Abstract
Human placental villi are surfaced by a multinucleated and terminally differentiated epithelium, the syncytiotrophoblast, with a subjacent layer of mononucleated cytotrophoblasts that can divide and fuse to replenish the syncytiotrophoblast. The objectives of this study were i) to develop an approach to definitively identify and distinguish cytotrophoblasts from the syncytiotrophoblast, ii) to unambiguously determine the relative susceptibility of villous cytotrophoblasts and syncytiotrophoblast to constitutive and stress-induced apoptosis mediated by caspases, and iii) to understand the progression of apoptosis in villous trophoblasts. Confocal microscopy with co-staining for E-cadherin and DNA allowed us to clearly distinguish the syncytiotrophoblast from cytotrophoblasts and identified that many cytotrophoblasts are deeply interdigitated into the syncytiotrophoblast. Staining for specific markers of caspase-mediated apoptosis indicate that apoptosis occurs readily in cytotrophoblasts but is remarkably inhibited in the syncytiotrophoblast. To determine if an apoptotic cell or cell fragment was from a cytotrophoblast or syncytiotrophoblast, we found co-staining with E-cadherin along with a marker for apoptosis was essential: in the absence of E-cadherin staining, apoptotic cytotrophoblasts would easily be mistaken as representing localized regions of apoptosis in the syncytiotrophoblast. Regions with perivillous fibrin-containing fibrinoid contain the remnants of trophoblast apoptosis, and we propose this apoptosis occurs only after physical isolation of a region of the syncytium from the main body of the syncytium. We propose models for the progression of apoptosis in villous cytotrophoblasts and for why caspase-mediated apoptosis does not occur within the syncytium of placental villi.
Introduction
The human placenta is a transient organ that mediates maternal–fetal exchange, the synthesis and secretion of pregnancy hormones, and the immunologic defense of the fetus (Kay et al. 2011). Placental dysfunction contributes to sub-optimal outcomes related to preeclampsia and intrauterine growth restriction (IUGR) and to long-term adverse health consequences for both the mother and offspring (Rampersad & Nelson 2007, Scifres & Nelson 2009, Longtine & Nelson 2011).
The chorioallantoic placenta develops from the trophectoderm of the blastocyst after implantation (Benirschke et al. 2006) and branching angiogenesis yields a series of villous trees that are surfaced by terminally differentiated syncytiotrophoblast. The syncytiotrophoblast is formed by fusion of underlying cytotrophoblasts. Cytotrophoblasts undergo a morphological transition in mid-pregnancy from a continuous, cuboidal epithelium to a discontinuous layer with extensive stellate processes (Jones et al. 2008), but retain the ability to proliferate and fuse with the overlying syncytium throughout pregnancy. Bathed in maternal blood, the syncytiotrophoblast is a unique epithelium and a true syncytium, with multiple nuclei in a common cytoplasm. Injury to the trophoblast layer occurs in localized areas of villi throughout pregnancy (Nelson 1996). Damaged regions of villi are denuded of syncytiotrophoblast, and fibrin-containing fibrinoid is deposited on the trophoblast basement membrane at these sites of injury (Benirschke et al. 2006) followed by repair by cytotrophoblasts which proliferate and fuse, re-establishing the syncytial epithelium over the fibrin matrix.
A key issue in placental biology is how villous trophoblasts respond to stressors, such as oxidative stress, hypoxia/re-oxygenation, dysregulated inflammation, or mechanical damage, among others (Burton et al. 2009). Notably, markers of oxidative and nitrative stress are higher in placentas of pregnancies complicated by preeclampsia, IUGR, or both (Benirschke et al. 2006, Desoye & Hauguel-de Mouzon 2007, Rampersad & Nelson 2007, Burton et al. 2009, Redman & Sargent 2009, Scifres & Nelson 2009, Burton & Jauniaux 2011). A second key issue is the mechanisms by which injury, repair, apoptosis, and overall epithelial turnover are regulated in the multinucleated syncytiotrophoblast that lacks lateral cell borders.
