Abstract
Advances in nanotechnology have demonstrated potential application of nanoparticles for effective and targeted drug delivery. Here, we investigated the antimicrobial and immunological properties and the feasibility of using nanoparticles to deliver antimicrobial agents to treat a cutaneous pathogen. Nanoparticles synthesized with chitosan and alginate demonstrated a direct antimicrobial activity in vitro against Propionibacterium acnes, the bacterium linked to the pathogenesis of acne. By electron microscopy imaging, chitosan-alginate nanoparticles were found to induce disruption of the P. acnes cell membrane, providing a mechanism for the bactericidal effect. The chitosan-alginate nanoparticles also exhibited anti-inflammatory properties as they inhibited P. acnes induced inflammatory cytokine production in human monocytes and keratinocytes. Furthermore, benzoyl peroxide, a commonly used anti-acne drug, was effectively encapsulated in the chitosan-alginate nanoparticles and demonstrated superior antimicrobial activity against P. acnes compared to benzoyl peroxide alone while demonstrating less toxicity to eukaryotic cells. Together, these data suggest the potential utility of topical delivery of chitosan-alginate nanoparticle encapsulated drug therapy for the treatment of dermatologic conditions with infectious and inflammatory components.
Keywords: Acne, Nanoparticle, Chitosan, Antimicrobial, Inflammation, Skin infection
Introduction
Nanoparticles (NPs) are currently being investigated and used in many areas of medicine to allow for specific drug delivery, lower dosing of active agents, combination therapy, minimizing side effects, and harnessing more potent drugs which cannot clinically be utilized by conventional drug delivery. In response to the growing threat of microbial drug resistance, nanotechnology has become a major area of interest due to its many unique characteristics. In particular, the physical and chemical properties of nanoparticles, including their high surface-tovolume ratio and small size, allows for the ability to surpass barriers and gain access to biological molecules and importantly, microorganisms (Blecher et al., 2011). This direct interaction with microbial cell membranes/walls and key proteins/enzymes can both inhibit pathogen growth and/or instigate cell death through mechanism different from our armament of antibiotics, to which resistance has developed. In addition, the size, shape, and chemical characteristics of nanoparticles may be manipulated in order to facilitate these molecular interactions, optimizing their ultimate action (Blecher et al., 2011; Kim et al., 2010).
It has become apparent that the development of nanotechnology for the treatment of infectious diseases, particularly skin disease, involves two major areas: The first utilizes materials that at the nanoscale have inherent antimicrobial properties (Hemmila et al., 2010), and the second incorporates known therapeutics into nano-vehicles in order to enhance delivery and improve efficacy (Cunha-Azevedo et al., 2011; Dominguez-Delgado et al., 2011; Kumar et al., 2011). The ideal approach to develop a topical therapy for microbial infection in skin would utilize a nanomaterial that has both inherent and potent antimicrobial properties and the ability to serve as a nano-vehicle would be ideal.
Chitosan is a natural polysaccharide biopolymer derived from chitin, the principal structural component of the crustacean exoskeleton. Depending on the degree of deacetylation, chitosan can have an extensive collection of C2 amino groups, having pKa values of ~ 6.5, which can become protonated in weakly acidic conditions. It is this polycationic character that confers chitosan’s antimicrobial properties, which favors interaction with negatively-charged microbial cell walls and cytoplasmic membranes. These interactions result in decreased osmotic stability, membrane disruption, and eventual leakage of intracellular elements (Banergee et al., 2010; Ma et al., 2008; Sanpui et al., 2008). In addition, chitosan may enter the nuclei of bacteria and fungi and inhibit mRNA and protein synthesis by binding to microbial DNA (Blecher et al., 2011; Ma et al., 2008; Qi et al., 2005). When nano-scaled, chitosan has a higher surface to volume ratio, translating into higher surface charge density, increased affinity to bacteria and fungi, and greater antimicrobial activity (Qi et al., 2005). Even more so, chitosan possesses some immunological functions including inhibition of pro-inflammatory cytokines, promotion of tissue granulation via fibroblast recruitment (Ueno et al., 2001), and production of type III collagen (Ueno et al., 1999). Together, these data suggest that chitosan can be utilized as an antimicrobial, anti-inflammatory and wound healing accelerant. However, while gaining a polycationic charge in a weakly acidic environment such as one supported by the epidermis, chitosan is weakly soluble in physiologic solvents, therefore limiting its clinical use to date (Chavez de Paz et al., 2011).
Given chitosan’s antimicrobial and immunological properties, we investigated whether chitosan-alginate NPs could be effectively generated, stored, and delivered in physiologic parameters ,demonstrate antimicrobial and anti-inflammatory activity against a clinically important cutaneous bacterium, P. acnes, and whether these NPs might be a useful approach to target acne therapy.
Results
Antimicrobial activity of chitosan-alginate nanoparticles
The primary goals of acne treatment are resolution of inflammatory lesions, prevention of future comedo formation and prevention of persistent inflammation (Lee, 2009). Therefore, for acne therapy, agents with both antimicrobial and anti-inflammatory properties are highly effective. As chitosan has demonstrated antimicrobial activity against various pathogens including S. aureus (Jia et al., 2001; Qi et al., 2005) and E. coli (Chen et al., 2002; Sanpui et al., 2008), chitosan-alginate NPs were evaluated for their antimicrobial activity against a common cutaneous bacterium, P. acnes.
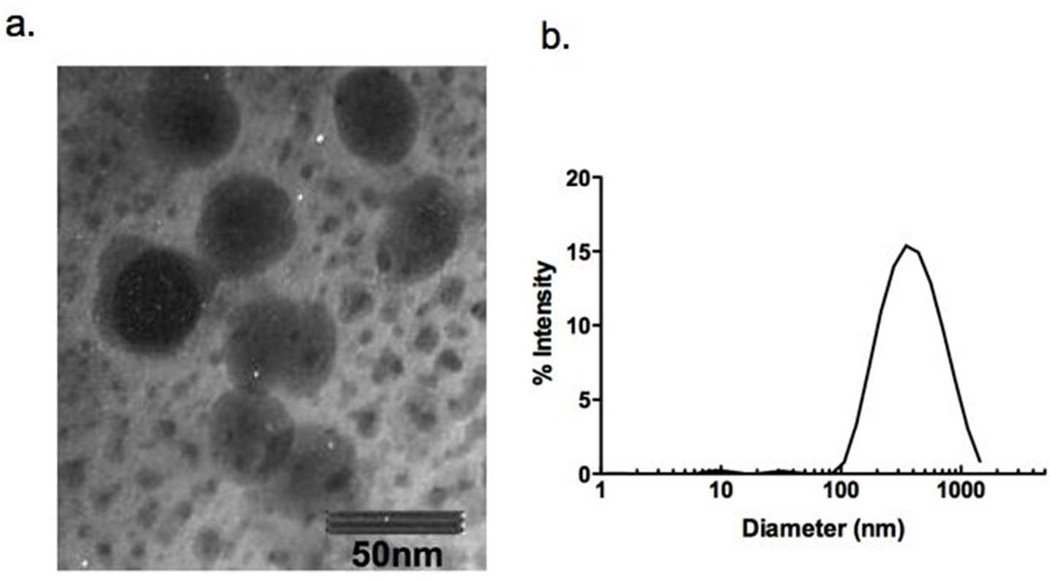
In order to synthesize nontoxic, biodegradable, and biocompatible NPs that can be used for the treatment of cutaneous infections, we chose a derivative of the biostructural crustacean shell polymer chitin, chitosan, along with the well known thermally stable gelling agent, alginate (Sayag et al., 1996). The synthesis of the chitosan-alginate nanoparticles was demonstrated to be successful using transmission electron microscopy (TEM) visualization that showed the majority of individual NPs were less than 50 nm diameter (Figure 1a). Using dynamic light scattering, particles were found to have an average hydrodynamic diameter of 341.6 ± 11.1nm (Figure 1b) with a polydispersity of 23.7. This is not surprising as measurements are taken in water and chitosan nanoparticles have been shown to swell rapidly in this setting (Gelfuso et al., 2011; Jayakumar et al., 2011)
Figure 1. Synthesis of chitosan-alginate nanoparticles.
