Abstract
Background and Aims
The germination test currently represents the most used method to assess seed viability in germplasm banks, despite the difficulties caused by the occurrence of seed dormancy. Furthermore, seed longevity can vary considerably across species and populations from different environments, and studies related to the eco-physiological processes underlying such variations are still limited in their depth. The aim of the present work was the identification of reliable molecular markers that might help in monitoring seed deterioration.
Methods
Dry seeds were subjected to artificial ageing and collected at different time points for molecular/biochemical analyses. DNA damage was measured using the RAPD (random amplified polymorphic DNA) approach while the seed antioxidant profile was obtained using both the DPPH (1,1-diphenyl, 2-picrylhydrazyl) assay and the Folin–Ciocalteu reagent method. Electron paramagnetic resonance (EPR) provided profiles of free radicals. Quantitative real-time polymerase chain reaction (QRT-PCR) was used to assess the expression profiles of the antioxidant genes MT2 (type 2 metallothionein) and SOD (superoxide dismutase). A modified QRT-PCR protocol was used to determine telomere length.
Key Results
The RAPD profiles highlighted different capacities of the two Silene species to overcome DNA damage induced by artificial ageing. The antioxidant profiles of dry and rehydrated seeds revealed that the high-altitude taxon Silene acaulis was characterized by a lower antioxidant specific activity. Significant upregulation of the MT2 and SOD genes was observed only in the rehydrated seeds of the low-altitude species. Rehydration resulted in telomere lengthening in both Silene species.
Conclusions
Different seed viability markers have been selected for plant species showing inherent variation of seed longevity. RAPD analysis, quantification of redox activity of non-enzymatic antioxidant compounds and gene expression profiling provide deeper insights to study seed viability during storage. Telomere lengthening is a promising tool to discriminate between short- and long-lived species.
Keywords: Antioxidant potential, ex situ seed longevity, QRT-PCR, RAPD, Silene vulgaris subsp. vulgaris, Silene acaulis subsp. acaulis, telomere
INTRODUCTION
Seed longevity is a relevant trait from an ecological perspective, playing a crucial role in safeguarding vulnerable plant species which are preserved ex situ in seed banks (Li and Pritchard, 2009; Probert et al., 2009). It is generally acknowledged that seeds stored for prolonged periods are subjected to severe oxidative damage, caused by the progressive accumulation of reactive oxygen species (ROS) and that loss of seed viability and reduced germination represent the undesired consequences of ageing (Kranner et al., 2010). Significant factors in seed longevity are the level of DNA damage and the DNA repair response, the amount of non-enzymatic antioxidants and activity of ROS-scavenging enzymes (Rajjou and Debeaujon, 2008; Ventura et al., 2012).
In order to preserve the high seed viability at the pre-emergence step, both the DNA repair functions and the overall antioxidant activities must be kept at an appropriate level in the embryo. Different DNA repair pathways are activated during the early phase of seed imbibition, as recently demonstrated by gene profiling studies (Macovei et al., 2010, 2011a, b; Balestrazzi et al., 2011a, 2012). The ability to carry out ROS scavenging, expressed as the seed antioxidant potential, is a critical requirement to withstand stress and improve germination (Liu et al., 2007). The cell antioxidant systems prevent ROS attack but, when ROS production exceeds the capacity of the antioxidant machinery, oxidative injury takes place.
Factors such as temperature and humidity are positively correlated with seed ageing and they must be strictly controlled during seed manipulation for long-term conservation in seed banks (Walters et al., 2005). To date, germination tests represent the most reliable method to assess seed viability (Smith et al., 2003) although it is a time-consuming and labour-intensive operation. For this reason, novel low-cost and equally reliable methods are required, which might speed up the seed viability analysis. Molecular and biochemical markers of seed ageing might be used for these purposes. A deeper understanding of the complex network of molecular events which control seed longevity is, however, required in order to select appropriate markers providing information on deterioration and germination potential of seed stocks collected for bank storage.
The effects of ageing on DNA integrity can be investigated using the random amplification of polymorphic DNA (RAPD), a simple and economical procedure for detecting DNA polymorphysms. Polymorphic RAPD profiles were detected in soybean (Glycine max) seeds of different ages (Bednarek et al., 1998), while no differences were observed between seeds subjected to artificial and natural ageing (Marcos-Filho and McDonald, 1998). More recently, Vijay et al. (2009) reported changes in DNA profiles of soybean and safflower (Carthamus tinctorius) seeds exposed to natural and accelerated ageing, respectively.
An intriguing aspect of ageing is related to the role played by telomeres, nucleoprotein structures located at the end of chromosomes, which are essential to preserve genome integrity. As in animal cells, plant telomere DNA is a dynamic structure subjected to shortening during differentiation and ageing (Kilian et al., 1995). Telomere monitoring has been suggested as a reliable marker of seed ageing. Bucholc and Buchowicz (1992) demonstrated significant differences in the length of telomere sequence between fresh and stored wheat (Triticum aestivum) seeds, despite their similarity in terms of germination percentage.
Several reports have highlighted the relevance of seed antioxidant ability as an indicator of longevity. According to Talai and Sen-Mandi (2010), the total antioxidant potential in fresh harvested seeds of different varieties is under direct genetic control, whereas environmental factors might act in aged seeds. Methodologies based on the use of the 1,1-diphenyl, 2-picrylhydrazyl (DPPH) radical are frequently utilized to assess the radical-scavenging activity of phenolic compounds (Hasan et al., 2009). The antioxidant activity of phenolic compounds is mainly due to their redox properties, which can play an important role in absorbing and neutralizing free radicals, quenching singlet and triplet oxygen or decomposing peroxides.
Antioxidant enzyme activities are also essential to protect the cellular components against oxidative injury. Superoxide dismutase (SOD) catalyses the dismutation of superoxide radicals to hydrogen peroxide and oxygen (Raychadhuri and Deng, 2000). The requirement for SOD activity, which plays a critical role in maintaining ROS at non-toxic levels during germination, has been reported in several plant species (Wojtyla et al., 2006), and in a recent work Lee et al. (2010) demonstrated that the overexpression of the SOD gene in Nicotiana tabacum resulted in protective effects against seed deterioration during ageing.
Metallothioneins (MTs) have recently been proposed to act as ROS scavengers and signal molecules outside and inside the nucleus, highlighting their possible interaction with the DNA repair machinery (Wang et al., 2010; Balestrazzi et al., 2011b). To date, there is only indirect evidence of the putative protective role played by MTs in the nucleus. Balestrazzi et al. (2009) demonstrated that expression of the PsMTA1 gene, encoding an MT-like protein from Pisum sativum, confers protection against oxidative stress in the nucleus, reducing the level of oxidative DNA damage. As for the possible role of MTs in seed physiology, the seed-specific type 4 MTs possess a high capacity to bind Zn ions, compared with other isoforms. In Arabidopsis thaliana, the MT4a and MT4b mRNAs are accumulated during late embryogenesis and disappear rapidly following seed imbibition (Kranner and Colville, 2010). Metallothioneins have also been investigated in the genus Silene, and copper-tolerant populations of Silene vulgaris have significantly higher levels of the SvMT2b transcript, compared with copper-sensitive populations (Mengoni et al., 2001).
Among seeds of different species stored under identical conditions there may be a wide inherent variation of seed longevity (Priestley et al., 1985). Such variation has been reported to be related to taxonomy, seed structure and climate at the geographic origin of the species (Probert et al., 2009; Mondoni et al., 2012a, b). Moreover, Mondoni et al. (2011) found that alpine plants have short-lived seeds in storage compared with those from lowland populations/related taxa, highlighting a significant concern for the successful ex situ conservation of these species. The reduced longevity of seeds of alpine plants was suggested to be caused by low selection pressure for seed resistance to ageing and/or damage occurring during seed development, due to the cool wet conditions of the alpine climate. More detailed studies are therefore required to better understand the processes responsible for the different degree of seed viability in species and populations originating from different environments.
The aim of this investigation was the identification of reliable markers of seed deterioration. The response to DNA damage induced by artificial ageing was compared in seeds of S. vulgaris and S. acaulis inhabiting low- and high-altitude locations of Northern Italy, respectively. A more in-depth investigation which included ROS accumulation profiles, antioxidant capacity and telomere length was carried out, focusing mainly on dry seeds and seeds subjected to rehydration. Previous investigations have demonstrated that these species differ in seed longevity (Mondoni et al., 2011), making them useful candidates to assess novel markers of seed deterioration.
MATERIALS AND METHODS
Seed material
Seeds of Silene vulgaris subsp. vulgaris (Moench) Garke and Silene acaulis (L.) Jacq. subsp. acaulis (http://ww2.bgbm.org/EuroPlusMed/PTaxonDetails.asp?Nameld=104374&PTRefFk=7200000) were collected at the point of natural dispersal from one lowland (44°33′N, 12°16′; 0 m a.s.l.) and one alpine location (46°2′N, 9°31′; 2213 m a.s.l.), respectively, in Northern Italy, between May and September 2010. After collection, seeds were held at the Lombardy Seed Bank (Rossi and Mondoni, 2006) under international seed bank standard conditions of – 20 °C after drying at 15 % RH (relative humidity), 15 °C (FAO/IPGRI, 1994) until use.
