Abstract
Background and Aims
Malpighiales are one of the largest angiosperm orders and have undergone radical systematic restructuring based on molecular phylogenetic studies. The clade has been recalcitrant to molecular phylogenetic reconstruction, but has become much more resolved at the suprafamilial level. It now contains so many newly identified clades that there is an urgent need for comparative studies to understand their structure, biology and evolution. This is especially true because the order contains a disproportionally large diversity of rain forest species and includes numerous agriculturally important plants. This study is a first broad systematic step in this endeavour. It focuses on a comparative structural overview of the flowers across all recently identified suprafamilial clades of Malpighiales, and points towards areas that desperately need attention.
Methods
The phylogenetic comparative analysis of floral structure for the order is based on our previously published studies on four suprafamilial clades of Malpighiales, including also four related rosid orders (Celastrales, Crossosomatales, Cucurbitales, Oxalidales). In addition, the results are compiled from a survey of over 3000 publications on macrosystematics, floral structure and embryology across all orders of the core eudicots.
Key Results
Most new suprafamilial clades within Malpighiales are well supported by floral structural features. Inner morphological structures of the gynoecium (i.e. stigmatic lobes, inner shape of the locules, placentation, presence of obturators) and ovules (i.e. structure of the nucellus, thickness of the integuments, presence of vascular bundles in the integuments, presence of an endothelium in the inner integument) appear to be especially suitable for characterizing suprafamilial clades within Malpighiales.
Conclusions
Although the current phylogenetic reconstruction of Malpighiales is much improved compared with earlier versions, it is incomplete, and further focused phylogenetic and morphological studies are needed. Once all major subclades of Malpighiales are elucidated, more in-depth studies on promising structural features can be conducted. In addition, once the phylogenetic tree of Malpighiales, including closely related orders, is more fully resolved, character optimization studies will be possible to reconstruct evolution of structural and biological features within the order.
Keywords: Androecium, Celastrales, COM clade, core eudicots, evolution, floral structure, gynoecium, Malpighiales, ovules, Oxalidales, phylogeny, rosids
INTRODUCTION
The eudicot order Malpighiales contains approx. 40 families and includes more than 16 000 species, making it one of the largest orders of the flowering plants (APG III, 2009; Wurdack and Davis, 2009; Xi et al., 2012). The sheer morphologial diversity in the group, including such extremes as tropical holoparasites with giant flowers and temperate trees and herbs with tiny, simple flowers, has long made its classification challenging. First taking shape in the early 1990s (Chase et al., 1993; APG I, 1998; Nandi et al., 1998), Malpighiales have undergone massive restructuring over the past 15 years as a result of the molecular phylogenetic revolution (Savolainen et al., 2000a, b; Soltis et al., 2000; Davis and Chase, 2004; APG III, 2009; Wurdack and Davis, 2009; Xi et al., 2012). A number of new families have been recognized (e.g. Peraceae, Centroplacaceae), others excluded (e.g. Medusandraceae, Peridiscaceae), some traditional families have been alternatively circumscribed (e.g. Salicaceae to also include Samydaceae and Scyphostegiaceae; Passifloraceae to also include Turneraceae and Malesherbiaceae), and new suprafamilial clades of families have been discovered (e.g. the parietal clade, the rhizophoroid clade and Rafflesiaceae + Euphorbiaceae; Schwarzbach and Ricklefs, 2000; Zhang and Simmons, 2006; Alford, 2007; Davis et al., 2007; Wurdack and Davis, 2009; Xi et al., 2012). Despite the extreme difficulty in resolving the order (Davis et al., 2005; Korotkova et al., 2009; Wurdack and Davis, 2009), however, recent attempts using phylogenomic approaches have made significant progress, although some relationships remain unresolved (Wurdack and Davis, 2009; Xi et al., 2012). In the most recent study by Xi et al. (2012) the 16 previously identified clades of Malpighiales, eight of which are suprafamilial (Wurdack and Davis, 2009), have been resolved into three large clades. Nine additional suprafamilial subclades have also been resolved within these three major clades. A new phylogenetic analysis of Malpighiales based on structural features at a more refined level is our ultimate goal but will only be possible to achieve once all families or suprafamilial clades have been equally studied. This endeavour will be of interest especially for the evaluation of fossil Malpighiales, for which DNA is unavailable. In combination with molecular analyses it may also improve the phylogenetic resolution of the group as has been shown in other studies (Nandi et al., 1998; Doyle and Endress, 2000).
These newly recognized Malpighiales subclades provide a foundation for conducting a comprehensive comparative analysis of their floral structure and biology, which to date has been sorely lacking. The reason for this vacuum in our knowledge is two-fold. First, the poor phylogenetic resolution (including sparse taxon sampling) within the order has hindered appropriate taxon selection to elucidate putative synapomorphies for major Malpighiales subclades, and second, as we outline below in greater detail, numerous groups within Malpighiales still lack sampling for morphological and anatomical features. To rectify this, the laboratory of P.K.E. has been conducting a series of comparative floral structural studies focusing on several newly identified Malpighiales clades (Endress and Matthews, 2006b, 2012; Matthews and Endress, 2008, 2011, 2013; Matthews et al., 2012). This research programme has also included the closest relatives of Malpighiales, Oxalidales and Celastrales (Matthews et al., 2001; Matthews and Endress, 2002, 2005a), plus two additional rosid orders, Cucurbitales and Crossosomatales (Matthews and Endress, 2004, 2005b). The main aim of this research has been to identify special floral features that characterize these clades based on the reconstruction of their outer and inner morphological surfaces using serial microtome sections plus scanning electron microscopy, and also anatomical and histological features. Concurrently, we have conducted a vast literature survey including over 3000 publications on macrosystematics, reproductive structure and embryology throughout all families of the core eudicots, which we have also used here.
To demonstrate the unbalanced sampling of structural features for Malpighiales we prepared a table (Table 1) listing the number of publications on aspects of ovule, embryo sac and floral development as well as the inner floral structure for each family of Malpighiales [clade circumscription sensu Wurdack and Davis (2009) and Xi et al. (2012)]. The clades treated in these two papers were chosen over the APG III (2009) list as they provide a more extensive representation of subclades for the euphorbioids, salicoids and ochnoids. Table 1 shows that 13 of the approx. 40 families (about 30 % of the families) are still unknown in their ovule and female gametophyte development, while the relatively large number of 132 for Euphorbiaceae is misleading because many of these studies treat only a small number of genera within the family (mainly Euphorbia, Croton and Acalypha). In the case of the latter, the ovule and female gametophyte development for most of the over 200 genera of Euphorbiaceae (Webster, 1994b) remain unknown. In addition, many of these publications are of varying quality and comprehensiveness, some relatively broad and others with only minor notes on single features from single species. The most useful publications are those with high-quality illustrations because they help with comparisons, especially when ambiguous terms are used. In our summary of this work we have tried to use all existing publications as much as possible; however, for the sake of brevity we cite only a small number of these. In particular, we focus on the newer and more comprehensive works whenever possible.
Table 1.
List of families in Malpighiales (following Xi et al., 2012)
| Achariaceae | (16/12) |
|---|---|
| Balanopaceae | (1/1) |
| Bonnetiaceae | (3/1) |
| Calophyllaceae | (4/1) |
| Caryocaraceae | (3/0) |
| Centroplacaceae | (0/0) |
| Chrysobalanaceae | (9/1) |
| Clusiaceae | (20/6) |
| Ctenolophonaceae | (5/0) |
| Dichapetalaceae | (4/1) |
| Elatinaceae | (6/6) |
| Erythroxylaceae | (12/8) |
| Euphorbiaceae | (172/132) |
| Euphroniaceae | (1/0) |
| Goupiaceae | (0/0) |
| Humiriaceae | (10/2) |
| Hypericaceae | (13/10) |
| Irvingiaceae | (3/1) |
| Ixonanthaceae | (3/0) |
| Lacistemataceae | (0/0) |
| Linaceae | (43/17) |
| Lophopyxidaceae | (1/0) |
| Malesherbiaceae | (1/0) |
| Malpighiaceae | (21/12) |
| Medusagynaceae | (3/0) |
| Ochnaceae | (15/7) |
| Pandaceae | (2/0) |
| Passifloraceae | (31/13) |
| Peraceae | (2/0) |
| Phyllanthaceae | (19/10) |
| Picrodendraceae | (8/2) |
| Podostemaceae | (70/36) |
| Putranjivaceae | (5/4) |
| Quiinaceae | (1/0) |
| Rafflesiaceae | (7/6) |
| Rhizophoraceae | (15/6) |
| Salicaceae | (26/13), |
| Samydaceae | (3/3) |
| Scyphostegiaceae | (2/0) |
| Trigoniaceae | (2/1) |
| Turneraceae | (13/5) |
| Violaceae | (28/14). |
The first number indicates the number of publications covering aspects of floral development and inner floral structure for members of each family (including male and female gametophytes and palynology). The second number indicates the number of publications on ovule development and/or female gametophyte development for members of each family (the second number is also included in the first number because the two topics often overlap).
A prominent character emphasized in premolecular classifications of Malpighiales family groups has been placentation type (Cronquist, 1981; Takhtajan, 1997). Specifically, ovaries with axile vs. parietal placentation have played a prominent early role in macrosystematics at the order and subclass rank (see Endress and Matthews, 2012). Part of the current Malpighiales, mainly families with parietal placentation, was earlier accommodated in the order Violales (or Parietales) [including Salicaceae, Samydaceae, Scyphostegiaceae, Lacistemataceae, Achariaceae, Violaceae, Turneraceae, Malesherbiaceae, Passifloraceae (of clade 1 in Xi et al., 2012), and Elatinaceae (clade 3)] and another part, in Guttiferales [including Medusagynaceae, Ochnaceae, Quiinaceae, Bonnetiaceae, Clusiaceae, Calophyllaceae, Hypericaceae (clade 2) and Caryocaraceae (clade 3)], which included mainly families with axile placentation (e.g. Melchior, 1964). Similarly, in Cronquist (1981), in the parietal Violales [including Salicaceae (as Flacourtiaceae), Lacistemataceae, Scyphostegiaceae, Violaceae, Turneraceae, Malesherbiaceae, Passifloraceae and Achariaceae (clade 1)] and the axile Theales [including Ochnaceae, Quiinaceae, Medusagynaceae, Clusiaceae, Hypericaceae, Calophyllaceae and Bonnetiaceae (clade 2), and Caryocaraceae and Elatinaceae (clade 3)]. These different placental forms are still of interest and appear to delineate some recently defined clades (e.g. the euphorbioids with axile placentation, and the parietal clade with parietal placentation) but are most appropriate at lower systematic levels than order.
With the advent of our more highly resolved phylogenetic tree of Malpighiales (Xi et al., 2012), it is appropriate to present an account on the current state of knowledge of the reproductive structures in the newly recognized suprafamilial clades of Malpighiales. Here, we combine our previous morphological studies within Malpighiales and related groups (Endress and Matthews, 2006b; Matthews and Endress, 2006, 2008, 2011, 2013; Matthews et al., 2012) with data available in the literature to produce a list of features and their distribution across the order. Because our database spans the entire core eudicots we also recognize features that are especially common in Malpighiales (i.e. occurring in many families and in a number of suprafamilial clades) compared with other orders, plus features that may be unique for the order. The latter are especially important as morphological characters that convincingly define the order have to-date been elusive. Gynoecial (including ovular) features appear especially promising in this regard (for other non-nucleotide features, see also Nandi et al., 1998; Stevens, 2001 onwards). The gynoecium is especially rich in this regard because it exhibits more structural complexity than the perianth and androecium by having an inner morphological surface and by also containing the ovules. Our work is a first major step to identify floral morphological features associated with the many newly identified clades within the order. Here, these serve as hypothetical synapomorphies. Obtaining a comprehensive picture of evolutionary changes of flowers within the major subclades of Malpighiales is a multi-step procedure, which we hope to continue to pursue in the near future, but which is beyond the scope of our present study. This goal is a vast undertaking because morphological analyses are time-consuming, plus it is more difficult to attain the necessary material than for molecular studies, for which any tissue of the plant can be used. Moreover, key features of the phylogeny remain unresolved (especially at the base), and numerous families are poorly sampled taxonomically in terms of molecular phylogenetics (e.g. Ochnaceae). Finally, in addition to presenting our current state of knowledge, our summary also serves to identify taxa for which focused floral morphological sampling is desperately needed.
For a reconstruction of the evolution of floral features in Malpighiales, or any such group for that matter, we need to establish (1) the position of the clade at a deeper phylogenetic level and (2) the phylogenetic structure within the clade of interest. It is also good to have an idea of the degree of stability of the selected floral features within the clade of interest at the outset of the study for the purpose of identifying putative synapomorphies. If we study a larger group more closely some features remain relatively constant (e.g. details of ovule structure in orders or supraordinal clades of angiosperms), whereas others fluctuate at low systematic levels (e.g. colour of flowers; ‘Brownian Motion’, Endress, 1994). This becomes ever more obvious by molecular phylogenetic studies with broad taxon sampling (e.g. Losos, 2011). Certain features are more prone to occur (and change) in one clade but not in another, perhaps due to the morphological and genetic preconditions for their appearance. In individual lineages they can disappear and reappear again, such that there appears to be rampant parallel evolution (Endress, 2010, 2011b), which may also apply at the genetic level (W.-H. Zhang et al., 2012). In our assessment here, we focus especially on features that are more common in Malpighiales than in other orders, and features that show a pattern of distribution within Malpighiales that may characterize larger or smaller subclades. Where the phylogenetic tree is not resolved for all subclades, our morphological summary also serves as a guide to possibly unite clades that may be identified in the future. At present, given the uncertainties explained in the following two paragraphs, we often cannot determine synapomorphies or plesiomorphies for subclades, but we can recognize special features that are characteristic for groups and thus hypothesize evolutionary trends within subclades.
With respect to the phylogenetic position of Malpighiales, our current knowledge suggests that within the rosid clade, Oxalidales + Huaceae represent the sister group of Malpighiales (Wurdack and Davis, 2009; see also Zhang and Simmons, 2006; Davis et al., 2007), with Celastrales sister to Malpighiales + (Oxalidales + Huaceae) (Wurdack and Davis, 2009). Unfortunately, the small family Huaceae (Hua and Afrostyrax) is poorly known in its floral structure (Baas, 1972; Bayer, 2007). In contrast, the floral structure of Oxalidales and Celastrales has been investigated in detail (Matthews and Endress, 2002, 2005a). These comparative studies are useful for a tentative evaluation of evolutionary trends in Malpighiales. The COM clade (coined by Endress and Matthews, 2006b, and consisting of Celastrales, Oxalidales and Malpighiales) has been shown to be more closely related to malvids based on floral structure (Endress and Matthews, 2006b) than to fabids, to which it was originally positioned based largely on plastid DNA phylogenies (Savolainen et al., 2000a; Hilu et al., 2003). Several recent molecular phylogenetic studies, however, especially those using mitochondrial and nuclear genes, indicate that the COM clade is sister to malvids (Zhu et al., 2007; Finet et al., 2010; Qiu et al., 2010; Shulaev et al., 2010; Lee et al., 2011; N. Zhang et al., 2012), or nested in malvids (Morton, 2011). These analyses suggest the possibility of a large-scale genomic incongruence involving the plastid placement of the COM clade, with morphology more consistent with the mitochondrial and nuclear gene phylogenies.
