Abstract
Background and Aims
Seeds of the moist temperate woodland species Galanthus nivalis and Narcissus pseudonarcissus, dispersed during spring or early summer, germinated poorly in laboratory tests. Seed development and maturation were studied to better understand the progression from developmental to germinable mode in order to improve seed collection and germination practices in these and similar species.
Methods
Phenology, seed mass, moisture content and ability to germinate and tolerate desiccation were monitored during seed development until shedding. Embryo elongation within seeds was investigated during seed development and under several temperature regimes after shedding.
Key Results
Seeds were shed at high moisture content (>59 %) with little evidence that dry mass accumulation or embryo elongation were complete. Ability to germinate developed prior to the ability of some seeds to tolerate enforced desiccation. Germination was sporadic and slow. Embryo elongation occurred post-shedding in moist environments, most rapidly at 20 °C in G. nivalis and 15 °C in N. pseudonarcissus. The greatest germination also occurred in these regimes, 78 and 48 %, respectively, after 700 d.
Conclusions
Seeds of G. nivalis and N. pseudonarcissus were comparatively immature at shedding and substantial embryo elongation occurred post-shedding. Seeds showed limited desiccation tolerance at dispersal.
Keywords: Amaryllidaceae, germination, Galanthus nivalis, Narcissus pseudonarcissus, seed development, desiccation tolerance, embryo growth, temperate woodland geophytes
INTRODUCTION
Orthodox seed (Roberts, 1973) development typically may be divided into three distinct phases following ovule fertilization: histodifferentiation, during which embryonic tissues are formed; seed filling, when reserve deposition takes place; and maturation drying, characterized by water loss and reduced metabolism (Kermode et al., 1986; Bewley and Black, 1994). Seed filling is terminated at mass maturity (Ellis and Pieta Filho, 1992), with maturation drying thereafter. During orthodox seed development, the ability to germinate (Kermode and Bewley, 1985), seed desiccation tolerance (Ellis et al., 1987) and potential longevity (Pieta-Filho and Ellis, 1991) develop and increase, usually in that order (Sanhewe and Ellis, 1996). In some species, drying promotes germinability; fresh seeds that have not undergone desiccation will not germinate or germinate only poorly (Adams and Rinne, 1981; Samarah et al., 2004). In recalcitrant seeds (Roberts, 1973), maturation drying is absent or much reduced; at dispersal, seeds show little water loss, are metabolically active and may still be accumulating dry weight (Farrant et al., 1993; Kermode and Finch-Savage, 2002).
Ideally, orthodox seeds should be collected when, for the majority of the seeds in the targeted population, germinability, desiccation tolerance and longevity are at their maximum. At this point, it is important that seeds are dried to low moisture contents to ensure that this potential for considerable longevity is maintained (Harrington, 1972; Roberts, 1973). Recommended conditions for the conventional long-term storage of orthodox seeds, after drying to 3–7 % moisture content (fresh mass basis), are in air-tight containers at –18 °C or less (Cromarty et al., 1982; FAO/IPGRI, 1994).
The Amaryllidaceae are extremely variable in fruit and seed characters, particularly among the American, African and Eurasian clades. Eurasian species usually have dry, wedge-shaped seeds that frequently have an elaiosome (oil-rich food body attractive to ants) at the chalazal end of the seed (Meerow et al., 1999). Seeds comprise a small, linear embryo embedded in endosperm (Church, 1908; Martin, 1946). Seed storage behaviour ranges from predominantly orthodox in the American clade to recalcitrant in the African clade (Priestley, 1986; Sershen et al., 2008). Seed storage behaviour in the Eurasian clade is unknown. The investigations reported here, of seed development in Galanthus nivalis L. (common snowdrop) and Narcissus pseudonarcissus L. (wild daffodil), arose from difficulties in germinating seeds from collections of European species of the Amaryllidaceae in the Millennium Seed Bank and concerns regarding survival of stored seed (R. J. Probert, RBG Kew, UK, pers. comm.).
Galanthus nivalis, indigenous to western, southern and central Europe, and N. pseudonarcissus, native to southern Europe and the Western Mediterranean, are typically found in temperate deciduous woodland growing in damp soil which, in the case of G. nivalis, may be prone to short periods of flooding (Church, 1908; Caldwell and Wallace, 1955; Budnikov and Kricsfalusy, 1994; Bishop et al., 2001). These plants are unique within these habitats as they are the first understorey plants to emerge and flower in late winter/early spring, with seed shed occurring near the end of spring. Rapid vegetative growth and flowering is possible in these species at these cold temperatures because shoots and flowers develop and differentiate internally in the bulbs during the previous summer (Church, 1908; Bishop et al., 2001). Light conditions in the understorey of deciduous woodland are maximal during the period between leaf fall in autumn and leaf development in spring, compared with shaded conditions during summer when trees are in leaf (Salisbury, 1916), light being the limiting factor for vegetative growth and reproduction in woodland herbs (Whigham, 2004). Hence, their strategy of early development enables G. nivalis and N. pseudonarcissus to take advantage of the availability of light by completing vegetative and reproductive development, including the major proportion of seed development, before tree canopy closure. Little is known about the phenology of seed development physiology in early spring-flowering temperate species. Consequently, these two species were appropriate study subjects to improve our understanding of the acquisition of germinability and desiccation tolerance at colder temperatures.
The main objective of this study was to develop an understanding of seed development in these species and so improve the management of the seeds of these and similar species in ex situ conservation. We describe the phenology of seed development and maturation, characterize seed germinability and desiccation tolerance during seed development and on shedding, and investigate embryo elongation pre- and post-shedding in order to test the hypothesis that seed development and maturation are complete prior to dispersal.
