Abstract
Human skin not only functions as a permeation barrier (mainly due to the stratum corneum layer), but also provides a unique delivery pathway for therapeutic and other active agents. These compounds penetrate via intercellular, intracellular and transappendageal routes, resulting in topical delivery (into skin strata) and transdermal delivery (to subcutaneous tissues and into the systemic circulation). Passive and active permeation enhancement methods have been widely applied to increase the cutaneous penetration.
The pathology, pathogenesis and topical treatment approaches of dermatological diseases, such as psoriasis, contact dermatitis, and skin cancer, are then discussed. Recent literature has demonstrated that nanoparticles-based topical delivery systems can be successful in treating these skin conditions. The studies are reviewed starting with the nanoparticles based on natural polymers specially chitosan, followed by those made of synthetic, degradable (aliphatic polyesters) and non-degradable (polyarylates) polymers; emphasis is given to nanospheres made of polymers derived from naturally occurring metabolites, the tyrosine-derived nanospheres (TyroSpheres™).
In summary, the nanoparticles-based topical delivery systems combine the advantages of both the nano-sized drug carriers and the topical approach, and are promising for the treatment of skin diseases. For the perspectives, the penetration of ultra-small nanoparticles (size smaller than 40 nm) into skin strata, the targeted delivery of the encapsulated drugs to hair follicle stem cells, and the combination of nanoparticles and microneedle array technologies for special applications such as vaccine delivery are discussed.
Keywords: polymeric nanospheres, topical delivery, psoriasis, skin, tyrosine-derived nanospheres
Introduction
The barrier function of skin can be attributed mostly to the stratum corneum layer of the epidermis, and this skin barrier also regulates the transport of compounds into the skin. Approaches that deliver drugs/active compounds through the skin barrier are referred to the topical route of administration (as opposed to the enteral and parenteral routes of administration). Passive and active skin penetration enhancement methods have been successfully used to improve the efficiency of either the topical delivery (the drugs/active compounds are delivered into skin strata), or transdermal delivery (drugs/active compounds are delivered into subcutaneous tissues and are taken up systemically into the body). Topically applied therapies are promising for the treatment of skin diseases such as psoriasis, contact dermatitis, and skin cancers, since the drugs are delivered directly into skin strata.
Nano-sized drug carriers have attracted much attention in the past decade as options in formulations for topical therapy. Nanoparticles (including nanospheres, solid lipid nanoparticles, micelles, and micellar-like nanoparticles), liposomes, and nanoemulsions are among the most studied systems. Inorganic and metal nanoparticles such as quantum dots and gold nanoparticles are widely used for diagnosis, and are not in the scope of this review. Rather, this paper focuses on topically applied, polymeric nanoparticle-based drug delivery systems. Nanoparticles made of natural polymers (e.g. chitosan) and synthetic biodegradable polymers (e.g. poly(lactide-co-glycolide) and poly(ε-caprolactone)) as well as non-degradable polymers (e.g. polyacrylates) are discussed. Special emphasis is given to the tyrosine-derived nanospheres since they consist of natural occurring metabolites and have been utilized in several products already marketed for patients. The most recent advances in the nanoparticle-based topical delivery systems elicited a few interesting questions: do the ultra-small nanoparticles penetrate into skin, can we manipulate hair follicle stem cells via drug-loaded nanoparticles that localize in the hair follicles, and will the less invasive vaccination using the combination of microneedle techniques and vaccine-loaded nanoparticles become routine clinical practice. All the above-mentioned topics are discussed in detail in this review paper.
SKIN BARRIER
Skin strata and stratum corneum as barrier
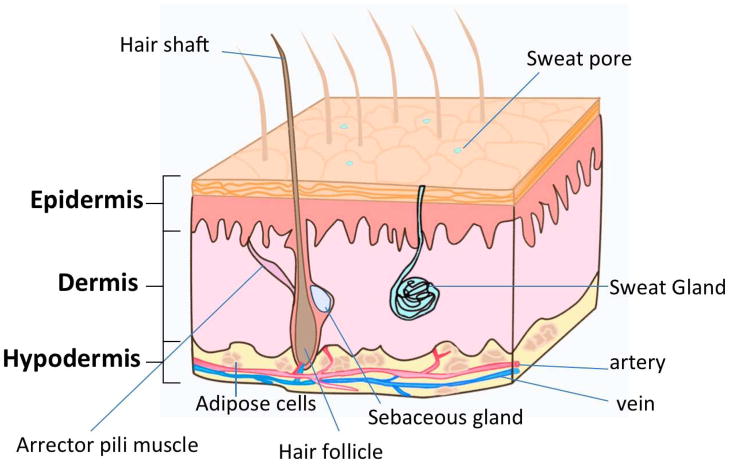
Human skin is the largest organ in the body with a surface area between 1.5 and 2.0 m2 for adults. The skin thickness varies over the body with the thinnest part of eyelids being less than 0.1 mm thick and the thickest on the palms, soles and upper back more than 5.0 mm. Not only is the skin a protective barrier against toxic substances, pathogens, and organisms, but it is also involved in many important physiological functions such as fluid homeostasis, thermoregulation, immune surveillance, and sensory detection1. These functions are related to the skin’s complex multiple layers with each layer associated with highly specified cells and structures. See Table 1 for details of the skin strata and Figure 1 for a schematic image of skin.
Table 1.
Nature of skin strata: cellular and molecular components and associated functions
| Anatomical feature | Cellular components | Molecular components | Function | ||
|---|---|---|---|---|---|
| Epidermis | |||||
| Stratum Corneum | A cornified layer possessing a “bricks and mortar” structure | Corneocytes, the dead cells lacking nuclei and organelles | Intercellular lamellar lipid bilayers | Contributes significantly to the permeation barrier properties of skin | |
| Stratum Lucidum | A thin and clear/translucent layer found only in certain areas of thick skin | The major cells of the viable epidermis are keratinocytes (90–95%). As the keratinocytes in the basal layer of epidermis proliferate, the daughter cells migrate superficially and differentiate, forming the shallower layers | Flattered and dead keratinocytes filled with eleiden, an intermediate form of keratin | Oily lamellar bodies containing lipids and proteins | Water proof barrier preventing fluid loss |
| Stratum Granulosum | A highly differentiated granular layer | Keratinocytes becoming granular cells that contain keratohyalin granules | |||
| Stratum Spinosum | A spinous layer where keratinization begins | Adjacent keratinocytes are joined together by desmosomes; Langerhans cells (the dendritic cells) |
Cytokeratins, the intermediate filaments of keratin, and desmosomes | Cytokeratin-desmosome networks provide structural support, helping the skin resist abrasion. Langerhans cells contribute to the immunological response of the skin |
|
| Stratum Basale | The deepest layer of epidermis | Melanocytes produce melanin that contributes to skin pigmentation and protects the skin from harmful ultraviolet radiation; Merkel-Ranvier cells (the oval receptor cells) contribute to sensory reception). | This is where the epidermal (keratinocytes) differentiation initiates | ||
| Dermis | |||||
| Papillary Dermis | A layer intertwined with the overlaying epidermis, forming rete pegs and rete ridges along the dermal-epidermal junction | Fibroblasts, macrophages, and adipocytes | Collagen, elastin, glycos-aminoglycans and glycoproteins | Provides tight contact between epidermis and dermis | |
| Reticular Dermis | A layer of dense collagen and elastic fiber matrix | Main source of the strength, flexibility and elasticity of the skin. | |||
Figure 1.
