Abstract
Nausea is associated with significant morbidity, and there is a wide range in the propensity of individuals to experience nausea. The neural basis of this heterogeneity in nausea susceptibility is poorly understood. Our previous functional magnetic resonance imaging (fMRI) study in healthy adults showed that a visual motion stimulus caused activation in the right MT+/V5 area, and that increased sensation of nausea due to this stimulus was associated with increased activation in the right anterior insula. For the current study, we hypothesized that individual differences in visual motion-induced nausea are due to microstructural differences in the inferior fronto-occipital fasciculus (IFOF), the white-matter tract connecting the right visual motion processing area (MT+/V5) and right anterior insula. To test this hypothesis, we acquired diffusion tensor imaging data from 30 healthy adults who were subsequently dichotomized into high and low nausea susceptibility groups based on the Motion Sickness Susceptibility Scale. We quantified diffusion along the IFOF for each subject based on axial diffusivity (AD); radial diffusivity (RD), mean diffusivity (MD) and fractional anisotropy (FA), and evaluated between-group differences in these diffusion metrics. Subjects with high susceptibility to nausea rated significantly (p<0.001) higher nausea intensity to visual motion stimuli and had significantly (p<0.05) lower AD and MD along the right IFOF compared to subjects with low susceptibility to nausea. This result suggests that differences in white-matter microstructure within tracts connecting visual motion and nausea-processing brain areas may contribute to nausea susceptibility or may have resulted from an increased history of nausea episodes.
Introduction
Susceptibility to the unpleasant visceral sensation of nausea varies among individuals, and the neural underpinnings of nausea susceptibility are poorly understood. Using functional magnetic resonance imaging (fMRI), we recently showed that primary (V1) and extrastriate (e.g., MT+/V5) visual areas were activated in response to a moving visual stimulus that resulted in motion sickness in some subjects. We further found that those subjects who experienced higher levels of nausea associated with the visual motion stimulus had greater activation in several non-visual gray matter regions, including the anterior insula and fronto-insula cortex (aIns/FIC) (1). These findings led us to hypothesize that microstructural differences in white-matter connecting visual motion and nausea-associated non-visual gray matter regions relate to differences in nausea susceptibility among individuals. Differences in brain microstructure might add to or underlie other factors known to contribute to nausea susceptibility, such as gender, race, and history of motion sickness (2).
Diffusion tensor imaging (DTI) is a sensitive, noninvasive technique for assessing white-matter microstructure in vivo. Here, we used DTI to evaluate whether differences in microstructure within white-matter tracts connecting visual motion and nausea-associated non-visual gray matter regions distinguishes subjects with high versus low susceptibility to motion sickness-induced nausea. Motion sickness models are commonly used to assess nausea physiology and susceptibility to motion sickness is known to predict susceptibility to clinical nausea states such as chemotherapy-induced and post-operative nausea, as well as hyperemesis gravitorum, suggesting shared etiology (2).
Methods
Thirty-two right-handed (Edinburgh Inventory, score>40) female subjects were recruited through advertisement and prescreened using the Motion Sickness Susceptibility Questionnaire (MSSQ, (3)) in order to determine susceptibility to nausea. Subjects with MSSQ scores <60 were categorized having low susceptibility to nausea (N = 11; MSSQ = 12.3±9.5, age = 25±2yrs,), while those with scores > 60 were categorized as high susceptibility (N = 19; MSSQ = 105.0±45.8; age = 28±9yrs, µ±σ). An MSSQ score of 60 was chosen because it has been shown that subjects with MSSQ scores > 60 reliably and more quickly experience moderate nausea to visual motion nauseogenic stimulation than those with lower scores (3). Subjects in the two groups did not differ on age (p=0.3). Exclusion criteria for both groups included epileptic and neurological disorders. Informed consent was obtained from all participants, and the Human Research Committee of Massachusetts General Hospital approved the protocol.