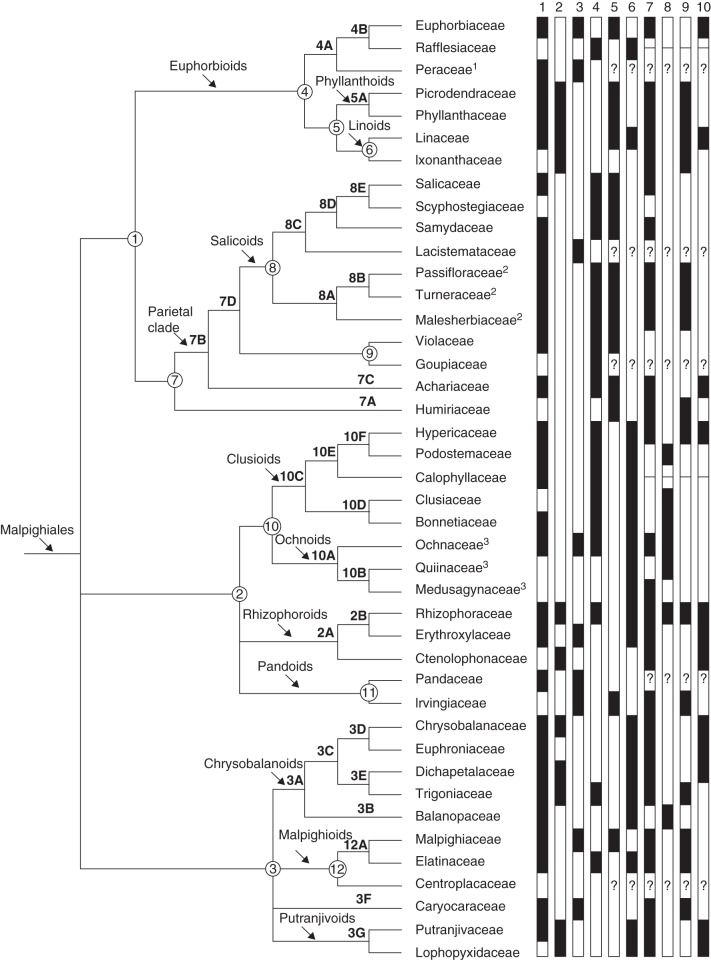
With respect to the phylogenetic structure within Malpighiales, Xi et al. (2012) found that the families of Malpighiales form three major subclades (clades 1–3) (Fig. 1); however, relationships between them are not resolved. Within these three clades are a number of lesser inclusive subclades, each with differing molecular phylogenetic support. Within clade 1, euphorbioids are weakly supported, but subclades within are strongly supported and include Euphorbiaceae, the phyllanthoids and the linoids (informal names following Xi et al., 2012). Mixed support is present in the subclade containing the parietal clade, salicoids and related families. Clade 2 contains the clusioids, ochnoids, rhizophoroids and pandoids, and all but the pandoids are well supported as subclades, although the resolution between them is poor. In clade 3, which contains the chrysobalanoids, malpighioids, putranjivoids and Caryocaraceae, all but the malpighioids form well-supported groups.
Fig. 1.
Phylogeny of Malpighiales reproduced from Xi et al. (2012) (Peraceae and Rafflesiaceae follow Wurdack and Davis, 2009). Suprafamilial clades are discussed in the main text (numbers 1–12 follow Xi et al., 2012). The following families are treated differently in APG III (2009): (1) Peraceae are circumscribed with Euphorbiaceae; (2) Passifloraceae, Turneraceae and Malesherbiaceae are circumscribed as Passifloraceae s.l.; (3) Ochnaceae, Medusagynaceae, and Quiinaceae are circumscribed as Ochnaceae s.l. The distribution of ten prominent features of gynoecium (incl. ovule) structure is plotted: 1. Flowers with mainly 3 carpels. 2. Carpels with 2 ovules, collateral, antitropous, with obturator. 3. Carpels with 1 ovule (median). 4. Carpels with more than 2 ovules. 5. Ovules crassinucellar. 6. Ovules with thin nucellus (incompletely tenuinucellar or weakly crassinucellar). 7. Inner integument thicker than outer at the mature embryo sac stage. 8. Outer integument thicker than inner at the mature embryo sac stage. 9. Outer integument only 2 cell layers thick at the mature embryo sac stage. 10. Inner integument 5 or more cell layers thick at the mature embryo sac stage
Using this molecular phylogenetic tree plus our present knowledge of the floral structure within these groups, we present the first broad synthesis of the potential floral features characterizing these subclades. In the first section we discuss floral structural features of interest that occur in the various families. In the second section we list features that potentially characterize the three main Malpighiales clades and the various subclades sensu Xi et al. (2012).
MORPHOLOGY OF FLOWERS AND THEIR PARTS
In this section the focus is on flowers, their parts and the position of specific floral features. The numbers given in parentheses (1–3) after family names or groups of families refer to the numbers of the three major subclades of Malpighiales to which they belong (sensu Xi et al., 2012; Fig. 1). Closely related families within each major subclade are connected with a + sign (but they do not necessarily represent sister families).
Flowers
Monosymmetric flowers occur especially in Violaceae (1), chrysobalanoids and Malpighiaceae (3) (oblique in the second two clades; Vogel, 1990; Matthews and Endress, 2008; W.-H. Zhang et al., 2012), otherwise only occasionally in predominantly polysymmetric families (Endress, 2012). Trimerous flowers are predominant in some Achariaceae + Salicaceae, part of Euphorbiaceae + Phyllanthaceae + Picrodendraceae (1), part of Podostemaceae (2), and in Elatinaceae (3). A long androgynophore occurs in some Passifloraceae (Bernhard, 1999b) + Malesherbiaceae (Harms, 1925a), and among euphorbioids (male flowers with reduced gynoecium) in Peraceae (Clutia; Pax and Hoffmann, 1931) + Euphorbiaceae (Caperonia; Michaelis, 1924) + Phyllanthaceae (Cleistanthus; Airy Shaw, 1974) (1). A floral cup is common and occurs in Passifloraceae (Bernhard, 1999b) + Turneraceae (González, 1993), occasionally in Salicaceae and Phyllanthaceae (1), in some Quiinaceae (2) (Matthews et al., 2012), in some Rhizophoraceae + Erythroxylaceae (Matthews and Endress, 2011), and at least partly in all families of the chrysobalanoids (except Balanopaceae) (Matthews and Endress, 2008) (3). Heterostyly is known from Linaceae (Matthews and Endress, 2011) and Turneraceae (1) (Arbo, 2007), as well as Hypericaceae (Lloyd and Webb, 1992; Robson, 2012) and Erythroxylaceae (2) (Ganders, 1979). Cleistogamous flowers occur in some Violaceae (1), Podostemaceae (2) and Elatinaceae + Malpighiaceae (3). Unisexual flowers are common in the euphorbioids (including Rafflesiaceae), and Salicaceae + Achariaceae p.p., are rare in Violaceae (1), and also occur in Clusiaceae, Medusagynaceae + Quiinaceae (2) and in putranjivoids (3). An abscission zone below the flowers is common in Malpighiales (Stevens, 2001 onwards), but is lacking or poorly developed in putranjivoids (Matthews and Endress, 2013).
Perianth
Synsepaly is scattered over the entire order but is never predominant in families or suprafamilial groups, e.g. Euphorbiaceae (Prenner et al., 2008a, b; De-Paula et al., 2011) + Peraceae (Bigio and Secco, 2012) (1); short synsepaly is present in Humiriaceae (Winkler, 1931), some Achariaceae (Sleumer, 1954) + Salicaceae (Kaul, 1995) (1), in all four families of Chrysobalanaceae sensu lato (s.l.) (except Balanopaceae) (Merino Sutter and Endress, 2003; Matthews and Endress, 2008), rhizophoroids (Matthews and Endress, 2011), ochnoids (Matthews et al., 2012), Pandaceae (Mildbraed, 1931) (2), Elatinaceae (Niedenzu, 1925) and Putranjivaceae (Matthews and Endress, 2013) (3). Lability may be expressed in that the male flowers of a species are synsepalous, but the female flowers are not (e.g. Manihot mcvaughii; Steinmann, 2005). Secretory structures (glands) on the outer surface of sepals are present in some Euphorbiaceae (Radcliffe-Smith, 2001), Linaceae (Narayana and Rao, 1969b), Passifloraceae (Knapp and Mallet, 1984) (1), Humiriaceae (2) (Winkler, 1931; Sabatier, 2004), most Malpighiaceae (e.g. Vogel, 1974; Anderson, 2007) + some Elatinaceae (Niedenzu, 1925), Caryocaraceae (Matthews and Endress, 2011), and some Chrysobalanaceae (Prance and White, 1988) (3). Secretory fimbriae on sepal margins are known from some Euphorbiaceae (1) (Dehgan and Webster, 1979), Ochnaceae (2) (Matthews et al., 2012), some Malpighiaceae (Anderson, 2006) and Putranjivaceae (Matthews and Endress, 2013) (3). Sepals of unequal size (outer two or three shorter than inner ones) are common in several groups, including the ochnoids (2) (Matthews et al., 2012), chrysobalanoids (Matthews and Endress, 2008) and putranjivoids (Matthews and Endress, 2013) (3). Special mucilage cells in the sepals are common in Malpighiales as in other rosids (Matthews and Endress, 2006) but are notably absent in the putranjivoids (Matthews and Endress, 2013).
Petals are larger than sepals in floral bud and cover the inner floral organs (sometimes combined with valvate aestivation) in several families, including Humiriaceae (Winkler, 1931), Achariaceae (Bernhard, 1999a), Goupiaceae (Mitchell, 2002) and Linaceae (Matthews and Endress, 2011) (1), Erythroxylaceae + Ctenolophonaceae, Irvingiaceae (Matthews and Endress, 2011), ochnoids (Matthews et al., 2012) (2), some chrysobalanoids (Matthews and Endress, 2008) and Caryocaraceae (Matthews and Endress, 2011) (3). Petal aestivation is contort in Humiriaceae (Narayana and Rao, 1969a), Turneraceae (González, 1993), and Linaceae (Matthews and Endress, 2011) + Ixonanthaceae (Steyermark and Luteyn, 1980) (1), many clusioids + ochnoids (Amaral, 1991; Matthews et al., 2012), some Rhizophoraceae + Ctenolophonaceae (Matthews and Endress, 2011) (2), and Euphroniaceae + Trigoniaceae (3) (Matthews and Endress, 2008). Petals with an often conspicuously narrow base are present in Linaceae (1) (Matthews and Endress, 2011), Rhizophoraceae + Erythroxylaceae (Matthews and Endress, 2011), many taxa in the three families of ochnoids (Matthews et al., 2012) (2), and in Chrysobalanaceae s.l. (Matthews and Endress, 2008). Petals with marginal elaborations are most pronounced in some Linaceae (1), many Rhizophoraceae (2) (Matthews and Endress, 2011) and Malpighiaceae (3) (Davis and Anderson, 2010). Petals with ventral elaborations occur in Linaceae (1) and some Erythroxylaceae (2) (Endress and Matthews, 2006a; Matthews and Endress, 2008).
Petals are postgenitally connected in various ways in some Linaceae (Matthews and Endress, 2011) and Chrozophora of Euphorbiaceae (Kapil, 1956) (1), and some Rhizophoraceae + Ctenolophonaceae (2) (Matthews and Endress, 2011). Sympetaly has been mentioned for some Euphorbiaceae (Pax and Hoffmann, 1931); however, it is not established whether this is real sympetaly (i.e. congenital union) or only postgenital union, and whether the perianth organs in these cases are even homologous with petals.
The unusual condition of three (instead of one) vascular traces in petals occurs in Caryocaraceae (3) and Irvingiaceae (2) (Matthews and Endress, 2011), and also in some taxa of all three families of ochnoids (2) (Matthews et al., 2012).
A corona (an elaboration between the corolla and the androecium) is known from part of the parietal clade (1) (Malesherbiaceae, Passifloraceae, Turneraceae, Salicaceae; Bernhard, 1999b; Arbo, 2007; Kubitzki, 2007a, b; Hemingway et al., 2011), from the clusioid-ochnoid clade (Clusiaceae, Gustafsson, 2000, and Ochnaceae, Amaral, 1991; Matthews et al., 2012), and from the rhizophoroids (Ctenolophonaceae, Matthews and Endress, 2011) (2).
Androecium
Constantly low stamen numbers (diplostemony or haplostemony) are found in Linaceae, the parietal clade (most Passifloraceae, Turneraceae + Malesherbiaceae, Violaceae + Goupiaceae, Lacistemataceae, and some Salicaceae) (1), pandoids (Pandaceae + Irvingiaceae) (2) and malpighioids (Malpighiaceae + Elatinaceae) (3). Haplostemony is especially present in part of the parietal clade (Malesherbiaceae, Passifloraceae + Turneraceae, and Violaceae + Goupiaceae) (1). However, there are multiple trends to polystemony (e.g. from haplostemony in Passifloraceae; Bernhard, 1999b; Krosnick et al., 2006). Stamen numbers with broad ranges occur in several larger groups, such as Humiriaceae, Achariaceae, Salicaceae and Euphorbiaceae (1), Clusiaceae + Podostemaceae (2), and Putranjivaceae (3). Representatives with especially large stamen numbers (up to several hundred) include Humiriaceae (200; Sabatier, 2002), Salicaceae (400; Alford, 2008), Euphorbiaceae (up to 1000 in Ricinus; Radcliffe-Smith, 2001) (1), Caryocaraceae (750; Prance and da Silva, 1973), Chrysobalanaceae (300; Prance and White, 1988), some ochnoids + clusioids, such as Medusagynaceae (480; Matthews et al., 2012) + Quiinaceae (600; Matthews et al., 2012), and Calophyllaceae (more than 400; Stevens, 1976) + Hypericaceae (650; Robson, 1996) (2). Stamens united in fascicles (or phalanges) occur especially in the clusioids (2), such as in Clusiaceae (Leins and Erbar, 1991; Bittrich and Amaral, 1996; Stevens, 2007; Sweeney, 2008), and Hypericaceae (Leins, 1964; Baas, 1970; Ronse Decraene and Smets, 1991) + Podostemaceae (a single phalanx) (Rutishauser, 1997). One-sided (monosymmetric) union of groups of stamens is prominent in Chrysobalanaceae s.l. (Prance and White, 1988; Matthews and Endress, 2008) (3). Synandry of all stamens (at least for a short distance) is known from many clades, e.g. some euphorbioids (1), rhizophoroids, few Ochnaceae (2), most chrysobalanoids, Caryocaraceae and few Putranjivaceae (3) (Matthews and Endress, 2008, 2011, 2013; Matthews et al., 2012). Reduction to a single stamen has repeatedly occured and is known from several families, including Euphorbiaceae (Prenner and Rudall, 2007), Lacistemataceae (Agostini, 1973; Endress, 1999) (1), Ochnaceae (Matthews et al., 2012) and Podostemaceae (Thiv et al., 2009) (2).
