Abstract
Background and Aims
The adaptive plastic reactions of plant populations to changing climatic factors, such as winter temperatures and photoperiod, have changed during range shifts after the last glaciation. Timing of flowering is an adaptive trait regulated by environmental cues. Its genetics has been intensively studied in annual plants, but in perennials it is currently not well characterized. This study examined the genetic basis of differentiation in flowering time, morphology, and their plastic responses to vernalization in two locally adapted populations of the perennial Arabidopsis lyrata: (1) to determine whether the two populations differ in their vernalization responses for flowering phenology and morphology; and (2) to determine the genomic areas governing differentiation and vernalization responses.
Methods
Two A. lyrata populations, from central Europe and Scandinavia, were grown in growth-chamber conditions with and without cold treatment. A QTL analysis was performed to find genomic regions that interact with vernalization.
Key Results
The population from central Europe flowered more rapidly and invested more in inflorescence growth than the population from alpine Scandinavia, especially after vernalization. The alpine population had consistently a low number of inflorescences and few flowers, suggesting strong constraints due to a short growing season, but instead had longer leaves and higher leaf rosettes. QTL mapping in the F2 population revealed genomic regions governing differentiation in flowering time and morphology and, in some cases, the allelic effects from the two populations on a trait were influenced by vernalization (QTL × vernalization interactions).
Conclusions
The results indicate that many potentially adaptive genetic changes have occurred during colonization; the two populations have diverged in their plastic responses to vernalization in traits closely connected to fitness through changes in many genomic areas.
Keywords: Arabidopsis lyrata; Brassicaceae; growth chamber; local adaptation; phenotypic plasticity; QTL; vernalization, flowering, morphology
INTRODUCTION
Range shifts and colonization of new areas have occurred frequently in the history of most plant species. These have been accompanied by natural selection for adaptive differentiation of the populations in the different parts of the range. However, the underlying genetics of the trait differences are still poorly understood, and will be examined here. Plant populations have colonized the northern areas of Europe since the last glaciation (Comes and Kadereit, 1998). The source of colonization for many species has been central Europe (Willis and van Andel, 2004), where most refugia were located during the last glaciation. Range shifts have involved adaptation of populations to different climatic conditions, e.g. colder winter temperatures and longer photoperiods. The new parts of the range may have new optimal phenotypes for many traits. Because plants tend to use environmental signals such as photoperiod or winter temperatures as cues for timing of developmental transitions, plant populations have evolved genetic changes that result in plastic responses that are adaptive in new environments (Nicotra et al., 2010). In plants, selection has influenced, for example, flowering time and morphological traits such as leaf size or water-use efficiency, which may be important for adaptation to altered resource availability in new temperature or moisture regimes (Geber and Griffen, 2003). However, evolution of quantitative traits may be constrained by lack of genetic variation (Pujol and Pannell, 2008), by canalization or pleiotropic effects (Kalisz and Kramer, 2008).
A requirement for a long cold period prevents many plants from flowering before spring (Michaels and Amasino, 2000). Vernalization shortens the time to flowering in winter annual accessions of Arabidopsis thaliana in growth-chamber conditions and enables overwintered rosettes to flower after winter in the wild (e.g. Wilczek et al., 2009). In contrast, summer annual accessions flower early without vernalization in the growth chamber and complete their life cycle during one growing season in natural field conditions. The genetic basis of the vernalization requirement for flowering has been studied thoroughly in A. thaliana (see Kim et al., 2009). The effect of vernalization in A. thaliana is mainly mediated by the FLOWERING LOCUS C (FLC) gene, a repressor of flowering that is epigenetically silenced during the cold period (Shindo et al., 2005). Variants of major genes such as FLC and FRI (FRIGIDA) account for natural variation in vernalization requirement in A. thaliana, but other genes with minor effects have also been found (Atwell et al., 2010; Brachi et al., 2010; Strange et al., 2011). In other plant groups, non-orthologous genes have convergently evolved as vernalization genes (see Hemming and Trevaskis, 2011). Some of the genes known to be involved in flowering and vernalization response have also been shown to affect development of leaves (Willmann and Poethig, 2011).
In annual plants such as A. thaliana, all resources are used for reproduction after the flowering decision is made, but in polycarpic perennials some meristems are retained for future growth (Amasino, 2009a). In the perennial Arabis alpina, an orthologue of FLC has an analogous function to that in A. thaliana, enabling flowering transition in some (cold exposed) meristems and thus seasonal flowering (Wang et al., 2009). An indel polymorphism in FRI has been found to be associated with population differentiation in vernalization response also in the perennial Arabidopsis lyrata (Kuittinen et al., 2008). However, details of the molecular basis for vernalization requirement and natural variation in perennials are still lacking.
Arabidopsis lyrata is a close perennial self-incompatible relative of A. thaliana (O'Kane and Al-Shehbaz, 1997), which has recently become a model for ecological population genetics and genome evolution (Mitchell-Olds, 2001; Clauss and Koch, 2006; Hu et al., 2011; Savolainen and Kuittinen, 2011). Its current distribution consists of scattered populations in low-competition habitats with large differences in growing season length in Europe, North America and north-eastern Russia. The level of neutral genetic diversity in Scandinavia is approximately half of the diversity in the central European populations. Both Germany and the Austrian Voralps have been suggested as refugial areas (Muller et al., 2008; Ross-Ibarra et al., 2008; Schmickl and Koch, 2011). It is assumed that the Scandinavian populations have lost much of the diversity due to reduction in effective population size during postglacial colonization (even if exact routes are not known).
The European A. lyrata populations have diverged in their flowering responses to photoperiod and vernalization (Riihimäki and Savolainen, 2004; Riihimäki et al., 2005; Kuittinen et al., 2008). More specifically, flowering was promoted, especially in the Scandinavian populations, by long photoperiods and vernalization (Riihimäki and Savolainen, 2004; Riihimäki et al., 2005; Kuittinen et al., 2008). Further, Sandring et al. (2007) observed selection for early flowering in a natural population of A. lyrata from Norway, providing direct evidence for the role of flowering phenology in adaptation. Reciprocal transplant experiments have shown that Norwegian and German populations are adapted to their local sites (Leinonen et al., 2009). However, the genetic basis of flowering, leaf morphology traits and their responses to vernalization is still unknown in A. lyrata.
The aims of the present study are to examine between-population differentiation in flowering time and morphological traits and in their plastic reactions to vernalization, and to associate these differing responses with particular regions of the A. lyrata genome via a QTL analysis. The study is conducted in two A. lyrata populations: a southern population from Plech (Germany) and a northern from Spiterstulen (Norway), in a growth-chamber experiment. The German population originates from the area of the presumed Pleistocene refugia of the north European populations (Clauss and Mitchell-Olds, 2006; Ansell et al., 2010; Schmickl et al., 2010); the Norwegian population from a formerly ice-covered region. Based on earlier results by Kuittinen et al. (2008), vernalization should accelerate flowering, especially in the Norwegian population. Short day length in turn should delay flowering in the Norwegian population (Riihimäki et al., 2005). Earlier field studies have shown that the Norwegian population has fewer inflorescence shoots and larger vegetative size than the German population (Leinonen et al., 2009). Here, we study the plasticity of these traits in the two populations using a vernalization treatment and a control treatment. Further, we search for the genomic areas [quantitative trait loci (QTL)] underlying differences in flowering traits and leaf morphology as well as those involved in different vernalization responses of the populations (QTL × vernalization interaction).
Specifically, we aimed at answering the following questions. (1) Do the parental populations differ in their vernalization responses for flowering phenology and morphology? We examined flowering phenology at different stages of inflorescence development and morphological traits. (2) What are the genomic areas governing differentiation in these traits (i.e. are there additive QTL?) and governing differentiation in the vernalization response (i.e. are there QTL × vernalization interactions?)? Then we explored putative candidate genes in the QTL regions identified.
