Abstract
Background and Aims
Eversporting eudicots were sought to see if they behave like gymnosperms. Behaviour of eversporting gymnosperm chimeras indicates a single apical cell is present in SAM and it would be of interest to see if eudicot chimeras have the same behaviour.
Methods
Four eversporting spireas, the pineapple mint and the Silver King euonymus were inspected for the fate of the yellow (mutant)–green (wild type) chimeras.
Key Results
As with gymnosperms, unstable eudicot chimeras in the four spireas, the pineapple mint and the Silver King euonymus became stable yellow about 80 % or more of the time and 20 % or less became stable green.
Conclusions
The statistically significant preponderance of chimeric fates becoming all yellow suggests that a single apical cell resides in the yellow tunica. As with gymnosperms, descendent cells of the yellow replacement corpus cell eventually take over the corpus. Here is the first chimeric set of data to support the hypothesis of a one-celled meristem in eudicots rather than the traditional view of a muticellular meristem.
Keywords: Apical cell, chimeras, euonymus, shoot apical meristem, pineapple mint, Spiraea, spirea, Rosaceae, Mentha, Lamiaceae, Euonymus, Celastraceae
INTRODUCTION
The vegetative stage of the shoot apical meristem (SAM) has been investigated by various techniques, including classical staining for identifying histological zonation and noting rates of cell division (Lyndon, l973), producing periclinal cytochimeras for recognizing stable cell layers (Satina et al., 1940), evaluating sectorial chimeras for proliferation patterns (Derman, l945), autoradiography for noting which cells are dividing (Steeves et al., l969), surgical examination for leaf initiation (Snow and Snow, 1931), exposure to high-energy radiation for determining regeneration capacities (Langenaur and Davis, 1973), and molecular biology for localizing RNA transcripts (Kelly and Meeks-Wagner, 1995), among others. Arguably the most direct approach to understanding the organization of SAM is from analysis of merigonal (sectorial) chimeras in which the size of mutant sector is taken as the reciprocal of the number of apical initial cells. For example, a one-third yellow sector of a green shoot as seen in transverse view is interpreted as coming from three cells in the corpus at the apex of SAM, two green and one yellow mutant (Derman, 1945). The reason for claiming that this approach is the most direct is the necessity to identify cell types first according to proliferation pattern because SAM is essentially a growth region and a sector is a clone of proliferating cells. By this criterion of proliferation, periclinal (layer) chimeras are next in importance to merigonal chimeras in understanding the zonation constraints of proliferating cell types as they indicate the number of permanent layers in SAM (Tilney-Bassett, 1963).
The author has criticized this assumption that the number of apical initials is the reciprocal of the sector fraction on three counts (Korn, 2001a). First, the concept of the apical initial is vague as to cause and effect, namely, are apical initials the founts of all other cells in the apex because of their position alone, since one (some) cell(s) has (have) to be at the summit, or is a cell an apical initial because it is a differentiated cell that regulates regional growth and so assumes its apogean position by dictating its own growth rate along with those of other cells. Secondly, most sectorial chimeras are temporary and vary in length and circumferential fraction from node to node, in some cases as small as 1/24 of the girth (Korn, 2002). Thirdly, a better explanation of the fractional expression is that the eversporting event occurs in a flank cell leading to an unstable fraction of the stem. Hence, fractions are expressions of secondary rather than primary features of the apex (Korn, 2001a). These three criticisms about the proliferation patterns of SAM shift the parameter for determining the nature of apical organization from sector fractions to the ultimate fate of a chimeric branch.
Hejnowicz (1956) first explained the origin of eversporting chimeras in gymnosperms as coming from a periclinal division of an epidermal cell. If the tunica is genetically yellow (L1Y) and the corpus is green (L2G) then the lower daughter cell takes over the corpus which then becomes yellow. Derman (1960) called this periclinal division a replacement division if one daughter enters a lower layer and a displacement division if a daughter cell enters a layer above The replacement event in several juniper species, plants having only two layers with L1Y, L2G → L1Y, L2Y produces a temporary green–yellow sectorial chimeric shoot that eventually converts to either wild type green or mutant yellow type (Korn, 2001a, 2002.) The ratio of conversion is 90 % : 10 % in favour of the mutant type, indicating that a single mutant cell occurring in L2 from a replacement event proliferates into many cells that entirely take over the stem corpus. Hence, a single apical initial exists in L1 that occasionally divides periclinally, leading to one daughter cell in L1 and the other in L2. To realize this 90 % takeover by a mutant cell introduced into L2 requires a stability of the apical initial cell above it in L1, and this apical initial cell forms a lineage of apical cell initials that remain at the top by their control over growth rates and division pattern. Hence this apical initial is actually an apical cell because it satisfies the criteria of regulating growth in the region and expressing apical dominance, i.e. inhibiting other cells from becoming apical cells (Korn, 1993). A lineage of apical cells maintains this stability of tissue location over a long period of time, namely, over the formation of many nodes.