The response of human villous trophoblasts to stress and the mechanisms involved cannot be studied directly in vivo, but in vitro studies using cultured primary human trophoblasts (PHTs) have generally been consistent, concluding that cytotrophoblasts more readily undergo apoptosis than syncytiotrophoblasts (Levy et al. 2000, Crocker et al. 2001, 2003, Yusuf et al. 2002, Chen et al. 2010). However, data on the level of apoptosis under constitutive or stress conditions in the cytotrophoblasts and syncytiotrophoblasts in freshly fixed villi, and from villous explants in short-term culture, have yielded conflicting results. Some studies have not identified the trophoblast phenotype to undergo apoptosis (Allaire et al. 2000, Kadyrov et al. 2001, Tomas et al. 2011). Other studies that have attempted to identify the trophoblast phenotype have concluded that there are higher levels of apoptosis in cytotrophoblasts, compared with syncytiotrophoblasts (Burton et al. 2003, Huppertz et al. 2003), while others conclude that apoptosis is highest in localized regions within the syncytiotrophoblast (Nelson 1996, Smith et al. 1997, Austgulen et al. 2002, Hung et al. 2002, Ishihara et al. 2002, Heazell et al. 2008a,b, 2011).
In the course of our studies of apoptosis using confocal immunofluorescence microscopy, we found that many cases of caspase-mediated substrate cleavage that initially appeared to be within the syncytiotrophoblast were actually from apoptosis of cytotrophoblasts that were highly interdigitated into the syncytium. This ambiguity, and the above noted discordance in the literature, stimulated the three objectives of our study: i) to develop an approach by light microscopy that definitively distinguished the cytotrophoblasts from the syncytiotrophoblast on villi, ii) to unambiguously determine the relative susceptibility of cytotrophoblasts and syncytiotrophoblast in explants of term villi to constitutive and stress-induced apoptosis mediated by caspases, and iii) to develop a model for the progression of apoptosis in term villous trophoblasts. We hypothesized that the apoptosis in term villi results predominantly from apoptosis of villous cytotrophoblasts, not from localized regions of apoptosis in the syncytiotrophoblast.
Results
Validation of antibodies
E-cadherin, an epithelial-cell specific, plasma-membrane protein, is expressed in placental villi only by the trophoblast cells (Lecuit et al. 2004, Tabata et al. 2007, Aplin et al. 2009). Anti-native E-cadherin antibody recognized a single band of ∼130 kDa present in primary trophoblasts but not HeLa cells, as expected (Supplementary Figure 1A, see section on supplementary data given at the end of this article). Antibodies against native cytokeratins 7 and 18 recognized single bands of the molecular weight of the intact proteins in extracts from untreated HeLa cells and primary trophoblasts (Supplementary Figure 1A). Cytokeratin 18 is cleaved at multiple sites by caspases during apoptosis (Ku et al. 1997, Leers et al. 1999, Tao et al. 2008) and after exposure to staurosporine the monoclonal anti-native cytokeratin 18 antibody recognized the ∼50 kDa full-length protein as well as a ∼30 kDa cleavage product (Supplementary Figure 1A and B). The M30 MAB recognizes an epitope on cytokeratin 18, reported to be generated by caspase cleavage at Asp396 (Leers et al. 1999, Schutte et al. 2004, Tao et al. 2008), which is an early event in caspase-mediated apoptosis that generates a cleaved fragment of cytokeratin 18 (clCyt18) of ∼43 kDa. Subsequent caspase cleavage of this 43 kDa fragment at Asp238 resulted in a shorter (∼20 kDa) fragment recognized by the M30 antibody. Both of these fragments were detected by the M30 antibody in HeLa cells and trophoblasts exposed to staurosporine (Supplementary Figure 1A and B). Antibodies specific for the caspase-cleaved, active form of caspase 8 (clCASP8) and for caspase-cleaved poly-ADP-ribose polymerase (clPARP) recognized cleavage products of the expected sizes after staurosporine exposure (Supplementary Figure 1A). The antibodies to human chorionic gonadotropin (hCG), and to the Ki67 antigen that is expressed only in proliferating cells, have been validated previously (Corless et al. 1987, Scholzen & Gerdes 2000). Together, these results indicate that the antibodies we used identified the specific proteins and protein fragments of interest.