Nanoparticles were synthesized using chitosan and alginate. The structure was analyzed using (a) TEM (bar = 50 nm) and analytical sizing perform using DLS.
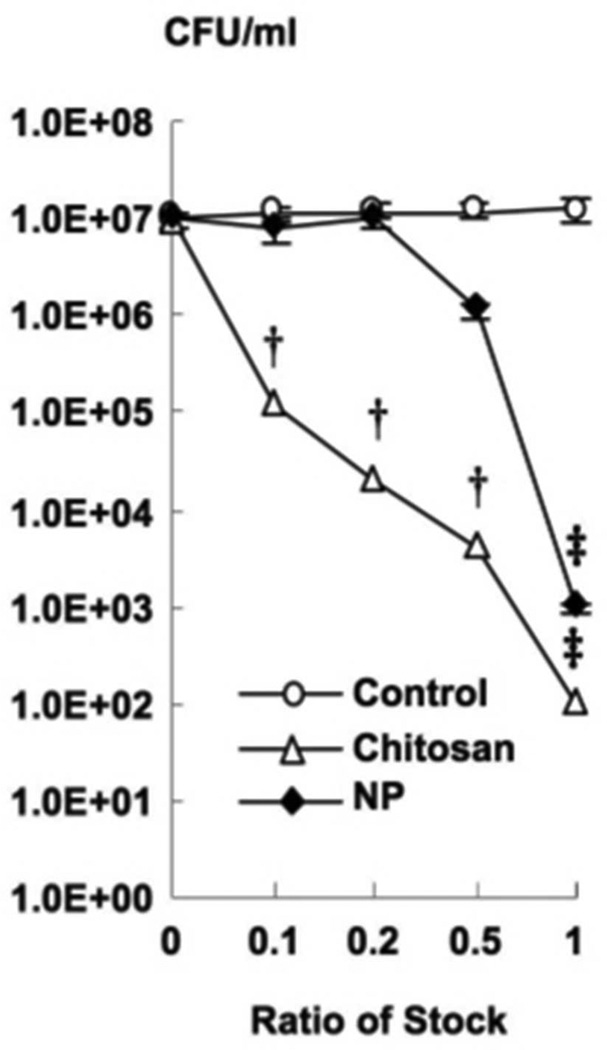
To measure the antimicrobial activity against P. acnes, we incubated the bacteria with varying doses of chitosan-alginate NPs, as well as with chitosan and alginate control alone for four hours. Subsequently, the bacteria were plated and the viability was determined by colony forming unit (CFU) assay. The chitosan-alginate NPs were effective at inhibiting the growth of P. acnes in a dose-dependent manner and by approximately four logs at the most concentrated dose of NPs tested (Figure 2). We also demonstrated that chitosan alone could inhibit the growth of P. acnes by a 5.0 log decrease but alginate had no effect on the growth of P. acnes. These data demonstrate that chitosan-alginate NPs have antimicrobial activity against P. acnes, and that the antimicrobial activity of the NPs was due to the chitosan and not the alginate.
Figure 2. Antimicrobial effects of chitosan-alginate nanoparticles.
Various concentrations (1%, .5%, .2%, .1%) of chitosan-alginate NPs were incubated with P. acnes for 4 h and tested for antimicrobial activity using CFU assay (mean CFU/ml) and compared to chitosan and alginate as controls. These data are derived from eight independent experiments ± SEM (p-values: † ≤ 0.005, ‡ ≤ 0.001).
Anti-inflammatory effects of the chitosan-alginate nanoparticles
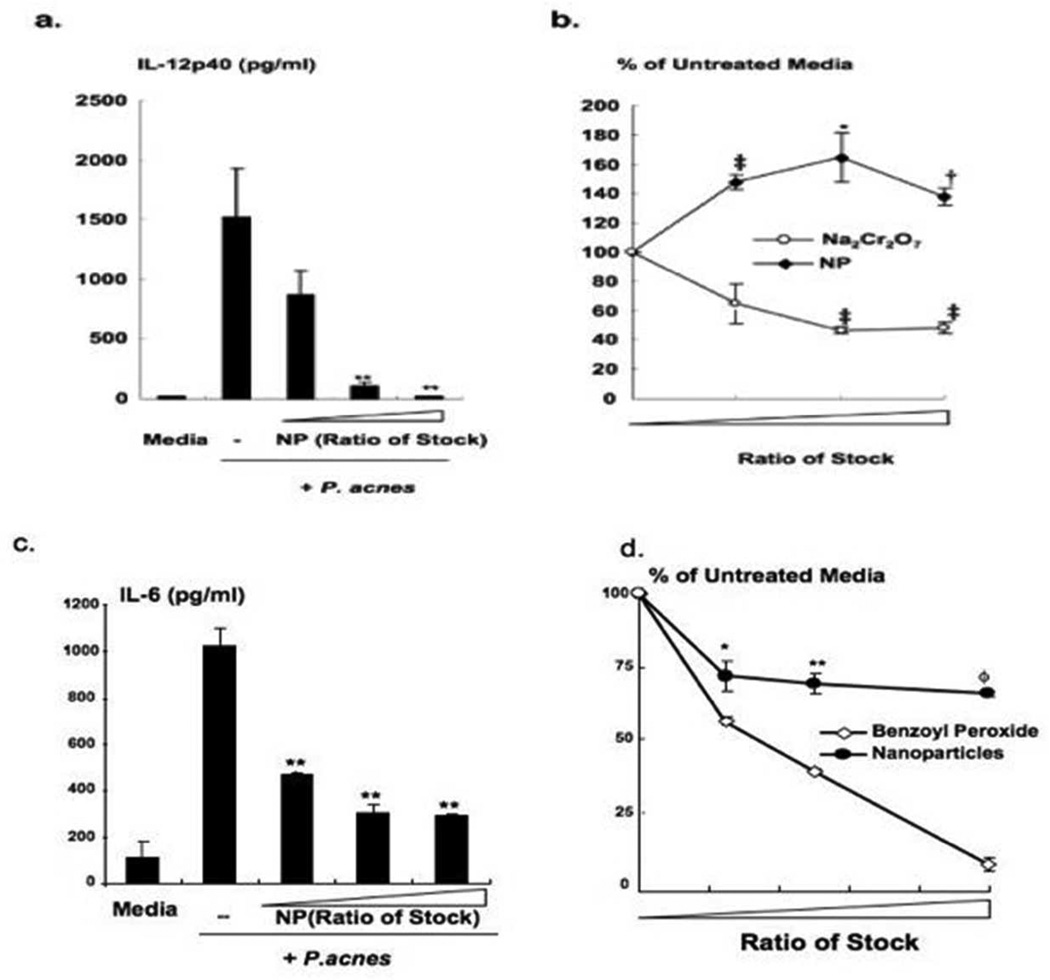
Since chitosan has been shown to have various anti-inflammatory properties (Kim et al., 2004)), we specifically investigated whether the inflammatory cytokines and chemokines induced by P. acnes could be modulated in the presence of chitosan-alginate NPs. Human monocytes were isolated from peripheral blood and stimulated cells with P. acnes in the presence of various concentrations of chitosan-alginate NPs. As shown in Figure 3a, P. acnes induction of cytokine IL-12p40, previously shown to be involved in the inflammatory response in acne, was inhibited by the chitosan-alginate NPs in a dose-dependent manner, demonstrating almost complete reduction IL-12 protein at the highest concentration of chitosan-alginate NPs tested. Similarly, human keratinocytes HaCaT cells were cultured, stimulated with P. acnes in the presence of various concentrations of chitosan-alginate NPs. We found that the induction of IL-6 by P. acnes in keratinocytes were inhibited in the presence of chitosan-alginate NPs almost completely, even at a low dose concentration (Figure 3c). Chitosan-alginate NPs did not have a toxic effect on human monocytes as demonstrated in the MTT assay (Figure 3b) while sodium chromate, a positive control, had a significant cytotoxic effect on human monocytes. On the other hand, there was mild toxicity to HaCaT cells at higher concentration of NPs, however when compared to subclinical concentrations of benzoyl peroxide, this impact was insignificant (Figure 3d). Therefore, our data suggest that the chitosan-alginate NPs can inhibit P. acnes induced cytokine production in human monocytes and keratinocyte and this is not simply due to the release of cytokines at cell death.