There are relatively few floral developmental studies in Malpighiales, and thus broad comparisons of androecium development are not yet possible. However, in polystemonous Salicaceae, stamen initiation is centrifugal, while it is centripetal or more or less synchronous in Achariaceae (Bernhard and Endress, 1999; both families formerly included in Flacourtiaceae, Chase et al., 2002) (1). Centrifugal stamen initiation is also present in the clusioid families Clusiaceae (Hochwallner and Weber, 2006), and Hypericaceae (Leins, 1964) + Podostemaceae (Rutishauser and Grubert, 1999) (2), but in Ochnaceae (2) both centrifugal (Luxemburgia; Amaral and Bittrich, 1998) and centripetal (Ochna, Pauzé and Sattler, 1978) initiation is present. In Euphorbiaceae there is centripetal stamen initiation in Garcia (Ronse Decraene and Smets, 1992) but probable centrifugal fascicle initiation in Ricinus (Euphorbiaceae; Prenner et al., 2008b) (1).
In all chrysobalanoid families (except Balanopaceae) the anthers are extremely introrse, with the thecae nearly in one plane, and the anthers have a dorsal pit (Matthews and Endress, 2008) (3). To have the thecae in one plane is also characteristic of Podostemaceae (Kapil, 1970) (2).
Gynoecium
Trimery is most common in the gynoecium of families within Malpighiales and is especially prominent in the euphorbioids and the parietal clade (1), and in the malpighioids (3). An increase of carpel number to more than five has occurred numerous times in various families without any obvious concentration in a single subclade. Also, pseudomonomery (gynoecia with a single fertile carpel and reduced sterile carpels) is known from several distantly related clades, including Euphorbiaceae (1), Chrysobalanaceae and Putranjivaceae (3) (Matthews and Endress, 2008, 2013).
Angiospermy type 4 (for typology, see Endress and Igersheim, 2000) was found in all chrysobalanoids studied (3) (Matthews and Endress, 2008) and in most rhizophoroids (2) (Matthews and Endress, 2011), whereas type 2 is predominant in putranjivoids (3) (Matthews and Endress, 2013). In ochnoids (2) types 2, 3 and 4 occur (Matthews et al., 2012). Postgenital union of free carpel parts appears to be rare; it was found in two distantly related families: Trigoniaceae (3) (Trigoniastrum; Matthews and Endress, 2008) and Ochnaceae (2) (Ochnoideae; Matthews et al., 2012). In groups that have free carpel tips they tend to be unifacial, especially in Linaceae (Baum-Leinfellner, 1953; Matthews and Endress, 2011) and Passifloraceae (Bernhard, 1999b) (1), the rhizophoroid families Ctenolophonaceae (Matthews and Endress, 2011) + Erythroxylaceae (Baum-Leinfellner, 1953; Matthews and Endress, 2011), perhaps Podostemaceae (Ameka et al., 2002; Sobral-Leite et al., 2011) (2) and Caryocaraceae (Matthews and Endress, 2011) (3). Bulged ovaries occur in taxa of Euphorbiaceae (Pax and Hoffmann, 1931) + Picrodendraceae (Berg, 1975) (1), Ochnaceae (Matthews et al., 2012), and Podostemaceae (Jäger-Zürn, 2003) (2), Caryocaraceae (Matthews and Endress, 2011), Chrysobalanaceae (Juel, 1915; Prance and White, 1988; Matthews and Endress, 2008) and Elatinaceae (Niedenzu, 1925) + Malpighiaceae (Simpson, 1989) (3).
Parietal placentae are predominant in the ‘parietal clade’, including the families Achariaceae, Goupiaceae + Violaceae, Malesherbiaceae, Turneraceae + Passifloraceae, Lacistemataceae, Samydaceae, and Salicaceae + Scyphostegiaceae (1). Parietal placentae also tend to go hand-in-hand with several (or at least more than two) ovules per carpel. More than two ovules per carpel are predominant in the parietal clade (1) and in clusioids (2) and are present in only few families of other Malpighiales subclades. The low ovule number in the parietal Lacistemataceae (Krause, 1925) probably arose by reduction and is correlated with flower miniaturization. Extreme placentation forms are the free central placentae in some Podostemaceae (Moline et al., 2007; Thiv et al., 2009), and the nearly basal placentae in Scyphostegiaceae (1) (van Heel, 1967) and some Sauvagesia species of Ochnaceae (2) (Sastre, 1970). A notable trend is the advent of false septa developing from the dorsal part of each carpel [e.g. Linaceae (Matthews & Endress, 2011), and weakly (incipient) in Ixonanthaceae (Link, 1992), Picrodendraceae (Merino Sutter et al., 2006)] (1), some Rhizophoraceae (Matthews and Endress, 2011), Hypericaceae (Eliaea, Baas, 1970) + some Calophyllaceae (Stevens, 2007) (2), and Chrysobalanaceae (Juel, 1915; Matthews and Endress, 2008) (3).
Bifurcated stigmatic branches are especially known from many Euphorbiaceae (incl. Peraceae) + Phyllanthaceae (1). It is of interest that they appear to be especially concentrated also in some other families with reduced flowers and wind pollination, including Balanopaceae (Merino Sutter and Endress, 2003), Putranjivaceae [in the wind-pollinated genus Putranjiva (!); Matthews and Endress (2013)] (3), Salicaceae (Kaul, 1995) + some Achariaceae (Bernhard, 1999a) (1), and Podostemaceae (Podostemum, also wind-pollinated; Rutishauser, 1997; Philbrick and Novelo, 2004) (2). A correlation of bifurcate stigmatic branches with wind pollination also appears in other orders (Rosales, Cucurbitales, Fagales, pers. obs. by P.K.E.). This feature may be selectively advantageous for maximizing pollen capture.
Ovules
The presence of two collateral pendant antitropous ovules (Endress, 2011a) with obturators and axile placentae in each carpel is a unique syndrome in the COM clade among eudicots. This syndrome is most prominent in Malpighiales and less so in Celastrales (Lepidobotryaceae, Matthews and Endress, 2005a) and Oxalidales (Brunelliaceae, Matthews and Endress, 2002). Among Malpighiales it occurs in several larger subclades, notably in the phyllanthoids (incl. Linaceae) (1), rhizophoroids (2), chrysobalanoids and putranjivoids (3). Among these groups the phyllanthoids stand out from the rest due to their crassinucellar ovules (Tokuoka and Tobe, 2001; Merino Sutter et al., 2006), often with nucellar caps (and sometimes nucellar beaks), whereas the other clades have ovules with thin (slender) nucelli, mostly incompletely tenuinucellar or weakly crassinucellar (Endress, 2011a), commonly with an endothelium and early disintegration of the nucellus around the embryo sac (Boesewinkel and Bouman, 1980; Tobe and Raven, 1984; Matthews and Endress, 2008, 2011, 2013). In Centroplacaceae (Bhesa) and Balanopaceae (3) the two ovules are not pendant but upright (Loesener, 1942; Merino Sutter and Endress, 2003). A rarer variant is carpels with more or less collateral placentae but the ovules are curved in a way that they are positioned one upon the other, such as in Humiriaceae (1) (Cuatrecasas, 1961; Boesewinkel, 1985), the ochnoids Quiinaceae + Medusagynaceae (2) (Matthews et al., 2012), and in the chrysobalanoids Balanopaceae + Euphroniaceae (3) (Merino Sutter and Endress, 2003; Matthews and Endress, 2008). Another variant is carpels with just a single ovule, which occurs in Peraceae (Bigio and Secco, 2012) + Euphorbiaceae (Pax and Hoffmann, 1931), Lacistemataceae (in part; Krause, 1925) (1), Irvingiaceae (Matthews and Endress, 2011; Tobe and Raven, 2011) + Pandaceae (Mildbraed, 1931; Pax and Hoffmann, 1931), Ochnoideae of Ochnaceae (Matthews et al., 2012) (2), Caryocaraceae (Dickison, 1990a; Matthews and Endress, 2011) and Malpighiaceae (Singh, 1959; Souto and Oliveira, 2008) (3). In Euphorbiaceae with their uniovulate carpels, the obturator develops as a double structure (Schweiger, 1905), as in the biovulate euphorbioids. It would be of interest to know whether there are obturators and antitropous ovule curvature in the other uniovulate families. As in phyllanthoids the ovules in Euphorbiaceae are crassinucellar and often have nucellar beaks (Bor and Bouman, 1975; Tokuoka and Tobe, 1995, 1998, 2002, 2003).
Ovules with slender nucelli (incompletely tenuinucellar or weakly crassinucellar) and an endothelium (without collateral placentation and pendant ovules) also characterize other subclades of Malpighiales. They are predominant in ochnoids (Matthews et al., 2012) and clusioids (Bonnetiaceae, Prakash and Lau, 1976; Clusiaceae, Lim, 1984; Calophyllaceae, Mourão and Beltrati, 2000; Hypericaceae, Rao, 1957, + Podostemaceae, Arekal and Nagendran, 1977) (2); and they are especially thin in the sister families Hypericaceae + Podostemaceae. Among euphorbioids (1) incompletely tenuinucellar ovules occur as an exception in the parasitic Rafflesiaceae, where they are quite big presumably due to their large cell size (Igersheim and Endress, 1998). The unusual combination of crassinucellar ovules with an endothelium is present in some Linaceae + Ixonanthaceae (1) (Narayana, 1970; Matthews and Endress, 2011) and in some Rhizophoraceae + Erythroxylaceae (2) (Matthews and Endress, 2011). Although these ovules are technically crassinucellar they tend to have relatively thin, slender nucelli. The presence of an endothelium (or integumentary tapetum) has long been known mainly from asterid families with their thin nucelli (Kapil and Tiwari, 1978). Our analysis shows that the endothelium is also an important feature of the COM clade in rosids. Here, it is nearly absent in fabids, but occurs in Brassicales of malvids. This further suggests that more recent analyses using nuclear and mitochondrial genes, but not plastid ones, may better reflect the phylogenetic placement of the COM clade.
Crassinucellar ovules are also concentrated in some larger subclades, especially in the parietal clade (Achariaceae, Steyn et al., 2003, Violaceae, Singh, 1963, Passifloraceae, Kloos and Bouman, 1980, Turneraceae, Kloos and Bouman, 1980, + Malesherbiaceae, Ricardi, 1967, and Samydaceae, Narayanaswami and Sawhney, 1959, Scyphostegiaceae, van Heel, 1967, + Salicaceae, Steyn et al., 2005), Humiriaceae (Boesewinkel, 1985), and in euphorbioids (Euphorbiaceae, Phyllanthaceae + Picrodendraceae, Corner, 1976; Tokuoka and Tobe, 1998, 2001, 2002, 2003; Merino Sutter et al., 2006; but not in the related linoids and Rafflesiaceae) (1), and in Malpighiaceae (3). These euphorbioids (and also Malpighiaceae) are further characterized by a nucellar beak, which develops by excessive growth of the nucellar apex, and is then often not covered by a micropyle (e.g. Z.-G. Zhang et al., 2012), an otherwise rare feature in eudicots. The opposite kind of excessive nucellus elongation – elongation at the base (and lack of micropyle) – occurs in Podostemaceae (e.g. Jäger-Zürn, 1967) (2), and a similar basal elongation is also known from Elatinaceae (Dathan and Singh, 1971) (3). In families with parietal placentation, such as Passifloraceae, the ovules are syntropous (e.g. Endress, 2011a). However, from fig. 8 in Singh (1963) it appears that in Violaceae antitropous ovules do occur. Thus, it would be of interest to know whether antitropous ovules are not restricted to such groups with a single ovule or a collateral pair of ovules per carpel, but have a wider distribution in Malpighiales.
A feature that has been shown to be of great macrosystematic interest in core eudicots is the thickness of the two integuments at anthesis (Endress and Matthews, 2006b). That the inner integument is thicker (with more cell layers) than the outer is practically absent in the fabids, but is predominant in malvids and the COM clade. Within Malpighiales subclades there are more refined patterns. In Humiriaceae and part of the parietal clade, the inner integument is likewise thicker than the outer, but both integuments are in general thinner than in other subclades: the outer often with only two cell layers, the inner with often only three (Humiriaceae, Boesewinkel, 1985, Passifloraceae, Turneraceae, Kloos and Bouman, 1980, + Malesherbiaceae, Ricardi, 1967), while in Violaceae both integuments are three cell layers thick (Singh, 1963) (1). The clusioid clade (2) is an exception in having predominantly the outer integument thicker than the inner (Bonnetiaceae, Prakash and Lau, 1976, Clusiaceae, Lim, 1984, + Podostemaceae, Jäger-Zürn, 1967), with the inner only 2–3 cell layers thick. Also in the ochnoids (2) there is a weak tendency towards the outer integument being thicker than the inner (Matthews et al., 2012).
Almost all Malpighiales have bitegmic ovules. Unitegmic ovules are present only in rare, scattered cases, such as Populus and Salix in Salicaceae (Nikolayeva, 1983; Steyn et al., 2004) (1), Mammea in Calophyllaceae (Mourão and Beltrati, 2000), and rarely in Ochnoideae, Ochnaceae (Matthews et al., 2012) (2) and Anthodiscus in Caryocaraceae (Dickison, 1990a, b).