Artificial ageing and germination tests
Due to logistical and time constraints in comparing storage life span under seed bank conditions, seed longevity was determined using a standard rapid ageing protocol (Probert et al., 2009), to raise the moisture content of the seeds prior to ageing. In order to minimize the subsequent adjustment of moisture content when samples were transferred to the ageing conditions, ten samples of 50 seeds each were rehydrated at 47 % RH at 20 °C in open glass vials or Petri dishes. The vials/dishes were placed over a non-saturated solution of LiCl (VWR International, Milan, Italy) in distilled water held in a sealed 300 × 300 × 130 mm electrical enclosure box (Ensto UK Ltd, Southampton, UK). At the end of the rehydration period (14 d), seed equilibrium relative humidity (eRH) was checked using a sample of the equilibrating seeds. The eRH was evaluated using a water activity measuring instrument which comprised a hygrometer sensor housed in an AW-DI0 water activity probe, in conjunction with a HygroPalm 3 display unit (Simens VDO, Milan, Italy). Once the test species was judged to have reached equilibrium, samples were transferred to a second electrical enclosure box, over a non-saturated solution of LiCl at 60 % RH placed in a compact incubator (Binder FD53, VWR International) without light at 45 ± 2 °C. One sample (50 seeds for each seed lot) was removed after 1, 2, 5, 9, 20, 30, 50, 75, 100, 120 and 150 d and used for germination tests. Seeds were sown on 1 % distilled water agar held in 90 mm diameter Petri dishes and placed in an LMS 250A cooled incubator (LMS Ltd, Sevenoaks, UK) at a temperature regime previously found to be optimal for germination of that accession. Plates were checked weekly for germination and seeds scored as germinated once the radicle had reached 2 mm. At the completion of each germination test, ungerminated seeds were cut-tested to confirm that they were not viable.
Probit analysis
Probit analysis was carried out on the data obtained from germination tests using GenStat Release 11·1 (VSN International Ltd, Oxford, UK) to estimate the time for viability to fall to 50 % (p50) by fitting the viability equation (Ellis and Roberts, 1980):
where v is the viability (in normal equivalent deviates, NED) of the seed lot after p days in storage, Ki is the initial viability (NED) of the seed-lot and σ is the time (d) for viability to fall by 1 NED (i.e. the standard deviation of the normal distribution of seed deaths over time). Analysis of residual deviance (variance ratio test following the F-distribution) was used to test for significance when constraining survival curve data for multiple seed lots to common estimates for Ki and/or σ.
Electron paramagnetic resonance (EPR)
Samples (15 seeds) of S. vulgaris and S. acaulis (8·5 and 4·3 mg, respectively) were inserted in quartz tubes and the EPR spectra were recorded with a Bruker EMX/12 spectrometer (Bruker BioSpin GmbH, Karlsruhe, Germany) operating in X-band, at room temperature and under RH conditions dictated by the silica gel used as a desiccant. Spectra were recorded with the same instrumental settings, under conditions far from saturation and performing accumulations for low intensity signals. Power saturation curves were obtained by plotting the EPR signal intensity against the square root of microwave power applied to the sample. The comparison between the two species was done by considering the areas of the spectra recorded at 0·01 mW, well away from saturation conditions. The areas of the EPR signals were calculated by double integration and normalized to seed weight and number of accumulations. The g-values were calculated with reference to a standard of Cr3+/MgO with g = 1·9797.
DNA extraction and RAPD analysis
Genomic DNA was extracted and purified from dried, rehydrated and artificially aged Silene seeds using the NucleoSpin® Plant II kit (Macherey-Nagel, Duren, Germany), according to the manufacturer's instructions. For each sample, 100 mg aliquots (corresponding to approx. 100 seeds) were used. RAPD analysis was performed as follows. Twenty Operon primers (Eurofins MWG Operon, Ebersberg, Germany) were used to screen the variation among differently aged groups of Silene seeds. The RAPD–polymerase chain reaction (PCR) mixture consisted of 50 ng of template DNA, 1× PCR buffer [7·5 mm Tris–HCl pH 9·0, 5·0 mm KCl, 2 mm (NH4)2SO4], 400 µm dNTPs, 0·80 µm 10-mer random primer, 3 mm MgCl2 and Taq polymerase (1 U; Biotools, Madrid, Spain) in a total volume of 25 µL. DNA amplification was carried out on a T Gradient apparatus (Biometra, Goettingen, Germany). Template DNA was initially denaturated at 94 °C for 3 min, followed by 40 cycles (1 min at 94 °C, 1 min at 36 °C and 2 min at 72 °C). Finally, extension was further prolonged for 10 min at 72 °C. For each reaction, three replicated samples were used in two independent experiments. Amplification products were separated by electrophoresis on 1·5 % agarose gels that were stained with ethidium bromide (0·5 µg L−1; Sigma-Aldrich, Milan, Italy) and visualized under UV light. The 100 bp DNA Ladder and 1 kb DNA Ladder (GeneRuler, Fermentas, Burlington, Canada) were used as molecular standards.
The reproducible RAPD bands were scored in binary characters and coded accordingly (presence = 1, absence = 0). The binary data obtained were used to estimate Nei's unbiased genetic identity and genetic distance (Nei, 1972). This matrix was used to cluster samples into a dendrogram built with unweighted pair group method analysis (UPGMA). The analysis was performed using the POPGENE32 software.
Seed extract, DPPH test and determination of phenolic content
Seed extracts were prepared as described by Li et al. (2008). Seeds (60 mg) were reduced to fine powder using a pestle and a mortar; the sample was recovered by adding 1·2 mL of acetone:water (70:30; v/v) and then transferred to a microtube and incubated overnight at 23 °C under gentle shaking. Subsequently, the sample was centrifuged and the supernatant was recovered and stored at –20 °C.
DPPH (1,1-diphenyl-2-picrylhydrazyl) and the reference antioxidant (standard) ascorbic acid were obtained from Sigma-Aldrich (Milan, Italy). The free radical-scavenging activity of seed extracts was determined as described by Braca et al. (2001). The seed extract (100 µL) was added to 3 mL of a solution containing 100 µm DPPH dissolved in methanol. The reaction was carried out in the dark for 20 min at room temperature. For each sample, absorbance at λ = 517 nm was measured using a V-530 spectrophotometer (Jasco Europe S.r.l., Cremella, Italy). Reduction of DPPH was calculated from [(A0 – A1)/A0] × 100 where A0 is the absorbance of the control without seed extract and A1 is the absorbance of the extract/standard. A standard curve was built using ascorbic acid with concentrations in the 0·1–1·5 mm range, and the total antiradical capacity (TAC) was expressed as ascorbate equivalents (AEs) mg−1 d. wt.
The amount of total phenolic compounds was determined using the Folin–Ciocalteu reagent (Spanos and Wrolstad, 1990) and reported as gallic acid equivalents (GAEs) mg−1 d. wt by reference to a standard curve. The seed extract (20 µL) was mixed with deionized H2O (1·58 mL) and with the Folin–Ciocalteu reagent (100 µL; Sigma-Aldrich). The sample was incubated for 1–8 min and the reaction was then neutralized with 300 µL of a sodium carbonate solution (w/v) (Na2CO3, 20 %; Sigma-Aldrich). The sample was incubated for 30 min at 40 °C in the dark. The absorption of the resulting blue colour was measured at λ = 765 nm, using a V-530 spectrophotometer (Jasco Europe S.r.l.). A calibration curve was built, using gallic acid with concentrations in the 50–400 mg L−1 range. The specific antioxidant capacity, defined as the ratio between the total antiradical capacity and the total phenolic content, was expressed as μg AE mg−1 GAE.
Quantitative real-time PCR (QRT-PCR) for gene expression analysis
RNA extraction was carried out using the Aurum Total RNA Fatty and Fibrous Tissue kit (Bio-Rad, Milan, Italy) and cDNA synthesis was then performed with the iScript cDNA Synthesis kit (Bio-Rad). QRT-PCR was carried out using the SsoFast™ EvaGreen® Supermix (Bio-Rad) and a Rotor-Gene 6000 PCR apparatus (Corbett Robotics, Brisbane, Australia). The Silene housekeeping gene EF1α (elongation factor 1α) (GenBank accession no. GH294012) was used as standard control in the QRT-PCRs since it was reported to have the most stable expression among a wide range of conditions represented by different tissues and treatments (Nicot, 2005; Han et al., 2012). To analyse the expression profiles of the Silene SOD (GenBank accession no. GH293101) and MT2 (type 2 MT) (GenBank accession no. AF101825) genes, the oligonucleotide primers were designed using the Real Time PCR Primer Design program from GenScript (Table 1). QRT-PCR conditions were as follows: denaturation at 94 °C for 2 min, cycling at 94 °C (15 s), 58 °C (15 s), 72 °C (30 s). For each primer set, a no-template control was used. The QRT-PCR outputs of three biological replicates per sample and gene were analysed using the LinRegPCR and REST 2009 computer software (Pfaffl et al., 2002; Ramakers et al., 2003). For each set of PCRs, the logarithms of the initial fluorescence (No) was calculated based on the individual PCR efficiency. For each reaction, the No values were normalized to the mean value of untreated replicated samples and then used for graphic representation.
