assay
Cells were washed three times with ice-cold PBS and suspended in cold lysis buffer containing 10 mM K2HPO4, 100 mM NaF, 50 mM KCl, 2 mM MgCl2, 1 mM EGTA, 0.2 mM dithiothreitol, 0.5% Triton X-100, 1 M sucrose, pH 7.0. The monomeric (G) and total (T) actin contents were determined as described previously (Papakonstanti et al., 1996).
RESULTS
Anti-Apoptotic Response of OK Cells to TNF-α and the Involvement of NF-κB and Actin Cytoskeleton
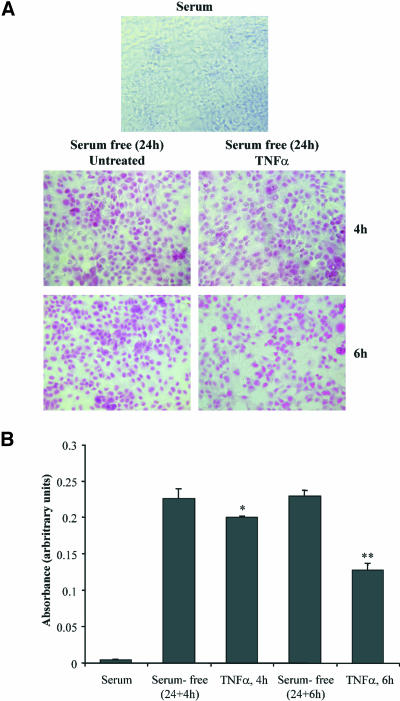
To determine whether TNF-α exerts an apoptotic or anti-apoptotic cell response in OK cells, we performed a colorimetric apoptosis assay in cells cultured in the presence or absence (deprived cells) of serum for 24 h and then incubated with TNF-α for the indicated times (Figure 1). As shown in Figure 1A, cell culture in serum-free condition resulted in the appearance of many apoptotic cells that were progressively decreased by the addition of TNF-α. Quantification of these results revealed that exposure of deprived cells to TNF-α induced a significant decrease of arbitrary absorbance units (Figure 1B), suggesting that TNF-α exerts antiapoptotic effect in OK cells. Indeed, the absorbance units were decreased by 12% when cells were incubated with TNF-α for 4 h compared with untreated cells (0.226 ± 0.014 compared with 0.200 ± 0.002, n = 6, p < 0.05), whereas 6-h incubation with TNF-α resulted in a more substantial decrease of absorbance units by 44% compared with the respective untreated cells (0.229 ± 0.008 compared with 0.128 ± 0.007, n = 6, p < 0.01) (Figure 1B). As has been published for most effects of TNF-α, its antiapoptotic effect in OK cells was found to be also transient because the absorbance units after treatment of cells with TNF-α for 24 h were almost equal to that measured in the respective deprived untreated cells (0.200 ± 0.003 compared with 0.205 ± 0.002, n = 6).
Figure 1.
Antiapoptotic effects of TNF-α in OK cells. Cells in complete medium or cells that had been exposed to serum-free medium for 24 h and then incubated with TNF-α for the indicated times were assessed for apoptosis using the colorimetric APOPercentage apoptosis assay. Apoptotic cells were photographed using an inverted microscope (A) or quantified by measuring the absorbance by using a microplate colorimeter (B). Mean + SEM from two separate experiments performed in triplicate (significance level *p < 0.05, **p < 0.01).
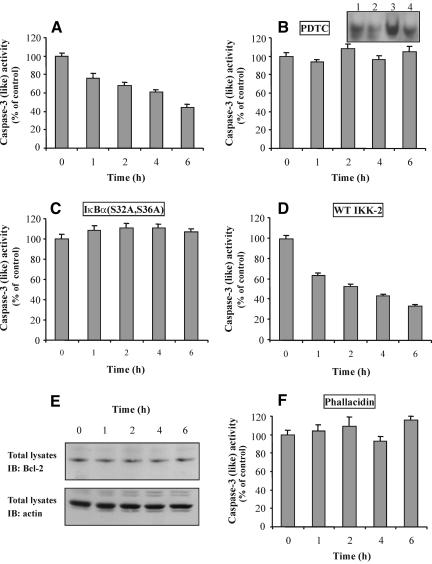
To confirm the results obtained above, we also examined the effect of TNF-α on caspase-3 activity, a central molecule that is early activated in the apoptotic pathway. As shown in Figure 2A, TNF-α induced a significant decrease in the protease activity, compared with control (deprived) cells, which became evident 1 h after TNF-α treatment and remained at low levels during the next 6 h after the addition of TNF-α. In an effort to identify the upstream molecule(s) that directly involved in the protease inhibition, cells were preincubated with the NF-κB inhibitor PDTC and then exposed to TNF-α. As shown in Figure 2B, PDTC blocked the inhibition of caspase-3, indicating the involvement of NF-κB in the survival effect of TNF-α. To confirm the involvement of NF-κB and the inhibitory action of PDTC, cells were incubated for 1 h with TNF-α in the absence or presence of PDTC and the NF-κB binding activity was determined by EMSA. As shown in Figure 2B (inset), the NF-κB binding activity was induced by TNF-α (compare lane 1 with lane 3), whereas in the presence of PDTC it was significantly decreased (compare lane 3 with lane 4). To obtain more direct evidence that NF-κB is involved in the inhibition of caspase-3, we used the double mutant IκBα(S32A/S36A), which is not phosphorylated because of the S32 and S36 substitutions resulting in prevention of degradation of IκBα and the subsequent translocation of NF-κB into the nucleus (Whiteside et al., 1995). Additionally, we determined the caspase-3 activity in cells that had been transfected with the wild-type (WT) IKK-2, which induces the TNF-α-stimulated NF-κB-mediated gene activation (Mercurio et al., 1997). We observed that, indeed, IκBα(S32A/S36A) blocked the protease inhibition (Figure 2C), whereas the WT IKK-2 induced a more drastic inhibition of caspase-3 (Figure 2D). Although the IKK-2(S177E, S181E) mutant generates a highly active IκBα kinase activity, we do not present data for this mutant because we observed that IKK-2(S177E, S181E) induces the activity of NF-κB in the absence of cell stimulation, as it has been also described by others (Mercurio et al., 1997).
Figure 2.
Involvement of NF-κB and actin in the inhibition of caspase-3 by TNF-α. Extracts of TNF-α-treated cells (A) or cells that had been preincubated with PDTC (B), or had been transfected with IκBα(S32A/S36A) (C) or WT IKK-2 (D), or had been preincubated with phallacidin (F) and then treated with TNF-α, for the indicated times, were incubated with the caspase-3 substrate DEVD-p-nitroanilide, and the protease activity was analyzed as described in MATERIALS AND METHODS. Data are presented as percentage of control cells' activity by measuring the OD per milligram of protein per minute (mean + SEM from two to three separate experiments performed in duplicate). (B, inset) Cells were incubated with TNF-α for 1 h in the presence or absence of PDTC. Nuclear extracts were subjected to EMSA as described in MATERIALS AND METHODS. Lane 1, untreated cells; lane 2, cells treated with PDTC; lane 3, cells treated with TNF-α; lane 4, cells treated with TNF-α in the presence of PDTC. (E) Cells were stimulated with TNF-α for the indicated times. Total cell lysates were prepared and immunoblotted using anti-Bcl-2 (top) or anti-actin antibody (bottom).
Because the Bcl-2 family proteins function to inhibit also caspase-3, we examined the expression of Bcl-2 by immunoblot analysis. This experiment revealed that Bcl-2 protein remained unchanged after TNF-α treatment (Figure 2E, top). Immunoblotting of the same nitrocellulose membrane for total actin (Figure 2E, bottom) confirmed that there are no differences in the total Bcl-2 protein levels. It is well known that actin acts as a sensor and/or mediator of various signals. Accordingly, we examined its possible involvement in the inhibition of caspase-3 activity induced by TNF-α. Interestingly, when cells were preincubated with phallacidin, an actin-stabilizing agent that prevents actin depolymerization induced by extracellular stimuli in these cells (Papakonstanti et al., 1996), the caspase-3 activity was inhibited (Figure 2F), suggesting that actin polymerization state plays a key role in antiapoptotic signaling triggered by TNF-α. To confirm the inhibitory action of phallacidin on actin depolymerization and therefore the involvement of actin in the inhibition of caspase-3, we determined the G/T actin ratio in TNF-α-treated cells in the presence or absence of phallacidin. Using the DNase I inhibition assay, we found that the G/T actin ratio was significantly increased in cells exposed to TNF-α for 1 h compared with untreated cells (0.25 ± 0.02 compared with 0.31 ± 0.01, n = 4, p < 0.05), indicating actin depolymerization, whereas when cells were preincubated with phallacidin this actin ratio remained at basal levels (0.26 ± 0.03, n = 4).