Imaging was conducted using a Siemens 1.5T Avanto MRI scanner and custom 23-channel head coil. DTI was performed using a twice-refocused echo planar spin-echo sequence (TR/TE=9970/82ms; voxel size=2x2x2mm; 60 images with non-collinear directions at b0 = 700 s/mm2; 10 images at b0 = 0 s/mm2). Functional MRI was conducted with a nauseogenic visual motion stimulus, as previously described (1). Concurrently, subjects rated nausea symptoms on a 5 point scale (0–4, 4 = the most nausea ever experienced, approaching the urge to vomit).
Diffusion tensor images were motion and eddy current corrected. Eigenvalues (λ1, λ2, and λ3) were calculated with a tensor model using FSL software (http://www.fmrib.ox.ac.uk/fsl). In white-matter, the axial diffusivity (AD, λ1), reflects diffusion parallel to axon bundles and is a marker of axonal integrity (4). The mean of diffusivities perpendicular to axon bundles, λ2 and λ3, is known as radial diffusivity (RD) and is modulated by axonal membranes and myelination. Additionally, mean diffusivity (MD) and fractional anisotropy (FA) were computed to provide summary measures of overall white-matter diffusivity and relative difference in AD and RD, respectively.
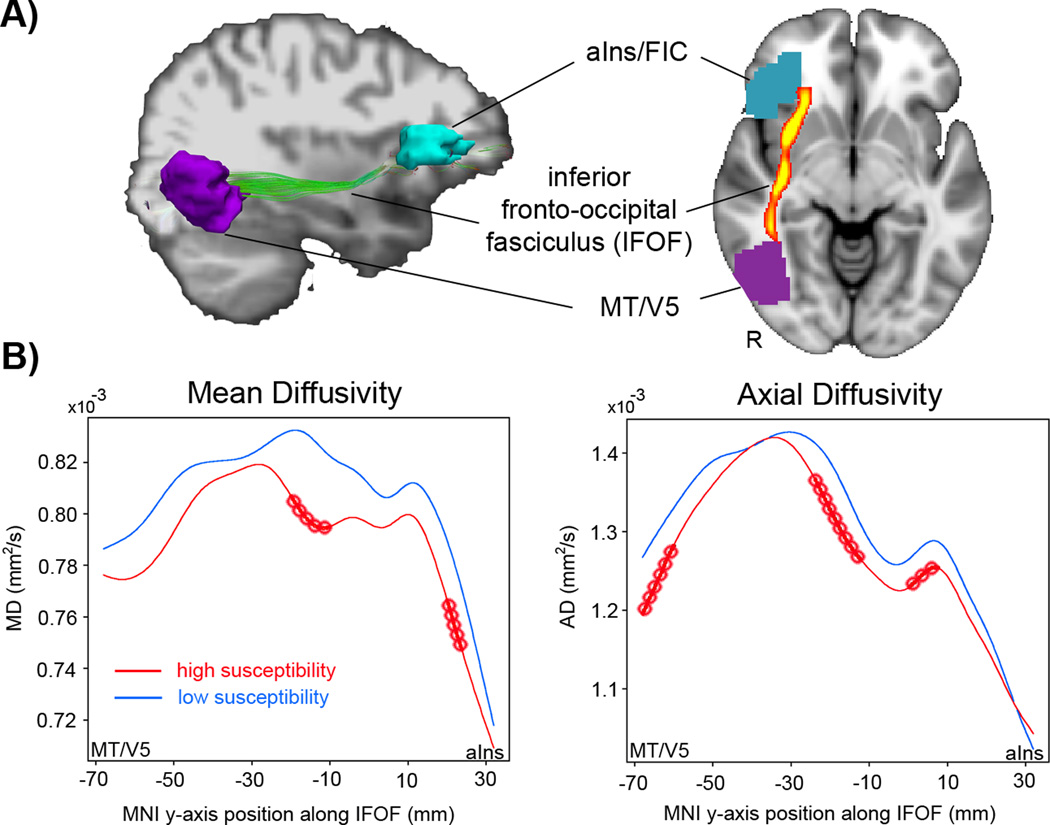
Tractography was performed on DTI data from each subject with an interpolated streamline algorithm implemented using the Diffusion Tool Kit (DTK, TrackVis.org). Tractography involved launching streamlines from every brain voxel, propagating the streamlines in 0.5 mm steps, and terminating them where FA was ≤ 0.20 or the angle between successive steps was ≥ 33°. To test our hypothesis, we isolated streamlines connecting right MT+/V5 and right aINS/FIC from each subject’s tractography data. The right MT+/V5 region was identified in each subject by intersecting the individual’s fMRI activation response to visual motion with an atlas-defined MT+/V5 label (Julich Histological Atlas, FSL). The right aINS/FIC region was identified by the cluster showing correlation between increasing activation and nausea in our prior fMRI study (1). These clusters were localized in each subject’s diffusion space (FMRIB58 FA-MNI template, FNIRT, FSL). In addition, these gray-matter clusters were dilated (2 voxels radially) to assure overlap with apposed white-matter streamlines. Of note, the number, density and location of streamlines between right MT+/V5 and aINS/FIC varied slightly among subjects. Therefore, we created a group tract consisting of voxels common to > 90% of subjects after nonlinearly warping each subject’s streamlines into standard MNI space. The group tract extended along the MNI Y axis from −70mm to +30mm, and was verified by neuroanatomist (NM) and atlas (5) as being consistent with inferior fronto-occipital fasciculus (IFOF) (Figure 1A). Furthermore, we accounted for slight inter-individual variability in IFOF location by defining each subject’s core IFOF as the five voxels at each coronal slice along its anterior-posterior length with the highest density of streamline intersections. DTI metrics were averaged over these five voxels.
Figure 1.