Table 1.
Sequences of oligonucleotide primers utilized in QRT-PCR
| Gene | Forward primer | Reverse primer | Efficiency* |
|---|---|---|---|
| SOD | 5'-GAAGGAGATGGTCCAACAACT-3' | 5'-TGTTTCCAAGGTCACCAGCA-3' | 1·79 |
| MT | 5'-TGTGGATCTGCCTGCAAGT-3' | 5'-ACACCCATTTCCATCTCTGC-3' | 1·70 |
| EF1 | 5'-TAACGGTTATGCCCCAGTTC-3' | 5'-AGAAGGTCTCGACAACCATG-3' | 1·66 |
*Efficiency of the primer pair in QRT-PCR
Quantitative real-time PCR for telomere length analysis
Telomere length was evaluated with a QRT-PCR assay as described by Cawthon (2002) with the following modifications. In order to calculate the telomere absolute length, a standard curve was established using a 98-mer oligonucleotide containing 14 repeats of the plant telomere sequence TTTAGGG which was serially diluted from 10 ng to 10 pg. Telomere absolute length was expressed as log[TL(kb)], logarithm of telomere length (kb). Each reaction was performed in triplicate in a total volume of 25 µL containing 50 ng of DNA, 1× Maxima SybrGreen Master Mix (Fermentas, Burlington, Canada), 500 nm telomere forward primer (5′-CGGTTTGTTGTGGGTTGTGGGTTGTGGGTTGTGGGTTGTGGGTT-3′), 600 nm telomere reverse primer (5′- GGCTTGTCCTGACCCTTGACCCTTGACCCTTGACCCTTGACCCT-3′) and 0·5 % dimethylsulfoxide (DMSO; Sigma-Aldrich). The following cycle conditions were used: denaturation at 95 °C for 10 min; 15 s at 95 °C, 30 s at 56 °C and 1 min at 72 °C (40 cycles). Reactions were carried out in triplicate in a Rotor-Gene 6000 PCR apparatus (Corbett Robotics, Brisbane, Australia) and results were interpreted using the LinRegPCR computer software (Ramakers et al., 2003).
Statistical analysis
Experiments were repeated three times and carried out in triplicate. Data are expressed as means ± standard deviation (s.d.) values. Differences observed in the S. vulgaris seeds as concerns the free radical-scavenging activity, total phenolic content, specific antioxidant activity, SOD and MT transcript accumulation, and telomere length, compared with the S. acaulis seeds were evaluated statistically. Statistical significance of differences was determined using Student's t-test (*P < 0·05, **P < 0·01, ***P < 0·001).
RESULTS
Effects of artificial ageing on the germination efficiency of S. vulgaris and S. acaulis seeds
When S. vulgaris and S. acaulis seeds were subjected to artificial ageing, significant differences were observed in terms of germination. In both the tested seed lots, germination declined as the period of experimental storage increased (Fig. 1). The two species differed in the time period required for seed germination to drop to 50 % of the T0 value (p50) (approx. 35 compared with 63 d, Fig. 1). Variation in p50 values between the seed lots of S. vulgaris and S. acaulis was explained by differences in the initial seed viability (Ki; F1,9 = 15·71; P < 0·01) and by differences in the rate at which seeds lost the ability to germinate (1/σ, F1,8 = 34·89; P < 0·01). The reported data show the reduced seed germinability of the S. acaulis species.
Fig. 1.
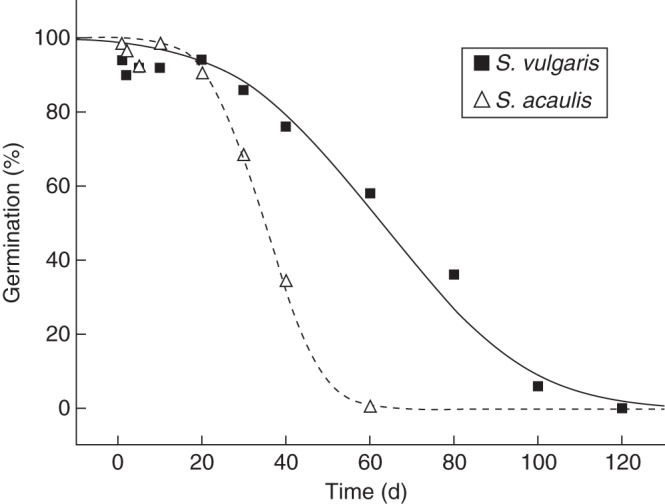
Survival curves fitted by probit analysis for Silene acaulis and S. vulgaris seed lots. Seeds were subjected to experimental storage conditions at 45 °C, 60 % RH. All non-germinated seeds were cut-test at the end of the test and found to be dead.
ROS profiles in Silene dry seeds collected from low and high altitude locations
Electron paramagnetic resonance provides a unique opportunity for studying the reactive radical species, since it allows information to be acquired not only about ROS levels but also about the structure of the molecular environment surrounding the radical core. The EPR spectra of S. acaulis and S. vulgaris dry seeds are shown in Fig. 2A. Both spectra show a single peak characterized by a weak axial anisotropy with the peak to peak line width of 0·7 mT and g// = 2·0025 g⊥ = 2·0046 for S. acaulis and the line width of 0·5 mT and g// = 2·0027 g ⊥ = 2·0047 for S. vulgaris. The comparison of normalized areas indicates that the concentration of radicals in S. acaulis is about 64 % of that found in S. vulgaris.
Fig. 2.
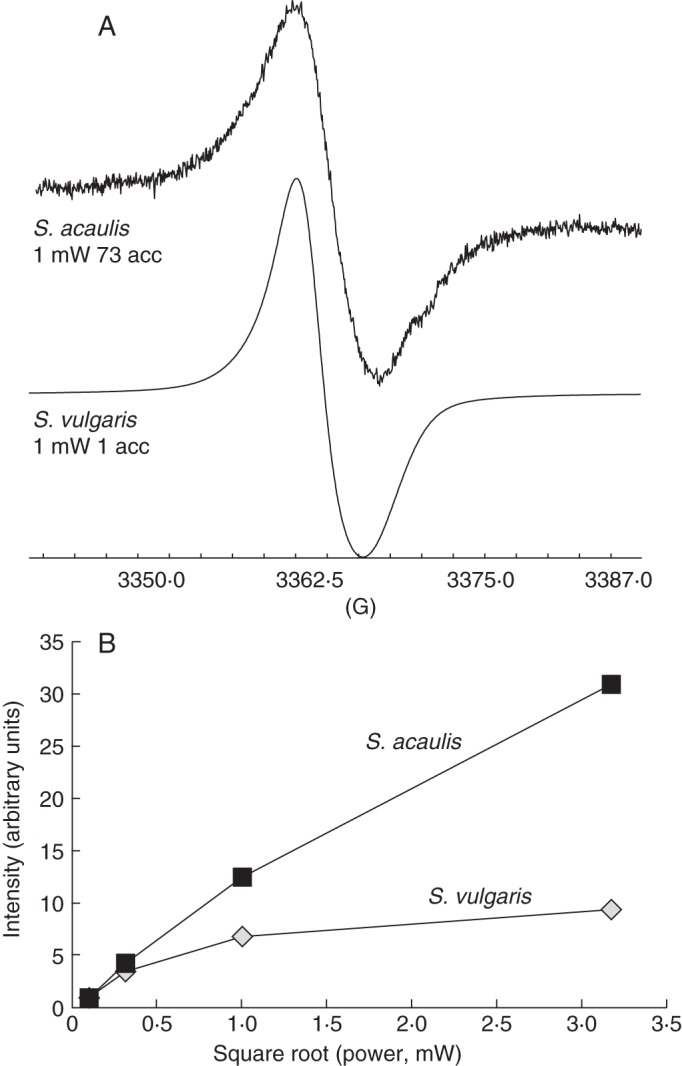
(A) EPR spectra of Silene acaulis and Silene vulgaris dry seeds. The instruments settings were 1 mW for microwave power, a modulation amplitude of 0·2 mT, and ten and five accumulations for S. acaulis and S. vulgaris, respectively. (B) Power saturation curves of S. acaulis and S. vulgaris dry seeds. The areas of the spectra at 0·01 mW have been normalized to unity.
In order to better characterize the profiles of S. acaulis and S. vulgaris dry seeds, the samples were exposed to increasing microwave power and the resulting saturation behaviour was analysed (Fig. 2B). The areas of the EPR signals of S. acaulis and S. vulgaris dry seeds at the lowest microwave power were normalized for direct comparison of the power saturation curves. As shown in Fig. 2B, the signal recorded with the S. acaulis seeds increased progressively with the increasing microwave power, reaching an intensity of 5·0, 12·5 and 30·0 a.u. (arbitrary units) when 0·1, 1·0 and 10·0 mW power was provided. In contrast, the signal recorded with the S. vulgaris seeds increased up to 7·0 a.u. in response to 1·0 mW power and subsequently the signal intensity remained almost constant when a microwave power in the range 1–10 mW was applied. According to EPR analysis, the two Silene species showed a different saturation behaviour.