A schematic image of epidermis, dermis and hypodermis structure. The appendages such as hair shaft and hair follicle, sweat gland, sebaceous gland, and arrector pili muscle are illustrated.
The permeation barrier properties of human skin are mostly attributed to the top layer of the epidermis, the stratum corneum. The barrier function is related to the unique structure in the stratum corneum layer that is composed of “bricks (corneocytes) and mortar (intercellular lamellar lipid bilayers)” 2.
Allied with the epidermis and dermis are skin appendages possessing different functions. The features of the common skin appendages and their functions are described in box 1.
Box 1. Skin appendages.
Hair follicles are formed by the infolding of the epidermis into the dermal layer, and are composed of papilla, matrix, root sheath, hair fiber and bulge. Besides producing hair, the hair follicles contain several types of stem cells that play an important role in wound healing and re-epithelialization 3. Associated with hair follicles are arrector pili muscles that pull hairs straight and sebaceous glands that secrete sebum to moisturize the skin and hair. Other skin appendages include eccrine sweat glands which secrete sweat and therefore regulate body temperature, apocrine glands involved with hair follicles in certain restricted regions of the body (e.g. axillae), and nails.
Topical and transdermal delivery across skin barrier
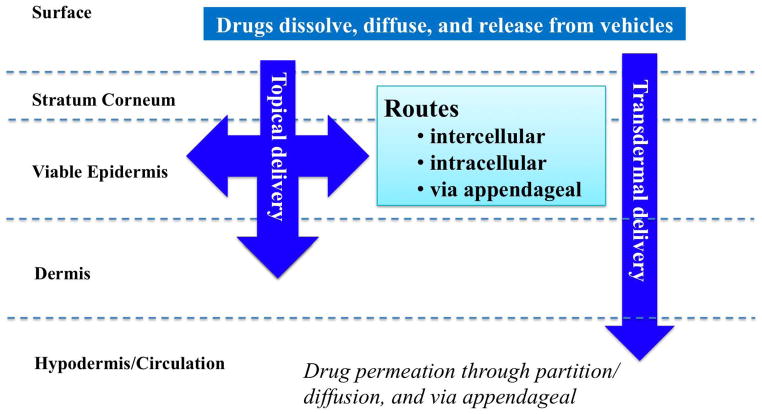
The permeation barrier properties of human skin elicit challenging but exciting delivery avenues for drugs and other compounds into the skin strata (topical delivery) and/or to the systemic exposure (transdermal delivery). Currently, there are more than 30 transdermal products in the US market, and it is expected that the topical and transdermal drug delivery market will reach $32 billion in 2015; formulations of a number of low molecular weight drugs and macromolecules have been developed and some are currently under clinical trial 4.
Compared to the other delivery routes, topical and transdermal delivery approaches have the unique advantages: a) for skin diseases, topical delivery approaches directly deliver drugs to the site of the diseases cells/tissues; b) smaller amounts of drugs are needed to produce a therapeutic effect; c) plasma level peaking of drugs will be avoided; d) increase bioavailability due to elimination of hepatic first-pass metabolism; and e) greatly enhanced patient compliance by eliminating frequent dosing.
The cutaneous penetration pathways are a) through stratum corneum via intercellular/intracellular routes, followed by the viable epidermis and dermis via partitioning/diffusion; and b) through the appendageal pathway 5. These routes result in topical and transdermal delivery.
Passive and active skin penetration enhancement methods
It was not until 1960s – 1970s that the scientists reached the consensus that a) the stratum corneum is the rate-limiting barrier against percutaneous drug penetration, and b) the specific content, composition and structure of the stratum corneum lipids selectively and effectively inhibit the penetration of chemicals2. In addition, not all the compounds can penetrate through the stratum corneum barrier; the ones with moderate lipophilicity (octanol-water partition coefficient between 10 and 1000) and molecular weight less than 500 Daltons are able to permeate the stratum corneum and penetrate into deeper layers of skin. Box 2 illustrates the percutaneous drug penetration hypothesis.
Box 2. Percutaneous drug penetration.
Generally, the permeation of drug molecules into skin is guided by passive diffusion in one dimension and can be described most simply by Fick’s first law (Equation 1):
| Equation 1 |
where J, the diffusion flux, is the amount of the substance going through a unit area during a small interval of time; D is the diffusion coefficient (or diffusivity) of the compound in the membrane; is concentration gradient which is the driving force that leads to molecular movement into skin; A is barrier surface area; M is mass of drug 6.
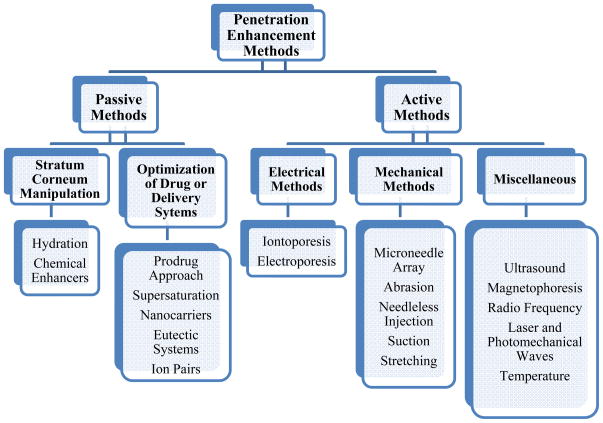
Even for the compounds that favor the penetration requirements, additional means are usually implemented to enhance the transport across the skin 7. A variety of strategies have been studied in the past three decades to overcome the barrier and optimize the cutaneous delivery of drugs, and they can be categorized into passive and active penetration enhancement methods (Figure 2).
Figure 2.
An overview of passive and active skin penetration enhancement methods.
Passive skin penetration enhancement methods
The diffusion flux, J, can be further described by Equation 26:
| (Equation 2) |
Where Dm represents the diffusion coefficient of the drug in the membrane (skin), cs,m represents solubility of the drug in the membrane, cv and cs,v are respectively the concentration and the solubility of the drug in the vehicle, and L the diffusion path length across the membrane.
In a recent proposed solute structure-skin transport model, permeation rates were linked to both partitioning of the drug between stratum corneum and the topically applied formulation and the diffusivity (the “D” in Equation 1, or the “Dm ” in Equation 2) of the drug into the skin strata. The former is related to octanol-water partition coefficient and the latter to solute size and hydrogen bonding if applicable 8.
Passive penetration enhancement methods entail optimization of formulation and drug carriers to increase the flux. As can be seen from Equation 2, the strategies for passive penetration enhancement include increasing the diffusion coefficient Dm (increase the diffusivity) and solubility of the drug in the skin (increase cs,m, and thus increase the partition coefficient) by adding chemical penetration enhancers to the formulation or using eutectic system9; chemical penetration enhancers (such as alcohols, sulfoxides, fatty acids, etc.) cause temporary disorder of the intercellular lipids structure in the stratum cornuem and reduce skin resistance 10. Another strategy is to increase the ratio cv/cs,v and this can be realized by a supersaturation approach and using nano-sized carriers11.
