Abstract
Background and Aims
Most genera of the neotropical Galipeinae (tribe Galipeeae, Rutoideae) exhibit several forms and degrees of fusion between the floral organs, including the union of petals into an apparently sympetalous corolla, the joining of the stamens among themselves and to the corolla, and the partial to complete connation of carpels. Though these and others floral traits are currently used in the circumscription of species in Galipeinae, few studies have shown in detail in which way (postgenital or congenital) and to what extent these fusions occur. To elucidate these anatomical conditions, a structural study of the flowers of the Galipeinae species was carried out.
Methods
Flowers of six species from three genera of Galipeinae were studied in their morphology, anatomy and development with stereomicroscopy, light microscopy and scanning electron microscopy (SEM).
Key Results
The floral tube is formed by synorganization of stamens with petals in all species, and exhibits three main patterns: (1) Conchocarpus heterophyllus and C. minutiflorus have a floral tube formed by marginal coherence/adherence of petals and filaments due to interwining trichomes (postgenital connection); (2) Erythrochiton brasiliensis has a tube formed by congenital fusion of petals and filaments; and (3) Galipea jasminiflora and Conchocarpus macrophyllus have a tube formed distally with the first pattern, and proximally with the second pattern. Although floral tubes seem to be homologous within Galipeinae, this is not true at the level of the family: the floral tube of Correa (from an only distantly related clade of the family) is formed by postgenital union of the petals representing a convergent structure. The gynoecium of the studied species of Galipeinae shows a great variability in the extent of fusion of carpel flanks. Even though different structures for the mature gynoecium were found in each genus, all genera show postgenitally fused carpel apices, which is related to the formation of a compitum, as described earlier for other members of Rutaceae.
Conclusions
The degree and diversity of fusions of floral organs in Galipeinae is unique within the order Sapindales. A study of the amount of diversification of Galipeinae in South America and comparison with other clades of Rutaceae would be of interest.
Keywords: Floral anatomy, floral tubes, floral morphology, false sympetaly, partial apocarpy, syncarpy, postgenital union, floral development
INTRODUCTION
Galipeinae are one of the two subtribes in the tribe Galipeeae [formerly Cuspariinae and Cusparieae in Engler (1931); invalid names according to Kallunki and Pirani (1998)] of the subfamily Rutoideae (Rutaceae). Galipeinae comprise approx. 26 exclusively Neotropical genera and 130 species (Groppo et al., 2008; Kubitzki et al., 2011). The group is distinguished from the rest of the subfamily by its flowers, which are usually tubular and slightly to pronouncedly zygomorphic, with a variable number of staminodes (usually three), and mostly two stamens with anthers commonly bearing basal appendages (Morton and Kallunki, 1993; Kallunki and Pirani, 1998). In many genera, the fertile stamens (usually two) are located on the posterior side of the monosymmetric flower. Since corolla aestivation is ascending cochlear, these two anthers are adjacent to the innermost petal or the innermost two petals (Kubitzki et al., 2011).
Several forms and degrees of fusion between the floral organs have been reported in Galipeinae and these features are currently used to distinguish genera and species in the group. Notably the union of petals into a sympetalous corolla, the union of the stamens among themselves and their fusion with the petals, the union of anthers and their basal appendages, and the partial to complete connation of carpels have been described (e.g. Engler, 1874, 1931; Ramp, 1988; Kallunki, 1992, 1998; Kallunki and Pirani, 1998; Pirani, 1999, 2004; Pirani and Kallunki, 2007; Pirani et al., 2010). In several systematic treatments for genera of Galipeinae, Kallunki and Pirani have reported the participation of trichomes in the connection of floral organs and have used a special terminology for it (e.g. Kallunki, 1992, 1994, 2009; Kallunki and Pirani, 1998; Pirani et al., 2010). In addition to the traditional terms applied for types of union of floral whorls (connation and adnation), they use the term ‘coherent’ when segments of the same whorl are joined only through intertwining trichomes, and ‘adherent’ for the same condition between organs of different whorls. Because in most of these studies the observations were based on stereomicroscopic analysis alone, for the majority of species it is not yet clear how and to what extent the floral organs are really united, and whether or not there is fusion of the organs.
Although not explicitly stated in the works of Kallunki and Pirani, it is clear by their descriptions that in some species the coherence and adherence of perianth organs in Galipeinae are forms of union with a combination of congenital fusion and postgenital coherence. Tube formation in flowers with postgenital coherence was reported from some other eudicots, such as Correa (Rutaceae) (Hartl, 1957). Even a case of tube formation without any coherence was shown for Geranium robertianum (Endress, 2010a). The anatomy of the floral tube of some Galipea species has been described previously (Pirani et al., 2010). Thus it would be of interest to know how such tubes in other genera of Galipeinae are formed.
Recent phylogenetic studies based on molecular data indicate that floral features in Galipeinae are important for the circumscription of some clades emerging from the analyses. Groppo et al. (2008) found that even though the subtribe as currently circumscribed is not monophyletic, the group of species with tubular flowers form a robust clade, suggesting that this feature may be a morphological synapomorphy for this group. Groppo et al. (2008) also showed that Engler's (1931) subfamilial circumscription in Rutaceae based mainly on the degree of carpel connation and fruit dehiscence is inadequate because these characters have been reconstructed as having evolved multiple times in the family. However, their discussion was limited by the lack of detailed information on the kind of union of organs for most species studied. For example, the clade in which tubular corolla species emerged was characterized by the presence of ‘a more or less tubular corolla’, and the optimization of gynoecium characters in the phylogeny was made in the same way: ‘ovary with some degree of apocarpy vs. full syncarpy’ (Groppo et al., 2008). This lack of precision in the morphological states of the analysis is a consequence of the lack of detailed floral studies in Galipeinae.
The only studies that have demonstrated these unions in some detail for a few species of Galipeinae were those by Ramp (1988) and Pirani et al. (2010), who both reported different types of unions between floral whorls. Ramp (1988) studied gynoecium development and anatomy of several members of Rutaceae, including only a single member of Galipeinae, Erythrochiton brasiliensis, in which postgenital fusion of carpel apices was described, but only for anthetic flowers. Pirani et al. (2010) analysed the floral structure (based on microtome sections) of five species of Galipea, and found that the floral tube is formed by adnation of stamens to petals in their basal third and by coherence of petals and adherence of stamens to petals in their upper part. They also found that the gynoecium in these species is not completely syncarpous as previously described. These findings indicate that the fusion within and between whorls in Galipeinae flowers is quite complex. The need for studies of more taxa in order to better understand flower structure and its variation among related groups, and consequently its evolution, motivated the present study. Our aim is to investigate how and to what extent the floral organs are united in five selected species of three different genera of Galipeinae, using macroscopic and microscopic analysis, and to discuss the evolutionary implications of the results.
MATERIALS AND METHODS
Flowering material of the following taxa was studied: Conchocarpus heterophyllus (A.St.-Hil.) Kallunki and Pirani, Conchocarpus minutiflorus Groppo and Pirani, Conchocarpus macrophyllus J.C. Mikan, Erythrochiton brasiliensis Nees and Mart. and Galipea jasminiflora (A.St.-Hil.) Engl. Materials were collected in the Atlantic Forest of Espírito Santo, Brazil (C. minutiflorus, collection number Groppo 1617 and Pirani 6126; C. macrophyllus, Zuntinni 151, El Ottra 96; G. jasminiflora, El Ottra 137), Minas Gerais (G. jasminiflora, Pirani 4923) and São Paulo (G. jasminiflora, El Ottra 233). Flowers of C. heterophyllus (El Ottra 11) and E. brasiliensis (El Ottra 236) were collected from cultivated plants at the garden of the Instituto de Biociências da Universidade de São Paulo (São Paulo, Brazil). Voucher specimens are deposited in the herbarium of the Universidade de São Paulo (SPF).
Young floral buds and mature flowers were fixed in 50 % FAA (Johansen, 1940), and stored in 70 % ethanol. The morphology of the flowers, especially with respect to the fusion of organs, was analysed using a Leica M125 stereomicroscope. For light microscopy, the material was dehydrated in an ethanol–butanol series and then infiltrated and embedded in paraffin (following the protocol of Johansen, 1940). The embedded material was sectioned using a rotary microtome and a standard microtome knife D. The sections were stained with 1 % astra blue and 1 % safranin in 50 % ethanol (following the protocol of Bukatsch, 1972), and mounted in Canada balsam. The slides were analysed using a Leica DM 4000B microscope, and photomicrographs were taken with a Leica DFC 425 digital camera. Additionally, diagrams of the outline of floral organs and the main vascularization patterns of the serial microtome sections of floral buds were illustrated.
For scanning electron microscopy (SEM) studies, the fixed material was dissected, dehydrated in an ethanol series and critical-point dried. The floral organs were mounted on stubs and sputter-coated with gold. Observations were made with a Zeiss DMS-940 scanning electron microscope.