Confocal microscopy and immunofluorescence for E-cadherin distinguishes cytotrophoblasts from syncytiotrophoblast
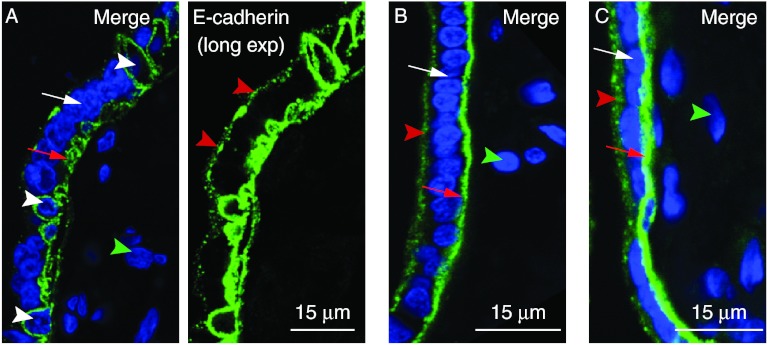
Placental villi collected from term normotensive pregnancies were fixed rapidly after delivery and co-stained for DNA and for E-cadherin. Confocal imaging using ≤0.5 μm spaced optical Z-sections showed that E-cadherin outlined the apical and basal plasma membranes of the syncytiotrophoblast and the entire plasma membranes of adjacent mononucleated cells (Fig. 1). Terminal villi, with a maximal diameter of ≤80 μm, contained both syncytiotrophoblast and mononucleated cells outlined with E-cadherin (Fig. 1A; Supplementary Movie 1A, see section on supplementary data given at the end of this article) while intermediate villi, with a maximal diameter of >80 μm without tunica media, had both regions of syncytiotrophoblast with detectable mononucleated cells outlined with E-cadherin and many regions with uniform thickness of syncytiotrophoblast with few or no mononucleated cells outlined with E-cadherin (Fig. 1B and C; Supplementary Movie 1B and C). As expected, cells and connective tissue in the villous stroma did not stain with E-cadherin.
Figure 1.
E-cadherin staining identifies villous syncytiotrophoblast and mononucleated cells. Villous tissue was co-stained for E-cadherin (green) and DNA (blue). (A) Terminal villous with mononucleated cells outlined by E-cadherin. (B and C) Intermediate villous with uniformly thick syncytiotrophoblast and few mononucleated cells outlined by E-cadherin. White arrowheads: mononucleated cells defined by E-cadherin staining. White arrows: syncytiotrophoblast nuclei. Red arrows: E-cadherin staining at syncytiotrophoblast basal plasma membrane. Green arrowheads: nuclei of stromal cells. Red arrowheads: E-cadherin staining at syncytiotrophoblast apical plasma membrane. In this and all subsequent figures, images are oriented with the syncytiotrophoblast and intervillous space at the left of the image.
We used two approaches to verify that the mononucleated cells outlined by E-cadherin in the term, normotensive pregnancies were cytotrophoblasts. First, we co-stained villi for E-cadherin, for hCG, which is expressed at high levels by syncytiotrophoblast and is either not expressed or expressed at low levels by cytotrophoblasts (Hoshina et al. 1982, Kliman et al. 1986, Daoud et al. 2005) and for DNA. HCG was well expressed in the syncytiotrophoblast but not in the mononucleated cells outlined by E-cadherin (Fig. 2A). Secondly, we co-stained villi for E-cadherin, DNA, and for cytokeratin 7 or 18, which are intermediate filament proteins expressed only by epithelial cells. Both cytokeratins were abundantly present in filaments in the cytoplasm of the syncytiotrophoblast and of the mononucleated cells outlined by E-cadherin, but not by villous stromal cells (Fig. 2B and C). We were able to visualize the cytokeratin filaments (Fig. 2A and B; Supplementary Figure 2A, see section on supplementary data given at the end of this article) and spatially discriminate between the E-cadherin in the plasma membrane (Fig. 2C, inset) and the cytokeratin filaments in the cytoplasm (Fig. 2B and C; Supplementary Figure 2A and B). Together, these results show that confocal microscopy with co-staining for DNA and E-cadherin permits the clear identification of syncytiotrophoblast and cytotrophoblasts, with excellent resolution of subcellular structure.