DTI analysis demonstrated altered microstructure along the inferior fronto-occipital fasciculus (IFOF) in adults susceptible to visual motion-induced nausea. (A) The IFOF was localized between the extrastriate areas MT+/V5 with the anterior insula / fronto-insular cortex (alns/FIC) using diffusion tensor tractography in each subject (left) and streamlines common to 90% of subjects defined at the group level IFOF (right). (B) Subjects with high nausea susceptibility had lower mean diffusivity (MD) and axial diffusivity (AD) along the IFOF compared to those with low nausea susceptibility. Note: red circles denote significant two-tailed differences (p<0.05, FDA). For ease of visualization, plots do not include the variability at each point along the curve.
Functional data analysis (FDA) was used to compare diffusion metrics (www.r-project.org) and considers data points along an experimentally obtained curve as samples from a continuous curve in function space. A statistically significant difference between two sets of curves can be examined without adjusting for the number of pairs in each experimentally determined curve (6). Diffusion data were fit using B-splines and smoothed to achieve 15 degrees-of-freedom (6). Functional unpaired Students’ t-tests compared diffusion parameter curves along the IFOF between subjects with low and high nausea susceptibility. Curves were considered significantly different if they differed over any interval at the two-tailed 0.05 level.
RESULTS
Subjects with high nausea susceptibility, based on self-report using the MSSQ, rated their visual stimulus-induced nausea experienced during fMRI as significantly stronger (3.47 ± 0.77) than low susceptibility subjects (0.36 ± 0.81, p<0.001, unpaired t-test). Subjects with high nausea susceptibility were also found to have lower MD (MNI Y -22 to -14 and 18 to 20 mm; p < 0.05, FDA, unpaired t-test) and AD (MNI Y -70 to -64, -26 to -16, and -2 to 4 mm, p < 0.05), while RD and FA did not show significant differences between groups along the IFOF (Figure 1).
Discussion
Nausea is a subjective experience of unease typified by epigastric discomfort with the urge to vomit. While this aversive symptom is quite common and is a source of significant morbidity, very little is known about the neurobiology of nausea perception in humans or the neuroanatomical substrate underlying the known heterogeneity for susceptibility to motion-induced nausea (2). Based on our previous human fMRI study (1), we hypothesized that susceptibility to motion-induced nausea would be associated with microstructural differences in white-matter tracts (i.e. IFOF) connecting brain regions known to process visual motion stimuli (i.e., MT+/V5) with regions parametrically activated by increasing nausea sensation (i.e., aIns/FIC). Subjects were dichotomized into high and low nausea susceptibility groups based on self-report, verified by rating nausea induced by visual nauseogenic stimuli. DTI analysis found that subjects with high nausea susceptibility had lower MD and AD along IFOF than subjects with low nausea susceptibility. In white-matter, the AD reflects diffusion parallel to axon bundles and is a marker of axonal integrity (4), while MD provides a summary measure of overall white-matter diffusivity. Differences in MD and AD suggests that IFOF microstructure may contribute to inter-individual susceptibility to motion-induced nausea, or may have resulted from an increased history of nausea episodes.
