Abstract
Background and Aims
In bryophytes the sporophyte offspring are in contact with, nourished from, and partially surrounded by the maternal gametophyte throughout their lifespan. During early development, the moss sporophyte is covered by the calyptra, a cap of maternal gametophyte tissue that has a multilayered cuticle. In this study the effects on sporophyte offspring fitness of removing the maternal calyptra cuticle, in combination with dehydration stress, is experimentally determined.
Methods
Using the moss Funaria hygrometrica, calyptra cuticle waxes were removed by chemical extraction and individuals were exposed to a short-term dehydration event. Sporophytes were returned to high humidity to complete development and then aspects of sporophyte survival, development, functional morphology, and reproductive output were measured.
Key Results
It was found that removal of calyptra cuticle under low humidity results in significant negative impacts to moss sporophyte fitness, resulting in decreased survival, increased tissue damage, incomplete sporophyte development, more peristome malformations, and decreased reproductive output.
Conclusions
This study represents the strongest evidence to date that the structure of the calyptra cuticle functions in dehydration protection of the immature moss sporophyte. The investment in a maternal calyptra with a multilayered cuticle increases offspring fitness and provides a functional explanation for calyptra retention across mosses. The moss calyptra may represent the earliest occurance of maternal protection via structural provisioning of a cuticle in green plants.
Keywords: Bryophyte, calyptra, cuticle, dehydration stress, fitness, Funaria hygrometrica, functional morphology, maternal effects, offspring protection, sporophyte development
INTRODUCTION
As the ancestrally dominant generation that both protected and nourished the zygote, the gametophyte played a significant role in the evolution of the multicellular sporophyte in embryophytes. Subsequent reductions in the gametophyte have decreased its influence in the vast majority of plants. However, in bryophytes the gametophyte generation remains influential; sporophyte offspring are in contact with, nourished from, and partially surrounded by the maternal gametophyte throughout their lifespan (Ligrone et al., 1993; Graham and Wilcox, 2000). This prolonged interaction may result in maternal effects that play a significant role in the survival, development, and fitness of the offspring sporophytes.
Protection against the desiccative effects of life above water is critical for the survival of terrestrial plants. An array of adaptations has evolved that decrease water loss from plant bodies (e.g. cuticle, bark, trichomes, scales; Esau, 1977). Of these structures, the plant cuticle is ubiquitous, occurring in taxa from all major lineages of land plants [e.g. mosses (Proctor, 1979), cheilanthoid ferns (Sigel et al., 2011), gymnosperms, Ginkgo (Taylor et al., 1989) and angiosperms, Agave (Wattendorff and Holloway, 1980)]. The cuticle consists of layers of the biopolymers cutin/cutan and waxes that cover and permeate the outermost layers of the cell wall (Holloway, 1982), decreasing intracellular water loss. Cuticle function relating to water loss has been studied primarily in angiosperms and less so in other tracheophyte lineages (e.g. Riederer and Schreiber, 2001; Schreiber and Schönherr, 2009). Cuticles on the leafy gametophytes of mosses may also function in decreasing water loss (i.e. endohydric taxa; Proctor, 1979). Herein we demonstrate experimentally that the cuticle on the maternal moss gametophyte plays a critical role in reducing dehydration stress on developing sporophyte offspring.
The moss sporophyte apex is covered throughout much of its development by the calyptra, a cap of maternal gametophytic tissue. For decades the calyptra has been known to be critical for both sporangium differentiation and sporogenesis (Herzfelder, 1923; Bopp, 1954; French and Paolillo, 1975). The calyptra has been hypothesized to influence the sporophyte in various ways including, mechanical constraint (physical), hormonal secretion (physiological), and desiccation protection. In terms of desiccation protection, we know that young sporophytes survive removal of the calyptrae only when grown at high humidities (Bopp, 1957; French and Paolillo, 1975) and a dye solution ascends sporophytes faster without calyptrae compared with those with calyptrae on the apices (Bopp and Stehle, 1957), suggesting a greater rate of water loss from sporophytes lacking a calyptra. A role in controlling internal sporophyte water balance suggests that the calyptra is covered by a cuticle, a hypothesis raised as early as 1884 by Hy, and only recently confirmed (Hy, 1884; Budke et al., 2011). Determining whether the calyptra cuticle contributes to dehydration protection of the developing sporophyte may add further support to the calyptra's role in maternal protection.
In Funaria hygrometrica the calyptra is covered by a multilayered cuticle that is significantly thicker than that covering the sporophyte or leafy gametophyte, which is fully developed prior to elongation of the underlying sporophyte (Budke et al., 2011). Furthermore, the development of a cuticle on the sporophyte apex is completed only during the maturation of the sporogenous capsule (Budke et al., 2012). Thus water loss from the developing sporophyte is likely to be prevented by the maternal calyptra rather than by structures of the sporophyte itself. While the significance of the calyptra to sporophyte maturation has been demonstrated, the effects on different components of the maturation process remain obscure.
Despite its small size, the calyptra and its cuticle are reasonably amenable to experimentation. The calyptra can be physically removed from the sporophyte, the cuticle waxes chemically extracted, and then replaced on the sporophyte apex. Calyptra vitality is most likely unnecessary for later stages of sporophyte development (Bopp, 1957; French and Paolillo, 1975), and thus any cellular damage caused by the chemical removal of the cuticle is experimentally acceptable. To examine the importance of the maternal calyptra cuticle for the sporophyte offspring, we experimentally removed the calyptra cuticle of the moss F. hygrometrica and then exposed the sporophytes to a short-term dehydration event. If the function of the calyptra cuticle is to prevent dehydration, then we predict that components of sporophyte fitness (i.e. survival, development, functional morphology, reproductive output) will be negatively affected when the calyptra cuticle has been removed compared with those with the calyptra cuticle intact.
MATERIALS AND METHODS
Study taxon
Funaria hygrometrica Hedw. is a pioneer species growing on soil in exposed habitats (Miller and Miller, 2007). Gametangia production is triggered by cool temperatures (Dietert, 1980) and sporophytes typically complete their development from March to April in Connecticut, USA. Sporangia are elevated above the substrate prior to sporogenesis on a relatively long seta (i.e. sporophytes 20–45 mm tall; Miller and Miller, 2007).