As the comparative frequencies of fates of chimeras in gymnosperms leads to the suggestion that SAM is regulated by an apical cell (Korn, 2001a), it would be of interest to inspect similar features in flowering plants. Generally, chimeras in angiosperms differ from those in gymnosperms in three important ways (Tilney-Basset, l986). First, each chimera in dicots usually comes from a rare mutational event (approx. 10−6/node) whereas in gymnosperms the eversporting pattern is from many cell replacement events per shoot, approx. 2 %/node, involving a persistent periclinal chimera. Secondly, chimeras in dicots are relatively stable and in gymnosperms they are unstable, usually reverting to the mutant type. Thirdly, an angiosperm meristem in most cases has three layers, L1, L2 and L3, whereas many gymnosperm meristems usually have only two layers, L1 and L2. Consequently chimeras in dicots are usually different from those in gymnosperms.
What can cell replacement in a typical three-layered angiosperm apex reveal about the composition of SAM? An L1Y–L2G–L3G leaf is all green because a yellow L1 is undetected as it only gives rise to the epidermis and if it were to experience a cell replacement event in L1 to form an L2 yellow cell, an L2 yellow margin would appear and the desired analysis would be possible; however, such cases are rare and difficult to evaluate (Derman, 1960; Tilney-Bassett, 1963). Replacement of an L2 cell into L3, as in many eudicots, suggests nothing about the behaviour of L1 where critical fount cells reside, so analysis here is of no value. Tilney-Bassett (l963) described the gymnosperm eversporting pattern in two eudicots, Spiraea japonica ‘Anthony Waterer’ and Salvia officinalis variegata. Pohlheim (l971) found the same types of chimeras in Mentha arvense ‘Variegata’ and again in Spiraea japonica ‘Anthony Waterer’ as well as histologically finding apices in these plants with only two layers with no chloroplasts in stomata (in L1), as is usually found in gymnosperms. It would then be of interest to determine the fates of chimeras in these angiosperms because they are similar to those in junipers where analysis leads to meaningful ideas about apical meristem organization. The search for eversporting Spiraea and Mentha cultivars led to finding several such cultivars on which the following study is based.
MATERIALS AND METHODS
Three bushes of Spiraea japonica ‘Normandii’ (Rosaceae) growing in the Bernheim Arboretum (accession numbers 4285, 4286, 4287; originally received from Carroll Gardens, MD in l980), Clarmont, KY, were examined by scoring the number of chimeric branches of various types. A few were permitted to be removed for photography and dissection. Mutant leaves are cream coloured with colourless plastids and here will be described as yellow. Several bushes of S. japonica ‘Gold Flame’ growing next to the horticultural building on the campus of Oregon State University, Corvallis, OR, were examined. Shrubs of S. japonica ‘Gold Flame’ adjacent to the computer science building on the campus of Marquette University, Milwaukee, WI, were also collected and analysed. Two large bushes of S. japonica ‘Anthony Waterer’ at 1212 Bardstown Road, Louisville, KY, were also evaluated. Two plants of the pineapple mint, Mentha suaveolens ‘Variegata’ Ehrh. (Lamiaceae ), were purchased from Monrovia Nursery, Azusa, CA and were grown outdoors for 4 years. Nine plants of Euonymus japonica ‘Silver King’ (Celastraceae) from Frank Otte Nursery, Louisville, KY were inspected for eversports.
Terms used here are the apical initial which is the proliferative fount of other cells in a tissue and the apical cell which is a special apical initial that regulates various growth rates in different zones in the meristem and also expresses apical dominance by inhibiting other cells from becoming apical cells (Korn, l993). Also, replacement is the insertion of a cell from one layer into a deeper layer, i.e. L1 → L2 and L2 → L3, whereas a displacement is the insertion of a cell from one layer to a more outside layer, i.e. L3 → L2 and L2 → L1 (Derman, l960). Cell layers and their phenotypes are abbreviated as, for example, a green L1 tunica, yellow L2 tunica and a green L3 corpus combination is noted as L1G–L2Y–L3G.
Some confusion exists as to the identification of Spiraea plants. Some large plants of Spiraea japonica ‘Goldflame’ have eversporting chimeras such as those bushes at Oregon State University, whereas many other shrubs of this cultivar found elsewhere have no chimeric branches, including 17 large bushes at Bernheim Arboretum. The taxon S. japonica ‘Normandii’ studied here has not been described in the literature. Hence, for practical reasons it is important to include their location and, if possible, their origin rather than by their name alone.