Vascular bundles in the outer or inner integument (never in both) are present in Euphorbiaceae (inner or outer; Tokuoka and Tobe, 1995, 2002, 2003), Phyllanthaceae (outer; Tokuoka and Tobe, 2001) + Picrodendraceae (inner; Merino Sutter et al., 2006), Salicaceae (outer; Dathan and Singh, 1979) (1), Irvingiaceae (outer; Matthews and Endress, 2011; Tobe and Raven, 2011), Rhizophoraceae (outer; Nikiticheva and Yakovlev, 1985; Matthews and Endress, 2011) (2) and Putranjivaceae (outer; Tokuoka and Tobe, 1999; Matthews and Endress, 2013) (3). These integumentary bundles usually depart from the ovular bundle at the chalaza. However, bundles of the outer integument may already depart from the raphe, which is the case in the big-seeded mangrove genera of Rhizophoraceae (e.g. Rhizophora), in Irvingiaceae (Matthews and Endress, 2011), some Ochnoideae (Ochnaceae; Matthews et al., 2012) (2), Chrysobalanaceae (Matthews and Endress, 2008), Caryocaraceae (Matthews and Endress, 2011) and rarely in Putranjivaceae (Matthews and Endress, 2013) (3). The mature embryo sac grows out of the nucellus tip in Samydaceae (Narayanaswami and Sawhney, 1959) + Salicaceae (Mauritzon, 1936; Steyn et al., 2005), which is also found in Indorouchera of Linaceae (Narayana, 1970) (1).
Another highly unusual feature within angiosperms is the Penaea-type of embryo sac development. It is almost only known in Malpighiales across all angiosperms [apart from Penaea in Myrtales, where it was first described by Stephens (1909), and Biebersteinia of Sapindales (Kamelina and Koonova, 1990)]. Among Malpighiales it occurs in some Euphorbiaceae (e.g. Modilewski, 1910; Tateishi, 1927; Mukherjee, 1958) (1) and Malpighiaceae (e.g. Stenar, 1937; Rao, 1940, 1941) (3). Another shared feature in ovules between Euphorbiaceae and Malpighiaceae is the (occasional) presence of several (more than two) meiocytes in an ovule, as recorded in Euphorbia (Modilewski, 1911) and Codiaeum (Singh, 1965), as well as Malpighia and Tristellateia (Rao, 1941).
Seeds with arils of various differentiation occur predominantly in the parietal clade (Achariaceae, Violaceae, Malesherbiaceae + Passifloraceae + Turneraceae, and Samydaceae + Scyphostegiaceae + Salicaceae), in Linaceae + Ixonanthaceae, and in Euphorbiaceae + Picrodendraceae (1), and also in Rhizophoraceae + Ctenolophonaceae (2) (e.g. Corner, 1976).
Floral biology
Trends to wind pollination (including flower reduction) are known from several subclades of Malpighiales: salicoids (Sacchi and Price, 1988), euphorbioids (1), chrysobalanoids (Balanopaceae; Merino Sutter and Endress, 2003) and putranjivoids (Matthews and Endress, 2013) (3). Among euphorbioids the transition to wind-pollination has occurred many times, in Phyllanthaceae + Picrodendraceae (Webster, 1994a), and among Euphorbiaceae in Acalyphoideae + Crotonoideae (Webster, 1994a), and perhaps several times in each family and subfamily. The general presence of small, unisexual flowers without a corolla in euphorbioids may have been a favourable precondition for this trend.
A unique pollination mode is via resin collection from resin-producing flowers (Armbruster, 1984). Among eudicots such flowers are only known in Malpighiales, and here in two unrelated subclades: in euphorbioids (1), among Euphorbiaceae (Dalechampia; Armbruster, 1984), and in clusioids (2), among Clusiaceae (Clusia, Chrysochlamys, Tovomitopsis; Bittrich et al., 2003) + Calophyllaceae (Clusiella; Bittrich and Amaral, 1997). Even in clusioids they most likely evolved more than once, as indicated by their occurrence in the phylogenetic tree (Gustafsson and Bittrich, 2002; Ruhfel et al., 2011). Other floral biological differentiations vary at lower systematic levels and are therefore not mentioned here.
FLORAL MORPHOLOGICAL CHARACTERIZATION OF MALPIGHIALES SUBCLADES
In this section we focus on the suprafamilial subclades of Malpighiales and how they are characterized by floral features. All suprafamilial subclades sensu Xi et al. (2012) are addressed (see also Fig. 1). In addition, subclades consisting of a single family are also considered if the family has an unresolved position or if its sister consists of more than two families. The subclades are numbered and named as above, following Xi et al. (2012). Additional subclades recognized but not informally named in Xi et al. (2012) are numbered here with the number of the inclusive subclade and an additional specifiying letter. Characters given for the most inclusive clades are not repeated for the subordinated clades, unless the emphasis differs. Only a few publications are cited here. More can be found in the preceding section. The molecular support values from Xi et al. (2012) shown below are given in parentheses after each grouping (maximum-likelihood bootstrap percentages/Bayesian posterior probabilities). The unequal length of each description below generally reflects our unequal knowledge of these groups.
(1) Euphorbioids + Humiriaceae + parietal clade (85/1)
Androgynophore. Ovules mostly crassinucellar. (Pax and Hoffmann, 1931; Winkler, 1931; Cuatrecasas, 1961; Kapil and Bhatnagar, 1994; Tokuoka and Tobe, 2003, 2006).
(2) Clusioids + ochnoids + rhizophoroids + pandoids (83/1)
Centrifugal stamen initiation in polystemonous flowers of clusioids and ochnoids. Ovules with thin nucellus (weakly crassinucellar or incompletely tenuinucellar). (Matthews and Endress, 2008; Matthews et al., 2012).
(2A) Rhizophoroids (Ctenolophonaceae + Erythroxylaceae + Rhizophoraceae) (100/1)
Sepals with fewer than 3 vascular traces. Stamens forming a short basal androecial tube; filament attachment of one or both of the two stamen whorls not on the rim of the androecial tube but slightly inside the tube. Short (andro)gynophore. Angiospermy type 4 predominant; placentation axile; ovary septum with tendency to be thin and disintegrating during development leading to a secondarily unilocular ovary. Ovules antitropous and pendant, with zigzag-shaped micropyle; inner integument more than 5 cell layers thick; lobes of outer integument especially cytoplasm-rich; nucellus long and slender and early disintegrating; endothelium present. Nectaries on androecial tube. Seeds often with aril. (Matthews and Endress, 2011).
(2B) Rhizophoraceae + Erythroxylaceae (100/1)
Floral cup present. Free parts of sepals postgenitally connected in bud, sepals with distinctive layer of large idioblasts in the mesophyll. Petals with conspicuously narrow attachment zone, valvate at least close to the base and postgenitally connected for some distance above the base, conduplicate and enwrapping stamens or parts of stamens in bud. Antepetalous stamens longer than antesepalous ones. Carpels often 3; tendency of carpel bulging and apical septum in the ovary. (Matthews and Endress, 2011).
(3) Chrysobalanoids + malpighioids + putranjivoids + Caryocaraceae (81/1)
Tendency to (oblique) floral monosymmetry in chrysobalanoids and Malpighiaceae. Tendency to bulging of ovaries; placentation mostly axile. (Vogel, 1990; Matthews and Endress, 2008, 2011, 2013; W.-H. Zhang et al., 2012).
(3A) Chrysobalanoids (Balanopaceae + Trigoniaceae + Dichapetalaceae + Euphroniaceae + Chrysobalanaceae) (100/1)
Flowers obliquely monosymmetric (not in Balanopaceae). Sepals basally united, with special mucilage cells (not in Balanopaceae). Petals of unequal size (not in Balanopaceae). Fertile stamens united to some degree into a strap on the anterior side of the flower; anthers with basal pit, strongly introrse (with thecae nearly in one plane) (not in Balanopaceae). Carpels mostly 3; angiospermy type 4. Ovules with thin nucellus (incompletely tenuinucellar or weakly crassinucellar); inner integument more than 5 cell layers thick (only in Trigoniaceae fewer than 5 cell layers thick), with endothelium. (Litt and Chase, 1998; Merino Sutter and Endress, 2003; Matthews and Endress, 2008).
(3B) Balanopaceae (support not indicated, single species sampled for terminal clade)
Flowers unisexual, simple, wind-pollinated. Sepals lacking or minute. Petals lacking. Stamens with short filaments. Gynoecium 2–3-carpellate, stigmatic branches multiply bifurcate, stigma large. Ovules 2 per carpel, collateral but curved in such a way that one is above the other, weakly crassinucellar, notably bitegmic (not unitegmic as stated in earlier publications), both integuments equally thick. (Merino Sutter and Endress, 2003).
(3C) Trigoniaceae + Dichapetalaceae + Euphroniaceae + Chrysobalanaceae (100/1)
See 3A for characteristics. (Matthews and Endress, 2008).
(3D) Euphroniaceae + Chrysobalanaceae (100/1)
Floral cup distinctive, forming a spur. Petals widely spaced on floral cup, distinctly clawed, with lignified hairs. Style with distinct longitudinal furrow between carpels; symplicate zone present in ovary. Nectary smooth, unlobed. Cells with oxalate crystals present in all floral organs. (Matthews and Endress, 2008).
(3E) Trigoniaceae + Dichapetalaceae (100/1)
Floral cup absent. Anthers almost basifixed. Ovary and lower style completely synascidiate. Ovules pendant, with obturator. Nectary with distinct scales or lobes. (Matthews and Endress, 2008).
(3F) Caryocaraceae (100/1)
Flowers large. Pronouncedly polystemonous, with up to 500 and more stamens per flower. Styles free, unifacial. A single antitropous ovule per carpel, hemitropous or slighly campylotropous at anthesis, weakly crassinucellar, without endothelium, without obturator, tendency of the outer integument to be only 2 cell layers thick. (Dickison, 1990a; Matthews and Endress, 2011).
(3G) Putranjivoids (Putranjivaceae + Lophopyxidaceae) (100/1)
Flowers unisexual. Petals lacking or small. Stamens in one whorl, antesepalous (or probably secondarily increased). Angiospermy type 2 predominant; placentation axile. Ovules 2 per carpel, antitropous, pendant, with obturator, with thin nucellus (weakly crassinucellar or incompletely tenuinucellar), inner integument more than 5 cell layers thick. Mucilage cells and special mucilage cells in floral organs lacking. Fruits indehiscent, drupes. (Pax and Hoffmann, 1931; Matthews and Endress, 2006, 2013).
(4) Euphorbioids (Peraceae + Rafflesiaceae + Euphorbiaceae + phyllanthoids + linoids) (64/0·61)
Tendency to unisexual, trimerous flowers with reduced petals (not in Linaceae + Ixonanthaceae; the giant flowers of Rafflesiaceae being different and difficult to compare with other euphorbioids, except for unisexuality). Petals, if present, often contort. Placentation mostly axile. Ovules 2 (more rarely 1) per carpel, antitropous, pendant, with obturator, vascular bundles present in outer or inner integument (not in Linaceae + Ixonanthaceae). (Sutter and Endress, 1995; Bouman and Meijer, 1994; Merino Sutter et al., 2006; Matthews and Endress, 2011).
(4A) Peraceae + Rafflesiaceae + Euphorbiaceae (Rafflesiaceae not included in Xi et al., 2012, thus support not indicated here)
Flowers unisexual. Sepals lacking in some Peraceae and some Euphorbiaceae. Petals mostly lacking. Flowers with a single ovule per carpel (these features not in Rafflesiaceae). (Pax and Hoffman, 1931; Igersheim and Endress, 1998).
(4B) Euphorbiaceae + Rafflesiaceae (Rafflesiaceae not included, thus support not indicated here)
Characterization difficult. (Pax and Hofmann, 1931; Bouman and Meijer, 1994; Igersheim and Endress, 1998)
(5) Phyllanthoids + linoids (84/1)
Tendency to false septa (in addition to normal septa) in the ovary. Tendency of outer integument to be only 2 cell layers thick. Fruits predominantly capsules. (Pax and Hoffmann, 1931; Sutter and Endress, 1995; Merino Sutter et al., 2006; Matthews and Endress, 2011).
(5A) Phyllanthoids (Phyllanthaceae + Picrodendraceae) (100/1)
Flowers with tendency to trimery. Petals, if present, not conspicuous. (Pax and Hoffmann, 1931; Merino Sutter et al., 2006).
(6) Linoids (Linaceae + Ixonanthaceae) (96/1)
Flowers bisexual; mostly diplostemonous. Carpels with false septa (in addition to normal septa) in the ovary; placentation axile. (Link, 1992; Matthews and Endress, 2011).
(7) Humiriaceae + parietal clade (62/0·79)
Flowers mostly haplostemonous (polystemonous in many Humiriaceae, Achariaceae and salicoids). Carpels often 3 (not in Humiriaceae); placentation parietal (not in Humiriaceae). Ovules often more than 2 per carpel (not in Humiriaceae), crassinucellar, without endothelium. Seeds often with aril (not in Humiriaceae). Some families representing basal lineages (including Humiriaceae, Goupiaceae, Violaceae) have a conspicuous anther appendage. (Winkler, 1931; Cuatrecasas, 1961; Bernhard, 1999a, b).
(7A) Humiriaceae (100/1)
Diplostemonous to highly polystemonous. Stamens basally united into a tube, anthers with conspicuous broad appendage. Carpels often 5, forming a united style; placentation axile. Ovules 1 or 2 per carpel, antitropous (without obturator?), pendant, often at unequal levels (one upon the other); integuments relatively thin (outer 2 layers, inner 3 layers). (Winkler, 1931; Mauritzon, 1934; Cuatrecasas, 1961; Boesewinkel, 1985).
(7B) Parietal clade (Achariaceae + Violaceae + Goupiaceae + salicoids) (100/1)
Placentation parietal. (Gilg, 1925; Bernhard, 1999a, b; Bernhard and Endress, 1999).
(7C) Achariaceae (100/1)
Sepals often 3. Petals often more than 5. Often polystemonous. Androecium initiation centripetal or synchronous. Fruits mostly indehiscent. (Gilg, 1925; Lemke, 1988; Bernhard and Endress, 1999).
(7D) Violaceae + Goupiaceae + salicoids (75/0·56)
See 7B. Characterization difficult. (Melchior and Becker, 1925; Loesener, 1942; Bernhard, 1999a; Bernhard and Endress, 1999).
(8) Salicoids (Passifloraceae alliance + Salicaceae alliance) (96/1)
Mostly haplostemonous (not in many Salicaceae and not in many Samydaceae). Corona present in some families. Ovules mostly more than 2 per carpel, crassinucellar (ovule structure in Lacistemataceae unknown). Seeds often with aril. (Gilg, 1925; Harms, 1925a, b; Narayanaswami and Sawhney, 1959; van Heel, 1967; Bernhard, 1999a, b; Bernhard and Endress, 1999).
(8A) Passifloraceae alliance (Malesherbiaceae + Turneraceae + Passifloraceae) (100/1)
Floral cup present. Corona present. Androgynophore present (in Malesherbiaceae and Passifloraceae). Integuments thin (outer 2 cell layers, inner 3 cell layers). Heterostyly in Malesherbiaceae and Turneraceae. (Harms, 1925a, b; Ricardi, 1967; González, 1993; Bernhard, 1999b; Arbo, 2007; Kubitzki, 2007a, b).