Sporophyte production in laboratory populations
Collections were made of F. hygrometrica from five Connecticut populations with developing sporophytes (CONN – Budke #142 East Hartford, #144 Mansfield, #145 Willimantic, #163 Coventry; Goffinet #9027 Canaan). Spores from several sporophytes per site were used to establish laboratory populations for each locality. Two gametophytes from each laboratory population were isolated, grown independently, and the resulting plants were used to create two genetically uniform lineages per population. These gametophytes were grown in the laboratory for 4 months at room temperature (20–25 °C) with 14–16 h of light (50–70 µmol m−2 s−1) in PlantCon containers (MP Biomedicals, Solon, OH, USA) on a rich sandy–loam soil mix, then placed into a cold chamber at 10 °C with 8 h of light (65 µmol m−2 s−1) for 2 months to stimulate gametangia production. Cultures were flooded with deionized water for 24 h, drained and left in the cold chamber for 1 week. Plants were then moved to room temperature with 14–16 h of light (50–70 µmol m−2 s−1) to facilitate sporophyte development.
Experimental design
Calyptra manipulations
Sporophytes <10 mm tall rarely form sporangia when their calyptrae are removed (French and Paolillo, 1975) and are easily damaged when manipulated. Thus, older sporophytes approx. 15–20 mm tall (Fig. 1A), at developmental stage 4 (Table 1), were used in this manipulation experiment. These spear-shaped sporophytes with undifferentiated sporangia were randomly assigned to one of three calyptra manipulations: (1) unmanipulated control; (2) calyptra physically removed from the sporophyte apex then rinsed in water followed by replacement (manipulation control, calyptra cuticle intact); or (3) calyptra removed then rinsed in chloroform followed by replacement (calyptra cuticle-removal). These manipulations were performed using a dissecting microscope and fine forceps in water. For the two removal–replacement manipulations, calyptrae were removed and sporophytes were maintained in the dark in water for 5 min, while the calyptrae were rinsed. Individual calyptrae were either rinsed in a single change of deionized water for 30 s (manipulation control), or in chloroform for 1 min, two changes of 95 % ethanol for 30 s each, and three changes of deionized water for 30 s each, to remove the chloroform-soluble waxes and lipids of the calyptra cuticle.
Fig. 1.
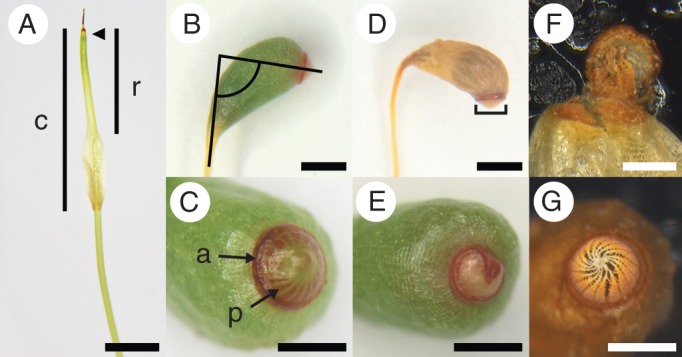
Sporophytes of Funaria hygrometrica. (A) Manipulation stage, spear-shaped sporophyte, sporangium unexpanded, 15 − 20 mm tall, covered by a gametophytic calyptra (c) with narrow rostrum (r) region at the apex (Table 1, developmental stage 4); arrow indicates sporophyte apex beneath calyptra. (B, C) Sporophyte with fully expanded sporangium and red annulus (a) (Table 1, developmental stage 9); (B) lines illustrate angle of sporangium inclination; (C) red peristome teeth (p) visible through the sporangium lid. (D) Air-dried sporophyte with a darker spore-filled region visible through the sporangium wall; lines illustrate measure of annulus diameter. (E) Annulus malformed and with a smaller diameter; the annulus is non-circular, penetrating the centre of the sporangium lid. (F) Peristome malformation with some peristome teeth fused to the inside of the sporangium lid; disabling sporangium dehiscence. (G) Sporangium lid naturally dehisced with lid now absent; revealing mature, properly formed, peristome teeth. Scale bars: (A, B, D) = 1 mm; (C, E, G) = 0·5 mm; (F) = 0·25 mm.
Table 1.
Sporophyte developmental stages of Funaria hygrometrica prior to sporangium dehiscence
| Developmental stage | Sporophyte |
|---|---|
| 4 | Sporangium unexpanded, spear-shaped |
| 5 | Sporangium expanding |
| 6 | Sporangium filling the calyptra inflated base |
| 7 | Annulus visible and green |
| 8 | Annulus pink |
| 9 | Peristome teeth visible and red, annulus red |
Stages 4 and 9 shown in Fig. 1. All other stages are illustrated in Budke et al. (2012, fig. 1).
Transmission electron microscopy (TEM)
The anatomical effects of chloroform rinsing were examined for calyptrae from unexpanded sporophytes (Fig. 1A and Table 1) identical to the individuals used in the experiment. Calyptrae were air-dried (n = 3), rinsed in a single change of deionized water for 30 s (n = 3), or rinsed with a sequence of chloroform (1 × 1 min), 95 % ethanol (2 × 30 s), and deionized water (3 × 30 s; n = 3). Calyptrae were then split longitudinally, embedded in Spurrs resin (Pelco, Redding, CA, USA), sectioned transversely at 100 µm thick using a Ultrotome III (LKB Produktor, Stockholm, Sweden), stained (potassium permanganate, uranyl acetate, lead citrate), and examined using a Technai Botwin (FEI Electron Optics, Eindhoven, The Netherlands) as outlined in Budke et al. (2011).
Dehydration treatments
Post-rinsing, calyptrae were carefully replaced on the original sporophyte apex. Some sporophytes expanded slightly during the 5 min of calyptra removal (pers. obs.). Only individuals with sporophytes fitting back into the calyptrae and filling at least two-thirds of the rostrum length (Fig. 1A) were retained in the experiment. Individuals were randomly assigned a dehydration treatment, PlantCon container, and location in a container (16 individuals were spaced in a grid pattern in each 10 × 10 cm container). Any individuals that were damaged during the manipulation and did not survive overnight were removed from the experiment. The following day, individuals underwent a dehydration treatment for 6 h at 50 %, 70 %, or 99 % relative humidity (RH) at 20·5 °C in an Enconair GR-27 growth chamber (Enconair, Winnipeg, Manitoba, Canada). Individuals were then returned to 99 % RH at room temperature with 16 h light. Plant containers were randomly assigned a bench location during both the dehydration treatment and when returned to 99 % RH. Containers were rotated every 3 or 4 d and watered with 25 mL of water every 6 or 7 d after the dehydration treatment.
Morphological and developmental observations
Sporophyte height, sporophyte apex shape (pointed or rounded), calyptra length, and number of maternal gametophyte leaves were determined for all individuals prior to manipulation. The sporophyte developmental age and the size of the maternal gametophyte may influence the sporophyte response variables and thus were analysed as covariates. Sporophytes were evaluated at 10, 21, and 35 d post-manipulation for survival, dehydration damage (sporophyte tissue brown, categories: proximal [1], distal [2], proximal and distal [3], entire [4]), and sporophyte developmental stage (Table 1).