MATERIALS AND METHODS
Plant material
We studied two populations of Arabidopsis lyrata: one from an alpine habitat at a northern latitude in Norway, Spiterstulen (61°38′N 8°24′E, 1106 m a.s.l.); the other from a continental southern latitude, lowland site at Plech, Germany (49°39′N 11°29′E approx. 400 m a.s.l.), where plants grow on large boulders and rocky outcrops (Clauss and Mitchell-Olds, 2006; Balañá-Alcaide et al., 2006). The environments at Plech and Spiterstulen differ fundamentally with regard to climatic aspects such as winter and summer temperatures (Table 1).
Table 1.
Locations (latitude, longitude and altitude) of Arabidopsis lyrata populations and climate overview
| Norway |
Germany |
|||
|---|---|---|---|---|
| Climatic variable | Spiterstulen | Juvvashoie | Plech | Nürnberg |
| Latitude | 61°38′N | 61°38′N | 49°39′N | 49°30′N |
| Longitude | 8°24′E | 8°24′E | 11°29′E | 11°03′E |
| Altitude (m. a.s.l.) | 1106 | 1894 | 400 | 314 |
| Day length in July (h) | 20 | 20 | 16 | 16 |
| Mean annual precipitation (mm) | 478* | _ | _ | 693 |
| Mean annual temperature (°C) | _ | –6·09 | _ | 4·08 |
| Mean January temperature (°C) | _ | –9·98 | _ | -0·8 |
| Mean June temperature (°C) | _ | 2·16 | _ | 16·1 |
| Length of the thermal growing season (days)† | _ | 49 | _ | 166 |
Climatic data were recorded at Juvvashoie near Spiterstulen (Norwegian Meteorological Institute) for 2002–2010 and at Nürnberg near Plech (Germany's National Meteorological Service) for 1998–2007.
* Recorded at Fokstua (62°07′N 09°16′ E, 951 m a.s.l.).
† Period that begins at the start of a period of five successive days where the daily average temperature is >5·0°C and ends on the day before of a period of five successive days when the daily average temperature is <5·0 °C.
The F2 mapping population was derived from crosses between the Plech and Spiterstulen populations (Kemi et al., 2013). To avoid inbreeding depression and problems with self-incompatibility two random individuals from Spiterstulen population as mothers were crossed with two random individuals from Plech population as fathers to obtain two unrelated F1 plants. These F1 plants were crossed with each other reciprocally to obtain F2.
We included plants from the parental populations as well as F1 and F2 individuals in our study. The Plech and Spiterstulen populations were represented by seven and six laboratory-grown full sib families, respectively.
Growing conditions
To examine between-population differentiation in response to vernalization and genomic regions underlying this differentiation, vernalized and non-vernalized A. lyrata plants were grown in day-length conditions of 14 h light/10 h dark (LD14:10) in a controlled environment in a climate chamber at the University of Oulu, Oulu, Finland. The light sources in the chamber were metal halide lamps (Osram Power Star HQI-T 400 W/DH; Osram, Germany; measured light intensity 8000 lux).
The F1 and F2 seeds from Spiterstulen, Plech were put to germinate on moist filter paper in Petri dishes at 20 °C and 60 % humidity. Seeds for non-vernalized controls were sown with 2 months delay. Two weeks after sowing, the seedlings (nPlechV– = 23; nSpiterstulen V– = 43; nF1V– = 32; nF2V– = 118) were transferred into pots filled with peat–gravel (1 : 1), and the plants were grown at 20 °C ± 2 °C with 75–85 % humidity in LD14:10. For the vernalized treatment, the seedlings (nPlechV+ = 44; nSpiterstulen V+ = 42; nF1V+ = 42; nF2V+ = 168) were grown for 1 month in LD14:10 and then transferred to 4 °C for 2 months of vernalization (V+) in LD8:16. After vernalization, the plants were randomized among the non-vernalized control plants in LD14:10. Plants were watered daily and commercial fertilizer was added once per week (N : P : K; 17 : 4 : 25; 10 % solution).
The relatively short photoperiod, LD14:10 (which is the approximate day length in Germany at midsummer), was predicted to delay flowering in the Spiterstulen population.
Phenotypic measurements
To quantify phenotypic differentiation and the extent of vernalization response and plasticity, the emergence of visible flower buds (‘bud emergence date’), start of elongation of the reproductive shoot (1 cm long; ‘inflorescence shoot elongation date’) and the start of flowering date (the day of opening of the first flower) were scored daily. The corresponding dates were calculated as number of days from the end of vernalization. From these observations the probability of flowering was computed and called ‘flowering propensity’. We also calculated the number of days from bud emergence to inflorescence shoot elongation and from inflorescence shoot elongation to the start of flowering, because inflorescence buds may remain dormant in perennials even after they are visually observable. At the start of flowering, we scored morphological traits related to reproductive investment: number of inflorescences, the length of the main inflorescence and number of secondary inflorescences (axillary branches in the main inflorescence). Height of the leaf rosette was also measured as a surrogate for leaf angle. Length and width of the longest leaf were measured and ratio of leaf length : width was calculated as an estimate of leaf shape. Three weeks after first flowering, the number of inflorescences and the total number of flowers opening during the 3 weeks were counted for each plant.
Molecular marker analysis and linkage map construction
DNA was extracted with FastDNA® kit (Qbiogene) or DNeasy® 96 Plant Kit (Qiagen). The F2 plants from both experiments were genotyped with 40 microsatellite, CAPS and dCAPS markers. Twenty-two of them were microsatellite or indel length variation markers obtained from studies by Bell and Ecker (1994), Clauss et al. (2002), Kuittinen et al. (2004) and Kemi et al. (2013). Details on markers are available in Kemi et al. (2013 – see ‘notes S2′ and ‘table S2′ in their supporting information).
The linkage map was constructed with JoinMap v.3·0 (Van Ooijen and Voorrips, 2001) from a total of 529 F2 plants. See Kemi et al. (2013) for details. The resulting map with eight linkage groups (LG1 to LG8) corresponding to the eight chromosomes covers 575 cM with an average 18-cM interval between markers.
Adding locations of candidate genes to the linkage map
To enable examining locations of putative candidate genes in the linkage map of the present study, full A. thaliana genomic sequences for a total of 88 candidate genes from recent reviews (Parcy, 2005; Kobayashi and Weigel, 2007; Turck et al., 2008; Fornara et al., 2009; Horvath, 2009; Lagercrantz, 2009) were searched from the Arabidopsis Information Resource (TAIR, http://www.Arabidopsis.org/). Approximately 1 kb from the beginning of the genes was blasted to the A. lyrata genome sequence assembly v1·0 at JGI (http://genome.jgi-psf.org/Araly1/Araly1.home.html) to obtain their exact locations in the A. lyrata genome. To calculate the relative locations of the candidate genes as cM in the linkage map of the current study, the genomic position of each marker in our A. lyrata cross was also determined.
Statistical analysis of phenotypic differentiation and vernalization response
To examine between-population differentiation in vernalization responses, we tested the significance of vernalization × population interaction with likelihood ratio tests of generalized linear models (GLM) in R (R Development Core Team, 2009). We used the binomial model for flowering propensity and Gaussian family for the others. If the interaction was not significant, we tested for main effects of population and vernalization. In cases where residuals were not normally distributed, we used nonparametric Wilcoxon rank sum test to test for significance of between-population differences within each vernalization treatment and the effect of vernalization within each population. In these cases, we used the Bonferroni correction (significant result when P < 0·0125). We used a continuity correction for discrete traits such as the number of inflorescences. Analyses were performed with the statistical software R version 2·9·2 (R Development Core Team, 2009).