RESULTS
The three shrubs of S. japonica ‘Normandii’ were estimated to have about 4920 leafy branches of all types. Most branches were all green and another type of branch has only yellow leaves with 46 shoots of this type found; they were discounted here as coming from chimeric events from past years' activity. Another type of chimeric branch begins with all green leaves but terminates with all yellow leaves (Fig. 1A). The sectorial leaves have large green–yellow symmetric regions not like the typical eudicot sectors that have either marginal or central mutant areas. Twenty such branches of this type, G → chimera → Y, were found (Table 1). Another type of branch begins like the previous type as all green, becomes chimeric and terminates with all green leaves (Fig. 1B); only nine cases of this kind of branch, G → chimera → G, were found. The final type of branch has sectorial leaves extending to the stem tip or to the point where flowers formed, so the fate of these chimeras remains undetermined; they were included in this study only for determining the frequency of eversporting. Ninety-four branches of this type were observed for a total of 94 + 20 + 9, or 123 chimeras. Additional data are 9·2 ± 1·7 leaves per branch (n = 25) and 3·5 ± 0·7 leaves per sectored region (the chimeric state in G → chimera → G) chimera (n = 9) with a maximum of five leaves.
Fig. 1.

Spirea chimera types: (A) branch of Spiraea japonica ‘Normandii’ that passes through a sectorial region ending as a stable yellow type (×1·1); (B) branch of Spiraea japonica ‘Normandii’ chimeric sector shifting to stable green (×1·1); (C) spray of S. japonica ‘Anthony Waterer’ passing from chimera type to stable chlorotic type (×1·1); (D) branch of S. japonica ‘Anthony Waterer’ with chimera reverting back to stable green (×1·1). Scale bar = 1 cm.
Table 1.
Frequencies of chimeric shoot shifts for the cultivars studied
| Stem fate |
|||
|---|---|---|---|
| Cultivar | Wild type → wild type | Wild type → mutant | Probability of fitting a 2 : 1 ratio |
| S. japonica ‘Normandii’ | 9 | 20 | <0·001 |
| S. japonica ‘Gold Flame’* | 1 | 49 | <0·001 |
| S. japonica ‘Gold Flame’† | 19 | 57 | <0·05 |
| S. japonica ‘Anthony Waterer† | 6 | 80 | <0·001 |
| Mentha suaveolens ‘Variegata’ | 1 | 12 | <0·0001 |
| Euonymus japonica ‘Silver King’ | 1 | 14 | <0·00001 |
| Σ | 37 (0·137) | 232 (0·862) | |
* From Corvallis; † from Milwaukee; ‡ from Louisville.
The total number of chimeric branches is 20 + 9 + 94, or 123 of an estimated 4920 – 46, or 4874, branches for a frequency of about 0·025/branch. The number of chimeric branches terminating as yellow is 20, whereas the number ending green is nine for a ratio of green to yellow of 1 : 2·22. According to the fraction hypothesis of Derman (1945) with three cells at the apex, two wild-type and one mutant, the expected ratio from a shift is 2 : 1 (66 : 33), so by the χ2 method with one degree of freedom the χ2 value is 16·59 and the P-value is <0·0001. Clearly, a sectorial branch is most likely to end yellow-leaved (Table 1).
The Corvallis S. japonica ‘Gold Flame’ spirea which normally has green leaves had a ratio of 1 : 49 of green shoots remaining green (wild type) in contrast to green shoots becoming yellow; Table 1), and the data for the Milwaukee spirea chimeras were 19 green shoots remaining green to 57 green shoots becoming yellow green (Table 1). Material of S. japonica ‘Anthony Waterer’ collected from Louisville was different from the other material as far as the mutant expression is concerned. Wild-type green leaf areas were the same as for those in other cultivars but the mutant leaf area was chlorotic with yellow intercostal areas and slightly green vein regions (Fig. 1C). The number of chimeras was 86 chimeras in an estimated 2842 branches, or the frequency of chimeras per branch is 1·6 % (Table 1) not that different from that of the estimated 2·5 % for S. japonica ‘Normandii’. Of the 86 chimeras, six were revertants to green (Fig. 1D) and 80 switched to yellow Fig. 1C). Although the chlorotic area is typical of viral infection, this feature appears to be a true genetic trait as in the other cultivars, in that it occurs at about the same frequency (approx. 1·6 % compared with 2·5 %), the frequency of reversion back to green is about the same the same (6/86 or approx. 7 %) and the initial sectored length is about the same (approx. 3 plastochrons).