Active skin penetration enhancement methods
For high molecular weight (>500 Da) polar and hydrophilic molecules such as plasmid DNA, peptides, and vaccines, passive skin permeation enhancement methods are not sufficient. Active skin penetration enhancement methods have been developed and categorized into electrical, mechanical and other energy-related techniques (see Figure 2, the active methods). The mechanisms involved in active penetration enhancement methods are mostly as follows: a) bypassing the stratum corneum using techniques such as microneedle array12; b) removing stratum corneum by abrasion13 c) disrupting lipid packing in stratum corneum by ultrasound waves14; and d) generating transient aqueous pores in the lipid bilayers of stratum corneum by electroporation15. Among these, significant efforts have been devoted to developing microneedle-based techniques. A brief discussion on microneedles is given below:
Microneedles are micro-scaled structures designed in an array on patches that can painlessly pierce and deliver active ingredients into skin. Various materials such as metal, silicon and degradable polymers have been fabricated into microneedles. Microneedles have been widely applied to improve skin delivery of various small molecules as well as macromolecules such as proteins16, peptides17, nucleic acids18 and virus-like particles19 to enhance its therapeutic values or vaccination efficacies. The improvement of delivery is the result of the formation of microsized channels that are opened throughout the stratum corneum allowing the drug access to the viable epidermis.
DERMATOLOGICAL DISEASES: PATHOLOGY, PATHOGENESIS AND TOPICAL TREATMENT APPROACHES
Major challenges for the treatment of skin diseases
In this section, the pathology and pathogenesis of several skin diseases i.e. psoriasis, contact dermatitis and skin cancer are discussed. The major challenges in treatment of these skin diseases include poor efficiency of drug delivery into the disease site and risks of increased toxicity associated with approaches used to improve the drug delivery efficiency. Despite the achievements in the passive and active skin penetration enhancement methods, many skin disease patients are still waiting for treatment based on safer and more efficient cutaneous delivery of therapeutics across the stratum corneum barrier.
Psoriasis
Pathology and pathogenesis
Psoriasis is a chronic, autoimmune disease affecting as many as 7.5 million Americans and 2% to 3% of the world population (data from National Psoriasis Foundation website). The most prevalent form of psoriasis, plaque psoriasis, is recognized by psoriatic plaques of red lesions covered by silvery white flakes on top of the skin. The pathogenesis of psoriasis is associated with both genetics and the immune system20. As the current understanding of psoriasis, a stimulation of dermal dendritic cells activates the immune system including macrophages and T-cells and promotes the interaction between epidermal keratinocytes and the immune system. These events result in the up-regulated production of cytokines, which in turn causes the over-proliferation of keratinocytes starting from the basal layer of the epidermis and the overall skin inflammation associated with psoriasis lesion formation 21, 22.
Topical Treatment for psoriasis
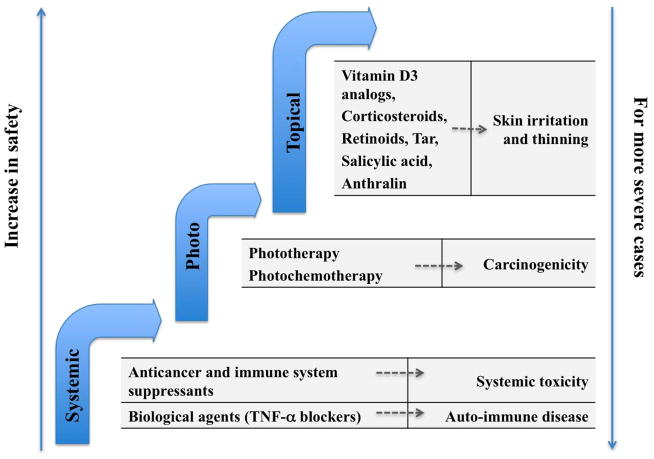
The standard of care for psoriasis can be categorized into systemic treatment, phototherapy and topical treatment. Figure 3 illustrates these approaches and the associated side effects; the general concept applies for the treatment of other dermatological conditions as well.
Figure 3.
Standard of care for psoriasis categorized as systemic approach, phototherapy and topical approach. Under each category, the current treatment options (on the left columns) and the side effects (on the right columns) are given.
The application of systemic treatment and phototherapy is limited to treating severe psoriasis patients only, and this is due to the toxicity and carcinogenicity of these approaches. Topical treatment approaches are safer, and the drugs are targeted to the basal layer of epidermis where psoriasis originates. A number of topically applied formulations are available for psoriasis patients upon prescription: anti-inflammatory agents such as corticosteroids are the most frequently applied; examples of other agents are anthralin, synthetic Vitamin D3 and Vitamin A. Over the counter (OTC) formulations containing salicylic acid and coal tar are also available 23. The most common side effects of topical treatments are skin irritation, thinning of the skin, and dilated blood vessels 24. Controlled drug encapsulation and release systems that not only decrease the side effects of the applied agents, but also enhance drug permeation into deeper epidermis layers would be ideal for psoriasis treatment.
Contact dermatitis
Pathology and pathogenesis
Contact dermatitis is a common dermatological disorder caused by repeated direct contact of skin with external factors (allergens and irritants). This skin disease is characterized by a wide range of clinical features, such as inflammation, redness, erythema, burning, pruritus, and the formation of vesicles (a circumscribed, fluid-containing, epidermal elevation) and pustules 25. There are two types of contact dermatitis: irritant (ICD) and allergic (ACD) contact dermatitis. ICD represents about 70% of the contact dermatitis, and it is a non-specific, local and immediate inflammatory reaction of the innate immune system to low molecular weight chemicals. ACD is a delayed-type hypersensitivity response with a skin inflammation mediated by hapten-specific T-cells (adaptive immune response)26.
Topical treatment for dermatitis
Topical products marketed for contact dermatitis may contain a combination of ingredients in various forms including creams, ointments, gels, lotions, and sprays. The most commonly used drugs are topical formulations of hydrocortisone, oral antihistamines, and other antipruritic agents such as calamine. Topical astringent products such as aluminum acetate and zinc oxide also serve as a protective covering for inflamed skin and promote drying of moist, wet, and oozing lesions27.
Skin Cancer
Pathology and pathogenesis
Skin cancer is malignant growth of cells that forms in the tissue of the skin. Most common skin cancers are keratinocyte cancers (basal cell carcinoma that starts in the basal layer of epidermis and squamous cell carcinoma that starts in the upper part of epidermis) and melanoma characterized as the malignant tumor of melanocytes. Ultraviolet radiation, particularly UVB is the major risk factor in the development of squamous and basal cell carcinoma28; the formation of cancer cells is triggered by DNA damage29, loss of activity of tumor suppressor genes such as P5330 and activation of oncogenes31. Other risk factors such as HPV (human papillomavirus) infection and immunosuppression disease were also reported to be associated with skin cancer32.
Topical treatment for skin cancer
Creams and solution formulations of fluorouracil (5-FU)33 and imiquimod34 are the most widely applied topical treatments for squamous and basal carcinoma. 5-FU, a uracil analog, is an anti-metabolite competing with normal metabolites. It acts as a thymidylate synthase inhibitor and ultimately leads to the thymine-less death of the rapidly dividing cancerous cells35. Imiquimod is an immune-enhancing drug that specifically binds to TLRs (toll-like receptors) such as TLR-7 and TLR-8 on macrophage, Langerhans and dendritic cells. The binding of imiquimod with TLRs results in the up-regulation and secretion of proinflammatory cytokines such as TNF-α, IL-12 and interferons, and ultimately the activation of TH1 dominant immune response. Examples of FDA approved topical drugs for squamous and basal carcinoma treatments are Efudex (5%, 2% 5-FU) and Aldara (5% imiquimod).