Thirty-five days post-manipulation, sporophyte height was measured to the nearest millimetre using a ruler and dissecting scope. Lateral photos were taken of each sporangium using a Leica WILD M10 dissecting microscope (Leica, Vienna, Austria) and MicroPublisher 3·3 RTV camera (QImaging Corp., Burnaby, British Columbia, Canada). The following observations and measurements were made from these images using Adobe Photoshop CS3 (Adobe Systems Inc., San Jose, CA, USA): sporangium inclination (angle between a line through the centre of the seta and one perpendicular to the annulus; Fig. 1B); peristome formation (absent or present, red colored and visible through the operculum; Fig. 1C); annulus formation (categories: absent, immature green, mature red; Fig. 1C) and diameter (Fig. 1D). Sporangia were cut with 1 mm of the seta attached and dried for at least 2 weeks in paper envelopes at room temperature under 25 % RH. Dried sporangia (Fig. 1D) were weighed using a Cahn C-33 microbalance (Thermo Electron Corp., Beverly, MA, USA) to the nearest microgram. We dissected sporophytes with expanded sporangia to determine whether or not any spores were produced and to assess any malformations of the operculum, annulus, and peristome teeth.
For the spore-producing capsules spore number per sporangium (subset of individuals only), spore size, and spore germination rate were assessed. Spores were emptied into a clear 1·5-mL plastic tube in 0·5 mL of sterile deionized water and vortexed for 30 s. Immediately after mixing, 30 µL of the spore suspension was added to a Hausser counting chamber (Hausser Scientific, Horsham, PA, USA) and both the obviously shrivelled/empty spores (inviable) and viable spores in the gridded field were counted (Shaw, 1991). Mixing and counting was repeated 3× for each sporangium to assess average spore number per sporangium. Additionally the diameters of 60 spores per sporangium were measured using a Leica DMLB compound microscope and ocular micrometer to the nearest 1·5 µm to assess average spore size. Twenty-five microlitres of the spore suspension and 600 µL of sterile deionized water were added to and spread across each of three replicate Petri dishes containing a modified Knop sterile media (Collier and Hughes, 1982) with 7·5 g L−1 of Gelrite (Research Products Intl. Corp., Mount Prospect, IL, USA) used in place of agar. Dishes of spores were grown at 20·5 °C with 16 h light in an Enconair GR-27 growth chamber at a randomly assigned bench location. After 4 d, germination rates were assessed based on the first 90 spores encountered along two parallel transects across the dish. A spore was scored as having germinated when a protonemal filament was visible (Shaw, 1990).
Statistical analyses
A series of linear and generalized linear mixed models were used to assess the main effects of the experimental manipulations and dehydration treatments on the response variables of sporophyte development, morphology, and reproductive output. In addition, the pre-manipulation measures (see above) were included as covariates and were tested for correlations. All of these were considered fixed effects, with population and manipulation date included as random effects. Both linear and quadratic interactions between the main effects were included when the model fit (AIC) was significantly improved (Littell et al., 2006). Analyses were performed using Proc MIXED or Proc GLIMMIX in the program SAS v. 9·1·3 (SAS Institute, Cary, NC, USA). Significance tests were performed using Kenward–Roger approximations for degrees of freedom (SAS Institute, 2005). Response variables were continuous, categorical, or binary, and modelled as outlined below. A cumulative logit model was used for ordered categorical response variables with three or more categories (SAS Institute, 2005). Whenever we detected a significant manipulation by dehydration interaction (linear or quadratic) we used Tukey-adjusted least-squares means and the associated 95 % confidence intervals to determine whether the manipulations differed by dehydration level. This test corrects for multiple comparisons and accounts for unequal sample sizes, which occurred when analysing reduced datasets of only individuals that developed a sporangium or only individuals that made spores.
Sporophyte development
Sporophyte survival (binary) was assessed using a survival analysis (Proc LIFEREG in SAS) to account for both right and interval censored data. Sporophyte dehydration damage and sporophyte developmental stage (both ordered categorical variables) were analysed initially as repeated measures from 10, 21, and 35 d post-manipulation to explore the effect of time on the results and then at only the last time step to examine the main experimental effects at the end of sporophyte development.
Sporophyte functional morphology
Sporophyte growth, annulus diameter, and sporangium inclination were analysed using MANOVA to account for intertrait correlations between these continuous variables. Sporophyte growth was calculated as the difference in height between the sporophytes measured pre-manipulation and 35 d post-manipulation; these were log-transformed to normalize the data prior to analysis. For a significant MANOVA, we performed protected ANOVAs on individual response variables.
Annulus and peristome formation (categorical and binary, respectively), as well as, annulus, operculum, and perisome malformations (all binary) were analysed for a subset of the data composed of only the individuals that produced a sporangium. Correlations among the formation variables and malformation variables were tested prior to further analysis.
Reproductive output
Spore production (binary) was analysed for all individuals and a subset that produced sporangia. Spore number per sporangium was examined relative to sporangium weight using a simple linear regression model to determine the strength and significance of the relationship between these variables. Sporangium weight (continuous) was subsequently analysed for a subset of the data composed of only the individuals that produced a sporangium. The percentage spore germination (categorical) was arcsin transformed to account for this data being bounded at 0 and 100 %. Spore germination and average spore size (both continuous) were analysed for only the individuals that produced spores.
RESULTS
Sporophyte production in laboratory populations
Of the ten genetically uniform gametophytic lines produced, only six produced sufficient numbers of sporophytes to be used in preliminary versions of this experiment (data not shown). For the experimental results presented here, three genetically uniform lines from three independent collections (Cannan, Mansfield, Willimantic), were used. These laboratory-grown populations produced 171 sporophytes, between 13·0 and 21·5 mm in height, that were included in the experiment over five manipulation days. The two-way factorial complete block design (manipulation × dehydration treatment) resulted in sample sizes of n = 39 for the unmanipulated control with calyptra intact, n = 66 for the removal then replacement with calyptra cuticle intact, n = 66 for the removal then replacement with the calyptra cuticle-removed, with each of these groups divided evenly between the three dehydration levels (50, 70, 99 % RH). The three genetically uniform lines composed 32 % (Cannan), 45 % (Mansfield) and 23 % (Willimantic) of the individuals in the experiment and were represented in these proportions across all treatments [e.g. 99 % RH with calyptra cuticle-removed n = 7 (Cannan), n = 10 (Mansfield), n = 5 (Willimantic)].
