Abstract
Background
Captive elephants infected with tuberculosis are implicated as an occupational source of zoonotic tuberculosis. However, accurate estimates of prevalence and incidence of elephant tuberculosis from well-defined captive populations are lacking in the literature. Studies published in recent years contain a wide range of prevalence estimates calculated from summary data. Incidence estimates of elephant tuberculosis in captive elephants are not available.
Objective
This study estimated the annual point prevalence, annual incidence, cumulative incidence, and incidence density of tuberculosis in captive elephants within the USA during the past 52 years.
Animals and Methods
We combined existing elephant census records from captive elephants in the USA with tuberculosis culture results obtained from trunk washes or at necropsy. This data set included 15 years where each elephant was screened annually.
Results
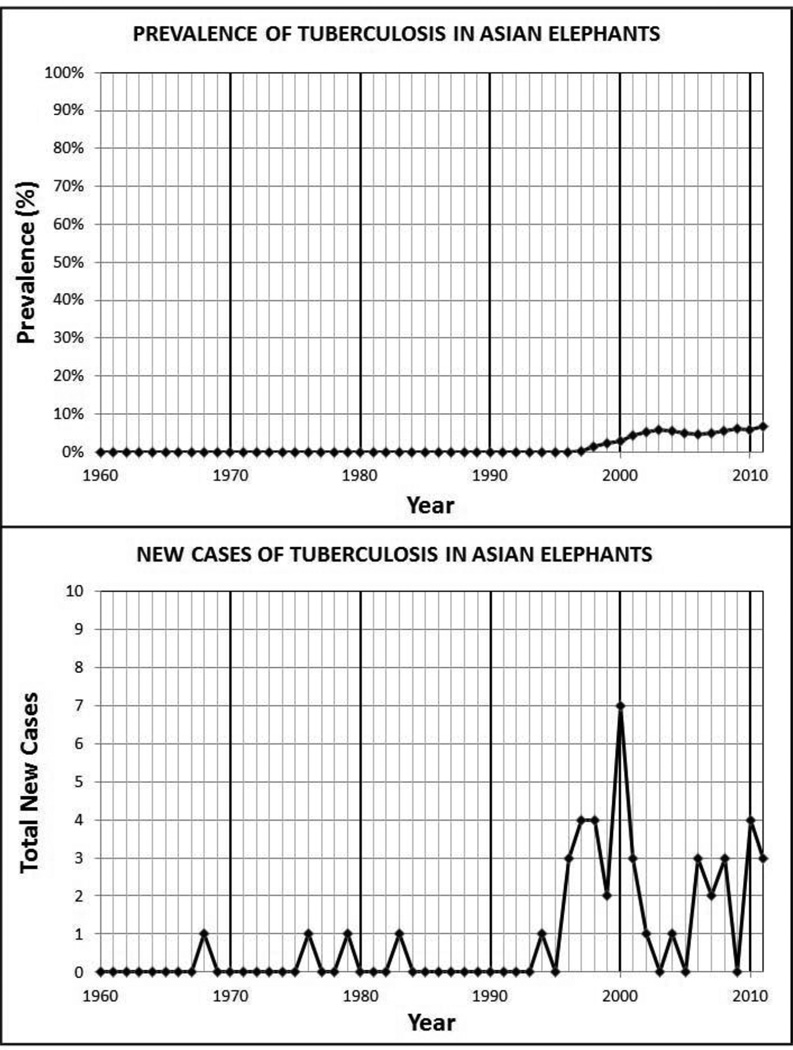
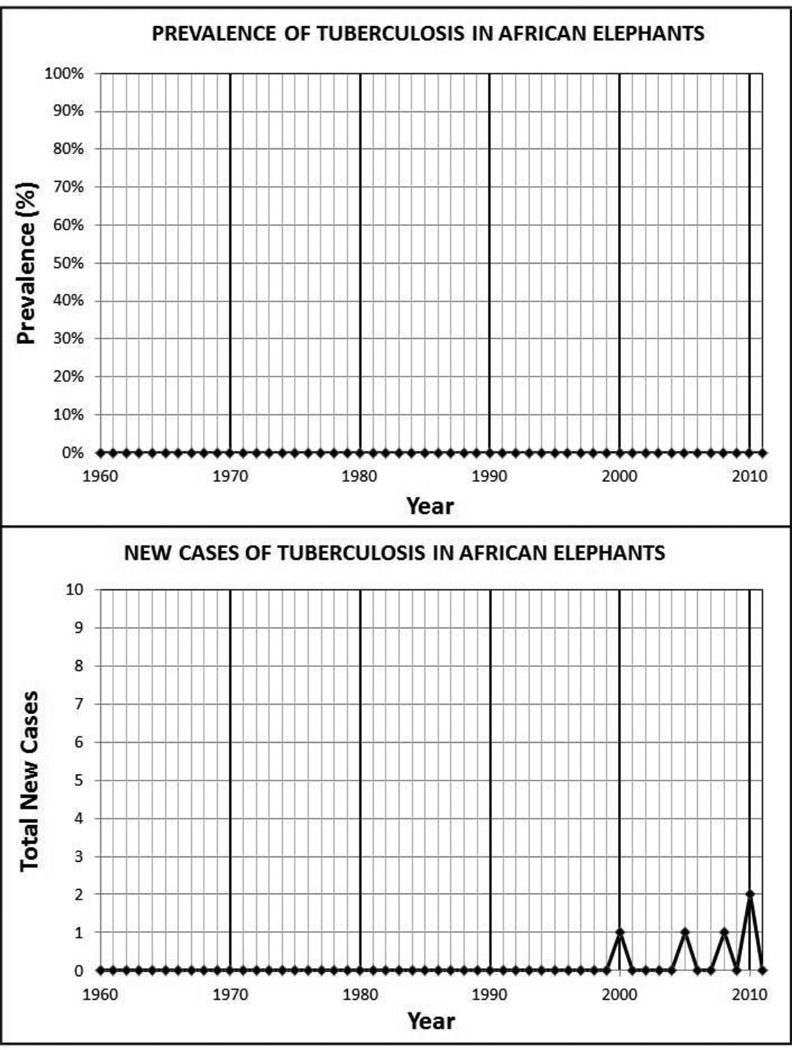
Between 1960 and 1996, the annual point prevalence of tuberculosis complex mycobacteria for both species was 0. From 1997 through 2011, the median point prevalence within the Asian elephant population was 5.1%, with a range from 0.3% to 6.7%. The incidence density was 9.7 cases/1000 elephant years (95% CI: 7.0–13.4). In contrast, the annual point prevalence during the same time period within the African elephant population remained 0 and the incidence density was 1.5 cases/1000 elephant years (95% CI: 0.7–4.0).
Conclusions
The apparent increase in new cases noted after 1996 resulted from a combination of both index cases and the initiation of mandatory annual tuberculosis complex (MTBC) screening in 1997 for all the elephants. This study found lower annual point prevalence estimates than previously reported in the literature. These discrepancies in prevalence estimates are primarily due to differences in terminology and calculation methods. Using the same intensive testing regime, the incidence of tuberculosis differed significantly between Asian and African elephants.