Effect of TNF-α on the Organization of Actin Microfilaments
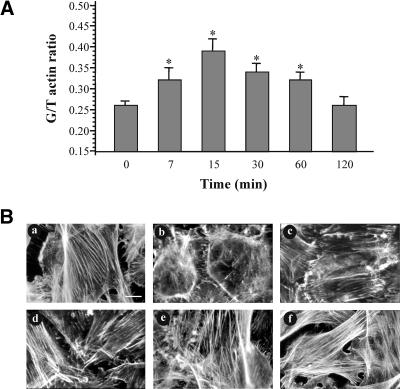
The results obtained above suggest that TNF-α affects the organization of actin and moreover that this alteration is involved in cell response to TNF-α. Indeed, using the well-established DNase I inhibition assay, we found that TNF-α induced a time-dependent increase of G/T actin ratio consistent with a decrease in the proportion of filamentous actin (Figure 3A). The G/T actin ratio was increased to a maximum (50 ± 5%, n = 5) within 15 min of TNF-α treatment, whereas it reached the basal levels at 2 h. To determine whether the TNF-α-induced microfilament depolymerization was accompanied by actin reorganization, we performed direct immunofluorescence experiments. As shown in Figure 3B, cells exposed to TNF-α for short periods (7-30 min) (Figure 3B, b-d) exhibited a clear redistribution of actin filaments, including the loss of stress fibers as well as the formation of many peripheral filopodia and microspikes, which were accompanied by intense lamellipodia. In longer incubation times (60-120 min) (Figure 3B, e and f), these peripheral structures were progressively disappeared, whereas stress fibers reappeared.
Figure 3.
Actin redistribution and depolymerization in response to TNF-α. (A) Cells were incubated for the indicated times with 10 ng/ml TNF-α and then the monomeric (G) or total (T) actin levels was measured as described in MATERIALS AND METHODS. Data are presented as G/T actin ratio (mean + SEM from five separate experiments; significance level *p < 0.05). (B) Cells were incubated with TNF-α for 7 (b), 15 (c), 30 (d), 60 (e), or 120 (f) min and then the redistribution of filamentous actin was determined with rhodamine-phalloidin staining by immunofluorescence microscopy. (a) Untreated cells. Bar, 20 μm. Similar results were obtained in three independent experiments.
TNF-α Activates Cdc42 and Rac1 Downstream of PI-3 Kinase
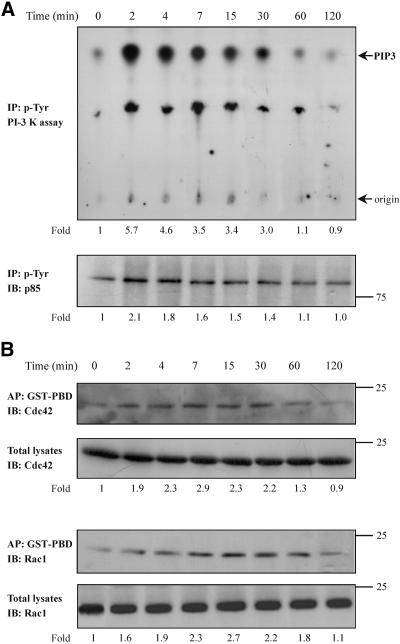
In an effort to identify the signaling mechanism triggered by TNF-α and leads to actin reorganization, we focused on key signal transduction molecules. PI-3 kinase, a signal transduction molecule that is activated upstream or downstream of small GTPases (Tolias et al., 1995; Krasilnikov 2000; Vanhaesebroeck et al., 2001; Papakonstanti et al., 2003), influences the organization of actin cytoskeleton. To examine whether PI-3 kinase is activated by TNF-α, an in vitro kinase assay was performed on anti-phosphotyrosine immune complexes isolated from equal amounts of proteins of control and TNF-α-treated cells. We found that the lipid kinase activity of PI-3 kinase (Figure 4A, top) as well as the tyrosine phosphorylation of its p85 regulatory subunit (Figure 4A, bottom) was induced to a maximum within 2 min of TNF-α treatment, and it had been reached the control levels 2 h later.
Figure 4.
Effect of TNF-α on the PI-3 kinase and Cdc42/Rac1 activity. Cells were incubated for the indicated times with TNF-α (10 ng/ml). Equal amount of proteins of cell lysates were immunoprecipitated with an anti-phosphotyrosine antibody and subjected to an in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, by using PIP2 as substrate. The number below each lane indicates the fold amount of phosphatidylinositol-3,4,5-trisphosphate (PIP3) product, with that of untreated cells taken as 1 (top). The phosphorylation of the p85 regulatory subunit of PI-3 kinase that was immunoprecipitated in the kinase assay was assessed by immunoprecipitation (IP) with an anti-phosphotyrosine antibody and immunoblotting (IB) with anti-PI-3 kinase (p85) antibody. The number below each lane indicates the fold phosphorylation of p85, with that of untreated cells taken as 1 (bottom). (B) Equal volume of cell lysates from untreated or TNF-α-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-agarose beads. Precipitated GTP-Cdc42 or GTP-Rac1 was detected by IB with anti-Cdc42 or anti-Rac1 antibody, respectively. Equal volume of total lysates from untreated and TNF-α-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and IB with monoclonal anti-Cdc42 or anti-Rac1 antibody, respectively. The number below each lane indicates the normalized fold activation of Cdc42 or Rac1, with that of untreated cells taken as 1. Results shown are representative of three independent experiments with similar results.
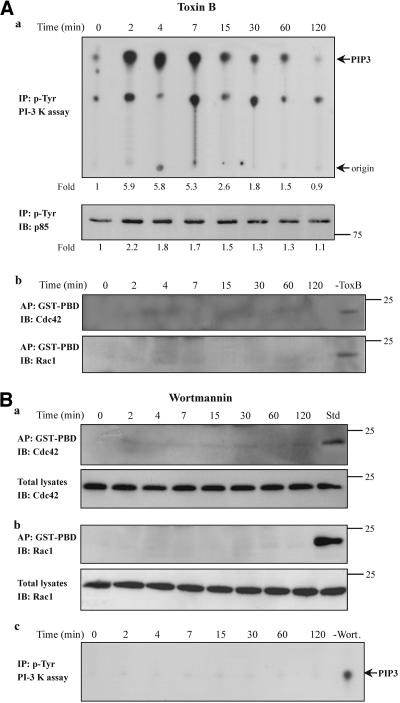
Because the small GTPases Cdc42/Rac1 have been implicated in the formation of filopodia, microspikes, and membrane ruffles (Ridley and Hall, 1992), and such morphological changes were observed in our system, we examined next whether these GTPases are activated in response to TNF-α. Equal volumes of cell lysates from control and TNF-α-treated cells were affinity precipitated with a GST-fusion protein corresponding to the p21-binding domain of PAK1 (GST-PBD) that specifically binds to and precipitates the Cdc42-GTP and Rac1-GTP (Benard et al., 1999). The presence of each GTPase was assessed with a specific antibody. To normalize the results, equal volumes of cell extracts were also analyzed by immunoblotting for total amounts of Cdc42 and Rac1. As shown in Figure 4B, the ratio of GTP-Cdc42 or GTP-Rac1 to total Cdc42 or Rac1 was significantly and time dependently increased in cells exposed to TNF-α. The activation of Cdc42 and Rac1 was induced to a maximum within 7 and 15 min, respectively, of TNF-α incubation, suggesting that it would be a relatively proximal consequence of PI-3 kinase activation. To confirm this, we performed the in vitro PI-3 kinase assay in cells that had been pretreated with the C. difficile toxin B. As shown in Figure 5A, a, both the lipid kinase activity (top) as well as the tyrosine phosphorylation of p85 regulatory subunit of PI-3 kinase (bottom) were significantly induced by TNF-α indicating that the small GTPases are activated downstream of the PI-3 kinase. On the other hand, pretreatment of cells with toxin B abolished the formation of GTP-Cdc42 or GTP-Rac1 (Figure 5A, b), confirming the inhibition of the small GTPases. Similar results were obtained when PI-3 kinase activity was determined after transfection of cells with inactive Cdc42(T17N) or Rac1(T17N) (our unpublished data). Additionally, when the activated form of Cdc42 or Rac1 was determined in cells that had been pretreated with the specific PI-3 kinase inhibitor wortmannin no specific signal was detected implying that the activation of the small GTPases is dependent on PI-3 kinase activity (Figure 5B, a and b). The inhibitory action of wortmannin on PI-3 kinase activity was confirmed by the in vitro kinase assay performed in cells that had been preincubated with wortmannin and then exposed to TNF-α for the indicated times (Figure 5B, c).
Figure 5.
PI-3 kinase is activated upstream of the small GTPases Cdc42/Rac1 in TNF-α-treated cells. (A) Cells were pretreated with toxin B (50 ng/ml, 2 h) and then incubated with TNF-α (10 ng/ml) for the indicated times. (a) Equal amount of proteins of cell lysates were immunoprecipitated with an anti-phosphotyrosine antibody and subjected to an in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, by using PIP2 as substrate. The number below each lane indicates the fold amount of PIP3 product, with that of untreated cells taken as 1 (top). The phosphorylation of the p85 regulatory subunit of PI-3 kinase that was immunoprecipitated in the kinase assay was assessed by immunoprecipitation (IP) with an anti-phosphotyrosine antibody and immunoblotting (IB) with anti-PI-3 kinase (p85) antibody. The number below each lane indicates the fold phosphorylation of p85, with that of untreated cells taken as 1 (bottom). (b) The activated forms of Cdc42 (top) or Rac1 (bottom) in the presence or absence (-ToxB) of toxin B was determined by affinity precipitation (AP) with GST-PBD and then by IB with the respective antibodies. (B) Cells were pretreated with wortmannin (100 nM, 30 min) and then with TNF-α. Equal volume of cell lysates from untreated or TNF-α-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-agarose beads. Precipitated GTP-Cdc42 (a, top) or GTP-Rac1 (b, top) was detected by immunoblot (IB) with anti-Cdc42 or anti-Rac1 antibody, respectively. Equal volumes of total lysates from untreated and TNF-α-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and IB with monoclonal anti-Cdc42 (a, bottom) or anti-Rac1 (a, bottom) antibody, respectively. (c) The lipid kinase activity of PI-3 kinase in the presence or absence (-Wort.) of wortmannin was determined by the in vitro PI-3 kinase assay, as described in MATERIALS AND METHODS, by using PIP2 as substrate. Results shown are representative of three similar experiments. PIP3, phosphatidylinositol-3,4,5-trisphosphate.