Manipulation experiment
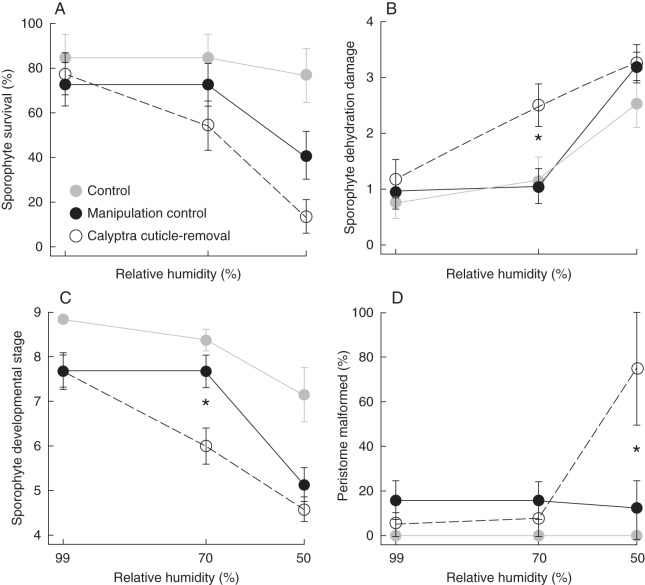
Correlations between the pre-manipulation measures of the sporophyte, calyptra, and maternal leafy gametophyte (covariates) were tested (Pearson product–moment correlation). The gametophyte traits were significantly correlated with each other, and also the sporophyte characters were significantly correlated with each other (P < 0·001 for both); however, the correlation coefficients were not large (0·39 and 0·31, respectively). Thus, all pre-manipulation measures were retained as independent covariates in the models. Calyptra removal and replacement alone had a negative effect on sporophyte survival (Fig. 2A). Therefore we present the results for the unmanipulated control (Figs 2–4, in grey) but we compare and analyse only the manipulation control (removal then replacement with calyptra cuticle intact) and the calyptra cuticle-removal (removal then replacement with the chloroform-rinsed calyptra).
Fig. 2.
Developmental and morphological results from 35 d post-manipulation of the manipulation treatments across three dehydration levels with ± s.e. Unmanipulated control with calyptra intact (grey circles); calyptra removal then replacement with cuticle intact (manipulation control, black circles); removal then replacement with the calyptra cuticle-removed (open circles). Statistically significant differences between the manipulation control and calyptra cuticle-removal within dehydration level are indicated with an asterisk. (A) Percentage sporophyte survival; (B) average sporophyte dehydration damage (proximal [1], distal [2], proximal and distal [3], entire [4]); (C) average sporophyte developmental stage; (D) percentage of sporophytes that formed a sporangium with a malformed peristome.
Fig. 4.
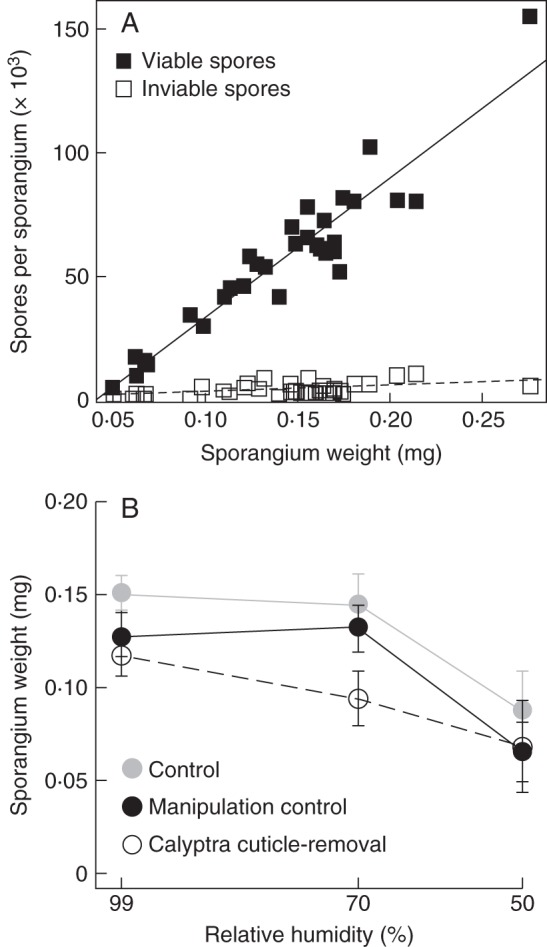
Reproductive results from 35 d post-manipulation. (A) Sporangium weight and average number of spores per sporangium for 31 sporophytes. Average number of viable spores (black squares, regression line continuous) and inviable spores (white squares, regression line dashed). (B) Average sporangium weight of the manipulation treatments across three dehydration levels with ± s.e. for sporophytes that formed a sporangium. Unmanipulated control with calyptra intact (grey circles); calyptra removal then replacement with cuticle intact (manipulation control, black circles); removal then replacement with the calyptra cuticle-removed (open circles).
Cuticle removal
When rinsed with only water, the calyptra cuticle retained all of the anatomical layers observed in unrinsed specimens (Fig. 5A, compare with figs 4B and 5B in Budke et al., 2011). Calyptrae rinsed with chloroform, then ethanol and water to remove the chloroform completely, often lack the exterior-most cuticle layer, the electron-dense cuticle proper, and all epicuticular waxes (Fig. 5B). Additional intracuticular waxes may have been chemically altered or extracted by the rinses, but these changes if present were not visible anatomically.
Fig. 5.
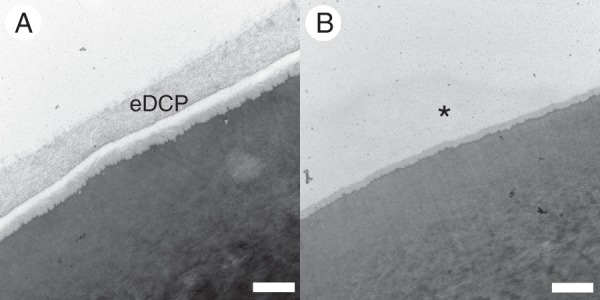
Transmission electron micrographs of transverse sections through the calyptra rostrum of Funaria hygrometrica. The outermost edge of the epidermal cells are shown. The upper edges are facing the exterior environment. (A) Rinsed with water only prior to processing for TEM; the calyptra cuticle is completely intact; the electron-dense cuticle proper (eDCP) layer is present. (B) Rinsed with chloroform, ethanol and water; a portion of the calyptra cuticle has been removed; the eDCP layer is absent and noted by an asterisk. Scale bars = 500 nm.
Sporophyte development
Sporophyte survival (Fig. 2A) is significantly affected by the cuticle treatment (Wald χ2 = 4·522, d.f. = 1, P < 0·05), the dehydration treatment (Wald χ2 = 24·190, d.f. = 1, P < 0·0001), and the covariate calyptra length (Wald χ2 = 5·407, d.f. = 1, P < 0·05). A significant interaction between the two main experimental effects was lacking, and thus these data were not analysed further.