QTL analysis
QTL analyses were performed with R/qtl version 1·02-2 (Broman et al., 2003), an add-on package to the general statistical software R. R/qtl allows adding additional simulated pseudo-markers based on the observed marker data, enabling the search for QTL effects between markers. We performed standard interval mapping (Lander and Botstein, 1989) using the scanone function in R/qtl. We used normal and binary models depending on the traits. The most appropriate logarithm of odds (LOD) threshold at P = 0·05 was determined separately for each trait by analysing 1000 permutations of the data.
To find QTL governing differentiation in the measured traits and to find QTL × vernalization interactions, a similar approach was used as in Leinonen et al. (2013). Two scanone and permutation runs with identical seeds for the random number generator (to obtain identical permutations for the two runs) were performed for each trait: a run with vernalization as an additive covariate and a second run with vernalization as both additive and interactive covariate. The result from the first run indicated QTL with significant effect (difference between genotypes) in the combined dataset, after taking into account the effect of vernalization. To find QTL × vernalization interactions, the LOD scores of the first run was subtracted from the LOD scores of the second run. This approach allows detection of interactions where the QTL effects are opposite in the two treatments and would remain undetected in the combined analysis. It also allows overcoming the lack of power when analysing the two treatments separately. Because some traits had skewed distributions or in some cases the number of observations between the two treatments was unbalanced, we used a nonparametric approach in R/qtl to obtain additional QTL scans separately for the vernalized and non-vernalized F2 that are more robust but are also less powerful.
We focused on the QTL that are most relevant for explaining between-population differences: the ones with significant additive effect (different effects of alleles originating from different populations). The significance of additive effects was estimated using a custom script from Leinonen et al. (2013) based on generalized linear models (GLM in R) and taking into account dominance (d) and the difference between the two heterozygous classes (i). For QTL with vernalization interactions, we identified the cases where the alleles from the two populations had different additive effects in the two treatments. We also estimated the size and significance of the additive component (2a) with the GLM approach separately for each treatment at each QTL. Because the analysis requires that the residuals are normally distributed, we examined the model fit with residual plots.
RESULTS
Between-population differentiation in vernalization response
We found that vernalization influenced significantly differently the flowering propensity of the two populations (Table 2). Although plants from both populations flowered more when vernalized, Plech plants showed such an increase of flowering propensity when vernalized (from 0·39 to 1) that the ranking of the two populations was reversed between the two treatments (Table 3). Vernalization reduced the number of days from inflorescence elongation start to flowering only in the Plech population (Table 2), from 17 to 11, but the interaction could not be tested due to non-normality. Plants from both populations had earlier bud emergence, inflorescence elongation and the start of flowering dates when vernalized (Table 2). These results are presented in Supplementary Data Fig. S1.
Table 2.
Between-population differentiation of vernalization response in flowering phenology for the two parental Arabidopsis lyrata populations Plech (Germany) and Spiterstulen (Norway) in the growth chamber in LD14:10 (light : dark)
| Factor |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean1 |
Vernalization × population |
Population |
Vernalization |
||||||||
| Trait | Pl | Sp | Deviance | P | W | P | W | P | |||
| Flowering propensity* | V– | 0·39 (0·2–0·6) | 0·53 (0·4–0·7) | 26·8 | <0·0001 | – | – | – | – | ||
| V + | 1 (0·9–1) | 0·79 (0·6–0·9) | – | – | – | – | |||||
| Bud emergence date† | V– | 31·5 (2·2) | 31·4 (3·3) | – | – | V– | 34·5 | 0·6519 | Pl | 176·0 | <0·0001 |
| V + | 2·7 (0·3) | 11·9 (1·8) | V + | 1229·0 | <0·0001 | Sp | 459·5 | <0·0001 | |||
| Inflorescence shoot elongation date† | V– | 30·5 (2·4) | 37·5 (3·9) | – | – | V– | 37·5 | 0·1910 | Pl | 176·0 | 0·0009 |
| V + | 5·0 (0·26) | 18·9 (2·2) | V + | 1178 | <0·0001 | Sp | 310·0 | 0·0003 | |||
| The start of flowering date† | V– | 71·2 (7·1) | 61·6 (3·7) | – | – | V– | 79·0 | 0·3043 | Pl | 396·0 | <0·0001 |
| V + | 16·1 (0·3) | 36·6 (4·1) | V + | 1313·5 | <0·0001 | Sp | 619·5 | <0·0001 | |||
| Days from bud emergence to inflorescence shoot elongation† | V– | 3·25 (0·5) | 8·6 (1·0) | – | – | V– | 47·0 | 0·0051 | Pl | 85·0 | 0·4784 |
| V + | 2·8 (0·2) | 10·2 (1·4) | V + | 794·0 | <0·0001 | Sp | 144·0 | 0·8452 | |||
| Days from inflorescence shoot elongation to flowering start† | V– | 17·0 (2·1) | 8·1 (0·6) | – | – | V– | 0·0 | 0·0033 | Pl | 172·0 | 0·0001 |
| V + | 11·1 (0·2) | 8·7 (0·6) | V + | 264·0 | <0·0001 | Sp | 140·0 | 0·4992 | |||
1 For each trait and cold treatment, population means are given, with 95 % confidence interval for flowering propensity, and ± s.e. for other traits. Significance of vernalization × population interaction based on likelihood ratio tests of generalized linear models (GLM) is shown. In cases where the residuals were not normally distributed and interaction could not be tested, we show between-population differences within each treatment and effect of vernalization within each population based on Wilcoxon rank sum test instead.
W, Wilcoxon rank sum test statistic; P, P-value; V–, non-vernalized; V + , vernalized; Pl, Plech (Germany); Sp, Spiterstulen (Norway).
* GLM; † Wilcoxon rank sum test.
Table 3.
Total number of plants and number of plants which flowered in each treatment
| V– |
V + |
|||
|---|---|---|---|---|
| Pop | Total | Flowered | Total | Flowered |
| Sp | 43 | 23 | 42 | 33 |
| Pl | 23 | 9 | 44 | 44 |
| F1 | 32 | 8 | 42 | 42 |
| F2 | 118 | 65 | 168 | 163 |
V–, Non-vernalized; V + , vernalized; Pl, Plech (Germany); Sp, Spiterstulen (Norway).
Vernalization responses differed between populations in the number of inflorescence shoots at the start of flowering and the number of inflorescences and flowers after 3 weeks from the start of flowering. Vernalization responses differed also in inflorescence length, rosette height and leaf width. Indeed, Plech plants showed stronger vernalization responses than Spiterstulen plants by producing more and longer inflorescences (the means were 14·1 and 3·2 cm for Plech and Spiterstulen, respectively when vernalized, against 8·9 and 2·4 cm without vernalization) and wider leaves when vernalized, compared with the non-vernalized controls. Only rosette height showed a plastic response in the Spiterstulen population: the leaf rosettes were more erect when vernalized compared with the control plants, whereas for this trait, the Plech population did not respond. The results are presented in Table 4 and Supplementary Data Fig. S2.
Table 4.
