Abstract
A role for WNT signalling in gastric carcinogenesis has been suggested due to two major observations. First, patients with germline mutations in adenomatous polyposis coli (APC) are susceptible to stomach polyps and second, in gastric cancer, WNT activation confers a poor prognosis. However, the functional significance of deregulated WNT signalling in gastric homoeostasis and cancer is still unclear. In this study we have addressed this by investigating the immediate effects of WNT signalling activation within the stomach epithelium. We have specifically activated the WNT signalling pathway within the mouse adult gastric epithelium via deletion of either glycogen synthase kinase 3 (GSK3) or APC or via expression of a constitutively active β-catenin protein. WNT pathway deregulation dramatically affects stomach homoeostasis at very short latencies. In the corpus, there is rapid loss of parietal cells with fundic gland polyp (FGP) formation and adenomatous change, which are similar to those observed in familial adenomatous polyposis. In the antrum, adenomas occur from 4 days post-WNT activation. Taken together, these data show a pivotal role for WNT signalling in gastric homoeostasis, FGP formation and adenomagenesis. Loss of the parietal cell population and corresponding FGP formation, an early event in gastric carcinogenesis, as well as antral adenoma formation are immediate effects of nuclear β-catenin translocation and WNT target gene expression. Furthermore, our inducible murine model will permit a better understanding of the molecular changes required to drive tumourigenesis in the stomach.
Keywords: β-catenin, gastric cancer, APC, GSK3
Introduction
Stomach cancer is the fourth most common cancer worldwide and the second most common cause of death with a very poor 5-year survival rate (usually of under 15%) due to the late stage at time of diagnosis.1, 2 Over 90% of gastric cancers are adenocarcinomas and, based on histological features, can be divided into two types: intestinal and diffuse.3, 4, 5 The incidence of both types varies greatly between groups with differences linked to age of diagnosis and gender; however, the intestinal type is slightly more common overall.6 In intestinal-type gastric cancer, the sequence of patho-physiological changes is well established with Helicobacter pylori infection causing chronic inflammation of the mucosa followed by gastric atrophy, intestinal metaplasia, dysplasia and ultimately adenocarcinoma.7, 8, 9 However, <1% of individuals infected with H. pylori will go on to develop gastric cancer.10 Diffuse-type stomach cancer is characterised by the downregulation or loss of the cell adhesion molecule E-cadherin.11, 12 This is an early event in tumourigenesis and individuals carrying germline mutations in the CDH1 gene develop hereditary diffuse gastric cancer.13
Deregulation of the WNT signalling pathway has been implicated in numerous cancers; however, only in colorectal cancer (CRC) it has been shown to be the driving mutation.14, 15, 16, 17, 18 In CRC, adenomatous polyposis coli (APC) is the key tumour suppressor protein and is mutated in ∼80% of sporadic cancers. Moreover, germline heterozygosity of this gene leads to familial adenomatous polyposis (FAP).19, 20, 21 FAP patients acquire numerous colonic and rectal polyps early in life, which will then progress to carcinoma by 40–50 years of age.22, 23 Deregulated WNT signalling is thought to be oncogenic through the upregulation of T-cell factor (TCF)/lymphoid enhancer factor (LEF) transcriptional targets. Briefly, in the absence of WNT ligand, β-catenin is targeted for degradation by a complex containing APC, glycogen synthase kinase 3 (GSK3) and axin.24 In this situation, GSK3 phosphorylates β-catenin, which is then degraded by the proteasome. In the presence of a WNT signal, this complex is destabilised and β-catenin translocates to the nucleus.24, 25 Together, β-catenin and TCF/LEF activate a large number of WNT target genes including C-MYC, SOX9 and CYCLIN D1.26, 27, 28 Thus, inactivating mutations of APC or activating mutations in β-catenin (which prevent its phosphorylation by GSK3) lead to the induction of WNT signalling and target gene activation. Indeed, we have shown that within the murine small intestine APC loss alone is sufficient to drive adenoma formation when targeted to the intestinal stem cell.29
Outwith CRC, FAP patients also develop fundic gland polyps (FGPs) and these can rarely progress to carcinoma.30, 31, 32 Importantly, these FGPs have been shown to lose the remaining wild-type (WT) APC allele, a process also responsible for initiation of intestinal and colonic adenomas in the same patients.33, 34 Furthermore, sporadic FGPs tend to have β-catenin activating mutations, suggesting that deregulation of the WNT signalling pathway may be central to both hereditary and sporadic tumour formation.33, 34, 35, 36, 37
In gastric carcinogenesis, APC mutations have been found in both benign and malignant stomach tumours (of the intestinal as well as diffuse types) with up to 25% of gastric adenomas exhibiting somatic mutations in this gene.38, 39, 40, 41 β-Catenin activating mutations are also found in intestinal- and diffuse-type gastric cancer with 30% of tumours showing nuclear accumulation.35, 38, 42, 43, 44 Furthermore, nuclear β-catenin correlated with an invasive phenotype in intestinal-type tumours.45 However, the mechanism by which WNT signalling promotes tumour progression is poorly understood.
Therefore, in order to dissect out the functional role of the WNT pathway in this cancer, we have directly activated WNT signalling in the gastric epithelium either through inactivation of either APC or GSK3 or expression of a constitutively active β-catenin allele. In all cases, activation of WNT signalling had a marked impact on stomach homoeostasis. Our results demonstrate that nuclear β-catenin accumulation is sufficient for antral adenoma formation, while within the corpus activation of WNT signalling resulted in the loss of the parietal cell population with FGP formation as well as adenomatous change. Therefore, our data definitively prove that WNT activation can act as an initiating step in gastric tumourigenesis.
Results
The AHCreER transgene drives Cre recombinase expression within the gastric epithelium
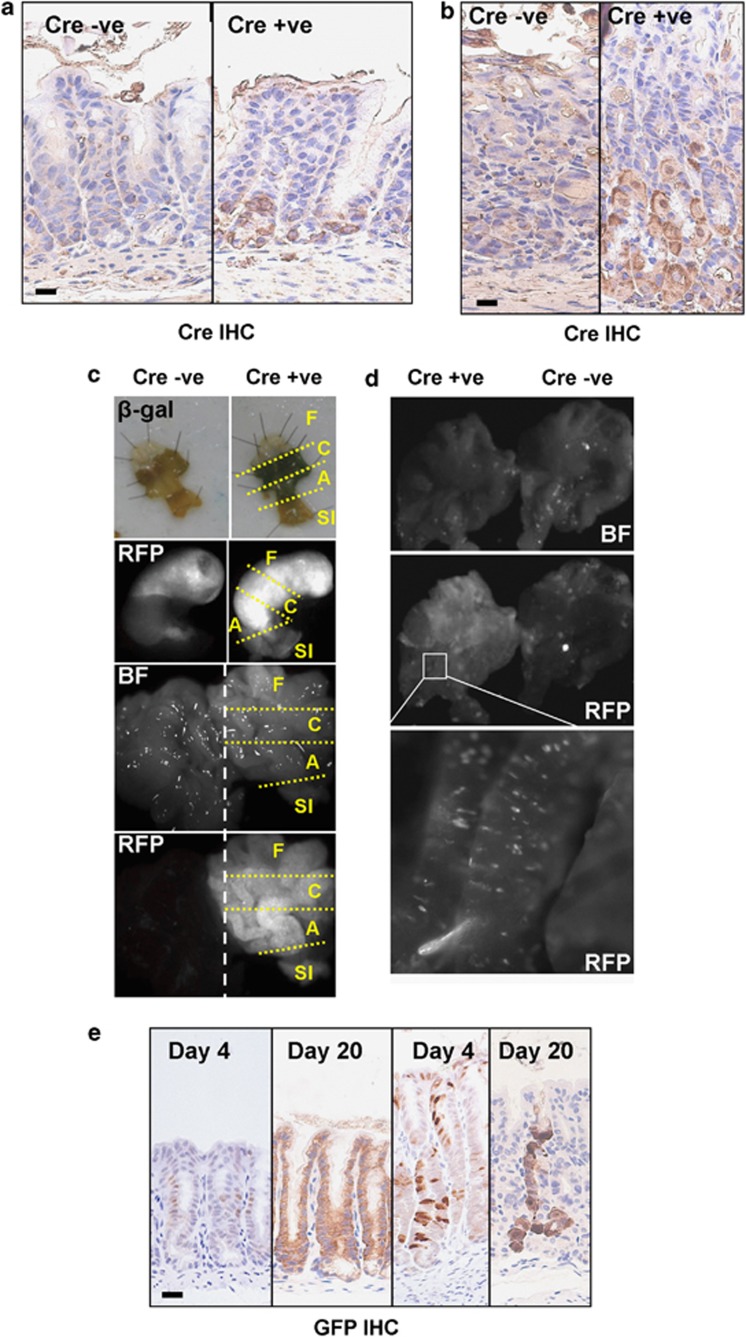
To obtain deregulation of WNT signalling within the mouse adult stomach, we used a Cre recombinase under the control of the Cyp1A1 promoter fused to the OEstrogen Receptor (ER), the AHCreER construct.46 This Cre is inducible by an i.p. (intraperitoneal) injection of β-naphthoflavone and tamoxifen. To obtain maximum recombination efficiency four i.p. injections were administered over 2 days. Figures 1a and b shows Cre immunohistochemistry (IHC) in stomach tissue taken at day 1 post-induction, which confirms Cre expression within the gastric epithelium.
