Abstract
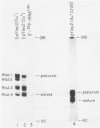
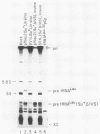
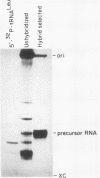
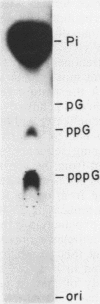
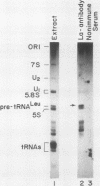
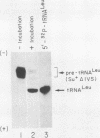
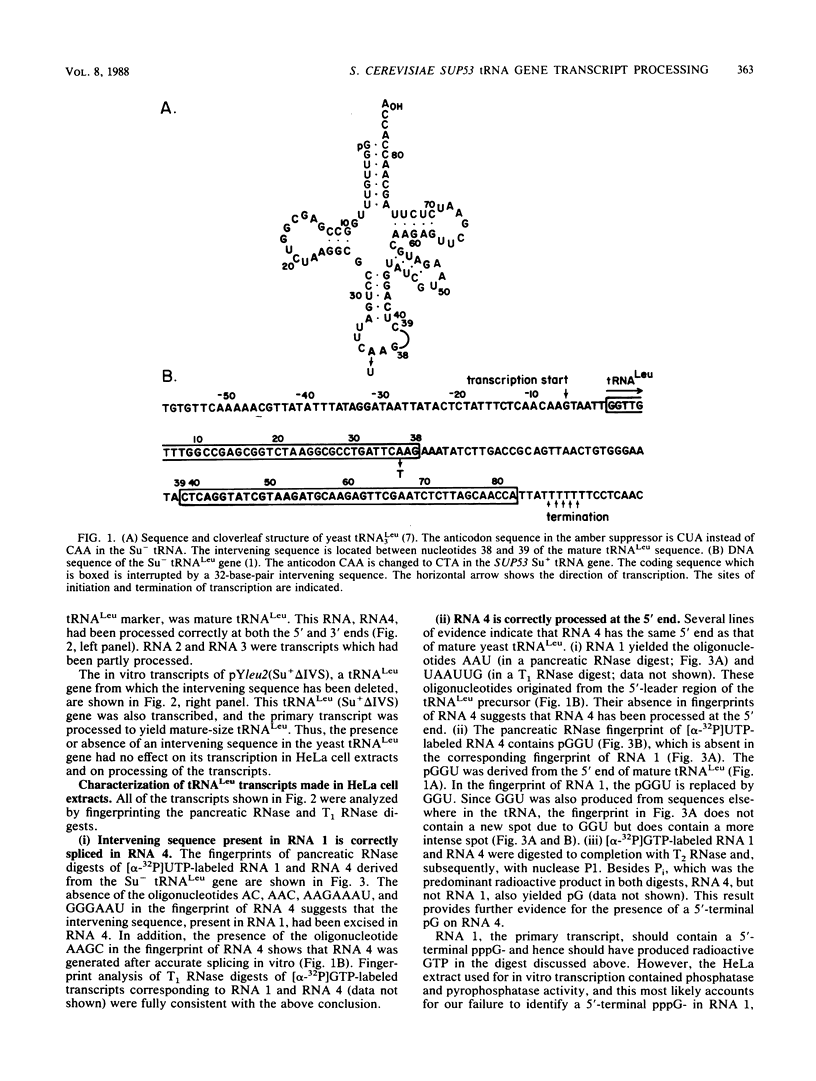
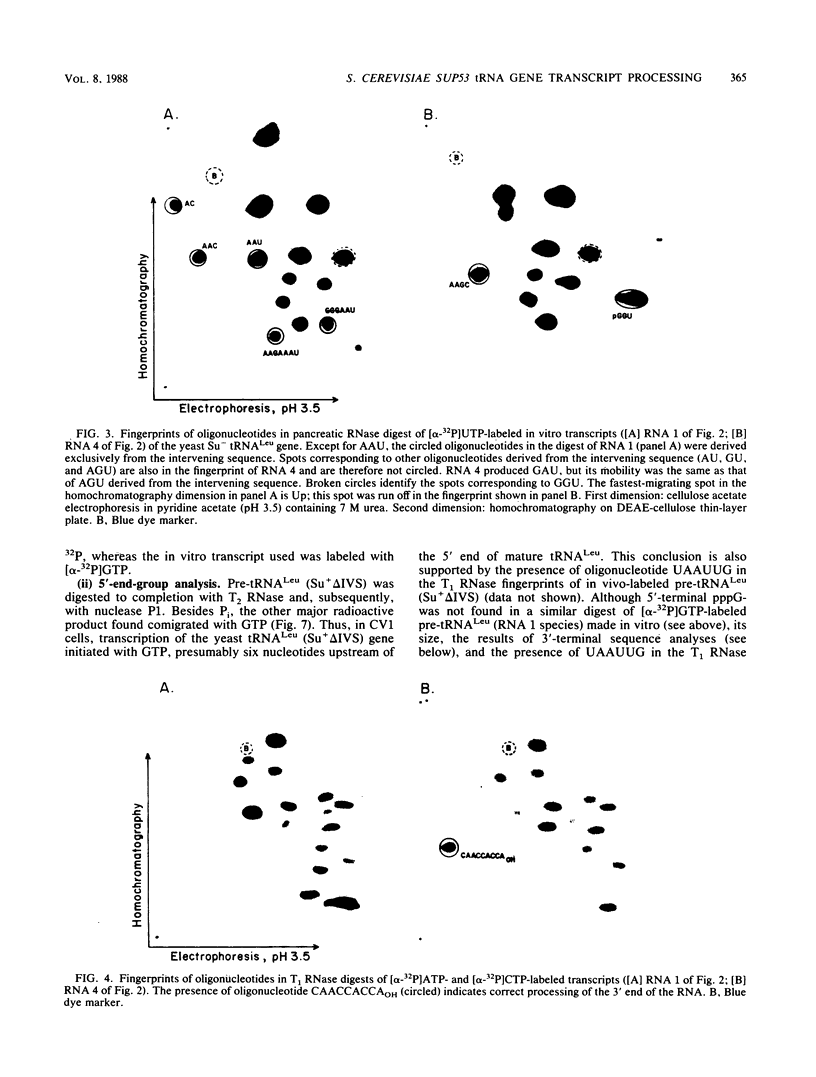
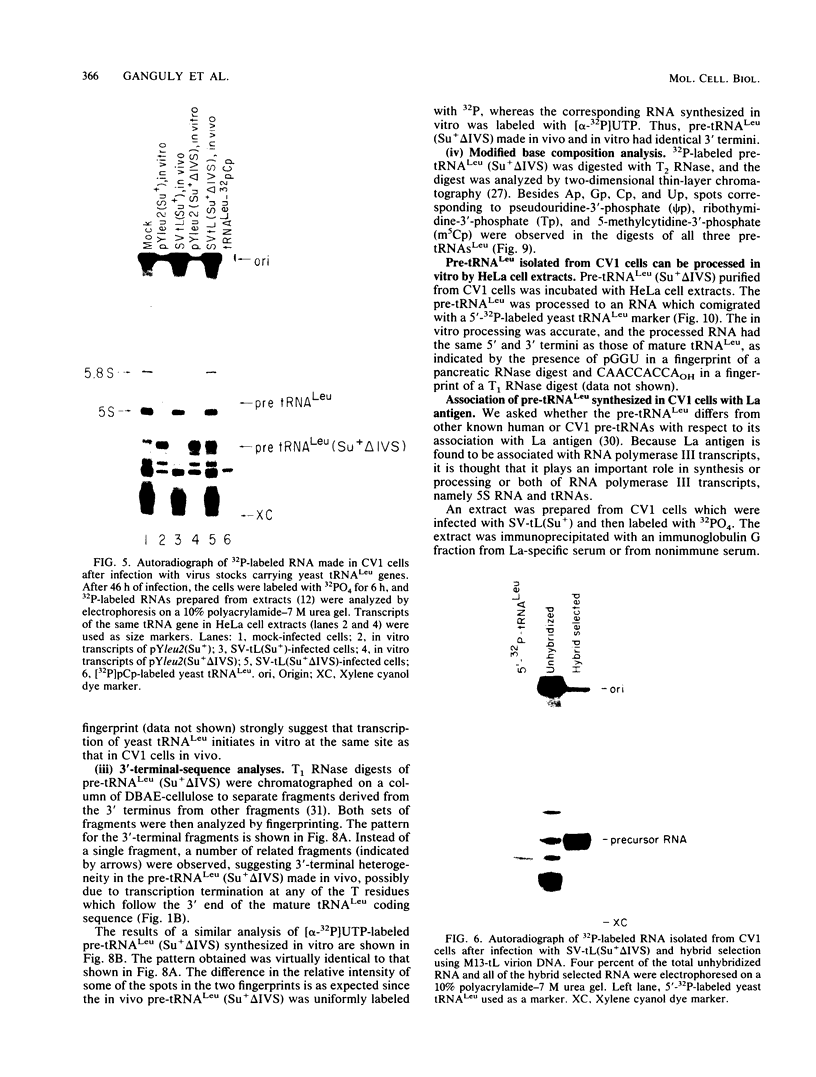
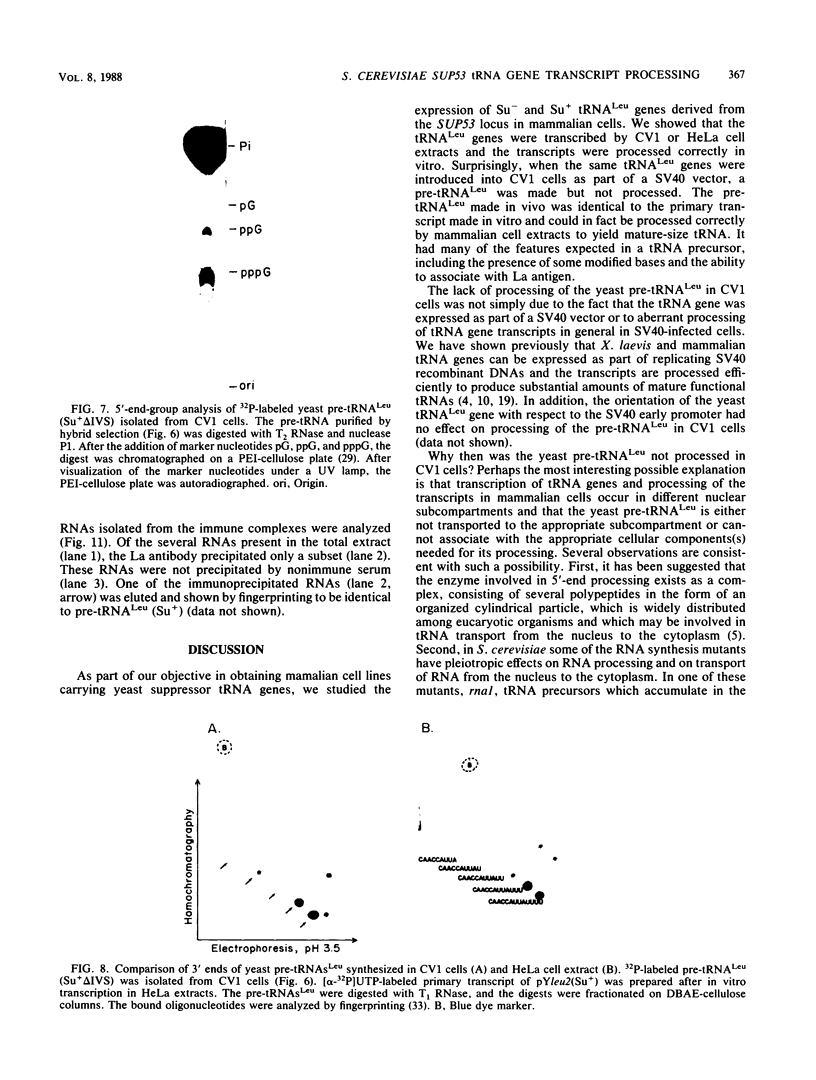
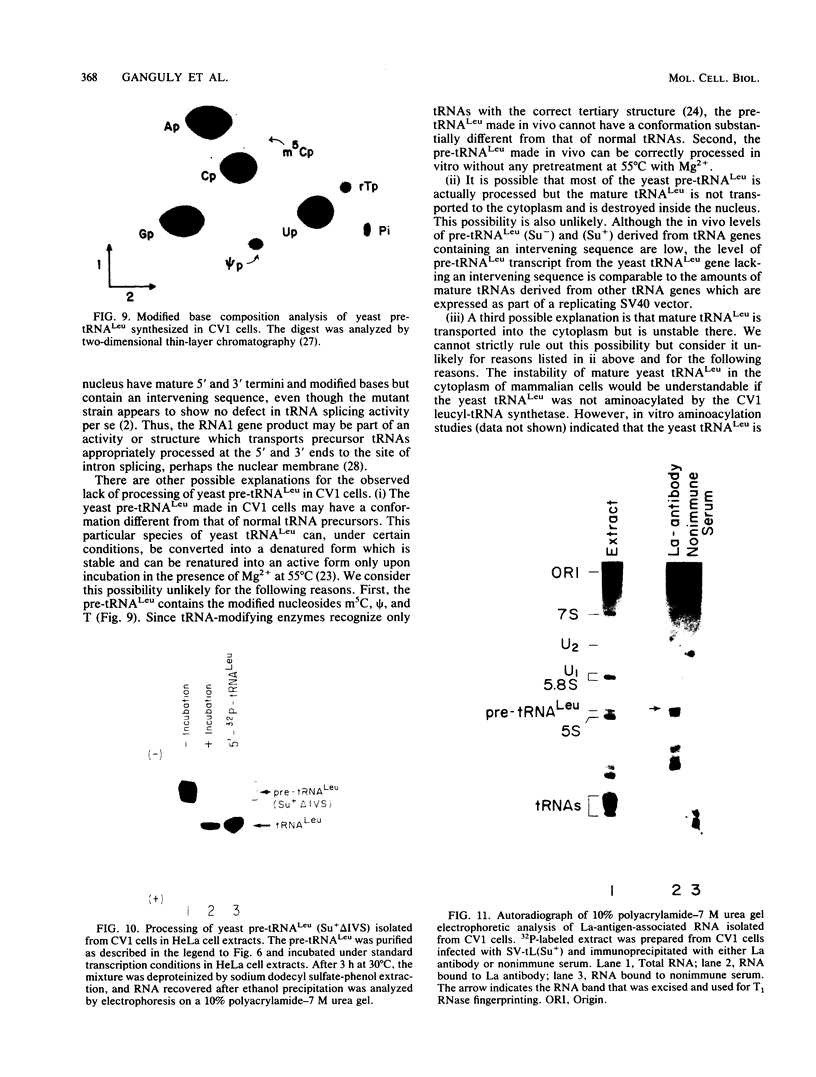
We describe the results of our studies of expression of a Saccharomyces cerevisiae amber suppressor tRNA(Leu) gene (SUP53) in mammalian cells in vivo and in cell extracts in vitro. Parallel studies were carried out with the wild-type (Su-) tRNA(Leu) gene. Extracts from HeLa or CV1 cells transcribed both tRNA(Leu) genes. The transcripts were processed correctly at the 5' and 3' ends and accurately spliced to produce mature tRNA(Leu). Surprisingly, when the same tRNA(Leu) genes were introduced into CV1 cells, only pre-tRNAs(Leu) were produced. The pre-tRNAs(Leu) made in vivo were of the same size and contained the 5'-leader and 3'-trailer sequences as did pre-tRNAs(Leu) made in vitro. Furthermore, the pre-tRNAs(Leu) made in vivo were processed to mature tRNA(Leu) when incubated with HeLa cell extracts. A tRNA(Leu) gene from which the intervening sequence had been removed yielded RNAs that also were not processed at either their 5' or 3' termini. Thus, processing of pre-tRNA(Leu) in CV1 cells is blocked at the level of 5'- and 3'-end maturation. One possible explanation of the discrepancy