Terminology
Floral tube: an architectural term; tubular part of the flower through which the floral centre can be reached. A floral tube may be formed by different parts of the flower, mostly by sepals and/or petals (and sometimes also stamens).
Congenital fusion: connection of organs by confluence of their primary meristems.
Postgenital coherence: connection of floral organs of a whorl by intertwining trichomes of their contiguous surface.
Postgenital adherence: connection of floral organs of different whorls by intertwining trichomes of their contiguous surface.
Postgenital fusion: connection of contiguous organs by epidermal fusion.
RESULTS
Floral tube
In all species studied, a shorter or longer floral tube is formed by the petals and stamens. Antesepalous stamens are present in all studied species, but antepetalous stamens only in G. jasminiflora and C. macrophyllus. Interestingly, the construction of the floral tube is not uniform in the group.
Conchocarpus heterophyllus and C. minutiflorus show a similar floral morphology and anatomy (Figs 1 and 5A, E, F). Although they have a floral tube, the petals and stamens are free from each other along the entire length (Fig. 1A–I). However, transverse sections of the region of the floral tube show that the tube is formed by the coherence of petals and adherence of filaments to petals by intertwining trichomes (Fig. 5E, F). This connection is also facilitated by the form and position of the petals and stamens; in transverse section, they have a somewhat triangular flat shape, with both whorls precisely alternating (Fig. 1F–H). Higher up, the petals and stamens are free, and the petals form five blades (Fig. 5A). Thus, even though there is no fusion between petals or filaments, the corolla is seemingly sympetalous due to the intertwining trichomes of the margins of the alternating petals and filaments.
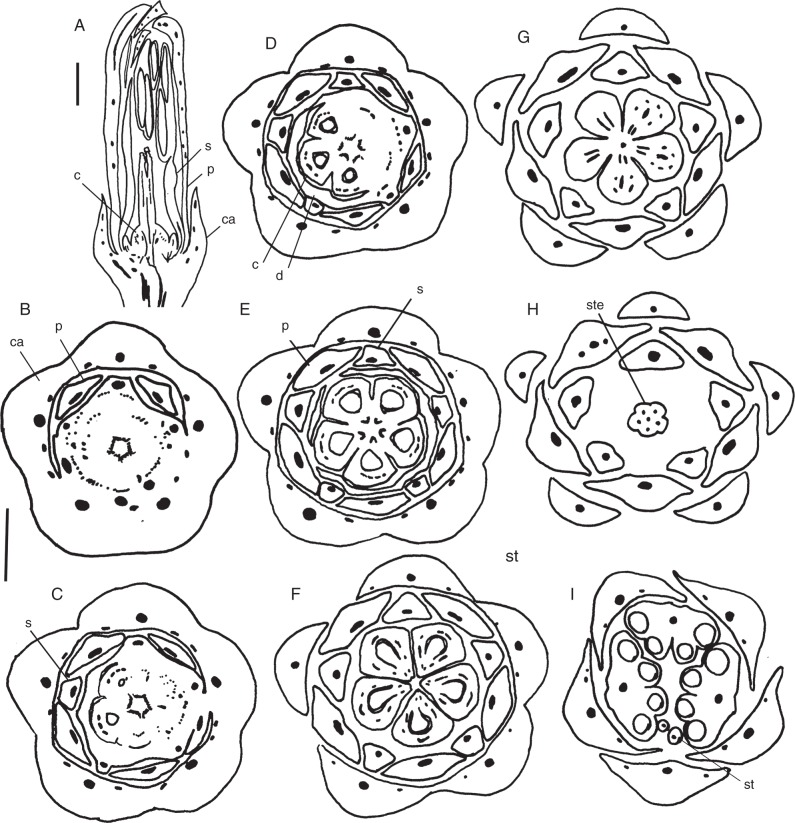
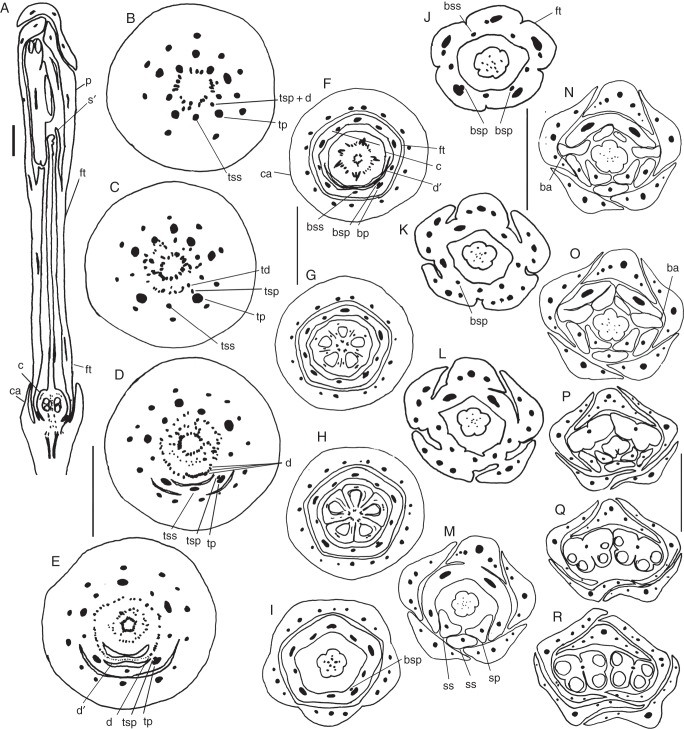
Fig. 1.
Conchocarpus heterophyllus and C. minutiflorus. Sections of floral buds. (A) Longisection of C. heterophyllus. (B–I) Transections of C. minutiflorus, successive levels, from the base upwards. (B–E) Transition from floral base to ovary. (F) Mid-level of ovary. (G) Uppermost level of ovary. (H) Basal level of style. (I) Level of the anthers. Abbreviations: ca, calyx; c, carpel; d, disc; s, stamen; p, petal; st, staminode; ste, style. Scale bars: (A) = 1 mm; (B–I) = 0·5 mm.
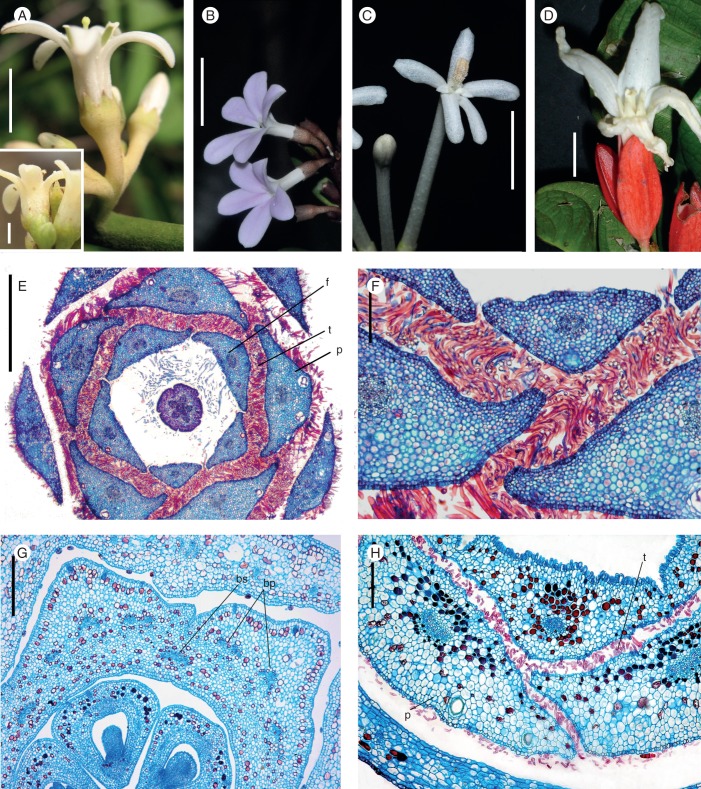
Fig. 5.