Figure 2.
Mononucleated cells outlined by E-cadherin are cytotrophoblasts. Villous tissue was co-stained for DNA, E-cadherin, and (A) hCG, (B) native cytokeratin 7, or (C) native cytokeratin 18. Yellow arrow: hCG in syncytiotrophoblast cytoplasm. White arrowheads: cytotrophoblast nuclei. White arrows: syncytiotrophoblast nuclei. Green arrowheads: stromal cell nuclei. Insets: red arrowhead indicates spatial separation of cytoplasmic cytokeratins and plasma membrane-associated E-cadherin.
Previous electron microscopic and immunohistochemical studies reported that ∼90% of trophoblast nuclei in villi were within the syncytium, with ∼10% in the cytotrophoblasts (Simpson et al. 1992, Mayhew & Simpson 1994, Mayhew et al. 1994). Because confocal microscopy allows a larger sample size than electron microscopy, and because E-cadherin and DNA co-staining allowed greater resolution of the two trophoblast phenotypes than routine immunohistochemical analyses, we asked if our approach reproduced the proportion of nuclei in syncytiotrophoblasts and cytotrophoblasts of term villi reported by the limited sampling of electron microscopy. We scored >1000 trophoblast nuclei from term, normotensive placentas (n=6), and found 91±3% of trophoblast nuclei in syncytiotrophoblast and 9±1% in cytotrophoblasts. These proportions were not significantly different from those reported by electron microscopic studies. We also used E-cadherin and DNA staining to score >200 cytotrophoblasts per placenta (n=6 placentas), and found 30±7% of cytotrophoblasts were interdigitated into the syncytiotrophoblast and 70±8% of cytotrophoblasts were subjacent, and not interdigitated.
Can interdigitated cytotrophoblasts provide a paracellular route of transport through the syncytia?
Paracellular transfer of molecules from the maternal to fetal circulations by a route dependent on the diffusion coefficient of molecules in water has been shown to occur in regions of villi denuded of their syncytiotrophoblast layer (Edwards et al. 1993, Brownbill et al. 1995, 2000). We tested the hypothesis that a morphological pathway for paracellular transfer was present in undamaged regions of the syncytiotrophoblast where the cytotrophoblasts were highly interdigitated. We co-stained villi for E-cadherin, DNA, and for native cytokeratin 7 (n=20 cytotrophoblasts) or native cytokeratin 18 (n=20 cytotrophoblasts), and obtained high-resolution confocal images (0.3 μm spaced Z-stacks at a zoom of 3×), targeting deeply interdigitated cytotrophoblasts. For all 40 cytotrophoblasts, cytokeratin staining showed that syncytiotrophoblast cytoplasm always overlaid the cytotrophoblast plasma membrane, identified by E-cadherin staining (Supplementary Figure 2B; Supplementary Movie 2, see section on supplementary data given at the end of this article). Thus, cytotrophoblasts did not penetrate the syncytiotrophoblast apical microvillous plasma membrane and did not reach the intervillous space. We concluded that cytotrophoblast interdigitations into the apical cytoplasm of syncytiotrophoblast were not a site of paracellular transfer through undamaged syncytiotrophoblast, although the syncytiotrophoblast cytoplasm overlying a given interdigitated cytotrophoblast was often highly attenuated (at ∼0.5–1 μm).
Are interdigitated and non-interdigitated villous cytotrophoblasts distinct sub-populations?
Previous electron microscopy studies have indicated that villous cytotrophoblast nuclei are typically larger and more euchromatic than the smaller syncytiotrophoblast nuclei, which contain more condensed chromatin (Burton et al. 2003, Crocker et al. 2004). Cytotrophoblast nuclei likely exit the cell cycle and undergo chromatin condensation before, or during, fusion (Genbacev et al. 2000, Crocker et al. 2007). Our observation that a subset of cytotrophoblasts were interdigitated into the syncytiotrophoblast led to the hypothesis that these cytotrophoblasts have exited the cell cycle and were in the process of differentiation and fusion, while the non-interdigitated cytotrophoblasts were continuing to progress through the cell cycle.