MATERIALS AND METHODS
Study sites and environmental conditions during seed development
Narcissus pseudonarcissus seeds were collected from a wild population within a 200 × 200 m area in the Loder Valley Nature Reserve, West Sussex, UK (51°03′29″N, 00°05′33″W). Galanthus nivalis seeds were collected from an introduced population at Wakehurst Place, West Sussex, UK (51°04′07″N, 00°05′16″W). The latter population of approx. 20 000 plants was established from bulbs planted in September 2005 supplied by The Boston Bulb Company (Cradge Bank, Spalding, UK) from a UK grower who collected the naturalized plants under licence and with permission from landowners in Norfolk, Yorkshire and Scotland. A Tiny Tag PT 100 logger [Gemini Loggers (UK) Ltd, Chichester, UK], positioned approx. 100 mm above the ground within the study sites, recorded relative humidity (RH) and temperature every 20 min during the period of capsule collection; the means of which were 75·1 % (±0·47 s.e.) and 15·5 °C (± 0·12 s.e.), respectively. Daily maximum and minimum temperatures, obtained from the Horticulture Section at Wakehurst Place, were used to calculate the weighted mean daily temperature (taking photoperiod into account) during seed development.
Capsule collection
Flowers of N. pseudonarcissus (in 2006) and G. nivalis (in 2008) were tagged every few days (1–3 times a week) from when they first opened in March and January, respectively. At least twice as many flowers were tagged as capsules collected due to flower and fruit loss during flowering and seed development. All flowers that had opened in the case of the first tagging, or had opened subsequent to the previous tagging, were tagged. This was continued within the designated sampling area until all flowers had opened. Capsules were sampled regularly to monitor seed development (from both species and all flower cohorts, where tagged), from the beginning of May (Fig. 1) until capsule opening and seed shed. Closed G. nivalis capsules, in which the flower stalk had started to disintegrate (typically around mid-May), thereby cutting the vascular connection to the mother plant, were collected and arranged in a monolayer on blotting paper in a plastic tray at 15 °C and 75 % RH in order to mimic the natural environment for continued ripening ex planta. The majority of seed development data for G. nivalis were obtained from capsules ripened ex planta in these conditions for 1–20 d. A few G. nivalis capsules dried without dehiscing; seeds from these capsules were excluded from analyses. As the flower stalk did not disintegrate in N. pseudonarcissus, capsules were left to ripen naturally on the plant throughout seed development.
Fig. 1.
Phenology of flowering, fruit and seed development of (A) Galanthus nivalis (2008) and (C) Narcissus pseudonarcissus (2006), and maximum and minimum daily temperatures at Wakehurst Place in West Sussex in (B) 2008 and (D) 2006, respectively.
Capsule and seed measurements
In total, 47 N. pseudonarcissus capsules were collected in 2006 and 46 in 2009; 260 G. nivalis capsules were collected in 2008. Capsule colour was recorded and seeds from individual capsules were divided equally into five groups (n ≤ 10 seeds per group) to determine: (1) individual seed fresh weight, dry weight and hence moisture content; (2) the ability of fresh (i.e. not dried) seeds to germinate; (3) the ability of dried seeds to germinate (and so desiccation tolerance); (4) embryo length and viability of fresh seeds; and (5) embryo length and viability of dried seeds. To estimate desiccation tolerance, seeds from individual capsules were rapidly dried by placing in a monolayer in 10 mL clear, open glass vials (Scientific Laboratory Supplies Ltd, Nottingham, UK) at 15 °C and 15 % RH for 28 d prior to germination testing. Data from individual capsules were then combined for analysis.
The effect of drying at 15 °C and 75 % RH (reflecting the mean ambient temperature and RH at the study site at the time of capsule opening) on seed moisture content was also determined for separate samples of freshly dispersed N. pseudonarcissus and G. nivalis seeds.
Seed weight and moisture content determination
Seed moisture content (and also fresh and dry weight) was determined gravimetrically according to the low-constant-temperature-oven method (ISTA, 2005) for 482 seeds from 47 capsules, 380 seeds from 39 capsules, and 347 seeds from 189 capsules for N. pseudonarcissus 2006, N. pseudonarcissus 2009, and G. nivalis 2008, respectively. Fresh weight was obtained by weighing individual seeds on a seven-place balance (Mettler Toledo, Beaumont Leys, UK). Seeds were then dried for 17 ± 1 h at 103 ± 2 °C in a Genlab 100 L ventilated oven (Jencons-PLS, Forest Row, UK), and reweighed to determine dry weight. Moisture content was calculated as a percentage of fresh seed mass.
Embryo length and viability
Dried seeds were suspended over water (100 % RH) for 24 h to rehydrate before placing onto 1 % distilled water agar for 24 h to imbibe. Rehydrated and fresh seeds were dissected longitudinally near the mid-section (avoiding damaging the embryo), using a Cobra light microscope (Vision Engineering, Woking, UK) and placed into a 1 % aqueous solution of tetrazolium chloride (ISTA, 2005) for 72 h at 30 °C in the dark [method for the genus Amaryllis, modified from Moore (1985)]. Seeds in which the entire embryo and endosperm stained a uniform bright or dark red colour were scored as viable. After staining, seeds were dissected to record embryo length using a Nikon SMZ645 dissecting microscope with a C-W10X/22 micrometer (Southern Microscopes, Maidstone, UK): 167 embryos from N. pseudonarcissus 2006, 412 embryos from N. pseudonarcissus 2009, and 117 embryos from G. nivalis 2008 seeds were measured.
Germination testing
Fresh or dried seeds (one seed per well) were sown on 1 % distilled water agar (Microbiological Agar Granules, Merck, Darmstadt, Germany) in 10 mm Nunc multiwell dishes (Scientific Laboratory Supplies Ltd), which allowed individual seeds to be monitored. Narcissus pseudonarcissus seeds collected in 2006 were placed in cooled incubators (LMS Ltd, Sevenoaks, UK) at 25/10 °C for 84 d then moved to 15/5 °C for 84 d (8/16 h light/dark photoperiod). Improved germination conditions were later determined and so, for G. nivalis seeds collected in 2008 and N. pseudonarcissus seeds collected in 2009, dishes were double-wrapped in aluminium foil, sealed in clear plastic bags and placed for 56 d at each of, in sequence, 25, 20, 15 and 10 °C (with fresh agar every 56 d). In total, 612 dried N. pseudonarcissus (2006), 374 fresh and 356 dried N. pseudonarcissus (2009) and 351 fresh and 297 dried G. nivalis (2008) seeds were tested for germination. Seeds dried before germination testing were placed at 15 % RH and 15 °C for 28 d, attaining a mean moisture content of 7·1 ± 0·13 % (s.e.) for G. nivalis and 6·9 ± 0·33 % (s.e.) for N. pseudonarcissus. Seeds were assessed for germination at regular intervals under a dim, safe, green light comprising three 15–20 W cool white fluorescent tubes covered by three layers of no. 39 (primary green) Cinemoid as described in Probert and Smith (1986). Seeds were scored as germinated when the radicle was at least 2 mm long. Seeds that had not germinated at the end of a test were dissected to determine whether they remained firm (and so assumed viable).