The pineapple mint, Mentha suaveolens ‘Variegata’, is a periclinal chimera of L1W tunica–L2G corpus with the L1W showing as some white serrations along the leaf margin (Fig. 2A). Of 187 branches with ten or more leaf pairs, three were all green and two all white, both presumably coming from previous years' changes and 182 were mosaic with green leaves having an occasional white serration. Of these 182 mosaic branches, 95 ended with flowers, 74 remained mosaic (Fig. 2A), 12 were terminally all white (Fig. 2B) and one ended as all green (Fig. 2C) (Table 1). Discounting the floral branches, the frequencies of the L1W–L2G → L1G–L2G transition was 1/87 (74 + 12 + 1) or 0·011 and the L1WL2G → L1WL2W shift was 12/87, or 0·137, or 12 times greater. Supporting the assumption that all leaves on a branch with no occasional white serrations is L1G–L2G comes from finding stomata with chloroplasts (Fig. 2D), while that for leaves with occasional white serration are L1W–L2G is confirmed by finding stomata with no chloroplasts (Fig. 2E).
Fig. 2.
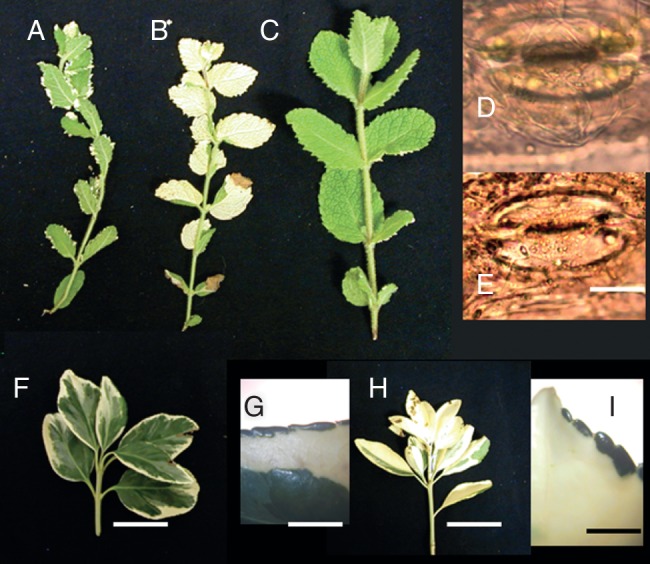
Mentha suaveolens ‘Variegata’: (A) periclinal chimera with white serrations on green leaves that would be L1W–L2G; (B) white shoot that would be L1W–L2W, in which four chimeric leaves formed before all white took over shoot; (C) all green shoot that would be L1G–L2G; (D) stoma with chloroplasts from wild-type plant, L1G–L2G; (E) stoma with no chloroplasts in L1W–L2G leaf. (F) Euonymus green-white chimera. (G). Close-up in a somewhat rare chimeric leaf with green margin. (H) Shift to all white-leafed branch. (I) Somewhat rare leaf with green margin indicating three layers are present with composition GWW. Scale bars: (A–C) = 1 cm; (D) = 50 µm; (E) = 40 µm, (F, H) = 3 cm; (G, I) = 300 µm.
Nine plants of Euonymus japonica ‘Silver King’ had a total of 711 branches with five or more mature leaves. Leaves are centrally green and submarginally white (Fig. 2F) with an occasional green margin (Fig. 2G), or are L1G–L2W–L3G. Fourteen branches became all white (Fig. 2H) with an occasional green margin (Fig. 2I) and are then L1G–L2W–L3W most likely by a replacement division in L2 to insert a white daughter cell into the L3 layer that then takes over the corpus. This event has a frequency of 14/711, or 0·020, similar to that of 0·025 for S. japonica ‘Normandii’ (Table 1). One branch was found with at least three chimeric leaves that switched to all green leaves.
The ratio of all chimeras in the six cultivars examined changing to all green or to all white branches is 0·135 : 0·865 (Table 1, bottom line) as a meta-analysis that clearly favours all white. As these six cultivars of eversports are the only ones examined, the meta-analysis is not biased in any direction.
DISCUSSION
The patterns of leaf sectoring in the spireas, a mint and a euonymus examined here parallel those found in six gymnosperms (Korn, 2001a), suggesting that these eudicots examined are periclinal chimeras as noted by Derman (l960) and Pohlheim (l971). Their similarity to the gymnosperms reported is 3-fold: (1) they are eversporting, (2) the yellow mutant-green wild-type chimera is asymmetrically located over a leaf and (3) most chimeras become stable mutant in type. Tilney-Bassett (l963) was the first to note that the sectorial patterns in S. japonica ‘Anthony Waterer’ and to some extent in Salvia offinalis variegatis differ from the typical marginal eudicot pattern and were similar to those of the eversporting patterns in gymnosperms. Pohlheim (l971) also found in Spiraea japonica ‘Anthony Waterer’ as well as in Mentha arvense ‘Variegata’ many branches that were interpreted as L1Y–L2G and, by microscopic observation, SAM histologically appeared to be two-layered with no chloroplasts in L1. The chimeric branches in five of the six cultivars studied here also seem to come from two-layered meristems because green–yellow sectors often cover much of the leaf, not just the margin. The euonymus seems to have three layers because chimeric regions were marginal, sub-marginal and central. Dulieu (1968) induced chlorophyll mutants with ethyl methyl sulfonate in species of Nicotiana, Cosmos, Vicia, Lupinus and Linum and described sectors that covered much of the leaf and were probably L3W, although he did not note whether these sectors extended exactly to the margin or not. Poethig (l984) found in tobacco that induced mutants expressed in individual leaves are either isodiametric spots of various sizes or elongated sectors along the margin, features that can be explained by the former having mutations occurring in L3 cells and the latter from mutations in L2 cells.