We tested this hypothesis in three ways using villi from term, normotensive pregnancies. First, we compared the average nuclear volume of interdigitated and non-interdigitated cytotrophoblasts (n=25 for each group) with each other and with syncytiotrophoblast nuclei (n=45). The nuclear volume of interdigitated cytotrophoblasts was 1605±692 μm3, of non-interdigitated cytotrophoblasts was 1372±404 μm3, and of syncytiotrophoblast nuclei was 1097±401 μm3. The nuclear volumes of each cytotrophoblast group were greater than that of syncytiotrophoblast nuclei (P<0.05), but there was no difference between the two cytotrophoblast groups (P=0.15).
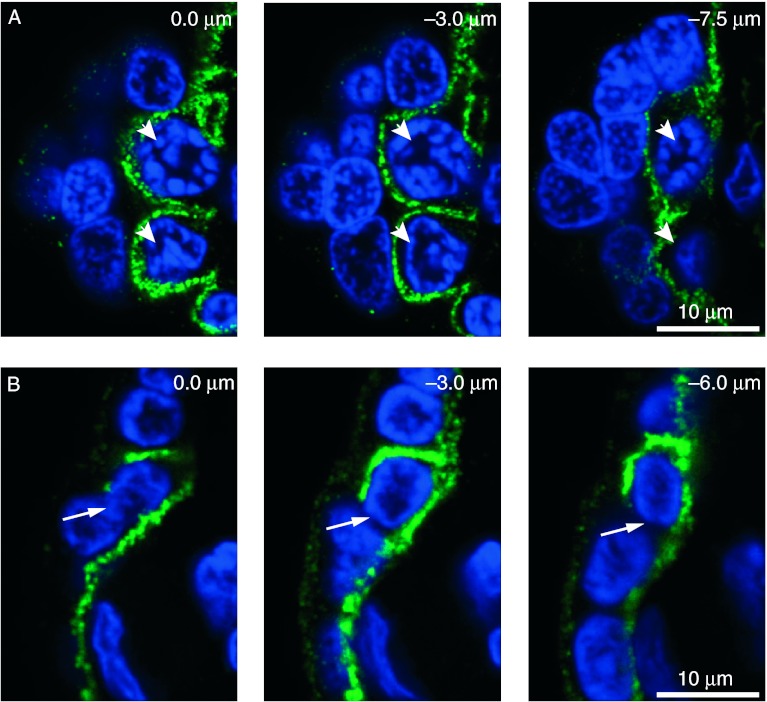
Secondly, we co-stained villi for E-cadherin and DNA and obtained 0.25 μm spaced confocal Z-stacks of interdigitated (n=75) and non-interdigitated (n=75) cytotrophoblasts. Of these, 147 displayed clear continuity of E-cadherin staining (Fig. 3A; Supplementary Movie 3A, see section on supplementary data given at the end of this article), indicating they were unlikely to be in the process of fusion with the syncytiotrophoblast. There was a discontinuity in E-cadherin staining, consistent with ongoing fusion, in only two interdigitated cytotrophoblasts and one non-interdigitated cytotrophoblast (Fig. 3B; Supplementary Movie 3B).
Figure 3.
Continuous and discontinuous plasma membrane staining of cytotrophoblasts. Villous tissue was co-stained for DNA (blue) and E-cadherin (green). (A) Two cytotrophoblast nuclei surrounded by continuous E-cadherin (arrowheads), (B) Discontinuous E-cadherin (arrow) around an interdigitated cytotrophoblast. Shown are selected confocal Z-stack images of the depths indicated.