Post-shedding germination testing and embryo elongation
Germination tests (n = 50 seeds per species and temperature) were set up on fresh seeds from naturally dehiscing capsules. Poorly developed seeds were excluded from samples. Seeds were placed into Nunc multiwell dishes containing 1 % distilled water agar; N. pseudonarcissus seeds collected in 2006 were placed at three alternating (8/16 h) temperatures (10/0, 15/5 or 25/10 °C) and N. pseudonarcissus (2007) and G. nivalis (2007) seeds at six constant temperatures (0, 5, 10, 15, 20 or 25 °C). Narcissus pseudonarcissus seeds were exposed to light (8/16 h light/dark cycle), while seeds of G. nivalis were kept in the dark. Selection of these environments, and those above for germination tests, were based on developing experience with these species in the Millennium Seed Bank (data not shown). Germination was scored as above.
Duplicate samples were placed onto agar and into identical conditions to assess post-shedding embryo elongation. At each sampling (initially every 30 d, subsequently less frequently), ten seeds were randomly removed for embryo length measurements and the remaining seeds placed onto fresh agar and returned to the incubators. Seeds were dissected and placed into tetrazolium to assess viability (as described above). Only non-germinated, viable seeds were assessed for embryo length.
Additional seeds incubated in the dark at 20 °C for 84 d and then moved to either 10 °C (N. pseudonarcissus 2007) or 15 °C (G. nivalis 2007) were checked every 1–3 d for germination. Embryo lengths of germinated seeds (n = 20 seeds per species) were measured as described above as soon as the radicle was first observed. The germination embryo length measurement did not include the section of radicle that protruded beyond the seed coat.
Data analysis
All analyses were done in Genstat for Windows Eleventh Edition (VSN International Ltd, Hemel Hempstead, UK). Seed dry weight, fresh weight and moisture content were initially regressed against each of the days after flowering, thermal time from flower opening and sample day (calculated by setting the last sample date, which occurred within the period during which most seed was being dispersed, to zero) to determine which of these explanatory variables best accounted for the variation observed in seed development. Sample day was selected as the explanatory variable that enabled full and representative presentation of the results.
Variance increased with seed development. Logarithmic transformation of individual capsule means of seed dry weight, fresh weight and moisture content satisfied the requirement for normality and equal variance (with the exception of moisture content for N. pseudonarcissus). Regressions were weighted by the number of observations for capsule means. Given the expected patterns of seed development, linear and split-line regressions (dry weight), linear and quadratic regressions (fresh weight), and linear regressions (moisture content) were fitted to capsule means.
Pre-shedding embryo lengths of fresh and fully rehydrated dried seeds were not significantly different in either G. nivalis 2008 (Mann–Whitney U-test, P = 0·137) or N. pseudonarcissus 2009 (Mann–Whitney U-test, P = 0·138). Embryo lengths for both cohorts were thus combined for analysis.
Linear, split-line or quadratic regressions were fitted to embryo length measurements against post-shedding duration at each temperature. Multiple linear regression analysis was performed on natural log-transformed post-shedding embryo length data to determine the relative importance of duration of incubation and temperature on elongation.
RESULTS
Phenology
Development from shoot emergence to seed dispersal occupied the first 5 months of the calendar year for both species (Fig. 1). Not surprisingly, given the cool temperatures, the duration between flower formation and ovary expansion or capsule swelling (i.e. seed formation) was considerable, especially in G. nivalis. In contrast, subsequent fruit ripening and seed development was more rapid (Fig. 1). Capsule colour progressed from green through light green then yellow to brown as seeds matured in both G. nivalis and N. pseudonarcissus. In G. nivalis, individual flower life span was much reduced in later-opening flowers. There were no differences, otherwise, in seed development among different flowering cohorts. By mid-May, some seeds within capsules of both species had aborted. The number of seeds per capsule was substantially greater in N. pseudonarcissus than in G. nivalis (Table 1).
Table 1.
Developing seeds per capsule, seed moisture content (MC) on shedding or after a further 14 d at 75 % RH and 15 °C (simulating the environment into which seeds are shed), and dry weight at shedding in Narcissus pseudonarcissus and Galanthus nivalis (where N is the number of capsules and n is the number of seeds; all values are means ± s.e.)
| Species and year | No. of seeds per capsule | MC (%) on shedding | MC (%) after 14 d at 75 % RH, 15 °C | Dry weight (mg) |
|---|---|---|---|---|
| N. pseudonarcissus 2006 | 43 ± 4·2 (N = 33) | 66·8 ± 1·96 (n = 50) | 16·7 ± 0·48 (n = 3 × 20) | 4·10 ± 0·199 (n = 50) |
| N. pseudonarcissus 2009 | 45 ± 2·8 (N = 46) | 59·1 ± 1·35 (n = 58) | – | 6·00 ± 0·247 (n = 58) |
| G. nivalis 2008 | 6·2 ± 0·34 (N = 260) | 60·1 ± 1·65 (n = 12) | 22·0 ± 0·73 (n = 10) | 7·08 ± 0·696 (n = 12) |
Dry mass accumulation, moisture content and embryo elongation during seed development
As expected, seed moisture content declined while seed fresh weight, dry weight and embryo length increased during seed development (Fig. 2), with considerable variability in each. Seeds were shed at the comparatively high moisture content of 59–67 % (Fig. 2A–C). Seed filling (Fig. 2D–I, Table 1) and embryo elongation (Fig. 2J–L) continued until, or almost until, seed dispersal. When dispersed, the seeds had not equilibrated with ambient RH [75·1 % (±0·47 s.e.)], as freshly collected seeds of G. nivalis and N. pseudonarcissus held at 75 % RH and 15 °C dried within 14 d to 22 and 17 % moisture content (Table 1), respectively.
Fig. 2.