The cuticle treatment did not have a significant effect on either sporophyte dehydration damage or sporophyte developmental stage in a repeated measure analysis of data from 10, 21, and 35 d post-manipulation. Examined separately, dehydration damage at 35 d was significantly explained by the cuticle (F1,120 = 5·36, P < 0·05) and dehydration treatments (F1,120 = 47·50, P < 0·0001), including a quadratic interaction between these main effects (c × d × d: F1,120 = 5·73, P < 0·05). Using differences in least squares means, a significant difference in the level of dehydration damage was found between the cuticle treatments at the 70 % RH dehydration level (Fig. 2B). Sporophytes with their calyptra cuticle removed have significantly higher levels of dehydration damage. Sporophyte developmental stage at 35 d was also significantly explained by the cuticle (F1,118 = 5·97, P < 0·05) and dehydration treatments (F1,118 = 57·16, P < 0·0001), including a quadratic interaction between these main effects (c × d × d: F1,118 = 4·76, P < 0·05). A significant difference in the sporophyte developmental stage was found between the cuticle treatments at the 70 % RH dehydration level (Fig. 2C). Sporophytes with their calyptra cuticle intact progressed to a significantly later developmental stage (Table 1 and Fig. 1B, C).
Sporophyte functional morphology
Sporangia were produced under both cuticle treatments and at all dehydration levels. The dehydration treatment significantly affected sporophyte growth, annulus diameter, and sporangium inclination (MANOVA, dehydration treatment F3,67 = 5·17, P < 0·005); the covariate apex shape was also significant (F3,67 = 2·76, P < 0·05). In subsequent univariate analyses, the following factors were significant: sporophyte growth was affected by apex shape (F = 7·29, d.f. = 1, P < 0·001); annulus diameter and sporangium inclination were significantly affected by the dehydration treatment (F = 13·46, d.f. = 1, P < 0·001; F = 7·91, d.f. = 1, P < 0·01, respectively). Quadratic and linear interactions between the cuticle and dehydration treatments were not significant.
Mature annulus and peristome formation were significantly correlated (r = 0·94, d.f. = 29, P < 0·0001); individuals lacking or with an immature annulus never formed a peristome, whereas the vast majority of individuals with a mature annulus developed a peristome. Therefore these responses were combined into a single categorical variable, with annulus and peristome fully formed added as a fourth category to the three categories of annulus formation (see Materials and methods above). The combined variable of annulus and peristome formation had a significant quadratic interaction between the main effects (c × d × d: F1,72 = 4·29, P = 0·042). Despite this interaction, the least squares means between cuticle treatments within dehydration levels did not differ significantly (data not shown).
Malformations to the annulus, operculum, and/or perisome teeth occurred in 15 % of the individuals that produced sporangia. Malformations of the annulus and operculum were due to incomplete separation from the underlying peristome teeth, which may prevent sporangia from opening at maturity (Fig. 1E, F). Hence, annulus and peristome, as well as operculum and peristome malformations were significantly correlated (r = 0·62, d.f. = 80, P < 0·0001 and r = 0·55, d.f. = 80, P < 0·0001, respectively). Annulus and operculum malformations were not significantly correlated (data not shown). Peristome malformation was significantly explained by a linear interaction between the main effects (c × d: F1,71 = 5·61, P < 0·05). Significant differences in peristome malformation using the least squares means were found between cuticle treatments at 50 % RH (Fig. 2D). Sporophytes with their calyptra cuticle removed when exposed to a relatively severe dehydration event are more likely to have peristome malformations (Fig. 1F).
Reproductive output
Spore production is significantly affected by both cuticle (F1,116 = 6·60, P < 0·05), and dehydration treatments (F1,116 = 57·61, P < 0·0001), including a quadratic interaction between these main effects (c × d × d: F1,116 = 5·13, P < 0·05). A significant difference in the proportion of individuals that produced spores was found between the cuticle treatments at 70 % RH (Fig. 3A); sporophytes with their calyptra cuticle removed are significantly less likely to produce spores (36 % compared with 77 %). When only the individuals that produced sporangia are considered, only the dehydration treatment significantly affected spore production (F1,72 = 13·78, P < 0·001). Though not significantly different using the least squares means, a similar trend emerges from this subset of the data; a lower proportion of sporangia produce spores when their calyptra cuticle is removed at the 70 % RH dehydration level (Fig. 3B).
Fig. 3.
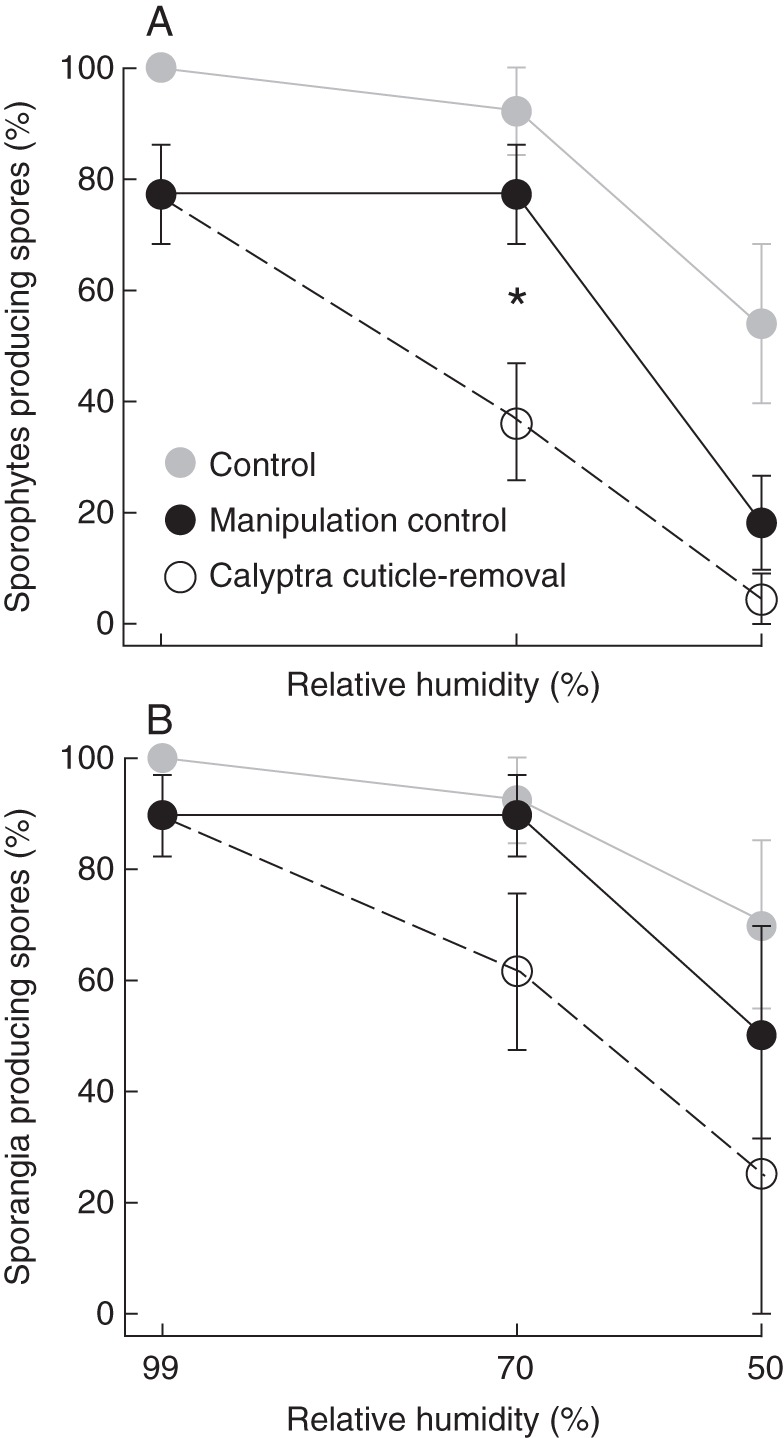
Reproductive results from 35 d post-manipulation of the manipulation treatments across three dehydration levels with ± s.e. Unmanipulated control with calyptra intact (grey circles); calyptra removal then replacement with cuticle intact (manipulation control, black circles); removal then replacement with the calyptra cuticle-removed (open circles). Statistically significant differences between the manipulation control and calyptra cuticle-removal within dehydration level are indicated with an asterisk. (A) Percentage of sporophytes that produced spores; (B) percentage of sporophytes with sporangia that produced spores.