(8B) Passifloraceae + Turneraceae (100/1)
Flowers ephemeral (open one morning). Sepals mucronate. Stigma with conspicuous multicellular papillae. (Endress, 1994; Bernhard, 1999b; Arbo, 2007).
(8C) Salicaceae alliance (Lacistemataceae + Samydaceae + Scyphostegiaceae + Salicaceae) (100/1)
Petals, if present, not conspicuous. (Gilg, 1925; Narayanaswami and Sawhney, 1959; van Heel, 1967; Bernhard and Endress, 1999).
(8D) Samydaceae + Scyphostegiaceae + Salicaceae (100/1)
Petals often absent (perhaps not Scyphostegiaceae). Often polystemonous (not Scyphostegiaceae). (Gilg, 1925; Narayanaswamy and Sawhney, 1959; van Heel, 1967; Bernhard and Endress, 1999; Alford, 2003, 2007).
(8E) Scyphostegiaceae + Salicaceae (100/1)
Androecial initiation centrifugal in polystemonous groups of Salicaceae. (Gilg, 1925; van Heel, 1967; Bernhard and Endress, 1999).
(9) Violaceae + Goupiaceae (75/0·62)
Flowers haplostemonous. Anthers with conspicuous apical appendage. Nectary, if present, at outer base of stamens. Ovules more than 2 per carpel, antitropous, crassinucellar (ovule structure in Goupiaceae unknown). (Melchior and Becker, 1925; Loesener, 1942; Singh, 1963).
(10) Clusioids + ochnoids (70/0·81)
Petals often contort. Mostly polystemonous. Placentation mostly axile. Nucelli often thin (ovules often incompletely tenuinucellar), with endothelium, outer integument tends to be thicker than inner (most clusioides, part of ochnoids). (Matthews et al., 2012).
(10A) Ochnoids (Ochnaceae + Medusagynaceae + Quiinaceae) (100/1)
Sepals often of very different size within a flower; sepals with more than 3 vascular traces. Petals not delayed in floral bud development but forming the protective organs of advanced buds; petal aestivation predominantly contort; petals reflexed at anthesis, with 3 vascular traces. Androecium mostly polystemonous (diplostemonous or haplostemonous in some Ochnaceae); anthers basifixed; anthers x-shaped; short androgynophore and gynophore. Tendency to more than 5 carpels per flower; stigmas more or less suction-cup-shaped; vasculature forming a dorsal band of bundles in the upper stylar region; micropyle formed by both integuments; gynoecium epidermis with large, radially elongate cells. Flowers nectarless. Special mucilage cells in floral organs; tanniferous tissue and sclerenchyma abundant in floral organs. (Dickison, 1990b; Amaral, 1991; Matthews et al., 2012).
(10B) Medusagynaceae + Quiinaceae (75/0·98)
Flowers (at least partly) functionally and morphologically unisexual. Polystemonous. Massive thecal septum that persists after dehiscence. Very similar gynoecium with more than 5 carpels, stigmatic lobes radiating outward from the ovary, stigmas capitate (suction-cup-shaped), large ovary roof, ovary surface conspicuously sculpted with longitudinal ribs. Ovules 2 (or more) per carpel, superposed, with a ‘false endothelium’ on the nucellus surface. (Matthews et al., 2012).
(10C) Clusioids (Bonnetiaceae + Clusiaceae + Calophyllaceae + Podostemaceae + Hypericaceae) (100/1)
Stamens often in phalanges (groups of united stamens). Placentation axile. Nucelli always thin, outer integument thicker than inner (not in Hypericaceae). (Engler, 1925; Rao, 1957; Prakash and Lau, 1976; Arekal and Nagendran, 1977; Lim, 1984; Rutishauser, 1997; Mourão and Beltrati, 2000; Sweeney, 2008).
(10D) Clusiaceae + Bonnetiaceae (85/0·92)
The 5 (or 4) sepals are preceded by several bracts. (Engler, 1925; Dickison and Weitzman, 1998; Sweeney, 2008).
(10E) Calophyllaceae + Podostemaceae + Hypericaceae (100/1)
Difficult to characterize. The only studied species of Calophyllaceae (in Mammea) has unitegmic ovules (in contrast to the other families within this clade). (Mourão and Beltrati, 2000).
(10F) Podostemaceae + Hypericaceae (100/1)
Stamens in phalanges (in Podostemaceae a single phalanx). Ovules with extremely thin nucelli, tendency of the outer integument to be only 2 cell layers thick. Podostemaceae are highly specialized water plants with reduced flowers. Thus, the two families are morphologically very different. (Rao, 1957; Arekal and Nagendran, 1977; Rutishauser, 1997).
(11) Pandoids (Pandaceae + Irvingiaceae) (64/0·97)
Placentation axile. Ovule 1 per carpel, antitropous, crassinucellar. (Merino Sutter et al., 2006; Matthews and Endress, 2011; Tobe and Raven, 2011).
(12) Malpighioids (Centroplacaceae + Malpighiaceae + Elatinaceae) (63/0·51)
Low stamen number (haplostemonous or diplostemonous). Placentation axile. Sepals persistent in fruit. (Gilg, 1908; Niedenzu, 1925; Loesener, 1942; Anderson, 1990).
(12A) Malpighiaceae + Elatinaceae (100/1)
Sepals with secretory structures on dorsal side. Mostly diplostemonous. Carpels mostly 3; tendency to have dorsally bulged ovaries; placentation axile. Ovules without endothelium, tendency of outer integument to be only 2 cell layers thick. Presence of cleistogamous flowers in some representatives of both families. However, it should also be mentioned that the two families markedly differ in showy vs. minute, inconspicuous flowers, monosymmetry vs. polysymmetry, uniovular vs. pluriovular carpels, crassinucellar vs. weakly crassinucellar ovules (Niedenzu, 1925; Frisendahl, 1927; Raghavan and Srinivasan, 1940; Rao, 1940; Dathan and Singh, 1971; Anderson, 1980, 1990; Vogel, 1990; Davis and Chase, 2004; Davis and Anderson, 2010; W.-H. Zhang et al., 2012).
ASSOCIATIONS OF FAMILIES BASED MERELY ON FLORAL STRUCTURAL SIMILARITIES (NOT FORMING CLADES IN MOLECULAR STUDIES)
There also appear to be groups of families, which share structural similarities, that are not always closely related according to recent molecular phylogenetic studies. In fact, in some cases they appear quite distantly related. It is of interest to address these groups because they may tell us something about parallel evolutionary trends within Malpighiales or of future relationships yet to be resolved. Numbers in parentheses refer to the three main clades and the subclade numbers in the preceding section, to which the families belong.
Malesherbiaceae, Passifloraceae, Turneraceae (1: 8A), Violaceae (1: 9)
Five stamens, 3 carpels, parietal placenta, corona, aril, crassinucellar ovules, several ovules per carpel. Aril lacking in Malesherbiaceae (Gengler-Nowak, 2003), but chalazal seed appendage present (Harms, 1925a), as in Viola; corona lacking in Violaceae. Thin integuments: Malesherbiaceae, Passifloraceae, Turneraceae (outer 2, inner 3), Violaceae (both integuments 3) (Singh, 1963; Ricardi, 1967; Kloos and Bouman, 1980). Androgynophore in Malesherbiaceae and Passifloraceae (not in Turneraceae and Violaceae). Pollen with more than three colporate apertures and thick two-layered or channelled apertural intine in Passifloraceae and Violaceae (Furness, 2011).
Euphorbiaceae (1: 4B), Malpighiaceae (3: 12A)
Anderson (1990) considered Euphorbiaceae and Malpighiaceae as close possible relatives. In Soltis et al. (2011) Malpighiaceae plus Elatinaceae appear as sister to Phyllanthaceae plus Picrodendraceae but Euphorbiaceae are far apart. This alternative topology, however, is not well supported. Secretory structures on the dorsal surface and secretory fimbriae on margins of sepals, 3 carpels, tendency to dorsally bulged ovaries, axile placenta (the combination of 3 carpels and axile placenta is unusual at the level of Malpighiales), a single ovule per carpel, ovules crassinucellar, at least partly campylotropous, nucellar beak protruding out of at least the inner integument, gaps between inner integument and nucellus and between the integuments, lack of an endothelium; occasional presence of several meiocytes in the ovules. Penaea type of embryo sac development. (Modilewski, 1911; Rao, 1941; Singh, 1965; Sutter and Endress, 1995). Zigzag micropyles occasionally present (e.g. Bouharmont, 1962; Singh, 1959). Malpighian hairs (Euphorbiaceae, Argythamnia, Correll and Correll, 1982; see also Pax and Hoffmann, 1931). Presence of latex (Vega et al., 2002).
Linaceae (1: 6), Humiriaceae (1: 7A), Erythroxylaceae, Rhizophoraceae (2: 2B), Ochnaceae (2: 10A), Chrysobalanaceae, Trigoniaceae (3: 3C), Lophopyxidaceae (3: 3G)
These families show tendencies to diplostemony, stamens basally united, nectaries at outer stamen base (or at inner petal base), axile placenta, two collateral antitropous ovules per carpel, with obturator, ovules incompletely tenuinucellar or weakly crassinucellar, with endothelium. This may be a plesiomorphic combination of features.
Podostemaceae, Hypericaceae (2: 10F), Elatinaceae (3: 12A)
Water plants (among Hypericaceae, Hypericum elodes), many ovules per carpel, placenta only basally axile (in Podostemaceae entirely axile), nucellus conspicuously elongating at its base in Elatinaceae and Podostemaceae.
Achariaceae (1: 7C), Samydaceae (1: 8D), Scyphostegiaceae, current Salicaceae (1: 8E) (i.e. former Flacourtiaceae pro parte)
Lacerate arils, constriction of the funicle in Scyphostegia and Salix, Populus; embryo sac growing out of ovules in Casearia, Idesia, Oncoba and Populus; oligostemony combined with synandry in Scyphostegia and some Salix species.
Euphorbiaceae (1: 4B), Phyllanthaceae, Picrodendraceae (1: 5A)
Unisexual flowers. Petals mostly lacking. Ovules antitropous, with obturator, crassinucellar, with prominent nucellar cap, sometimes nucellar beak (ovules unknown in Peraceae) (Kapil and Bhatnagar, 1994; Sutter and Endress, 1995; Merino Sutter et al., 2006), aril differentiated as a caruncle (for Picrodendraceae, Berg, 1975), fruits explosive, falling into pieces.
A CASE STUDY: EVOLUTIONARY CHANGES IN OVULES IN MALPIGHIALES
We have demonstrated here that features of the ovules represent a rich source of macroevolutionary information. A conspicuous feature in many Malpighiales are ovules with thin, slender nucelli and an endothelium. They are either weakly crassinucellar or even incompletely tenuinucellar (Fig. 1). If we first focus on the entire COM clade, we see that this feature is not restricted to Malpighiales but is common in closely related Celastrales (Celastraceae and Parnassiaceae, Matthews and Endress, 2005a) and also in a number of Oxalidales [Cephalotaceae, some Oxalidaceae, some Elaeocarpaceae s.l. (Tremandraceae); Matthews and Endress, 2002)]. Thus, it is probably not a synapomorphy for just Malpighiales; it may, however, be a synapomorphy for the entire COM clade. The feature dominates in clade 2 (clusioids + ochnoids + rhizophoroids + pandoids; but does not occur in Ctenolophonaceae and Irvingiaceae, and is unknown in Pandaceae) and in clade 3 (chrysobalanoids + malpighioids + putranjivoids; but does not occur in Malpighiaceae and Caryocaraceae, and is unknown in Centroplacaceae). Another interesting exception are the derived mangrove genera of Rhizophoraceae (e.g. Rhizophora) with most likely secondarily crassinucellar ovules because of their large seeds concomitant with their specialized fruit biology. Conversely, thin nucelli occur as exceptions in Rafflesiaceae and some Linaceae among clade 4 (euphorbioids), which otherwise have crassinucellar ovules.
BIG GAPS IN OUR KNOWLEDGE
In 13 families of Malpighiales development of ovules and embryology are largely unknown, such as in Caryocaraceae, Centroplacaceae, Euphroniaceae, Goupiaceae, Ixonanthaceae, Lacistemataceae, Lophopyxidaceae, Malesherbiaceae, Medusagynaceae, Pandaceae, Peraceae and Quiinaceae (Table 1). In most families there are at least some rudimentary data on internal floral morphology and floral anatomy. But even these are unstudied in Centroplacaceae, Goupiaceae and Lacistemataceae (Fig. 1). These are obvious areas where future inquiry would be fruitful.
CONCLUSIONS
The distribution of floral characters in Malpighiales shows that, as expected, variation of features is at different systematic levels. For instance, fusion or non-fusion of sepals is variable within families (e.g. Euphorbiaceae and Ochnaceae), whereas nucellus thickness is constant in families and even in suprafamilial clades (e.g. thick in Euphorbiaceae and the parietal clade, and thin in clusioids, ochnoids and chrysobalanoids; Fig. 1). In this paper we have focused on features that are relatively constant within families but vary among families, providing information on suprafamilial clades. Such features mainly occur in gynoecium and ovule structure. Notably, placentation form, direction of ovule curvature in combination with presence or absence of an obturator, thickness of the nucellus and relative thickness of the two integuments are features of special interest.
Our original studies in several subclades of Malpighiales identified many new potentially significant features for families of the order, especially in gynoecium and ovule structure. Together with the literature these studies have begun to reveal patterns of structural characteristics for the newly recognized major suprafamilial subclades in recent molecular studies. They also begin to give us an idea of how evolution may have proceeded within the large clade Malpighiales. As in other organisms, complexes of characters can disappear but reappear because the genetic structure for the complex has not been lost but rather persists in a cryptic state so that the character or character complex can easily reappear under certain circumstances. This is expressed by trends of evolution and parallel evolution. We are still only at the beginning of understanding extreme evolutionary pathways, such as in euphorbioids with the advent of the giant flowers of Rafflesiaceae and the tiny reduced flowers of Euphorbia (Davis et al., 2007, 2008), or in Malpighiaceae with the evolution of floral monosymmetry (W.-H. Zhang et al., 2012).
Thus, a number of subclades of Malpighiales newly found in molecular phylogenetic studies can also be recognized as related by shared floral structural features. Nevertheless, there are still big lacunae in our structural knowledge of the other subclades at the suprafamilial level, and a lack of resolution in parts of the Malpighiales phylogeny. Knowledge of the structure of the families and larger subclades of Malpighiales is important for reconstruction of the evolution of the group and a better understanding of its biological properties, and also for the placement of fossil flowers (Endress and Friis, 2006; Schönenberger and von Balthazar, 2006; Friis et al., 2011). The future looks bright!