(A–C) Mean moisture content (logarithmic scale), (D–F) fresh weight (logarithmic scale), (G–I) dry weight (logarithmic scale), and (J–L) embryo length of seeds (± s.e.) from individual capsules during seed development plotted against sample day (where seed dispersal occurred mainly on day zero). A total of 482 seeds from 47 capsules (Narcissus pseudonarcissus 2006), 380 seeds from 39 capsules (N. pseudonarcissus 2009) and 347 seeds from 189 capsules (Galanthus nivalis 2008) were sampled. All results, including outliers, are shown. For linear regression analysis (C–I, K) and split-line regression analysis (J), one outlier was removed from the N. pseudonarcissus 2006 data set, two from the N. pseudonarcissus 2009 data set and three from the G. nivalis 2008 data set. In (A), (B) and (L), trend lines are shown where regression analysis was not possible.
Acquisition of ability to germinate and tolerate rapid desiccation
In N. pseudonarcissus (2009), some seeds were able to germinate 19 d before dispersal, when mean seed moisture content was 84 % (Fig. 3A). Ability to germinate increased progressively thereafter, reaching 88 % when moisture content had declined to 66 %, 3 d before most seeds were dispersed. The ability to germinate was first detected in fresh G. nivalis seeds somewhat earlier, 29 d prior to dispersal, when mean seed moisture content was 77 % (Fig. 3B). It then increased gradually to a maximum of 79 % when moisture content had declined to 67 %, 12 d before dispersal, with a subsequent slight decline at dispersal (Fig. 3B).
Fig. 3.
Changes in viability and ability to germinate during seed development of (A) fresh N. pseudonarcissus 2009 (n = 374), (B) fresh G. nivalis 2008 (n = 351), (C) dried N. pseudonarcissus 2006 (n = 612), (D) dried N. pseudonarcissus 2009 (n = 356) and (E) dried G. nivalis 2008 (n = 297) seeds. Mean moisture content (MC, fresh weight basis) is shown for seeds from the same set of capsules. Seeds were sampled from 12 May 2009 (day –19) until observed natural seed dispersal on 31 May 2009 (day 0) for N. pseudonarcissus (A, D), from 25 May 2006 (day –13) until 7 June 2006 (day 0) for N. pseudonarcissus (C) and from 1 May 2008 (day –34) until 4 June 2008 (day 0) for G. nivalis (B, E). Seeds were incubated for a total of 224 d at decreasing constant temperatures of 25–20–15–10 °C (56 d each) in the dark.
The proportion of seeds that were fully formed (i.e. solid) on dissection (for embryo measurement) was positively associated with that which germinated immediately following collection in parallel tests for both G. nivalis 2008 (r = 0·91, P = 0·001) and N. pseudonarcissus 2009 (r = 0·93, P = 0·004). The seed populations at dispersal were not homogeneous in either species, with some seeds failing to accumulate dry matter, possibly the result of seed abortion. This observation tallies with the substantial proportion of non-viable seeds at dispersal (e.g. open bars in Fig. 3A, B at the final sample day).
The development of desiccation tolerance lagged behind that of germinability (Fig. 3). Desiccation tolerance (here the ability to germinate following rapid drying at 15 % RH) was first observed in N. pseudonarcissus 12 d (2009) and 13 d (2006) before dispersal when moisture content was 73–78 %, about 7 d after germinability was first detected. Fewer than half the seeds at dispersal were viable after rapid drying (Fig. 3C, D). Desiccation tolerance was not detected in G. nivalis until seed moisture content had declined to 63 %, 23 d after the ability to germinate was first observed and only 6 d before dispersal (Fig. 3E). A maximum of 36 % of seeds close to dispersal germinated following rapid desiccation, with 44 % potentially viable.
Capsule and seed characteristics
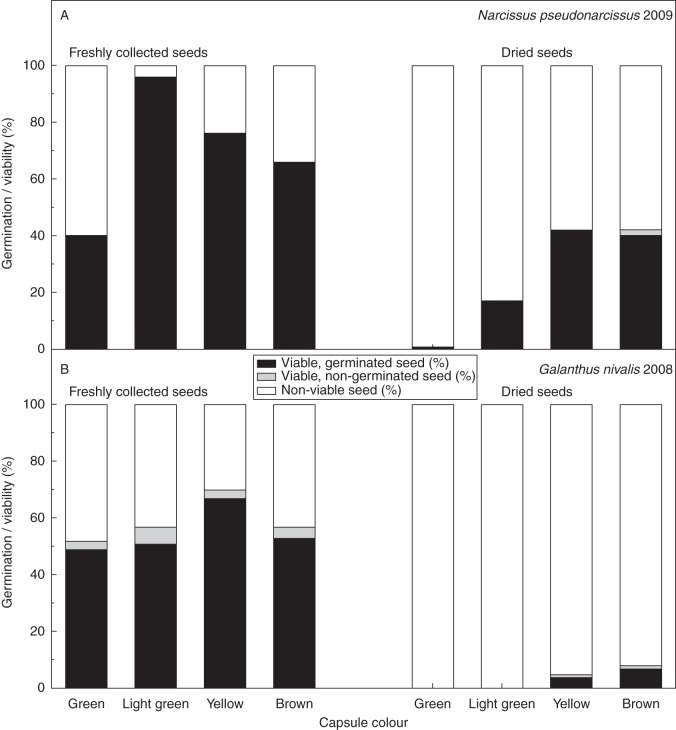
In N. pseudonarcissus, fewer than half of fresh seeds from green capsules were potentially germinable (Fig. 4A). Most seeds in light green capsules were germinable, with a greater proportion of non-viable seeds on dissection in yellow and brown capsules (Fig. 4A). A similar pattern of increasing then decreasing germinability with capsule colour progression occurred in G. nivalis, but with maximum germinability in seeds from yellow capsules (Fig. 4B).
Fig. 4.
The ability of fresh and dried seeds to germinate from different coloured capsules sampled during seed development in (A) Narcissus pseudonarcissus 2009 (n = 374 for fresh and n = 356 for dried seeds) and (B) Galanthus nivalis 2008 (n = 351 for fresh and n = 297 for dried seeds). This analysis is drawn from the same overall data set as that in Fig. 3; for further details, see Fig. 3.