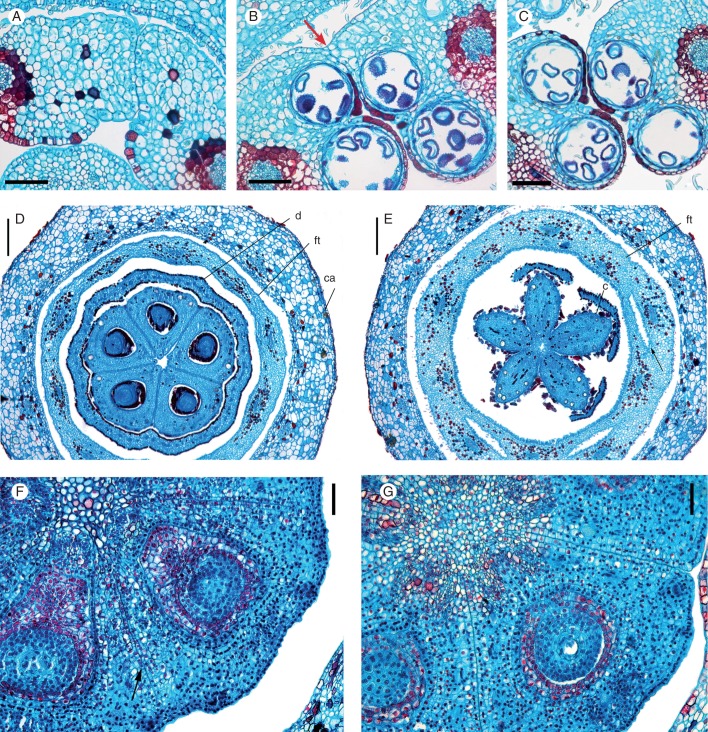
Galipeinae. Photographs of flowers at anthesis. (A) Conchocarpus heterophyllus and C. minutiflorus (bottom-left); (B) C. macrophyllus; (C) Galipea jasminiflora; (D) Erythrochiton brasiliensis. Microtome transections of floral buds: (E) Region of floral tube in Conchocarpus heterophyllus. (F) Detail of E, showing the intertwining trichomes in petals and filaments. (G) Detail of the floral tube of E. brasiliensis, showing the congenital fusion of stamens and petals. (H) Detail of the upper half of the floral tube of C. macrophyllus, showing the intertwining trichomes in petals and filaments. Abbreviations: f, filament; bs, vascular bundle of stamen; bp, vascular bundle of petal; p, petal; st, staminal tube; t, trichomes. Scale bars: (A) = 1 cm, inset = 2 mm; (B–D) = 1·5 cm; (E, H) = 200 µm; (F) = 400 µm, (G) = 300 µm.
In E. brasiliensis the floral tube is formed by congenital union of the stamen filaments and their congenital fusion with the petals as shown in transverse section series (Figs 2 and 5D, G). In the tube, the stamen vascular bundles alternate with the median vascular bundles of the petals (Figs 2B–E and 5G). Higher up, the petals are free, forming five spreading blades (Figs 2F–I and 5D). The staminal tube extends slightly higher up than the stamen–petal tube (Fig. 2F, G). In the distal part of the androecium, the stamens are free (Fig. 2G–I). In young stages seen from the dorsal side, petals and stamens appear free (Fig. 8G, H). However, on the ventral side, there is fusion from the beginning between petals and stamens, and this becomes apparent later also on the dorsal side (Fig. 8I).
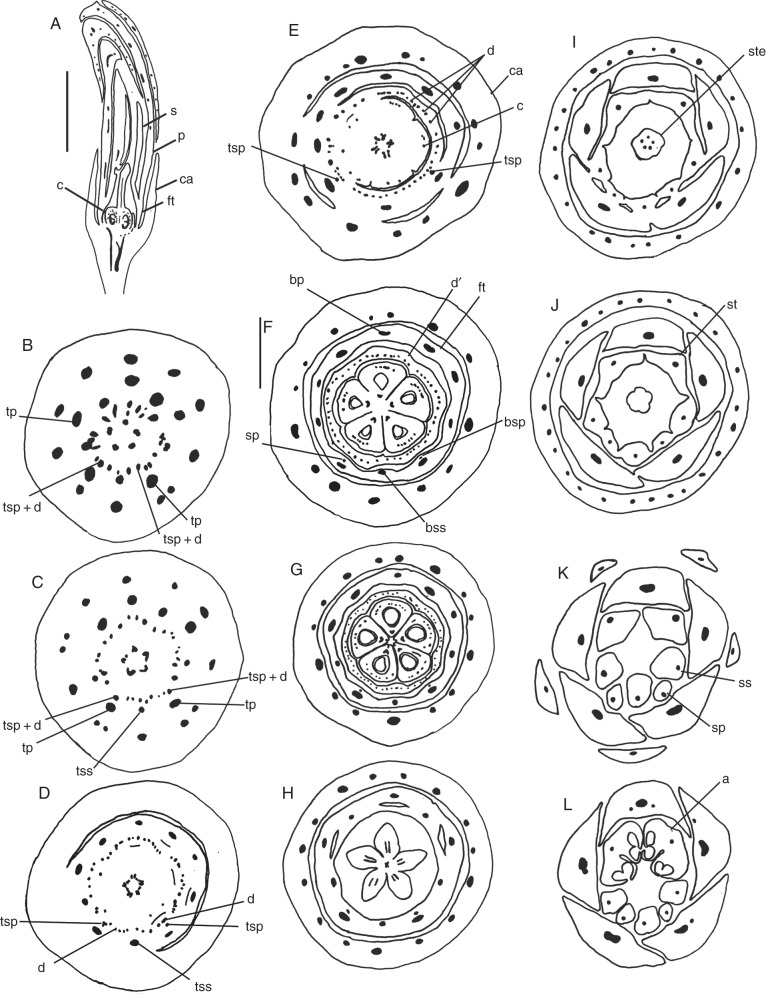
Fig. 2.
Erythrochiton brasiliensis. Sections of floral buds. (A) Longisection. (B–I) Transections: successive levels, from the base upwards. (B) Transition from floral base to ovary. (C) Basal level of ovary. (D) Mid-level of ovary. (E) Uppermost level of ovary. (F) Level of staminal tube. (G, H) Level of free petals and stamens. (I) Level of anthers. (J) Distal region of bud. Abbreviations: ca, calyx; c, carpel; d, disc; fl, floral tube; p, petal; bp, vascular bundle of petal; bs, vascular bundle of stamen; s, stamen; st, staminal tube. Scale bars: (A) = 1 mm; (B–D) = 1 mm; (E–H) = 1 mm; (I) = 1 mm; (J) = 0·5 mm.
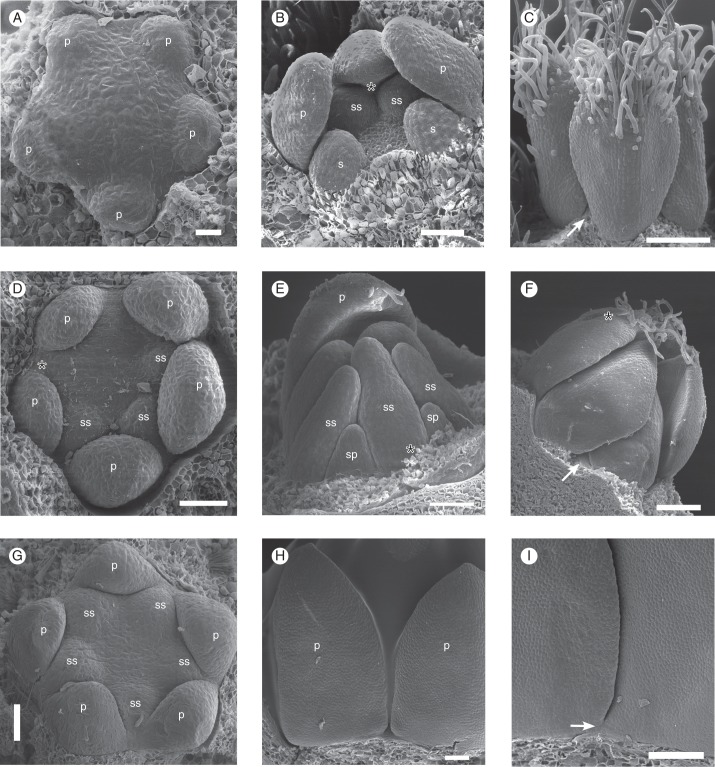
Fig. 8.