Cell replacement (Derman, 1960) appears to be the event by which an eversport chimera arises when an L1Y cell divides periclinally to insert a yellow daughter cell into the L2G tissue. Clearly, chimera formation in S. japonica ‘Normandii’ is eversporting as it occurred in 123 instances, or at a frequency of about 2·5 %,/branch in the three shrubs examined over 1 year and in S. japonica ‘Anthony Waterer’ where it occurred 46 times in 2842 branches for a frequency of 0·16/branch. In mint the frequency is considerably higher, 12/87, or about 14 %/branch. The spirea values are similar to rates in junipers (approx. 2 %) where branches are often longer and leaves are perennial (Korn 2001a) and it is many orders of magnitude greater than expected for mutation.
The chimera (L1G–L2W–L3G) of E. japonica ‘Silver King’ has been described in many unrelated cultivars (Tilney-Basset, 1986) and explained histogenically by Derman, (1950) but finding trichimeric eversports sports (L1G–L2W–L3G → L1G–L2W–L3W) is new.
The yellow takeover of the corpus by an L2Y cell is significant. Derman (1945) suggested there are several, perhaps two to four cells (x = 2, 3, 4), at the apex and if one becomes mutant it will give rise to a clone of mutant cells observed as a 1/x sector when there are x apical initial cells. He explained a totally mutant apex occurs when there was a shift in the centre of the apex to the centre of the L2 mutant cell. This interpretation is difficult to accept on two counts. First, if the apex is composed of one mutant and two wild-type cells, a shift would most likely be over a wild-type cell two-thirds of the time and over the mutant cell one-third of the time but the actual ratio is not 2/3 wild type to 1/3 mutant but just the opposite of 1/7 wild type to 6/7 mutant (35 : 206; Table 1). The ratio of green to white for the pineapple mint is 1 : 12 (Table 1). With small numbers a binomial test can be made and, expecting a 2 : 1 ratio for green to white for 13 trials, the chance of this ratio is 13 × (0·333)12 × (0·666)1 or about 1 in 62 000. In euonymus with an obtained ratio of 14 white to one green the expected 1 : 2 ratio is 15 × (0·333)14 × (0·666)1, or about one in 485 500 trials.
The second problem with the fractional interpretation is that the sectored green–yellow stem segment before becoming all green or all yellow is somewhat stable and should be fairly long, much greater than the average of 3·5 plastochrons found here. Ruth et al. (1985) explained how the majority of stems become mutant by proposing diplontic selection where wild-type cells are at a disadvantage in proliferation. This idea was discounted for two reasons: (1) it would seem that the wild type is then more likely the trait that is at an advantage in competition; and (2) there is insufficient time in cell generations in the shoot apex, about five, for such effective elimination to occur by competitive selection.
How is the asymmetrical chimera in the G → chimera → Y sequence formed? This chimera begins as a replacement division of the L1 apical cell to form a mutant daughter cell in L2 which, in turn, proliferates to take over the entire L2 corpus (Fig. 3G–K). Constructing a computer model of this cell proliferation (Korn, 2001b) allows one to follow this L2 daughter cell as it proliferates into a clone of cells with a shape that is asymmetric with respect to the location of the cell beneath the apical cell (Fig. 3L–Q).
Fig. 3.