Less than 1 % of seeds from green capsules were desiccation tolerant in N. pseudonarcissus, but seeds from light green and yellow capsules were progressively better able to withstand rapid desiccation (Fig. 4A). Narcissus pseudonarcissus seeds from yellow and brown capsules showed similar desiccation tolerance (Fig. 4A). No G. nivalis seeds from green or light green capsules tolerated desiccation; however, a small fraction from yellow capsules and a marginally larger fraction from brown capsules survived drying (Fig. 4B).
Post-shedding embryo elongation
At dispersal, embryos of N. pseudonarcissus were longer than those of G. nivalis (Figs 2J–L and 5), but broadly comparable amongst years within species, ranging from 1·1 to 1·4 mm in N. pseudonarcissus and from 0·6 to 0·7 mm in G. nivalis. Considerable embryo elongation (almost doubling in N. pseudonarcissus and more than trebling in G. nivalis) occurred post-shedding in hydrated seeds over prolonged periods in vitro at different temperatures (Fig. 5A–C). Rates of embryo elongation were ten times slower in N. pseudonarcissus and three times slower in G. nivalis than pre-shedding elongation (at the equivalent temperature).
Fig. 5.
Embryo length (mean ± s.e., n = 10 per temperature) of viable, non-germinated (A) Narcissus pseudonarcissus 2006, (B) N. pseudonarcissus 2007 and (C) Galanthus nivalis 2007 seeds, and cumulative germination (n = 50) (D–F, respectively) following incubation at alternating or constant temperatures in the light (8/16 h light/dark photoperiod) for N. pseudonarcissus or darkness for G. nivalis. Solid horizontal lines (± s.e., dotted lines) in A–C are mean embryo length at shedding. For comparison, mean embryo length (horizontal broken line, ±s.e., dotted line) of seeds subsequently able to germinate at 15 °C (G. nivalis 2007) and 10 °C (N. pseudonarcissus 2007) following incubation at 20 °C for 84 d, are shown. No regression could be fitted for embryo length at 0 °C in N. pseudonarcissus.
Embryo elongation was temperature dependent: embryos in seeds of both species held at warmer temperatures (≥15 °C) elongated more rapidly than those in cooler (<15 °C) conditions (Fig. 5A–C). Multiple regression analysis for both species at constant temperatures indicated that temperature (P < 0·001) and incubation period (P < 0·001) both affected embryo length, as did their interaction (P < 0·001). The models explained a greater proportion of the variance for G. nivalis (R2 = 0·68, P < 0·001) than N. pseudonarcissus (R2 = 0·36, P < 0·001), with temperature more influential, especially in G. nivalis. The germination embryo length was similar in both species, at 2·10–2·36 mm (Fig. 5B, C). In warmer temperatures (≥10 °C in N. pseudonarcissus and ≥15 °C in G. nivalis), the mean germination embryo length was eventually exceeded or attained, while embryos at cooler temperatures elongated slowly throughout the investigation without reaching the germination embryo length. Embryo elongation in N. pseudonarcissus appeared to cease once the germination embryo length was reached; in G. nivalis, elongation continued, albeit more slowly.
Temperature regimes in which more rapid elongation was detected were also those at which germination occurred more readily (Fig. 5D–F). The greatest germination was observed in N. pseudonarcissus at 25/10 °C (2006) and 15 °C (2007) and in G. nivalis at 20 °C, starting around the same time as germination embryo length was reached. Germination occurred to a lesser extent at slightly cooler temperatures (10 °C in N. pseudonarcissus and 15 °C in G. nivalis) (Fig. 5E, F), commencing before embryos at these temperatures had reached the mean germination embryo length (Fig. 5B, C).
DISCUSSION
The hypothesis that seeds are shed when they are mature is rejected for both of these moist temperate woodland species: seed dispersal occurred whilst seeds were immature and considerable embryo development occurred after shedding. At dispersal, there was no strong evidence of prior cessation of dry weight accumulation (Fig. 2G–I) in seeds of G. nivalis and N. pseudonarcissus. Furthermore, maturation drying was absent (Fig. 2D–F), i.e. no loss in fresh weight, and similarly seeds had not equilibrated with ambient RH prior to shedding (Table 1). Instead, most of the seed development period comprised seed filling, with seeds being shed at high moisture content (>59 %) into the environment (Fig. 2A–C, Table 1), similar to that of the temperate woodland herb Anemone nemorosa [66 % moisture content, fresh weight basis, 1·97 g (g d. wt)−1] dispersed in late spring (Ali et al., 2007). This pattern is unusual compared with most non-fleshy orthodox seeded species, which typically undergo maturation drying before dispersal (Ellis and Pieta-Filho, 1992; Hay and Probert, 1995; Samarah et al., 2004), equilibrating with the RH of the environment after maximum dry weight is attained (Probert and Hay, 2000), or compared with fleshy fruited orthodox species where seeds continue to mature in planta for several weeks after seed filling until fruit drop and seeds are shed at high moisture contents (Welbaum and Bradford, 1989; Berry and Bewley, 1991; Demir and Ellis, 1992).
Seeds are shed at the end of May (Fig. 1) into moist, shady habitats (Church, 1908), with continuous hydration in the soil seed bank probable (Fenner and Thompson, 2005). Indeed, species from damp temperate habitats may not have been exposed to the selection pressure of seed able to withstand considerable desiccation before germination (Ali et al., 2007). It is therefore reasonable to assume that the linear embryos of G. nivalis and N. pseudonarcissus, which elongated during seed development (Fig. 2) and continued to develop in vitro for a considerable period of time post-shedding (Fig. 5), may do so similarly in the natural environment, albeit at a slower rate. Several other temperate plant species, including A. nemorosa (Ali et al., 2007; Mondoni et al., 2008), A. ranunculoides (Mondoni et al., 2009) and Scilla bifolia (Vandelook and van Assche, 2008) possess embryos that grow continuously within the seed following dispersal without an extended period of developmental arrest.