Figure 1.
The AHCreER transgene drives recombination in the gastric epithelium. (a) IHC detecting Cre expression in the antrum of AhCreER+ versus AhCreER− mice at day 1 post-induction. Scale bar, 10 μm. (b) IHC detecting Cre expression in the corpus of AhCreER+ versus AhCreER− mice at day 1 post-induction. Scale bar, 10 μm. (c) Top: β-Galactosidase staining of whole-mount stomachs from AhCreER− Rosa26lacZ (left) and AhCreER+ Rosa26lacZ (right) mice at day 4 post-induction. Bottom: In-vivo RFP fluorescence imaging of AhCreER− Rosa26tdRFP (left) and AhCreER+ Rosa26tdRFP (right) mice at day 4 post-induction (unopened stomach) and at day 10 post-induction (opened stomach). F, forestomach; C, corpus; A, antrum; SI, small intestine; BF, bright field. Forestomach tissue is auto-fluorescent. (d) In-vivo RFP fluorescence imaging of AhCreER+ Rosa26tdRFP (left) and AhCreER− Rosa26tdRFP (right) mice at day 60 post-induction following a single injection of the inducing agent. Individual gastric pits are positive for RFP (bottom high magnification panel). (e) IHC detecting GFP expression in the stomach of AhCreER+ Z/EG mice at day 4 and 20 post-induction. Left two panels, antrum. Right two panels, corpus. Scale bar, 20 μm.
To assess the recombination efficiency using this regime in mice carrying the AHCreER transgene, these mice were crossed to mice carrying at the Rosa26 locus either a lacZ or red fluorescent protein (RFP) reporter construct. Figure 1c shows stomach whole-mount staining for β-galactosidase activity 4 days following Cre induction as well as in-vivo imaging of RFP expression at the same time point (day 4) and a later time point (day 10).
To determine the precise cell lineages where recombination was occurring, we crossed the AHCreER transgenic mice to the Z/EG reporter mice. The Z/EG transgenic reporter contains a loxP flanked lacZ cassette followed by a GFP reporter gene.47 Thus, following Cre-mediated excision, GFP is expressed. Figure 1e shows GFP IHC staining at an early time point, day 4 post-induction, and at a later time point, day 20 post-induction. GFP expression was found in individual cells in both the fundic and antral glands at day 4 post-induction. Furthermore, by day 20 following recombination, whole gastric units were now GFP positive, demonstrating that Cre expression occurred in precursor or stem cells capable of generating the entire range of differentiated cell types.
Stem cell recombination was also achieved using a much lower induction regime of only one i.p. injection of β-naphthoflavone and tamoxifen with individual gastric pits still expressing RFP 60 days after the single injection (Figure 1d).
Activation of WNT signalling in the antral glands leads to rapid adenoma formation
As both APC and β-catenin are mutated in a large number of gastric cancers, we next characterised the effect of WNT activation within the adult stomach epithelium. For this we used three different ways of driving deregulated WNT signalling: either by deleting the WNT negative regulators APC or GSK3 or activating β-catenin by deleting exon 3, which contains the GSK3 phosphorylation sites hence preventing its degradation. Therefore, we crossed the AhCreER+ mice to either APCfl/fl, Catnbexon3fl/exon3fl or GSK3 alphafl/fl betafl/fl mice and obtained the following experimental mice: AhCreER+ APCfl/fl, AhCreER+ Catnbexon3fl/exon3fl and AhCreER+ GSK3 alphafl/fl betafl/fl. Table 1 contains an overview of the incidence and phenotype observed in experimental versus control mice along with time points and number of mice used per experiment.
Table 1. Incidence of β-catenin-positive lesions at different time points.
| Genotype | Time point |
Number of mice with nuclear β-catenin-positive lesions |
|
|---|---|---|---|
| Antrum—microadenoma (MA) and adenoma(A) | Corpus—fundic gland polyps (FGP) and adenomatous change (AC) | ||
| Experimental mice | |||
| AhCreER+ APCfl/fl | Day 4 | MA 8 out of 8 | 0 out of 8 |
| Day 6 | MA 3 out of 3 | 0 out of 3 | |
| Day 12 | A 3 out of 3 | FGP and AC 3 out of 3 | |
| AhCreER+ Catnbexon3fl/exon3fl | Day 4 | MA 4 out of 4 | 0 out of 4 |
| Day 11 | A 5 out of 5 | FGP and AC 5 out of 5 | |
| AhCreER+ GSK3 | Day 4 | MA 8 out of 8 | 0 out of 8 |
| Day 6 | MA 3 out of 9 | FGP 2 out of 9 | |
| alphafl/fl betafl/fl | A 6 out of 9 | AC 3 out of 9 | |
| Day 8 | A 6 out of 6 | FGP and AC 6 out of 6 | |
| Day 12 | A 5 out of 5 | FGP and AC 5 out of 5 | |
| Control mice | |||
| AhCreER+ | Day 4 | 0 out of 4 | 0 out of 4 |