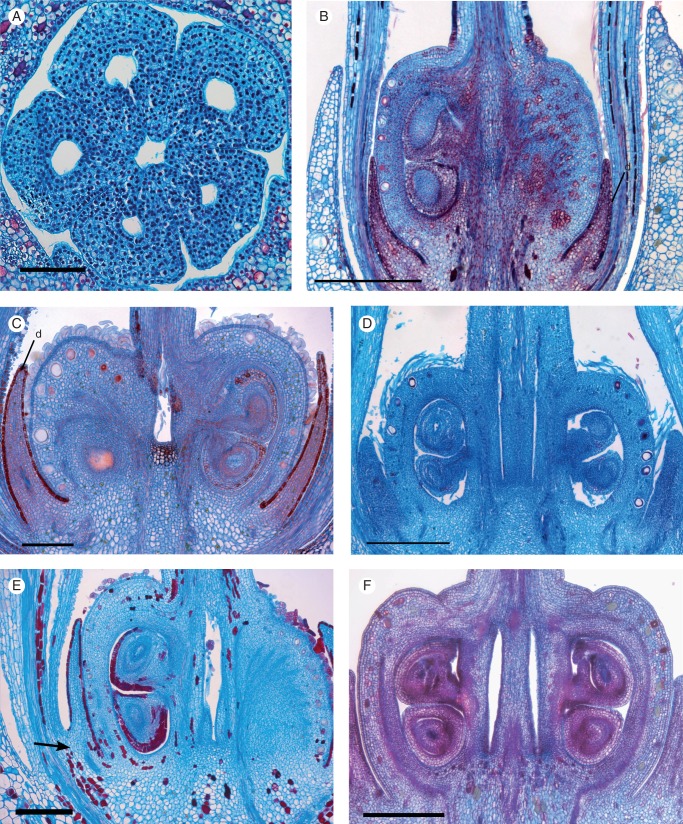
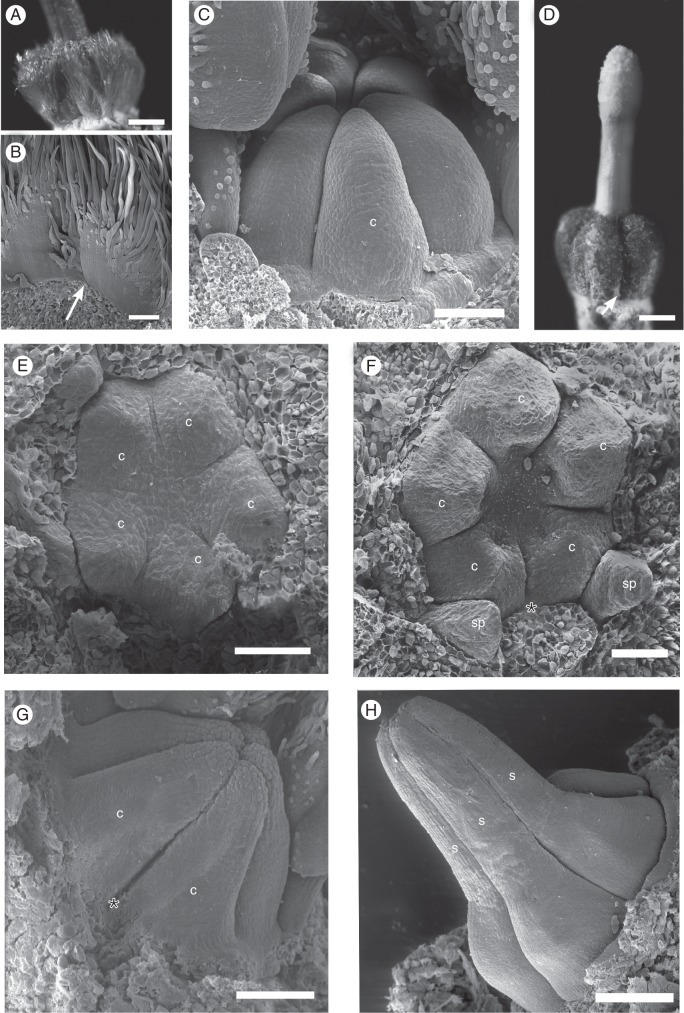
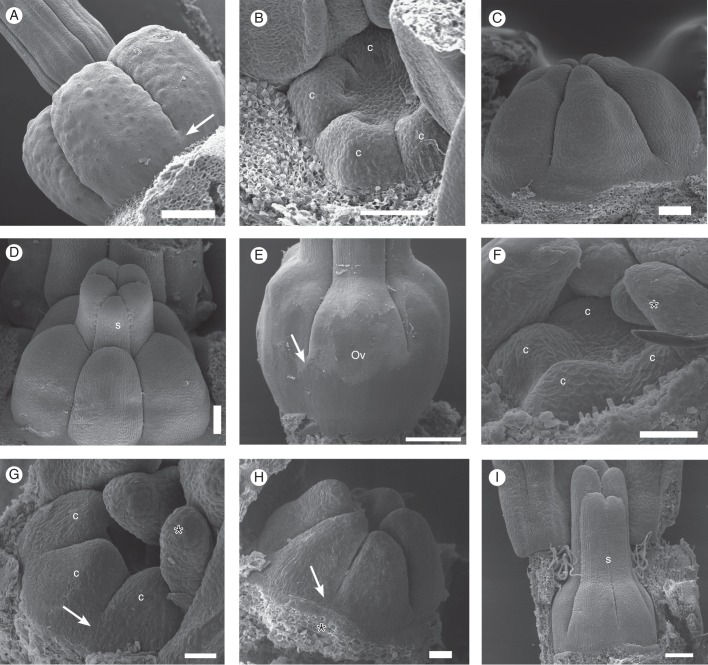
SEM micrographs of young floral buds. (A–C) Galipea jasminiflora. (A) View from above, with sepals removed, showing young free petals. (B) View from the side, with sepals, one petal and stamen removed, showing young free stamens. (C) Lateral view of young free petals (arrow points to free base), later stage than B (calyx removed). (D–F) Conchocarpus macrophyllus. (D) View from above, with sepals removed, showing young free petals and emergence of three stamens. (E) View from the side, with young free stamens in a later stage than D. (F) Young free petals (arrow points to free base), in a later stage than D. (G–I) Erythrochiton brasiliensis. (G) View from above, with sepals removed, showing young free petals and five stamens. (H) View from the side, with sepals removed, showing young free petals, later stage than G. (I) Later stage, where basal congenital fusion of petals becomes visible from the surface (arrow). Abbreviations: p, petal; sp, antepetalous stamen; ss, antesepalous stamen; asterisk indicates posterior side of the flower. Scale bars: (A) = 20 µm; (B, D) = 50 µm; (C, E–I) = 100 µm.
In C. macrophyllus (Fig. 5B) and G. jasminiflora (Fig. 5C), the floral tube is formed by adnation of the staminal filaments to the petals, in Galipea, up to their upper half, and in C. macrophyllus up to their lower half (Figs 4A–L and 3A–H). In the floral tube, the staminal vascular bundles alternate with the median petal bundles (Figs 3B–G, 4B–I and 6D, E). Higher up, the floral tube is formed by the coherence of petals and adherence of petals and filaments by intertwining trichomes in both taxa (Figs 4M, N and 5H). In C. macrophyllus, the nectary disc and the floral tube have a common base for a short distance (approx. 150 µm; Fig. 7E). In their distal portion, the petals are free, forming five spreading blades (Fig. 5B, C), and the filaments are free from the corolla. In C. macrophyllus, the filaments form a short tube (Fig. 3J), but they are free just above (Fig. 3K). In this same region, the filaments of G. jasminiflora are gradually released from their union and fusion to the corolla. This begins with the staminodes, and proceeds to the filaments of the two fertile anthers (Fig. 4L–O).
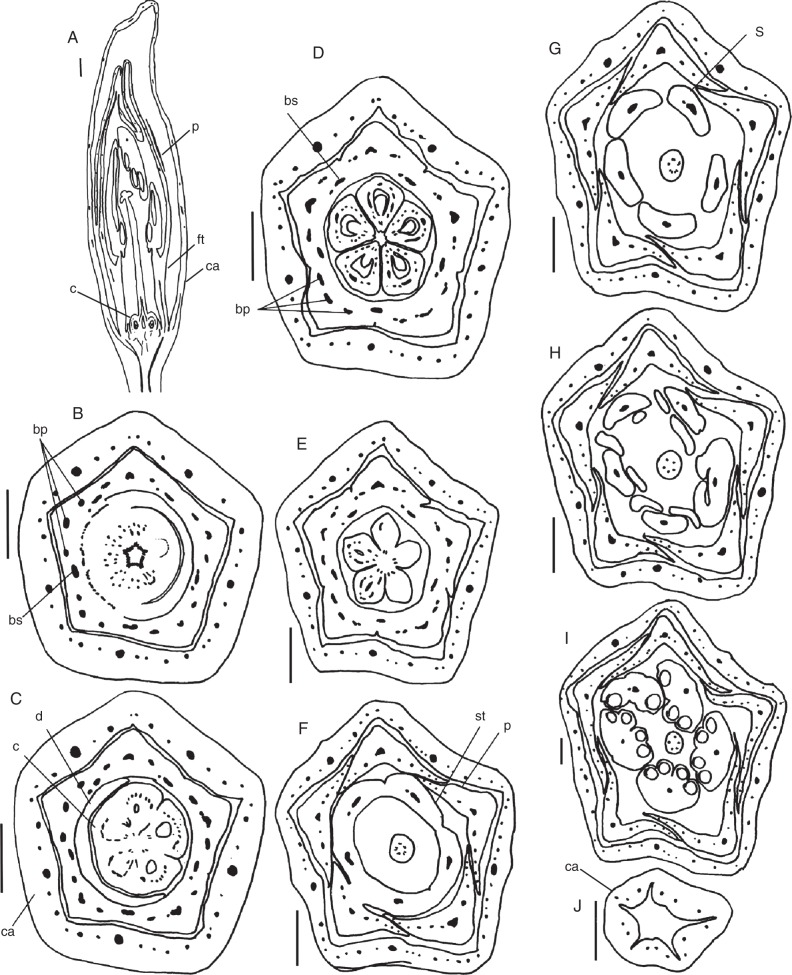
Fig. 4.