Effects of rehydration and artificial ageing on the RAPD profiles of S. vulgaris and S. acaulis seeds
Silene vulgaris and S. acaulis seeds collected from low- and high-altitude locations, respectively, were subjected to artificial ageing, and samples collected at the indicated time points (0, 5, 10, 20, 30 and 60 d) were used for molecular analyses. Dry seeds were also analysed, and the results were compared with those obtained from both the rehydrated and artificially aged seeds.
The RAPD profiles of S. vulgaris and S. acaulis seeds were analysed using five and four primers, respectively, out of 20 random primers screened individually in both species. In S. vulgaris, five primers (OPA-9, OPA-16, OPA-17, OPA-18 and OPA-19) resulted in the amplification of bands when tested with DNA extracted from dry seeds and they produced polymorphic profiles. In the case of S. acaulis, four out of 20 primers (OPA-9, OPA-16, OPA-18 and OPA-19) revealed polymorphisms with dry and artificially aged seeds. Only four primers (OPA-9, OPA-16, OPA-18 and OPA-19) were able to amplify DNA bands in both species. The RAPD primers generated a total of 32 and 26 polymorphic fragments in S. vulgaris and S. acaulis, respectively (Table 2).
Table 2.
RAPD primers producing bands with the Silene seed DNAs
| Species | Bands | OPA-9 | OPA-16 | OPA-17 | OPA-18 | OPA-19 |
|---|---|---|---|---|---|---|
| S. vulgaris | Amplified | 16 | 7 | 8 | 9 | 8 |
| Polymorphic | 10 | 3 | 7 | 6 | 6 | |
| S. acaulis | Amplified | 12 | 6 | – | 7 | 4 |
| Polymorphic | 12 | 6 | – | 4 | 4 |
For each primer, the number of amplified bands and the number of bands resulting from polymorphic sites are indicated.
As for S. vulgaris, the UPGMA tree (Nei 1972) calculated from the RAPD data, divided the seed samples into three clusters (Fig. 3A). Both the dry seeds and the rehydrated seeds are grouped into one cluster, indicating that in S. vulgaris the RAPD profiles were not affected by rehydration, despite the fact that this step was carried out over 15 d. This finding might suggest that low levels of DNA damage and genomic instability are accumulated during rehydration in the Silene species originating from a low-altitude environment.
Fig. 3.
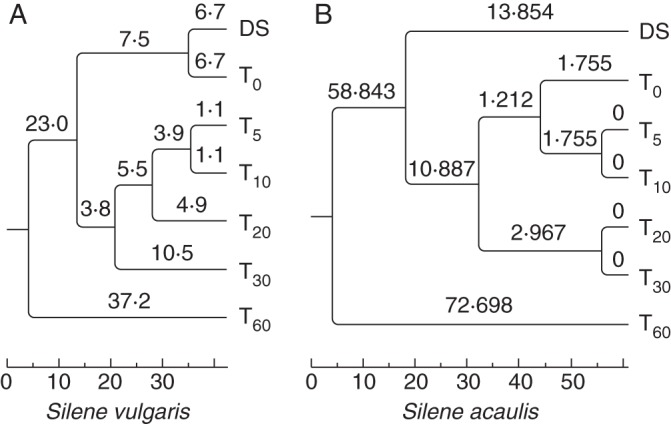
UPGMA dendrograms corresponding to the RAPD profiles of dry seeds (DS) and artificially aged seeds belonging to (A) Silene vulgaris and (B) S. acaulis. Following rehydration, analyses were carried out at different time points (days) during artificial ageing (T0, T5, T10, T20, T30 and T60).
When considering artificially aged seeds, samples collected at 5, 10, 20 and 30 d are all grouped in the second cluster (Fig. 3A). This result is expected, since the level of DNA damage was enhanced as a consequence of artificial ageing. DNA alterations progressively accumulate during artificial ageing and, although the T5 and T10 samples are grouped together within the second cluster, later on the genetic distance further increases since T20 and T30 samples are represented as single groups.
Also in the case of S. acaulis, the UPGMA tree calculated from RAPDs data separated the seed samples into three cluster (Fig. 3B). However, the dry seeds are grouped in a single cluster, while the rehydrated seeds and the artificially aged seeds are grouped in the second cluster. This finding suggests that the S. acaulis seeds undergo severe DNA rearrangements during the prolonged rehydration period. The results suggest that the Silene species originating from the high-altitude environment is less effective in controlling DNA damage, compared with S. vulgaris. When considering the artificially aged seeds, the T5 and T10 samples are grouped together within the second cluster and subsequently the genetic distance further increases. In contrast to S. vulgaris, the T20 and T30 samples are grouped together. As previously reported for S. vulgaris, the extent of DNA damage was more pronounced at 60 d, as also indicated by the RAPD analysis, resulting in a visible smear after agarose gel electrophoresis (data not shown).
Cladograms of genetic distance obtained by RAPD analyses carried out in S. vulgaris and S. acaulis indicate distinct trends associated with rehydration and artificial ageing, showing the different capacities of the two species to overcome the problem of DNA damage. The reported data strengthen the use of RAPD as a reliable tool for investigating seed quality as affected by prolonged storage.
Correlation between the radical-scavenging ability and the content of total phenolics in Silene seed extracts
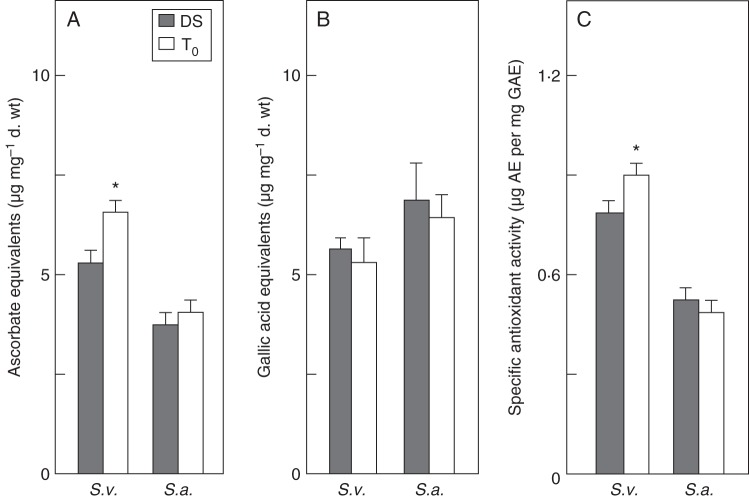
Besides the basal antioxidant features of the dehydrated seed, enhanced protection against radical species also results from the activation of ROS-scavenging mechanisms during imbibition. For this reason, the antioxidant profile of Silene seeds was analysed with biochemical assays in the dry and rehydrated seed samples. The radical-scavenging ability of S. vulgaris and S. acaulis seed extracts was measured using the DPPH test, expressed as μg AE mg−1 d. wt. For each species, extracts from dry seeds and rehydrated seeds were analysed and compared, as shown in Fig. 4A. In the case of S. vulgaris, the estimated DPPH-scavenging activity of dry seed and rehydrated seed extracts was 5·62 ± 0·12 and 6·43 ± 0·11 µg AE mg−1 d. wt, respectively. When compared with S. vulgaris, both the dry and rehydrated seed extracts of S. acaulis, revealed significantly (P < 0·0025) lower values of DPPH-scavenging activity, corresponding to 4·24 ± 0·01 and 4·33 ± 0·22 µg AE mg−1 d. wt, respectively (Fig. 4A). This might suggest that the S. acaulis seeds, collected at high altitude, possess a limited antioxidant response while, interestingly, the highest DPPH-scavenging ability was found in the extracts from rehydrated seeds of the Silene species living at low altitude. Furthermore, in the case of S. acaulis, no significant differences in the radical-scavenging activity of dry and rehydrated seeds were detected.
Fig. 4.
Antioxidant properties of Silene vulgaris (S.v.) and S. acaulis (S.a.) seed extracts. (A) DPPH radical-scavenging activity expressed as μg of ascorbate equivalents per mg of dry weight. (B) The total phenolics content, evaluated using the Folin–Ciocalteu reagent method, expressed as gallic acid equivalents per mg of dry weight. (C) Specific antioxidant activity is expressed as μg ascorbate equivalents per mg gallic acid equivalents. Values are expressed as means ± s.d. of three independent experiments. Statistical significanc: *P < 0·05; **P < 0·01; ***P < 0·001, compared with S. acaulis. DS, dry seeds. T0, rehydrated seeds.
Since the reduction of DPPH can result from the radical-scavenging activity of phenolic compounds, such as flavonoids, polyphenols, tannins and phenolic terpenes, the total content of phenolic compounds was measured in the Silene seed extracts. Results from this analysis are shown in Fig. 4B. The estimated content of total phenolic compounds in S. vulgaris seed extracts in the dry and rehydrated state was 6·59 ± 0·00 and 6·46 ± 0·36 µg GAE mg−1 d. wt, respectively. Apparently, no significant differences were observed in the amount of total phenolics in both dry and rehydrated seed extracts of the species from the low-altitude location. As for S. acaulis, similar results were obtained, since the content of total phenolic compounds in the dry seed extract (7·31 ± 0·93 µg GAE mg−1 d. wt) did not differ significantly from that measured in the rehydrated seed extract (6·86 ± 0·25 µg GAE mg−1 d. wt) (Fig. 4B).