| Day 6 | 0 out of 3 | 0 out of 3 | |
| Day 12 | 0 out of 3 | 0 out of 3 | |
| AhCreER+ Catnbexon3fl/+ | Day 4 | 0 out of 3 | 0 out of 3 |
| Day 6 | 0 out of 5 | 0 out of 5 | |
| Day 21 | 0 out of 3 | 0 out of 3 | |
| AhCreER+ GSK3 alphafl/fl betafl/+ | Day 6 | 0 out of 3 | 0 out of 3 |
| Day 8 | 0 out of 3 | 0 out of 3 | |
| Day 12 | 0 out of 5 | 0 out of 5 | |
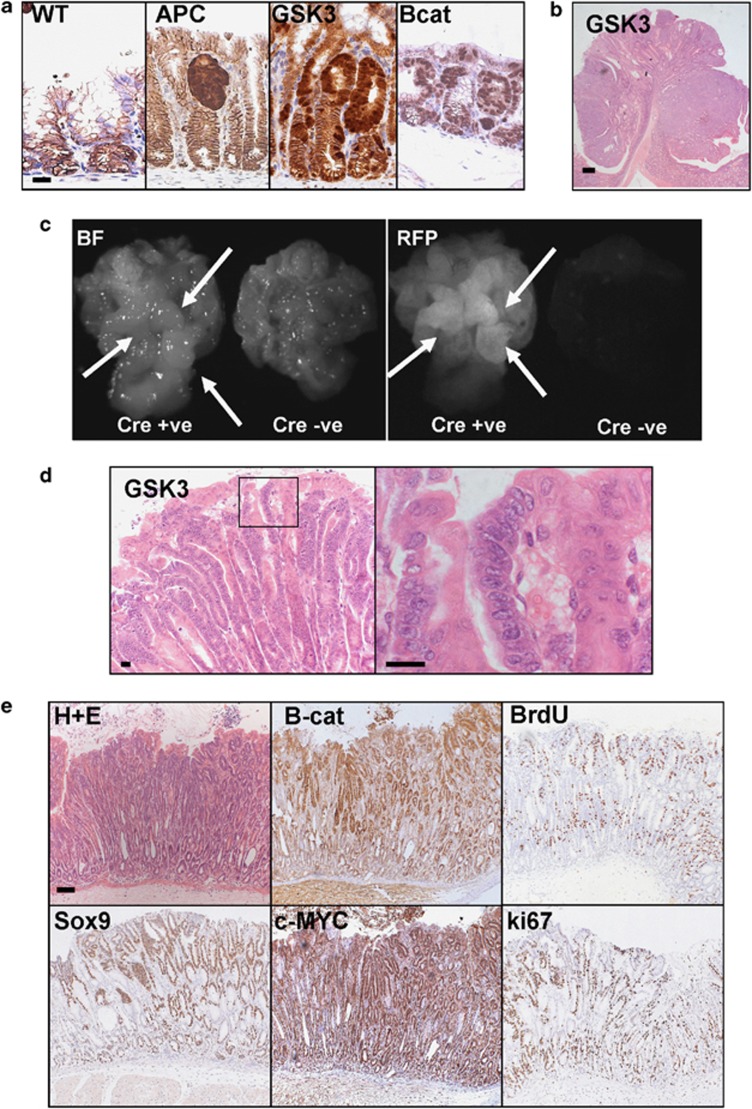
First, we investigated the immediate phenotype of WNT signalling deregulation (using the maximal Cre induction detailed above) at day 4 post-induction in the stomach antrum. Figure 2a shows that in all three mouse models there were individual cells with nuclear β-catenin showing deregulation of the WNT pathway as WT antrum lacks nuclear β-catenin. Remarkably, even at this very early time point, there are already small hyperproliferative microadenomas. This indicates that the antral region is very sensitive to nuclear β-catenin accumulation.
Figure 2.
Activation of WNT signalling in the antral glands leads to rapid adenoma formation. (a) IHC detecting β-catenin showing nuclear localisation in antrum tissue from AhCreER+ APCfl/fl (APC), AhCreER++ Catnbexon3fl/exon3fl (Bcat) and AhCreER+ GSK3 alphafl/fl betafl/fl (GSK3) mice versus AhCreER+ (WT) only mice at day 4 post-induction. Scale bar, 10 μm. (b) H+E stain of an antral tumour from AhCreER+ GSK3 alphafl/fl betafl/fl (GSK3) mouse at day 136 post-induction (single inducer injection). Scale bar, 200 μm. (c) In-vivo RFP fluorescence imaging of AhCreER+ Rosa26tdRFP APCfl/fl (left) and AhCreER− Rosa26tdRFP APCfl/fl (right) mice at day 10 post-induction. Arrows indicate thickened areas of glandular stomach. BF, bright field. (d) Haematoxylin and eosin (H+E) stain of an antral tumour from AhCreER+ GSK3 alphafl/fl betafl/fl (GSK3) mouse at day 8 post-induction with a high magnification panel (right). Scale bar, 20 μm. (e) Antral neoplasia in AhCreER+ GSK3 alphafl/fl betafl/fl mice at day 8 post-induction. IHC staining reveals high levels of nuclear β-catenin with corresponding c-MYC and Sox9 staining, as well as high levels of proliferation (BrdU and ki67-positive cells). Scale bar, 50 μm.
These mice were then aged until they showed signs of illness. As shown in Figures 2b–e, the small lesions observed at day 4 progress to large adenomas, which due to their proximity to the small intestine can cause obstruction. These adenomas are reminiscent of intestinal adenomas observed when APC or β-catenin are mutated and can be classified as intestinal-type gastric adenoma.48, 49 Furthermore, when stomachs from mice where WNT activation had occurred were collected and opened, the glandular region was greatly thickened compared with Cre negative controls (Figure 2c).
To confirm the activation of the WNT pathway, we looked by IHC at the expression of a number of WNT target genes in these lesions. Compared with normal tissue, these adenomas express high levels of c-MYC and Sox9 as well as markers of proliferation like Mcm2 and ki67, which are now localised on the surface of the glands (Figure 2e; Supplementary Figure 1).
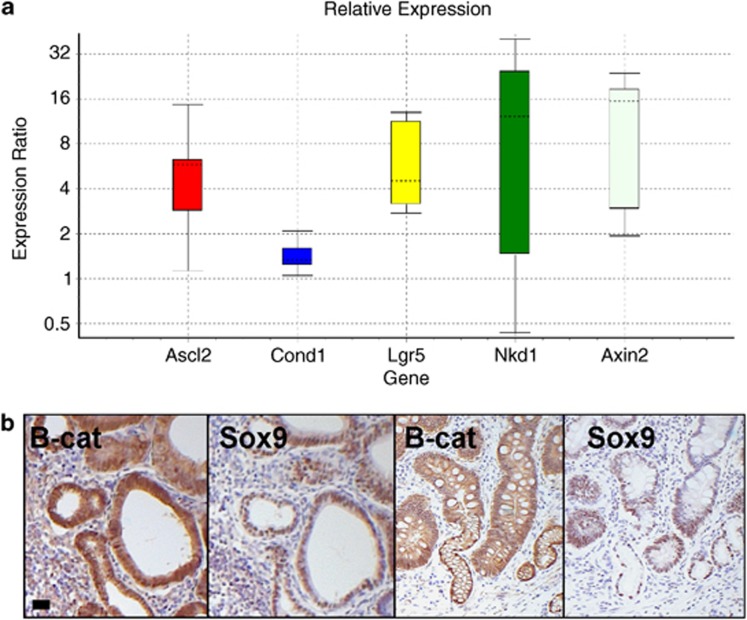
We also looked at mRNA levels in whole stomach tissue and found WNT targets Achaete scute-like 2 (Ascl2), Cyclin D1 (CCnd1), Leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5) and Axin2 were significantly upregulated (Figure 3a).
Figure 3.
Activation of WNT signalling in the stomach correlates with the phenotype observed in FAP patients with intestinal-type adenomas. (a) Relative expression (qRT–PCR levels) of WNT target genes Achaete scute-like 2 (Ascl2), Cyclin D1 (Ccnd1), Leucine-rich-repeat-containing G-protein-coupled receptor 5 (Lgr5), Naked cuticle homologue 1 (Nkd1) and Axin2. mRNA levels were measured in whole stomach tissue from AhCreER+ GSK3 alphafl/fl betafl/fl (experimental) compared with AhCreER+ GSK3 alphafl/fl betafl/+ (control) mice at day 6 post-induction. P<0.001 apart from Nkd1 P<0.097. (b) IHC staining for β-catenin and Sox9 in gastric lesions from FAP patients. Left two panels, intestinal-type adenoma; right two panels, intestinal-type metaplasia. Scale bar, 10 μm.
To confirm that nuclear β-catenin accumulation is present in stomach lesions from FAP patients, we also performed β-catenin IHC on this tumour tissue. Biopsy samples from patients with intestinal-type gastric adenomas indeed confirm that nuclear β-catenin accumulation and expression of the WNT target Sox9 is a feature of this type of neoplasia (Figure 3b).