ACKNOWLEDGEMENTS
This study is part of the project ‘Flower diversity and evolution in rosids’ of P.K.E., supported by grant # 31003A_129804 of the Swiss National Foundation (SNF). In addition, studies for the phylogenetic component for this research was funded by the United States National Science Foundation (NSF) Assembling the Tree of Life Grant DEB-0622764 (to C.C.D.), and NSF DEB-1120243 (to C.C.D.). For helpful information on floral structure in Malpighiales we thank Maria do Carmo E. Amaral, Volker Bittrich and Rolf Rutishauser. For comments on the manuscript we thank Joel Nitta and Zhenxiang Xi. For critical reviews of the manuscript we thank Louis Ronse De Craene and an anonymous reviewer.
LITERATURE CITED
- Agostini G. El genero Lozania Mutis (Lacistemaceae) Acta Botanica Venezuelica. 1973;8:167–175. [Google Scholar]
- Airy Shaw HK. Cleistanthus insignis Airy Shaw. Hooker's Icones Plantarum. 1974;38(1) Tabula 3711. [Google Scholar]
- Alford MH. Claves para los géneros de Flacourtiaceae de Perú y del Nuevo Mundo. Arnaldoa. 2003;10:19–38. [Google Scholar]
- Alford MH. Samydaceae. 2007 Tolweb.org/Samydaceae/68361/2007·02·06. [Google Scholar]
- Alford MH. Revision of Neosprucea (Salicaceae) Systematic Botany Monographs. 2008;85:1–62. [Google Scholar]
- Amaral MCE. Phylogenetische Systematik der Ochnaceae. Botanische Jahrbücher für Systematik. 1991;113:105–195. [Google Scholar]
- Amaral MCE, Bittrich V. Ontogenia inicial do androceu de espécies de Ochnaceae subfam. Sauvagesioideae através de análise em microscopia eletrônica de varredura. Revista Brasileira de Botânica. 1998;21:269–273. [Google Scholar]
- Ameka KG, Pfeifer E, Rutishauser R. Developmental morphology of Saxicolella amicorum and S. submersa (Podostemaceae: Podostemoideae) from Ghana. Botanical Journal of the Linnean Society. 2002;139:255–273. [Google Scholar]
- Anderson C. Revision of Galphimia (Malpighiaceae) Contribution from the University of Michigan Herbarium. 2007;25:1–82. [Google Scholar]
- Anderson WR. Cryptic self-fertilization in the Malpighiaceae. Science. 1980;207:892–893. doi: 10.1126/science.207.4433.892. [DOI] [PubMed] [Google Scholar]
- Anderson WR. The origin of the Malpighiaceae – The evidence from morphology. Memoirs of the New York Botanical Garden. 1990;64:210–224. [Google Scholar]
- Anderson WR. Eight segregates from the neotropical genus Mascagnia (Malpighiaceae) Novon. 2006;16:168–204. [Google Scholar]
- APG I (The Angiosperm Phylogeny Group) An ordinal classification for the families of flowering plants. Annals of the Missouri Botanical Garden. 1998;85:531–553. [Google Scholar]
- APG III. An update of the Angiosperm Phylogeny Group classification for the orders and families of flowering plants: APG III. Botanical Journal of the Linnean Society. 2009;161:105–121. [Google Scholar]
- Arbo MM. Turneraceae. In: Kubitzki K, editor. The families and genera of vascular plants, 9. Berlin: Springer; 2007. pp. 458–466. [Google Scholar]
- Arekal GD, Nagendran CR. The female gametophyte in two Indian genera of Tristichoideae (Podostemaceae) – a reinvestigation. Proceedings of the Indian Academy of Sciences B. 1977;86:287–294. [Google Scholar]
- Armbruster WS. The role of resin in angiosperm pollination: ecological and chemical considerations. American Journal of Botany. 1984;71:1149–1160. [Google Scholar]
- Baas P. Anatomical contributions to plant taxonomy I. Floral and vegetative anatomy of Eliaea from Madagascar and Cratoxylum from Indo-Malesia (Guttiferae) Blumea. 1970;18:369–391. [Google Scholar]
- Baas P. Anatomical contributions to plant taxonomy II. The affinities of Hua Pierre and Afrostyrax Perkins et Gilg. Blumea. 1972;20:369–391. [Google Scholar]
- Baum-Leinfellner H. Über unifaziale Griffel und Narben. Planta. 1953;42:452–460. [Google Scholar]
- Bayer C. Huaceae. In: Kubitzki K, editor. The families and genera of vascular plants, 9. Berlin: Springer; 2007. pp. 191–193. [Google Scholar]
- Berg R. Fruit, seed, and myrmecochorous dispersal in Micrantheum (Euphorbiaceae) Norwegian Journal of Botany. 1975;22:173–194. [Google Scholar]
- Bernhard A. Floral structure and development of Ceratiosicyos laevis (Achariaceae) and its systematic position. Botanical Journal of the Linnean Society. 1999a;131:103–113. [Google Scholar]
- Bernhard A. Flower structure, development, and systematics in Passifloraceae and in Abatia (Flacourtiaceae) International Journal of Plant Sciences. 1999b;160:135–150. [Google Scholar]
- Bernhard A, Endress PK. Androecial development and systematics in Flacourtiaceae s.l. Plant Systematics and Evolution. 1999;215:141–155. [Google Scholar]
- Bigio NC, Secco RS. As espécies de Pera (Euphorbiaceae s.s.) in Brazilian Amazon. Rodriguésia. 2012;63(1):163–207. [Google Scholar]
- Bittrich V, Amaral MCE. Pollination biology of Symphonia globulifera (Clusiaceae) Plant Systematics and Evolution. 1996;200:101–110. [Google Scholar]
- Bittrich V, Amaral MCE. Floral biology of some Clusia species from Central Amazonia. Kew Bulletin. 1997;52:617–635. [Google Scholar]
- Bittrich V, Amaral MCE, Machado SMF, Marsaioli AJ. Floral resin of Tovomitopsis saldanhae (Guttiferae) and 7-Epi-nemorosone: structural revision. Zeitschrift für Naturforschung. 2003;58c:643–648. doi: 10.1515/znc-2003-9-1008. [DOI] [PubMed] [Google Scholar]
- Boesewinkel FD. The ovule and seed of Humiria balsamifera (Aubl.) St. Hil. Acta Botanica Neerlandica. 1985;34:183–191. [Google Scholar]
- Boesewinkel FD, Bouman F. Development of ovule and seed-coat of Dichapetalum mombuttense Engl. with notes on the species. Acta Botanica Neerlandica. 1980;29:103–115. [Google Scholar]
- Bor J, Bouman F. Development of ovule and integuments in Euphorbia milii and Codiaeum variegatum. Phytomorphology. 1975;24:280–296. [Google Scholar]
- Bouharmont J. Fécondation de l'ovule et développement de la graine après croisement et autopollinisation chez Hevea brasiliensis. La Cellule. 1962;62:119–130. [Google Scholar]
- Bouman F, Meijer W. Comparative structure of ovules and seeds in Rafflesiaceae. Plant Systematics and Evolution. 1994;193:187–212. [Google Scholar]
- Chase MW, Soltis DE, Olmstead RG, et al. Phylogenetics of seed plants: an analysis of nucleotide sequences from the plastid gene rbcL. Annals of the Missouri Botanical Garden. 1993;80:528–580. [Google Scholar]
- Chase MW, Zmarzty S, Lledó MD, Wurdack KJ, Swensen SM, Fay MF. When in doubt, put it in Flacourtiaceae: a molecular phylogenetic analysis based on plastid DNA sequences. Kew Bulletin. 2002;57:141–181. [Google Scholar]
- Corner EJH. The seeds of dicotyledons. Cambridge: Cambridge University Press; 1976. [Google Scholar]
- Correll DS, Correll HB. Flora of the Bahama archipelago. Vaduz: Cramer; 1982. [Google Scholar]
- Cronquist A. An integrated system of classification of flowering plants. New York: Columbia University Press; 1981. [Google Scholar]
- Cuatrecasas J. A taxonomic revision of the Humiriaceae. Contributions from the United States National Herbarium. 1961;35:25–214. [Google Scholar]
- Dathan ASR, Singh D. Embryology and seed development in Bergia L . Journal of the Indian Botanical Society. 1971;50:362–370. [Google Scholar]
- Dathan ASR, Singh D. Structure and development of female gametophyte and seed in Hydnocarpus laurifolia (Dennst.) Sleumer. Journal of the Indian Botanical Society. 1979;58:256–263. [Google Scholar]
- Davis CC, Anderson WR. A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. American Journal of Botany. 2010;97:2031–2048. doi: 10.3732/ajb.1000146. [DOI] [PubMed] [Google Scholar]
- Davis CC, Chase MW. Elatinaceae are sister to Malpighiaceae; Peridiscaceae belong to Saxifragales. American Journal of Botany. 2004;91:262–273. doi: 10.3732/ajb.91.2.262. [DOI] [PubMed] [Google Scholar]
- Davis CC, Webb CO, Wurdack KJ, Jaramillo CA, Donoghue MJ. Explosive radiation of Malpighiales supports a mid-Cretaceous origin of modern tropical rain forests. American Naturalist. 2005;165:E36–E65. doi: 10.1086/428296. [DOI] [PubMed] [Google Scholar]
- Davis CC, Latvis M, Nickrent DL, Wurdack KJ, Baum DA. Floral gigantism in Rafflesiaceae. Science. 2007;315:1812. doi: 10.1126/science.1135260. [DOI] [PubMed] [Google Scholar]
- Davis CC, Endress PK, Baum DA. Evolution of floral gigantism. Current Opinion in Plant Sciences. 2008;11:49–57. doi: 10.1016/j.pbi.2007.11.003. [DOI] [PubMed] [Google Scholar]
- De-Paula OC, Sajo MG, Prenner G, Cordeiro I, Rudall PJ. Morphology, development and homologies of the perianth and floral nectaries in Croton and Astraea (Euphorbiaceae-Malpighiales) Plant Systematics and Evolution. 2011;292:1–14. [Google Scholar]
- Dehgan B, Webster GL. Morphology and infrageneric relationships of the genus Jatropha (Euphorbiaceae) University of California Publications, Botany. 1979;74:1–73. [Google Scholar]
- Dickison WC. A study of the floral morphology and anatomy of the Caryocaraceae. Bulletin of the Torrey Botanical Club. 1990a;117:123–137. [Google Scholar]
- Dickison WC. The morphology and relationships of Medusagyne (Medusagynaceae) Plant Systematics and Evolution. 1990b;171:27–55. [Google Scholar]
- Dickison WC, Weitzman AL. Floral morphology and anatomy of Bonnetiaceae. Journal of the Torrey Botanical Society. 1998;125:268–286. [Google Scholar]
- Doyle JA, Endress PK. Morphological phylogenetic analysis of basal angiosperms: comparison and combination with molecular data. International Journal of Plant Sciences. 2000;161:S121–S153. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Endress PK. Diversity and evolutionary biology of tropical flowers. Cambridge: Cambridge University Press; 1994. [Google Scholar]
- Endress PK. Symmetry in flowers – diversity and evolution. International Journal of Plant Sciences. 1999;160:S3–S23. doi: 10.1086/314211. [DOI] [PubMed] [Google Scholar]
- Endress PK. Flower structure and trends of evolution in eudicots and their major subclades. Annals of the Missouri Botanical Garden. 2010;97:541–583. [Google Scholar]
- Endress PK. Angiosperm ovules: diversity, development, evolution. Annals of Botany. 2011a;107:1465–1489. doi: 10.1093/aob/mcr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endress PK. Evolutionary diversification of the flowers in angiosperms. American Journal of Botany. 2011b;98:370–396. doi: 10.3732/ajb.1000299. [DOI] [PubMed] [Google Scholar]
- Endress PK. The immense diversity of floral monosymmetry and asymmetry across angiosperms. Botanical Review. 2012;78:345–397. [Google Scholar]
- Endress PK, Friis EM. Rosids – reproductive structure, fossil and extant, and their bearing on deep relationships. Introduction. Plant Systematics and Evolution. 2006;260:83–85. [Google Scholar]
- Endress PK, Igersheim A. Gynoecium structure and evolution in basal angiosperms. International Journal of Plant Sciences. 2000;161:S211–S223. doi: 10.1086/314241. [DOI] [PubMed] [Google Scholar]
- Endress PK, Matthews ML. Elaborate petals and staminodes in eudicots: structure, function, evolution. 6 Organisms, Diversity and Evolution. 2006a;6:257–293. [Google Scholar]
- Endress PK, Matthews ML. First steps towards a floral structural characterization of the major rosid subclades. Plant Systematics and Evolution. 2006b;260:223–251. [Google Scholar]
- Endress PK, Matthews ML. Progress and problems in the assessment of flower morphology in higher-level systematics. Plant Systematics and Evolution. 2012;298:257–276. [Google Scholar]
- Engler A. Guttiferae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925. pp. 154–237. 21. [Google Scholar]
- Finet C, Timme RE, Delwiche CF, Marlétaz F. Multigene phylogeny of the green lineage reveals the origin and diversification of land plants. Current Biology. 2010;20:2217–2222. doi: 10.1016/j.cub.2010.11.035. [DOI] [PubMed] [Google Scholar]
- Friis EM, Crane PR, Pedersen KR. Early flowers and angiosperm evolution. Cambridge: Cambridge University Press; 2011. [Google Scholar]
- Frisendahl A. Über die Entwicklung chasmogamer und kleistogamer Blüten bei der Gattung Elatine. Acta Horti Goetheborgensis. 1927;3:99–142. [Google Scholar]
- Furness CA. Comparative structure and development of pollen and tapetum in Malpighiales, with a focus on the parietal clade. International Journal of Plant Sciences. 2011;172:980–1011. [Google Scholar]
- Ganders FR. Heterostyly in Erythroxylum coca (Erythroxylaceae) Botanical Journal of the Linnean Society. 1979;78:11–20. [Google Scholar]
- Gengler-Nowak KM. Molecular phylogeny and taxonomy of Malesherbiaceae. Systematic Botany. 2003;28:333–344. [Google Scholar]
- Gilg E. Flacourtiaceae africanae. Botanische Jahrbücher für Systematik. 1908;40:444–518. [Google Scholar]
- Gilg E. Flacourtiaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925. pp. 377–457. 25. [Google Scholar]
- González AM. Anatomía y vascularización floral de Piriqueta racemosa, Turnera hassleriana y Turnera joelii (Turneraceae) Bonplandia. 1993;7:143–184. [Google Scholar]
- Gustafsson MHG. Floral morphology and relationshipsof Clusia gundlachii with a discussion of floral organ identity and diversity in the genus Clusia. International Journal of Plant Sciences. 2000;161:43–53. doi: 10.1086/314229. [DOI] [PubMed] [Google Scholar]