For melanoma, no topical treatment has been approved by FDA since it is often hindered by the high metastatic properties of the cancer; surgery in combination with systemic treatment of monoclonal antibody such as Yervoy™ (Ipilimumab) or oral uptake of Zelboraf® (Vemurafenib) tablets were more preferred.
POLYMERIC NANOPARTICLES-BASED TOPICAL DELIVERY SYSTEMS
Nano-sized carriers have been extensively studied in the past decades for various biomedical applications including disease diagnostics, drug delivery, gene delivery and vaccination. The using of nano-sized carriers benefits in (a) sustained and controlled release of drugs; (b) stabilization of compounds by providing chemical and physical protection; (c) higher concentration in tumors due to the Enhanced Permeation and Retention (EPR) effect 36; (d) cell and tissue specific targeting by conjugating antibodies and peptides to carrier surfaces; and (e) gene delivery via preparing drug-vehicle complexes that can be internalized. Great efforts have been devoted to the commercialization of nanomedicine technologies. Several nanoparticle-based treatments have already been approved by FDA, such as Estrasorb (micellar nanoparticles) for topical menopause therapy and Abraxane (albumin-bounded paclitaxel nanoparticles) for breast cancer treatment.
Nano-sized carriers such as polymeric nanoparticles, solid lipid nanoparticles, liposomes, and nano-emulsions have been widely applied as topical formulations to enhance cutaneous delivery37, 38. Among these nano-sized carriers, polymeric nanoparticles with readily-tunable chemical and physical features can effectively protect unstable drugs from degradation/denaturation, decrease the side effects of toxic drugs by producing controlled release, and enhance the cutaneous penetration of the drugs across the skin barrier by increasing the concentration gradient. Here, we focus on polymeric nanoparticles-based topical delivery systems.
Natural polymeric nanoparticles
Natural polymeric nanoparticles are composed of polymers occurring in nature such as chitosan, alginate, gelatin and albumin. These natural polymers are usually obtained from extraction followed by various purification procedures. The tendency of these natural polymers to form hydrogels makes them ideal carriers for oligonucleotides, peptides, proteins and water-soluble drugs. For example, Pilocarpine hydrochloride-loaded gelatin nanoparticles were reported for topical ophthalmic use 39; oxybenzone-loaded gelatin microspheres, although not in the nano-sized range, were used for sunscreen applications40. Table 2 shows a summary of topical delivery systems based on natural polymeric nanoparticles.
Table 2.
Topical delivery systems based on natural polymeric nanoparticles.
| Natural Polymeric Nanoparticles | Encapsulated Drug | Target treatment or effect | Particle size | Ref |
|---|---|---|---|---|
| Gelatin | Pilocarpine HCL | Ophthalmic use | 300~500 nm | 39 |
| Chitosan | Retinol | Wrinkled skin, acne | 50–200 nm | 41 |
| Chitosan-TPP | Antisense oligonucleotides | Overexpressed genes | 221 nm | 42 |
| Aciclovir | Anti-viral in herpes infections | 350–700 nm | 43 | |
| Plasmid DNA | Genetic immunization | 200–287 nm | 44 | |
| Lecithin-chitosan | Clobetasol-17-propionate | Antineoplastic, anti-inflammatory | 250 nm | 45 |
| Quercetin | Antioxidant, delay UV mediated oxidant | 95–168 nm | 46 |
Among various natural polymeric nanoparticles, chitosan-based nanoparticles have been most frequently applied for topical skin delivery. Chitosan, the N-deacetylated derivative of chitin, is a natural biodegradable, cationic polymer composed of mainly glucosamine units. Its anti-oxidant, anti-inflammatory and anti-microbial properties make chitosan a suitable vehicle for delivering therapeutics to treat dermatological disorders. Moreover, at physiological pH, the primary amine groups of chitosan are protonated, and therefore chitosan is positive-charged. The positive charge can be used to form nanoparticles in solution via cross-linking with polyanions, to efficiently encapsulate negative-charged drugs via electrostatic interaction, and to promote cellular internalization of drug-containing chitosan nanoparticles. A few examples of topically applied, chitosan-based nanoparticle systems are given below.
Kim et al.41 encapsulated retinol, a vitamin A derivative in chitosan nanoparticles. The encapsulation of retinol in chitosan nanoparticles minimized the irritation and toxicity of retinol, and the retinol-loaded chitosan nanoparticles can be potentially used for acne and anti-wrinkle treatment. Nanoparticle fabrication based on the cross-linking between chitosan and polyanions such as tripolyphosphate (TPP) was developed. Hasanovic et al.43 demonstrated that the encapsulation of aciclovir (an anti-viral drug) into chitosan-TPP nanoparticles resulted in significantly increased drug stability, decreased photo degradation, and enhanced drug penetration through porcine skin.
Moreover, chitosan nanoparticles and chitosan-TPP nanoparticles were shown to deliver macromolecules such as antisense oligonucleotides and plasmid DNA for topical gene therapy. Ozbas-Turan et al.42 reported that topically applied antisense oligonucleotide-loaded chitosan nanoparticles at a dose range of 15–90 μg showed the highest inhibition of beta-gal expression at 6 days post-transfection in rats. The same group also reported that chitosan-TPP nanoparticles exhibited high loading efficiency for plasmid DNA pSV-β-Gal and that the DNA-containing nanoparticles showed transfection in baby rat skin44.
The electrostatic interaction between chitosan and lecithin was also used to prepare nanoparticles for the purpose of skin delivery. Şenyiğit et al.45 reported that chitosan-lecithin nanoparticles in a chitosan gel formulation delivered a corticosteroid clobetasol-17-propionate to epidermis and dermis as effectively as a commercial cream. In another study, Tan et al.46 demonstrated that under in vitro and in vivo conditions, quercetin as loaded in chitosan-lecithin nanoparticles had higher skin permeation and increased accumulation in epidermis as compared to quercetin applied in propylene glycol solution.
In addition to topical application onto skin, chitosan-based nanoparticles were capable of delivering drugs to nasal47 and ocular mucosa48, and intestinal epithelia49.
Synthetic polymeric nanoparticles
The most widely used polymeric nanoparticles are prepared from synthetic polymers. Since natural polymers vary in purity and often lack the batch-to-batch consistency, it is hard to obtain reproducible particles and controlled release pattern for the encapsulated drug(s). On the contrary, synthetic polymers can be supplied with good purity and batch-to-batch reproducibility; and the drug release from synthetic polymeric nanoparticles can be also be modeled50. Compared to nanoparticles based on natural polymers, nanoparticles from synthetic polymers have been applied predominantly for hydrophobic/lipophilic drugs. Hydrophilic, biologically active molecules can be loaded into synthetic polymer-based nanoparticles by using the double emulsion method; however, since the volatile organic solvents are often applied, it is a challenge to maintain the biological activity of the molecules.
Commonly used synthetic polymers for drug delivery applications include biodegradable aliphatic polyesters such as polylactides (PLA), poly(lactide-co-glycolide) copolymers (PLGA), and poly(ε-caprolactone) as well as non-degradable polymers such as poly(methyl methacrylate) and polyacrylates. Table 3 lists the topically applied synthetic nanoparticle systems.
Table 3.