Temperature was the most influential factor in post-shedding embryo elongation, with more rapid elongation rates at warmer temperatures (Fig. 5A–C), similar to embryo elongation patterns reported in Daucus carota (Gray et al., 1988), N. hispanicus (Copete et al., 2011) and by other authors also for N. pseudonarcissus (Vandelook and van Assche, 2008) seeds. Germination was delayed and sporadic (Fig. 5D–F). Several authors have suggested a minimum embryo length for germination in species where embryos must elongate before radicle emergence (van der Toorn and Karssen, 1992; Hidayati et al., 2000; Kondo et al., 2004; Copete et al., 2011). Although the mean embryo length at germination was determined for N. pseudonarcissus (Fig. 5B) and G. nivalis (Fig. 5C) from move-along germination experiments (warm followed by cool temperatures as may be experienced in the natural environment), attainment of this embryo length neither guaranteed, nor was required for, germination. Little germination occurred in seeds at 25 °C (Fig. 5E, F), in which the apparent germination embryo length was attained or exceeded, while germination at cooler temperatures (10 °C in N. pseudonarcissus and 15 °C in G. nivalis) commenced in some seeds before the germination embryo length was reached (Fig. 5B, C). Hence, while embryo elongation is necessary, the notion of a critical embryo length for germination in these species is questionable. This is in agreement with Karlsson et al. (2003), who showed that the embryo length at which Argemone ochroleuca germinated differed with temperature.
As embryos elongated before radicle emergence (Fig. 5A–C) and the latter did not occur within 30 d of dispersal (Fig. 5D–F), seeds may be said to possess morphophysiological dormancy (Baskin and Baskin, 1998, 2004). An increasing number of studies suggest, however, that if embryo elongation occurs continuously from dispersal to radicle emergence, seeds should be classified as non-dormant (e.g. Ali et al., 2007; Mondoni et al., 2008; Carasso et al., 2011). Similarly, the classification of species with shoot (‘epicotyl’) dormancy, where delayed emergence is a result of continuous slow shoot development at cold temperatures rather than developmental arrest, has been challenged (Vandelook and van Assche, 2008). Alternative definitions of dormancy include ‘ … the temporary suspension of visible growth of any plant structure containing a meristem’ (Lang, 1987) and ‘ … an arrest in development of seed embryos … in conditions otherwise suited for growth’ (Taylorson and Hendricks, 1977). Under these definitions, seeds in which embryo elongation continues after shedding would be considered non-dormant.
Substantial variation in dry weight, fresh weight and moisture content during G. nivalis and N. pseudonarcissus seed development (Fig. 2A–I) is not unique. In a recent study on Trifolium ambiguum, seed-to-seed variability in final dry weight and mass maturity was considerable (Hay et al., 2010). A reason for this variability during seed development may be that flowering occurs during the cold part of the year, especially in G. nivalis. Not only do flowers of G. nivalis open over an extended 7 week period (Fig. 1), but individual flowers may stay open for up to 8 weeks (Church, 1908), with the potential for pollination to occur throughout this period.
During G. nivalis and N. pseudonarcissus seed development, the ability to germinate and to withstand rapid drying increased as seeds matured, reaching a maximum just prior to seed release, with the former being acquired before the latter (Fig. 3), similar to that of other species such as Digitalis purpurea (Hay and Probert, 1995) and Acer platanoides (Hong and Ellis, 1990). The ability to tolerate rapid drying developed relatively late in seed development, especially in G. nivalis, and in less than half of the seed population in both species (Fig. 3C–E).
The slight decline in germination of fresh seeds before dispersal from more mature capsules (Figs 3A, B and 4) may have been confounded by dormancy induction in a proportion of seeds. An alternative and more plausible explanation for this, as well as the low incidence of desiccation tolerance at dispersal (Figs 3C–E and 4), however, may be the premature breaking of the connection of the seed to the placenta, or perhaps selective abortion, before germinability and desiccation tolerance, respectively, were acquired. Ovule abortion can take place at any stage before seeds reach maturity (Lersten, 2004). Substantial seed abortion can occur in both N. pseudonarcissus (Balatková et al., 1977) and G. nivalis (Budnikov and Kricsfalusy, 1994). Hence, the considerable variation within capsules (Fig. 2) may in part be due to seed abortion.
The ability of seeds to tolerate desiccation may not be essential in these species in situ, particularly in G. nivalis, as it is highly unlikely that seeds would experience conditions in their natural environment that would result in rapid drying to low moisture contents; yet the latter is recommended for ex situ seed conservation (FAO/IPGRI, 1994). As most seeds were unable to germinate following this desiccation procedure at the point of natural dispersal (Fig. 3C–E), conventional collection and drying protocols may not be suitable for producing high quality seed collections of these species. That desiccation tolerance increases right up until seed dispersal in both species highlights the importance of not collecting seeds until evidence of seed maturity is observed (Hay and Smith, 2003), such as yellow or brown capsule colour (Fig. 4) and natural dispersal (e.g. capsules starting to split).
Given that some seeds of both species withstand desiccation to approx. 6–7 % (Fig. 3C–E), it is unlikely that they possess intermediate (Ellis et al., 1990) or recalcitrant storage behaviour, but neither do we have firm evidence that they are orthodox. Their poor seed germination and storability record in the Millennium Seed Bank may be due to failure of a large proportion of seeds to complete maturation in planta and therefore become fully desiccation tolerant at the time of seed collection (shedding), especially in G. nivalis (Fig. 3). Improvement of desiccation tolerance (and also longevity) in immature or prematurely collected seeds may be achieved by slow drying rates or delayed drying, which is thought to allow the continuation of natural ripening events (Hay and Probert, 1995, 2011; Hay et al., 1997; Lima et al., 1998; Probert et al., 2007). Delayed drying improved the ability of seeds of A. nemorosa to survive drying as embryos developed to the desiccation-tolerant heart shape (Ali et al., 2007). However, such procedures cannot benefit seeds that aborted early in their development.
The similarity of this evidence across several species suggests a distinct typology of seed development in early spring-flowering temperate woodland understorey plants which matches their ecological niche, whereby seeds are dispersed close to the end of seed filling without substantial developmental arrest or significant maturation drying (i.e. whilst immature) and with continued seed development occurring naturally on the woodland floor or in the soil seed bank under the tree canopy. In ex situ conservation practice, therefore, fruits of these species may need to be placed in similar, simulated environments after collection. Investigation of the post-collection sequence of environments that enable high ability to germinate is the subject of further research.