Activation of WNT signalling in the corpus of the stomach leads to fundic gland polyposis and adenomatous change
In contrast to the antrum, within the corpus region of the stomach resides the parietal cell lineage. These cells are responsible for the acidic pH of the stomach via the activity of their membrane-bound proton pumps.50 A common pre-neoplastic lesion found in the corpus region in FAP patients is the FGP. This lesion is characterised by enlarged glands with cystic transformation lined by parietal cell and chief cells.51
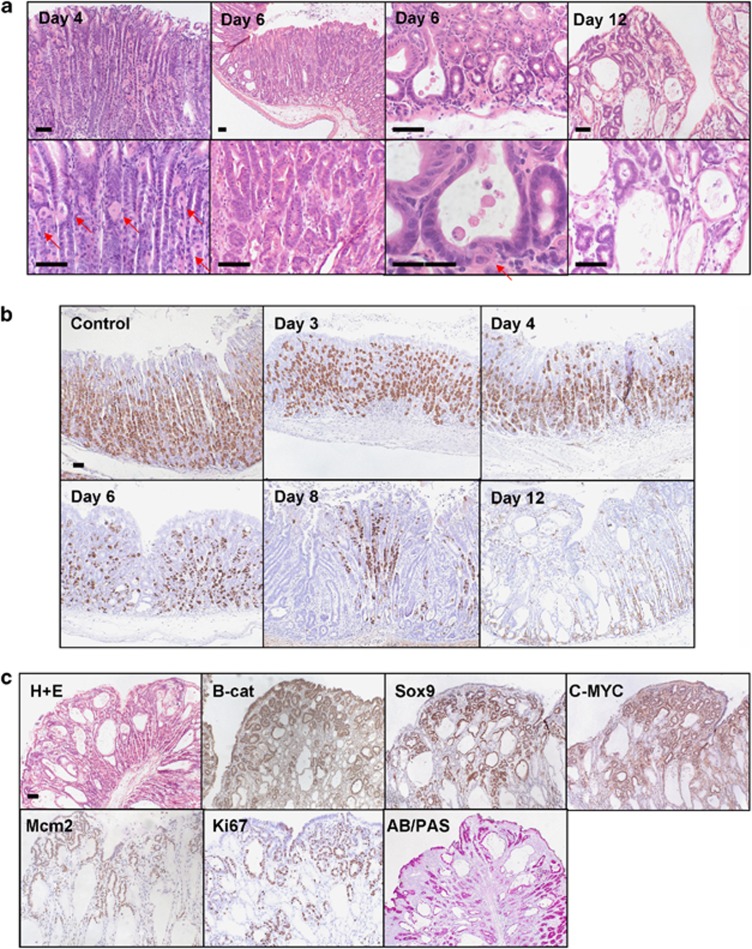
Deregulation of WNT signalling (through either APC or GSK3 loss of β-catenin activation) led to progressive loss of the parietal cell population concomitant with FGP formation interspersed with adenomatous change. Figures 4a and b show wide spread fundic gland cysts, characteristic of fundic gland polyposis, together with tubular adenomatous structures identical to the adenomas observed in the antrum. These lesions show a dramatic increase in nuclear β-catenin accumulation throughout (Figure 4c). Importantly, this was in contrast to the WT corpus where nuclear β-catenin was absent (Supplementary Figure 2). Similar to the antrum, these cells also expressed c-MYC and Sox9 WNT target genes as well as Mcm2 and ki67 proliferation markers (Figure 4c).
Figure 4.
Activation of WNT signalling in the corpus of the stomach leads to FGPs with progressive parietal cell loss. (a) H+E stain of corpus region from AhCreER+ GSK3 alphafl/fl betafl/fl (GSK3) mice at days 4, 6 and 12 post-induction (top, low magnification; bottom, high magnification). Day 4—areas of the corpus show a reduction in the number of parietal cells (red arrows). Day 6—both FGPs and regions of adenomatous change are present in the corpus (red arrow marks parietal cells lining a fundic cyst). Day 12—widespread fundic gland polyposis and adenomatous change in the corpus. Scale bar, 50 μm. (b) IHC detecting H+–K+–ATPase expression marking parietal cells in AhCreER+ GSK3 alphafl/fl betafl/fl versus AhCreER+ (Control) mice at days 4, 6, 8 and 12 post-induction. Scale bar, 50 μm. (c) FGPs and adenomatous change in AhCreER+ GSK3 alphafl/fl betafl/fl mice at day 12 post-induction. IHC staining for β-catenin, c-MYC, Sox9, Mcm2, ki67 and Alcian Blue Periodic Acid Schiff (AB/PAS). Scale bar, 50 μm.
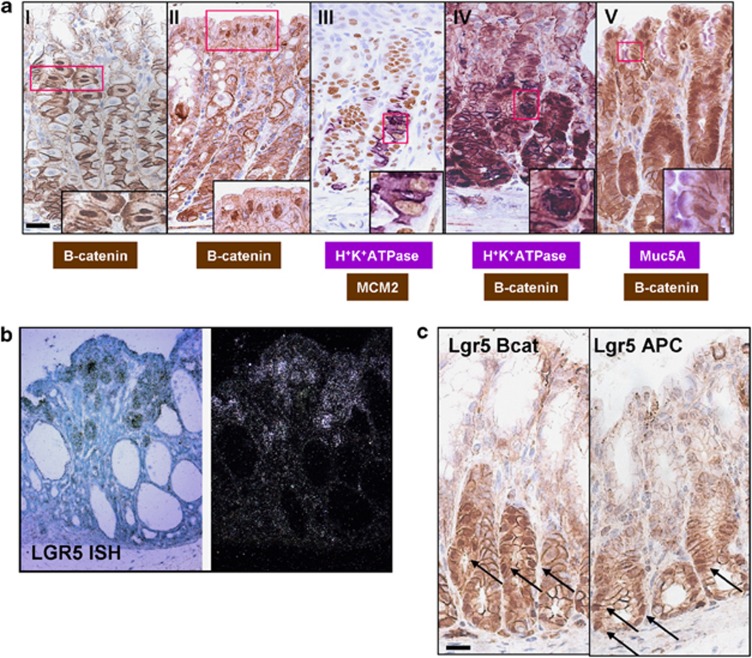
It is well known that loss of parietal cell function through acid suppression therapy can lead to fundic gland polyposis;52, 53 however, we wanted to investigate the mechanistic basis of how WNT activation led to a marked reduction in the number of the parietal cells. First, we investigated if recombination was occurring in the parietal cell population. Figure 5a shows at day 3 post-induction nuclear β-catenin accumulation in parietal cells. To confirm this, we used double staining with a parietal cell specific marker, the proton pump. We observe that terminally differentiated parietal cells can now display nuclear β-catenin accumulation. At the same time, double staining for the proliferation marker Mcm2 and the proton pump highlights the fact that these cells are now primed to enter the cell cycle. However, very few cells stained positive for both Mcm2 and the proton pump showing that this potential re-entry into the cell cycle is a rare event.
Figure 5.
Recombination occurs within multiple cell types in the corpus. (a) IHC detecting β-catenin showing nuclear localisation in parietal cells (I) and foveolar cells (II) from AhCreER+ APCfl/fl mice at day 3 post-induction. Double IHC staining detecting Mcm2 (brown) and H+K+ATPase (violet) in parietal cells (III). β-Catenin (brown) and H+K+ATPase (violet) in parietal cells (IV). β-Catenin (brown) and Muc5A (violet) in foveolar cells (V). Scale bar, 10 μm. (b) ISH for Lgr5 in corpus tissue from AhCreER+ GSK3 alphafl/fl betafl/fl mice at day 12 post-induction. Bright-field (left) and dark-field microscopy (right) images. (c) IHC detecting nuclear β-catenin in Lgr5creERT2+ Catnbexon3fl/exon3fl (Lgr5Bcat) and Lgr5creERT2+ APCfl/fl (Lgr5 APC) mice at day 28 and 50 post-induction, respectively. Arrows indicate cells with nuclear β-catenin that have not progressed. Scale bar, 20 μm.