Figure 3. Anti-inflammatory effect of chitosan-alginate nanoparticles.
Chitosan-alginate NPs at various concentrations (6.5, 25 and 50 percent of stock, respectively) were incubated with primary human monocytes or HaCaT cells which were subsequently stimulated with P. acnes. (a) IL-12p40 and (c) IL-6 levels were determined by ELISA expressed by mean cytokine concentration (pg/ml) and are representative of four independent experiments + SEM (p-values: ** ≤ 0.01). (b,d) Cells were collected and evaluated for cell viability using a MTT (b) and MTS (d) assay. Data is expressed by mean percentage as compared to untreated cells and is composite of four independent experiments ± SEM (b. pvalues: * ≤ 0.05, † ≤ 0.005, ‡ ≤ 0.001; d. p-values: * ≤ 0.05, ** ≤ 0.01, ≤ 0.0001).
Chitosan-alginate nanoparticles as a delivery vehicle for topical therapeutics
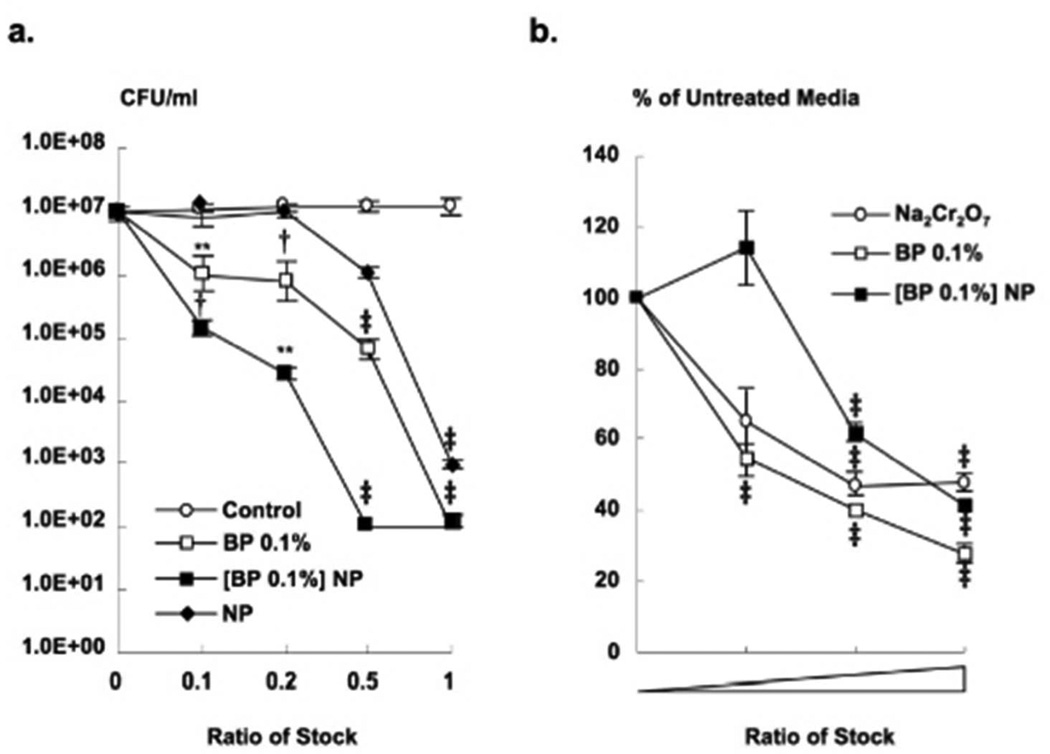
While chitosan alone shows antimicrobial activity against P. acnes, the use of chitosan-alginate NPs provides the benefit of incorporating multi-therapeutics. Benzoyl peroxide (BP) has both a mild keratolytic and potent bactericidal effect to which P. acnes has yet to demonstrate resistance (Dutil, 2010). Although an effective acne therapy, skin irritation is an expected but an unwanted adverse event, and is frequent at efficacious doses. Therefore, encapsulation in nanoparticles could be one approach to improving efficacy by reducing the side effects associated with topical application and ultimately improving patient compliance. In addition, benzoyl peroxide and chitosan together may provide superior antimicrobial effect against P. acnes when combined in this format, each providing a different mechanism of action. Benzoyl peroxide (0.1%) was encapsulated into chitosan-alginate NPs and incubated with P. acnes prior to plating and determination of bacterial viability. Encapsulation of BP into NPs demonstrated enhanced antimicrobial activity against P. acnes at several concentrations tested (Figure 4a). The encapsulated BP exhibited a synergistic antimicrobial activity against P. acnes in comparison to NPs and BP alone at several concentrations tested. Furthermore, encapsulated BP demonstrated less toxicity against eukaryotic cells than BP alone (Figure 4b), suggesting that the encapsulation of BP within the chitosan-alginate NPs may provide protection for eukaryotic cells.
Figure 4. Synergistic activity of benzoyl peroxide encapsulated chitosan-alginate nanoparticles.
(a) Benzoyl peroxide encapsulated into chitosan-alginate NPs ([BP 0.1%] NP) were incubated with P. acnes for 4 h and tested for antimicrobial activity using CFU assay (mean CFU/ml) and compared to chitosan-alginate NPs, BP 0.1%, and alginate as a negative control. These data are composite of eight distinct experiments ± SEM (p-values: ** ≤ 0.01, † ≤ 0.005, ‡ ≤ 0.001). (b) Primary human monocytes were stimulated with BP 0.1%, [BP 0.1%] NP, and Na2Cr2O7 as a positive control for toxicity, and evaluated for cell viability using MTT assay. Data is expressed by mean percentage as compared to untreated cells and is composite of three independent experiments ± SEM (p-value: ‡ ≤ 0.001).