Between-population differentiation of vernalization response in morphology for the two parental Arabidopsis lyrata populations Plech (Germany) and Spiterstulen (Norway) in the growth chamber in LD14:10 (light : dark)
| Factor |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean (s.e.) |
Vernalization × population |
Population |
Vernalization |
||||||||
| Trait | Pl | Sp | Deviance | P | W | P | W | P | |||
| No. of inflorescence shoots† | V– | 1 (0) | 1 (0) | – | – | V– | – | – | Pl | 99 | 0·0088 |
| V + | 2 (0·2) | 1 (0) | V + | 363·0 | <0·0001 | Sp | – | – | |||
| Inflorescence shoot length (cm)* | V– | 8·9 (1·7) | 2·4 (0·3) | 131·0 | 0·0008 | – | – | – | – | ||
| V + | 14·1 (0·6) | 3·2 (0·4) | – | – | – | – | |||||
| No. of secondary inflorescence shoots† | V– | 2·0 (2·0) | 0 (0) | – | – | V– | 4·0 | 0·0801 | Pl | 8·5 | 0·0529 |
| V + | 5·2 (0·2) | 0·1 (0·1) | V + | 14·5 | <0·0001 | Sp | 100·0 | 0·4640 | |||
| Rosette height (cm)* | V– | 0·6 (0·1) | 1·4 (0·1) | 4·7 | 0·0135 | – | – | – | – | ||
| V + | 0·5 (0·0) | 2·4 (0·2) | – | – | – | – | |||||
| Leaf length (cm)* | V– | 3·7 (0·4) | 5·0 (0·2) | 1·6 | 0·7371 | 46·4 | <0·0001 | 69·7 | <0·0001 | ||
| V + | 4·6 (0·1) | 5·7 (0·2) | |||||||||
| Leaf width (cm)* | V– | 0·9 (0·1) | 1·0 (0) | 0·6 | 0·0078 | – | – | – | – | ||
| V + | 1·2 (0) | 1·0 (0) | – | – | – | – | |||||
| Leaf ratio (width : length)* | V– | 0·25 (0·0) | 0·2 (0·0) | 0·01 | 0·0720 | 0·2 | <0·0001 | 0·01 | 0·0087 | ||
| V + | 0·27 (0·0) | 0·17 (0·0) | |||||||||
| No. of inflorescence shoots after 3 weeks† | V– | 1 (0) | 1 (0) | – | – | V– | – | – | Pl | 25·0 | 0·0042 |
| V + | 2·9 (0·2) | 1 (0) | V + | 160·5 | <0·0001 | Sp | 203·0 | 0·5259 | |||
| No. of flowers after 3 weeks† | V– | 8·6 (1·3) | 8·4 (0·9) | – | – | V– | 34·5 | 0·9628 | Pl | 0·5 | 0·0003 |
| V + | 53·8 (4) | 10·7 (0·9) | V + | 14·5 | <0·0001 | Sp | 144·5 | 0·0978 | |||
For each trait and cold treatment, population means are given. Significance of vernalization × population interaction – and significance of main effects if the interaction was not significant – based on likelihood ratio tests of generalized linear models (GLM) is shown. In cases where the residuals were not normally distributed and interaction could not be tested, we show between-population differences within each treatment and effect of vernalization within each population based on the Wilcoxon rank sum test instead.
W, Test statistic; P, P-value; V–, non-vernalized; V + , vernalized; Pl, Plech (Germany); Sp, Spiterstulen (Norway).
* GLM; † Wilcoxon rank sum test.
In addition to traits showing differences in response to vernalization between the two populations other traits showed differences between populations but with similar or no response to vernalization (Tables 2 and 3). Especially the time from bud emergence to inflorescence elongation, a potentially important trait, particularly in perennials, was shorter for Plech plants compared with Spiterstulen plants (the means were 3·0 and 9·2 d, respectively). However, it did not show a significant response to vernalization in either population (Table 3 and Supplementary Data Fig. S1). Plech plants had more inflorescences, secondary branches and flowers in general compared with Spiterstulen plants, while Spiterstulen plants had higher rosettes and longer leaves (Table 3 and Supplementary Data Fig. S2).
QTL for differentiation in vernalization response
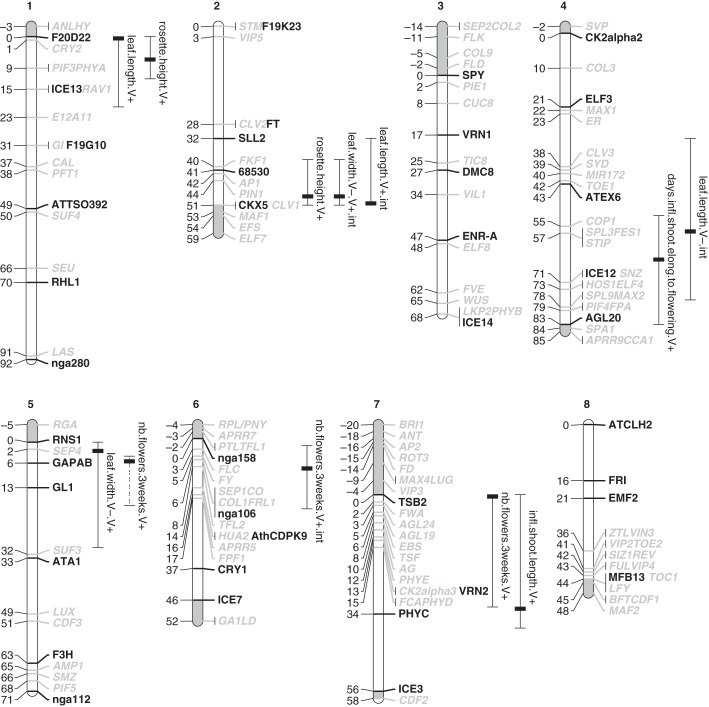
A total of six genomic regions with additive allelic effects were found to govern phenotypic differentiation between A. lyrata populations from Norway and Germany, when the plants were grown in LD14:10 in the growth chamber (Fig. 1 and Supplementary Data Table S1 and Fig. S4). At four of these QTL there was an effect for more than one trait.
Fig. 1.
Linkage-group locations of QTL governing between-population differentiation in flowering traits and leaf morphology in Arabidopsis lyrata from Plech (Germany) and Spiterstulen (Norway) grown in LD14:10 (light:dark) with (V+) and without (V–) vernalization. Lines to the right of linkage groups indicate Bayesian credible intervals, and the black boxes indicate QTL peaks (significant at 5 % level). For each QTL, the name (in black) refers to the trait and ‘V–’ and ‘V + ’ indicate that the additive effect based on GLM was significant in that treatment; ‘int’ indicates that the QTL × vernalization interaction was significant. Candidate genes from A. thaliana are shown in grey in their expected locations. Areas where the candidate genes were located outside the map are shaded grey. The QTL in LG5 that was only found with nonparametric analysis is indicated with a dashed line.