Cdc42 Induces PLC-γ1 Activity
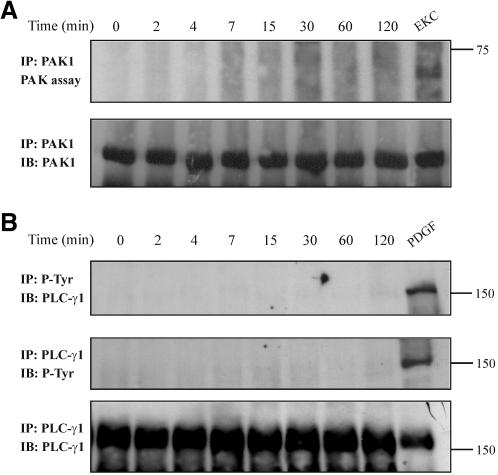
Among the downstream targets of activated Cdc42/Rac1 are the PAKs (Bagrodia et al., 1995; Manser et al., 1995), the activation of which results in depolymerization of stress fibers and peripheral actin reorganization (Daniels and Bokoch, 1999). To explore whether PAK1 activation is responsible for actin redistribution induced by TNF-α, we performed the PAK1 kinase assay on immunoprecipitates obtained from equal amounts of protein from untreated or TNF-α-treated cells. Ethylketocyclazocine (EKC)-treated cells were used as positive control because we have seen previously that this opioid agonist induces the PAK1 kinase activity in these cells (Papakonstanti and Stournaras, 2002). Although PAK1 was activated in cells exposed to EKC, no activation was observed in TNF-α-treated cells (Figure 6A, top), indicating that actin reorganization occurs through a PAK1-independent mechanism. The presence of PAK1 in the immunoprecipitates was confirmed by immunoblotting of the nitrocellulose membrane with an anti-PAK1 antibody (Figure 6A, bottom).
Figure 6.
TNF-α has no effect on PAK1 activity and on PLC-γ1 phosphorylation. (A) Cells were stimulated with TNF-α for the indicated times or with 10-8 M EKC for 30 min (positive control), lysed, and anti-PAK1 immune complexes were assayed for kinase autophosphorylation by an in vitro kinase assay as described in MATERIALS AND METHODS. The reaction products were separated by SDS-PAGE, transferred to nitrocellulose membrane, and phosphorylation was visualized by autoradiography (top). The amount of PAK1 protein that was immunoprecipitated in the kinase assay was assessed by immunoblotting (IB) with anti-PAK1 antibody (bottom). (B) Cells were stimulated with TNF-α for the indicated times or with 40 ng/ml platelet-derived growth factor for 15 min (positive control), lysed, and then equal amount of proteins were immunoprecipitated (IP) with an anti-phosphotyrosine antibody. The tyrosine-phosphorylated PLC-γ1 was detected by IB with a specific anti-PLC-γ1 antibody (top). PLC-γ1 phosphorylation was also examined by its immunoprecipitation that was followed by immunoblotting with an anti-phosphotyrosine antibody (middle). Stripping and reprobing of the nitrocellulose membrane confirmed the presence of PLC-γ1 protein on immunoprecipitates (bottom). Results shown are representative of three similar experiments.
PLC-γ1 is a signaling molecule that is involved in regulation of cytoskeletal organization (Yu et al., 1998; Nojiri and Hoek 2000; Papakonstanti et al., 2000). Accordingly, we examined whether PLC-γ1 was phosphorylated and/or activated in our system. Tyrosine-phosphorylated proteins were immunoprecipitated from untreated or TNF-α-treated cells with a specific antibody, and the nitrocellulose membrane was probed with an anti-PLC-γ1 antibody. PDGF-treated cells were used as positive control. As shown in Figure 6B (top) no phosphorylation of PLC-γ1 was observed in TNF-α-treated cells. The same result was obtained when PLC-γ1 was immunoprecipitated from untreated or TNF-α-treated cells, and the nitrocellulose membrane was probed with an anti-phosphotyrosine specific antibody (Figure 6B, middle). Reprobing of the nitrocellulose membrane with the anti-PLC-γ1 antibody confirms its presence in the immunoprecipitates (Figure 6B, bottom).
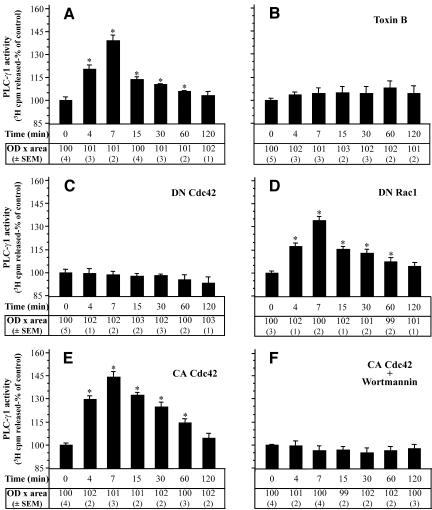
Although these results may indicate that PLC-γ1 was not phosphorylated after treatment of cells with TNF-α, they do not imply that PLC-γ1 was not activated because it is well known that PLC-γ isoenzymes are also activated in the absence of tyrosine phosphorylation (Sekiya et al., 1999). To test this, we performed the PLC activity assay on PLC-γ1 immunoprecipitates and indeed, we determined a time-dependent increase of PLC-γ1 activity. As shown in Figure 7A, the maximum stimulation of PLC-γ1 was observed 7 min after TNF-α treatment, whereas it returned to control levels during the next 2 h after the addition of TNF-α. To examine whether PLC-γ1 activation is dependent on small GTPases, we performed the PLC-γ1 activity assay in cells that had been preincubated with C. difficile toxin B or in cells that had been transfected with inactive Cdc42(T17N) or Rac1(T17N). As shown in Figure 7, B and C, both toxin B and Cdc42(T17N) prevented the increase of PLC-γ1 activity induced by TNF-α. Interestingly, transfection of cells with inactive Rac1(T17N) did not affect the activation of PLC-γ1 (Figure 7D). Because the transfection efficiency with the Rac1(T17N) was almost the same as that with Cdc42(T17N), we consider the above-mentioned finding meaningful. These results indicate that the activated Cdc42 is selectively essential for PLC-γ1 activation. However, it has been shown that PLC-γ1 is activated by PI(3,4,5)P3 (phosphatidylinositol-3,4,5-triphosphate) the product of the reaction catalyzed by the PI-3 kinase even in the absence of the tyrosine phosphorylation of the phospholipase (Bae et al., 1998; Falasca et al., 1998). The next experiment was designed to examine whether Cdc42 alone is sufficient to activate PLC-γ1. Cells were transfected with constitutively active Cdc42V12 and then incubated with the PI-3 kinase inhibitor wortmannin before their exposure to TNF-α. As shown in Figure 7F, wortmannin blocked the increase of PLC-γ1 activity in Cdc42V12-transfected cells, implying that Cdc42 is essential, but not sufficient, to activate PLC-γ1. Cdc42V12 alone did not negatively affect the phospholipase activation. In contrast, it caused PLC-γ1 activity to return at basal levels more slowly than in untransfected cells (Figure 7E).
Figure 7.
PLC-γ1 activity depends on Cdc42 activation in TNF-α-treated cells. PLC-γ1 was immunoprecipitated from equal amount of proteins from TNF-α-treated cells (A) or cells that had been preincubated with toxin B (B) or had been transfected with Cdc42(T17N) (C), Rac1(T17N) (D), Cdc42V12 (E), or with Cdc42V12 followed by exposure to wortmannin (100 nM, 30 min) (F) and then incubated with TNF-α for the indicated times. PLC-γ1 was analyzed for hydrolytic activity toward [3H]phosphatidylinositol 4,5-bisphosphate as described in MATERIALS AND METHODS. PLC-γ1 activity is presented as percentage of control cells' activity by measuring the cpm [3H]inositol 1,4,5-triphosphate released (mean + SEM from three separate experiments performed in duplicate, *p < 0.05). To confirm that equal amounts of PLC-γ1 protein were assayed under each condition separate immunoblot experiments were performed. The “OD × area” (as percentage of control) (±SEM) corresponding to bands of PLC-γ1 loaded was measured by PC-based image analysis and presented in table below each panel.
Separate immunoblots for all of the above-mentioned experiments confirmed that equal amounts of PLC-γ1 were assayed under each condition (table data below each panel in Figure 7).