Another question that arose from this observation was whether the parietal cells were the only cells in the corpus where gene deletion occurred. To investigate this, we used double staining to look at other cell types and their β-catenin expression pattern. Co-staining with Muc 5A, a marker of foveolar cells, indicated that recombination occurs also in this cell type (Figure 5a). However, the fate of these recombined foveolar cells is not known.
In conclusion, the nuclear β-catenin-positive cells present in the FGPs may have originated from a terminally differentiated cell population within the corpus, which have lost their cell type specific markers and are now able to proliferate.
It is interesting to note that when we activated one copy of β-catenin in our AhCreER+ Catnbexon3fl/+ and examined the parietal cell population, we now found many cells expressing various degrees of nuclear β-catenin accumulation (Supplementary Figure 3). These cells persisted up to 20 days post-induction without losing their parietal cell specific properties. This may suggest that the precise level of nuclear β-catenin determines the fate of these cells. Deletion of both copies of APC or GSK3 α and β or activation of both copies of β-catenin will induce much higher levels of nuclear β-catenin, since the destruction complex cannot form without either of these proteins. However, the activated β-catenin in the AhCreER+ Catnbexon3fl/+ mice cannot be degraded but it may still bind this destruction complex therefore slowing down the kinetics of its nuclear translocation.
In our experimental setup, low-level expression of the Cre recombinase in other tissues precluded aging of these mice sufficiently to observe the progression of these parietal cells with lower nuclear β-catenin. However, a recently described mouse model allows targeting gene deletion specifically to parietal cells, using a sequence from the promoter of the parietal specific proton pump.54 In future, this may allow the study of the effects of low/intermediate levels of nuclear β-catenin in parietal cells over the long term.
Although the tumours express the intestinal stem cell marker Lgr5, tumour formation is greatly accelerated when compared with microadenomas initiated by WNT signalling deregulation in the Lgr5 lineage
It has recently been reported that the orphan G-protein-coupled receptor Lgr5 (GPR49), a marker of intestinal stem cells, is expressed in the adult antral stomach but not in the glands of the corpus.55, 56 Lgr5+ve cells can give rise to all cell types in the antrum and they represent a gastric stem cell population. In the intestine, deletion of APC in the Lgr5+ve cells gives rise to rapid tumourigenesis, while APC loss in the Lgr5−ve population does not result in cancer progression.29 This argues for a requirement to have a stem cell as a cancer-initiating cell.
To investigate whether our gastric adenomas also displayed stem cell origins we examined Lgr5 expression. Interestingly, in situ hybridisation (ISH) shows that the adenomas found in the corpus express high levels of Lgr5 (Figure 5b). This is unexpected since Lgr5 is only present during the developmental stage of the corpus. This indicates that these cells are now adopting a ‘progenitor-cell' signature, which may highlight de-differentiation as a potential tumour initiation process in the corpus.
However, if we are to compare the latency of antral tumour formation between AhCreER+ APCfl/fl or AhCreER+ Catnbexon3fl/exon3fl mice to Lgr5creERT2+ APCfl/fl and Lgr5creERT2+ Catnbexon3fl/exon3fl, this is much more rapid in mice carrying the AHCreER transgene (Figure 5c). Indeed, the Lgr5creERT2 mice only developed microadenomas in the antral stomach in mice aged up to 100 days (these mice had to be killed due to intestinal adenoma formation) while AHCreER mice had small adenomas from day 4 post-induction, which progress to whole stomach lesions by days 10–12 post-induction. Therefore, unlike in the intestine, the Lgr5 antral stem cell population seems less sensitive to WNT pathway activation. Lack of Lgr5 expression in the corpus makes it impossible to use the Lgr5creERT2+ construct for a similar comparison in the corpus.
Discussion
Our data show that deregulation of the WNT signalling pathway strongly perturbs gastric homoeostasis in both the corpus and the antrum. In many ways, the antral phenotype recapitulates previous findings in the small intestine where deregulation of WNT signalling alone is sufficient for benign tumourigenesis.29, 48, 57 Indeed, the phenotype of hyperproliferation is also reminiscent of APC deletion in the small intestine. In the corpus, the loss of parietal cells appears to predispose to FGP formation, a situation akin to humans, where both proton pump inhibitors (which block parietal cell function) and germline heterozygosity for APC predispose to fundic gland polyposis.52 However, in both the antrum and the corpus, we failed to see conversion to malignant gastric cancer. This argues that activation of WNT signalling is not sufficient to drive malignancy.
In contrast to CRC, the mutations that drive gastric cancer are much more unclear. It has been suggested that the two major predisposing factors to gastric cancer, H. pylori infection or germline mutations in E-cadherin, both may drive tumourigenesis in part through activation of WNT signalling.58, 59 In-vitro H. pylori infection of cells leads to increased nuclear levels of β-catenin as well as TCF/LEF transactivation.60, 61 This has been suggested to be due to inhibition of GSK3 activity via the PI3K (phosphatidylinositol 3-kinase) pathway, which results in β-catenin stabilisation.62 However, linking this inhibitory phosphorylation of GSK3 by v-akt murine thymoma viral oncogene homologue (AKT) to WNT signalling pathway activation is controversial as it appears that this pool of GSK3 is functionally distinct to the pool of GSK3 that binds APC.63 Instead, it is likely that this phosphorylation of GSK3 is marking high PI3K activity, which itself can activate WNT signalling through an activating phosphorylation of β-catenin.64 H. pylori also induces phosphorylation of the WNT co-receptor low-density lipoprotein receptor-related protein 6, which plays a crucial role in WNT signal transduction.65 This is dependent on Dishevelled (Dvl) 2 and 3 proteins, which recruit GSK3 and Axin2 away from the β-catenin destruction complex. Moreover, another study has suggested that H. pylori may induce WNT signalling in a met proto-oncogene (c-Met)-dependent manner.66 Despite these multiple ways in which H. pylori can induce WNT signalling, our phenotypes following APC or GSK3 loss are distinct from the inflammatory response and gastritis induced by H. pylori. There are probably multiple reasons for this but the levels of deregulated WNT target gene expression induced by APC mutations are likely to be much greater than the levels induced by H. pylori infection. It is thus interesting to note from our results that activation of a single copy of β-catenin does not induce such a robust phenotype as two copies or loss of APC or GSK3. Thus, while activation of one copy of β-catenin leads to its nuclear accumulation from very early stages, progression to adenoma is much slower compared with APC or GSK3 deletion. Again this may be a direct result of β-catenin levels in the nucleus not being sufficient for malignant transformation, at least not at this early stage after gene mutation. It is probable that in the human context, a variety of genetic and environmental factors contribute to the β-catenin nuclear presence in high enough quantity to induce tumourigenic WNT activation. In agreement with this, premise studies have shown that GSK3 β phosphorylation and inactivation also correlates with cancer progression in human tumours.67 Furthermore, another WNT pathway component upregulated in advanced gastric cancer is the Wnt1 ligand.68 Elegant mouse studies by the Oshima group have shown that in the mouse co-activation of WNT (via expression of the WNT1 ligand) and the PGE2 (prostaglandin E2) pathway in a constitutive manner results in invasive gastric adenocarcinoma formation by 1 year.69 In contrast, WNT1 expression alone resulted in pre-neoplastic lesions at 7–18 weeks of age but never tumourigenesis. This is in good agreement with our data that shows that activation of a single copy of β-catenin does not result in histological changes up to 3 weeks after activation. In spite of the fact that both our AhCreER+ Catnbexon3fl/+ mice and the CK19 Cre-Wnt1 mice show higher levels of β-catenin with nuclear accumulation this is not sufficient to trigger adenoma formation. However, in our study when we activate both copies of β-catenin or loss of both copies of Apc this now reaches the required WNT threshold to initiate fundic gland polyposis and adenomatous change. For sporadic gastric cancer, it is much more likely that WNT activation will cooperate with other pathways (for example WNT and PGE2) in driving tumour progression.