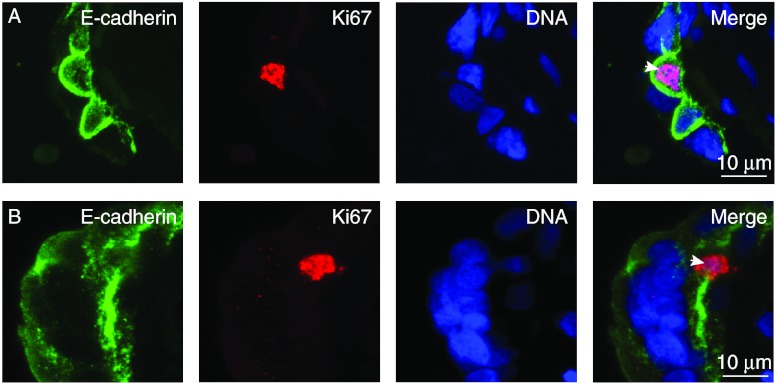
Finally, to determine if there was a difference in cell-cycle progression of the interdigitated and non-interdigitated cytotrophoblasts, we co-stained villi for DNA, E-cadherin, and for the Ki67 antigen, which is present in cells in G1, S, G2, and mitosis, but not in cells in G0 (Scholzen & Gerdes 2000). We used random screening to identify >25 Ki67-positive cytotrophoblasts per placenta (n=4 placentas). Ki67-positive cytotrophoblasts were found in both the interdigitated and non-interdigitated populations (Fig. 4A and B), with 33% being interdigitated, which was not significantly different from the 30% interdigitated trophoblasts in the population (χ2 test, P>0.3). These results do not support the hypothesis that interdigitated and non-interdigitated cytotrophoblasts reflect differences in differentiation or cell-cycle status, by the examined criteria of nuclear volume, hCG expression and Ki67 staining. However, we recognize that there may be other markers of villous trophoblast differentiation that might be different that we have not examined.
Figure 4.
Staining of villi for E-cadherin and Ki67. Villous tissue was co-stained for DNA, E-cadherin, and Ki67. Ki67-positive nuclei (arrowheads) were found in interdigitated (A) and non-interdigitated (B) cytotrophoblasts. Shown are maximal pixel value projections of confocal Z-stacks.
Caspase activation occurs in cytotrophoblasts of explants and term villi but does not occur in syncytiotrophoblast
Results from other labs (Crocker et al. 2003, Hu et al. 2006) and ours (Yusuf et al. 2002, Hu et al. 2006, Chen et al. 2010) indicate that cultured primary human cytotrophoblasts are more susceptible than syncytiotrophoblasts to caspase-mediated apoptosis induced by hypoxia, hypoxia mimetics, and staurosporine. Therefore, we predicted that in placental villous explants staurosporine would induce a greater level of apoptosis in cytotrophoblasts than in the syncytiotrophoblast.
To test this prediction, we cultured placental villous explants in 20% oxygen and exposed them to vehicle control or 100 nM staurosporine for 12 h. Explants were co-stained for E-cadherin, DNA, and for clCyt18, clPARP, or clCASP8 (as markers of caspase-mediated apoptosis) and the presence of these markers was scored by Z-stack confocal microscopy. Staurosporine exposure significantly (P<0.05) increased caspase activation in cytotrophoblasts, as indicated by expression of clCyt18 by 24% of cytotrophoblasts, compared with 8% in vehicle control (Table 1). Notably, the signal/noise ratio was high in clCyt18-positive cytotrophoblasts, with clCyt18 easily detectable even after a tenfold reduction in laser power. Importantly, in both control and staurosporine-exposed explants, ∼90% of cytotrophoblasts that expressed clCyt18 also expressed clCASP8 and clPARP, with few expressing clCASP8 but lacking clCyt18 (Table 1). Conversely, neither control nor staurosporine-exposed explants expressed detectable clCyt18, clCASP8, or clPARP in the syncytiotrophoblast (Table 1), as defined by a region with multiple nuclei outlined by E-cadherin on an apical and basal surface. Thus, in villous explants, caspase-mediated apoptosis occurred in cytotrophoblasts, but not in the syncytiotrophoblast, even in the presence of staurosporine, a potent inducer of apoptosis.
Table 1.