The average number of spores per sporangium was estimated for a subset of the individuals that produced spores (n = 31). Sporangium weight and number of viable spores per sporangium were highly correlated (r2 = 0·88, d.f. = 29, P < 0·0001; Fig. 4A). Thus sporangium weight was used as a proxy for the number of viable spores per sporangium. For individuals that produced a sporangium, the dehydration treatment significantly contributed to differences in sporangium weight (F1,72 = 10·39, P < 0·005); additionally the pre-manipulation number of gametophyte leaves and sporophyte length significantly contribute to differences in sporangium weight (F1,72 = 4·85, P < 0·05; F1,72 = 5·86, P < 0·05, respectively). Despite the lack of interaction between the cuticle and dehydration treatments in the model, a pattern similar to spore production was present in the data; the largest difference in mean sporangium weight occurred between the cuticle treatments at 70 % RH (Fig. 4B). Using the regression to calculate viable spore number from sporangium weight, sporophytes at 70 % RH with the calyptra cuticle intact have on average 52 006 viable spores per sporangium whereas sporophytes without the calyptra cuticle have on average 30 523 viable spores. This represents a 40 % decrease in the reproductive output of sporophytes when cuticles were experimentally removed from calyptrae and individuals were exposed to an intermediate level of dehydration.
For only individuals that produced spores, none of the main effects or covariates significantly affected spore germination. Spore size, however, was significantly explained by a cuticle treatment by dehydration treatment interaction (c × d: F1,53 = 4·16, P = 0·046), as well as a quadratic interaction of dehydration (d × d: F1,53 = 4·16, P = 0·046). Additionally the pre-manipulation number of gametophyte leaves significantly contributes to differences in spore size (F1,53 = 4·07, P = 0·049). Despite these interactions, the least squares means across the treatments did not differ significantly (data not shown).
DISCUSSION
The plant cuticle is a critical structure for desiccation protection of sporophytes in vascular plants (Riederer and Schreiber, 2001). Here we demonstrate that the cuticle plays a similar role in bryophytes but, unlike in vascular plants, this cuticle is produced by a maternal gametophyte tissue. Our experiment demonstrates a direct connection between a maternal gametophyte structure (i.e. the calyptra cuticle) and its functional importance for sporophyte offspring. Without the calyptra cuticle, dehydration during early development disrupts sporophyte maturation, resulting in decreased survival, increased tissue damage, incomplete sporophyte development, more peristome malformations, and decreased spore production. Thus the protection of the calyptra cuticle is crucial for maximizing sporophyte fitness and provides a functional explanation for calyptra retention across mosses.
The effect of removing the calyptra cuticle strongly differs across the humidity levels (Figs 2–4). Under high relative humidity (99 %), the calyptra cuticle is unnecessary for dehydration protection, since little water loss is occurring, and thus a cuticle treatment effect at this dehydration level is never significant (Figs 2–4). At lower relative humidities (50 % and 30 %, the latter used in preliminary experiments) even sporophytes covered by a calyptra with an intact cuticle suffered negative effects (Figs 2–4), suggesting that the calyptra cuticle did not provide sufficient protection against water loss under extreme dry conditions. The presence of an intact cuticle on the calyptra makes the most difference for sporophyte development and fitness at the intermediate relative humidity (70 %; Figs 2–4). Sporophytes of F. hygrometrica develop during the relatively, milder and wetter months of early spring (pers. obs.); however, variation in microenvironments may significantly effect the daily humidity fluxuations that the mosses experience (e.g. Sonnleitner et al., 2009). How closely these experimental conditions approximate the levels of dehydration stress sporophytes of F. hygrometrica experience in the wild have yet to be determined.
Sexual reproduction begins with fertilization but is only truly successful when spores are dispersed from the sporophyte and establish new populations. The annulus and peristome are two specialized structures that aid in moss spore dispersal: the annulus is a ring of inflated cells that enables the sporangium to open and then the peristome controls spore release via humidity-dependent movement [i.e. arthrodontous mosses (Crum, 2001); e.g. F. hygrometrica]. When the calyptra is retained but the cuticle is removed under high dehydration stress, more sporangia have malformed peristome teeth (Fig. 2D), which are regularly fused with the lid or annulus, disabling sporangium dehiscence (Fig. 1F). The undifferentiated cells that will form these structures are located at the apex of the sporophyte (Fig. 1A), which is covered by the calyptra and its cuticle, but lacks its own cuticle early during development (Budke et al., 2012). The sporophyte exhibits conspicuous malformations when the entire calyptra is removed (Herzfelder, 1923; Bopp, 1954), and it is now clear that the calyptra cuticle is also required for proper sporophyte development by protecting these structures from dehydration.
Two other sporangial features important for spore dispersal were significantly affected by lower relative humidity, independent of the calyptra cuticle. Sporophytes have a narrower mouth through which spores are released (annulus diameter; compare parts C and E in Fig. 1) and form more upright sporangia, which may slow passive spore release compared with an oblique or nearly horizontal mouth (sporangium inclination; compare parts B and D in Fig. 1). Sporangium inclination results from differential elongation of cells on one side of the sporangium and, as previously mentioned, the annulus is composed of inflated cells (Crum, 2001), thus both of these features rely on cell expansion for proper development. The lower relative humidity and increased evaporation from the sporophyte may result in decreased turgor pressure, which is required for cell expansion (Ray et al., 1972).