Clinical Importance
Accurate and species specific knowledge of prevalence and incidence will inform our efforts to mitigate occupational risks associated with captive elephants in the USA.
Keywords: Mycobacterium tuberculosis, Elephas maximus, Loxodonta Africana, prevalence, incidence
1. Introduction
Throughout the previous century, occasional case reports of Mycobacterium tuberculosis from North American captive elephants were reported in the veterinary literature (Mikota and Maslow 2011). It was not until 1996, however, when two elephants were found to be infected, that elephant tuberculosis became an apparent emerging disease with possible zoonotic implications (Mikota et al. 2001). Further attention was drawn to the occupational risks after three studies reported that 18–50% of elephant workers employed in the U.S.A. were PPD skin test reactors (Michalak et al. 1998; Oh et al. 2002; Murphree et al. 2011). To date, Michalak (1998) reported the only case of zoonotic transmission of tuberculosis between elephants and humans.
Starting in 1997, the United States Department of Agriculture required that all elephants be annually tested for tuberculosis complex (MTBC) with a regime of three trunk wash cultures within a week (Miller and Olea-Popelka 2012). As a result of this screening, Mikota reported fifty new cases of elephant tuberculosis in the USA between 1994 and 2010 (Mikota and Maslow 2011). Currently, published prevalence estimates range from 3.3 to 18%, with higher estimates frequently quoted in the non-scientific literature (Mikota 2001; Mikota 2011; Murphree 2011; Miller 2012). For public health and regulatory policy, accurate estimates of prevalence and incidence are essential parameters in assessing the disease burden, estimating occupational health risks, and appropriating resources to control the disease.
The objective of this study was to calculate the annual point prevalence, annual incidence, cumulative incidence, and incidence density of tuberculosis in captive elephants within the USA during the past 52 years based on culture from trunk washes and necropsies.