- Gustafsson MHG, Bittrich V. Evolution of morphological diversity and resin secretion in flowers of Clusia (Clusiaceae): insights from ITS sequence variation. Nordic Journal of Botany. 2002;22:183–203. [Google Scholar]
- Harms H. Malesherbiaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925a. pp. 467–470. 21. [Google Scholar]
- Harms H. Passifloraceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925b. pp. 470–507. 21. [Google Scholar]
- Hemingway CA, Christensen AR, Malcomber ST. B- and C-class gene expression during corona development of the blue passionflower (Passiflora caerulea, Passifloraceae) American Journal of Botany. 2011;98:923–934. doi: 10.3732/ajb.1100026. [DOI] [PubMed] [Google Scholar]
- Hilu KW, Borsch T, Müller K, et al. Angiosperm phylogeny based on matK sequence information. American Journal of Botany. 2003;90:1758–1776. doi: 10.3732/ajb.90.12.1758. [DOI] [PubMed] [Google Scholar]
- Hochwallner H, Weber A. Flower development and anatomy of Clusia valerioi, a Central American species of Clusiaceae offering floral resin. Flora. 2006;201:407–418. [Google Scholar]
- Igersheim A, Endress PK. Gynoecium diversity and systematics of the paleoherbs. Botanical Journal of the Linnean Society. 1998;127:289–370. [Google Scholar]
- Jäger-Zürn I. Embryologische Untersuchungen an vier Podostemaceen. Österreichische Botanische Zeitschrift. 1967;114:20–45. [Google Scholar]
- Jäger-Zürn I. The occurrence of apical septum in the ovary of Rhyncholacis, Apinagia, Marathrum, and Mourera (Podostemoideae-Podostemaceae): a taxonomic implication. Botanische Jahrbücher für Systematik. 2003;124:303–324. [Google Scholar]
- Juel O. Über den Bau des Gynoeceums bei Parinarium. Arkiv för Botanik. 1915;14:1–12. [Google Scholar]
- Kamelina OP, Konnova VA. Embryological characters of Biebersteinia. Dokladhoi Akademijai Fanhoi RSDS Togikis. 1990;33:193–195. [Google Scholar]
- Kapil RN. A further contribution to the morphology and life history of Chrozophora Neck. Phytomorphology. 1956;6:278–288. [Google Scholar]
- Kapil RN. Podostemaceae. Bulletin of the Indian National Science Academy. 1970;41:104–109. [Google Scholar]
- Kapil RN, Bhatnagar AK. The contribution of embryology to the systematics of Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994;81:145–159. [Google Scholar]
- Kapil RN, Tiwari SC. The integumentary tapetum. Botanical Review. 1978;44:457–490. [Google Scholar]
- Kaul RB. Reproductive structure and organogenesis in a cottonwood, Populus deltoides (Salicaceae) International Journal of Plant Sciences. 1995;156:172–180. [Google Scholar]
- Kloos A, Bouman F. Case studies in aril development. Passiflora suberosa L. and Turnera ulmifolia L. Beiträge zur Biologie der Pflanzen. 1980;55:49–66. [Google Scholar]
- Knapp S, Mallet J. Two new species of Passiflora (Passifloraceae), with comments on their natural history. Annals of the Missouri Botanical Garden. 1984;71:1068–1074. [Google Scholar]
- Korotkova N, Schneider JV, Quandt D, Worberg A, Zizka G, Borsch T. Phylogeny of the eudicot order Malpighiales: analysis of a recalcitrant clade with sequences of petD group II intron. Plant Systematics and Evolution. 2009;282:201–228. [Google Scholar]
- Krause K. Lacistemaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925. pp. 321–323. 21. [Google Scholar]
- Krosnick SE, Harris EM, Freudenstein JV. Patterns of anomalous floral development in the Asian Passiflora (subgenus Decaloba; supersection Disemma) American Journal of Botany. 2006;93:620–636. doi: 10.3732/ajb.93.4.620. [DOI] [PubMed] [Google Scholar]
- Kubitzki K. Introduction to the Passifloraceae alliance (“Passiflorales”=Malpighiales) In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer, 12; 2007a. 9. [Google Scholar]
- Kubitzki K. Malesherbiaceae. In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer; 2007b. pp. 247–249. 9. [Google Scholar]
- Lee EK, Cibrian-Jaramillo A, Kolokotronis SO, et al. A functional phylogenomic view of the seed plants. PLoS Genetics. 2011;7:e1002411. doi: 10.1371/journal.pgen.1002411. http://dx.doi.org/10.1371/journal.pgen.1002411 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leins P. Die frühe Blütenentwicklung von Hypericum hookerianum Wight et Arn. und H. aegypticum L. Berichte der Deutschen Botanischen Gesellschaft. 1964;77:112–123. [Google Scholar]
- Leins P, Erbar C. Fascicled androecia in Dilleniidae and some remarks on the Garcinia androecium. Botanica Acta. 1991;104:336–344. [Google Scholar]
- Lemke DE. A synopsis of Flacourtiaceae. Aliso. 1988;12:29–43. [Google Scholar]
- Lim AL. The embryology of Garcinia mangostana L. (Clusiaceae) Gardens' Bulletin Singapore. 1984;37:93–103. [Google Scholar]
- Link DA. The floral nectaries of the Geraniales and their systematic implications: VI. Ixonanthaceae Exell and Mendonça. Botanische Jahrbücher für Systematik. 1992;114:81–90. [Google Scholar]
- Litt A, Chase MW. The systematic position of Euphronia, with comments on the position of Balanops: an analysis based on rbcL sequence data. Systematic Botany. 1998;23:401–409. [Google Scholar]
- Lloyd DG, Webb CJ. The evolution of heterostyly. In: Barrett SCH, editor. Evolution and function of heterostyly. Berlin: Springer; 1992. pp. 151–207. [Google Scholar]
- Loesener T. Celastraceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1942. pp. 87–197. 20b. [Google Scholar]
- Losos JB. Seeing the forest for the trees: the limitation of phylogenies in comparative biology. American Naturalist. 2011;177:709–727. doi: 10.1086/660020. [DOI] [PubMed] [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Oxalidales (Oxalidaceae, Connaraceae, Cephalotaceae, Brunelliaceae, Cunoniaceae, Elaeocarpaceae, Tremandraceae) Botanical Journal of the Linnean Society. 2002;140:321–381. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Cucurbitales (Corynocarpaceae, Coriariaceae, Datiscaceae, Tetramelaceae, Begoniaceae, Cucurbitaceae, Anisophylleaceae) Botanical Journal of the Linnean Society. 2004;145:129–185. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Celastrales (Celastraceae, Parnassiaceae, Lepidobotryaceae) Botanical Journal of the Linnean Society. 2005a;149:129–194. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Crossosomatales (Crossosomataceae, Stachyuraceae, Staphyleaceae, Aphloiaceae, Geissolomataceae, Ixerbaceae, Strasburgeriaceae) Botanical Journal of the Linnean Society. 2005b;147:1–46. [Google Scholar]
- Matthews ML, Endress PK. Floral structure and systematics in four orders of rosids, including a broad survey of floral mucilage cells. Plant Systematics and Evolution. 2006;260:199–221. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Chrysobalanaceae s.l. (Chrysobalanaceae, Dichapetalaceae, Euphroniaceae, Trigoniaceae; Malpighiales) Botanical Journal of the Linnean Society. 2008;157:249–309. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics in Rhizophoraceae, Erythroxylaceae and the potentially related Ctenolophonaceae, Linaceae, Irvingiaceae and Caryocaraceae (Malpighiales) Botanical Journal of the Linnean Society. 2011;166:331–416. [Google Scholar]
- Matthews ML, Endress PK. Comparative floral structure and systematics of the clade of Lophopyxidaceae and Putranjivaceae. Botanical Journal of the Linnean Society. 2013 in press. [Google Scholar]
- Matthews ML, Endress PK, Schönenberger J, Friis EM. A comparison of floral structures of Anisophylleaceae and Cunoniaceae and the problem of their systematic position. Annals of Botany. 2001;88:439–455. [Google Scholar]
- Matthews ML, Amaral MEC, Endress PK. Comparative floral structure and systematics in Ochnaceae s.l. (Ochnaceae, Quiinaceae, and Medusagynaceae; Malpighiales) Botanical Journal of the Linnean Society. 2012;170:299–392. [Google Scholar]
- Mauritzon J. Etwas über die Embryologie der Zygophyllaceen sowie einige Fragmente über die der Humiriaceen. Botaniska Notiser. 1934;1934:409–422. [Google Scholar]
- Mauritzon J. Zur Embryologie einiger Parietales-Familien. Svensk Botanisk Tidskrift. 1936;30:79–113. [Google Scholar]
- Melchior H. A. Engler's Syllabus der Pflanzenfamilien. 12th edn. Berlin-Nikolassee: Borntraeger; 1964. 2. [Google Scholar]
- Melchior H, Becker W. Violaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1925. pp. 329–377. 21. [Google Scholar]
- Merino Sutter D, Endress PK. Structure of female flowers and cupules in Balanopaceae, an enigmatic rosid family. Annals of Botany. 2003;92:459–469. doi: 10.1093/aob/mcg158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino Sutter D, Forster PI, Endress PK. Female flowers and systematic position of Picrodendraceae (Euphorbiaceae s.l., Malpighiales) Plant Systematics and Evolution. 2006;261:187–215. [Google Scholar]
- Michaelis P. Blütenmorphologische Untersuchungen an den Euphorbiaceen unter besonderer Berücksichtigung der Phylogenie der Angiospermenblüte. Botanische Abhandlungen. 1924;3:1–150. [Google Scholar]
- Mildbraed J. Pandaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn, 19a. Leipzig: Engelmann; 1931. pp. 1–3. [Google Scholar]
- Mitchell JD. Mori SA, Cremers G, Gracie CA, de Granville J-J, Heald SV, Hoff M, Mitchell JD, editors. Goupiaceae. Memoirs of the New York Botanical Garden. 2002;76:199–202. [Google Scholar]
- Modilewski J. Weitere Beiträge zur Embryobildung einiger Euphorbiaceen. Berichte der Deutschen Botanischen Gesellschaft. 1910;28:413–418. [Google Scholar]
- Modilewski J. Über die anomale Embryosackentwicklung bei Euphorbia palustris L. und anderen Euphorbiaceen. Berichte der Deutschen Botanischen Gesellschaft. 1911;29:430–436. [Google Scholar]
- Moline P, Thiv M, Ameka GK, Ghogue J-P, Pfeifer E, Rutishauser R. Comparative morphology and molecular systematics of African Podostemaceae-Podostemoideae with emphasis on Dicraeanthus and Ledermanniella from Cameroon. International Journal of Plant Sciences. 2007;168:159–180. [Google Scholar]
- Morton CM. Newly sequenced nuclear gene (Xdh) for inferring angiosperm phylogeny. Annals of the Missouri Botanical Garden. 2011;98:63–89. [Google Scholar]
- Mourão KSM, Beltrati CM. Morphology and anatomy of developing fruits and seeds of Mammea americana L. (Clusiaceae) Revista Brasileira de Biologia. 2000;60:701–711. doi: 10.1590/s0034-71082000000400023. [DOI] [PubMed] [Google Scholar]
- Mukherjee PK. The female gametophyte of Acalypha malabarica Muell. with a brief discussion on the Penaea type of embryo-sac. Journal of the Indian Botanical Society. 1958;37:504–508. [Google Scholar]
- Nandi OI, Chase MW, Endress PK. A combined cladistic analysis of angiosperms unsing rbcL and non-molecular data sets. Annals of the Missouri Botanical Garden. 1998;85:137–212. [Google Scholar]
- Narayana LL. Linaceae. Bulletin of the Indian National Science Academy. 1970;41:127–132. [Google Scholar]
- Narayana LL, Rao D. Contributions to the floral anatomy of Humiriaceae 1. Journal of Japanese Botany. 1969a;44:328–335. [Google Scholar]
- Narayana LL, Rao D. Contributions to the floral anatomy of Linaceae (1) Journal of Japanese Botany. 1969b;44:289–294. [Google Scholar]
- Narayanaswami S, Sawhney S. Microsporogenesis and embryo sac development in Casearia tomentosa Roxb. Phyton (Buenos Aires) 1959;13:133–144. [Google Scholar]
- Niedenzu F. Elatinaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Vol. 21. Leipzig: Engelmann; 1925. pp. 270–276. [Google Scholar]
- Nikiticheva ZI, Yakovlev MS. Rhizophoraceae. In: Batygina TB, Yakovlev MS, editors. Comparative embryology of flowering plants – Brunelliaceae-Tremandraceae. Leningrad: Nauka; 1985. pp. 120–125. [Google Scholar]
- Nikolayeva ES. Salicaceae. In: Yakovlev MS, editor. Comparative embryology of flowering plants – Phytolaccaceae-Thymelaeaceae. Leningrad: Nauka; 1983. pp. 188–192. [Google Scholar]
- Qiu YL, Li L, Wang B, et al. Angiosperm phylogeny inferred from sequences of four mitochondrial genes. Journal of Systematics and Evolution. 2010;48:391–425. [Google Scholar]
- Pauzé F, Sattler R. L'androcées centripète d'Ochna atropurpurea. Canadian Journal of Botany. 1978;56:2500–2511. [Google Scholar]
- Pax F, Hoffmann K. Euphorbiaceae. In: Engler A, Prantl K., editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann, 11–233, 240; 1931. 19c. [Google Scholar]
- Philbrick CT, Novelo M. Monograph of Podostemum (Podostemaceae) Systematic Botany Monographs. 2004;70:1–106. [Google Scholar]
- Prakash N, Lau YY. Morphology of Ploiarium alternifolium and the taxonomic position of Ploiarium. Botaniska Notiser. 1976;129:279–285. [Google Scholar]
- Prance GT, da Silva MF. Caryocaraceae. Flora Neotropica Monograph. 1973;12:1–77. [Google Scholar]
- Prance GT, White F. The genera of Chrysobalanaceae – A study in practical and theoretical taxonomy and its relevance to evolutionary biology. Philosophical Transactions of the Royal Society London B. 1988;320:1–184. [Google Scholar]