Galipea jasminiflora. Sections of floral buds. (A) Longisection. (B–R) Transections: successive levels, from the base upwards. (B–F) Transition from floral base to ovary. (F) Below the locules. (G) Basal level of ovary. (H) Mid-level of ovary. (I) Basal level of style. (J) Mid-level of style. (K–M) Levels of gradual separation of petals from stamens. (N, O) Level of basal appendages of anthers. (P, Q) Level of postgenitally united anthers. (R) Region of free anthers. Abbreviations: a, anther; ba, basal appendage of anther; bp, vascular bundle of petal; bsp, vascular bundle of antepetalous stamen; bss, vascular bundle of antesepalous stamen; c, carpel; ca, calyx; d, vascular bundles of disc; d', disc; fl, floral tube; p, petal; s', staminode; sp, antepetalous stamen; ss, antesepalous stamen; st, staminal tube; ste, style; td, traces of disc; tp, trace of petal; tsp, trace of antepetalous stamen; tss, trace of antesepalous stamen; tsp + d, trace complex of antepetalous stamen plus disc. Scale bars: (A) = 1 mm; (B–E) = 1 mm; (F–I) = 1 mm; (J–O) = 1 mm; (P–R) = 1 mm.
Fig. 3.
Conchocarpus macrophyllus. Sections of floral buds. (A) Longisection. (B–L) Transections: successive levels, from the base upwards. (B–F) Transition from floral base to ovary. (F) Basal level of ovary. (G) Mid-level of ovary. (H) Uppermost level of ovary. (I) Level of separation of petals from stamens. (J) Level of staminal tube. (K) Level of free petals and stamens. (L) Level of anthers. Abbreviations: a, anther; bp, vascular bundle of petal; bsp, vascular bundle of antepetalous stamen; bss, vascular bundle of antesepalous stamen; c, carpel; ca, calyx; d, vascular bundle of disc; d', disc; fl, floral tube; p, petal; s, stamen; sp, antepetalous stamen; ss, antesepalous stamen; st, staminal tube; ste, style; tp, trace of petal; tsp, trace of antepetalous stamen; tss, trace of antesepalous stamen; tsp + d, trace complex of antepetalous stamen plus disc. Scale bars: (A) = 2 mm; (B–L) = 1 mm.
Fig. 6.
Photomicrographs of microtome sections of floral buds. (A–C) Transections of the anthers of Galipea jasminiflora. (A, B) Detail of anthers, below the thecae (A) and at mid-level of thecae (B), where postgenital fusion is evident (arrow). (C) Upper half of thecae, showing free anthers. (D, E) Conchocarpus macrophyllus. (D) Apocarpous zone of ovary and floral tube. (E) Uppermost level of ovary, showing postgenitally united carpels; beginning of separation of stamens from petals (arrow). (F, G) Galipea jasminiflora, carpels. (F) Basal region of ovary, showing the morphological surfaces of adjacent carpels as narrow slits (arrow). (G) Upper half of ovary, showing postgenital union of carpels in the centre of the gynoecium. Abbreviations: a, anther; ba, basal appendage; c, carpel; ca, calyx; d, disc; ft, floral tube. Scale bars: (A, B, D, E) = 100 µm; (C) = 200 µm; (F, G) = 500 µm.
Fig. 7.
Photomicrographs of microtome sections of floral buds. (A) Galipea jasminiflora, transection of young ovary, above the level of placenta, showing postgenital union of carpel flanks. (B–F) Longitudinal sections of base of floral buds, focusing on the median plane of carpels. (B) Galipea jasminiflora. (C) Conchocarpus minutiflorus. (D) Conchocarpus heterophyllus. (E) Conchocarpus macrophyllus (arrow indicates the oblique base of the disc at the floral tube). (F) Erythrochiton brasiliensis. Abbreviation: d, disc. Scale bars: (A) = 100 µm; (B, D, F) = 500 µm; (C, E) = 200 µm.
Confluence of the meristems of the young floral organs, resulting in congenital union of their lower parts, occurs relatively late, when the upper free parts of the organs are already visible (Fig. 8A–F). Thus, the floral tube in C. macrophyllus and Galipea species is partially formed by congenital fusion of petals and stamens, and partially by postgenital coherence and adherence of these organs.
Anthers
The anthers of all species studied are free, except for those of G. jasminiflora. Galipea has only two fertile stamens, whose thecae bear a basal sterile appendage; the two fertile stamens are firmly attached to each other laterally, from the region of the basal appendages up to the middle of the anthers (Figs 4P, Q and 6A–C). Anther fusion is postgenital as the epidermis of the two organs is still apparent in advanced stages at the base of the anthers. The epidermal surfaces are interlocked, with epidermal cells undifferentiated and similar to hypodermal cells (Fig. 6A, B).
Gynoecium
In all species studied, the carpels are congenitally and postgenitally united to various degrees. In C. minutiflorus, C. heterophyllus and C. macrophyllus, the carpels are connate in the centre at the base of the ovary. Five shallow furrows at the periphery of the ovary between adjacent carpels delineate the flanks of the five carpels at their bases (Figs 1C–E and 3E, F). This zone of basal congenital union varies in extent between species, comprising approximately one-third of the total length of the ovary in C. minutiflorus, approximately one-quarter in C. heterophyllus and approximately one-seventh in C. macrophyllus (Fig. 7C–E). This zone is not seen externally (Fig. 9A–C). Above this zone, the ovaries are completely free from each other (Figs 1F, 3D, 6D, 7C–E and 9E, F). However, in the distal zone of the ovary, close to the base of the style, and up to the stigma, the carpels are again united in the centre. However, here the union is postgenital (Fig. 1G, H, 3H, I and 6E). That this upper fusion zone is postgenital is also evident from young stages in which the carpels are free in the uppermost part (Fig. 9C, G). As they widen and elongate, they become postgenitally united to form a single style and stigma (Fig. 9D, H). Thus the gynoecium is apocarpous for most of its length but the carpels are postgenitally united in the upper apocarpous zone.
Fig. 9.
Carpel development of Conchocarpus heterophyllus (A–C) and C. macrophyllus (D–H). (A) Photograph of mature gynoecium (ovary and style base). (B, C) SEM micrographs. (B) Detail of (A), at the base of ovary (arrow indicates free carpel flanks). (C) Young free carpels. (D) Photograph of mature gynoecium; arrow indicates base of ovary with free carpel flanks. (E–H) SEM micrographs. (E) View from above, showing five young carpels. (F) Later stage than E. (G) Carpels elongating (lateral view). (H) Style elongating. Abbreviations: c, carpel; sp, antepetalous stamen; s, style; asterisk indicates posterior side of the flower. Scale bars: (A–D, F–H) = 100 µm; (G) = 50 µm.
In E. brasiliensis, the gynoecium is similar to that of Conchocarpus. The postgenital union in the apocarpous zone extends from immediately above the ovary up to the stigma (Figs 2A, E–I, 7F and 10A, D). Postgenital union of carpels becomes apparent from the surface when the uniform style develops (Fig. 10D). Also here, in early development, the five carpels appear to be free (Fig. 10B–C); they are congenitally united at their bases only in the centre, for a short extent (approximately one-fifth of the length of the ovary; Figs 2B, C and 7F). However, in contrast to Conchocarpus, the gynoecium has a short gynophore (Figs 2B, C and 10A).
Fig. 10.
SEM micrographs of carpel development of Erythrochiton brasiliensis (A–D) and Galipea jasminiflora (E–I). (A) Mature gynoecium (side view); arrow indicates where carpels are congenitally united at base. (B) Young carpels, from above. (C) Young carpels, from the side. (D) Carpels with beginning differentiation of style, from the side. (E) Galipea jasminiflora, mature gynoecium from the side (arrow indicates the upper end of congenital carpel union). (F) Carpel primordia. (G) Side view of young carpels, showing the congenital union of their bases (arrow). (H) Farther advanced stage than ‘G’ (arrow points to basal congenital union). (I) Carpels with beginning differentiation of style, from the side. Abbreviations: c, carpel; ov, ovary; s, style; asterisk indicates posterior side of the flower. Scale bars: (A) = 500 µm; (B–E) = 100 µm; (F–I) = 50 µm.