Within human gastric cancer, loss of function mutations in APC or activating mutations in β-catenin are often associated with poor prognosis. The Wnt1 ligand is also overexpressed at the later stages of gastric carcinoma as opposed to early on. These data are in contrast to our data presented here where mice develop benign tumours. It is thus likely that in human gastric cancers, mutations that deregulate WNT signalling occur post-initiation and it will be of interest to combine APC loss or β-catenin activation in the gastric epithelium with other mutations that occur in gastric cancer to see if we can develop a representative model of the later stages of gastric carcinoma.
In conclusion, our data demonstrate that WNT activation can act as an initiator in gastric neoplasia. Both deletion of APC or GSK3 α and β lead to immediate nuclear β-catenin accumulation and WNT gene expression. Intestinal-type adenomas and FGPs were the results of this WNT activation. These closely mimic the lesions observed in FAP patients. Activating one copy of β-catenin also led to β-catenin nuclear translocation but this seemed to be a much slower process. In the future, our inducible mouse model may be used to dissect the importance of other genes in gastric carcinogenesis as well as assessing the efficacy of new anti-cancer therapies.
Materials and methods
Ethics statement
Mouse experiments: all mouse experiments were performed under the UK Home Office guidelines. Human samples: formalin-fixed paraffin-embedded biopsy samples of intestinal-type adenomas of the stomach with low-grade intraepithelial neoplasia were recovered from the archives of the Institute of Pathology, Ludwig-Maximilians-Universität, München, Germany. The corresponding clinico-pathological data sets were obtained from the database. The study was performed according to the recommendations of the local ethics committee of the Medical Faculty of the Ludwigs-Maximilians-Universität München, Germany.
Mouse colonies
Outbred mice segregating for the C57BL6J and S129 genomes (five generations C57Bl6J) were used from 8 to 12 weeks of age. The following alleles were used: APC580Sfl 57, AhCreER,46 GSK3 alphafl and GSK3 betafl,70 Catnbexon3fl,48 Z/EG,47 Rosa26lacZ,71 Lgr5-EGFP–IREScreERT2,55 Rosa26tdRFP.72
Gene deletion, tissue isolation and β-galactosidase reporter staining
To induce recombination using the AhCreER+ construct, mice were given four i.p. injections over 2 days of β-naphthoflavone (Sigma, Dorset, UK) and tamoxifen (Sigma) in corn oil at 80 mg/kg concentration each. To induce recombination Lgr5-EGFP–IREScreERT2+ mice were given 1 i.p. injection of 3 mg of tamoxifen in sunflower oil. At the appropriate time point or when showing signs of illness, the mice were killed and the stomach removed and inflated with 10% neutral buffered formaldehyde.73 The forestomach was removed and the glandular stomach was cut into three sections to give representative areas of corpus and antrum. For whole-mount β-galactosidase (β-gal) staining, stomachs were prepared by opening, flushing with ice-cold PBS and pinning out on a wax plate. The tissue was then fixed and incubated overnight with X-gal substrate (Promega, Southampton, UK) as described previously.74 For each time point and genotype a minimum of three mice was used.
In-vivo imaging
In-vivo imaging of RFP fluorescence within stomach tissue was performed as follows: whole stomachs from AhCreER+ Rosa26tdRFP mice were imaged using the Olympus OV100 molecular imaging system (Olympus, Southend-on-Sea, UK).
Immunohistochemistry
IHC was performed as described previously.49, 75 Primary antibodies used for IHC were anti-β-catenin (mouse monoclonal, 1:50; Transduction Labs, Oxford, UK), anti-BrdU (mouse monoclonal, 1:500, BD Biosciences, Oxford, UK), anti-MCM2 (rabbit, 1:500, Cell Signalling Technology, Danvers, MA, USA), anti-H+–K+–ATPase (mouse monoclonal, 1:1000, MBL International Corporation, Woburn, MA, USA), anti-muc 5AC (mouse monoclonal, 1:500, Novocastra, Milton Keynes, UK), anti-Sox9 (rabbit, 1:500, Chemicon, Watford, UK), anti-c-MYC (rabbit, 1:100, Santa Cruz, Santa Cruz, CA, USA), anti-GFP (rabbit, 1:1000, Abcam, Cambridge, UK), anti-ki67 (rabbit, 1:250, Thermo Fisher Scientific, Loughborough, UK), anti-Cre (rabbit, 1:1000, Covance Research Products, Berkeley, CA, USA). For detection, the Envision+ system HRP Mouse and Envision+ system HRP Rabbit (DAKO, Cambs, UK) were used according to the manufacturer's instructions. For the double IHC staining, the visualisation step was performed using the VECTOR VIP Substrate Kit and VECTOR SG Substrate Kit (Vector Laboratories, Cambs, UK). IHC imaging was performed using a Zeiss Axio Imager A1 microscope (EC PLAN-NEOFLUAR 40 × /0.75 ∞/0.17), AxioCam MRC camera (Zeiss, Luton, UK) and Axiovision Rel 4.7 (Zeiss) acquisition software.
Quantitative reverse-transcriptase PCR (qRT–PCR) and ISH
Details can be found in Supplementary Information.
Acknowledgments
This work was funded by the CR-UK project and programme grants and AICR grant 06-0614 to Owen Sansom. Help from Colin Nixon with histology and from Derek Miller and Tom Hamilton with transgenic work was much appreciated. This work was funded by AICR and CRUK grants.