2. Materials and Methods
For this analysis we combined the North American Regional Studbooks for both Asian elephants (Elephas maximus) and African elephants (Loxodonta africana) into a single database (Keele 2010; Olson 2011). We then entered the MTBC positive culture results from the elephants into the database. These results were gathered from multiple sources including laboratory results, elephant culture results reported to regulatory agencies, and personal communications. The elephant populations studied included all known living elephants in the USA from 1960 through 2011. A living elephant was defined as an elephant listed in the studbook, over the age of 6 months, and documented to be alive at a specific location within the USA. With these criteria, the combined data set contained a total of 684 Asian elephants and 459 African elephants. The annual populations were calculated as the number of living elephants on the first day of each year. Positive elephant cases were M. tuberculosis or M. bovis culture positive animals that were identified within the studbooks. For this analysis, the first isolation of MTBC was defined as the index isolation and the elephant was considered positive from that time forward, regardless of treatment, until its death.
Annual point prevalences were calculated by dividing the number of living culture positive elephants by the total number of living elephants at the start of the year. The annual incidences were calculated by counting the number of new elephant MTBC cases diagnosed within a given calendar year. The cumulative incidence was calculated from the Kaplan-Meier curve for the period from 1997 through 2011. The incidence density for the same period was calculated by tabulating the total incidence during the study period divided by the total number of elephant years contributed by the living elephants during that period.
3. Results
The annual point prevalence and number of new MTBC cases were plotted for each elephant species (Figures 1 and 2). Between 1960 and 1996, the annual point prevalence of MTBC for both species was 0 and the incidence of new cases in Asian elephants was sporadic. From 1997 through 2011, the median point prevalence within the Asian elephant population increased to 5.1%, with a range from 0.3% to 6.7%. Similarly, the average annual incidence was 2.4 cases per year, with a range from 0 to 7. The period cumulative incidence from 1997 through 2011 was 13.5% (SE: 2.1%) with an incidence density of 9.7 cases/1000 elephant years (95% CI: 7.0–13.4). In contrast, within the same time period and under the same testing regime, the annual point prevalence within the African elephant population remained 0 and the average annual incidence was 0.4 cases per year, with a range from 0 to 2. For the African elephants, the period cumulative incidence from 1997 through 2011 was 2.7% (SE: 1.2%) and the incidence density was 1.5 cases/1000 elephant years (95% CI: 0.7–4.0).
Figure 1.
Prevalence and New Cases of TB in Captive Asian Elephants (Elephas maximus) in the United States of America.
Figure 2.
Prevalence and New Cases of TB in Captive African Elephants (Loxodonta africana) in the United States of America.
Since 1960, a total of 45 cases of MTBC (45 M. tuberculosis) were tabulated in Asian elephants in comparison to only 5 cases in African elephants (4 M. tuberculosis and 1 M. bovis), suggesting a difference in species susceptibility. Between 1997 and 2011 when both species were sampled annually, the incidence density was significantly different (P>0.0001 Log-Rank test of equality over strata).
4. Conclusions
The availability of 52 years of census data in the elephant studbooks provided an opportunity to calculate prevalence and incidence from a well-defined population. For the first 37 years, the incidence of elephant MTBC in both populations was artificially low because the elephants were not subjected to annual ante-mortem MTBC screening; thus new cases were identified only at necropsy. The apparent increase in new cases noted after 1996 resulted from a combination of both index cases and the initiation of mandatory annual MTBC screening in 1997 for all the elephants. The prevalence subsequently increased due to a combination of this ante-mortem screening and the initiation of treatment of many of the infected elephants. Because of this annual cross-sectional screening, the data set from 1997–2011 provided the best estimates of both prevalence and incidence.
This study defined MTBC infected elephants as only those elephants that were culture positive for MTBC from trunk washes or at necropsy. As noted in several papers and in the USDA guidelines, the trunk wash culture is a definitive diagnostic test for the identification of elephants that are actively shedding MTBC, but lacks sensitivity due to negative culture results when the elephants are not shedding mycobacteria in sufficient numbers needed to obtain a positive culture (Mikota and Maslow 2011; Miller and Olea-Popelka 2012; USDA Guidelines 2008). The causes of negative culture results in an infected elephant include intermittent or paucibacillary shedding of mycobacteria, periods of latency where the elephants are not shedding mycobacteria, and laboratory error that fails to culture mycobacteria from a trunk wash sample containing viable MTBC. Given the inherent poor sensitivity of the trunk wash, it is possible that this analysis of the population may under estimate the true incidence and prevalence of MTBC, however, the repeated annual sampling of every individual in this population for a period of 15 years probably identified most of the actively shedding cases in the population.