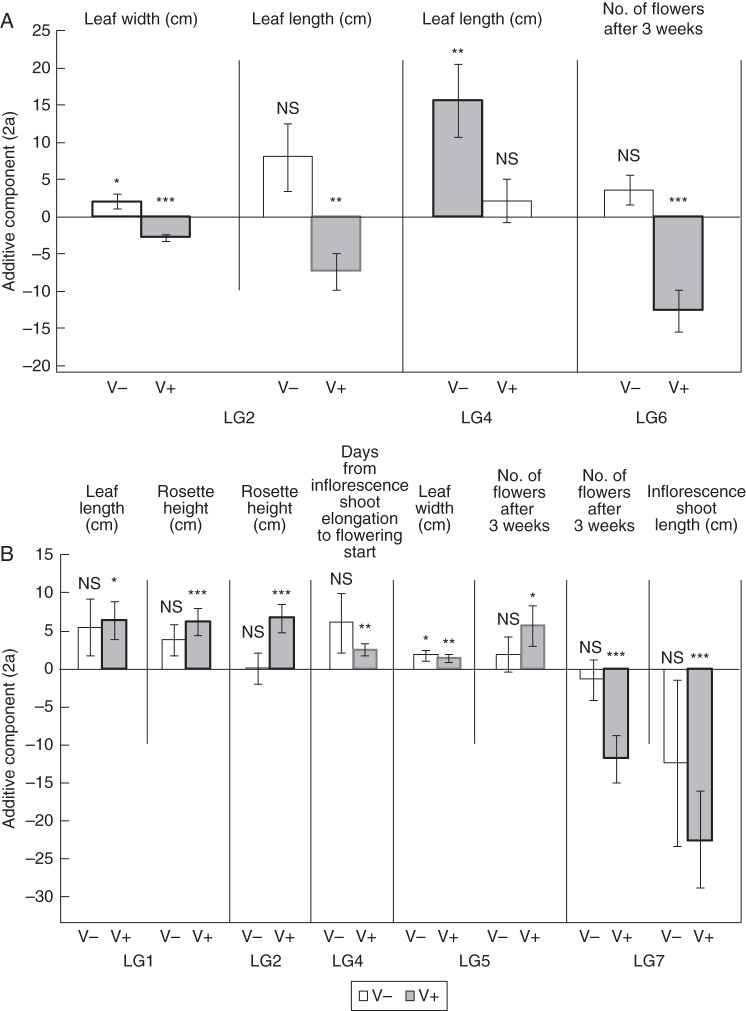
Three genomic areas (LG2, LG4 and LG6) had significant QTL × vernalization interactions (Fig. 2A). At the interactive QTL in LG2 and LG4, QTL alleles from the Spiterstulen population were associated with longer or wider leaves when the plants were not vernalized. Another QTL × vernalization interaction in LG6, near the candidate gene FLC, controlled the number of flowers 3 weeks after the start of flowering. The plants with Spiterstulen alleles at this locus had fewer flowers than the plants with Plech alleles, when vernalized (Fig. 2A). Interestingly, there was also a putative QTL peak just under the genome-wide significance threshold at this locus for the number of days from bud emergence to inflorescence shoot elongation; Spiterstulen alleles delayed inflorescence elongation when not vernalized (Supplementary Data Fig. S3). At the QTL in LG2 for leaf width, LG4 for leaf length and LG6 for flower number, the direction of additive effects corresponded to between-population differences documented in our study (Table 3).
Fig. 2.
Direction and significance of additive effects (± 2 s.e., difference between homozygotes) for (A) QTL with significant QTL × vernalization interactions and (B) QTL detected with the effect of vernalization taken into account as an additive covariate (plants from the two treatments analysed jointly). Positive values: Spiterstulen alleles promote flowering (earlier flowering and greater inflorescence or flower number) and leaf size (greater leaf dimensions). NS, P > 0·05; *, P < 0·05; **, P < 0·01; ***, P < 0·001; ****, P < 0·0001. Non-vernalized (V–) and vernalized (V+) as indicated. Whether the additive effect is in the same (thick black line) or different (thick grey line) direction as in the parental populations is indicated when significant.
In addition, five regions with significant QTL (LG1, LG2, LG4, LG5 and LG7) were detected when the plants from the two treatments were analysed jointly with the effect of vernalization taken into account as an additive covariate (Figs 1 and 2B). We detected again the QTL in LG2 for leaf width and the QTL for flower number in LG6 that were also found in the interaction analysis. In LG1, there were overlapping QTL for leaf length and rosette height for which the Spiterstulen alleles were associated with longer leaves and higher rosettes (Fig. 2B). In LG2, there was a QTL for rosette height that overlapped with the two vernalization interaction QTL for leaf length and width (Fig. 1). In LG4, the Spiterstulen QTL alleles delayed the time from inflorescence elongation start to flowering. There was also a QTL in LG7 where the Spiterstulen alleles were associated with shorter inflorescences and fewer flowers (LG7) after 3 weeks from the start of flowering. The direction of additive effects was concordant with the differences found in parental phenotypic means at the QTL in LG1, LG2 and LG7 (Table 4). Nonparametric QTL scans revealed an additional QTL in LG5 for flower number that showed significant additive effects (the QTL with a dashed line in Fig. 1).
DISCUSSION
Differentiation in plastic responses in life-history and morphology
Adaptive phenotypic plasticity evolves most easily in heterogeneous environments with reliable signals from environmental conditions (Nicotra et al., 2010). Photoperiod and winter cold are such predictable signals that are used for flowering initiation in plants (Bernier and Périlleux, 2005). The vernalization requirement synchronizes flowering of plants with favourable conditions in the spring after winter. The strength of the vernalization requirement in a population is assumed to correspond to the local winter conditions (Amasino, 2009b; King and Heide, 2009). In A. thaliana, late-flowering strains respond most to vernalization (Karlsson et al., 1993; Nordborg and Bergelson, 1999) and, within the 21 European accessions tested by Stinchcombe et al. (2005), southern accessions were significantly more sensitive to vernalization than northern ones. Evidence for a latitudinal cline in flowering time and vernalization responses has also been found by Lempe et al. (2005) in a study of 150 accessions.
In the perennial A. lyrata, earlier studies have shown that vernalization at the rosette stage increased the probability to flower and advanced the the start of flowering date, especially in the northern populations and long days (Riihimäki et al., 2005; Kuittinen et al., 2008). In the present study, we documented significant vernalization responses for flowering phenology in both populations. Interestingly, the number of days from bud emergence to inflorescence elongation did not show a vernalization response, and inflorescence development was slow despite vernalization in the northern Spiterstulen population. The 14-h day length used in our study likely delayed flowering of the northern Spiterstulen population, and this effect could not be overcome with vernalization (Table 2). Further studies in different day lengths have been conducted to take into account the effects of photoperiod at this stage. Indeed, day length can be important especially in the case of perennial plants, preventing them from flowering before spring (Leinonen, 2011; Kemi et al., 2013). For example, inflorescence development of the Spiterstulen population was shown to be delayed and flowering propensity reduced in short day length (14-h day length) compared with long day length (20-h day length) conditions (Leinonen, 2011).
Here we also examined the effect of vernalization on reproductive effort and morphology. We expected that the German Plech plants would show more plasticity in inflorescence production according to results from Leinonen et al. (2009). Indeed we found that rosette vernalization strongly increased reproductive effort (e.g. number of inflorescence shoots and flowers) in the southern Plech population. In contrast, in the northern Spiterstulen population, the investment in sexual reproduction was very limited and highly constrained.
Vernalization reduced flowering-ime variability. The strong vernalization responses in the southern population could be due to selection for synchronization of flowering before the onset of drought in the summer. In the southern population, most seedlings are established in the autumn, and flowering occurs from March/April until September, but drought and high temperatures suppress flowering (Clauss and Koch, 2006). Because of the weak vernalization requirement for the start of flowering (Riihimäki et al., 2005), plants that germinate in the spring may be able to reproduce before the winter. However, these plants would not receive the induction for abundant flowering by cold temperatures. The life history of the northern population is not as well known. The timing of germination in the Spiterstulen field has not been studied. Plants may reproduce vegetatively by rhizomes to some extent, but the limited spatial extent of clones and the high genetic diversity observed imply extensive propagation by seeds (Lundemo et al., 2010). The requirement for a long cold period for flowering in the Spiterstulen population (Riihimäki et al., 2005; Kuittinen et al., 2008; this study) may be related to preventing the newly established rosettes from flowering before the next spring. However, vernalization did not increase the reproductive investment in this population as it did in the Plech plants. The low investment to reproduction is likely to be strictly canalized in this alpine environment due to limitations of the short growing season and a need to allocate resources to a large energy reserve (Körner, 1999); it is also possible that there are constraints due to pleiotropy of the underlying genes.