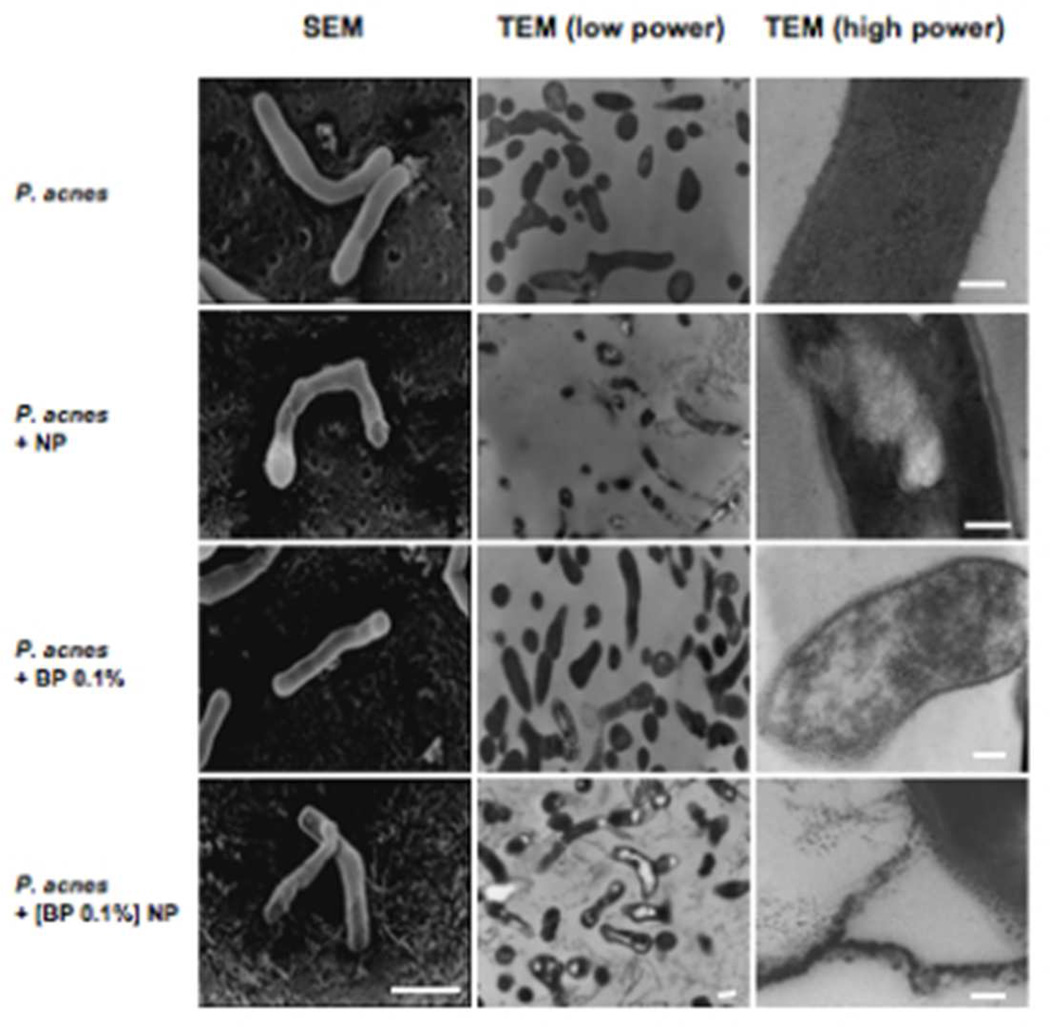
Chitosan-alginate nanoparticles and nanoparticle encapsulated benzoyl peroxide induce cell wall disruption of P. acnes
To determine the mechanism by which the chitosan-alginate NP induces the killing of P. acnes, bacteria that were treated with chitosan-alginate NPs were examined using both scanning eletron microscopy (SEM) and transmission electron microscopy (TEM). The SEM photographs of untreated P. acnes illustrated the bacterium's normal structure demonstrating the smooth and intact cell wall (Figure 5). A higher magnification TEM image revealed the normal cell wall structure of P. acnes with well-demarcated outer and inner dark lipophilic layers and a lighter hydrophilic peptidoglycan layer in the center. When the bacteria were treated with chitosan-alginate NPs, the P. acnes’ cell wall appeared light, suggesting loss of lipids. Higher magnification TEM imaging demonstrated that the cell membranes lost their lipophilic “sandwich-like” normal appearance. In addition, the low TEM and SEM imaging revealed damage to the cytoplasm.
Figure 5. Effects of chitosan-alginate NP, benzoyl peroxide and NP encapsulated benzoyl peroxide on the cell structure of P. acnes.
SEM (bar = 1 µm), low power TEM (bar = 1 µm) and high power TEM (bars = 100 nm) demonstrated the effect of chitosan-alginate NP, benzoyl peroxide alone (BP 0.1%) and NP encapsulated BP ([BP 0.1%] NP) on P. acnes after 4 h of incubation with the bacteria. EM studies represent data from at least three different experiments.
The BP treated bacteria also experienced some damage to the cell wall and membrane. There was loss of the normal cell wall structure and clustering of the membrane’s lipophilic components into droplets with loss of normal cytoplasmic features. However, the addition of NP encapsulated BP to P. acnes resulted in greater perturbation of the cell as compared to native BP (0.1%). The TEM and SEM images revealed severe disruption and destruction and “peeling” of the cell wall. Many cells appeared to have lost their cytoplasmic contents. The lipophilic portion of the membrane showed alteration of the structure and formation of relatively larger lipophilic globules as well as a wider, edematous space within the cell wall. These images suggest that the cell damage is due to an osmotic disturbance.
Discussion
In this paper we synthesized chitosan-alginate NPs and demonstrated that they are therapeutically multi-faceted. Chitosan-alginate NPs demonstrated potent antimicrobial activity against P. acnes. Chitosan-alginate NPs were shown to induce bacterial cell wall membrane disruption as demonstrated by electron microscopy, suggesting a mechanism for NP antimicrobial activity. The chitosan-alginate NPs also exhibited anti-inflammatory properties as they inhibited P. acnes induced inflammatory cytokine IL-12 production in human monocytes and IL-6 production in human keratinocytes. While chitosan alone exhibits potent antimicrobial activity against P. acnes, it’s therapeutic use can be challenging due to the solubility limitations of native chitosan. The chitosanalginate NP can therefore overcome this limitation yet still provide potent antimicrobial activity and furthermore can be utilized as a delivery system to incorporate additional therapeutics. Thus, the NP encapsulation of benzoyl peroxide, a common yet clinically irritating acne therapeutic, resulted in increased antimicrobial activity in comparison to benzoyl peroxide alone, while at the same time reducing toxicity to eukaryotic cells. Our data suggest that the chitosan-alginate NPs may be useful for the treatment of dermatologic conditions with infectious and inflammatory components such as acne vulgaris.
The treatment of cutaneous infections has been complicated by the emergence of microbial species that are resistant to standard antibiotic regimens(Spellberg, 2011; Spellberg et al., 2011). This evolving medical challenge is of particular significance in the treatment of one of the most common cutaneous skin disease, acne vulgaris. The gram-positive bacteria P. acnes and to a lesser extent P. granulosum and P. parvum are present and contribute to acne lesions (Zaenglein and Thiboutot, 2003). They produce enzymes and lipases that promote comedo rupture, comedogenic and proinflammatory factors, and glycocalyx polymers, which acts as adhesive that causes further aggregation of keratinocytes (Bellew et al., 2011). In addition, P. acnes activates immune cells to produce inflammatory mediators. Therefore, targeting both P. acnes and its associated inflammation is key to optimal therapy. In the setting of increasing resistance to standard antimicrobial therapy, innovative antimicrobial agents and approaches are needed (Kinney et al., 2010). The nanoparticle platform described was formed by combining the biostructural polymer chitosan with sodium alginate. Alginate is a family of polysaccharides composed of α-l-guluronic acid (G) and β-d-mannuronic acid (M) residues, arranged in homopolymeric blocks of each type (MM, GG) and heteropolymeric blocks (MG). Alginates form strong complexes with polycationic material, which stabilize this gel like material and reduce its porosity. Among those polycations, chitosan has received considerable attention due to its cationic characteristics as chitosan has the ability to gel on contact with counter anions (Coppi and Iannuccelli, 2009; Coppi et al., 2001). Chitosan’s amino groups are able to interact with an anionic polymer that has carboxylic groups, such as alginate by ionic binding. Using electron microscopy, we demonstrated that nanoparticles measuring approximately 50 nm in diameter were formed.
Interestingly, when suspended in water, the particles swell to almost seven times their initial size, similarly witnessed by previous investigators (Gelfuso et al., 2011; Luo et al., 2011; Murugeshu et al., 2011). As chitosan is a hydrophilic polymer that presents amine groups in its chemical structure, it can form hydrogen bonds with water. Therefore, in the presence of water, chitosan nanoparticles can undergo swelling, forming a gel layer around the particles due to chitosan hydration. This swelling increases the diameter of these particles likely influencing the mechanism of drug release. For example, the benzoyl peroxide can diffuse through the swollen particle. Therefore, consideration must be given when translating this technology to the vehicle in which these nanoparticles are suspended in order to effectively control the rate of swelling and therefore release of therapeutics (Martinac et al., 2005).