The gynoecium of G. jasminiflora differs from that of the other taxa in the degree of syncarpy. There is no zone where the carpels are free (Fig. 7B). They are congenitally or postgenitally united along their entire length. In the ovary, the carpels are congenitally united in the floral centre but they appear free at the flanks (Figs 4H and 6G). This zone of free flanks ends at the base as short pockets between the flanks when carpels are also united at the periphery for a short distance (Figs 4G and 6F). These pockets look like the slits of septal nectaries in monocots; however, here they are not nectariferous. As seen from the surface, the lateral connation of the carpels appears to reach up to half the length of the ovary (Fig. 10E). However, the lowermost part is below the locules and thus is a short gynophore (Figs 4F and 7B). The uniform style is formed by the upper part of the five postgenitally united carpels (Fig. 4I). In G. jasminiflora, in early development, the carpels appear to arise as five independent organs, but they soon become united laterally at the base (Fig. 10F–H). This united zone elongates during gynoecium development (Fig. 10E, I) compared with E. brasiliensis (Fig. 10A). The carpels also appear united in the centre of the gynoecium (Fig. 7B). The free apical parts later become postgenitally united and differentiate into the apical portion of the ovary and the united style and stigma (Figs 7A and 10E, I).
DISCUSSION
Floral tube in Galipeinae: structure and possible evolutionary implications
All Galipeinae species studied have a floral tube formed by the synorganization of the stamen filaments with the petals. However, the connection of these organs is conspicuously diverse in detail in the studied genera, and three main patterns were found.
In the pattern found in C. heterophyllus and C. minutiflorus, the floral tube is formed by postgenital connection of stamens and petals by intertwining trichomes. A case of postgenital connection of petals was reported for another member of Rutaceae, Correa speciosa Donn ex Andrews (Boronieae, Rutoideae), by Hartl (1957), in which the tubular corolla is formed by the close interlocking of epidermal papillate projections and by cuticular protuberances from the petal magins. Hartl (1957) called this postgenital connection of petals ‘false sympetaly’. With regard to histological details, the term ‘dentonection’ was used (Weberling, 1989). However, Conchocarpus differs from Correa in the participation of filaments, and the coherence of petals by interwining trichomes, instead of interlocking epidermal cells and cuticular projections. Postgenital connection of petals also occurs in some other rosids, such as some members of Oxalidales (Matthews and Endress, 2002), Celastraceae (Matthews and Endress, 2005a), Rhizophoraceae, Erythroxylaceae and Linaceae (Matthews and Endress, 2011).
A second pattern of tube formation was found in E. brasiliensis, in which the petals are congenitally united via the filaments of the neighbouring stamens. This kind of tube is also known from other angiosperm groups, such as Bruniaceae (Leinfellner, 1964; Quint and Claßen-Bockhoff, 2006), Commelinaceae (Rohweder, 1969) and, among rosids, from Dichapetalaceae (Matthews and Endress, 2008). Leinfellner (1964) also called this pattern ‘false sympetaly’. However, it should be emphasized that this is completely different from how the term was used by Hartl (1957). The term ‘stapet’ is used to designate congenital fusion of stamens to petals (Ritterbusch, 1991), which is found commonly in association with sympetaly in core eudicots. However, this phenomenon is rarely found in rosids (Endress and Matthews, 2012).
The third pattern, observed in C. macrophyllus and G. jasminiflora, is a mixture of the first two patterns. In its basal portion, the floral tube is similar to that of E. brasiliensis, and in the upper portion it is similar to C. heterophyllus and C. minutiflorus. Such a mixed pattern of congenital and postgenital fusion is also known from other angiosperms (some Gentianales; Fallen, 1986; Robbrecht, 1988; Endress, 2010b). The features described for the floral tube of G. jasminiflora have been reported earlier for other Galipea species (G. carinata Pirani, G. ciliata Engl., G. dasysperma Gómez-Laur. and Q. Jiménez, G. laxiflora Engl.; Pirani et al., 2010). As all 14 species of Galipea have flowers of a remarkably similar general aspect, it is likely that a floral tube with a mixed pattern is a general characteristic of this genus. The three Conchocarpus species studied show an intriguing heterogeneity in the structure of the floral tube: pattern 1 (with only postgenital coherence) in C. heterophyllus and C. minutiflorus, and a mixed pattern in C. macrophyllus. This heterogeneity probably reflects the putative non-monophyly of the genus. Conchocarpus is the largest genus of Galipeinae (48 species) and also the most heterogeneous for other mainly floral morphological features, specifically with differences in number of fertile stamens and staminodes, in floral symmetry and in the shape of the sepals (Kallunki and Pirani, 1998; Pirani et al., 2012). The results of a systematic treatment of the genera of Galipeinae (Kallunki and Pirani, 1998) and recent molecular phylogenetic studies (Kallunki and Groppo, 2007; Bruniera, 2010; Groppo, 2010) suggest that Conchocarpus is not monophyletic and that its circumscription should be re-evaluated. Thus the study of floral structure may contribute with more characters to elucidate the systematic relationships between Conchocarpus species and to help in the new circumscription of monophyletic genera in Galipeinae.
The three main structural patterns found in the floral tube of Galipeinae species studied seem to be inter-related because of the presence of an intermediate ‘mixed’ pattern. Thus one may reconstruct the hypothetical pathway of evolution of these structures. Since the majority of Galipeinae possess tubular flowers, which are absent from most other members of Rutaceae, the presence of tubes is here considered a synapomorphy of Galipeinae, and the floral tubes are thus homologous between species. The following structural transformations during the evolution of Galipeinae may be assumed: (1) the ancestral state in the group was a floral tube formed by mere postgenital coherence of petals and stamens by intertwining trichomes (the pattern observed in C. heterophyllus and C. minutiflorus); (2) congenital fusion of petals and stamens at the base of the tube, but retaining postgenital coherence higher up, would have led to the mixed pattern of Galipea species and C. macrophyllus; and (3) complete loss of the trichomes from the margins of petals and stamen filaments and congenital fusion along the entire length of the tube would finally form the tube pattern found in E. brasiliensis. However, this evolutionary hypothesis has to be considered with caution as our subtribal taxonomic sampling was limited (nine species; five from this study and four additional ones from Pirani et al., 2010) and as a robust phylogeny of Galipeinae is still not available (M. Groppo, unpubl. res., pers. comm.). The evolutionary shift may have occurred in the reverse direction and/or with additional transitions (if other, unknown floral tube structures would be found in genera not yet studied).
At a broader systematic scale, in the context of the family, the postgenitally united floral tubes in Correa, as mentioned above, are only superficially similar to those of Galipeinae. In Correa, the tube is formed by the postgenital interlocking of papillate epidermal cells and cuticular projections, whereas in Galipeinae it is formed by the intertwining trichomes and congenital fusion of petals and stamens. Considering these morphological differences, together with phylogenetic studies which show that Correa and Galipeinae belong to distantly related clades (Groppo et al., 2008, 2012), it may be assumed that the floral tubes of C. speciosa and Galipeinae are not clearly homologous and their resemblance is a case of convergence.
Assuming that convergent evolution occurred, it can be speculated that similar selective pressures may have acted upon Correa and Galipeinae flowers, and that they might have been associated with pollination. The majority of cases reported for fusion of floral whorls comes from studies of petals and carpels, which are often associated with reproductive success (Stebbins, 1950; Verbeke, 1992; Endress, 2006). A possible reproductive advantage of having a floral tube is the restriction of the access to the floral reward for nectar robbers (Faegri and van der Pijl, 1966); in addition, the accumulation of nectar in the bottom of the tube may reduce nectar evaporation and dilution by rain (Endress, 1994). One may expect that the flowers of Galipeinae and C. speciosa offer nectar as a reward and are pollinated by nectar-seeking insects with a long proboscis and/or long-beaked birds. This has been shown by field studies for both groups: Armstrong (1979) reported that ten Correa species are pollinated by several Meliphagidae birds; G. jasminiflora is pollinated by species of Lepidoptera (Piedade and Ranga, 1993), and E. brasiliensis is pollinated by the hummingbird Glaucis hirsuta (Lopes, 2002). In addition, we observed pollination by two butterfly species and by the hummingbird Phaethornis idaliae in Almeidea rubra A.-St.Hil. (J. H. L. El Ottra et al., unpubl. res.), and several butterfly visits in C. macrophyllus and Angostura bracteata (J. H. L. El Ottra and E. Pansarin, pers. obs.; all Galipeinae). However, the paucity of studies about pollination of Galipeinae limits our evolutionary inferences about the factors that might have had an influence on the generation of the different floral tubes present in the subtribe.
Partially apocarpous gynoecium and its consequences: compitum and the fruit stage
The comparative gynoecium analysis of Galipeinae shows a conspicuous variability in the extent of carpel union. In the mature gynoecium of G. jasminiflora, carpels are connate along most of their length. Pirani et al. (2010) obtained the same results for four other species of Galipea. Conversely, carpels of C. heterophyllus, C. minutiflorus, C. macrophyllus and E. brasiliensis have an unfused zone, which comprises a large part of the ovary, but carpels are congenitally united basally in the centre of the ovary. Additionally, in G. jasminiflora and E. brasiliensis, the carpels are completely united below the locule, forming a short, inconspicuous gynophore, as is common in Rutaceae (Gut, 1966; Ramp, 1988), but has not yet been described for these genera. Therefore, the mature gynoecium is different in each of the three genera as to the extent of the syncarpous zone and the postgenitally united part of the apocarpous zone.