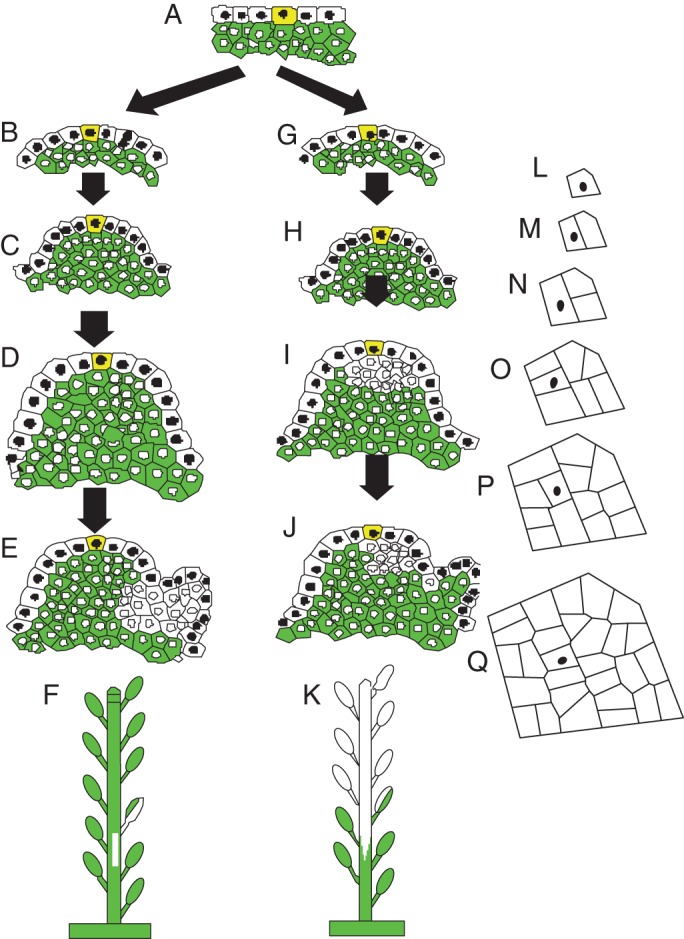
Diagrams of origin of different types of chimeric branches with yellow-coloured apical cells (L1 cells have black nuclei and L2 cells are green with white nuclei): (A–F) cell replacement in an L1 flank cell that produces an L2 white clone that is swept downward with the stem reverting back to a stable green status; (G–L) replacement division in the L1 apical cell with daughter mutant L2 cell proliferating into a stable yellow stem; (L–Q) computer model of cell proliferation (Korn, 2001b) where the L2 cell (black dot) beneath apical cell proliferates into an asymmetrical clone with respect to location of L2 cells beneath an L1 apical cell lineage. Early cell plates are thick to give the appearance of independent cell clones.
By the single apical cell hypothesis the sectored chimera (G → chimera–G) would come from a replacement division in a cell on the flank of the apex (Fig. 3B–F). The closest division of these divisions to the apical cell would give the longest chimera, that of five leaves. That this division extends over about five leaves allows a conversion from plastochron number to replacement cell number. Given the presence of an apical cell that infrequently divides periclinally, the frequency of such a division can be determined. In S. japonica ‘Normandii’ there were 4874 branches scored with 9·2 leaves per branch for a total of 44 841 leaves. The longest sectored chimera (G → chimera → G) comes from a single cell division, or five leaves is equivalent to one apical cell division, so there were 44 841/5, or 8968 apical cell divisions. With 20 + 9 + 94 chimeras, the frequency of an apical cell displacement division is 120/8968 or 0·013 of L1 apical cell divisions.
The alternate hypothesis to the multicellular apical meristem is that of an apical cell as proposed here. Cell replacement is then the apical cell dividing periclinally and, if mutant, it will give rise to an L2 mutant daughter cell. This mutant cell will divide into a clone, a secondary clone, that will eventually take over the corpus as the daughter L1 apical cell above will form a primary clone as the entire L1 tunica layer. A reverted apex back to green would then be a replacement division of a cell along the flank of the apex, which gives rise to an unstable mutant L2 secondary clone, as it is replaced by some daughters from the proliferation of more apical L2 green cells.
Both Tilney-Bassett (l963) and Pohlheim (l971) noted that most periclinal chimeric L1Y–L1G branches in S. japonica ‘Anthony Waterer’ became stable L1Y–L2Y yellow shoots, and they also understood how is comes about by cell replacement as noted earlier by Hejnowicz (1956). Even without statistical data, they could have tentatively invoked the presence of a true apical cell in the L1 tissue by their finding that most sectorial shoots became mutant, but they were more interested in documenting cases of the eversporting phenomenon in angiosperms than in the regulatory aspects of tissue behaviour. The first reports of singular apical cells were by Hofmeister (l852) in Zostera and later for Acer and Fraxinus, also by Hofmeister (1857), and the idea of an apical cell similar to what is found in cryptograms was supported by others, including Nageli (1878). Eventually this idea fell into disrepute as reviewed by Schüepp (1926), mainly because it could not be seen in most plants. Sporadic reports of indirect evidence for apical cells include that of Naylor and Johnson (1937), in which observations on sectioned and stained petiolar material of the African violet, Saintpaulia ionantha, suggest that a new regenerative shoot arises from a single cell. Also, Mohr (l961) found many completely mutant plants arising from irradiated coffee seeds, a feature he concluded came from a single cell in the embryonic SAM. The author (Korn, l993) found one-hit kinetics when re-examining the published data of Langenaur and Davis (1973) on sunflower shoot inactivation by X-radiation; the one hit indicates only one target needs to be destroyed for cessation of apical growth, namely one cell. Since the unit of meristem construction is the cell, the target is a singularly unique cell in the meristem. More recently, the author (Korn, 2001a) found chimeras in six gymnosperms from cell replacement almost always led to completely mutant shoots, similar to the pattern found here in four Spiraea cultivars, and concluded a single apical cell resides at the apex in the L1 layer. While the evidence for a true apical cell is supported here for six cultivars, this is a fundamental developmental feature that is unlikely to vary within major groups of plants and so it is proposed that a true apical cell exists in stems of all seed plants.