in the results obtained in vivo and in vitro is that tRNA biosynthesis in mammalian cells involves transport of pre-tRNA from the site of its synthesis to a site or sites where processing takes place, and perhaps the yeast pre-tRNAs(Leu) synthesized in CV1 cells are not transported to the appropriate site.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreadis A., Hsu Y. P., Kohlhaw G. B., Schimmel P. Nucleotide sequence of yeast LEU2 shows 5'-noncoding region has sequences cognate to leucine. Cell. 1982 Dec;31(2 Pt 1):319–325. doi: 10.1016/0092-8674(82)90125-8. [DOI] [PubMed] [Google Scholar]
- Atkinson N. S., Dunst R. W., Hopper A. K. Characterization of an essential Saccharomyces cerevisiae gene related to RNA processing: cloning of RNA1 and generation of a new allele with a novel phenotype. Mol Cell Biol. 1985 May;5(5):907–915. doi: 10.1128/mcb.5.5.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone J. P., Sedivy J. M., Sharp P. A., RajBhandary U. L. Introduction of UAG, UAA, and UGA nonsense mutations at a specific site in the Escherichia coli chloramphenicol acetyltransferase gene: use in measurement of amber, ochre, and opal suppression in mammalian cells. Mol Cell Biol. 1986 Sep;6(9):3059–3067. doi: 10.1128/mcb.6.9.3059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capone J. P., Sharp P. A., RajBhandary U. L. Amber, ochre and opal suppressor tRNA genes derived from a human serine tRNA gene. EMBO J. 1985 Jan;4(1):213–221. doi: 10.1002/j.1460-2075.1985.tb02338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castaño