The development of structures that assist in spore dispersal is superfluous without spore production. When the calyptra cuticle is removed and sporophytes are exposed to a lower relative humidity, reproductive output is negatively impacted at two levels. Individuals regularly do not produce any spores (Fig. 3) and may not even initiate meiosis, and even when sporophytes do undergo meiosis, the average number of spores per sporangium is lower (Fig. 4B). Thus the calyptra and the dehydration protection that it provides are critical not only for the development of spore dispersal structures, but overall sporophyte fitness.
The calyptra is only one component of maternal care that may influence moss sporophyte development. In all bryophytes, maternal care includes both nutrient and water transfer from gametophyte to sporophyte via the placenta (Ligrone et al., 1993), and the size of the maternal leafy gametophyte may therefore affect sporophyte development (Shaw and Beer, 1997). As the sporophyte tissues are exposed from beneath the covering of the calyptra they develop a multilayered cuticle (Budke et al., 2012) that most likely limits direct water uptake from the surrounding environment. Thus the maternally supplied, internally transported water (Ligrone et al., 2000) must be essential for maintaining sporophyte hydration. Without the calyptra and its cuticle covering the apical tissues, which have yet to develop their own multilayered cuticle (Budke et al., 2012), this critical supply of water would be rapidly lost through transpiration. Both of these components of maternal care are required for successful development of the sporophyte offspring, and may be especially important for taxa growing in habitats with fluctuating atmospheric moisture.
In mosses, sporophytes may be poorly equipped to cope with abiotic stresses compared with their leafy gametophytes. As has been shown in other species, sporophytes are more sensitive to both desiccative (Tortula; Stark et al., 2007) and temperature (Microbryum; McLetchie and Stark, 2006) stresses than their associated gametophytes, suggesting that physiological mechanisms for desiccation tolerance (e.g. Oliver et al., 2005; Khandelwal et al., 2010) are not as well developed or are lacking in sporophytes. Since both generations contain identical genomes that only in differ in their ploidy level, these physiological differences are most likely due to differential gene expression. The relatively short-lived nature of the moss sporophyte and/or the protective abilities of the calyptra may have reduced the selective pressures on this life stage to evolve or co-opt physiological strategies to tolerate desiccation.
The ability of sporophytes to tolerate water stress and calyptrae to prevent it may also be environmentally plastic. Irmscher (1912) noted that wild-grown sporophytes were more robust to desiccation treatments than those that were grown in the laboratory. This may also extend to the calyptra with wild-developed calyptrae having even greater dehydration protection abilities. Thus the observations made during experimental studies, such as ours, may not represent the environmentally realized abilities of the sporophyte to survive and and the calyptra to prevent dehydration. Ecological developmental studies (i.e. eco-devo; Sultan, 2007), may bridge the gap between laboratory experiments and plants growing in the wild, but are currently underexplored in mosses.
In mosses, the undifferentiated apical region experiences higher levels of dehydration as it is elevated during seta elongation, escaping the buffering effects of the boundry layer that surrounds the leafy gametophyte (Rice and Schneider, 2004). The protective abilities of the maternal calyptra allow sporangium differentiation to be delayed and may have enabled the evolution of taller sporophyte offspring with permanent seta. Without the calyptra, it may have been necessary for the sporangium to complete its development prior to seta elongation, as occurs in liverworts. Liverworts elevate their fully differentiated sporangia on an ephemeral seta, via cell elongation, and spore dispersal occurs as a single event over a relatively short period of time (Crum, 2001). The ability of mosses to build a permanent seta that enables spore dispersal over an extended time period represents a distinct evolutionary advantage, which may have contributed to the greater number of moss species compared with liverworts [9000–13 000 (Magill, 2010) to 5000–6000 (von Konrat et al., 2010), respectively].
In conclusion, this study demonstrates that removal of the maternal calyptra cuticle negatively impacts development and reproductive output of the sporophyte offspring. The experimental evidence shown here and our observations of a specialized cuticle on the calyptra that develops precociously relative to the sporophyte cuticle (Budke et al., 2011, 2012), support the hypothesis that the calyptra cuticle is a structural adaptation that functions to prevent dehydration of the immature sporophyte apex. This role in dehydration prevention is in addition to any mechanical or physiological roles that the calyptra may play. The cuticle on the maternal calyptra is analogous to the cuticle layers provided by the maternal sporophyte of angiosperms on fruits and seeds that protect these organs from dehydration (Esau, 1977). Thus the moss calyptra may represent the earliest occurrence of maternal protection via structural provisioning of a cuticle in green plants.
ACKNOWLEDGEMENTS
We thank L.A. Lewis and members of the Goffinet and Jones laboratories for comments on earlier versions of this manuscript. We appreciate the assistance of L. R. Lewis, K. Mocko, K. H. Sinclair and M. A. Wynne with preliminary trials of this experiment and data collection. We thank J. E. Carlson for statistical advice. This research was supported by grants from the National Science Foundation (DEB 0919284 to B.G. and C.S.J.), H. N. Andrews Endowment Fund to the Connecticut Museum of Natural History, International Association of Bryologists, Microscopical Society of America, and Botanical Society of America. This research represents a portion of the doctoral dissertation of J.M.B. at the University of Connecticut.