Such diversity in the degree of carpel union, including congenital and postgenital union, is well known in general for Rutaceae. In the extreme, the carpels may be completely congenitally united, such as in Aurantioideae, or completely free (not even with postgenital union), such as in part of the genus Zanthoxylum (Engler, 1931; Tilson and Bamford, 1938; Gut, 1966; Guédès, 1973; Ramp, 1988; Beurton, 1994; Kubitzki et al., 2011). Molecular phylogenetic studies (Groppo et al., 2008) indicate that the change in carpel fusion extent is labile in evolution. However, more structural studies are needed to clarify the complex evolutionary history of the gynoecium in Rutaceae.
As the floral apex in Galipeinae is convex when the carpels are initiated, the area of the base of the carpels is oblique (Figs 6F, 7C–F, 9E, F and 10F, G). Therefore, sections of the gynoecium base perpendicular and parallel to the longitudinal axis of the flower show an area of larger celled tissue in the centre that is not involved in carpel formation. The same is present in other Rutaceae and was discussed earlier (Gut, 1966; Ramp, 1988).
A shared feature of all three genera studied here is the presence of postgenitally united carpel tips (style and stigma), as known from most other Rutaceae (Endress et al., 1983; Ramp, 1988; Caris et al., 2006; Wei et al., 2012). In Galipeinae, it was first observed in E. brasiliensis by Ramp (1988). The occurrence of gynoecia that are more or less apocarpous but with a postgenitally united upper part is associated with the presence of a compitum at anthesis, which is assumed to provide the advantage of centralized pollen tube selection as opposed to a gynoecium without a compitum (Endress et al., 1983; Endress, 2011). Families with this gynoecium architecture are especially common in Sapindales. In addition to Rutaceae, they also occur in Simaroubaceae (Endress et al., 1983; Ramp, 1988), Kirkiaceae (Bachelier and Endress, 2008) and occasionally in Anacardiaceae (Bachelier and Endress, 2009). They were also found in other core eudicots, such as Malvaceae, Loganiaceae and Apocynaceae (Endress et al., 1983), and more recently in some Crossosomatales (Matthews and Endress, 2005b) and some Ochnaceae (Matthews et al., 2012).
Apocarpous gynoecia with a postgenitally united upper part and compitum are features that in combination have been assumed to be secondarily apocarpous in terms of evolution in Rutaceae and other groups because of their phylogenetic position within eudicots (Endress et al., 1983). However the extreme variability in the extent of carpel fusion found in the crown group of the Rutaceae makes this hypothesis difficult to corroborate in a phylogenetic context (Kubitzki et al., 2011). For many clades within the family, detailed structural data on the gynoecium are still lacking, which makes the analysis of evolutionary pathways of gynoecium traits difficult. The study of smaller clades may help gradually to untangle the evolutionary history of the gynoecium in Rutaceae. Also the apparent lability of carpel fusion indicates that secondary apocarpy may have evolved multiple times in the family (Ramp, 1988).
During fruit development, the postgenitally united style breaks off at its base, and the carpels in the apical part of the ovary become separated in the postgenitally united zone. This was observed in this study and is commonly found in other Rutoideae (Gut, 1966; Ramp, 1988; Pirani et al., 2010). However, we found that this separation varies according to the extent of the apocarpous zone (regardless of whether or not it is postgenitally united). Thus the apical parts of the ovaries of Conchocarpus and Erythrochiton become much more divergent at fruit maturity than those of Galipea. This is a consequence of the greater extent of syncarpy in Galipea.
Postgenital union of the anthers in Galipea
The postgenital union of the anthers and their sterile basal appendages found in this study only for G. jasminiflora was similar to the observations of Pirani et al. (2010) for three other species of Galipea. Thus this feature may occur in the majority of the species of the genus, except for G. dasysperma and G. panamensis, which have free anthers. The only difference between our study and that of Pirani et al. (2010) was in the histology of the fusion of the anthers. In G. ciliata, the histological union between adjacent anther appendages and thecae was complete: the epidermal layers of both anthers were no longer visible along the suture region at anthesis (Pirani et al., 2010), whereas G. jasminiflora still shows vestiges of the suture between the epidermis of adjacent anthers in mature buds (Fig. 6B). These differences could be a consequence of the timing with which both anthers come into close contact. Sutures tend to remain evident at maturity only when the organs to be fused come into contact relatively late in floral development (Verbeke, 1992). Whether in the two fertile stamens of G. ciliata the fusion process begins earlier than in the other Galipea species in which less complete fusion occurs, needs developmental investigation.
Although only five species were anatomically analysed, the presence of two connate anthers and sterile basal appendages is a conspicuous feature in Galipea (Pirani, 2004), and occurs less frequently in other genera of Galipeinae. This feature may have a functional importance for the floral biology of the group (Pirani et al., 2010). The presence of only two fertile, connate stamens, with an upright position at anthesis, has been traditionally associated with pollen economy and precise pollen deposition on the dorsal parts of the body of pollinators, as in some Gesneriaceae and Labiatae (Faegri and van der Pijl, 1979; Westerkamp and Claßen-Bockhoff, 2007). The study of floral biology and pollination of G. jasminiflora indicates that nototribic pollination actually occurs as expected by their anther display (Piedade and Ranga, 1994). Additionally, the fusion of part of the anthers and its basal appendages may contribute to the stabilization of the whole structure during foraging of pollinators.
Conclusions
The structural floral features studied here are shared by groups of genera and species of Galipeinae, which could be used in future studies of character evolution. They may also represent possible synapomorphies for clades in these groups. However, the limited taxonomic sampling still prevents accurate testing at the subtribal level. Further investigation of floral features of a greater number of species and genera of Galipeinae, as well as interpretation of the data based on a phylogenetic framework, are needed in order to evaluate the findings and to better understand their role in the evolutionary history of this interesting neotropical group of Rutaceae.
ACKNOWLEDGEMENTS
Thanks are due to the technicians of the Plant Anatomy Laboratory (IB-USP), Gisele R. O. Costa, Tássia C. Santos and Irwandro R. Pires, for assisting the first author. The first author also thanks Maria F. Calió and José E. A. R. Marian for assistance with SEM techniques, Diego Demarco for assistance with histological techniques, Milton Groppo and Alexandre Zuntinni for pickled plant material, and João Semir, Marcelo M. Egea and Julie H. A. Dutilh for their assistance in the collection of G. jasminiflora. We thank Mary Endress for her linguistic suggestions. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo [grants nos 06/609900, 09/54569-9 and 09/08764-4] to J.H.L.E.O. and J.R.P.; and Conselho Nacional de Desenvolvimento e Pesquisa productivity grant to J.R.P.