Finally, results from the DPPH test and from the Folin–Ciocalteu reagent method were combined to calculate the specific antioxidant activity of the Silene seeds, expressed as μg AE mg−1 GAE (Fig. 4C). Based on this calculation, the specific antioxidant activity of seed extracts was significantly enhanced in the S. vulgaris species found in a low-altitude location, compared with the species collected at high altitude. As shown in Fig. 4C, in S. vulgaris the specific antioxidant activity was 0·86 ± 0·00 µg AE mg−1 GAE in the dry seed extract and it further increased, up to 0·98 ± 0·03 µg AE mg−1 GAE, in rehydrated seeds. On the other hand, the dry seed extract of S. acaulis revealed a specific antioxidant activity of 0·62 ± 0·02 µg AE mg−1 GAE and no significant differences were observed in rehydrated seeds which showed an estimated value of 0·58 ± 0·03 µg AE mg−1 GAE.
The reported data suggest that the two Silene species possess different antioxidant capacities, the high-altitude species being characterized by a lower radical-scavenging activity. Furthermore, it seems that the high content of total phenolic compounds observed in the dry seed extract of S. acaulis does not support the radical-scavenging ability.
Distinct expression profiles of the SOD and MT2 genes in S. vulgaris and S. acaulis seeds
The expression profiles of the SOD and MT2 genes encoding the cytosolic SOD and a type 2 MT, respectively, were chosen as molecular indicators of the antioxidant response in Silene seeds. The expression patterns of the SOD and MT2 genes were investigated only in dry seeds and rehydrated seeds since imbibition appears to be a critical step for the activation of antioxidant genes that provide protection during germination. Results from these experiments are shown in Fig. 5. As regards S. vulgaris, both genes were up-regulated during seed rehydration. The level of SOD transcript was significantly (P < 0·05) increased (up to 15-fold) in rehydrated seeds, compared with dry seeds, while the MT2 gene expression was enhanced up to 100-fold (Fig. 5). In S. acaulis, no fluctuations in the amount of the SOD mRNA was observed in dry seeds, compared with rehydrated seeds, while the level of MT transcript significantly increased (up to 58-fold ) in the rehydrated seeds of S. acaulis (Fig. 5).
Fig. 5.
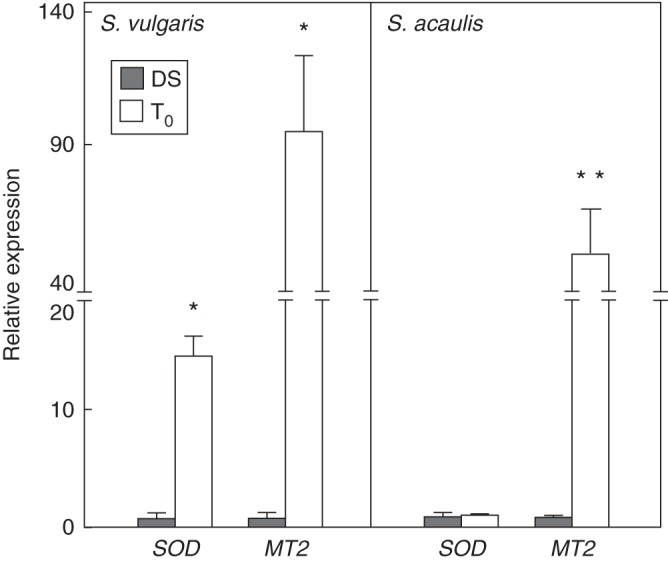
Expression profiles of the SOD and MT genes in Silene seeds were evaluated by QRT-PCR. Values are expressed as means ± s.d. of three independent experiments. Statistical significance: *P < 0·05; **P < 0·01; ***P < 0·001, compared with S. acaulis. DS, dry seeds; T0, rehydrated seeds.
The QRT-PCR analysis provided useful information concerning the ability of Silene seeds to activate the antioxidant response at the transcriptional level, and both SOD and MT2 genes might be used to screen other members of the Silene genus.
Telomere length homeostasis in S. vulgaris and S. acaulis seeds
The usefulness of telomere length as a parameter for assessing seed deterioration is still debated, while the current knowledge needs to be expanded. For this reason. telomere length was measured not only in dry and rehydrated seeds of S. vulgaris and S. acaulis but also during artificial ageing, using QRT-PCR. Results from these analyses are shown in Fig. 6.
Fig. 6.
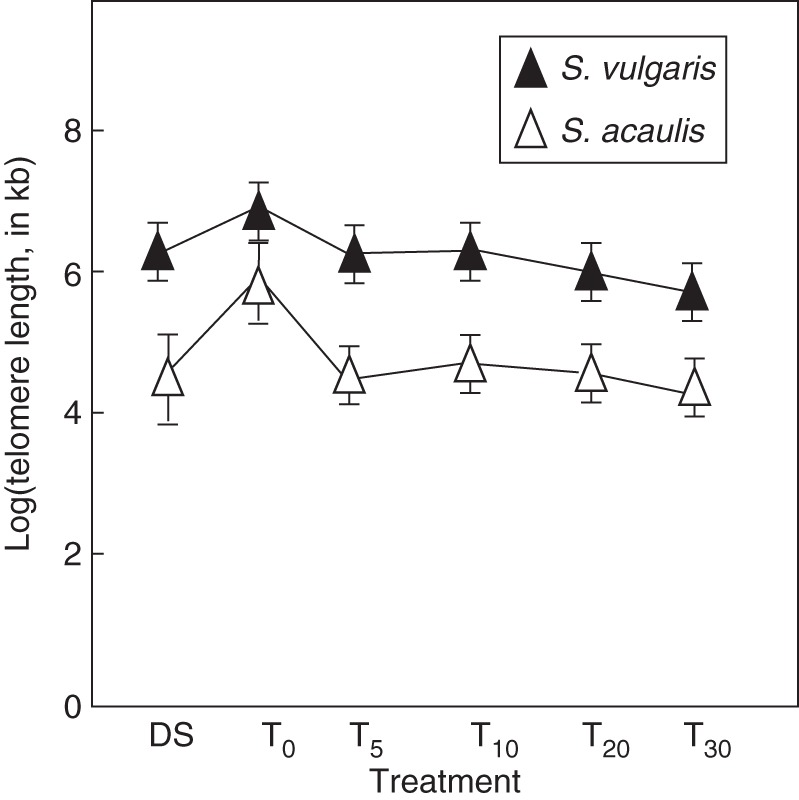
Telomere length measurement by a QRT-PCR-based approach in Silene vulgaris and S. acaulis dry seeds (DS), rehydrated seeds (T0) and artificially aged seeds collected at different time points (5, 10, 20 and 30 d; T5, T10, T20 and T30). Data were log transformed, and values are expressed as means ± s.d. of three independent experiments.
The estimated telomere length in dry seeds was 6·09 ± 0·05 and 5·31 ± 0·19 log[TL(kb)] per reaction in S. vulgaris and S. acaulis, respectively. This finding highlights a significant difference between the two species in telomere length. In both S. vulgaris and S. acaulis, telomere length significantly increased following rehydration. As shown in Fig. 6, the estimated telomere length in S. vulgaris rehydrated seeds was 6·30 ± 0·04 log[TL(kb)], 2-fold higher compared with dry seeds. In the case of S. acaulis, the observed enhancement in telomere length was 5·99 ± 0·13 log[TL(kb)] in rehydrated seeds, 3-fold higher compared with dry seeds.
During artificial ageing, a significant reduction in telomere length was observed in both species compared with rehydrated seeds (Fig. 6). After 5 d of artificial aging (T5), S. vulgaris showed an average telomere length of 6·08 ± 0·05 log[TL(kb)], and subsequently values of 6·19 ± 0·05, 6·14 ± 0·01 and 5·98 ± 0·01 log[TL(kb)] were measured at 10, 20 and 30 d of treatment (T10, T20 and T30). In the case of S. acaulis, the average telomere length was 5·33 ± 0·05 log[TL(kb)] at T5. Subsequently, values of 5·46 ± 0·03, 5·40 ± 0·01 and 5·34 ± 0·04 log[TL(kb)] were measured at 10, 20 and 30 d of treatment (T10, T20 and T30).
The results presented here highlight significant species-specific differences in telomere lengthening between S. vulgaris and S. acaulis. Seed rehydration resulted in telomere lengthening in both Silene species. The dynamics of telomere shortening in relation to seed ageing represent an interesting and novel aspect of seed physiology that deserves more in-depth investigation.
DISCUSSION
The present work addresses some molecular aspects related to the ex situ preservation of short-lived seeds, particularly those originating from high-altitude environments. For seeds stored under identical conditions (60 % RH, 45 °C), there was a significant variation in seed longevity between S. vulgaris and S. acaulis inhabiting low- and high-altitude locations, respectively. The estimated p50 values ranged between 35 d (S. acaulis) and 63 d (S. vulgaris), confirming the findings of Mondoni et al. (2011, 2012) that alpine species are significantly shorter lived than lowland counterparts. Although seeds of both species were collected at the moment of natural seed dispersal and then stored under identical conditions, caution is needed when interpreting differences in seed longevity solely due to the role of species and habitat, as initial seed quality may also play a role.