ACKNOWLEDGEMENTS
We thank Darren Murray (VSN International Ltd) for advice on development regression analyses, John Adams and Nicola Keogh for laboratory support, David Hardman and Stephen Robinson for permission to collect seeds, and Robin Probert for helpful discussions. This work was supported by the Millennium Commission, The Wellcome Trust and Orange plc. The Royal Botanic Gardens, Kew receives grant-in-aid from Defra, UK.
LITERATURE CITED
- Adams CA, Rinne RW. Seed maturation in soybeans (Glycine max L. Merr.) is independent of seed mass and of the parent plant, yet is necessary for production of viable seeds. Journal of Experimental Botany. 1981;32:615–620. [Google Scholar]
- Ali N, Probert R, Hay F, Davies H, Stuppy W. Post-dispersal embryo growth and acquisition of desiccation tolerance in Anemone nemorosa L. seeds. Seed Science Research. 2007;17:155–163. [Google Scholar]
- Balatková V, Tupý J, Hrabětová E. Seed formation in Narcissus pseudonarcissus L. after placental pollination in vitro. Plant Science Letters. 1977;8:17–21. [Google Scholar]
- Baskin CC, Baskin JM. Seeds: ecology, biogeography, and evolution of dormancy and germination. London: Academic Press; 1998. [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Science Research. 2004;14:1–16. [Google Scholar]
- Berry T, Bewley JD. Seeds of tomato (Lycopersicon esculentum Mill.) which develop in a fully hydrated environment in the fruit switch from a developmental to a germinative mode without a requirement for desiccation. Planta. 1991;186:27–34. doi: 10.1007/BF00201494. [DOI] [PubMed] [Google Scholar]
- Bewley JD, Black M. Seeds: physiology of development and germination. 2nd edn. New York: Plenum Press; 1994. [Google Scholar]
- Bishop M, Davis A, Grimshaw J. Snowdrops: a monograph of cultivated Galanthus. Maidenhead, UK: The Griffin Press; 2001. [Google Scholar]
- Budnikov G, Kricsfalusy V. Bioecological study of Galanthus nivalis L. in the East Carpathians. Thaiszia – Journal of Botany. 1994;4:49–75. [Google Scholar]
- Caldwell J, Wallace TJ. Biological flora of the British Isles: Narcissus pseudonarcissus L. Journal of Ecology. 1955;43:331–341. [Google Scholar]
- Carasso V, Hay FR, Probert RJ, Mucciarelli M. Temperature control of seed germination in Fritillaria tubiformis subsp. moggridgei (Liliaceae) a rare endemic of the South-west Alps. Seed Science Research. 2011;21:33–38. [Google Scholar]
- Church AH. Types of floral mechanism. Part 1. Oxford: Clarendon Press; 1908. [Google Scholar]
- Copete E, Herranz JM, Ferrandis P, Baskin CC, Baskin JM. Physiology, morphology and phenology of seed dormancy break and germination in the endemic Iberian species Narcisssus hispanicus (Amaryllidaceae) Annals of Botany. 2011;107:1003–1016. doi: 10.1093/aob/mcr030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromarty AS, Ellis RH, Roberts EH. Rome: International Board for Plant Genetic Resources; 1982. The design of seed storage facilities for genetic conservation. Handbook for genebanks: no. 1. Revised 1985. [Google Scholar]
- Demir I, Ellis RH. Changes in seed quality during seed development and maturation in tomato. Seed Science Research. 1992;2:81–87. [Google Scholar]
- Ellis RH, Pieta Filho C. The development of seed quality in spring and winter cultivars of barley and wheat. Seed Science Research. 1992;2:9–15. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. The development of desiccation-tolerance and maximum seed quality during seed maturation in six grain legumes. Annals of Botany. 1987;59:23–29. [Google Scholar]
- Ellis RH, Hong TD, Roberts EH. An intermediate category of seed storage behaviour? I. Coffee. Journal of Experimental Botany. 1990;41:1167–1174. [Google Scholar]
- FAO/IPGRI. Genebank standards. Rome: Food and Agriculture Organization of the United Nations and International Plant Genetic Resources Institute; 1994. [Google Scholar]
- Farrant JM, Pammenter NW, Berjak P. Seed development in relation to desiccation tolerance: a comparison between desiccation-sensitive (recalcitrant) seeds of Avicennia marina and desiccation-tolerant types. Seed Science Research. 1993;3:1–13. [Google Scholar]
- Fenner M, Thompson K. The ecology of seeds. Cambridge: Cambridge University Press; 2005. [Google Scholar]
- Gray D, Steckel JRA, Dearman J, Brocklehurst PA. Some effects of temperature during seed development on carrot (Daucus carota) seed growth and quality. Annals of Applied Biology. 1988;112:367–376. [Google Scholar]
- Harrington JF. Seed storage and longevity. In: Kozlowski TT, editor. Seed biology. Volume III. Insects, and seed collection, storage, testing, and certification. New York: Academic Press; 1972. pp. 145–245. [Google Scholar]
- Hay FR, Probert RJ. Seed maturity and the effects of different drying conditions on desiccation tolerance and seed longevity in foxglove (Digitalis purpurea L.) Annals of Botany. 1995;76:639–647. [Google Scholar]
- Hay FR, Probert RJ. Collecting and handling seeds in the field. In: Guarino L, Ramanatha Rao V, Goldberg E, editors. Collecting plant genetic diversity: technical guidelines – 2011 update. Rome: Bioversity International; 2011. [Google Scholar]
- Hay FR, Smith RD. Seed maturity: when to collect seeds from wild plants. In: Smith RD, Dickie JB, Linington SH, Pritchard HW, Probert RJ, editors. Seed conservation: turning science into practice. Kew, London: Royal Botanic Gardens, Kew; 2003. pp. 97–133. [Google Scholar]
- Hay FR, Probert RJ, Coomber SA. Development of desiccation tolerance and longevity in seeds from detached capsules of foxglove (Digitalis purpurea L.) Annals of Botany. 1997;79:419–427. [Google Scholar]