Topical delivery systems based on synthetic polymeric nanoparticles.
| Synthetic polymers | Encapsulated drug/model compounds | Target treatment or effect | Particle size | Ref. |
|---|---|---|---|---|
| Biodegradable | ||||
| PLGA | Hinokitol, 6-benzyaminopurin | Hair growth | 182–210 nm | 51 |
| PLGA | indomethacin | Analgesic for transdermal delivery | 100 nm | 52 |
| PLGA-chitosan | spantide II and ketoprofen | Anti-inflammatory | 169 nm | 53 |
| Poly(ε-caprolactone) | Octyl methoxycinnamate | Sunscreen effect | 255–427 nm | 54 |
| Poly(ε-caprolactone)-block-PEG | minoxidil | Anti-hairloss | 40 &130 nm | 55 |
| Non-biodegradable | ||||
| Polystyrene | Nile red | Hydrophobic dye (to study release mechanism) | 28–31 nm | 7 |
| Poly(methyl methacrylate) | Nile red | Hydrophobic dye (to study release mechanism) | 68–100 nm | 56 |
| Polyacrylate | N-thiolated β-lactam | Antibiotic for wound healing | 40 nm | 57 |
Nanoparticles made of biodegradable polymers
PLGA copolymers are biocompatible and biodegradable; in the body, the final degradation compounds i.e. lactic acid and glycolic acid are eventually removed by citric acid cycle50. These copolymers are the most commonly applied synthetic polymers for nanoparticle preparation, and PLGA nanoparticles have been widely employed for topical delivery.
Tomado et al.52 prepared spherical PLGA nanoparticles that encapsulated with indomethacin by an emulsion solvent evaporation method and applied these indomethacin-loaded nanoparticles for transdermal delivery. An In vitro permeation study using rat skin showed that transdermal delivery of indomethacin was enhanced when it was loaded in PLGA nanoparticles. Since the surface of these nanoparticles was negatively charged, iontophoresis could be used to enhance drug penetration through the skin.
Shah et al.53 utilized PLGA and chitosan to prepare surface modified bilayered nanoparticles with oleic acid. Two anti-inflammatory compounds, spantide II and ketoprofen were incorporated into PLGA inner core and chitosan outer layer, respectively. Nano gel formulation of these particles was created using hydroxypropyl methylcellulose (HPMC) and Carbopol to further enhance drug delivery to the deeper skin layers by improving the skin contact time and avoiding the water loss from the skin. In vitro human skin permeation study of this nano gel system exhibited significant increase in penetration of spantide II and ketoprofen in epidermis and dermis layers as compared to the control groups. In vivo studies were performed using an allergic contact dermatitis model and a psoriatic plaque like model; in both cases, drug-loaded nano gel formulation obtained better treatment effect as compared to the control groups.
Poly(ε-caprolactone) is another synthetic polymer that is widely employed for the preparation of nanoparticle formulations due to its biocompatibility, biodegradability and mechanical properties. In addition, due to its semi-crystalline structure, its degradation is delayed compared with amorphous polyesters. Alvarez-Roman et al. 54 reported the preparation of octyl methoxycinnamate-loaded poly(ε-caprolactone) nanoparticles for epidermal delivery of the loaded sunscreen agent; the results suggested better sun protection and partial protection against erythema.
Nanoparticles made of non-degradable polymers
Nanoparticles formulated with non-degradable polymers have also been studied for cutaneous delivery of active compounds. For example, Turos el al.57 developed a water-based emulsion system using non-degradable polyacrylate polymers. This new nano-formulation contains antibiotic-conjugated polyacrylate nanoparticles in which the drug monomer (an N-thiolated β-lactam antibiotic) is incorporated into the polymeric matrix by emulsion polymerization. In vitro screens illustrated antimicrobial activity of this nanomedicine against methicillin-resistant Staphylococcus aureus and also proved that this dosage form was nontoxic to human dermal broblasts. This group also performed an in vivo study of their penicillin-conjugated nanoparticle emulsion on a dermal abrasion model and observed an accelerated wound healing process by an average of 3 to 5 days58.
Tyrosine-derived nanospheres
A unique type of nanospheres, made of tyrosine-derived polymers from naturally occurring metabolites, has been developed at the New Jersey Center for Biomaterials, Rutgers University. The chemical structure of a typical ABA block copolymer used to prepare such nanospheres is shown in Figure 4: the A blocks, poly(ethylene glycol) is hydrophilic, and the B block made from tyrosine-derived diol and suberic acid is hydrophobic. In aqueous medium, the block copolymers self-assemble to nanospheres with size approximately 70 nm59. The nanospheres are trademarked as Tyrospheres™.
Figure 4.
Tyrosine-derived, ABA-type poly(ethylene glycol)-b-oligo(desaminotyrosyl-tyrosine octyl ester suberate)-b-poly(ethylene glycol) triblock copolymer used in the preparation of Tyrospheres™.
TyroSpheres™ have demonstrated their ability to (a) self-assemble together with a number of lipophilic drugs exemplified by paclitaxel59, 60; (b) retain the activity of the encapsulated drug59,61; (c) efficiently deliver a wide range of hydrophobic compounds into the skin 62, 63, 60; (d) cause no detrimental effects to skin62; (e) process into more viscous formulation using HPMC, a pharmaceutically acceptable thickening agent 60, 62; (f) control the over-proliferation of keratinocytes by sustained release of encapsulated paclitaxel60; and (g) preferentially deposit paclitaxel into the epidermis, thus eliminating the adverse side effects associated with systemic exposure 60. These results demonstrate the applicability of Tyrospheres™ in the treatment of skin diseases such as psoriasis.
SUMMARY AND PERSPECTIVES
Topically applied, polymeric nanoparticles-based drug delivery systems for the treatment of skin diseases have a number of advantages including the fact they:
effectively enhance the skin permeation of therapeutics, especially the poorly water-soluble lipophilic drugs, via increasing the drug concentration gradient across the skin;
improve the drug stability;
decrease the side effects such as skin irritation of applied drugs;
deliver drugs directly to the disease site and minimize the systemic exposure.
However, compared to the formulations developed in the industrial setting, the nanoparticles-based drug delivery systems have, in general, shown only limited degree of enhancement in skin permeation. Any “game changing” techniques enabling rapid, more effective, and targeted delivery of therapeutics would be the “holy grail” for the pharmaceutical and skin care society. Below, we offer our perspectives on potential breakthroughs in the nanoparticles-based skin delivery systems are:
Skin penetration of nanoparticles
Several recent studies using various nanoparticles demonstrated the penetrating feasibility of nanoparticles penetrating across the mucus membranes 64, 65 and the skin barrier 66. In one study, the biodistribution and elimination of a series of near-infrared (NIR) fluorescent nanoparticles, either inorganic/organic hybrid (INP) or the organic ones (ONP), with varying chemical composition, shape, size, and surface charge, were quantified in rat models after lung instillation 65. It was demonstrated that nanoparticles with hydrodynamic diameter in the rage of approximately 6 to 34 nm and a non-cationic surface charge translocated rapidly from the lung to mediastinal lymph nodes. Nanoparticles with hydrodynamic diameter < 6 nm could traffic rapidly from the lungs to lymph nodes and the bloodstream, and then be subsequently cleared by the kidneys 65. A most recent paper reported that spherical nucleic acid-nanoparticle conjugates (SNA-NCs), featuring of 13 nm gold cores surrounded by a dense shell of highly oriented, covalently immobilized siRNA, freely penetrated keratinocyte layer in vitro, mouse skin, and human epidermis within hours after application 66.