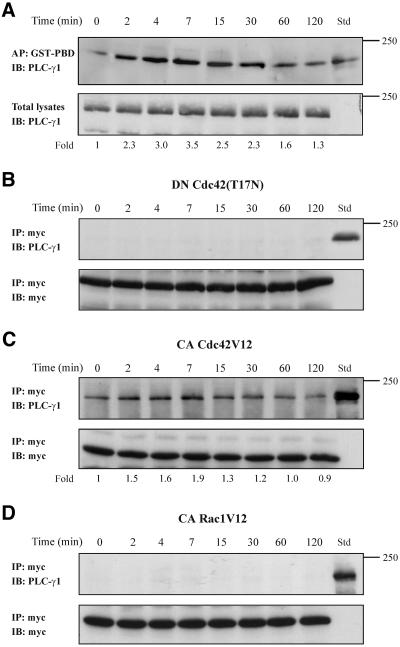
The Activated Cdc42 Associates In Vivo with PLC-γ1
The above-mentioned results suggest that Cdc42 is activated upstream of PLC-γ1 and that it is essential for the activation of the latter in the signaling cascade triggered by TNF-α. Therefore, we explored whether these proteins are associated in vivo. To this end, equal volumes of cell lysates from untreated and TNF-α-treated cells were affinity precipitated with the GST-PBD that specifically binds to and precipitates the Cdc42-GTP and Rac-GTP (Benard et al., 1999). In addition, total lysates were used as standard (Std) to compare the mobility of the total PLC-γ1 with that coprecipitated with activated GTPase(s) (Figure 8A, top). The presence of the PLC-γ1 was assessed with a specific antibody. To normalize the results, equal volumes of cell extracts from untreated or TNF-α-treated cells were also analyzed by immunoblotting for total amounts of PLC-γ1 (Figure 8A, bottom). As shown in Figure 8A (top), PLC-γ1 was coprecipitated with the activated forms of the small GTPases, and it was migrated with the same mobility (∼150 kDa) as the PLC-γ1 in total lysates (Std). The ratio of coprecipitated PLC-γ1 (Figure 8A, top) to total PLC-γ1 (Figure 8A, bottom), which represents the normalized measurements, was significantly and time dependently increased in cells exposed to TNF-α. The association of the activated GTPase(s) with PLC-γ1 was induced to a maximum within 7 min of TNF-α incubation. Interestingly, the kinetics of activated GTPase(s)-PLC-γ1 association was shown to correlate with the increase in Cdc42 activity, which was induced to a maximum within 7 min (Figure 4B) as well as with the increase in PLC-γ1 activity, which also was induced to a maximum within 7 min of TNF-α treatment (Figure 7A). This result indicates that the association of GTP-Cdc42 with PLC-γ1 could play an important role in PLC-γ1 activation. To further confirm the interaction between GTP-Cdc42 and PLC-γ1, we performed coimmunoprecipitation experiments in cells that had been transfected either with the inactive myc-tagged Cdc42(T17N) or with the constitutively active myc-tagged Cdc42V12. As shown in Figure 8B, immunoprecipitation experiments with anti-myc antibody did not reveal any association between the inactive Cdc42(T17N) and PLC-γ1. On the contrary, the constitutively active Cdc42V12 was effectively associated with PLC-γ1 (Figure 8C). Under the later experimental conditions, the Cdc42V12-PLC-γ1 interaction in TNF-α-treated cells was also induced compared with that of untreated cells, but to a lesser extent than the respective interaction in untransfected cells. This could be explained by the fact that cells expressing Cdc42V12 exhibited an induction in Cdc42V12-PLC-γ1 association under control conditions compared with Cdc42-PLC-γ1 association in untransfected control cells (compare Figure 8A with C). In addition to GTP-Cdc42, GST-PBD precipitates also the GTP-Rac. Accordingly, we examined whether GTP-Rac is involved in the active GTPase(s)-PLC-γ1 complex observed in Figure 8A. For this, we performed immunoprecipitation experiments in cells that had been transfected with constitutively active myc-tagged RacV12. As shown in Figure 8D, no interaction was observed between RacV12 and PLC-γ1, indicating that the effect on PLC-γ1 activation is specific for Cdc42. This result is in line with that observed in Figure 7D, whereby inactive Rac1(T17N) did not affect the activity of PLC-γ1.
Figure 8.
PLC-γ1 associates in vivo with Cdc42. (A) Equal volumes of cell lysates from untreated or TNF-α-treated cells were affinity precipitated (AP) with GTP-PBD bound to glutathione-agarose beads. Coprecipitated PLC-γ1 was detected by immunoblot (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). Equal volume of total lysates from untreated and TNF-α-treated cells were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with anti-PLC-γ1 antibody (bottom). The number below each lane indicates the normalized fold amount of PLC-γ1 precipitated with GST-PBD, with that of untreated cells taken as 1. (B) Cells were transfected with the inactive Cdc42(T17N) and then stimulated with TNF-α for the indicated times. Myc-tagged Cdc42(T17N) was immunoprecipitated (IP) with mouse monoclonal anti-myc epitope antibody, and Western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Cdc42(T17N) in the immunoprecipitates (bottom). (C) Cells were transfected with the constitutively active Cdc42V12 and then stimulated with TNF-α for the indicated times. Myc-tagged Cdc42V12 was IP with mouse monoclonal anti-myc epitope antibody and western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Cdc42V12 in the immunoprecipitates (bottom). The number below each lane indicates the fold amount of PLC-γ1 coimmunoprecipitated with Cdc42V12, with that of untreated cells taken as 1. (D) Cells were transfected with the constitutively active Rac1V12 and then stimulated with TNF-α for the indicated times. Myc-tagged Rac1V12 was IP with mouse monoclonal anti-myc epitope antibody and Western blot of immunoprecipitates was probed (IB) with anti-PLC-γ1 antibody. Total lysates were used as Std to assess the mobility of precipitated PLC-γ1 (top). The blot was stripped and reprobed with anti-myc antibody to confirm the presence of Rac1V12 in the immunoprecipitates (bottom). The experiments were repeated four times with similar results.
Actin Cytoskeleton Alterations Induced by TNF-α Depend on Cdc42 and PLC-γ1 Activation
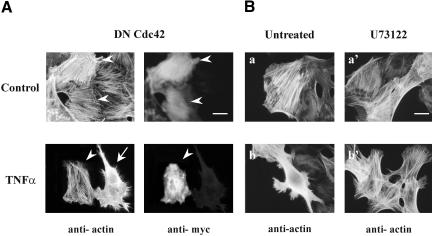
To determine the involvement of Cdc42 and/or PLC-γ1 in actin redistribution induced by TNF-α, cells were transfected with inactive Cdc42(T17N) or preincubated with the PLC-γ1 inhibitor U73122 and TNF-α was added for 15 min. To confirm the inhibitory action of U73122, PLC-γ1 activity was measured in TNF-α-treated cells in the presence or in the absence of U73122. We found that the phospholipase activity was significantly increased in cells exposed to TNF-α for 15 min compared with untreated cells (2490 ± 28 compared with 3011 ± 23 3H cpm released, n = 4, p < 0.05), whereas when cells were preincubated with U73122 and then exposed to TNF-α this activity remained at basal levels (2530 ± 32, 3H cpm released, n = 4). As shown in Figure 9A, in cells expressing the Cdc42(T17N) the formation of filopodia as well as the dissolution of stress fibers induced by TNF-α were completely blocked (arrowhead). On the contrary, these cytoskeletal changes were observed in untransfected cells (arrow). On the other hand, when cells were preincubated with U73122 the formation of filopodia still occurred in TNF-α-treated cells but the dissolution of stress fibers was suppressed (compare Figure 9B, b′ with B, b). These observations indicate that Cdc42 is responsible for filopodia formation and PLC-γ1 for stress fibers depolymerization. Moreover, these data suggest that Cdc42 acts upstream of PLC-γ1 and influences the activation of the latter.
Figure 9.
The TNF-α-induced actin remodeling occurs through a Cdc42- and PLC-γ1-dependent mechanism. Cells were transfected with Myc-tagged dominant negative Cdc42(T17N) (A) or preincubated with U73122 (B) and then incubated with TNF-α for 15 min. Transfected cells were identified by double immunofluorescence as described in MATERIALS AND METHODS. Cells expressing Myc-tagged Cdc42(T17N) are indicated by an arrowhead; arrows show untransfected cells. Bar, 30 μm. Similar results were obtained in three independent experiments.
NF-κB Translocation to the Nucleus and Caspase-3 Activity Depend on Actin Reorganization
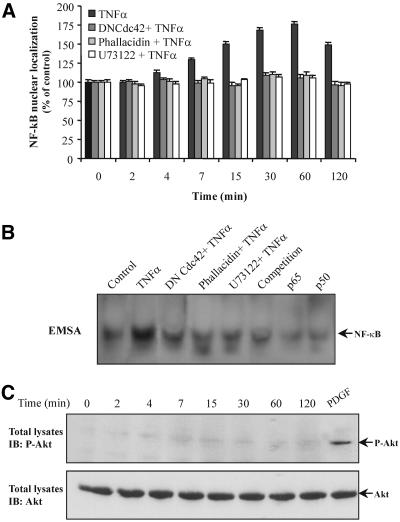
To examine the involvement of the signaling molecules described above in the activation of NF-κB in TNF-α-treated cells, we determined the NF-κB translocation to the nucleus as well as its binding activity. Using the anti-NF-κB (p65) antibody, immunoblotting of nuclear extracts from untreated or TNF-α-treated cells revealed a time-dependent translocation of NF-κB that was induced to a maximum within 30-60 min of TNF-α treatment (Figure 10A). Interestingly, no significant nuclear NF-κB translocation was seen when cells were transfected with the inactive Cdc42(T17N) or preincubated with U73122 or phallacidin. In agreement with these results, the NF-κB binding activity, as it was determined by EMSA, was induced after 30 min of TNF-α treatment, whereas it remained unchanged in Cdc42(T17N)transfected cells as well as in cells that had been preincubated with U73122 or phallacidin (Figure 10B). According to these data, the stabilization of actin affected the antiapoptotic process so far as it was occurred by the inhibition of the upstream signaling molecules. This result indicates that the translocation of NF-κB to the nucleus and its subsequent activation depend ultimately on the polymerization state of actin.