The Silene seeds showed EPR spectra composed of only one weak singlet line which is due to the presence of radicals with a quinoide nature (Yordanov et al., 2004). The Q-band EPR study carried out by Yordanov et al. (2004) demonstrated the anisotropic nature of this species having g-values compatible with radicals from polyphenols, normally found as antioxidant compounds within plant tissues (Swartz et al., 1972; Nandi et al., 1997). When high and low vigour rice (Oryza sativa L.) dry seeds stored in natural environment were analysed by EPR spectroscopy, loss in viability due to high temperature and humidity correlated with a decrease in the level of free radicals, possibly proposed to be carbon-based quinone derivatives (Nandi et al., 1997). It was hypothesized that cell deterioration in the rice embryonic axis depends on the balance between free radical accumulation and the activity of ROS scavengers, e.g. the antioxidant enzyme SOD. The saturation curves, obtained by challenging the Silene dry seeds with increasing microwave power, provided valuable information on both the radical structure and their surrounding environments. In fact if the radical species are free to move and interact with the other molecules, they are able to absorb more microwave power, thus reaching saturation at higher energy values. In contrast, if the radical species are in a stabilized form, the saturation level is rapidly reached. In the case of S. acaulis, no saturation was observed in the tested power range and this might be due to the highly efficient energy transfer occurring from the excited state to the environment surrounding the radical species. Moreover, the efficiency of energy transfer increases with the number of molecular interactions between the radical species and the surrounding environment. When considering the phenolic compounds and their radicals, the most relevant interaction is mediated by hydrogen bonds and it is directly related to the polarity of the medium, thus to the water content as well as to the presence of polar structures. Increased water content inevitably leads to enhanced radical mobility and decay, and this might explain the finding highlighted by EPR that the S. acaulis dry seeds show a lower radical content, compared with S. vulgaris. On the other hand, the S. vulgaris seeds exposed to increasing microwave power rapidly reached saturation in terms of signal intensity. Such a response might be due to the presence of a non-polar environment surrounding the radical species and, possibly to a reduced water content. The reported data are in agreement with the biochemical profiles provided by the analysis of the radical-scavenging ability and the content of total phenolics in the Silene seeds. They also highlight the potential of EPR technology as an effective and low-cost tool for investigating the radical profiles in seeds and the surroundings of the radicals. A more detailed EPR analysis carried out with individual seeds might help in clarifying the spatial distribution of free radicals as demonstrated for several legume and Brassica species (Hepburn et al., 1986).
In accordance with the observed variation of seed longevity, the additional analyses reported here demonstrate the occurrence of variation in the RAPD profiles of genomic DNA extracted from dry, rehydrated and artificially aged seeds of both species. Thus, the molecular approaches used in the present work allowed discrimination between the seed response to rehydration and artificial ageing.
At the moment, we can only speculate about the extent of DNA repair in the Silene seeds and we do not know how ageing could affect these crucial functions, paving the way to increased global damage. As already highlighted by Vijay et al. (2009), different responses have been reported concerning the qualitative and quantitative distribution of DNA lesions during seed ageing. This is the case of artificially aged rye embryos in which Boubriak et al. (1997) showed an accumulation of DNA nucleosome multimers rather than random fragmentation. Besides this, the occurrence of chromatin remodelling events, e.g. histone modifications (Tanaka et al., 2008; Filkenstein et al., 2008), that might be responsible for the observed RAPD profiles, cannot be ruled out.
In the case of S. vulgaris, both dry and rehydrated seeds are grouped in the same cluster, leaving the artificially aged seeds in a separate cluster. This means that the Silene species originating from a low-altitude environment produces seeds with the ability to withstand the oxidative injury that inevitably takes place during imbibition. This is not the case for S. acaulis, the species from high elevations, since consistent changes in DNA profiles were detected when dry seeds were compared with rehydrated seeds. The relationship between DNA damage and the seed moisture content has been recently established by El-Maarouf et al. (2011) who used RAPD analysis to demonstrate the threshold moisture value at which DNA laddering, a typical hallmark of programmed cell death, occurred. In the artificial ageing experiments, the aim of the rehydration step is to raise the moisture content of the seeds prior to ageing and to minimize the subsequent adjustment of moisture content when samples are transferred to the ageing conditions (Davies and Probert, 2004). In our study, in place of seed moisture content measurement, we have considered the eRH (equilibrium relative humidity) as an alternative, non-destructive method to monitor the level of seed imbibition, when seeds were moved from the drying room (15 % RH) to the rehydration environment (47 % RH).
The present work demonstrates that seeds of S. acaulis from alpine locations are particularly vulnerable to oxidative damage when imbibition is carried out, indicating that seed viability was already affected in this species, before transfer to the artificial ageing environment. Based on this evidence, the timing and/or conditions of the rehydration phase should be reconsidered when comparing short-lived seed lots. From this point of view, the RAPD markers tested here might be used to work out improved rehydration protocols.
It has also been demonstrated that germination efficiency positively correlates with the level of free radical scavenging or antioxidant potential, used as an indicator of seed longevity (Bailly et al., 1998). More recently, Talai and Sen-Mandi (2010) have evaluated the total antioxidant potential of rice (O. sativa L.) seeds and found that freshly harvested seeds with fast germination possess a higher total antioxidant potential, compared with aged seeds. Birtic et al. (2011) demonstrated that the half-cell reduction potentials of low molecular weight thiols, cysteine, cysteinyl-glycine and γ-glutamyl-cysteine, can be used as markers of seed ageing.
According to the reported data, the highest antioxidant activity was recorded in extracts of rehydrated seeds of the low-altitude species S. vulgaris. Furthermore, the high antioxidant activity of the S. vulgaris seed extracts correlated with the amounts of total phenolic compounds, suggesting that, in this species, phenols might act as antioxidant molecules. In contrast, the analyses carried out on the S. acaulis seed extracts revealed that high levels of total phenolic compounds, found in dry seeds, were not associated with increased antioxidant properties. This might be related to the chemical nature of the phenolic molecules accumulated in the S. acaulis seeds. This aspect of seed physiology has been recently highlighted by Mhamdi et al. (2010), who found that the antioxidant activity of seed extracts of Borago officinalis depends on the composition rather than content of total phenolics. Accumulation of phenolics is more generally considered as a protective mechanism of plants against adverse environments, and increased levels of phenolics have been measured during the last stages of seed maturation in relation to increasing temperature (Mhamdi et al., 2010). According to the reported data, the specific antioxidant activity of seed extracts might be a promising viability marker.
The seed antioxidant response can also be evaluated at the molecular level, by monitoring the expression profiles of antioxidant genes. The SOD enzyme, involved in the early scavenging of superoxide radicals, is an essential component of the seed antioxidant system and, accordingly, the SOD gene expression is responsive to seed imbibition under physiological conditions and osmotic stress (Balestrazzi et al., 2010; Macovei et al., 2011b). Moreover Mylona et al. (2007) demonstrated that exposure of maize scutella to ROS-generating xenobiotics results in the accumulation of SOD transcript, which correlates with the increase in the enzyme activity. Recently, Yao et al. (2012) reported the presence of SOD transcript in pea dry seed and accumulation during imbibition. The same authors also observed a decline in SOD expression as a consequence of seed ageing which correlated with reduction in seed germinabilty and viability. Thus they suggest a key role for SOD in mediating the deleterious effects of ageing. In accordance with these observations, the level of SOD transcript is indicative of the antioxidant response in Silene seeds, since significant accumulation was detected only in the low-altitude species. The MT2 gene, encoding a type 2 MT, typically induced by oxidative stress, turned out to be upregulated, even more than the SOD gene, in both species. Although there are no reports on the expression profiles of MT genes in Silene seeds, it is known that the genus Silene includes several heavy metal-tolerant species characterized by high levels of MT transcript (van Hoof et al. 2001; Mengoni et al., 2001). In a recent work, Zhou et al. (2012) demonstrated that transgenic arabidopsis seeds overexpressing the NnMT2a and NnMT3 genes from sacred lotus (Nelumbo nucifera Gaertn.) show improved resistance to accelerated ageing, thus indicating the protective role of MTs.
The two Silene species showed significant differences in their average telomere length. This finding is in agreement with the current literature which highlights the involvement of species-specific factors in the regulation of telomere length. Ecotype-specific telomere lengths have been reported in arabidopsis as a result of genetic and/or epigenetic differences affecting the balance between telomere shortening/extension (Maillet et al., 2006).
A few studies carried out in wheat and rye seeds have shown the correlation between telomere length and seed ageing, demonstrating the fragmentation of chromosomal telomeric sequences during ageing (Bucholc and Buchowicz, 1995; Boubriak et al., 2007). Moreover, it has been reported that restoration of telomeres at chromosome ends takes place early during seed imbibition, possibly due to the presence of telomerase activity in dry seeds (Riha et al., 1998).