LITERATURE CITED
- Armstrong JA. Biotic pollination mechanism in the Australian flora – a review. New Zealand Journal of Botany. 1979;17:467–508. [Google Scholar]
- Bachelier JB, Endress PK. Floral structure of Kirkia (Kirkiaceae) and its position in Sapindales. Annals of Botany. 2008;102:539–550. doi: 10.1093/aob/mcn139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelier JB, Endress PK. Comparative floral morphology and anatomy of Anacardiaceae and Burseraceae (Sapindales), with a special focus on gynoecium structure and evolution. Botanical Journal of the Linnean Society. 2009;159:499–571. [Google Scholar]
- Beurton C. Gynoecium and perianth in Zanthoxylum s.l. (Rutaceae) Plant Systematics and Evolution. 1994;189:165–191. [Google Scholar]
- Bruniera CP. Estudos filogenéticos e sistemáticos em Rutaceae: análise cladística e posicionamento de Almeidea A. St.-Hil. entre as Galipeinae (Galipeae, Rutoideae) com o uso de dados morfológicos e moleculares. Brazil: Universidade de São Paulo; 2010. MSc Thesis. [Google Scholar]
- Bukatsch F. Bemerkungen zur Doppelfärbung Astrablau-Safranin. Mikrokosmos. 1972;61:255. [Google Scholar]
- Caris P, Smets E, De Coster K, Ronse De Craene LP. Floral ontogeny of Cneorum tricoccon L. (Rutaceae) Plant Systematics and Evolution. 2006;257:223–232. [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Angiosperm floral evolution: morphological developmental framework. Advances in Botanical Research. 2006;44:1–61. [Google Scholar]
- Endress PK. Synorganisation without organ fusion in the flowers of Geranium robertianum (Geraniaceae) and its not so trivial obdiplostemony. Annals of Botany. 2010a;106:687–695. doi: 10.1093/aob/mcq171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden. 2010b;97:541–583. [Google Scholar]
- Endress PK. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany. 2011;98:370–396. doi: 10.3732/ajb.1000299. [DOI] [PubMed] [Google Scholar]
- Endress PK, Matthews ML. Progress and problems in the assessment of flower morphology in higher-level systematics. Plant Systematics and Evolution. 2012;298:257–276. [Google Scholar]
- Endress PK, Jenny M, Fallen ME. Convergent elaboration of apocarpous gynoecia in higher advanced angiosperms (Sapindales, Malvales, Gentianales) Nordic Journal of Botany. 1983;3:293–300. [Google Scholar]
- Engler A. Rutaceae. In: Martius CPF, Eichler AG, editors. Flora brasiliensis. Leipzig: Friedrich Fleischer; 1874. 12: 75–196, tabs. 14–39. [Google Scholar]
- Engler A. Rutaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn, 19a. Leipzig: Wilhelm Engelmann; 1931. pp. 187–359. [Google Scholar]
- Faegri L, van der Pijl L. The principles of pollination ecology. 3rd edn. New York: Pergamon Press; 1979. [Google Scholar]
- Fallen ME. Floral structure in the Apocynaceae: morphological, functional and evolutionary aspects. Botanische Jahrbücher für Systematik. 1986;106:245–286. [Google Scholar]
- Groppo M. Simpósio de Sapindales. X Congresso Latinoamericano de Botánica. La Serena: Chile; 2010. Estudos filogenéticos em Rutaceae Neotropicais: presente e futuro. [Google Scholar]
- Groppo M, Pirani JR, Salatino MLF, Blanco SR, Kallunki JA. Phylogeny of Rutaceae based on two noncoding regions from cpDNA. American Journal of Botany. 2008;95:985–1005. doi: 10.3732/ajb.2007313. [DOI] [PubMed] [Google Scholar]
- Groppo M, Kallunki JA, Pirani JR, Antonelli A. Chilean Pitavia more closely related to Oceania and Old World Rutaceae than Neotropical groups: evidence from two cpDNA non-coding regions, with a new subfamilial classification of the family. PhytoKeys. 2012;19:9–29. doi: 10.3897/phytokeys.19.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guédès M. Carpel morphology and axis-sharing in syncarpy in some Rutaceae, with further comments on ‘New Morphology. Botanical Journal of the Linnean Society. 1973;66:55–74. [Google Scholar]
- Gut BJ. Beiträge zur Morphologie des Gynoeciums und der Blütenachse einiger Rutaceen. Botanische Jahrbücher für Systematik. 1966;85:151–247. [Google Scholar]
- Hartl D. Die Pseudosympetalie von Correa speciosa (Rutaceae) und Oxalis tubiflora (Oxalidaceae) Abhandlungen der Mathematisch-Naturwissenschaftlichen Klasse, Akademie der Wissenschaften und der Literatur Mainz. 1957;1957(2):1–13. [Google Scholar]
- Johansen DA. Plant microtechnique. New York: McGraw-Hill Book Company; 1940. [Google Scholar]
- Kallunki JA. A revision of Erythrochiton sensu lato (Cuspariinae, Rutaceae) Brittonia. 1992;44:107–139. [Google Scholar]
- Kallunki JA. Revision of Raputia Aubl. (Cuspariinae, Rutaceae) Brittonia. 1994;46:279–295. [Google Scholar]
- Kallunki JA. Validation of Neoraputia (Galipeae, Rutaceae) and description of two new species from Eastern Brazil. Brittonia. 2009;61:28–34. [Google Scholar]
- Kallunki JA, Groppo M. Phylogenetic analyses of the subtribe Galipeinae (Rutaceae) 2007 doi: 10.1371/journal.pone.0125650. http://2007.botanyconference.org/engine/search/index.php?func=detail&aid=1344 . Last accessed 12 October 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallunki JA, Pirani JR. Synopses of Angostura Roem. & Schult. and Conchocarpus J. C. Mikan. Kew Bulletin. 1998;53:257–334. [Google Scholar]
- Kubitzki K, Kallunki JA, Duretto M, Wilson PG. Rutaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Vol. 10. Berlin: Springer; 2011. pp. 276–356. [Google Scholar]
- Leinfellner W. Über die falsche Sympetalie bei Lonchostoma und anderen Gattungen der Bruniaceen. Österreichische Botanische Zeitschrift. 1964;111:345–353. [Google Scholar]
- Lopes AVF. Polinização por beija-flores em remanescentes da Mata Atlântica Pernambucana, nordeste do Brasil. Brazil: Universidade de Campinas; 2002. MSc Thesis. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Brunelliaceae, Cephalotaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae) Botanical Journal of the Linnean Society. 2002;140:321–381. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae) Botanical Journal of the Linnean Society. 2005a;149:129–194. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae) Botanical Journal of the Linnean Society. 2005b;147:1–46. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Chrysobalanaceae s.l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales) Botanical Journal of the Linnean Society. 2008;157:249–309. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Rhizophoraceae, Erythroxylaceae and the potentially related Ctenolophonaceae, Linaceae, Irvingiaceae and Caryocaraceae (Malpighiales) Botanical Journal of the Linnean Society. 2011;166:331–416. [Google Scholar]
- Matthews ML, Amaral MCE, Endress PK. Comparative floral structure and systematics in Ochnaceae s.l. (Ochnaceae, Quiinaceae, Medusagynaceae; Malpighiales) Botanical Journal of the Linnean Society. 2012;170:299–392. [Google Scholar]
- Piedade LH, Ranga NT. Ecologia da polinização de Galipea jasminiflora Engler (Rutaceae) Revista Brasileira de Botânica. 1993;16:151–157. [Google Scholar]
- Pirani JR. Three new species of Galipea (Rutaceae, Galipeinae) from Brazil. Botanical Journal of the Linnean Society. 2004;144:365–373. [Google Scholar]
- Pirani JR, Kallunki JA. Two new species of Galipea (Rutaceae, Galipeae) from Bolivia, Ecuador, and Peru. Brittonia. 2007;59:343–349. [Google Scholar]
- Pirani JR, El Ottra JHL, Menezes NL. Morfoanatomia de flores de cinco espécies de Galipea Aubl. e seu significado na evolução de flores tubulosas entre as Rutaceae neotropicais. Revista Brasileira de Botânica. 2010;33:301–318. [Google Scholar]
- Pirani JR, Groppo M, Kallunki JA. Two new species and a new combination in Conchocarpus (Rutaceae, Galipeeae) from eastern Brazil. Kew Bulletin. 2012;66:1–7. [Google Scholar]
- Quint M, Claßen-Bockhoff R. Floral ontogeny, petal diversity and nectary uniformity in Bruniaceae. Botanical Journal of the Linnean Society. 2006;150:459–477. [Google Scholar]
- Ramp E. Struktur, Funktion und systematische Bedeutung des Gynoeciums bei den Rutaceae und Simaroubaceae. Switzerland: Universität Zürich; 1988. PhD Thesis. [Google Scholar]
- Ritterbusch A. Morphologisches Beschreibungsmodell tubiflorer Kronen, ein Beitrag zur Terminologie und Morphologie der Asteriden-Blüte. Botanische Jahrbücher für Systematik. 1991;112:329–345. [Google Scholar]
- Robbrecht E. Tropical woody Rubiaceae. Opera Botanica Belgica. 1988;1:1–271. [Google Scholar]
- Rohweder O. Beiträge zur Blütenmorphologie und -anatomie der Commelinaceen mit Anmerkungen zur Begrenzung und Gliederung der Familie. Berichte der Schweizerischen Botanischen Gesellschaft. 1969;79:199–220. [Google Scholar]
- Tilson AH, Bamford R. The floral anatomy of the Aurantioideae. American Journal of Botany. 1938;25:780–793. [Google Scholar]
- Stebbins GL. Variation and evolution in plants. New York: Columbia University Press; 1950. [Google Scholar]
- Verbeke JA. Fusion events during floral morphogenesis. Annual Reviews of Plant Physiology and Plant Molecular Biology. 1992;43:583–598. [Google Scholar]
- Weberling F. Morphology of flowers and inflorescences. Cambridge: Cambridge University Press; 1989. [Google Scholar]
- Wei L, Wang YZ, Li ZY. Floral ontogeny of Ruteae (Rutaceae) and its systematic implications. Plant Biology. 2012;14:190–197. doi: 10.1111/j.1438-8677.2011.00475.x. [DOI] [PubMed] [Google Scholar]
- Westerkamp C, Claβen-Bockhoff R. Bilabiate flowers: the ultimate response to bees? Annals of Botany. 2007;100:361–374. doi: 10.1093/aob/mcm123. [DOI] [PMC free article] [PubMed] [Google Scholar]