Figure 10.
NF-κB nuclear translocation is dependent on actin polymerization state. (A) Nuclear extracts of cells incubated with TNF-α or cells that had been transfected with Cdc42(T17N) or that had been preincubated with phallacidin or U73122 and then exposed to TNF-α, for the indicated times, were prepared as described in MATERIALS AND METHODS. The nuclear levels of NF-κB (p65) protein were immunodetected using a specific anti-NF-κB (p65) antibody, and the band's intensity was quantified using a PC-based image analysis. Data are presented as percentage of NF-κB (p65) protein levels in control cells (mean + SEM from three separate experiments). (B) Cells were incubated with TNF-α for 30 min. Nuclear extracts were subjected to EMSA as described in MATERIALS AND METHODS. Specificity of the bands was determined using anti-p65 (p65) or anti-p50 (p50) antibodies or by competition with 25× excess of unlabeled probe. (C) Cells were treated with TNF-α, for the indicated times, or with platelet-derived growth factor (PDGF) (40 ng/ml, 15 min) that was used as positive control and then equal amount of proteins (50 μg) were subjected to SDS-PAGE, transferred to nitrocellulose membrane, and immunoblotted (IB) with an anti-phospho-Akt (top) or anti-Akt (bottom) antibody.
It is well known that Akt could be activated downstream of PI-3 kinase, and its phosphorylation is also involved in NF-κB activation. As shown in Figure 10C, however, stimulation of cells with TNF-α did not result in phosphorylation of Akt, because it was observed by using a specific anti-phospho Akt antibody, suggesting that NF-κB activation is not dependent on Akt in our system.
Finally, to determine whether the molecules, which were found to transduce the signal to the actin cytoskeleton, are involved in the inhibition of caspase-3 activity we tested specific inhibitors or dominant-negative mutants. Transfection of cells with inactive Cdc42(T17N) abrogated the antiapoptotic effects of TNF-α (our unpublished data). Similar results were obtained when cells were preincubated with the PLC-γ1 inhibitor U73122 (our unpublished data). In this case, we observed as well that the blockage of the signaling cascade leading to actin reorganization or the blockage of the actin depolymerization alone, using phallacidin (Figure 2F), prevented the antiapoptotic effects of TNF-α.
DISCUSSION
The role of inflammatory cytokines in the pathogenesis of renal diseases is well established (Baud and Ardaillou 1995; Lieberthal et al., 1998). Indeed, cytokines induce a variety of responses on renal cells, including toxic effects, and increased proliferation and release of inflammatory mediators. Moreover, proinflammatory cytokines are up-regulated during the course of progressive renal disease. It is also well known that the renal proximal tubular epithelial cells are targeted in a variety of immune and inflammatory diseases of the kidney as well as that TNF-α is produced by proximal tubule cells and acts on these cells (Jevnikar et al., 1991; Ortiz et al., 1994; Klahr, 1999; Luster et al., 1999; Meldrum and Donnahoo, 1999). However, the signaling mechanism by which TNF-α exerts its effects in proximal tubule cells remains unclear to date.
Cdc42 Is Essential for PLC-γ1 Activation in TNF-α Signaling That Leads to Actin Redistribution in OK Cells
In the present study, we have used the OK cell line to examine whether TNF-α elicits apoptotic or antiapoptotic effects on these cells and the possible involvement of actin cytoskeleton in the TNF-α-induced apoptosis or cell survival. We found that TNF-α promotes cell survival of OK cells by inhibiting the activity of caspase-3, and, indeed, this effect depends on actin redistribution. Consequently, we investigated the signaling mechanism controlling the reorganization of actin and the cell response to TNF-α. At the early stages after stimulation, TNF-α caused a significant depolymerization of stress fibers and the formation of extended filopodia, which were accompanied by lamellipodia and membrane ruffles. Gradually, the peripheral membrane structures disappeared, whereas stress fibers reformed. As expected by the morphological observations, we found that Cdc42 and Rac1 were activated early in response to TNF-α. It is likely that TNF-α induced the activation of a GTPase signaling cascade in OK cells similar to that observed in other cell systems according to which activation of Cdc42 leads to activation of Rac and subsequently to activation of Rho (Nobes and Hall, 1995; Wojciak-Stothard et al., 1998; Puls et al., 1999). However, TNF-α induced a significant dissolution of stress fibers in our cell system, in addition to peripheral membrane changes, indicating the involvement of signaling molecule(s) capable of leading to such actin redistribution. The best characterized downstream effector of Cdc42/Rac PAK1 was not activated in response to TNF-α. Accordingly, we examined the possible PLC-γ1 activation that could modulate the organization of actin cytoskeleton. PLC has three isoforms: β, γ, and δ. The activation mechanism of PLC-δ is unclear. PLC-β isoenzymes are stimulated by agonists whose receptors are coupled to heterotrimeric G proteins. Among the PLC-γ isoenzymes, the PLC-γ1 is expressed ubiquitously, whereas PLC-γ2 is expressed mainly in cells of hematopoietic origin (Rhee and Bae, 1997; Sekiya et al., 1999). Therefore, we focused on PLC-γ1 because it is the only PLC isoform that could be activated in our system. Indeed, we found that PLC-γ1 is time dependently activated in cells exposed to TNF-α. Interestingly, both C. difficile toxin B and the inactive mutant Cdc42(T17N) abolished the phospholipase activity implying that PLC-γ1 is activated downstream of the small GTPase Cdc42. It is noteworthy, that the inactive Rac1(T17N) did not influence the activity of PLC-γ1, indicating that only Cdc42 is essential for the phospholipase activation. Under the experimental conditions used, we were not able to demonstrate any phosphorylation of PLC-γ1. It has been shown previously that the product of the reaction catalyzed by PI-3 kinase, PI(3,4,5)P3, can induce PLC-γ1 translocation to the cell membrane, via interaction with its PH or SH2 domains, leading to phospholipase activation in the absence of its tyrosine phosphorylation (Bae et al., 1998; Falasca et al., 1998). Our experiments revealed that TNF-α induced a significant increase of PI-3 kinase activity, generating thus high levels of PI(3,4,5)P3, which was followed by activation of the small GTPases. Because Cdc42(T17N) prevented PLC-γ1 activation, it is likely that the PI(3,4,5)P3 was not sufficient to activate PLC-γ1. However, the activated Cdc42 alone was not sufficient to activate the phospholipase because wortmannin abolished the activation of the latter in cells that had been transfected with CA Cdc42V12 and then exposed to TNF-α. Together, these data indicate that GTP-Cdc42 is essential; however, both GTP-Cdc42 and PI(3,4,5)P3 are needed for efficient PLC-γ1 activation.
In Vivo Association of Cdc42 with PLC-γ1 in TNF-α Signaling
This study has also demonstrated an association of activated Cdc42 with PLC-γ1 in vivo, which could account for the essential role of the small GTPase to PLC-γ1 activation. The fact that GTP-Cdc42, but not GTP-Rac1, interacted with the phospholipase is consistent with the observed Rac1-indepentent activation of PLC-γ1 and further supports the notion that the Cdc42-PLC-γ1 association might be critical for the activation of the latter. It is of note that CA Cdc42V12 did not bind to the same extent to PLC-γ1 from unstimulated and stimulated cells. In contrast, TNF-α time dependently induced the Cdc42V12-PLC-γ1 interaction, indicating that factor(s) triggered by TNF-α enhance this association. It is reasonable to assume that such a factor could be the PI(3,4,5)P3, which is produced by the reaction catalyzed by PI-3 kinase. In particular, PI(3,4,5)P3 can induce PLC-γ1 translocation to the plasma membrane (Bae et al., 1998; Falasca et al., 1998), thus mediating the effective binding of Cdc42 with PLC-γ1.
Cdc42 and Rac have been previously characterized as stimulators of PLC-β2 in a study describing the in vitro activation of PLC-β2 by the complex Cdc42HsxLyGDI or by the direct association of Cdc42/Rac and PLC-β2 in a system of purified recombinant proteins (Illenberger et al., 1998). Another study demonstrates that Cdc42 and Rac activate the IP3/calcium pathway upon antigen stimulation in RBL-2H3 mast cells (Hong-Geller and Cerione, 2000). This study presents the in vitro association of recombinant GST-Cdc42 with PLC-γ1 in RBL-2H3 cells as well as the preferential association of Cdc42 with a form of PLC-γ1 that migrates slightly faster than the majority of the PLC-γ1 protein purified from insect cells, indicating that a posttranslational modification of PLC-γ1 might be necessary for efficient binding to Cdc42. Cdc42 did not stimulate PLC-γ1 activity in the system used in the above-mentioned study, consisting of the purified recombinant proteins, due to lack of a fully modified population of PLC-γ1. Moreover, an in vivo association between the two proteins was not observed, and it was attributed to the formation of an unstable complex between unmodified PLC-γ1 and Cdc42, which could not be detected by coimmunoprecipitation methods (Hong-Geller and Cerione, 2000). In our study, we observed that activated Cdc42 both associates with PLC-γ1 in vivo and is essential for the activation of the latter upon TNF-α stimulation. However, the differences regarding PLC-γ1 activation and its association with Cdc42 observed between OK and RBL-3H3 cells may very well reflect differentially modified PLC-γ1 proteins that could be explained by the different organ or species origin of the cells studied.