- Prenner G, Rudall PJ. Comparative ontogeny of the cyathium in Euphorbia and its allies: exploring the organ-flower-inflorescence boundary. American Journal of Botany. 2007;94:1612–1629. doi: 10.3732/ajb.94.10.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G, Hopper SD, Rudall PJ. Pseudanthium development in Calycopeplus paucifolius, with particular reference to the evolution of the cyathium in Euphorbieae (Euphorbiaceae-Malpighiales) Australian Systematic Botany. 2008a;21:153–161. doi: 10.1071/sb08010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenner G, Box MWS, Cunniff J, Rudall PJ. The branching stamens of Ricinus and the homologies of the angiosperm stamen fascicle. International Journal of Plant Sciences. 2008b;169:735–744. [Google Scholar]
- Radcliffe-Smith A. Genera Euphorbiacearum. Kew: Royal Botanic Gardens Kew; 2001. [Google Scholar]
- Raghavan TS, Srinivasan VK. A contribution to the life-history of Bergia capensis Linn. Journal of the Indian Botanical Society. 1940;19:283–291. [Google Scholar]
- Rao AMS. Studies in the Malpighiaceae. 1. Embryo-sac development and embryogeny in the genera Hiptage, Banisteria and Stigmaphyllum. Journal of the Indian Botanical Society. 1940;18:145–156. [Google Scholar]
- Rao AMS. Studies in the Malpighiaceae. 2. Structure and development of the ovules and embryo-sacs of Malpighia coccifera Linn. and Tristellateia australis Linn. Proceedings of the National Institute of Science India B. 1941;7:393–404. [Google Scholar]
- Rao AN. The embryology of Hypericum patulum Thunb. and H. mysorense Heyne. Phytomorphology. 1957;7:36–45. [Google Scholar]
- Ricardi SM. Revision taxonomica de las Malesherbiaceas. Gayana Bot. 1967;16:1–139. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Guttiferae). 6. Sections 20. Myriandra to 28. Elodes. Bulletin of the Natural History Museum. 1996;26:75–217. [Google Scholar]
- Robson NKB. Studies in the genus Hypericum L. (Hypericaceae). 9. Addenda, corrigenda, keys, lists and general discussion. Phytotaxa. 2012;72:1–111. [Google Scholar]
- Ronse Decraene LP, Smets E. Androecium and floral nectaries of Harungana madagascariensis. Plant Systematics and Evolution. 1991;178:179–194. [Google Scholar]
- Ronse Decraene LP, Smets E. Complex polyandry in the Magnoliatae: definition, distribution and systematic value. Nordic Journal of Botany. 1992;12:621–649. [Google Scholar]
- Ruhfel BR, Bittrich V, Bove CP, et al. Phylogeny of the clusioid clade (Malpighiales): evidence from the plastid and mitochondrial genomes. American Journal of Botany. 2011;98:306–325. doi: 10.3732/ajb.1000354. [DOI] [PubMed] [Google Scholar]
- Rutishauser R. Structural and developmental diversity in Podostemaceae (river-weeds) Aquatic Botany. 1997;57:29–70. [Google Scholar]
- Rutishauser R, Grubert M. The architecture of Mourera fluviatilis (Podostemaceae): developmental morphology of inflorescences, flowers, and seedlings. American Journal of Botany. 1999;86:907–922. [PubMed] [Google Scholar]
- Sabatier D. Vantanea ovicarpa (Humiriaceae), a new species from French Guiana. Brittonia. 2002;54:233–235. [Google Scholar]
- Sabatier D. Humiriaceae. In: Smith N, Mori SA, Henderson A, Stevenson DW, Heald SV, editors. Flowering plants of the Neotropics. Princeton: Princeton University Press; 2004. pp. 185–187. [Google Scholar]
- Sacchi CF, Price PW. Pollination of the arroyo willow, Salix lasiolepis: role of insects and wind. American Journal of Botany. 1988;75:1387–1393. [Google Scholar]
- Sastre C. Recherches sur les Ochnacées. II. Les espèces de Sauvagesia L. à placenta basale. Caldasia. 1970;10:497–516. [Google Scholar]
- Savolainen V, Chase MW, Hoot SB, et al. Phylogenetics of flowering plants based on combined analysis of plastid atpB and rbcL gene sequences. Systematic Biology. 2000a;49:306–362. doi: 10.1093/sysbio/49.2.306. [DOI] [PubMed] [Google Scholar]
- Savolainen V, Fay MF, Albach DC, et al. Phylogeny of the eudicots: a nearly complete familial analysis based on rbcL gene sequences. Kew Bulletin. 2000b;55:257–309. [Google Scholar]
- Schönenberger J, von Balthazar M. Reproductive structures and phylogenetic framework of the rosids – progress and prospects. Plant Systematics and Evolution. 2006;260:87–106. [Google Scholar]
- Schwarzbach AE, Ricklefs RE. Systematic affinities of Rhizophoraceae, based on chloroplast DNA, nuclear ribsomal DNA, and morphology. American Journal of Botany. 2000;87:547–564. [PubMed] [Google Scholar]
- Schweiger J. Beiträge zur Kenntnis der Samenentwicklung der Euphorbiaceen. Flora. 1905;94:339–379. [Google Scholar]
- Shulaev V, Sargent DJ, Crowhurst RN, et al. The genome of woodland strawberry (Fragaria vesca) Nature Genetics. 2010;43:109–116. doi: 10.1038/ng.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson BB. Pollination biology and taxonomy of Dinemandra and Dinemagonum (Malpighiaceae) Systematic Botany. 1989;14:408–426. [Google Scholar]
- Singh B. Studies in the family Malpighiaceae: I. Morphology of Thryallis glauca Kuntze. Horticultural Advance (Saharanpur) 1959;3:1–19. [Google Scholar]
- Singh D. Structure and development of ovule and seed of Viola tricolor L. and Ionidium suffruticosum Ging. Journal of the Indian Botanical Society. 1963;42:448–462. [Google Scholar]
- Singh RP. Structure and development of seeds in Codiaeum variegatum Blume. Journal of the Indian Botanical Society. 1965;44:205–210. [Google Scholar]
- Sleumer H. Flacourtiaceae. In: van Steenis CGGJ, editor. Flora Malesiana. Leiden: Noordhoff; 1954. pp. 1–106. Ser I, 5. [Google Scholar]
- Sobral-Leite M, Siqueira Filho JA, Erbar C, Machado IC. Anthecology and reproductive system of Mourera fluviatilis (Podostemaceae): pollination by bees and xenogamy in a predominantly anemophilous and autogamous family? Aquatic Botany. 2011;95:77–87. [Google Scholar]
- Soltis DE, Soltis PS, Chase MW, et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Botanical Journal of the Linnean Society. 2000;133:381–461. [Google Scholar]
- Soltis DE, Smith SA, Cellinese N, et al. Angiosperm phylogeny: 17 genes, 640 taxa. American Journal of Botany. 2011;98:704–730. doi: 10.3732/ajb.1000404. [DOI] [PubMed] [Google Scholar]
- Souto LS, Oliveira DMT. Morfoanatomia e ontogênese das sementes de espécies de Banisteriopsis C.B. Robinson e Diplopterys A. Juss. (Malpighiaceae) Acta Botanica Brasilica. 2008;22:733–740. [Google Scholar]
- Steinmann VW. New Euphorbiaceae from Mexico. II. Contributions from the University of Michigan Herbarium. 2005;24:173–187. [Google Scholar]
- Stenar H. Zur Embryosackentiwicklung einiger Malpighiazeen. Botaniska Notiser. 1937;1937:110–118. [Google Scholar]
- Stephens EL. The embryo-sac and embryo of certain Penaeaceae. Annals of Botany. 1909;23:363–378. [Google Scholar]
- Stevens PF. The Old World species of Calophyllum (Guttiferae). I. The Mascarene species. Journal of the Arnold Arboretum. 1976;57:167–184. [Google Scholar]
- Stevens PF. Angiosperm phylogeny website, version 12, July 2012 [and more or less continuously updated since] 2001 onwards Available at http://www.mobot.org/MOBOT/research/APweb/ [Google Scholar]
- Stevens PF. Clusiaceae-Guttiferae. In: Kubitzki K, editor. The families and genera of vascular plants. Berlin: Springer; 2007. pp. 48–66. 9. [Google Scholar]
- Steyermark JA, Luteyn JL. Revision of the genus Ochthocosmus (Linaceae) Brittonia. 1980;32:128–143. [Google Scholar]
- Steyn EMA, van Wyk AE, Smith GF. Embryology and systematic relationships of Kiggelaria (Flacourtiaceae) Bothalia. 2003;33:199–206. [Google Scholar]
- Steyn EMA, Smith GF, van Wyk AE. Functional and taxonomic significance of seed structure in Salix mucronata (Salicaceae) Bothalia. 2004;34:53–59. [Google Scholar]
- Steyn EMA, van Wyk AE, Smith GF. Ovule and seed structure in Scolopia zeyheri (Scolopieae) with notes on the embryology of Salicaceae. Bothalia. 2005;35:175–183. [Google Scholar]
- Sutter D, Endress PK. Aspects of gynoecium structure and macrosystematics in Euphorbiaceae. Botanische Jahrbücher für Systematik. 1995;116:517–536. [Google Scholar]
- Sweeney PW. Phylogeny and floral diversity in the genus Garcinia (Clusiaceae) and relatives. International Journal of Plant Sciences. 2008;169:1288–1303. [Google Scholar]
- Takhtajan A. Diversity and classification of flowering plants. New York: Columbia University Press; 1997. [Google Scholar]
- Tateishi S. On the development of the embryo sac and fertilization of Acalypha australis. Botanical Magazine Tokyo. 1927;41:477–485. [Google Scholar]
- Thiv M, Ghogue J-P, Grob V, Huber K, Pfeifer E, Rutishauser R. How to get off the mismatch at the generic rank in African Podostemaceae. Plant Systematics and Evolution. 2009;283:57–77. [Google Scholar]
- Tobe H, Raven PH. An embryological contribution to systematics of the Chrysobalanaceae I. Tribe Chrysobalaneae. Botanical Magazine Tokyo. 1984;97:397–411. [Google Scholar]
- Tobe H, Raven PH. Embryology of the Irvingiaceae, a family with uncertain relationships among the Malpighiales. Journal of Plant Research. 2011;124:577–591. doi: 10.1007/s10265-010-0393-7. [DOI] [PubMed] [Google Scholar]
- Tokuoka T, Tobe H. Embryology and systematics of Euphorbiaceae sens. lat.: a review and perspective. Journal of Plant Research. 1995;108:97–106. [Google Scholar]
- Tokuoka T, Tobe H. Ovules and seeds in Crotonoideae (Euphorbiaceae): structure and systematic implications. Botanische Jahrbücher für Systematik. 1998;120:165–186. [Google Scholar]
- Tokuoka T, Tobe H. Embryology of tribe Drypeteae, an enigmatic taxon of Euphorbiaceae. Plant Systematics and Evolution. 1999;215:189–208. [Google Scholar]
- Tokuoka T, Tobe H. Ovules and seeds in subfamily Phyllanthoideae (Euphorbiaceae): structure and systematic implications. Journal of Plant Research. 2001;114:75–92. [Google Scholar]
- Tokuoka T, Tobe H. Ovules and seeds in Euphorbioideae (Euphorbiaceae): structure and systematic implications. Journal of Plant Research. 2002;115:361–374. doi: 10.1007/s10265-002-0047-5. [DOI] [PubMed] [Google Scholar]
- Tokuoka T, Tobe H. Ovules and seeds in Acalyphoideae (Euphorbiaceae): structure and systematic implications. Journal of Plant Research. 2003;116:355–380. doi: 10.1007/s10265-003-0116-4. [DOI] [PubMed] [Google Scholar]
- Tokuoka T, Tobe H. Phylogenetic analyses of Malpighiales using plastid and nuclear DNA sequences, with particular reference to the embryology of Euphorbiaceae sens. str. Journal of Plant Research. 2006;119:599–616. doi: 10.1007/s10265-006-0025-4. [DOI] [PubMed] [Google Scholar]
- van Heel WA. Anatomical and ontogenetic investigations on the morphology of the flowers and the fruit of Scyphostegia borneensis Stapf (Scyphostegiaceae) Blumea. 1967;15:107–125. [Google Scholar]
- Vega AS, Castro MA, Anderson WR. Occurrence and phylogenetic significance of latex in the Malpighiaceae. American Journal of Botany. 2002;89:1725–1729. doi: 10.3732/ajb.89.11.1725. [DOI] [PubMed] [Google Scholar]
- Vogel S. Ölblumen und ölsammelnde Bienen. Tropische und Subtropische Pflanzenwelt. 1974;7:283–547. [Google Scholar]
- Vogel S. History of the Malpighiaceae in the light of pollination ecology. Memoirs of the New York Botanical Garden. 1990;55:130–142. [Google Scholar]
- Webster GL. Classification of the Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994a;81:3–32. [Google Scholar]
- Webster GL. Synopsis of the genera and suprageneric taxa of Euphorbiaceae. Annals of the Missouri Botanical Garden. 1994b;81:33–144. [Google Scholar]
- Winkler H. Linaceae. In: Engler A, Prantl K, editors. Die natürlichen Pflanzenfamilien. 2nd edn. Leipzig: Engelmann; 1931. pp. 82–130. 19a. [Google Scholar]
- Wurdack KJ, Davis CC. Malpighiales phylogenetics: gaining ground on one of the most recalcitrant clades in the angiosperm tree of life. American Journal of Botany. 2009;96:1551–1570. doi: 10.3732/ajb.0800207. [DOI] [PubMed] [Google Scholar]
- Xi Z-X, Ruhfel BR, Schaefer H, et al. Phylogenomics and a posteriori data partitioning resolve the Cretaceous angiosperm radiation Malpighiales. Proceedings of the National Academy of Sciences of the USA. 2012;109:17519–17524. doi: 10.1073/pnas.1205818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L-B, Simmons MP. Phylogeny and delimitation of the Celastrales inferred from nuclear and plastid genes. Systematic Botany. 2006;31:122–137. [Google Scholar]
- Zhang N, Zeng LP, Shan HY, Ma H. Highly conserved low-copy nuclear genes as effective markers for phylogenetic analyses in angiosperms. New Phytologist. 2012;195:923–937. doi: 10.1111/j.1469-8137.2012.04212.x. [DOI] [PubMed] [Google Scholar]
- Zhang W-H, Kramer EM, Davis CC. Similar genetic mechanisms underlie the parallel evolution of floral phenotypes. PLoS one. 2012;7:e36033. doi: 10.1371/journal.pone.0036033. http://dx.doi.org/10.1371/journal.pone.0036033 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z-G, Meng A-P, Li J-Q, Ye Q-G, Wang H-C, Endress PK. Floral development of Phyllanthus chekiangensis (Phyllanthaceae), with special reference to androecium and gynoecium. Plant Systematics and Evolution. 2012;298:1229–1238. [Google Scholar]
- Zhu XY, Chase MW, Qiu YL, Kong HZ, Dilcher D, Chen ZD. Mitochondrial matR sequences help to resolve deep phylogenetic relationships in rosids. BMC Evolutionary Biology. 2007;7:217. doi: 10.1186/1471-2148-7-217. http://dx.doi.org/10.1186/1471-2148-7-217 . [DOI] [PMC free article] [PubMed] [Google Scholar]