These recent discoveries may suggest that nanoparticles smaller than a certain size can penetrate efficiently through the epithelial barriers. The mechanisms of the penetration are yet to be elucidated. Whether or not the skin appendages such as hair follicles play a significant role in such rapid penetration is open to discussion. Safety concerns should also be addressed before any clinical trials begin.
Targeted delivery of active reagents to hair follicle stem cells
The localization of topically applied nanoparticles in hair follicles was reported by several investigators7, 55, 56, and a straight forward application would be the delivery of hair growth ingredients for the treatment of hair loss 51. Moreover, the localization of nanoparticles in the hair follicles may promote the delivery of active reagents that manipulate the proliferation and differentiation of hair follicle stem cells. The potential applications could be addressed to the treatment of dermatological conditions and wound healing.
Combining microneedles and nanoparticles
A number of studies have indicated the accumulation of topically applied nanoparticles on the surface of stratum corneum or in hair follicles, and the approach of combining nanoparticles and microneedle arrays could potentially facilitate the nanoparticle deposition or permeation into the deeper skin layers. Zhang et al.67 reported increased deposition of a fluorescent probe in both epidermis and dermis from 160 nm PLGA nanoparticles after microneedle treatment on skin. Coulman et al.68 confirmed the diffusion of 138 nm polystyrene nanoparticles to the interior surface of microchannels after the skin was treated with microneedles. These studies have shown the feasibility of using microneedles to enhance delivery of drug-loaded nanoparticles to deeper layers of skin where most dermatological diseases originate.
Moreover, combination of the microneedle and the targeted nanoparticles techniques can provide an alternative route to deliver drugs into specific cells/tissues. Specific targeting of mast cells and lymphocytes that lie in the dermis can improve the treatment of allergic and inflammatory associated skin diseases.
Besides treating dermatological diseases, another attractive application of combining microneedles and nanoparticles technologies lies in the area of vaccine delivery. Microneedles were shown to deliver antigen to epidermal and intradermal layers having large number of antigen presenting cells, such as Langerhans and dendritic cells 69.
Although many studies have shown the improvement of topical delivery via microneedles and nanoparticles, the mechanisms of colloid movement through micron-sized channels are still poorly understood and require future investigation. Safety issues such as transcutaneous penetration of nanoparticles and systemic uptake of drugs also need to be studied.
Acknowledgments
The authors thank our colleagues Drs. Larisa Sheihet and Brian E. Kilfoyle (The New Jersey Center for Biomaterials at Rutgers University) for their contributions on the development of tyrosine-derived nanospheres for the topical treatment of psoriasis. This work was supported by Grant Number 5R01AR056079 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS - NIH). The authors acknowledge editing assistance from Carole Kantor.
Footnotes
All authors have no conflict of interests.
References
- 1.Holbrook KA, Wolff K. The structure and development of skin. In: Fitzpatrick TB, Eisen AZ, Wolff K, Freedberg IM, Austen KF, editors. Dermatology in general medicine. McGraw-Hill; New York, USA: 1987. pp. 93–131. [Google Scholar]
- 2.Scheuplein RJ, Blank IH. Permeability of the skin. Physiol Rev. 1971;51:702–747. doi: 10.1152/physrev.1971.51.4.702. [DOI] [PubMed] [Google Scholar]
- 3.Cotsarelis G. Epithelial stem cells: a folliculocentric view. J Invest Dermatol. 2006;126:1459–1468. doi: 10.1038/sj.jid.5700376. [DOI] [PubMed] [Google Scholar]
- 4.Paudel KS, Milewski M, Swadley CL, Brogden NK, Ghosh P, Stinchcomb AL. Challenges and opportunities in dermal/transdermal delivery. Ther Deliv. 2010;1:109–131. doi: 10.4155/tde.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadgraft J, Guy RH. Feasibility assessment in topical and transdermal delivery: Mathematical models and in vitro studies. In: Guy RH, Hadgraft J, editors. Trandermal drug delivery. 2. Marcel Dekker, Inc; New York, USA: 2003. pp. 1–23. [Google Scholar]
- 6.Higuchi T. Physical chemical analysis of percutaneous absorption process from creams and ointments. J Soc Cosmet Chem. 1960;11:85–97. [Google Scholar]
- 7.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Skin penetration and distribution of polymeric nanoparticles. J Control Release. 2004;99:53–62. doi: 10.1016/j.jconrel.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q, Grice JE, Li P, Jepps OG, Wang GJ, Roberts MS. Skin solubility determines maximum transepidermal flux for similar size molecules. Pharm Res. 2009;26:1974–1985. doi: 10.1007/s11095-009-9912-4. [DOI] [PubMed] [Google Scholar]
- 9.Moser K, Kriwet K, Naik A, Kalia YN, Guy RH. Passive skin penetration enhancement and its quantification in vitro. Eur J Pharm Biopharm. 2001;52:103–112. doi: 10.1016/s0939-6411(01)00166-7. [DOI] [PubMed] [Google Scholar]
- 10.Barry BW. Penetration enhancer classification. In: Smith EW, Maibach HI, editors. Percutaneous penetration enhancers. 2. Taylor and Francis; New York, USA: 2006. pp. 3–16. [Google Scholar]
- 11.Alvarez-Roman R, Naik A, Kalia YN, Guy RH, Fessi H. Enhancement of topical delivery from biodegradable nanoparticles. Pharm Res. 2004;21:1818–1825. doi: 10.1023/b:pham.0000045235.86197.ef. [DOI] [PubMed] [Google Scholar]
- 12.Cormier M, Johnson B, Ameri M, Nyam K, Libiran L, Zhang DD, Daddona P. Transdermal delivery of desmopressin using a coated microneedle array patch system. J Control Release. 2004;97:503–511. doi: 10.1016/j.jconrel.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 13.Akomeah FK, Martin GP, Muddle AG, Brown MB. Effect of abrasion induced by a rotating brush on the skin permeation of solutes with varying physicochemical properties. Eur J Pharm Biopharm. 2008;68:724–734. doi: 10.1016/j.ejpb.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Alvarez-Roman R, Merino G, Kalia YN, Naik A, Guy RH. Skin permeability enhancement by low frequency sonophoresis: Lipid extraction and transport pathways. J Pharm Sci. 2003;92:1138–1146. doi: 10.1002/jps.10370. [DOI] [PubMed] [Google Scholar]
- 15.Mori K, Hasegawa T, Sato S, Sugibayashi K. Effect of electric field on the enhanced skin permeation of drugs by electroporation. J Control Release. 2003;90:171–179. doi: 10.1016/s0168-3659(03)00164-0. [DOI] [PubMed] [Google Scholar]