Role of Cdc42, PLC-γ1 and Actin in the Nuclear Translocation of NF-κB and the Subsequent Inhibition of Caspase-3
It is well established that the transcription factor NF-κB is a key regulator of inflammatory responses (Ghosh et al., 1998) and that it is activated by a mechanism involving phosphorylation of IκBα or IκBβ by the IκB kinases, leading to translocation of NF-κB to the nucleus where it binds to specific DNA sequences present in the promoter of target genes (Barnes and Karin, 1997; Takeda et al., 1999). The inhibitors of apoptosis proteins (IAPs) belong to the gene family that protect cells to undergo apoptosis by inhibiting procaspase-9 or the activated caspase-3 and -7 (Deveraux et al., 1997, 1998; Roy et al., 1997). It has been shown that members of IAP family are included in the NF-κB-regulated genes, which are induced upon TNF-α treatment, thus preventing cells from undergoing apoptosis during inflammation (Chu et al., 1997; Stehlik et al., 1998a,1998b; Wang et al., 1998). The TNF-α-induced signaling cascade described in this study was found to result in nuclear translocation of NF-κB with subsequent increase of its binding activity and in inhibition of caspase-3. Because the antiapoptotic Bcl-2 protein, which inhibits caspase-3, remained unchanged after exposure of cells to TNF-α, it could be assumed that the inhibition of caspase-3 observed is a consequence of up-regulated IAPs followed by the NF-κB activation. Regarding the upstream signaling events, we found that either inactive Cdc42(T17N), the PLC-γ1 inhibitor U73122, or the actin-stabilizing agent phallacidin equally blocked NF-κB activity and retained caspase-3 at basal levels. A number of studies have implicated the small GTPases or Akt in the induction of NF-κB activity through IKK activation (Perona et al., 1997; Montaner et al., 1998; Ozes et al., 1999; Romashkova and Makarov, 1999; Deshpande et al., 2000). In our study, we were not able to detect a marked phosphorylation of Akt after TNF-α stimulation, indicating that Akt is not involved in NF-κB activation in OK cells. This is in agreement with studies performed with other cell systems (Madge and Pober, 2000; Rauch et al., 2000). In contrast, we found that upon TNF-α treatment the small GTPases were activated and, moreover, the inactive Cdc42(T17N) abrogated NF-κB nuclear translocation and activation as well as the inhibition of caspase-3, suggesting that Cdc42 could account for cellular response to TNF-α. However, the PLC-γ1 inhibitor U73122 prevented also the effects of TNF-α on NF-κB and caspase-3 activity. PLC-γ1 hydrolyzes PI(4,5)P2 to form the two second messengers, IP3, which causes the release of Ca2+ from intracellular stores; and diacylglycerol, which activates the protein kinase C. It has been shown recently that inhibition of PLC and PKCδ blocks the activation of NF-κB in TNF-α-stimulating human neutrophils by inhibiting degradation of IκBa (Vancurova et al., 2001) as well as that PLC-γ2 and PKCα are involved in TNF-α-induced activation of NF-κB-inducing kinase and IKK1/2 in human lung epithelial cells (Chen et al., 2000). In addition, elevated intracellular Ca2+ levels can induce IKK, leading to increased activity of NF-κB (Todisco et al., 1999; Petranka et al., 2001). Thus, it is possible that either or both of the second messengers generated by PLC-γ1 could mediate the activation of NF-κB observed in our system.
The fact that inhibition of Cdc42 or PLC-γ1 prevented NF-κB translocation and activity could mean that Cdc42 affects NF-κB through PLC-γ1 or that each of the two signal transduction molecules mediates separately the effects of TNF-α on NF-κB. However, the ability of these proteins to induce the activity of NF-κB and thus the antiapoptotic process does not occur independently of the signaling to the actin cytoskeleton. Indeed, we found that activation of Cdc42 and PLC-γ1 leads to redistribution and depolymerization of actin filaments. Moreover, we demonstrate that the actin-stabilizing agent phallacidin completely blocked the nuclear translocation of NF-κB and the subsequent inhibition of caspase-3. Therefore, it is likely that a critical step for the activation of NF-κB and the subsequent inhibition of caspase-3 is the depolymerization of actin because even though the upstream NF-κB-related events could occur, as a result of Cdc42 and/or PLC-γ1 activity, the stabilization of actin is sufficient to abrogate the TNF-α-induced cell response. Because it has been shown that the p65 subunit of NF-κB interacts with actin structures, including focal contacts and stress fibers (Are et al., 2000), it seems reasonable that depolymerization of stress fibers and the redistribution of actin could facilitate the translocation of NF-κB to the nucleus. In the present study, we found that actin depolymerization occurred in short time periods and returned to control levels 2 h after TNF-α treatment, whereas a significant decrease in caspase-3 activity became evident 1 h after TNF-α treatment and remained at low levels during the next 6 h after the addition of TNF-α. As expected, the apoptotic cells had been decreased at 4 h and this effect became significantly stronger at 6 h after the addition of TNF-α. These data are compatible with the involvement of actin depolymerization in nuclear translocation of NF-κB because its nuclear localization was progressively increased early after TNF-α treatment, which is in line with the time course of the actin redistribution. The maximum nuclear localization of NF-κB at 30-60 min after the addition of TNF-α could be explained by the time that is essential for its gradual translocation. However, the nuclear localization of NF-κB was started to decrease 2 h after the addition of TNF-α, and it was expected, because in the same time period, actin had been already repolymerized. With respect to the decrease of apoptotic cells at 4 h and mainly at 6 h, it is compatible with the events that are necessary for the expression of cell response. These events may include the induction of the NF-κB target genes; the activation of IAPs, resulting in the inhibition of caspase-3; and finally the inhibition of apoptosis. The results presented here are in line with previously reported data. In particular, it has been shown that jasplakinilide, a molecule that stabilizes polymerized actin structures, increases apoptosis induced by cytokine deprivation (Posey and Bierer, 1999). Conversely, latrunculin, an inhibitor of actin polymerization reduced the ability of constitutively active GTPase mutants to stimulate apoptosis and blocked Fas-induced activation of caspase-3 (Subauste et al., 2000).
CONCLUSIONS
The above-mentioned results imply that TNF-α induces antiapoptotic effects in OK cells by activating key signaling molecules in a hierarchy of PI-3 kinase→ Cdc42→ PLC-γ1→actin reorganization→ NF-κB activation→inhibition of caspase-3. In addition to the identification of the TNF-α-induced antiapoptotic signal transduction pathway, our results provides novel mechanistic insights. Indeed, we demonstrate that PLC-γ1, in the absence of its tyrosine phosphorylation, is regulated in vivo not only by PI(3,4,5)P3 but also by the small GTPase Cdc42, which possibly occurs through the association of the two proteins. Our experiments provide also evidence that the depolymerization of actin is essential for the nuclear translocation of NF-κB and the subsequent inhibition of caspase-3.
Acknowledgments
We are indebted to Dr. A. Zantema for providing the double mutant IkBa(S32A/S36A) and to Drs. A. Rao and F. Mercurio for the WT IKK-2 and IKK-2(S177E, S181E) expression plasmids. We are grateful to Dr. A. Moustakas for providing the expression plasmids pCMV-Cdc42(T17N), pCMV-Cdc42V12, pEXV-Rac1(T17N), and pEXV-Rac1V12 and to Dr. A. Gravanis for APOPercentage apoptosis assay. We thank Ioanna Zarifi, a postgraduate student, for the great assistance during rotation in our laboratory. We also thank Drs. A. Gravanis, A. Moustakas, and D. Boumpas for the critical reading of the manuscript. This work was partially supported by grants from Greek Secreteriat for Research and Technology and the Biomedical Research Council of the Greek Ministry of Health.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-07-0491. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-07-0491.