Although the exact mechanism of how chitosan-alginate NPs kill microbes is unclear, we demonstrated through transmission and scanning electron microscopy osmotic disruption of the P. acnes cell membrane. Chitosan by itself is known to strongly adhere to negatively charged surfaces due to its high charge density at pH < 6.5. The interactions between the polycationic chitosan and the electronegative charges on the cell surfaces are thought to trigger perturbations in membrane integrity, leading to alterations in cell permeability (Chen et al., 1998). This interaction can lead to the leakage of intracellular electrolytes and proteinaceous components. Many endogenous cationic peptides, including granulysin, human beta-defensins, and cathelicidins, are thought to interact with the negatively charged microbe cell wall to induce killing. The advantage of using these molecules that target conserved membrane structures versus enzyme antagonists is the low possibility of microbe resistance, given the low probability of a single mutation leading to altered bacterial membrane structure. However, it has been shown that in certain environment, S. aureus can express surface protein IsdA, to become less hydrophobic and thus resistant to skin innate defenses (Clarke et al., 2007), underscoring the growing need for novel approaches to antimicrobial therapies.
Work by other investigators has shown that chitosan has antimicrobial activities against various pathogens including bacteria, fungi and viruses, suggesting a broad antimicrobial spectrum (Alburquenque et al., 2010; El-Sharif and Hussain, 2011; Jayakumar et al., 2011; Potara et al., 2011; Tayel et al., 2010). Despite distinctive differences between gram-negative and gram-positive bacteria, an antibacterial agent’s first interaction with its target will be at the cell surface, compromising either the cell wall or outer membrane. For gram-positive bacteria, lipoteichoic acid could provide a molecular linkage for chitosan at the cell surface, allowing it to disturb membrane functions (Raafat et al., 2008). Lipopolysacchride and proteins in the gram-negative bacteria outer membrane are held together by electrostatic interactions with divalent cations that are required for its integrity and stabilization. Chitosan’s polycations may compete with divalent metals for binding with polyanions as well as has the potential to chelate important biologic metals (Kong et al., 2008). Replacement of Mg2+ and Ca2+ ions present in the cell wall likely disrupt the integrity of the cell wall or influence the activity of degradative enzymes. These data underscore the additional benefit of utilizing chitosan-derived NPs for drug delivery.
The chitosan-alginate NPs were also found to have immunomodulatory activity, inhibiting the production of inflammatory cytokine IL-12p40 and IL-6 by P. acnes stimulated monocytes and keratinocytes, respectively. P. acnes is a potent stimulator of host immune responses and because inflammation is a key pathogenic element of acne, it is tempting to speculate that the anti-inflammatory activity of NPs is the result of inhibition of the TLR2 pathway, given the role of TLR2 activation in P. acnes induced cytokine and other pro-inflammatory mediators (Jugeau et al., 2005; Kim et al., 2002; Nagy et al., 2005). Past studies have provided insight into chitosan’s ability to inhibit inflammatory cytokines and chemokines. It was shown that chitosan can inhibit NF-κβ activation in human mast cell lines through downmodulation of CA2+ induced mast cell activation (Seo et a., 2003) and suppression of mRNA expression of membrane metalloproteinases 1 and 3 (Kim et al., 2004), suggesting that chitosan interferes with the Toll-like receptor signaling pathways. Additionally, it has been shown that IL-6 can play a role as a regulator of extracellular matrix deposition via MMPs and is involved in the immune response to P. acnes; as a consequence, it may be an important determinant of acne and therefore downregulation could be an important component of therapy(Pajulo et al., 1999). The anti-inflammatory effects of chitosan-alginate NPs suggest a possible role as immune modulating agents that could be translated to clinical use in infectious and other diseases with unwanted inflammatory reactions.
Due to chitosan’s polycationic nature as well as high affinity to metal, it has been employed as a carrier system and platform stabilizer for a variety of nanoparticle systems including metallic nanoparticles (Chadwick et al., 2010), nitric oxide-releasing nanoparticles (Friedman et al., 2008), and drug-containing nanoparticles which allows for targeted delivery of various medications (Qi et al., 2005). By combining this property with chitosan’s inherent antimicrobial action, using chitosan as a delivery platform for antimicrobial agents could potentially augment the antimicrobial properties of these agents. For example, the antimicrobial efficacy of silver-loaded membranes is enhanced with increasing chitosan contents of up to 70%, resulting in larger zones of inhibition against both S. aureus and E. coli (Ma et al., 2008). Here, we sought to encapsulate and enhance the efficacy of benzoyl peroxide, a common therapy used for acne, in the chitosan-alginate NPs since bacterial resistance to benzoyl peroxide has not been reported to date. We found that nano-encapsulation of benzoyl peroxide offers improved antimicrobial efficacy at lower and consequently less irritating and toxic concentrations, suggesting that the chitosan-alginate NP encapsulation of benzoyl peroxide may offer a highly effective therapeutic molecule with less irritation to the skin. In fact, the greatest damage to P. acnes cell wall occurred with NP encapsulated benzoyl peroxide. This is not surprising as benzoyl peroxide exerts its influence through oxidative stress and chitosan binds to the cellular membrane, altering permeability and structural stability.
Given the multifaceted impact of the NPs, the presented data inspire future in vivo studies to translate this technology to clinical practice. While future studies most certainly include in vivo safety testing such as clinical, histological, and immunohistochemical evaluation following topical application, the utility of studying efficacy in an animal model is not clear given the lack of a established animal models. While rhino mouse model is occasionally used in the literature, it only addresses hyperkeratotic effect of acne pathogenesis and is typically not used for new therapeutic development(Mirshahpanah and Maibach, 2007). Anti-inflammatory in vivo models with Propionibacterium acnes has been reported (Fan et al., 2012), however, this model is also limited in that it is an intradermal injection of P. acnes, measuring a local, granulomatous, inflammatory response rather than a true multifactorial acne model encompassing the pilosebaceous unit. Finally, and from a translational standpoint, the FDA does not require in vivo acne model for efficacy in the development of therapeutics for acne, rather in vivo safety data is required for pre-clinical safety data, which will be pursued as mentioned above.
The known benefits of nano-drug delivery, including size, stability, and encapsulation of a great range of therapeutics (Mu and Sprando, 2010; Nasir, 2010; Schroeter et al., 2010), combined with our data that chitosan-alginate NPs have both antimicrobial and anti-inflammatory properties suggest that chitosan-alginate NPs have the potential to serve as a topical class of antimicrobials for the treatment of acne vulgaris as well as other cutaneous infections and inflammatory conditions. Even more importantly, the combination of antimicrobial activities between chitosan and benzoyl peroxide limits the risk of the emergence of resistant species. Therefore, our findings suggest that the chitosan-alginate NP encapsulation, which offers enhanced antimicrobial and anti-inflammatory properties as well as the promise of controlled release, provides previously undescribed therapeutic opportunities including delivery of multidrug regimens to combat resistant microbes and inflammatory disease states.
Materials and Methods
Production of Nanoparticles
70.0 mg of alginic acid sodium salt (Sigma Aldrich, Inc., St. Louis, MO) was added to 100 ml of dH2O (0.7 mg/ml) and magnetically stirred until dissolved. 15 mg of calcium chloride (CaCl2, Acros Organics, Geel, Belgium) was then stirred into 20 ml of dH2O (0.75mg/ml). The 20 ml CaCl2 solution was added drop wise into the alginic acid solution via a 27.5 gauge needle while the alginic acid solution was concurrently sonicated. Following administration of the CaCl2, sonication was maintained for 1 min. The new solution was stirred for 30 min. Following this, 7 mg of chitosan (> 85% deacetylated, Sigma Aldrich), dissolved in 20 ml hydrochloric acid (0.35mg/ml, pH 1.4 – 2.0) (Fisher Scientific, Pittsburgh, PA) was added drop wise via a 27.5 gauge needle into the alginic acid-CaCl2 solution. The resulting mixture was stirred magnetically for 1 h and then allowed to settle over a 24 h time period.