The traditional multicellular view of SAM is based on very little evidence, most of which is negative or anecdotal. As noted earlier, SAM in seed plants was first assigned a single apical cell (Hofmeister, 1852, 1857; Nageli, 1878) but later given a multicellular status because of the absence of any typical apical cell morphology. This argument for a multicellular apex because no large triangular (in 2D) or tetrahedral (in 3D) central cell is present is invalid, because no such cells are seen at the tip in some young moss leaves and later stages of fern gametophytes (Korn, l993). Later, Ball (l960) observed living SAMs from the top view and found summit cells tend to be displaced off to the flanks, supporting the idea there are no permanent cells at the very apex. However, it is known that SAM tilts in position during a plastochron cycle, thereby leading to a shift in location of the geometric, not developmental, summit. Also, the behaviour of a single apex, over a period of 1 week after leaves were experimentally pushed around, opens the door to the criticism of atypical conditions present, such that his argument appears as more anecdotal than conclusive. With the many investigations on the organization of SAM it is more than curious that no scheme, much less a model, has been proposed to illustrate how a group of functionally interrelated cells is needed to form a dynamically stable dome-shaped apex during growth. The evidence for a multicellular meristem is hardly convincing and perhaps most developmentalists still hold to this idea while waiting for the appropriate evidence to appear, meanwhile the evidence for a single apical cell based on significant statistical data continues to mount. The presence of cell tiers as unequivocally demonstrated by Satina et al. (l940) implies at least one apical initial, not an apical cell, at each level at any one time so there is a multitude of fount cells present, one or more at each level. This argument can be dismissed as misleading to the conclusion of a multicellular apex (Korn, l993). A growing apex has a longitudinal axis running down centrally from the summit through the meristem and is physically real in that growing cells recede from this axis at all levels of the apex. Consequently, there is at least one cell at every level, or layer, through which the axis passes. These central cells are only special by their position and behave as fount cells circumstantially. That the axis would consistently pass exactly between two or three cells in each layer is unlikely; hence the explanation of Derman (l960) for sectorial fractions as the reciprocal of the number of fount cells is equally unrealistic. The argument favouring multicellularity in the stem apex is based on the fact that many cells in the dome are dividing. But many cells in the moss leaf and fern stem are dividing but only one in each case is regulatory and a true apical cell.
The initial appearance of induced mutations, expressed in dicots as leaf chimeras, are usually unstable and asymmetrically sectored (Dulieu, l968). By contrast, chimeras in nurseries are the stable sandwiched 1LG–2LY–3LG type that appears as green leaves with yellow margins (Tilney-Bassett, l986; Geneve, 1991). The resolution to this difference is that new sector mutant chimeras do not pass through the seed and cannot be vegetatively propagated [tobacco, Linum, common bean, Coleus (Dulieu, l968)] so are lost, whereas sandwich chimeras are vegetatively propagated [privet, apple, peach (Derman,1960)] and survive. Consequently one sees mostly sandwich (periclinal) chimeras and concludes that the apices have three layers and no single apical cell. However, a few exceptional eudicots (Spiraea and Mentha; Pohlheim, 1971) with two-layered meristems do reveal an apical cell in L1 and are not exceptional with respect to the presence of a meristem with an apical cell but as to the number of layers in the apex that allows the detection of this special cell.
The presence of an apical cell in one of two tissue layers has an interesting evolutionary interpretation. That it is the apical cell in L1 that (a) divides periclinally and (b) repeatedly at about a 1·3 % frequency per apical cell division suggests a developmental step instead of an error. A two-layered SAM, having an L1W and an L2G, can have two different fates, the L1 to form an epidermis with some features that contributes to the plant survival, while the other L2 layer forms reproductive cells among other specialized cells, i.e. each layer contributes differently to development. The true measure of the evolutionary survival value of some feature is when it is in both layers, otherwise the status for any success or failure is equivocal. With an occasional replacement division of an apical cell, the stem becomes genotypically pure and evolutionary testing is thorough. That 1 % of apical divisions are the correcting replacement event is a compromise between the gain in genotype uniformity of several layers and the cost of reorganization by the temporary upsetting of the two-layered organization.