J. G., Ornberg R., Koster J. G., Tobian J. A., Zasloff M. Eukaryotic pre-tRNA 5' processing nuclease: copurification with a complex cylindrical particle. Cell. 1986 Aug 1;46(3):377–385. doi: 10.1016/0092-8674(86)90658-6. [DOI] [PubMed] [Google Scholar]
- Chang S. H., Miller N. R., Harmon C. W. Nucleotide sequence of "renaturable" leucine transfer ribonucleic acid. FEBS Lett. 1971 Oct 1;17(2):265–268. doi: 10.1016/0014-5793(71)80161-8. [DOI] [PubMed] [Google Scholar]
- Dassarma S., Rajbhandary U. L., Khorana H. G. Bacterio-opsin mRNA in wild-type and bacterio-opsin-deficient Halobacterium halobium strains. Proc Natl Acad Sci U S A. 1984 Jan;81(1):125–129. doi: 10.1073/pnas.81.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Olson M. V. Transcription and processing of cloned yeast tyrosine tRNA genes microinjected into frog oocytes. Nature. 1979 Mar 8;278(5700):137–143. doi: 10.1038/278137a0. [DOI] [PubMed] [Google Scholar]
- Drabkin H. J., RajBhandary U. L. Expression in vivo of a mutant human initiator tRNA gene in mammalian cells using a simian virus 40 vector. J Biol Chem. 1985 May 10;260(9):5588–5595. [PubMed] [Google Scholar]
- Goff S. P., Berg P. Construction, propagation and expression of simian virus 40 recombinant genomes containing the Escherichia coli gene for thymidine kinase and a Saccharomyces cerevisae gene for tyrosine transfer RNA. J Mol Biol. 1979 Sep 25;133(3):359–383. doi: 10.1016/0022-2836(79)90398-x. [DOI] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Ho Y. S., Norton G. P., Palese P., Dozy A. M., Kan Y. W. Expression and function of suppressor tRNA genes in mammalian cells. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):1033–1040. doi: 10.1101/sqb.1986.051.01.119. [DOI] [PubMed] [Google Scholar]