After 24 h, the formed suspension was centrifuged at 4,000 rpm for 1 h. The supernatant was discarded with subsequent addition of 50 ml of dH2O. The suspension was again centrifuged at 4,000 rpm for 1 h and the past step was repeated twice. Due to aggregation of the particles, the suspension was extensively vortexed for 2 min then sonicated for 3.5 h.
Encapsulation of benzoyl peroxide
0.1 g of benzoyl peroxide (Sigma Aldrich) was dissolved into 100 ml of 20% ethanol (Sigma Aldrich) and spun for 1 h. 70.0 mg of alginic acid was added to the mixture and spun to dissolve. 1.5 mg of CaCl2 was stirred magnetically into 20 ml of ethanol (0.75 mg/ml). The 20 ml of CaCl2 solution was added drop wise into the alginic acid solution via a 27.5 gauge needle while the alginic acid solution was concurrently being sonicated. Following administration of the CaCl2, sonication was maintained for 1 min. The new solution was stirred for 30 min. Following this, 7 mg of chitosan dissolved in 20 ml hydrochloric acid (0.35 mg/ml, pH 1.4 – 2.0) was added drop wise via a 27.5 gauge needle into the alginic acid-CaCl2 solution. The resulting mixture was stirred for 1 h and then allowed to settle over a 24 h. After 24 h, the now formed suspension was centrifuged at 4,000 rpm for 1 h. The supernatant was discarded and 50 ml of dH2O was added. The suspension was again centrifuged at 4,000 rpm × 1 h and the past step was repeated twice. Due to aggregation of the particles, the suspension was vortexed for 2 min then sonicated for 3.5 h.
CFU assay
P. acnes strain ATCC 6919 (American Type Cell Culture, Manassas, Virginia) was grown under anaerobic conditions in Reinforced Clostridial Medium (Oxoid, Basingstroke, England) for 3 days and collected in mid-log phase. The bacteria were washed three times, suspended in 10 mM sodium phosphate, pH 7.2, supplemented with 0.03% trypticase soy broth (TSB, Becton-Dickinson, Cockeysville, Maryland), and enumerated by applying a conversion factor of 7.5 × 107 bacteria per ml=1 OD unit at 600 nm (Beckman DU® 640B Spectrophotometer, Beckman Coulter, Inc., Fullerton, CA). Various concentrations of alginate, chitosan, and chitosan-alginate NPs (1%, .5%, .2% .1%) were incubated with 3.75 × 105 bacteria in a final volume of 30 Wl at 37°C for 4 h. After incubation, 10-fold dilutions were prepared and plated onto Brucella Agar supplemented with 5% sheep blood, hemin, and vitamin K (Remel, Lenexa, KS). Plates were incubated for 4 days at 37°C under anaerobic conditions. Individual colonies were counted and the number of CFU was tabulated.
Electron Microscopy
P. acnes at a concentration of 2.08×107 bacteria per ml was suspended in 10 mM sodium phosphate buffer, pH 7.2, supplemented with 0.03% TSB and incubated with chitosan-alginate NPs, BP 0.1%, [BP 0.1%] NP, and alginate as a control. Samples were then fixed for 30 min with 2% gluteraldehyde in 1x PBS, pH 7.35 at room temperature, then washed three times and suspended in 1 ml of 1x PBS. Scanning EM samples were filtered onto a micropore filter. The filters with the sample on top were dehydrated in graded ethanol: 50%, 75%, 95%, and 100% for 15 min in each, followed by similar transfers in hexa-methyl-disilazane reagent: 50%, 75%, 95% for 30 min each followed by 100% hexa-methyl-disilazane reagent overnight. The scanning EM samples were gold coated then viewed and photographed on a Cambridge Scanning electron microscope. Transmission EM samples were dehydrated in graded ethanol as above, embedded in Epon, and sectioned on Sorvall MT6000 (RMC, Tucson Arizona). Thin sections (75 µm) were stained with uranyl acetate, viewed and photographed on Jeol XC100 at 80-kV.
Dynamic Light Scattering
A dilute suspension of chitosan alginate particles (0.1mg/ml) was sonicated and then the size of the particles were measured using dynamic light scattering (DLS). DLS measurement was performed using a DynaPro NanoStar (Wyatt Technology, Santa Barbara, CA) using an acquisition length of 5 seconds and a total of 40 acquisition attempts. An average particle hydrodynamic diameter (the diameter of the particle along with the shell of closely associated water molecules) and polydispersity was calculated from the results.
Primary human monocyte isolation and stimulation
Peripheral human blood was drawn from normal healthy volunteers into heparinized tubes according to a protocol approved by the Institutional Review Board at UCLA and gathered according to Declaration of Helsinki guidelines. Written consent was received prior to blood draw and use of material. PBMC were then isolated using Ficoll-Paque gradients (Amersham Biosciences, Piscataway, New Jersey) and plated (5 × 106 per well) in 24-well plates with RPMI media (Invitrogen, Grand Island, New York) containing 1% FCS (Omega Scientific, Tarzana, California) for 2 h at 37°C. Non-adherent cells were removed by washing three times with RPMI media. Adherent monocytes were cultured in RPMI containing 10% FCS and left untreated or incubated with alginate, chitosan, and chitosan-alginate NPs for 1 h before the addition of P. acnes sonicate (1µg/ml) for 24 h at 37°C. Supernatants were harvested 24 h later and assayed for IL-12p40 by ELISA (BD Pharmingen, San Diego, CA). Human keratinocyte cell line, HaCaT cells were cultured with keratinocyte medica containing 1% FCS (Omega Scientific, Tarzana, California). Cells were cultured in RPMI containing 10% FCS and left untreated or incubated with alginate, chitosan, and chitosan-alginate NPs for 1 h before the addition of P. acnes sonicate (1µg/ml) for 24 h at 37°C. Supernatants were harvested 24 h later and assayed for IL-6 by ELISA (BD Pharmingen, San Diego, CA).
MTT/MTS Toxicity Assay
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrasodium bromide tetrazolium (MTT, Sigma Aldrich) is a yellow water soluble compound processed by the mitochondria of live cells into a water insoluble purple formazan salt that can be measured at 570 nm. 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) assay reagent is used to measures dehydrogenase enzyme activity found in metabolically active cells. PBMC and HaCat cells were isolated as above and plated at 2x105 cells per well in a 96-well plate for 2 h. Non-adherent cells were removed by washing twice with RPMI. In addition, HaCaT keratinocytes were also plated for 2h. Chitosan-alginate NPs, BP 0.1%, and [BP 0.1%] NP were prepared in RPMI containing 10% FCS and 50 µl of each compound was added to the wells containing PBMC. Chitosan-alginate NPs and BP 0.1% were added to the wells containing HaCat. All compounds were evaluated in triplicates, including a positive control of sodium chromate (20–100 µM) and media as the negative control. The wells were incubated at 37°C overnight. Within 24 h, the wells were washed twice with 200 µl of sterile 1x PBS. For the MTT, 100 µl of RPMI containing 10% MTT (5 mg/ml) and 10% FCS were added to each well and incubated for 4 h. Without washing, 100 µl of 10% SDS were added to each well and incubated for 8 h. Absorbance was measured at 570 nm using a microplate reader. For the MTS, 20µL of MTS assay reagent (Promega) was added to each well, and cells were incubated for 4 hours at 37°C. The absorbances were read at 490nm, with the number of viable cells absorbance proportional to absorbance. Treated wells were compared to the media control as percent of untreated media.
Acknowlegement
This project was funded by the National Institute of Health K08 (AR48551) and R01 (AI059091). Dr. Friedman acknowledges the Dermatology Foundation for their funding support.