DTI has similarly been applied to evaluate white-matter microstructure supporting abnormal gray matter anatomy and physiology in other symptom-driven functional gastroenterological disorders such as irritable bowel syndrome (IBS) (7). In patients with IBS, FA in white-matter adjacent to the insula was found to correlate with severity of symptomatology, supporting our contention that altered white-matter microstructure within insula-related tracts accompanies aversive gastrointestinal symptomatology. While we did not find FA differences, MD differences were likely driven by differences in AD, reflecting axonal integrity (4), as RD was not significantly different between the two groups.
The IFOF links multiple fronto-temporo-parietal brain regions and lies adjacent to the medial wall of the insula (5). AIns/FIC has been postulated to integrate cognitive, affective, and interoceptive processing in order to engender autonomic outflow, which we previously found to also be significantly modulated by nausea in high susceptibility subjects (8). The findings of our prior and current studies suggest that altered white-matter microstructure for pathways that connect visual motion and insular brain areas is associated with increased autonomic outflow and susceptibility to motion sickness in response to a visual motion stimulus.
Disruption of white-matter integrity may underlie or, conversely, result from increased experience with nausea, and future longitudinal studies should explore the causal links between these variables as well as any altered white-matter microstructure in other regions of the brain. Furthermore, white-matter microstructure should also be compared between patients suffering from chronic nausea and nausea-prone (but otherwise healthy) adults. Such structural neuroplasticity may in fact be reversible and DTI metrics may serve as objective, complementary biomarkers in future clinical trials.
Acknowledgements
This work was supported by the National Institutes of Health (grant numbers K01-AT002166, P01-AT006663, and R01-AT004714 to VN, K23-DK069614 to BK, and R21-DK097499 to both VN and BK); the National Center for Research Resources (P41RR14075; CRC 1 UL1 RR025758, Harvard Clinical and Translational Science Center); and the International Foundation of Functional Gastrointestinal Disorders. The content is solely the responsibility of the authors and does not necessarily represent the official views of our sponsors.
References
- 1.Napadow V, Sheehan J, Kim J, LaCount L, Park K, Kaptchuk T, et al. The Brain Circuitry Underlying the Temporal Evolution of Nausea in Humans. Cerebral Cortex. 2012 doi: 10.1093/cercor/bhs073. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stern R, Koch K, Andrews P. Nausea: Mechanisms and Management. New York: Oxford University Press; 2011. [Google Scholar]
- 3.Golding JF. Motion sickness susceptibility questionnaire revised and its relationship to other forms of sickness. Brain Res Bull. 1998;47(5):507–516. doi: 10.1016/s0361-9230(98)00091-4. [DOI] [PubMed] [Google Scholar]
- 4.Budde MD, Xie M, Cross AH, Song SK. Axial diffusivity is the primary correlate of axonal injury in the experimental autoimmune encephalomyelitis spinal cord: a quantitative pixelwise analysis. J Neurosci. 2009;29(9):2805–2813. doi: 10.1523/JNEUROSCI.4605-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oishi K, Faria A, van Zijl P, Mori S. MRI Atlas of Human White Matter. 2nd ed. Amsterdam: Elsevier; 2010. [Google Scholar]
- 6.Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, et al. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30(11):3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 8.LaCount LT, Barbieri R, Park K, Kim J, Brown EN, Kuo B, et al. Static and dynamic autonomic response with increasing nausea perception. Aviat Space Environ Med. 2011;82(4):424–433. doi: 10.3357/asem.2932.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]