- Horowitz M., Cepko C. L., Sharp P. A. Expression of chimeric genes in the early region of SV40. J Mol Appl Genet. 1983;2(2):147–159. [PubMed] [Google Scholar]
- Hudziak R. M., Laski F. A., RajBhandary U. L., Sharp P. A., Capecchi M. R. Establishment of mammalian cell lines containing multiple nonsense mutations and functional suppressor tRNA genes. Cell. 1982 Nov;31(1):137–146. doi: 10.1016/0092-8674(82)90413-5. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Laski F. A., Alzner-DeWeerd B., RajBhandary U. L., Sharp P. A. Expression of a X. laevis tRNATyr gene in mammalian cells. Nucleic Acids Res. 1982 Aug 11;10(15):4609–4626. doi: 10.1093/nar/10.15.4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., Hudziak R. M., Capecchi M. R., Norton G. P., Palese P., RajBhandary U. L., Sharp P. A. Synthesis of an ochre suppressor tRNA gene and expression in mammalian cells. EMBO J. 1984 Nov;3(11):2445–2452. doi: 10.1002/j.1460-2075.1984.tb02154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Belagaje R., RajBhandary U. L., Sharp P. A. An amber suppressor tRNA gene derived by site-specific mutagenesis: cloning and function in mammalian cells. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5813–5817. doi: 10.1073/pnas.79.19.5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laski F. A., Fire A. Z., RajBhandary U. L., Sharp P. A. Characterization of tRNA precursor splicing in mammalian extracts. J Biol Chem. 1983 Oct 10;258(19):11974–11980. [PubMed] [Google Scholar]