Abbreviations
- NPs
Nanoparticles
- EM
Electron Microscopy
- CFU
Colony Forming Unit
- TLR
Toll-like receptor
- MMP
Membrane Metalloproteinases
- BP
Benzoyl Peroxide
- [BP 0.1%] NP
Benzoyl Peroxide 0.1% encapsulated into chitosan-alginate NPs
Footnotes
Conflict of Interest: The authors state no conflict of interest.
Works Cited
- Alburquenque C, Bucarey SA, Neira-Carrillo A, Urzua B, Hermosilla G, Tapia CV. Antifungal activity of low molecular weight chitosan against clinical isolates of Candida spp. Medical Mycology. 2010;48:1018–1023. doi: 10.3109/13693786.2010.486412. [DOI] [PubMed] [Google Scholar]
- Banergee M, Mallick S, Paul A, Chattopadhyay A, Ghosh S. Heightened Reactive Oxygen Species Generation in the Antimicrobial Activity of Three Component Iodinated Chitosan-Silver Nanoparticle composite. Langmuir : the ACS journal of surfaces and colloids. 2010;26:5901–5908. doi: 10.1021/la9038528. [DOI] [PubMed] [Google Scholar]
- Bellew S, Thiboutot D, Del Rosso JQ. Pathogenesis of Acne Vulgaris: What's New What's Interesting and What May Be Clinically Relevant. J Drugs Dermatol. 2011;10:582–585. [PubMed] [Google Scholar]
- Blecher K, Nasir A, Friedman A. The growing role of nanotechnology in combating infectious disease. Virulence. 2011;2:395–401. doi: 10.4161/viru.2.5.17035. [DOI] [PubMed] [Google Scholar]
- Chadwick S, Kriegel C, Amiji M. Nanotechnology solutions for mucosal immunization. Advanced drug delivery reviews. 2010;62:394–407. doi: 10.1016/j.addr.2009.11.012. [DOI] [PubMed] [Google Scholar]
- Chavez de Paz LE, Resin A, Howard KA, Sutherland DS, Wejse PL. Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms. Applied and environmental microbiology. 2011;77:3892–3895. doi: 10.1128/AEM.02941-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CS, Liau WY, Tsai GJ. Antibacterial effects of N-sulfonated and Nsulfobenzoyl chitosan and application to oyster preservation. J Food Prot. 1998;61:1124–1128. doi: 10.4315/0362-028x-61.9.1124. [DOI] [PubMed] [Google Scholar]
- Chen Y-M, Chung Y-C, Wang L-W, Chen K-T, Li S-Y. Antibacterial properties of chitosan in waterborne pathogen. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2002;37:1379–1390. doi: 10.1081/ese-120005993. [DOI] [PubMed] [Google Scholar]
- Clarke SR, Mohamed R, Bian L, Routh AF, Kokai-Kun JF, Mond JJ, et al. The Staphylococcus aureus surface protein IsdA mediates resistance to innate defenses of human skin. Cell Host Microbe. 2007;1:199–212. doi: 10.1016/j.chom.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Coppi G, Iannuccelli V. Alginate/chitosan microparticles for tamoxifen delivery to the lymphatic system. Int J Pharm. 2009;367:127–132. doi: 10.1016/j.ijpharm.2008.09.040. [DOI] [PubMed] [Google Scholar]
- Coppi G, Iannuccelli V, Leo E, Bernabei MT, Cameroni R. Chitosan-alginate microparticles as a protein carrier. Drug Dev Ind Pharm. 2001;27:393–400. doi: 10.1081/ddc-100104314. [DOI] [PubMed] [Google Scholar]
- Cunha-Azevedo EP, Silva JR, Martins OP, Siqueira-Moura MP, Bocca AL, Felipe MS, et al. In vitro antifungal activity and toxicity of itraconazole in DMSA-PLGA nanoparticles. J Nanosci Nanotechnol. 2011;11:2308–2314. doi: 10.1166/jnn.2011.3576. [DOI] [PubMed] [Google Scholar]
- De Young LM, Spires DA, Ballaron SJ, Cummins CS, Young JM, Allison AC. Acne-like chronic inflammatory activity of Propionibacterium acnes preparations in an animal model: correlation with ability to stimulate the reticuloendothelial system. The Journal of investigative dermatology. 1985;85:255–258. doi: 10.1111/1523-1747.ep12276732. [DOI] [PubMed] [Google Scholar]
- Dominguez-Delgado CL, Rodriguez-Cruz IM, Escobar-Chavez JJ, Calderon-Lojero IO, Quintanar-Guerrero D, Ganem A. Preparation and characterization of triclosan nanoparticles intended to be used for the treatment of acne. Eur J Pharm Biopharm. 2011 doi: 10.1016/j.ejpb.2011.01.017. [DOI] [PubMed] [Google Scholar]
- Dutil M. Benzoyl peroxide: enhancing antibiotic efficacy in acne management. Skin Therapy Lett. 2010;15:5–7. [PubMed] [Google Scholar]
- El-Sharif AA, Hussain MHM. Chitosan-EDTA New Combination is a Promising Candidate for Treatment of Bacterial and Fungal Infections. Current Microbiology. 2011;62:739–745. doi: 10.1007/s00284-010-9777-0. [DOI] [PubMed] [Google Scholar]
- Fan X, Xing YZ, Liu LH, Liu C, Wang DD, Yang RY, et al. Effects of 420-nm intense pulsed light in an acne animal model. Journal of the European Academy of Dermatology and Venereology : JEADV. 2012 doi: 10.1111/j.1468-3083.2012.04487.x. [DOI] [PubMed] [Google Scholar]
- Friedman AJ, Han G, Navati MS, Chacko M, Gunther L, Alfieri A, et al. Sustained release nitric oxide releasing nanoparticles: characterization of a novel delivery platform based on nitrite containing hydrogel/glass composites. Nitric Oxide. 2008;19:12–20. doi: 10.1016/j.niox.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Gelfuso GM, Gratieri T, Simao PS, de Freitas LA, Lopez RF. Chitosan microparticles for sustaining the topical delivery of minoxidil sulphate. Journal of microencapsulation. 2011;28:650–658. doi: 10.3109/02652048.2011.604435. [DOI] [PubMed] [Google Scholar]
- Hemmila MR, Mattar A, Taddonio MA, Arbabi S, Hamouda T, Ward PA, et al. Topical nanoemulsion therapy reduces bacterial wound infection and inflammation after burn injury. Surgery. 2010;148:499–509. doi: 10.1016/j.surg.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakumar R, Prabaharan M, Kumar PTS, Nair SV, Tamura H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnology Advances. 2011;29:322–337. doi: 10.1016/j.biotechadv.2011.01.005. [DOI] [PubMed] [Google Scholar]
- Jia Z, shen D, Xu W. Synthesis and antibacterial activities of quaternary ammonium salt of chitosan. Carbohydr Res. 2001;333:1–6. doi: 10.1016/s0008-6215(01)00112-4. [DOI] [PubMed] [Google Scholar]
- Jugeau S, Tenaud I, Knol AC, Jarrousse V, Quereux G, Khammari A, et al. Induction of toll-like receptors by Propionibacterium acnes. Br J Dermatol. 2005;153:1105–1113. doi: 10.1111/j.1365-2133.2005.06933.x. [DOI] [PubMed] [Google Scholar]
- Kim BYS, Rutka JT, WChan WCW. Nanomedicine. New England Journal of Medicine. 2010;363:2434–2443. doi: 10.1056/NEJMra0912273. [DOI] [PubMed] [Google Scholar]