AUTHOR CONTRIBUTIONS
Acquisition of data, analysis and interpretation of data, statistical analysis and drafting of the manuscript: SR; acquisition of data and analysis and interpretation of data: RR, JC and PS; acquisition of data, analysis and interpretation of data and statistical analysis: DA; acquisition of data, analysis and interpretation of data and critical revision of the manuscript for important intellectual content: RP; analysis and interpretation of data: JN; provided reagents: AJ, SP, JW and NB; critical revision of the manuscript for important intellectual content: MP, KO; study concept and design, drafting of the manuscript and obtained funding: OS.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Oncogene website (http://www.nature.com/onc)
Supplementary Material
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Gatta G, Ciccolallo L, Kunkler I, Capocaccia R, Berrino F, Coleman MP, et al. Survival from rare cancer in adults: a population-based study. Lancet Oncol. 2006;7:132–140. doi: 10.1016/S1470-2045(05)70471-X. [DOI] [PubMed] [Google Scholar]
- Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer. World J Gastroenterol. 2006;12:2979–2990. doi: 10.3748/wjg.v12.i19.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa P, Shiao YH. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994;54:1941s–1943s. [PubMed] [Google Scholar]
- Lauren P. The Two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31–49. doi: 10.1111/apm.1965.64.1.31. [DOI] [PubMed] [Google Scholar]
- Milne AN, Carneiro F, O'Morain C, Offerhaus GJ. Nature meets nurture: molecular genetics of gastric cancer. Hum Genet. 2009;126:615–628. doi: 10.1007/s00439-009-0722-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura N, Okamoto S, Yamamoto S, Matsumura N, Yamaguchi S, Yamakido M, et al. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345:784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- de Vries AC, van Grieken NC, Looman CW, Casparie MK, de Vries E, Meijer GA, et al. Gastric cancer risk in patients with premalignant gastric lesions: a nationwide cohort study in the Netherlands. Gastroenterology. 2008;134:945–952. doi: 10.1053/j.gastro.2008.01.071. [DOI] [PubMed] [Google Scholar]
- Parsonnet J, Friedman GD, Orentreich N, Vogelman H. Risk for gastric cancer in people with CagA positive or CagA negative Helicobacter pylori infection. Gut. 1997;40:297–301. doi: 10.1136/gut.40.3.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker KF, Hofler H. Frequent somatic allelic inactivation of the E-cadherin gene in gastric carcinomas. J Natl Cancer Inst. 1995;87:1082–1084. doi: 10.1093/jnci/87.14.1082. [DOI] [PubMed] [Google Scholar]
- Oda T, Kanai Y, Oyama T, Yoshiura K, Shimoyama Y, Birchmeier W, et al. E-cadherin gene mutations in human gastric carcinoma cell lines. Proc Natl Acad Sci USA. 1994;91:1858–1862. doi: 10.1073/pnas.91.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford PJ, Hopkins JB, Grady WM, Markowitz SD, Willis J, Lynch H, et al. E-cadherin germline mutations define an inherited cancer syndrome dominated by diffuse gastric cancer. Hum Mutat. 1999;14:249–255. doi: 10.1002/(SICI)1098-1004(1999)14:3<249::AID-HUMU8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Chan EF, Gat U, McNiff JM, Fuchs E. A common human skin tumour is caused by activating mutations in beta-catenin. Nat Genet. 1999;21:410–413. doi: 10.1038/7747. [DOI] [PubMed] [Google Scholar]
- Tycko B, Li CM, Buttyan R. The Wnt/beta-catenin pathway in Wilms tumors and prostate cancers. Curr Mol Med. 2007;7:479–489. doi: 10.2174/156652407781387118. [DOI] [PubMed] [Google Scholar]
- Guillen-Ahlers H. Wnt signaling in renal cancer. Curr Drug Targets. 2008;9:591–600. doi: 10.2174/138945008784911813. [DOI] [PubMed] [Google Scholar]
- Cavard C, Colnot S, Audard V, Benhamouche S, Finzi L, Torre C, et al. Wnt/beta-catenin pathway in hepatocellular carcinoma pathogenesis and liver physiology. Future Oncol. 2008;4:647–660. doi: 10.2217/14796694.4.5.647. [DOI] [PubMed] [Google Scholar]
- Segditsas S, Tomlinson I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene. 2006;25:7531–7537. doi: 10.1038/sj.onc.1210059. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y, Nagase H, Ando H, Horii A, Ichii S, Nakatsuru S, et al. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Nagase H, Miyoshi Y, Horii A, Aoki T, Petersen GM, Vogelstein B, et al. Screening for germ-line mutations in familial adenomatous polyposis patients: 61 new patients and a summary of 150 unrelated patients. Hum Mutat. 1992;1:467–473. doi: 10.1002/humu.1380010603. [DOI] [PubMed] [Google Scholar]
- Kinzler KW, Nilbert MC, Su LK, Vogelstein B, Bryan TM, Levy DB, et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Half E, Bercovich D, Rozen P. Familial adenomatous polyposis. Orphanet J Rare Dis. 2009;4:22. doi: 10.1186/1750-1172-4-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida M, Yao T, Itoh H, Watanabe H, Kohrogi N, Shigematsu A, et al. Natural history of fundic gland polyposis in patients with familial adenomatosis coli/Gardner's syndrome. Gastroenterology. 1985;89:1021–1025. doi: 10.1016/0016-5085(85)90203-3. [DOI] [PubMed] [Google Scholar]
- Barker N. The canonical Wnt/beta-catenin signalling pathway. Methods Mol Biol. 2008;468:5–15. doi: 10.1007/978-1-59745-249-6_1. [DOI] [PubMed] [Google Scholar]
- Shitashige M, Hirohashi S, Yamada T. Wnt signaling inside the nucleus. Cancer Sci. 2008;99:631–637. doi: 10.1111/j.1349-7006.2007.00716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetsu O, McCormick F. Beta-catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–426. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- Blache P, van de Wetering M, Duluc I, Domon C, Berta P, Freund JN, et al. SOX9 is an intestine crypt transcription factor, is regulated by the Wnt pathway, and represses the CDX2 and MUC2 genes. J Cell Biol. 2004;166:37–47. doi: 10.1083/jcb.200311021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Enjoji M, Yao T, Ohsato K. Gastric lesions in familial adenomatosis coli: their incidence and histologic analysis. Hum Pathol. 1978;9:269–283. doi: 10.1016/s0046-8177(78)80085-9. [DOI] [PubMed] [Google Scholar]
- Bianchi LK, Burke CA, Bennett AE, Lopez R, Hasson H, Church JM. Fundic gland polyp dysplasia is common in familial adenomatous polyposis. Clin Gastroenterol Hepatol. 2008;6:180–185. doi: 10.1016/j.cgh.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Garrean S, Hering J, Saied A, Jani J, Espat NJ. Gastric adenocarcinoma arising from fundic gland polyps in a patient with familial adenomatous polyposis syndrome. Am Surg. 2008;74:79–83. [PubMed] [Google Scholar]
- Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Fundic gland polyps in familial adenomatous polyposis: neoplasms with frequent somatic adenomatous polyposis coli gene alterations. Am J Pathol. 2000;157:747–754. doi: 10.1016/S0002-9440(10)64588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torbenson M, Lee JH, Cruz-Correa M, Ravich W, Rastgar K, Abraham SC, et al. Sporadic fundic gland polyposis: a clinical, histological, and molecular analysis Mod Pathol 200215718–723.12118109 [Google Scholar]
- Sekine S, Shibata T, Yamauchi Y, Nakanishi Y, Shimoda T, Sakamoto M, et al. Beta-catenin mutations in sporadic fundic gland polyps. Virchows Arch. 2002;440:381–386. doi: 10.1007/s004280100527. [DOI] [PubMed] [Google Scholar]
- Abraham SC, Nobukawa B, Giardiello FM, Hamilton SR, Wu TT. Sporadic fundic gland polyps: common gastric polyps arising through activating mutations in the beta-catenin gene. Am J Pathol. 2001;158:1005–1010. doi: 10.1016/s0002-9440(10)64047-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree M, Sieber OM, Lipton L, Hodgson SV, Lamlum H, Thomas HJ, et al. Refining the relation between 'first hits' and 'second hits' at the APC locus: the 'loose fit' model and evidence for differences in somatic mutation spectra among patients. Oncogene. 2003;22:4257–4265. doi: 10.1038/sj.onc.1206471. [DOI] [PubMed] [Google Scholar]
- Kim B, Byun SJ, Kim YA, Kim JE, Lee BL, Kim WH, et al. Cell cycle regulators, APC/beta-catenin, NF-kappaB and Epstein-Barr virus in gastric carcinomas. Pathology. 2010;42:58–65. doi: 10.3109/00313020903356392. [DOI] [PubMed] [Google Scholar]