- Lerner M. R., Steitz J. A. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman S. W., Srodulski Z., Reed C. R., Stewart J. W., Sherman F., Brennan G. Yeast amber suppressors corresponding to tRNA3Leu genes. J Mol Biol. 1984 Sep 15;178(2):209–226. doi: 10.1016/0022-2836(84)90140-2. [DOI] [PubMed] [Google Scholar]
- Lindahl T., Adams A., Fresco J. R. Renaturation of transfer ribonucleic acids through site binding of magnesium. Proc Natl Acad Sci U S A. 1966 Apr;55(4):941–948. doi: 10.1073/pnas.55.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman A. J., Ogden R. C., Abelson J. tRNA gene transcription in yeast: effects of specified base substitutions in the intragenic promoter. Cell. 1983 Nov;35(1):117–125. doi: 10.1016/0092-8674(83)90214-3. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Peebles C. L., Gegenheimer P., Abelson J. Precise excision of intervening sequences from precursor tRNAs by a membrane-associated yeast endonuclease. Cell. 1983 Feb;32(2):525–536. doi: 10.1016/0092-8674(83)90472-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Isolation and sequence determination of the 3'-terminal regions of isotopically labelled RNA molecules. Nucleic Acids Res. 1974 May;1(5):653–671. doi: 10.1093/nar/1.5.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Weissman S., deCrombrugghe B. Termination of transcription in bacteriophage lambda. Heterogeneous, 3'-terminal oligo-adenylate additions and the effects of rho factor. J Biol Chem. 1975 Jun 25;250(12):4755–4764. [PubMed] [Google Scholar]