- Kim J, Ochoa M-T, Krutzik SR, Takeuchi O, Uematsu S, Legaspi AJ, et al. Activation of toll-like receptor 2 in acne triggers inflammatory cytokine responses. J Immunol. 2002;169:1535–1541. doi: 10.4049/jimmunol.169.3.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M-S, You HJ, You MK, Kim N-S, Shim BS, Kim H-M. Inhibitory effect of water-soluble chitosan on TNF-alpha and IL-8 secretion from HMC-1 cells. Immunopharmacol Immunotoxicol. 2004;26:401–409. doi: 10.1081/iph-200026887. [DOI] [PubMed] [Google Scholar]
- Kinney MA, Yentzer BA, Fleischer AB, Jr, Feldman SR. Trends in the treatment of acne vulgaris: are measures being taken to avoid antimicrobial resistance? J Drugs Dermatol. 2010;9:519–524. [PubMed] [Google Scholar]
- Kong M, Chen XG, Liu CS, Liu CG, Meng XH, Yu le J. Antibacterial mechanism of chitosan microspheres in a solid dispersing system against E. coli. Colloids and surfaces B. Biointerfaces. 2008;65:197–202. doi: 10.1016/j.colsurfb.2008.04.003. [DOI] [PubMed] [Google Scholar]
- Kumar A, Agarwal SP, Ahuja A, Ali J, Choudhry R, Baboota S. Preparation, characterization, and in vitro antimicrobial assessment of nanocarrier based formulation of nadifloxacin for acne treatment. Pharmazie. 2011;66:111–114. [PubMed] [Google Scholar]
- Lee L. The clinical spectrum of neonatal lupus. Archives of Dermatological Research. 2009;301:107–110. doi: 10.1007/s00403-008-0896-4. [DOI] [PubMed] [Google Scholar]
- Luo Y, Zhang B, Whent M, Yu LL, Wang Q. Preparation and characterization of zein/chitosan complex for encapsulation of alpha-tocopherol, and its in vitro controlled release study. Colloids and surfaces B Biointerfaces. 2011;85:145–152. doi: 10.1016/j.colsurfb.2011.02.020. [DOI] [PubMed] [Google Scholar]
- Ma Y, Zhou T, Zhao C. Preparation of chitosan-nylon-6 blended membranes containing silver ions as antibacterial materials. Carbohydr Res. 2008;343:230–237. doi: 10.1016/j.carres.2007.11.006. [DOI] [PubMed] [Google Scholar]
- Martinac A, Filipovic-Grcic J, Perissutti B, Voinovich D, Pavelic Z. Spray-dried chitosan/ethylcellulose microspheres for nasal drug delivery: swelling study and evaluation of in vitro drug release properties. Journal of Microencapsulation. 2005;22:549–561. doi: 10.1080/02652040500098960. [DOI] [PubMed] [Google Scholar]
- Mirshahpanah P, Maibach HI. Models in acnegenesis. Cutaneous and ocular toxicology. 2007;26:195–202. doi: 10.1080/15569520701502815. [DOI] [PubMed] [Google Scholar]
- Mu L, Sprando RL. Application of Nanotechnology in Cosmetics. Pharmaceutical Research. 2010;27:1746–1749. doi: 10.1007/s11095-010-0139-1. [DOI] [PubMed] [Google Scholar]
- Murugeshu A, Astete C, Leonardi C, Morgan T, Sabliov CM. Chitosan/PLGA particles for controlled release of alpha-tocopherol in the GI tract via oral administration. Nanomedicine (Lond) 2011 doi: 10.2217/nnm.11.44. [DOI] [PubMed] [Google Scholar]
- Nagy I, Pivarcsi A, Koreck A, Szell M, Urban E, Kemeny L. Distinct strains of Propionibacterium acnes induce selective human beta-defensin-2 and interleukin-8 expression in human keratinocytes through toll-like receptors. J Invest Dermatol. 2005;124:931–938. doi: 10.1111/j.0022-202X.2005.23705.x. [DOI] [PubMed] [Google Scholar]
- Nasir A. Nanotechnology and dermatology: Part I-potential of nanotechnology. Clinics in Dermatology. 2010;28:458–466. doi: 10.1016/j.clindermatol.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Pajulo OT, Pulkki KJ, Alanen MS, Reunanen MS, Lertola KK, Mattila-Vuori AI, et al. Correlation between interleukin-6 and matrix metalloproteinase-9 in early wound healing in children. Wound Repair and Regeneration. 1999;7:453–457. doi: 10.1046/j.1524-475x.1999.00453.x. [DOI] [PubMed] [Google Scholar]
- Potara M, Jakab E, Damert A, Popescu O, Canpean V, Astilean S. Synergistic antibacterial activity of chitosan-silver nanocomposites on Staphylococcus aureus. Nanotechnology. 2011;22:135101. doi: 10.1088/0957-4484/22/13/135101. [DOI] [PubMed] [Google Scholar]
- Qi L, Xu Z, Jiang X, Hu C, Zou X. Preparation and antibacterial activity of chitosan nanoparticles. Carbohydrate Research. 2005;339:2693–2700. doi: 10.1016/j.carres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Raafat D, von Bargen K, Haas A, Sahl HG. Insights into the mode of action of chitosan as an antibacterial compound. Appl Environ Microbiol. 2008;74:3764–3773. doi: 10.1128/AEM.00453-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanpui P, Murugadoss A, Prasad PV, Ghosh SS, Chattopadhyay A. The antibacterial properties of a novel chitosan-Ag-nanoparticle composite. Int J Food Microbiol. 2008;124:142–146. doi: 10.1016/j.ijfoodmicro.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Sayag J, Meaume S, Bohbot S. Healing properties of calcium alginate dressings. J Wound Care. 1996;5:357–362. [PubMed] [Google Scholar]
- Schroeter A, Engelbrecht T, Neubert RHH, Goebel ASB. New Nanosized Technologies for Dermal and Transdermal Drug Delivery. A Review. Journal of Biomedical Nanotechnology. 2010;6:511–528. doi: 10.1166/jbn.2010.1149. [DOI] [PubMed] [Google Scholar]
- Seo S-B, Jeong H-J, Chung H-S, Lee J-D, You Y-O, Kajiuchi T, et al. Inhibitory effect of high molecular weight water-soluble chitosan on hypoxia-induced inflammatory cytokine production. Biol Pharm Bull. 2003;26:717–721. doi: 10.1248/bpb.26.717. [DOI] [PubMed] [Google Scholar]
- Spellberg B. The antibiotic crisis: can we reverse 65 years of failed stewardship? Arch Intern Med. 2011;171:1080–1081. doi: 10.1001/archinternmed.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spellberg B, Blaser M, Guidos RJ, Boucher HW, Bradley JS, Eisenstein BI, et al. Combating antimicrobial resistance: policy recommendations to save lives. Clin Infect Dis. 2011;52(Suppl 5):S397–S428. doi: 10.1093/cid/cir153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tayel AA, Moussa S, El-Tras WF, Knittel D, Opwis K, Schollmeyer E. Anticandidal action of fungal chitosan against Candida albicans. International Journal of Biological Macromolecules. 2010;47:454–457. doi: 10.1016/j.ijbiomac.2010.06.011. [DOI] [PubMed] [Google Scholar]
- Ueno H, Mori T, Fujinaga T. Topical formulations and wound healing applications of chitosan. Adv Drug Deliv Rev. 2001;52:105–115. doi: 10.1016/s0169-409x(01)00189-2. [DOI] [PubMed] [Google Scholar]
- Ueno H, Yamada H, Tanaka I, Kaba N, Matsuura M, Okumura M, et al. Accelerating effects of chitosan for healing at early phase of experimental open wound in dogs. Biomaterials. 1999;20:1407–1414. doi: 10.1016/s0142-9612(99)00046-0. [DOI] [PubMed] [Google Scholar]
- Zaenglein AL, Thiboutot DM. Chapter 37 – Acne Vulgaris. In: Bolognia J, Jorizzo JL, Rapini RP, editors. Dermatology. London; New York: Mosby; 2003. [Google Scholar]