LITERATURE CITED
- Ball E. Sterile culture of the shoot apex of Lupinus albus. Growth. 1960;24:91–110. [PubMed] [Google Scholar]
- Derman H. The mechanism of colchicine-induced cytohistological changes in cranberry. American Journal of Botany. 1945;32:387–394. [Google Scholar]
- Derman H. Pattern reversal in variegated plants. Journal of Heredity. 1950;41:325–328. [PubMed] [Google Scholar]
- Derman H. The nature of plant sports. Amererican Horticultural Magazine. 1960;39:123–173. [Google Scholar]
- Dulieu H. Emploi des chimères chlorophylliennes piur l'etude de l'ontogéne foliare. Bulletin scientifique de Bourgogne. 1968;25:1–25. [Google Scholar]
- Geneve R. Attainable chimeras. American Horticulturist. 1991;70:33–36. [Google Scholar]
- Hejnowicz Z. Eversporting periclinal chimeras. Recent Advances in Plant Science. 1956;2:1446–1448. [Google Scholar]
- Hofmeister W. Zur Entwickelungsgeschichte des Zostera. Botanische Zeitung. 1852;10:121–131. 137–149, 157–158. [Google Scholar]
- Hofmeister W. Beiträge zur Kenntniss der Gefässkryptogamen II. III. Über Entwickelung und Bau der Vegetationsorgane der Farrnkraeuter. K. Sächs. Gesell. Der Wiss. Abhandl. Math.-Phys. Cl. 1857;3:606–682. [Google Scholar]
- Kelly AJ, Meeks-Wagner DR. Characterization of a gene transcribed in the L2 and L3 of the tobacco shoot apical meristem. The Plant Journal. 1995;8:147–153. doi: 10.1046/j.1365-313x.1995.08010147.x. [DOI] [PubMed] [Google Scholar]
- Korn R. Apical cells as meristems. Acta Biotheoretica. 1993;41:175–189. [Google Scholar]
- Korn R. Analysis of shoot apical organization in six species of the Cupressaceae based upon chimeric behavior. American Journal of Botany. 2001a;88:1945–1952. [PubMed] [Google Scholar]
- Korn R. The geometry of proliferating dicot cell. Cell Proliferation. 2001b;34:43–54. doi: 10.1046/j.1365-2184.2001.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn R. Chimeric patterns in Juniperis chinensis ‘Torulosa variegata’ expressed during leaf and stem formation. American Journal of Botany. 2002;89:758–765. doi: 10.3732/ajb.89.5.758. [DOI] [PubMed] [Google Scholar]
- Langenaur HW, Davis EL. Helianthus annuus response to acute X-radiation. I. Damage and recovery in the vegetative apex and effects on development. Botanical Gazette. 1973;134:301–316. [Google Scholar]
- Lyndon RF. The cell cycle in the shoot apex. In: Balls M, FS Billett, editors. The cell cycle in development and differentiation. London: Academic Press; 1973. pp. 285–314. [Google Scholar]
- Mohr CC. Does a coffee plant develop from one initial cell in the shoot apex of an embryo? Radiation Botany. 1961;1:97–99. [Google Scholar]
- Nägeli CK. Über das Scheitel wachsthum der Phanerogamen. Botanische Zeitung. 1878;36:124–126. [Google Scholar]
- Naylor EE, Johnson B. A histological study of vegetative reproduction in Saintpaulia ionantha. American Journal of Botany. 1937;24:673–678. [Google Scholar]
- Poethig S. Cellular parameters of leaf morphogenesis. In: White RA, Dickison WA, editors. Contemporary problems in plant anatomy. New York, NY: Academic Press; 1984. pp. 235–259. [Google Scholar]
- Pohlheim F. Spiraea bunalda ‘Anthony Waterer’ und Mentha arvensis ‘Variegata’ – zwei immerspaltende Periklinalchimären unter den Angiospermen. Biologisches Zentralblatt. 1971;90:295–319. [Google Scholar]
- Ruth J, Klekowski J, Stein O. Impermanent initials of the shoot apex and diplontic selection in a juniper chimera. American Journal of Botany. 1985;72:1127–1135. [Google Scholar]
- Satina S, Blakeslee AF, Avery AG. Demonstration of the three germ layers in the shoot apex of Datura by means of induced polyploidy in periclinal chimeras. American Journal of Botany. 1940;27:895–905. [Google Scholar]
- Schüepp O. Meristeme. In: Linsbauer K, editor. Handbuch der Pflanzenanatomie. 1926. Band 4, Leif 16. [Google Scholar]
- Snow M, Snow R. Experiments on phyllotaxis. I. The effect of isolating a primordium. Philosophical Transactions of the Royal Society of. London Series B. 1931;221:1–43. [Google Scholar]
- Steeves TAM, Hicks A, Naylor JM, Rennie P. Analytical studies on the shoot apex of Helianthus annuus. Canadian Journal of Botany. 1969;47:1367–1375. [Google Scholar]
- Tilney-Bassett RAE. The structure of periclinal chimeras. Heredity. 1963;18:265–285. [Google Scholar]
- Tilney-Bassett RAE. Plant chimeras. London: Edward Arnold; 1986. [Google Scholar]