References
- Are, A.F., V. E., Galkin, T. V. Pospelova, and G. P. Pinaev. (2000). The p65/RelA subunit of NF-kappaB interacts with actin-containing structures. Exp. Cell Res. 256, 533-544. [DOI] [PubMed] [Google Scholar]
- Arkinstall, S., Payton, M., and Maundrell, K. (1995). Activation of phospholipase C gamma in Schizosaccharomyces pombe by coexpression of receptor or nonreceptor tyrosine kinases. Mol. Cell. Biol. 15, 1431-1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auger, K.R., Serunian, L.A., Soltoff, S.P., Libby, P., and Cantley, C. (1989). PDGF-dependent tyrosine phosphorylation stimulates production of novel polyphosphoinositides in intact cells. Cell 57, 167-175. [DOI] [PubMed] [Google Scholar]
- Bae, Y.S., Cantley, L.G., Chen, C.S., Kim, S.R., Kwon, K.S., and Rhe, S.G. (1998). Activation of phospholipase C-gamma by phosphatidylinositol 3, 4, 5-trisphosphate. J. Biol. Chem. 273, 4465-4469. [DOI] [PubMed] [Google Scholar]
- Bagrodia, S., Taylor, S.J., Creasy, C.L., Chernoff, J., and Cerione, R.A. (1995). Identification of a mouse p21Cdc42/Rac-activated kinase. J. Biol. Chem. 270, 22731-22737. [DOI] [PubMed] [Google Scholar]
- Barnes, P.J., and Karin, M. (1997). Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N. Engl. J. Med. 336, 1066-1071. [DOI] [PubMed] [Google Scholar]
- Baud, L., and Ardaillou, R. (1993). Involvement of reactive oxygen species in kidney damage. Br. Med. Bull. 49, 621-629. [DOI] [PubMed] [Google Scholar]
- Baud, L., and Ardaillou, R. (1995). Tumor necrosis factor in renal injury. Miner. Electrolyte Metab. 21, 336-341. [PubMed] [Google Scholar]
- Baud, V., and Karin, M. (2001). Signal transduction by tumor necrosis factor and its relatives. Trends Cell. Biol. 11, 372-377. [DOI] [PubMed] [Google Scholar]
- Benard, V., Bohl, B.P., and Bokoch, G.M. (1999). Characterization of Rac and Cdc42 activation in chemoattractant-stimulated human neutrophils using a novel assay for active GTPases. J. Biol. Chem. 274, 13198-13204. [DOI] [PubMed] [Google Scholar]
- Bergmann, M.W., Loser, P., Dietz, R., and von Harsdorf, R. (2001). Effect of NF-kappa B Inhibition on TNF-alpha-induced apoptosis and downstream pathways in cardiomyocytes. J. Mol. Cell Cardiol. 33, 1223-1232. [DOI] [PubMed] [Google Scholar]
- Bruijn, J.A., and de Heer, E. (1995). Adhesion molecules in renal diseases. Lab. Investig. 72, 387. [PubMed] [Google Scholar]
- Chang, H.Y., and Yang, X. (2000). Proteases for cell suicide: functions and regulation of caspases. Microbiol. Mol. Biol. Rev. 64, 821-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, C.C., Sun, Y.T., Chen, J.J., and Chiu, K.T. (2000). TNF-alpha-induced cyclooxygenase-2 expression in human lung epithelial cells: involvement of the phospholipase C-gamma 2, protein kinase C-alpha, tyrosine kinase, NF-kappa B-inducing kinase, and I-kappa B kinase 1/2 pathway. J. Immunol. 165, 2719-2728. [DOI] [PubMed] [Google Scholar]
- Chu, Z.L., McKinsey, T.A., Liu, L., Gentry, J.J., Malim, M.H., and Ballard, D.W. (1997). Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc. Natl. Acad. Sci. USA 94, 10057-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels, R.H., and Bokoch, G.M. (1999). p21-activated protein kinase: a crucial component of morphological signaling? Trends Biochem. Sci. 24, 350-355. [DOI] [PubMed] [Google Scholar]
- Deshpande, S.S., Angkeow, P., Huang, J., Ozaki, M., and Irani, K. (2000). Rac1 inhibits TNF-alpha-induced endothelial cell apoptosis: dual regulation by reactive oxygen species. FASEB J. 14, 1705-1714. [DOI] [PubMed] [Google Scholar]
- Deveraux, Q.L., Roy, N., Stennicke, H.R., Van Arsdale, T., Zhou, Q., Srinivasula, S.M., Alnemri, E.S., Salvesen, G.S., and Reed, J.C. (1998). IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 17, 2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deveraux, Q.L., Takahashi, R., Salvesen, G.S., and Reed, J.C. (1997). X-linked IAP is a direct inhibitor of cell-death proteases. Nature 388, 300-304. [DOI] [PubMed] [Google Scholar]
- Dignam, J.D., Lebovitz, R.M., and Roeder, R.G. (1983). Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 11, 1475-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnahoo, K.K., Shames, B.D., Harken, A.H., and Meldrum, D.R. (1999). Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J. Urol. 162, 196-203. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., Voelker, D.R., Campbell, P.A., Cohen, J.J., Bratton, D.L., and Henson, P.M. (1992). Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages J. Immunol. 148, 2207-2216. [PubMed] [Google Scholar]
- Falasca, M., Logan, S.K., Lehto, V.P., Baccante, G., Lemmon, M.A., and Schlessinger, J. (1998). Activation of phospholipase C gamma by PI 3-kinase-induced PH domain-mediated membrane targeting. EMBO J. 17, 414-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S., May, M.J., and Kopp, E.B. (1998). NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu. Rev. Immunol. 16, 225-260. [DOI] [PubMed] [Google Scholar]
- Healy, E., Leonard, M., Madrigal-Estebas, L., O'Farrelly, C., Watson, A.J., and Ryan, M.P. (1999). Factors produced by activated leukocytes alter renal epithelial cell differentiation. Kidney Int. 56, 1266-9. [DOI] [PubMed] [Google Scholar]
- Hong-Geller, E., and Cerione, R.A. (2000). Cdc42 and Rac stimulate exocytosis of secretory granules by activating the IP(3)/calcium pathway in RBL-2H3 mast cells. J. Cell Biol. 148, 481-494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idriss, H.T., and Naismith, J.H. (2000). TNF alpha and the TNF receptor superfamily: structure-function relationship(s). Microsc. Res. Tech. 50, 184-95. [DOI] [PubMed] [Google Scholar]
- Illenberger, D., Schwald, F., Pimmer, D., Binder, W., Maier, G., Dietrich, A., and Gierschik, P. (1998). Stimulation of phospholipase C-beta2 by the Rho GTPases Cdc42Hs and Rac1. EMBO J. 17, 6241-6249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jevnikar, A.M., Brennan, D.C., Singer, G.G., Heng, J.E., Maslinski, W., Wuthrich, R.P., Glimcher, L.H., and Kelley, V.E. (1991). Stimulated kidney tubular epithelial cells express membrane associated and secreted TNF alpha. Kidney Int. 40, 203-211. [DOI] [PubMed] [Google Scholar]
- Kim, B.C., Lee, M.N., Kim, J.Y., Lee, S.S., Chang, J.D., Kim, S.S., Lee, S.Y., and Kim, J.H. (1999). Roles of phosphatidylinositol 3-kinase and Rac in the nuclear signaling by tumor necrosis factor-alpha in rat-2 fibroblasts. J. Biol. Chem. 274, 24372-24377. [DOI] [PubMed] [Google Scholar]
- Klahr, S. (1999). Mechanisms of progression of chronic renal damage. J. Nephrol. 12, S53-S62. [PubMed] [Google Scholar]
- Knaus, U.G., Morris, S., Dong, H., Chernoff, J., and Bokoch, G.M. (1995). Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science (Wash. DC) 269, 221-223. [DOI] [PubMed] [Google Scholar]
- Koukouritaki, S.B., Vardaki, E.A., Papakonstanti, E.A., Lianos, E., Stournaras, C., and Emmanouel, D.S. (1999). TNF-a induces actin cytoskeleton reorganization in glomerular epithelial cells involving tyrosine phosphorylation of paxillin and focal adhesion kinase. Mol. Med. 5, 383-393. [PMC free article] [PubMed] [Google Scholar]
- Krasilnikov, M.A. (2000). Phosphatidylinositol-3 kinase dependent pathways: the role in control of cell growth, survival, and malignant transformation. Biochemistry 65, 59-67. [PubMed] [Google Scholar]
- Lieberthal, W., Koh, J.S., and Levine, J.S. (1998). Necrosis and apoptosis in acute renal failure. Semin. Nephrol. 18, 505-518. [PubMed] [Google Scholar]
- Luster, M.I., Simeonova, P.P., Gallucci, R., and Matheson, J. (1999). Tumor necrosis factor alpha and toxicology. Crit. Rev. Toxicol. 29, 491-511. [DOI] [PubMed] [Google Scholar]
- Madge, L.A., and Pober, J.S. (2000). A phosphatidylinositol 3-kinase/Akt pathway, activated by tumor necrosis factor or interleukin-1, inhibits apoptosis but does not activate NFkappaB in human endothelial cells. J. Biol. Chem. 275, 15458-15465. [DOI] [PubMed] [Google Scholar]
- Manser, E., Chong, C., Zhoa, Z., Leung, T., Michael, G., Hall, C., and Lim, L. (1995). Molecular cloning of a new member of the p21Cdc42/Rac-activated kinase (PAK) family. J. Biol. Chem. 270, 25070-25078. [DOI] [PubMed] [Google Scholar]