Vernalization also affected morphological traits, but differently in the two populations. The leaf rosettes of the Spiterstulen plants were more erect than those of Plech plants and vernalization increased the rosette height in the Spiterstulen population, while rosettes of the Plech plants remained flat. Rosette height in A. lyrata can be used as a surrogate for leaf angle. A high leaf angle increases the efficiency of photosynthesis when solar radiation is low and minimizes water loss when solar radiation is high. In support of this hypothesis, clines with more upright leaves in lower latitudes have been found, for example, in A. thaliana and Dryas octopetala (Herbert, 2003; Hopkins et al., 2008). In our experiment the leaf angle estimated by rosette height was higher in the northern Spiterstulen than in the southern Plech population, which is in contrast with the prediction based on the latitudinal correlation in these other species. Other environmental factors not correlated with latitude may have influenced evolution of leaf morphology in some populations of A. lyrata. In fact, the Plech population originates from a site shaded by trees, in contrast to the alpine valley in Spiterstulen above the tree line. Further studies on selection on leaf shape and leaf angle are needed to make inferences about evolution and adaptive significance of these traits.
Comparisons to field studies
Common-garden studies in controlled conditions are widely used to reveal whether population differences found in the field are genetically based. The use of ecologically meaningful controlled environments allows studying the effects of specific cues on trait responses and differentiation between populations in their sensitivity to these cues. Naturally, the possible range of conditions in a growth chamber is restricted, and gene effects may be different in the more complicated field conditions. In A. thaliana, environmental cues for flowering in the field (but absent in the growth chamber) overrode the inhibition of flowering posed by the functional FRI alleles (Wilczek et al., 2009). Further, the genetic architecture of flowering time was different in the field compared with the growth chamber (Brachi et al., 2010). Vergeer and Kunin (2011) found that natural populations of A. lyrata in Scandinavia were more likely to flower than populations growing in the south. This seems to be in contrast to our results and to the generalization that southern populations have a higher probability of flowering than northern populations. However, their southern populations were from a maritime Wales environment, in contrast to the more continental German population studied here. Differences in herbivore load may also have had an influence (Vergeer and Kunin, 2011).
Our results concerning between-population differences in flowering probability and number of inflorescences in the two populations are consistent with earlier results from field experiments with the same populations (Leinonen et al., 2009). In this former study the Plech population was more likely to flower and produced more inflorescences than Spiterstulen in a common-garden study at the original sites of the populations. In the same study, we also found that inflorescence production was an important fitness component for local adaptation in Germany, while vegetative size and survival were more important in Norway. The concordance of the results suggests that the growth-chamber conditions are robust. However, we acknowledge the restrictions of the simple growth-chamber conditions, and to complement these results QTL mapping in the field is needed.
Genetic basis of vernalization responses
Our study of the genetic basis of differences between the two populations revealed QTL × vernalization interactions, as well as QTL effects that were similar in the vernalized and non-vernalized treatments. In the former case, genes have different effects in different environments or a perceptible effect in only part of the environments. The relatively low and varying sample size in our experiment is likely to have influenced our ability to detect QTL with smallest effects, because most traits were measured only for plants that had flowered. Consequently, this reduced the sample size in the non-vernalized experiment where a large proportion of the plants remained vegetative. However, we were still able to detect interesting QTL × vernalization interactions.
At five QTL in our study, the direction of additive effect corresponded to those observed in the parents in at least one trait. The populations have adapted to their current sites after the last glaciation, and the traits we studied are related to flowering phenology and morphology. These have likely been under strong directional selection during the range expansion to current sites from refugial areas, and also near the refugia because of the warming climate. QTL effects mostly in the direction of parental mean differences (Orr, 1998) would support the hypothesis of strong directional selection (Yeaman and Whitlock, 2011). However, because the sizes of QTL effects in small or moderate size samples tends to be biased upwards (Beavis, 1998), inferences of strong directional selection based on our data are limited. Ongoing association studies will provide additional information on the role of selection for the QTL regions found here.
Putative candidate genes in the QTL regions
We found QTL for leaf dimensions with vernalization interaction at the bottom of LG2. In this region there are three candidate genes (MAF1, EFS, ELF7) that are all involved in mediating vernalization effects in A. thaliana (He et al., 2004). Another QTL for leaf length and rosette height (putatively correlated with leaf angle in A. lyrata), was found at the top of LG1 where PHYTOCHROME A (PHYA) is located. Phytochrome mutants in A. thaliana have inflated internode elongation (Mazzella et al., 2000; Halliday et al., 2003) which makes the plant more erect.
FLC is the central gene in the annual A. thaliana that mediates information on winter. Natural variation in vernalization requirement is governed mostly by allelic variation in the FLC or the FRI genes. Interestingly, one of the suggested roles of PEP1, an orthologue of FLC, in the perennial Arabis alpina is to prevent a part of the meristem from becoming reproductive (Wang et al., 2009). We detected QTL × vernalization interaction for the total number of flowers in the area containing FLC (and other flowering time and meristem identity candidate genes as well). We expected to find QTL effects especially in the non-vernalized treatment, where higher FLC expression would repress flowering of the Spiterstulen plants. In contrast, we found that when vernalized, the alleles from the Spiterstulen population at this locus were associated with low flower number. Interestingly, however, we also found a putative QTL near FLC for flowering phenology, where the Spiterstulen alleles delayed start of elongation of the reproductive shoot in the non-vernalized treatment, supporting our prediction (Supplementary Data Fig. S2). Kemi et al. (2013) studied expression differences of tandemly duplicated FLC genes between the Spiterstulen and Plech populations and found support that one of the genes can be responsible for mediating vernalization effects. However, FLC is not the only gene mediating the flowering response to vernalization in A. thaliana (Kim et al., 2009) and further investigation is required to determine whether FLC is involved in the QTL detected on LG6.
There were no QTL for any trait in the area of the FRI gene. A functional variant in FRI conferring a 15-d difference in bud emergence date in growth-chamber conditions is known to segregate in the studied Spiterstulen population (Kuittinen et al., 2008), but this polymorphism did not segregate in the cross studied here. Furthermore, although Plech is more likely to flower earlier than Spiterstulen in controlled conditions, the Plech population studied here harbours the late allele of FRI. Therefore, other genetic factors must be responsible for the flowering time differences between the populations (Kuittinen et al., 2008).
Conclusions
Our results indicate that the two populations have diverged in their plastic responses to vernalization in traits tightly connected to fitness. Those results are in accordance with previous studies in field conditions. This study also brings the first elements on the genetic basis of flowering and leaf morphology and their responses to vernalization in A. lyrata and highlights QTL × vernalization interactions.
The genetic differences described here are likely to have occurred during colonization, probably due to selection for climatic adaptation. An interesting extension of the framework would be to compare results of independent colonizations. For example, parallel changes at the FRI locus have been found in two continents (Kuittinen et al., 2008).
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
The authors thank M. Otsukka and S. Alatalo and the students A. Okuloff, S. Rönkönharju, K. Peltonen, T. Teräväinen, S. Sarlin, J. Kiiskilä and U. Kemi for their contributions to the laboratory work. We also thank Ulla Kemi for valuable comments on this manuscript. This research was financially supported by the Academy of Finland, INRA and the University of Oulu.