- Tahara E.Molecular biology of gastric cancer World J Surg 199519484–488.discussion 489–90. [DOI] [PubMed] [Google Scholar]
- Horii A, Nakatsuru S, Miyoshi Y, Ichii S, Nagase H, Kato Y, et al. The APC gene, responsible for familial adenomatous polyposis, is mutated in human gastric cancer. Cancer Res. 1992;52:3231–3233. [PubMed] [Google Scholar]
- Nakatsuru S, Yanagisawa A, Furukawa Y, Ichii S, Kato Y, Nakamura Y, et al. Somatic mutations of the APC gene in precancerous lesion of the stomach. Hum Mol Genet. 1993;2:1463–1465. doi: 10.1093/hmg/2.9.1463. [DOI] [PubMed] [Google Scholar]
- Clements WM, Wang J, Sarnaik A, Kim OJ, MacDonald J, Fenoglio-Preiser C, et al. beta-Catenin mutation is a frequent cause of Wnt pathway activation in gastric cancer. Cancer Res. 2002;62:3503–3506. [PubMed] [Google Scholar]
- Park WS, Oh RR, Park JY, Lee SH, Shin MS, Kim YS, et al. Frequent somatic mutations of the beta-catenin gene in intestinal-type gastric cancer. Cancer Res. 1999;59:4257–4260. [PubMed] [Google Scholar]
- Ebert MP, Fei G, Kahmann S, Muller O, Yu J, Sung JJ, et al. Increased beta-catenin mRNA levels and mutational alterations of the APC and beta-catenin gene are present in intestinal-type gastric cancer. Carcinogenesis. 2002;23:87–91. doi: 10.1093/carcin/23.1.87. [DOI] [PubMed] [Google Scholar]
- Miyazawa K, Iwaya K, Kuroda M, Harada M, Serizawa H, Koyanagi Y, et al. Nuclear accumulation of beta-catenin in intestinal-type gastric carcinoma: correlation with early tumor invasion. Virchows Arch. 2000;437:508–513. doi: 10.1007/s004280000283. [DOI] [PubMed] [Google Scholar]
- Kemp R, Ireland H, Clayton E, Houghton C, Howard L, Winton DJ. Elimination of background recombination: somatic induction of Cre by combined transcriptional regulation and hormone binding affinity. Nucleic Acids Res. 2004;32:e92. doi: 10.1093/nar/gnh090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak A, Guo C, Yang W, Nagy A, Lobe CG. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- Harada N, Tamai Y, Ishikawa T, Sauer B, Takaku K, Oshima M, et al. Intestinal polyposis in mice with a dominant stable mutation of the beta-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–1390. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forte JG, Forte TM, Black JA, Okamoto C, Wolosin JM. Correlation of parietal cell structure and function. J Clin Gastroenterol. 1983;5:17–27. doi: 10.1097/00004836-198312001-00003. [DOI] [PubMed] [Google Scholar]
- Park do Y, Lauwers GY. Gastric polyps: classification and management. Arch Pathol Lab Med. 2008;132:633–640. doi: 10.5858/2008-132-633-GPCAM. [DOI] [PubMed] [Google Scholar]
- Freeman HJ. Proton pump inhibitors and an emerging epidemic of gastric fundic gland polyposis. World J Gastroenterol. 2008;14:1318–1320. doi: 10.3748/wjg.14.1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spicer Z, Miller ML, Andringa A, Riddle TM, Duffy JJ, Doetschman T, et al. Stomachs of mice lacking the gastric H,K-ATPase alpha-subunit have achlorhydria, abnormal parietal cells, and ciliated metaplasia. J Biol Chem. 2000;275:21555–21565. doi: 10.1074/jbc.M001558200. [DOI] [PubMed] [Google Scholar]
- Zhao Z, Hou N, Sun Y, Teng Y, Yang X. Atp4b promoter directs the expression of Cre recombinase in gastric parietal cells of transgenic mice. J Genet Genomics. 2010;37:647–652. doi: 10.1016/S1673-8527(09)60083-7. [DOI] [PubMed] [Google Scholar]
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- Barker N, Huch M, Kujala P, van de Wetering M, Snippert HJ, van Es JH, et al. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. doi: 10.1016/j.stem.2009.11.013. [DOI] [PubMed] [Google Scholar]
- Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–123. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- Franco AT, Israel DA, Washington MK, Krishna U, Fox JG, Rogers AB, et al. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc Natl Acad Sci USA. 2005;102:10646–10651. doi: 10.1073/pnas.0504927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mimuro H, Suzuki T, Park M, Yamamoto T, Sasakawa C. Interaction of CagA with Crk plays an important role in Helicobacter pylori-induced loss of gastric epithelial cell adhesion. J Exp Med. 2005;202:1235–1247. doi: 10.1084/jem.20051027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolova O, Bozko PM, Naumann M. Helicobacter pylori suppresses glycogen synthase kinase 3beta to promote beta-catenin activity. J Biol Chem. 2008;283:29367–29374. doi: 10.1074/jbc.M801818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata-Kamiya N, Kurashima Y, Teishikata Y, Yamahashi Y, Saito Y, Higashi H, et al. Helicobacter pylori CagA interacts with E-cadherin and deregulates the beta-catenin signal that promotes intestinal transdifferentiation in gastric epithelial cells. Oncogene. 2007;26:4617–4626. doi: 10.1038/sj.onc.1210251. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Hisatsune J, Yamasaki E, Isomoto H, Kurazono H, Hatakeyama M, et al. Helicobacter pylori VacA-induced inhibition of GSK3 through the PI3K/Akt signaling pathway. J Biol Chem. 2009;284:1612–1619. doi: 10.1074/jbc.M806981200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SS, Mahmoudi T, Danenberg E, Bejaoui I, de Lau W, Korswagen HC, et al. Phosphatidylinositol 3-kinase signaling does not activate the wnt cascade. J Biol Chem. 2009;284:35308–35313. doi: 10.1074/jbc.M109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XC, Yin T, Grindley JC, Tian Q, Sato T, Tao WA, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnad T, Feoktistova M, Leverkus M, Lendeckel U, Naumann M. Helicobacter pylori-induced activation of beta-catenin involves low density lipoprotein receptor-related protein 6 and Dishevelled. Mol Cancer. 2010;9:31. doi: 10.1186/1476-4598-9-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Mimuro H, Kiga K, Fukumatsu M, Ishijima N, Morikawa H, et al. Helicobacter pylori CagA phosphorylation-independent function in epithelial proliferation and inflammation. Cell Host Microbe. 2009;5:23–34. doi: 10.1016/j.chom.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Zheng HC, Xu XY, Xia P, Yu M, Takahashi H, Takano Y. Involvement of inactive GSK3beta overexpression in tumorigenesis and progression of gastric carcinomas. Hum Pathol. 2010;41:1255–1264. doi: 10.1016/j.humpath.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang H, Xue Y. Wnt pathway is involved in advanced gastric carcinoma. Hepatogastroenterology. 2008;55:1126–1130. [PubMed] [Google Scholar]
- Oshima H, Matsunaga A, Fujimura T, Tsukamoto T, Taketo MM, Oshima M. Carcinogenesis in mouse stomach by simultaneous activation of the Wnt signaling and prostaglandin E2 pathway. Gastroenterology. 2006;131:1086–1095. doi: 10.1053/j.gastro.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR. Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol. 2008;28:6314–6328. doi: 10.1128/MCB.00763-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Luche H, Weber O, Nageswara Rao T, Blum C, Fehling HJ. Faithful activation of an extra-bright red fluorescent protein in ‘knock-in' Cre-reporter mice ideally suited for lineage tracing studies. Eur J Immunol. 2007;37:43–53. doi: 10.1002/eji.200636745. [DOI] [PubMed] [Google Scholar]
- Przemeck SM, Duckworth CA, Pritchard DM. Radiation-induced gastric epithelial apoptosis occurs in the proliferative zone and is regulated by p53, bak, bax, and bcl-2. Am J Physiol Gastrointest Liver Physiol. 2007;292:G620–G627. doi: 10.1152/ajpgi.00391.2006. [DOI] [PubMed] [Google Scholar]
- Ireland H, Kemp R, Houghton C, Howard L, Clarke AR, Sansom OJ, et al. Inducible Cre-mediated control of gene expression in the murine gastrointestinal tract: effect of loss of beta-catenin. Gastroenterology. 2004;126:1236–1246. doi: 10.1053/j.gastro.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Gregorieff A, Pinto D, Begthel H, Destree O, Kielman M, Clevers H. Expression pattern of Wnt signaling components in the adult intestine. Gastroenterology. 2005;129:626–638. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.