- 16.Harvey AJ, Kaestner SA, Sutter DE, Harvey NG, Mikszta JA, Pettis RJ. Microneedle-based intradermal delivery enables rapid lymphatic uptake and distribution of protein drugs. Pharm Res. 2011;28:107–116. doi: 10.1007/s11095-010-0123-9. [DOI] [PubMed] [Google Scholar]
- 17.Fukushima K, Ise A, Morita H, Hasegawa R, Ito Y, Sugioka N, Takada K. Two-layered dissolving microneedles for percutaneous delivery of peptide/protein drugs in rats. Pharm Res. 2011;28:7–21. doi: 10.1007/s11095-010-0097-7. [DOI] [PubMed] [Google Scholar]
- 18.Coulman SA, Barrow D, Anstey A, Gateley C, Morrissey A, Wilke N, Allender C, Brain K, Birchall JC. Minimally invasive cutaneous delivery of macromolecules and plasmid DNA via microneedles. Curr Drug Deliv. 2006;3:65–75. doi: 10.2174/156720106775197510. [DOI] [PubMed] [Google Scholar]
- 19.Quan FS, Kim YC, Compans RW, Prausnitz MR, Kang SM. Dose sparing enabled by skin immunization with influenza virus-like particle vaccine using microneedles. J Control Release. 2010;147:326–332. doi: 10.1016/j.jconrel.2010.07.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bowcock AM, Krueger JG. Getting under the skin: the immunogenetics of psoriasis. Nat Rev Immunol. 2005;5:699–711. doi: 10.1038/nri1689. [DOI] [PubMed] [Google Scholar]
- 21.Clark RA, Kupper TS. Misbehaving macrophages in the pathogenesis of psoriasis. J Clin Invest. 2006;116:2084–2087. doi: 10.1172/JCI29441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danilenko DM. Review paper: preclinical models of psoriasis. Vet Pathol. 2008;45:563–575. doi: 10.1354/vp.45-4-563. [DOI] [PubMed] [Google Scholar]
- 23.Van de Kerkhof PC, Vissers WH. Established treatments of psoriasis. Curr Drug Targets Inflamm Allergy. 2004;3:145–156. doi: 10.2174/1568010043343967. [DOI] [PubMed] [Google Scholar]
- 24.Naldi L, Griffiths CE. Traditional therapies in the management of moderate to severe chronic plaque psoriasis: an assessment of the benefits and risks. Br J Dermatol. 2005;152:597–615. doi: 10.1111/j.1365-2133.2005.06563.x. [DOI] [PubMed] [Google Scholar]
- 25.Andersen KE, Benezra C, Burrows D, Camarasa J, Dooms-Goossens A, Ducombs G, Frosch P, Lachapelle JM, Lahti A, Menné T, et al. Contact Dermatitis. A review. Contact Dermatitis. 1987;16:55–78. doi: 10.1111/j.1600-0536.1987.tb01382.x. [DOI] [PubMed] [Google Scholar]
- 26.Nosbaum A, Vocanson M, Rozieres A, Hennino A, Nicolas JF. Allergic and irritant contact dermatitis. Eur J Dermatol. 2009;19:325–332. doi: 10.1684/ejd.2009.0686. [DOI] [PubMed] [Google Scholar]
- 27.Plake KS, Darbishire PL. Chapter 34: Contact dermatitis. In: Krinsky DL, Berardi RR, Ferreri SP, Hume AL, Newton GD, Rollins CJ, Tietze KJ, editors. APhA Handbook of nonprescription drugs, an interactive approach to self-care. American Pharmacists Association; Washington D.C., USA: 2012. pp. 645–660. [Google Scholar]
- 28.Narayanan DL, Saladi RN, Fox JL. Ultraviolet radiation and skin cancer. Int J Dermatol. 2010;49:978–986. doi: 10.1111/j.1365-4632.2010.04474.x. [DOI] [PubMed] [Google Scholar]
- 29.Bendesky A, Michel A, Sordo M, Calderon-Aranda ES, Acosta-Saavedra LC, Salazar AM, Podoswa N, Ostrosky-Wegman P. DNA damage, oxidative mutagen sensitivity, and repair of oxidative DNA damage in nonmelanoma skin cancer patients. Environ Mol Mutagen. 2006;47:509–517. doi: 10.1002/em.20220. [DOI] [PubMed] [Google Scholar]
- 30.Kanjilal S, Strom SS, Clayman GL, Weber RS, el-Naggar AK, Kapur V, Cummings KK, Hill LA, Spitz MR, Kripke ML, et al. p53 mutations in nonmelanoma skin cancer of the head and neck: molecular evidence for field cancerization. Cancer Res. 1995;55:3604–3609. [PubMed] [Google Scholar]
- 31.Ro YS. Oncogene interaction in basal cell carcinomas of human skin. J Korean Med Sci. 1995;10:85–92. doi: 10.3346/jkms.1995.10.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dréau D, Culberson C, Wyatt S, Holder WD. Human Papilloma virus in Melanoma biopsy specimens and its relation to Melanoma progression. Ann Surg. 2000;231:664–671. doi: 10.1097/00000658-200005000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams AC. Topical 5-FU--a new approach to skin cancer. Ann Surg. 1971;173:864–871. doi: 10.1097/00000658-197106010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Urosevic M, Dummer R. Role of imiquimod in skin cancer treatment. Am J Clin Dermatol. 2004;5:453–458. doi: 10.2165/00128071-200405060-00010. [DOI] [PubMed] [Google Scholar]
- 35.Longley DB, Harkin DP, Johnston PG. 5-fluorouracil: mechanisms of action and clinical strategies. Nat Rev Cancer. 2003;3:330–338. doi: 10.1038/nrc1074. [DOI] [PubMed] [Google Scholar]
- 36.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in Cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent Smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 37.Guterres SS, Alves MP, Pohlmann AR. Polymeric nanoparticles, nanospheres and nanocapsules, for cutaneous applications. Drug Target Insights. 2007;2:147–157. [PMC free article] [PubMed] [Google Scholar]
- 38.Lee RW, Shenoy DB, Sheel R. Chapter 2: Micellar nanoparticles: applications for topical and passive transdermal drug delivery. In: Kulkarni VS, editor. Handbook of non-invasive drug delivery systems. Elsevier Inc; Burlington, MA, USA: 2010. pp. 37–58.pp. 37–58. [Google Scholar]
- 39.Vandervoort J, Ludwig A. Preparation and evaluation of drug-loaded gelatin nanoparticles for topical ophthalmic use. Eur J Pharm Biopharm. 2004;57:251–261. doi: 10.1016/S0939-6411(03)00187-5. [DOI] [PubMed] [Google Scholar]
- 40.Patel M, Jain SK, Yadav AK, Gogna D, Agrawal GP. Preparation and characterization of oxybenzone-loaded gelatin microspheres for enhancement of sunscreening efficacy. Drug Deliv. 2006;13:323–330. doi: 10.1080/10717540500398175. [DOI] [PubMed] [Google Scholar]
- 41.Kim DG, Jeong YI, Choi C, Roh SH, Kang SK, Jang MK, Nah JW. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int J Pharm. 2006;319:130–138. doi: 10.1016/j.ijpharm.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 42.Ozbas-Turan S, Akbuga J, Sezer AD. Topical application of antisense oligonucleotide-loaded chitosan nanoparticles to rats. Oligonucleotides. 2010;20:147–153. doi: 10.1089/oli.2009.0222. [DOI] [PubMed] [Google Scholar]
- 43.Hasanovic A, Zehl M, Reznicek G, Valenta C. Chitosan-tripolyphosphate nanoparticles as a possible skin drug delivery system for aciclovir with enhanced stability. J Pharm Pharmacol. 2009;61:1609–1616. doi: 10.1211/jpp/61.12.0004. [DOI] [PubMed] [Google Scholar]
- 44.Ozbas-Turan S, Akbuga J. Plasmid DNA-loaded chitosan/TPP nanoparticles for topical gene delivery. Drug Deliv. 2011;18:215–222. doi: 10.3109/10717544.2010.544688. [DOI] [PubMed] [Google Scholar]