The discrepancy between these reported prevalence estimates and previous estimates are primarily due to differences in terminology and calculation methods. The previous estimates were calculated by tabulating the total number of elephants (both live and deceased) ever diagnosed with MTBC, divided by the current number of live elephants in the population (Mikota et al. 2001; Mikota and Maslow 2011; Murphree et al. 2011). These prevalence calculations counted all incident cases, not living prevalent cases, and therefore approximated the cumulative incidence, not the prevalence. Additionally, the previous calculations also failed to account for an open cohort and changes that occurred in the denominator elephant population over their observed time periods. Unfortunately, these calculations produced inflated and potentially biased estimates of prevalence. To address these limitations, the current analysis used a combination of population census data, tabulations by year, and survival analysis as the basis of the calculations.
The significant species differences in prevalence and incidence noted in our results are important and useful because most previous prevalence estimates have combined the culture results from both species in to a single “elephant” estimate. These previous calculations ignored the large differences in prevalence between the species and produced biased in prevalence estimates for both species. We suggest that future prevalence and incidence calculations be reported as species-specific estimates.
The incidence density calculated during the last 15 years of the Asian elephant data set allows for an estimate of expected disease incidence within the population. With the current annual trunk wash testing regime, approximately 10 new cases can be expected for every thousand elephants cultured. Similar incidence densities for active tuberculosis have been documented in some high incident human populations, suggesting that this Asian elephant population also had a high incidence of MTBC (Baussano et al. 2011). However, this Asian elephant population was intensely screened annually since 1997 with cultures of each individual in the population. No large human populations are screened at this level and thus the elephant incidence density may be elevated in comparison to commonly reported human incidence densities.
The previously reported prevalence estimates of elephant MTBC have garnered media attention and discussion as a potential emerging disease. These findings demonstrate lower prevalence and provide an estimate of incidence density from an intensely screened population. Now more than ever, it is imperative to acquire accurate estimates of the burden of MTBC disease from captive elephants in order to accurately assess the occupational risks and appropriately allocate resources needed to control tuberculosis in these species.
Supplementary Material
Acknowledgements
This work supported in part by the National Institutes of Health Clinical and Translational Science Award program, grants UL1 TR000064 and KL2 TR000065.
References
- Baussano I, Williams BG, Nunn P, Beggiato M, Scano F. Tuberculosis Incidence in Prisons: A Systematic Review. PLoS Medicine. 2011;7(12):1–10. doi: 10.1371/journal.pmed.1000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keele M. Asian elephant (Elephas maximus) North American region studbook. 2010. Available from: http://www.elephanttag.org/professional/2010AsianElephantStudbook.pdf. [Google Scholar]
- Michalak K, Austin C, Diesel S, Bacon MJ, Zimmerman P, Maslow JN. Mycobacterium tuberculosis Infection as a Zoonotic Disease: Transmission between Humans and Elephants. Emerging Infectious Diseases. 1998;4(2):283–287. doi: 10.3201/eid0402.980217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikota KS, Peddie L, Peddie J, Isaza R, Dunker F, West G, Lindsay W, Larsen RS, Salman MD, Chatterjee D, et al. Epidemiology and Diagnosis ofMycobacterium Tuberculosis in Captive Asian Elephants (Elephas maximus) Journal of Zoo and Wildlife Medicine. 2001;32(1):1–16. doi: 10.1638/1042-7260(2001)032[0001:EADOMT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Mikota SK, Maslow JN. Tuberculosis at the human-animal interface: An emerging disease of elephants. Tuberculosis. 2011;9(1):208–211. doi: 10.1016/j.tube.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Miller M, Olea-Popelka One Health in the shrinking world: Experiences with tuberculosis at the human–livestock–wildlife interface. Comparative Immunology Microbiology Infectious Diseases. 2012 doi: 10.1016/j.cimid.2012.07.005. Available from: http://dx.doi.org/10.1016/j.cimid.2012.07.005. [DOI] [PubMed] [Google Scholar]
- Murphree R, Warkentin JV, Dunn JR, Schaffner W, Jones TF. Elephant-to-human transmission of tuberculosis, 2009. Emerging Infectious Diseases. 2009;17(3):366–371. doi: 10.3201/eid1703101668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh P, Granich R, Scott J, Sun B, Joseph M, Stringfield C, Thisdell S, Staley J, Workman-Malcolm D, Borenstein L, et al. Human exposure following Mycobacterium tuberculosis infection of multiple animal species in a metropolitan zoo. Emerging Infectious Diseases. 2002;8:1290–1293. doi: 10.3201/eid0811.020302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson D. The 2011 Edition; North American studbook for the African elephant. 2011. Available from: http://www.elephanttag.org/professional/2011_african_elephant_studbook_webversion.pdf. [Google Scholar]
- United States Department of Agriculture. Guidelines for the Control of Tuberculosis in Elephants 2008. The National Tuberculosis Working Group for Zoo and Wildlife Species. 2008 Available from: http://www.aphis.usda.gov/animal_welfare/downloads/elephant/elephant_tb.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.