- Sedivy J. M., Capone J. P., RajBhandary U. L., Sharp P. A. An inducible mammalian amber suppressor: propagation of a poliovirus mutant. Cell. 1987 Jul 31;50(3):379–389. doi: 10.1016/0092-8674(87)90492-2. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sompayrac L. M., Danna K. J. Efficient infection of monkey cells with DNA of simian virus 40. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7575–7578. doi: 10.1073/pnas.78.12.7575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring D. N., Venegas A., Rutter W. J. Yeast tRNA3Leu gene transcribed and spliced in a HeLa cell extract. Proc Natl Acad Sci U S A. 1981 Oct;78(10):5963–5967. doi: 10.1073/pnas.78.10.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel M. C., Abelson J. Effect of intron mutations on processing and function of Saccharomyces cerevisiae SUP53 tRNA in vitro and in vivo. Mol Cell Biol. 1986 Jul;6(7):2663–2673. doi: 10.1128/mcb.6.7.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple G. F., Dozy A. M., Roy K. L., Kan Y. W. Construction of a functional human suppressor tRNA gene: an approach to gene therapy for beta-thalassaemia. Nature. 1982 Apr 8;296(5857):537–540. doi: 10.1038/296537a0. [DOI] [PubMed] [Google Scholar]
- Tollervey D., Wise J. A., Guthrie C. A U4-like small nuclear RNA is dispensable in yeast. Cell. 1983 Dec;35(3 Pt 2):753–762. doi: 10.1016/0092-8674(83)90108-3. [DOI] [PubMed] [Google Scholar]