- Hay FR, Smith RD, Ellis RH, Butler LH. Developmental changes in the germinability, desiccation tolerance, hardseededness, and longevity of individual seeds of Trifolium ambiguum. Annals of Botany. 2010;105:1035–1052. doi: 10.1093/aob/mcq037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidayati SN, Baskin JM, Baskin CC. Morphophysiological dormancy in seeds of two North American and one Eurasian species of Sambucus (Caprifoliaceae) with underdeveloped spatulate embryos. American Journal of Botany. 2000;87:1669–1678. [PubMed] [Google Scholar]
- Hong TD, Ellis RH. A comparison of maturation drying, germination, and desiccation tolerance between developing seeds of Acer pseudoplantanus L. and Acer platanoides L. New Phytologist. 1990;116:589–596. [Google Scholar]
- ISTA. International rules for seed testing. Bassersdorf: The International Seed Testing Association; 2005. [Google Scholar]
- Karlsson LM, Tamado T, Milberg P. Seed dormancy pattern of the annuals Argemone ochroleuca and A. mexicana (Papaveraceae) Flora. 2003;198:329–339. [Google Scholar]
- Kermode AR, Bewley JD. The role of maturation drying in the transition from seed development to germination. I. Acquisition of desiccation-tolerance and germinability during development of Ricinus communis L. seeds. Journal of Experimental Botany. 1985;36:1906–1915. [Google Scholar]
- Kermode AR, Finch-Savage BE. Desiccation sensitivity in orthodox and recalcitrant seeds in relation to development. In: Black M, Pritchard HW, editors. Desiccation and survival in plants: drying without dying. Wallingford, UK: CAB International; 2002. pp. 149–184. [Google Scholar]
- Kermode AR, Bewley JD, Dasgupta J, Misra S. The transition from seed development to germination: a key role for desiccation? HortScience. 1986;21:1113–1118. [Google Scholar]
- Kondo T, Miura T, Okubo N, Shimada M, Baskin C, Baskin J. Ecophysiology of deep simple epicotyl morphophysiological dormancy in seeds of Gagea lutea (Liliaceae) Seed Science Research. 2004;14:371–378. [Google Scholar]
- Lang GA. Dormancy: a new universal terminology. HortScience. 1987;22:817–820. [Google Scholar]
- Lersten NR. Flowering plant embryology: with emphasis on economic species. Ames: Blackwell Publishing; 2004. [Google Scholar]
- Lima MDV, Jr, Ellis RH, Hong TD, Ferraz IDK. Drying method and subsequent desiccation tolerance and longevity of immature seeds of cedro (Cedrela odorata L. – Meliaceae) Seed Science and Technology. 1998;26:813–822. [Google Scholar]
- Martin AC. The comparative morphology of seeds. The American Midland Naturalist. 1946;36:513–660. [Google Scholar]
- Meerow AW, Fay MF, Guy CL, Li Q-B, Zaman FQ, Chase MW. Systematics of Amaryllidaceae based on cladistic analysis of plastid rbcL and trnL-F sequence data. American Journal of Botany. 1999;86:1325–1345. [PubMed] [Google Scholar]
- Mondoni A, Probert R, Rossi G, Hay F, Bonomi C. Habitat-correlated seed germination behaviour in populations of wood anemone (Anemone nemorosa L.) from northern Italy. Seed Science Research. 2008;18:213–222. [Google Scholar]
- Mondoni A, Probert R, Rossi G, Hay F. Habitat-related germination behaviour and emergence phenology in the woodland geophyte Anemone ranunculoides L. (Ranunculaceae) from northern Italy. Seed Science Research. 2009;19:137–144. [Google Scholar]
- Moore RP. Handbook on tetrazolium testing. Zurich: The International Seed Testing Association; 1985. [Google Scholar]
- Pieta Filho C, Ellis RH. The development of seed quality in spring barley environments. I. Germination and longevity. Seed Science Research. 1991;1:163–177. [Google Scholar]
- Priestley DA. Seed aging: implications for seed storage and persistence in the soil. Ithaca, NY: Cornell University Press; 1986. [Google Scholar]
- Probert RJ, Hay FR. Keeping seeds alive. In: Black M, Bewley JD, editors. Seed technology and its biological basis. Sheffield: Sheffield Academic Press; 2000. pp. 375–410. [Google Scholar]
- Probert RJ, Smith RD. The joint action of phytochrome and alternating temperatures in the control of seed germination in Dactylis glomerata. Physiologia Plantarum. 1986;67:299–304. [Google Scholar]
- Probert RJ, Adams J, Coneybeer J, Crawford A, Hay F. Seed quality for conservation is critically affected by pre-storage factors. Australian Journal of Botany. 2007;55:326–335. [Google Scholar]
- Roberts EH. Predicting the storage life of seeds. Seed Science and Technology. 1973;1:499–514. [Google Scholar]
- Salisbury EJ. The oak–hornbeam woods of Hertfordshire Parts I and II. Journal of Ecology. 1916;4:83–117. [Google Scholar]
- Samarah NH, Allataifeh N, Turk MA, Tawaha AM. Seed germination and dormancy of fresh and air-dried seeds of common vetch (Vicia sativa L.) harvested at different stages of maturity. Seed Science and Technology. 2004;32:11–19. [Google Scholar]
- Sanhewe AJ, Ellis RH. Seed development and maturation in Phaseolus vulgaris I. Ability to germinate and to tolerate desiccation. Journal of Experimental Botany. 1996;47:949–958. [Google Scholar]
- Sershen, Pammenter NW, Berjak P. Post-harvest behaviour and short- to medium-term storage of recalcitrant seeds and encapsulated embryonic axes of selected amaryllid species. Seed Science and Technology. 2008;36:133–147. [Google Scholar]
- Taylorson RB, Hendricks SB. Dormancy in seeds. Annual Review of Plant Physiology. 1977;28:331–354. [Google Scholar]
- van der Toorn P, Karssen CM. Analysis of embryo growth in mature fruits of celery (Apium graveolens) Physiologia Plantarum. 1992;84:593–599. [Google Scholar]
- Vandelook F, van Assche JA. Temperature requirements for seed germination and seedling development determine timing of seedling emergence of three monocotyledonous temperate forest spring geophytes. Annals of Botany. 2008;102:865–875. doi: 10.1093/aob/mcn165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welbaum GE, Bradford KJ. Water relations of seed development and germination in muskmelon (Cucumis melo L.) Journal of Experimental Botany. 1989;40:1355–1362. [Google Scholar]
- Whigham DF. Ecology of woodland herbs in temperate deciduous forests. Annual Review of Ecology, Evolution and Systematics. 2004;35:583–621. [Google Scholar]