LITERATURE CITED
- Amasino R. Floral induction and monocarpic versus polycarpic life histories. Genome Biology. 2009a;10:228–230. doi: 10.1186/gb-2009-10-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amasino R. Seasonal and developmental timing of flowering. The Plant Journal. 2009b;61:1001–1013. doi: 10.1111/j.1365-313X.2010.04148.x. [DOI] [PubMed] [Google Scholar]
- Ansell SW, Stenøien HK, Grundmann M, et al. Population structure and historical biogeography of European Arabidopsis lyrata. Heredity. 2010;105:543–553. doi: 10.1038/hdy.2010.10. [DOI] [PubMed] [Google Scholar]
- Atwell SY, Huang B, Vilhjálmsson G, et al. Genome-wide association study of 107 phenotypes in Arabidopsis thaliana inbred lines. Nature. 2010;465:627–631. doi: 10.1038/nature08800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balañá-Alcaide D, Ramos-Onsins SE, Boone Q, Aguadé M. Highly structured nucleotide variation within and among Arabidopsis lyrata populations at the FAH1 and DFR gene regions. Molecular Ecology. 2006;15:2059–2068. doi: 10.1111/j.1365-294X.2006.02918.x. [DOI] [PubMed] [Google Scholar]
- Beavis WD. QTL analyses: power, precision, and accuracy. In: Paterson AH, editor. Molecular dissection of complex traits. New York, NY: CRC Press; 1998. pp. 145–162. [Google Scholar]
- Bell CJ, Ecker JR. Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics. 1994;19:137–144. doi: 10.1006/geno.1994.1023. [DOI] [PubMed] [Google Scholar]
- Bernier G, Périlleux C. A physiological overview of the genetics of flowering time control. Plant Biotechnology Journal. 2005;3:3–16. doi: 10.1111/j.1467-7652.2004.00114.x. [DOI] [PubMed] [Google Scholar]
- Brachi B, Faure N, Horton M, et al. Linkage and association mapping of Arabidopsis thaliana flowering time in nature. PLoS Genetics. 2010;6:e1000940. doi: 10.1371/journal.pgen.1000940. http://dx.doi.org/10.1371/journal.pgen.1000940 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Wu H, Sen S, Churchill GA. R/qtl: QTL mapping in experimental crosses. Bioinformatics. 2003;19:889–890. doi: 10.1093/bioinformatics/btg112. [DOI] [PubMed] [Google Scholar]
- Clauss MJ, Koch MA. Poorly known relatives of Arabidopsis thaliana. Trends in Plant Science. 2006;11:449–459. doi: 10.1016/j.tplants.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Clauss MJ, Mitchell-Olds T. Population genetic structure of Arabidopsis lyrata in Europe. Molecular Ecology. 2006;15:2753–2766. doi: 10.1111/j.1365-294X.2006.02973.x. [DOI] [PubMed] [Google Scholar]
- Clauss MJ, Cobban H, Mitchell-Olds T. Cross-species microsatellite markers for elucidating population genetic structure in Arabidopsis and Arabis (Brassicaceae) Molecular Ecology. 2002;11:591–601. doi: 10.1046/j.0962-1083.2002.01465.x. [DOI] [PubMed] [Google Scholar]
- Comes HP, Kadereit JW. The effect of quaternary climatic changes on plant distribution and evolution. Trends in Plant Science. 1998;3:432–438. [Google Scholar]
- Fornara F, Panigrahi KCS, Gissot L, et al. Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Developmental Cell. 2009;17:75–86. doi: 10.1016/j.devcel.2009.06.015. [DOI] [PubMed] [Google Scholar]
- Geber MA, Griffen LR. Inheritance and natural selection on functional traits. International Journal of Plant Sciences. 2003;164:S21–S42. [Google Scholar]
- Halliday KJ, Salter MG, Thingnaes E, Whitelam GC. Phytochrome control of flowering is temperature sensitive and correlates with expression of the floral integrator FT. The Plant Journal. 2003;33:875–885. doi: 10.1046/j.1365-313x.2003.01674.x. [DOI] [PubMed] [Google Scholar]
- He Y, Doyle MR, Amasino RM. PAF1-complex-mediated histone methylation of FLOWERING LOCUS C chromatin is required for the vernalization-responsive, winter-annual habit in Arabidopsis. Genes & Development. 2004;18:2774–2784. doi: 10.1101/gad.1244504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemming MN, Trevaskis B. Make hay when the sun shines: the role of MADS-box genes in temperature-dependant seasonal flowering responses. Plant Science. 2011;180:447–453. doi: 10.1016/j.plantsci.2010.12.001. [DOI] [PubMed] [Google Scholar]
- Herbert TJ. A latitudinal cline in leaf inclination of Dryas octopetala and implications for maximization of whole plant photosynthesis. Photosynthetica. 2003;41:631–633. [Google Scholar]
- Hopkins RJ, Schmitt J, Stinchcombe JR. A latitudinal cline and response to vernalization in leaf angle and morphology in Arabidopsis thaliana (Brassicaceae) New Phytologist. 2008;179:155–164. doi: 10.1111/j.1469-8137.2008.02447.x. [DOI] [PubMed] [Google Scholar]
- Horvath D. Common mechanisms regulate flowering and dormancy. Plant Science. 2009;177:523–531. [Google Scholar]
- Hu TT, Pattyn P, Bakker EG, et al. The Arabidopsis lyrata genome sequence and the basis of rapid genome size change. Nature Genetics. 2011;43:476–81. doi: 10.1038/ng.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisz S, Kramer EM. Variation and constraint in plant evolution and development. Heredity. 2008;100:171–177. doi: 10.1038/sj.hdy.6800939. [DOI] [PubMed] [Google Scholar]
- Karlsson BH, Sills GR, Nienhuis J. Effects of photoperiod and vernalization on the number of leaves at flowering in 32 Arabidopsis thaliana (Brassicaceae) ecotypes. American Journal of Botany. 1993;80:646–648. [Google Scholar]
- Kemi U, Niittyvuopio A, Toivainen T, et al. Role of vernalization and of duplicated FLOWERING LOCUS C in the perennial Arabidopsis lyrata. New Phytologist. 2013;197:323–335. doi: 10.1111/j.1469-8137.2012.04378.x. [DOI] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. Vernalization: winter and the timing of flowering in plants. Annual Review of Cell and Developmental Biology. 2009;25:277–299. doi: 10.1146/annurev.cellbio.042308.113411. [DOI] [PubMed] [Google Scholar]
- King RW, Heide OM. Seasonal flowering and evolution: the heritage from Charles Darwin. Functional Plant Biology. 2009;36:1027–1036. doi: 10.1071/FP09170. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Weigel D. Move on up, it's time for change – mobile signals controlling photoperiod-dependent flowering. Genes & Development. 2007;21:2371–2384. doi: 10.1101/gad.1589007. [DOI] [PubMed] [Google Scholar]
- Körner C. Alpine plant life: functional plant ecology of high mountain ecosystems. Berlin: Springer; 1999. [Google Scholar]
- Kuittinen H, De haan AA, Vogl C. Comparing the linkage maps of the close relatives Arabidopsis lyrata and A. thaliana. Genetics. 2004;168:1575–1584. doi: 10.1534/genetics.103.022343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuittinen H, Niittyvuopio A, Rinne P, Savolainen O. Natural variation in Arabidopsis lyrata vernalization requirement conferred by a FRIGIDA indel polymorphism. Molecular Biology and Evolution. 2008;25:319–329. doi: 10.1093/molbev/msm257. [DOI] [PubMed] [Google Scholar]
- Lagercrantz U. At the end of the day: a common molecular mechanism for photoperiod responses in plants. Journal of Experimental Botany. 2009;60:2501–2515. doi: 10.1093/jxb/erp139. [DOI] [PubMed] [Google Scholar]
- Lander ES, Botstein D. Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics. 1989;121:185–199. doi: 10.1093/genetics/121.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinonen PH. Local adaptation and its genetic basis in Arabidopsis lyrata. Finland: University of Oulu; 2011. PhD Thesis. [Google Scholar]