LITERATURE CITED
- Bopp M. Die Wirkung von Maleinhydrazid und Kaliptraextrakt auf die Verdickung von Laubmoossporogonen. Naturwissenschaften. 1954;41:243–245. [Google Scholar]
- Bopp M. Entwicklungsphysiologische Untersuchungen an Mossmutanten. I. Zur Wirkung der Laubmooskalyptra. Zeitschrift für induktive Abstammungs- und Verebungslehre. 1957;88:600–607. [Google Scholar]
- Bopp M, Stehle E. Zur Frage der Wasserleitung in Gametophyten und Sporophyten der Laubmoose. Zeitschrift für Botanik. 1957;45:161–174. [Google Scholar]
- Budke JM, Goffinet B, Jones CS. A hundred-year-old question: is the moss calyptra covered by a cuticle? A case study of Funaria hygrometrica. Annals of Botany. 2011;107:1279–1286. doi: 10.1093/aob/mcr079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budke JM, Goffinet B, Jones CS. The cuticle on the gametophyte calyptra matures before the sporophyte cuticle in the moss Funaria hygrometrica (Funariaceae) American Journal of Botany. 2012;99:14–22. doi: 10.3732/ajb.1100311. [DOI] [PubMed] [Google Scholar]
- Collier PA, Hughes KW. Life cycle of the moss Physcomitrella patens, in culture. Journal of Tissue Culture Methods. 1982;7:19–22. [Google Scholar]
- Crum HA. Structural diversity of bryophytes. Ann Arbor, MI: University of Michigan Herbarium; 2001. [Google Scholar]
- Dietert MF. The effects of temperature and photoperiod on the development of geographically isolated populations of Funaria hygrometrica and Weissia controversa. American Journal of Botany. 1980;6:369–380. [Google Scholar]
- Esau K. Anatomy of seed plants. 2nd edn. New York, NY: John Wiley and Sons; 1977. [Google Scholar]
- French JC, Paolillo DJ., Jr On the role of the calyptra in permitting expansion of capsules in the moss Funaria. The Bryologist. 1975;78:438–446. [Google Scholar]
- Graham LKE, Wilcox LW. The origin of alternation of generations in land plants: a focus on matrotrophy and hexose transport. Philosophical Transactions of the Royal Society B. 2000;355:757–766. doi: 10.1098/rstb.2000.0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzfelder H. Experimente an Sporophyten von Funaria hygrometrica. Flora. 1923;116:476–490. [Google Scholar]
- Holloway PJ. Structure and histochemistry of plant cuticular membranes: an overview. In: Cutler D, Alvin K, Price C, editors. The plant cuticle. Vol. 10. London: Academic Press; 1982. pp. 1–32. [Google Scholar]
- Hy LF. Recherches sur l'archéchone et le développement du fruit des muscinées. Vol. 60. Paris, France: Librairie de l'Académe de Médecine; 1884. pp. 105–206. [Google Scholar]
- Irmscher E. Über die Resistenz der Laubmoose gegen Austrocknung und Kälte. Jahrbücher für wissenschaftliche Botanik. 1912;50:387–449. [Google Scholar]
- Khandelwal A, Cho SH, Marella H, et al. Role of ABA and AB13 in desiccation tolerance. Science. 2010;327:546. doi: 10.1126/science.1183672. [DOI] [PubMed] [Google Scholar]
- von Konrat M, Söderström L, Renner MAM, Hagborg A, Briscoe L. Early Land Plants Today (ELPT): how many liverwort species are there? Phytotaxa. 2010;9:22–40. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. The gametophyte–sporophyte junction in land plants. Advances in Botanical Research. 1993;19:231–317. [Google Scholar]
- Ligrone R, Duckett JG, Renzaglia KS. Conducting tissues and phyletic relationships of bryophytes. Philosophical Transactions of the Royal Society B. 2000;355:795–813. doi: 10.1098/rstb.2000.0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2nd edn. Carey, NC: SAS Institute Inc; 2006. [Google Scholar]
- McLetchie DN, Stark LR. Sporophyte and gametophyte generations differ in their thermotolerance response in the moss Microbryum. Annals of Botany. 2006;97:505–511. doi: 10.1093/aob/mcl011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magill RE. Moss diversity: new look at old numbers. Phytotaxa. 2010;9:167–174. [Google Scholar]
- Miller DH, Miller HA Flora of North America Editorial Committee. Flora of North America. Vol. 27. New York, NY: Oxford University Press; 2007. 12·3 Funaria; pp. 118–194. Bryophyta, Part 1. [Google Scholar]
- Oliver MJ, Velten J, Mishler BD. Desiccation tolerance in bryophytes: a reflection of the primitive strategy for plant survival in dehydrating habitats? Integrative and Comparative Biology. 2005;45:788–799. doi: 10.1093/icb/45.5.788. [DOI] [PubMed] [Google Scholar]
- Proctor MCF. Surface wax on the leaves of some mosses. Journal of Bryology. 1979;10:531–538. [Google Scholar]
- Ray PM, Green PB, Cleland R. Role of turgor in plant cell growth. Nature. 1972;239:163–164. [Google Scholar]
- Rice SK, Schneider N. Cushion size, surface roughness, and the control of water balance and carbon flux in the cushion moss Leucobryum glaucum (Leucobryaceae) American Journal of Botany. 2004;91:1164–1172. doi: 10.3732/ajb.91.8.1164. [DOI] [PubMed] [Google Scholar]
- Riederer M, Schreiber L. Protecting against water loss: analysis of the barrier properties of plant cuticles. Journal of Experimental Botany. 2001;52:2023–2032. doi: 10.1093/jexbot/52.363.2023. [DOI] [PubMed] [Google Scholar]
- SAS Institute. The GLIMMIX procedure. Cary, NC: SAS Institute; 2005. November 2005. [Google Scholar]
- Schreiber L, Schönherr J. Water and solute permeability of plant cuticles. Berlin: Springer; 2009. [Google Scholar]
- Shaw AJ. Intraclonal variation in morphology, growth rate, and copper tolerance in the moss, Funaria hygrometrica. Evolution. 1990;44:441–447. doi: 10.1111/j.1558-5646.1990.tb05212.x. [DOI] [PubMed] [Google Scholar]
- Shaw AJ. The genetic structure of sporophytic and gametophytic populations of the moss, Funaria hygrometrica Hedw. Evolution. 1991;45:1260–1274. doi: 10.1111/j.1558-5646.1991.tb04391.x. [DOI] [PubMed] [Google Scholar]
- Shaw AJ, Beer SC. Gametophyte–sporophyte variation and covariation in mosses. Advances in Bryology. 1997;6:35–63. [Google Scholar]
- Sigel EM, Windham MD, Huiet L, Yatskievych G, Pryer KM. Species relationships and farina evolution in the cheilanthoid fern genus Argyrochosma (Pteridaceae) Systematic Botany. 2011;36:554–564. [Google Scholar]
- Sonnleitner M, Dullinger S, Wanek W, Zechmeister H. Microclimatic patterns correlate with the distribution of epiphyllous bryophytes in a tropical lowland rain forest in Costa Rica. Journal of Tropical Ecology. 2009;25:321–330. [Google Scholar]
- Stark LR, Oliver MJ, Mishler BD, McLetchie ND. Generational differences in response to desiccation stress in the desert moss Tortula inermis. Annals of Botany. 2007;99:53–60. doi: 10.1093/aob/mcl238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultan S. Development in context: the timely emergence of eco-devo. Trends in Ecology and Evolution. 2007;22:575–582. doi: 10.1016/j.tree.2007.06.014. [DOI] [PubMed] [Google Scholar]
- Taylor WA, Taylor TN, Archangelsky S. Comparative ultrastructure of fossil and living gymnosperm cuticles. Review of Palaeobotany and Palynology. 1989;59:145–151. [Google Scholar]
- Wattendorff J, Holloway PJ. Studies on the ultrastructure and histochemistry of plant cuticles: the cuticular membrane of Agave americana L. in situ. Annals of Botany. 1980;46:13–28. [Google Scholar]