- 45.Senyigit T, Sonvico F, Barbieri S, Ozer O, Santi P, Colombo P. Lecithin/chitosan nanoparticles of clobetasol-17-propionate capable of accumulation in pig skin. J Control Release. 2010;142:368–373. doi: 10.1016/j.jconrel.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 46.Tan Q, Liu W, Guo C, Zhai G. Preparation and evaluation of quercetin-loaded lecithin-chitosan nanoparticles for topical delivery. Int J Nanomedicine. 2011;6:1621–1630. doi: 10.2147/IJN.S22411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shahnaz G, Vetter A, Barthelmes J, Rahmat D, Laffleur F, Iqbal J, Perera G, Schlocker W, Dunnhaput S, Augustijns P, et al. Thiolated chitosan nanoparticles for the nasal administration of leuprolide: bioavailability and pharmacokinetic characterization. Int J Pharm. 2012;428:164–170. doi: 10.1016/j.ijpharm.2012.02.044. [DOI] [PubMed] [Google Scholar]
- 48.de la Fuente M, Seijo B, Alonso MJ. Bioadhesive hyaluronan-chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008;15:668–676. doi: 10.1038/gt.2008.16. [DOI] [PubMed] [Google Scholar]
- 49.Chuah LH, Billa N, Roberts CJ, Burley JC, Manickam S. Curcumin-containing chitosan nanoparticles as a potential mucoadhesive delivery system to the colon. Pharm Dev Technol. doi: 10.3109/10837450.2011.640688. in press. [DOI] [PubMed] [Google Scholar]
- 50.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–347. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 51.Tsujimoto H, Hara K, Tsukada Y, Huang CC, Kawashima Y, Arakaki M, Okayasu H, Mimura H, Miwa N. Evaluation of the permeability of hairgrowing ingredient encapsulated PLGA nanospheres to hair follicles and their hairgrowing effects. Bioorg Med Chem Lett. 2007;17:4771–4777. doi: 10.1016/j.bmcl.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 52.Tomoda K, Terashima H, Suzuki K, Inagi T, Terada H, Makino K. Enhanced transdermal delivery of indomethacin-loaded PLGA nanoparticles by iontophoresis. Colloids Surf B Biointerfaces. 2011;88:706–710. doi: 10.1016/j.colsurfb.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 53.Shah PP, Desai PR, Patel AR, Singh MS. Skin permeating nanogel for the cutaneous co-delivery of two anti-inflammatory drugs. Biomaterials. 2012;33:1607–1617. doi: 10.1016/j.biomaterials.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alvarez-Roman R, Barre G, Guy RH, Fessi H. Biodegradable polymer nanocapsules containing a sunscreen agent: preparation and photoprotection. Eur J Pharm Biopharm. 2001;52:191–195. doi: 10.1016/s0939-6411(01)00188-6. [DOI] [PubMed] [Google Scholar]
- 55.Shim J, Seok Kang H, Park WS, Han SH, Kim J, Chang IS. Transdermal delivery of mixnoxidil with block copolymer nanoparticles. J Control Release. 2004;97:477–484. doi: 10.1016/j.jconrel.2004.03.028. [DOI] [PubMed] [Google Scholar]
- 56.Wu X, Price GJ, Guy RH. Disposition of nanoparticles and an associated lipophilic permeant following topical application to the skin. Mol Pharm. 2009;6:1441–1448. doi: 10.1021/mp9001188. [DOI] [PubMed] [Google Scholar]
- 57.Turos E, Shim JY, Wang Y, Greenhalgh K, Suresh Kumar Reddy GS, Dickey S, Lim DV. Antibiotic-conjugated polyacrylate nanoparticles: New opportunities for development of anti-MRSA agents. Bioorg Med Chem Lett. 2007;17:53–56. doi: 10.1016/j.bmcl.2006.09.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greenhalgh K, Turos E. In vivo studies of polyacrylate nanoparticle emulsions for topical and systemic applications. Nanomedicine. 2009;5:46–54. doi: 10.1016/j.nano.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 59.Sheihet L, Piotrowska K, Dubin RA, Kohn J, Devore D. Effect of tyrosine-derived triblock copolymer compositions on nanosphere self-assembly and drug delivery. Biomacromolecules. 2007;8:998–1003. doi: 10.1021/bm060860t. [DOI] [PubMed] [Google Scholar]
- 60.Kilfoyle BE, Sheihet L, Zhang Z, Laohoo M, Kohn J, Michniak-Kohn BB. Development of paclitaxel-TyroSpheres for topical skin treatment. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.06.021. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sheihet L, Garbuzenko OB, Bushman J, Gounder MK, Minko T, Kohn J. Paclitaxel in tyrosine-derived nanospheres as a potential anti-cancer agent: In vivo evaluation of toxicity and efficacy in comparison with paclitaxel in Cremophor. Eur J Pharm Sci. 2012;45:320–329. doi: 10.1016/j.ejps.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batheja P, Sheihet L, Kohn J, Singer AJ, Michniak-Kohn B. Topical drug delivery by a polymeric nanosphere gel: Formulation optimization and in vitro and in vivo skin distribution studies. J Control Release. 2011;149:159–167. doi: 10.1016/j.jconrel.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Sheihet L, Chandra P, Batheja P, Devore D, Kohn J, Michniak B. Tyrosine-derived nanospheres for enhanced topical skin penetration. Int J Pharm. 2008;350:312–319. doi: 10.1016/j.ijpharm.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 64.Tang BC, Dawson M, Lai SK, Wang YY, Suk JS, Yang M, Zeitlin P, Boyle MP, Fu J, Hanes J. Biodegradable polymer nanoparticles that rapidly penetrate the human mucus barrier. Proc Natl Acad Sci. 2009;106:19268–19273. doi: 10.1073/pnas.0905998106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi HS, Ashitate Y, Lee JH, Kim SH, Matsui A, Insin N, Bawendi MG, Semmler-Behnke M, Frangioni JV, Tsuda A. Rapid translocation of nanoparticles from the lung airspaces to the body. Nat Biotechnol. 2010;28:1300–1303. doi: 10.1038/nbt.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zheng D, Giljohann DA, Chen DL, Massich MD, Wang XQ, Iordanov H, Mirkin CA, Paller AS. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc Natl Acad Sci. 2012;109:11975–11980. doi: 10.1073/pnas.1118425109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang W, Gao J, Zhu Q, Zhang M, Ding X, Wang X, Hou X, Fan W, Ding B, Wu X, et al. Penetration and distribution of PLGA nanoparticles in the human skin treated with microneedles. Int J Pharm. 2010;402:205–212. doi: 10.1016/j.ijpharm.2010.09.037. [DOI] [PubMed] [Google Scholar]
- 68.Coulman SA, Anstey A, Gateley C, Morrissey A, McLoughlin P, Allender C, Birchall JC. Microneedle mediated delivery of nanoparticles into human skin. Int J Pharm. 2009;366:190–200. doi: 10.1016/j.ijpharm.2008.08.040. [DOI] [PubMed] [Google Scholar]
- 69.del Pilar Martin M, Weldon WC, Zarnitsyn VG, Koutsonanos DG, Akbari H, Skountzou I, Jacob J, Prausnitz MR, Compans RW. Local response to microneedle-based influenza immunization in the skin. MBio. 2012:3. doi: 10.1128/mBio.00012-12. [DOI] [PMC free article] [PubMed] [Google Scholar]