- Martin, S. J., Reutelingsperger, C.P., McGahon, A.J., Rader, J.A., van Schie, R.C., LaFace, D.M., and Green, D.R. (1995). Early redistribution of plasma membrane phosphatidylserine is a general feature of apoptosis regardless of the initiating stimulus: inhibition by overexpression of Bcl-2 and Abl. J. Exp. Med. 182, 1545-1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum, D.R., and Donnahoo, K.K. (1999). Role of TNF in mediating renal insufficiency following cardiac surgery: evidence of a postbypass cardiorenal syndrome. J. Surg. Res. 85, 185-199. [DOI] [PubMed] [Google Scholar]
- Mercurio, F., Zhu, H., Murray, B.W., Shevchenko, A., Bennett, B.L., Li, J., Young, D.B., Barbosa, M., Mann, M., Manning, A., and Rao, A. (1997). IKK-1 and IKK-2, cytokine-activated IkappaB kinases essential for NF-kappaB activation. Science 278, 860-866. [DOI] [PubMed] [Google Scholar]
- Montaner, S., Perona, R., Saniger, L., and Lacal, J.C. (1998). Multiple signalling pathways lead to the activation of the nuclear factor kappaB by the Rho family of GTPases. J. Biol. Chem. 273, 12779-12785. [DOI] [PubMed] [Google Scholar]
- Natoli, G., Costanzo, A., Guido, F., Moretti, F., and Levrero, M. (1998). Apoptotic, non-apoptotic, and anti-apoptotic pathways of tumor necrosis factor signalling. Biochem. Pharmacol. 56, 915-920. [DOI] [PubMed] [Google Scholar]
- Nobes, C.D., and Hall, A. (1995). Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell 81, 53-62. [DOI] [PubMed] [Google Scholar]
- Nojiri, S., and Hoek, J.B. (2000). Suppression of epidermal growth factor-induced phospholipase C activation associated with actin rearrangement in rat hepatocytes in primary culture. Hepatology 32, 947-957. [DOI] [PubMed] [Google Scholar]
- Ortiz, A., et al. (1994). The potential role of inflammatory and fibrogenic cytokines in the glomerular diseases. J. Lipid Mediat. Cell Signal. 9, 55-74. [PubMed] [Google Scholar]
- Ozes, O.N., Mayo, L.D., Gustin, J.A., Pfeffer, S.R., Pfeffer, L.M., and Donner, D.B. (1999). NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401, 82-85. [DOI] [PubMed] [Google Scholar]
- Papakonstanti, E.A., Kampa, M., Castanas, E., and Stournaras, C. (2003). A rapid, non-genomic, signaling pathway regulates the actin reorganization induced by membrane testosterone receptors' activation. Mol. Endocrinol. 17, 870-881. [DOI] [PubMed] [Google Scholar]
- Papakonstanti, E.A., and Stournaras, C. (2002). Association of PI-3 kinase with PAK1 leads to actin phosphorylation and cytoskeletal reorganization. Mol. Biol. Cell 13, 2946-2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti, E.A., Emmanouel, D.S., Gravanis, A., and Stournaras, C. (2000). PLC-γ 1 signaling pathway and villin activation are involved in actin cytoskeleton reorganization induced by Na+/Pi cotransport up-regulation. Mol. Med. 6, 303-318. [PMC free article] [PubMed] [Google Scholar]
- Papakonstanti, E.A., Emmanouel, A. Gravanis, and C. Stournaras. (1996). Na+/Pi co-transport alters rapidly cytoskeletal protein polymerization dynamics in opossum kidney cells. Biochem. J. 315, 241-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino, J.G., Tafan, M., and Farber, J.L. (1999). Tumor necrosis factor induces phosphorylation and translocation of BAD through a phosphatidylinositide-3-OH kinase-dependent pathway. J. Biol. Chem. 274, 19411-19416. [DOI] [PubMed] [Google Scholar]
- Perona, R., Montaner, S., Saniger, L., Sanchez-Perez, I., Bravo, R., and Lacal, J.C. (1997). Activation of the nuclear factor-kappaB by Rho, CDC42, and Rac-1 proteins. Genes Dev. 11, 463-475. [DOI] [PubMed] [Google Scholar]
- Petranka, J., Wright, G., Forbes, R.A., and Murphy, E. (2001). Elevated calcium in preneoplastic cells activates NF-kappa B and confers resistance to apoptosis. J. Biol. Chem. 276, 37102-37108. [DOI] [PubMed] [Google Scholar]
- Posey, S.C., and Bierer, B.E. (1999). Actin stabilization by jasplakinolide enhances apoptosis induced by cytokine deprivation. J. Biol. Chem. 274, 4259-4265. [DOI] [PubMed] [Google Scholar]
- Puls, A., Eliopoulos, A.G., Nobes, C.D., Bridges, T., Young, L.S., and Hall, A. (1999). Activation of the small GTPase Cdc42 by the inflammatory cytokines TNF(alpha) and IL-1, and by the Epstein-Barr virus transforming protein LMP1. J. Cell Sci. 112, 2983-2992. [DOI] [PubMed] [Google Scholar]
- Rabb, H.A. (1994). Cell adhesion molecules and the kidney. Am. J. Kidney Dis. 23, 155. [DOI] [PubMed] [Google Scholar]
- Rauch, B.H., Weber, A., Braun, M., Zimmermann, N., and Schror, K. (2000). PDGF-induced Akt phosphorylation does not activate NF-kappa B in human vascular smooth muscle cells and fibroblasts. FEBS Lett. 481, 3-7. [DOI] [PubMed] [Google Scholar]
- Rhee, S.G., and Bae, Y.S. (1997). Regulation of phosphoinositide-specific phospholipase C isozymes. J. Biol. Chem. 272, 15045-15048. [DOI] [PubMed] [Google Scholar]
- Ridley, A.J., and Hall, A. (1992). The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell 70, 389-399. [DOI] [PubMed] [Google Scholar]
- Romashkova, J.A., and Makarov, S.S. (1999). NF-kappaB is a target of AKT in anti-apoptotic PDGF signalling. Nature 401, 86-90. [DOI] [PubMed] [Google Scholar]
- Roy, N., Deveraux, Q.L., Takahashi, R., Salvesen, G.S., and Reed, J.C. (1997). The c-IAP-1 and c-IAP-2 proteins are direct inhibitors of specific caspases. EMBO J. 16, 6914-6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya, F., Bae, Y.S., and Rhee, S.G. (1999). Regulation of phospholipase C isozymes: activation of phospholipase C-gamma in the absence of tyrosine-phosphorylation. Chem. Phys. Lipids 98, 3-11. [DOI] [PubMed] [Google Scholar]
- Stehlik, C., de Martin, R., Binder, B.R., and Lipp, J. (1998b). Cytokine induced expression of porcine inhibitor of apoptosis protein (IAP) family member is regulated by NF-kappa B. Biochem. Biophys. Res. Commun. 243, 827-832. [DOI] [PubMed] [Google Scholar]
- Stehlik, C., de Martin, R., Kumabashiri, I., Schmid, J.A., Binder, B.R., and Lipp, J. (1998a). Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J. Exp. Med. 188, 211-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subauste, M.C., Von Herrath, M., Benard, V., Chamberlain, C.E., Chuang, T.H., Chu, K., Bokoch, G.M., and Hahn, K.M. (2000). Rho family proteins modulate rapid apoptosis induced by cytotoxic T lymphocytes and Fas. J. Biol. Chem. 275, 9725-9733. [DOI] [PubMed] [Google Scholar]
- Takeda, K., Takeuchi, O., Tsujimura, T., Itami, S., Adachi, O., Kawai, T., Sanjo, H., Yoshikawa, K., Terada, N., and Akira, S. (1999). Limb and skin abnormalities in mice lacking IKKalpha. Science 284, 313-316. [DOI] [PubMed] [Google Scholar]
- Todisco, A., Ramamoorthy, S., Pausawasdi, N., and Tacey, K. (1999). Carbachol activates IkappaB kinase in isolated canine gastric parietal cells. Biochem. Biophys. Res. Commun. 261, 877-884. [DOI] [PubMed] [Google Scholar]
- Tolias, K.F., Cantley, L.C., and Carpenter, C.L. (1995). Rho family GTPases bind to phosphoinositide kinases. J. Biol. Chem. 270, 17656-17659. [DOI] [PubMed] [Google Scholar]
- Vancurova, I., Miskolci, V., and Davidson, D. (2001). NF-kappa B activation in tumor necrosis factor alpha-stimulated neutrophils is mediated by protein kinase Cdelta. Correlation to nuclear Ikappa Balpha. J. Biol. Chem. 276, 19746-19752. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Leevers, S.J., Ahmadi, K., Timms, J., Katso, R., Driscoll, P.C., Woscholski, R., Parker, P.J., and Waterfield, M.D. (2001). Synthesis and function of 3-phosphorylated inositol lipids. Annu. Rev. Biochem. 70, 535-602. [DOI] [PubMed] [Google Scholar]
- Wang, C.Y., Mayo, M.W., Korneluk, R.G., Goeddel, D.V., and Baldwin, A.S., Jr. (1998). NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281, 1680-1683. [DOI] [PubMed] [Google Scholar]
- Whiteside, S.T., Ernst, M.K., LeBail, O., Laurent-Winter, C., Rice, N., and Israel, A. (1995). N- and C-Terminal sequences control degradation of MAD3/I kappa B alpha in response to inducers of NF-kappa B activity. Mol. Cell. Biol. 15, 5339-5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojciak-Stothard, B., Entwistle, A., Garg, R., and Ridley, A.J. (1998). Regulation of TNF-alpha-induced reorganization of the actin cytoskeleton and cell-cell junctions by Rho, Rac, and Cdc42 in human endothelial cells. J. Cell. Physiol. 176, 150-165. [DOI] [PubMed] [Google Scholar]
- Yu, H., Fukami, K., Itoh, T., and Takenawa, T. (1998). Phosphorylation of phospholipase Cgamma1 on tyrosine residue 783 by platelet-derived growth factor regulates reorganization of the cytoskeleton. Exp. Cell Res. 243, 113-122. [DOI] [PubMed] [Google Scholar]