An interesting aspect of the Silene seed response was the increased telomere length observed in the rehydrated seeds, particularly evident in S. acaulis, the species from a high altitude with lower antioxidant ability. This process might be related to seed repair mechanisms, which are activated during the early phase of seed imbibition to remove DNA fragmentation (Balestrazzi et al., 2010, 2011a). This hypothesis has been strongly supported by Bucholc and Buchowicz (1992) who demonstrated that dry wheat embryos subjected to long-term storage were depleted of telomeric repeats, while an enrichment was evident at the beginning of imbibition. On the other hand, there are reports describing the effects of altered telomere homeostasis on seed germinability that suggest the association between increased telomere length and genotoxic stress. The Arabidopsis rtbp1 (RICE TELOMERE BINDING PROTEIN1) mutant, characterized by increased telomere length compared with the wild type, was severely impaired in seed germination (Hong et al., 2007). Similarly, the lack of the k70 function required for telomere maintenance resulted in the marked expansion of telomere length and a high sentitivity to genotoxic agents (Riha et al., 2002). This is a very intriguing perspective which deserves additional investigations. A model has recently been proposed in animal cells, where telomere oxidative damage resulting from endogenous or exogenous sources can be differently recognized and induce different responses (Wang et al., 2010). According to these authors, moderate injuries in telomeric sequences caused by cellular metabolism are known to activate telomere lenghtening, while extensive damage caused by external factors result in telomere degradation. In agreement with the model, when considering seeds, telomere lenghtening might be expected after rehydration when there is resumption of metabolic activities, whereas telomere degradation might be associated with artificial ageing.
In the present work, we have selected different markers of seed deterioration that might be useful to predict seed viability not only in plant species from different altitudes but, possibly in a wide range of endangered species undergoing ex situ preservation. As evidenced, each molecular marker is related to specific cellular responses, including DNA damage/repair and the antioxidant system. RAPD analysis, quantitation of redox activity of non-enzymatic antioxidant compounds and gene expression profiling can be used to acquire more in-derpth information on the eco-physiological features of Silene species, as low-cost and time-saving reproducible procedures. Moreover, considering the limited knowledge currently available, telomere analysis proved to be a promising tool that can be applied to a large number of seed samples. A positive impact of the reported results could be envisaged within a relatively short time, since specific suggestions can be derived for improving the rehydration protocol of seeds from a high-altitude location.
ACKNOWLEDGEMENTS
This research was supported by Fondo di Ateneo per la Ricerca-University of Pavia and Centro Flora Autoctona of the Lombardy Region. The authors would like to express their thanks to Emanuele Vegini.
LITERATURE CITED
- Bailly C, Benamar A, Corbineau F, Come D. Free radical scavenging as affected by accelerated aging and subsequent priming in sunflower seeds. Physiologia Plantarum. 1998;104:646–652. [Google Scholar]
- Balestrazzi A, Botti S, Zelasco S, et al. Expression of the PsMTA1 gene in white poplar engineered with the MAT system is associated with heavy metal tolerance and protection against 8-hydroxy-2'-deoxyguanosine mediated-DNA damage. Plant Cell Reports. 2009;28:1179–1192. doi: 10.1007/s00299-009-0719-x. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Confalonieri M, Macovei A, Carbonera D. Seed imbibition in Medicago truncatula Gaertn.: expression profiles of DNA repair genes in relation to PEG-mediated stress. Journal of Plant Physiology. 2010;168:706–713. doi: 10.1016/j.jplph.2010.10.008. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Confalonieri M, Macovei A, Donà M, Carbonera D. Genotoxic stress and DNA repair in plants: emerging functions and tools for improving crop productivity. Plant Cell Reports. 2011;30:287–295. doi: 10.1007/s00299-010-0975-9. [DOI] [PubMed] [Google Scholar]
- Balestrazzi A, Macovei A, Tava A, Avato P, Raimondi E, Carbonera D. Unraveling the response of plant cells to cytotoxic saponins: role of metallothionein and nitric oxide. Plant Signaling and Behavior. 2011;6:1–4. doi: 10.4161/psb.6.4.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balestrazzi A, Confalonieri M, Donà M, Carbonera D. Genotoxic stress, DNA repair, and crop productivity. In: Tuteja N, Gill SS, editors. Crop improvement under adverse conditions. Berlin: Springer-Verlag; 2012. pp. 153–169. [Google Scholar]
- Bednarek PT, Chwdorzewska K, Puchalski J. Preliminary molecular studies on genetic changes in rye seeds due to long-term storage and regeneration. In: Gass T, Podyma W, Puchalski J, Eberhart SA, editors. Challenges in rye germplasm conservation. International Plant Genetic Resources Institute; 1998. pp. 54–61. [Google Scholar]
- Birtic S, Colville L, Pritchard HW, Pearce SR, Kranner I. Mathematically combined half-cell reduction potentials of the low-molecular-weight thiols as markers of seed aging. Free Radical Research. 2011;45:1093–1102. doi: 10.3109/10715762.2011.595409. [DOI] [PubMed] [Google Scholar]
- Boubriak I, Kargiolaki H, Lyne L, Osborne DJ. The requirement for DNA repair in desiccation tolerance of germinating embryos. Seed Science Research. 1997;7:97–105. [Google Scholar]
- Boubriak I, Polischuk V, Grodzinsky A, Osborne DJ. Telomeres and seeds banks. Cytology and Genetics. 2007;41:18–24. [PubMed] [Google Scholar]
- Braca A, Tommasi ND, Bari LD, Pizza C, Politi M, Morelli I. Antioxidant principles from Banhinia terapotensis. Journal of Natural Products. 2001;64:892–895. doi: 10.1021/np0100845. [DOI] [PubMed] [Google Scholar]
- Bucholc M, Buchowicz J. Synthesis and extrachromosomal DNA and telomere-related sequences in germinating wheat embryos. Seed Science Research. 1992;2:141–144. [Google Scholar]
- Cawthon RM. Telomere measurement by quantitative PCR. Nucleic Acid Research. 2002;30 doi: 10.1093/nar/30.10.e47. e47. http://dx.doi.org/10.1093/nar/30.10.e47 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies H, Probert R. Protocol for comparative seed longevity testing sheet. London: Royal Botanic Gardens, Kew; 2004. www.kew.org/msbp/scitech/publications/comparativelongevity.pdf . [Google Scholar]
- Ellis RH, Roberts EH. Improved equations for the prediction of seed longevity. Annals of Botany. 1980;45:13–30. [Google Scholar]
- El-Maarouf-Bouteau H, Mazuy C, Corbineau F, Bailly C. DNA alteration and programmed cell death during ageing of sunflower seed. Journal of Experimental Botany. 2011;62:5003–5011. doi: 10.1093/jxb/err198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO/IPGRI. Genebank standards. 1994. Rome: Food and Agriculture Organisation of the United Nations/International Plant Genetic Institute. [Google Scholar]
- Filkenstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annual Review of Plant Biology. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Han X, Lu M, Chen Y, Zhan Z, Cui Q, Wang Y. Selection of reliable reference genes for gene expression studies using real-time PCR in tung tree during seed development. PLoS One. 2012;8 doi: 10.1371/journal.pone.0043084. e43084. http://dx.doi.org/10.1371/journal.pone.0043084 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan SMR, Hossain MM, Akter R, Jamila M, Mazumder EH, Rahman S. DPPH free radical scavenging activity of some Bangladeshi medicinal plants. Journal of Medicinal Plants Research. 2009;3:875–879. [Google Scholar]
- Hepburn HA, Goodman BA, McPhail DB, Matthews S, Powell AA. An evaluation of EPR measurements of the organic free radical content of individual seeds in the non-destructive testing of seed viability. Journal of Experimental Botany. 1986;37:1675–1684. [Google Scholar]
- Hong J-P, Byun MY, Koo D-H, et al. Suppression of RICE TELOMERE BINDING PROTEIN 1 results in severe and gradual developmental defects accompanied by genome instability in rice. The Plant Cell. 2007;19:1770–1781. doi: 10.1105/tpc.107.051953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilian A, Stiff C, Kleinhofs A. Barley telomeres shorten during differentiation but grow in callus culture. Proceedings of the National Academy of Sciences, USA. 1995;92:9555–9559. doi: 10.1073/pnas.92.21.9555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kranner I, Colville L. Metals and seeds: biochemical and molecular implications and their significance for seed germination. Environmental & Experimental Botany. 2010;72:93–105. [Google Scholar]
- Kranner I, Minibayeva FV, Beckett RP, Seal CE. What is stress? Concepts, definitions and applications in seed science. New Phytologist. 2010;188:655–673. doi: 10.1111/j.1469-8137.2010.03461.x. [DOI] [PubMed] [Google Scholar]