- Leinonen PH, Sandring S, Quilot B, et al. Local adaptation in European populations of Arabidopsis lyrata (Brassicaceae) American Journal of Botany. 2009;96:1129–1137. doi: 10.3732/ajb.0800080. [DOI] [PubMed] [Google Scholar]
- Leinonen PH, Remington DL, Leppälä J, Savolainen O. Genetic basis of local adaptation and flowering time variation in Arabidopsis lyrata. Molecular Ecology. 2013;22:709–723. doi: 10.1111/j.1365-294X.2012.05678.x. [DOI] [PubMed] [Google Scholar]
- Lempe J, Balasubramanian S, Sureshkumar S, Singh A, Schmid M, Weigel D. Diversity of flowering responses in wild Arabidopsis thaliana strains. PLoS Genetics. 2005;1:e6. doi: 10.1371/journal.pgen.0010006. http://dx.doi.org/10.1371/journal.pgen.0010006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundemo S, Stenøien H, Savolainen O. Investigating the effects of topography and clonality on genetic structuring within a large Norwegian population of Arabidopsis lyrata. Annals of Botany. 2010;106:243–254. doi: 10.1093/aob/mcq102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Bertero D, Casal JJ. Temperature-dependent internode elongation in vegetative plants of Arabidopsis thaliana lacking phytochrome B and cryptochrome 1. Planta. 2000;210:497–501. doi: 10.1007/PL00008157. [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. Memories of winter: vernalization and the competence to flower. Plant, Cell & Environment. 2000;23:1145–1153. [Google Scholar]
- Mitchell-Olds T. Arabidopsis thaliana and its wild relatives: a model system for ecology and evolution. Trends in Ecology and Evolution. 2001;16:693–700. [Google Scholar]
- Muller MH, Leppälä J, Savolainen O. Genome-wide effects of postglacial colonization in Arabidopsis lyrata. Heredity. 2008;100:47–58. doi: 10.1038/sj.hdy.6801057. [DOI] [PubMed] [Google Scholar]
- Nicotra AB, Atkin OK, Bonser SP, et al. Plant phenotypic plasticity in a changing climate. Trends in Plant Science. 2010;15:684–692. doi: 10.1016/j.tplants.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Nordborg M, Bergelson J. The effect of seed and rosette cold treatment on germination and flowering time in some Arabidopsis thaliana (Brassicaceae) ecotypes. American Journal of Botany. 1999;86:470–475. [PubMed] [Google Scholar]
- O'Kane SL, Al-Shehbaz IA. A synopsis of Arabidopsis (Brassicaceae) Novon. 1997;7:323–327. [Google Scholar]
- Orr HA. Testing natural selection vs. genetic drift in phenotypic evolution using quantitative trait locus data. Genetics. 1998;149:2099–2104. doi: 10.1093/genetics/149.4.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcy F. Flowering: a time for integration. International Journal of Developmental Biology. 2005;49:585–593. doi: 10.1387/ijdb.041930fp. [DOI] [PubMed] [Google Scholar]
- Pujol B, Pannell JR. Reduced responses to selection after species range expansion. Science. 2008;321:96. doi: 10.1126/science.1157570. [DOI] [PubMed] [Google Scholar]
- R development Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2009. http://www.r-project.org . [Google Scholar]
- Riihimäki M, Savolainen O. Environmental and genetic effects on flowering differences between northern and southern populations of Arabidopsis lyrata (Brassicaceae) American Journal of Botany. 2004;91:1036–1045. doi: 10.3732/ajb.91.7.1036. [DOI] [PubMed] [Google Scholar]
- Riihimäki M, Podolsky R, Kuittinen H, Koelewijn H, Savolainen O. Studying genetics of adaptive variation in model organisms: flowering time variation in Arabidopsis lyrata. Genetica. 2005;123:63–74. doi: 10.1007/s10709-003-2711-7. [DOI] [PubMed] [Google Scholar]
- Ross-Ibarra J, Wright SI, Foxe JP, et al. Patterns of polymorphism and demographic history in natural populations of Arabidopsis lyrata. Plos One. 2008;3:e2411. doi: 10.1371/journal.pone.0002411. http://dx.doi.org/10.1371/journal.pone.0002411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandring S, Riihimäki M, Savolainen O, Ågren J. Selection on flowering time and floral display in an alpine and a lowland population of Arabidopsis lyrata. Journal of Evolutionary Biology. 2007;20:558–567. doi: 10.1111/j.1420-9101.2006.01260.x. [DOI] [PubMed] [Google Scholar]
- Savolainen O, Kuittinen H. In: Arabidopsis lyrata genetics. Bancroft I, Schmidt R, editors. New York, NY: Springer Verlag; 2011. [Google Scholar]
- Schmickl R, Jørgensen MH, Brysting A, Koch M. The evolutionary history of the Arabidopsis lyrata complex: a hybrid in the amphi-beringian area closes a large distribution gap and builds up a genetic barrier. BMC Evolutionary Biology. 2010;10:98. doi: 10.1186/1471-2148-10-98. http://dx.doi.org/10.1186/1471-2148-10-98 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmickl R, Koch M. Arabidopsis hybrid speciation processes. Proceedings of the National Academy of Sciences of the USA. 2011;108:14192–14197. doi: 10.1073/pnas.1104212108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shindo C, Lister C, Crevillen P, Nordborg M, Dean C. Variation in the epigenetic silencing of FLC contributes to natural variation in Arabidopsis vernalization response. Genes & Development. 2005;20:3079–3083. doi: 10.1101/gad.405306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JR, Caicedo A, Hopkins R, et al. Vernalization sensitivity in Arabidopsis thaliana (Brassicaceae): the effects of latitude and FLC variation. American Journal of Botany. 2005;92:1701–1707. doi: 10.3732/ajb.92.10.1701. [DOI] [PubMed] [Google Scholar]
- Strange A, Li P, Lister C, et al. Major-effect alleles at relatively few loci underlie distinct vernalization and flowering variation in Arabidopsis accessions. PloS One. 2011;6:e19949. doi: 10.1371/journal.pone.0019949. http://dx.doi.org/10.1371/journal.pone.0019949 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annual Review of Plant Biology. 2008;59:573–594. doi: 10.1146/annurev.arplant.59.032607.092755. [DOI] [PubMed] [Google Scholar]
- Van Ooijen JW, Voorrips RE. Joinmap® 3·0, software for the calculation of genetic linkage maps. Wageningen, The Netherlands: Plant Research International; 2001. [Google Scholar]
- Vergeer P, Kunin WE. Life history variation in Arabidopsis lyrata across its range: effects of climate, population size and herbivory. Oikos. 2011;120:979–990. [Google Scholar]
- Wang RH, Farrona S, Vincent C, et al. PEP1 regulates perennial flowering in Arabis alpina. Nature. 2009;459:423–427. doi: 10.1038/nature07988. [DOI] [PubMed] [Google Scholar]
- Wilczek A, Roe JL, Knapp MC, et al. Effects of genetic perturbation on seasonal life history plasticity. Science. 2009;323:930–934. doi: 10.1126/science.1165826. [DOI] [PubMed] [Google Scholar]
- Willis KJ, van Andel TH. Trees or no trees? The environments of Central and Eastern Europe during the last glaciation. Quaternary Science Review. 2004;23:2369–2387. [Google Scholar]
- Willmann MR, Poethig RS. The effect of the floral repressor FLC on the timing and progression of vegetative phase change in Arabidopsis. Development. 2011;138:677–685. doi: 10.1242/dev.057448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeaman S, Whitlock MC. The genetic architecture of adaptation under migration–selection balance. Evolution. 2011;65:1897–1911. doi: 10.1111/j.1558-5646.2011.01269.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.