- Lee YP, Baek K-H, Lee H-S, Kwak S-S, Bang J-W, Kwon S-Y. Tobacco seeds simultaneously overexpressing Cu/Zn-superoxide dismutase and ascorbate peroxidase display enhanced seed longevity and germination rates under stress conditions. Journal of Experimental Botany. 2010;61:2499–2506. doi: 10.1093/jxb/erq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li BY, Cheng M, Gao HQ, et al. Back-regulation of six oxidative stress proteins with grape seed proanthocyanidin extracts in rat diabetic nephropathy. Journal of Cell Biochemistry. 2008;104:668–679. doi: 10.1002/jcb.21658. [DOI] [PubMed] [Google Scholar]
- Li DZ, Pritchard HW. The science and economics of ex situ plant conservation. Trends in Plant Science. 2009;14:614–621. doi: 10.1016/j.tplants.2009.09.005. [DOI] [PubMed] [Google Scholar]
- Liu X, Xing D, Li L, Zhang L. Rapid deterioration of seed vigour based on the level of superoxide generation during early imbibition. Photochemistry and Photobiology Science. 2007;6:767–774. doi: 10.1039/b704337f. [DOI] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Carbonera D. The Tdp1 (tyrosyl-DNA phosphodiesterase) gene family in Medicago truncatula Gaertn.: bioinformatic investigation and expression profiles in response to copper- and PEG-mediated stress. Planta. 2010;232:393–407. doi: 10.1007/s00425-010-1179-9. [DOI] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Buttafava A, Carbonera D. he TFIIS and TFIIS-like genes from Medicago truncatula are involved in oxidative stress response. Gene. 2011;470:20–30. doi: 10.1016/j.gene.2010.09.004. [DOI] [PubMed] [Google Scholar]
- Macovei A, Balestrazzi A, Confalonieri M, Faè M, Carbonera D. New insights on the barrel medic MtOGG1 and MtFPG functions in relation to oxidative stress response in planta and during seed imbibition. Plant Physiology and Biochemistry. 2011;49:1040–1050. doi: 10.1016/j.plaphy.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Maillet G, White CI, Galleg ME. Telomere-length regulation in inter-ecotype crosses of Arabidopsis. Plant Molecular Biology. 2006;62:859–866. doi: 10.1007/s11103-006-9061-7. [DOI] [PubMed] [Google Scholar]
- Marcos-Filho J, McDonald MB. RAPD profiles, germination and vigour of naturally and artificially aged soybean seeds. Seed Science and Technology. 1998;26:141–156. [Google Scholar]
- Mengoni A, Gonnelli C, Hakvoort HWJ, et al. Evolution of copper-tolerance and increased expression of a 2b-type metallothionein gene in S. paradoxa L. populations. Plant and Soil. 2001;257:451–457. [Google Scholar]
- Mhamdi B, Aidi Wannes W, Sriti J, Jellali I, Ksouri R, Marzouk B. Effects of harvesting time on phenolic compounds and antiradical scavenging activity of Borago officinalis seed extracts. Industrial Crops and Products. 2010;31 e1–e4. http://dx.doi.org/doi:10.1016/j.indcrop.2009.07.002 . [Google Scholar]
- Mondoni A, Probert RJ, Rossi G, Vegini E, Hay FR. Seeds of alpine plants are short-lived: implications for long-term conservation. Annals of Botany. 2011;107:171–179. doi: 10.1093/aob/mcq222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Rossi G, Orsenigo S, Probert R. Climate warming could shift the timing of seed germination in alpine plants. Annals of Botany. 2012;110:155–1564. doi: 10.1093/aob/mcs097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondoni A, Rossi G, Probert R. Temperature controls seed germination and dormancy in the European woodland herbaceous perennial Erythronium dens-canis (Liliaceae) Plant Biology (Stuttggart, Germany) 2012;14:475–480. doi: 10.1111/j.1438-8677.2011.00517.x. [DOI] [PubMed] [Google Scholar]
- Mylona PV, Polidoros AN, Scandalios JG. Antioxidant gene responses to ROS-generating xenobiotics in developing and germinated scutella of maize. Journal of Experimental Botany. 2007;6:1301–1312. doi: 10.1093/jxb/erl292. [DOI] [PubMed] [Google Scholar]
- Nandi S, Sen-Mandi S, Sinha TP. Active oxygen and their scavengers in rice seeds (Oryza sativa cv. IET 4094) aged under tropical environmental conditions. Seed Science Research. 1997;7:253–260. [Google Scholar]
- Nei M. Genetic distance between populations. American Naturalist. 1972;106:283–292. [Google Scholar]
- Nicot N, Hausman JF, Hoffmann L, Evers D. Housekeeping gene selection for realtime RT-PCR normalization in potato during biotic and abiotic stress. Journal of Experimental Botany. 2005;56:2907–2914. doi: 10.1093/jxb/eri285. [DOI] [PubMed] [Google Scholar]
- Pfaffl MW, Horgan GW, Dempfle L. Relative expression software tool (REST) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Research. 2002;9 doi: 10.1093/nar/30.9.e36. e36. http://dx.doi.org/10.1093/nar/30.9.e36 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priestley DA, Cullinan VI, Wolfe J. Differences in seed longevity at the species level. Plant, Cell and Environment. 1985;8:557–562. [Google Scholar]
- Probert RJ, Daws MI, Hay FR. Ecological correlates of ex situ seed longevity: a comparative study on 195 species. Annals of Botany. 2009;104:57–69. doi: 10.1093/aob/mcp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. NeuroScience Letters. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Rajjou L, Debeaujon I. Seed longevity: survival and maintenance of high germination ability of dry seeds. Comptus Rendus Biology. 2008;331:796–805. doi: 10.1016/j.crvi.2008.07.021. [DOI] [PubMed] [Google Scholar]
- Raychadhuri SS, Deng XW. The role of superoxide dismutase in combating oxidative stress in higher plants. Botanical Review. 2000;66:89–98. [Google Scholar]
- Riha K, Fajkus J, Siroky S, Vyskot B. Developmental control of telomere lengths and telomerase activity in plants. The Plant Cell. 1998;10:1691–1698. doi: 10.1105/tpc.10.10.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riha K, Watson JM, Parkey J, Shippen DE. Telomere length deregulation and enhanced sensitivity to genotoxic stress in Arabidopsis mutants deficient in Ku70. EMBO Journal. 2002;21:2819–2826. doi: 10.1093/emboj/21.11.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G, Mondoni A. The Lombardy Seed Bank (LSB), the youngest seed bank in ENSCONET. The European Native Seed Conservation Newsletter 1. 2006 [Google Scholar]
- Smith RD, Dickie JD, Linington SH, Pritchard HW, Probert RJ. Seed conservation: turning science into practice. London: Royal Botanic Gardens, Kew; 2003. [Google Scholar]
- Spanos GA, Wrolstad RE. Influence of processing and storage on the phenolic composition of Thompson seedless grape juice. Journal of Agricultural Food Chemistry. 1990;38:1565–1571. [Google Scholar]
- Swartz HM, Bolton JR, Borg DC. Biological applications of electron spin resonance. New York: Wiley Interscience; 1972. [Google Scholar]
- Talai S, Sen-Mandi S. Seed vigour-related DNA marker in rice shows homology with acetyl CoA carboxylase gene. Acta Physiologia Plantarum. 2010;32:153–167. [Google Scholar]
- Tanaka M, Kikuchi A, Kamada H. The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiology. 2008;146:149–161. doi: 10.1104/pp.107.111674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoof NA, Hassinen VH, Hakvoort HW, Ballintijn KF, Schat H, Verkleij JA, Ernst WH, Karenlampi SO, Tervahauta AI. Enhanced copper tolerance in Silene vulgaris (Moench) Garcke populations from copper mines is associated with increased transcript levels of a 2b-type metallothionein gene. Plant Physiology. 2001;126:1519–1526. doi: 10.1104/pp.126.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura L, Donà M, Macovei A, et al. Understanding the molecular pathways associated with seed vigor. Plant Physiology and Biochemistry. 2012;60:196–206. doi: 10.1016/j.plaphy.2012.07.031. [DOI] [PubMed] [Google Scholar]
- Vijay D, Dadlani M, Ananda Kumar P, Panguluri SK. Molecular marker analysis of differentially aged seeds of soybean and safflower. Plant Molecular Biology Reporter. 2009;27:282–291. [Google Scholar]
- Walters C, Wheeler LM, Grotenhuis JM. Longevity of seeds stored in a genebank: species characteristics. Seed Science Research. 2005;15:1–20. [Google Scholar]
- Wang Z, Khee DB, Lu J, Bohr CT, Zhou F, Vallabhaneni H, de Souza-Pinto NC, Liu Y. Characterization of oxidative guanine damage and repair in mammalian telomeres. PLoS Genetics. 2010;6 doi: 10.1371/journal.pgen.1000951. e1000951. http://dx.doi.org/10.1371/journal.pgen.1000951 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtyla L, Garnczarska M, Zalewski T, Bednarski W, Ratajezak L, Jurga S. A comparative study of water distribution, free radical production and activation of antioxidative metabolism in germinating pea seeds. Journal of Plant Physiology. 2006;163:1207–1220. doi: 10.1016/j.jplph.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Yao Z, Liu L, Gao F, et al. Developmental and seed aging mediated regulation of antioxidative genes and differential expression of proteins during pre- and post-germinative phases in pea. Journal of Plant Physiology. 2012;15:1477–1488. doi: 10.1016/j.jplph.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Yordanov N, Aleksieva K. X- and Q-band EPR studies on fine powders of irradiated plants. New approach for detection of their radiation history by using Q-band EPR spectrometry. Radiation Physics and Chemistry. 2004;69:59–64. [Google Scholar]
- Zhou Y, Chu P, Chen H, et al. Overexpression of Nelumbo nucifera metallothioneins 2a and 3 enhances seed germination vigor in Arabidopsis. Planta. 2012;235:523–537. doi: 10.1007/s00425-011-1527-4. [DOI] [PubMed] [Google Scholar]