Percent trophoblast apoptosis in explants and villi from human term, normotensive pregnancies.
| clCyt18+ (cyto) | clCyt18+ (syn) | clCyt18+/clCASP8+ | clCyt18+/clCASPp8− | clCyt18−/clCASP8+ | clCyt18+/clPARP+ | |
|---|---|---|---|---|---|---|
| Explants | ||||||
| Vehicle control | 8±3* | ND | 91±3 | 9±2 | 5±3 | 90±3 |
| Staurosporine | 24±6* | ND | 92±4 | 8±3 | 6±3 | 94±6 |
| Tissue | ||||||
| Term, normotensive | 1±0.9* | ND | 90±6 | 10±4 | 3±3 | 88±8 |
Cyto, cytotrophoblast; Syn, syncytiotrophoblast; clCyt18, cleaved cytokeratin 18; clCASP8, cleaved caspase 8; clPARP, cleaved poly(ADP-ribose) polymerase. Vehicle control (n=3), staurosporine (n=4), term, normotensive (n=10). ND, not detectable. *P<0.05 by the Student's t-test, compared with vehicle control or normotensive, as appropriate.
We next investigated caspase activation in villi from term, normotensive pregnancies. Tissues were rapidly retrieved and fixed after delivery and co-stained for E-cadherin, DNA, and markers of caspase activation, and then examined by Z-stack confocal microscopy. clCyt18 was detected in 1% of cytotrophoblasts in the freshly fixed tissue (Table 1), which was significantly lower than the ∼8% in control explants (Table 1), indicating that caspase activation occurred in cytotrophoblasts during explant culture, even in the absence of specific inducers of apoptosis. As in explants, in the freshly fixed tissues the cytotrophoblasts that expressed clCyt18 co-expressed clCASP8 and clPARP, with few expressing clCASP8 but lacking clCyt18 (Table 1). In the rapidly harvested and fixed specimens, we did not detect any regions of syncytiotrophoblast that expressed clCyt18, clCASP8, or clPARP (Table 1) in the absence of fibrin-containing fibrinoid. Together, these results indicate that in placentas from term, normotensive pregnancies, caspase-mediated apoptosis is restricted to the cytotrophoblasts, with no detectable signs of apoptosis in the syncytiotrophoblast outside of regions of fibrin-containing fibrinoid.
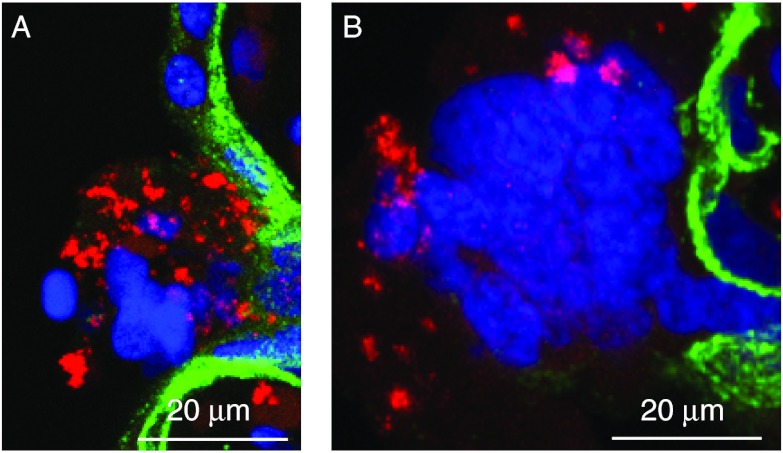
Light microscopy indicated a qualitatively similar frequency of regions of villi with fibrin-containing fibrinoid in the term, normotensive tissue as in the vehicle-control and staurosporine-exposed explants. This was expected as fibrin deposition depends on components in the maternal blood in the intervillous space and the majority of maternal blood was removed by washes before explant culture. Previous studies indicated that the cleavage products derived from caspase-mediated apoptosis occurred in fibrin-containing fibrinoid deposits where there is commonly denudation of syncytiotrophoblast (Nelson 1996, Austgulen et al. 2002). Consistent with these observations, 90% of the fibrin-containing fibrinoid regions from both rapidly fixed tissues and villous explants contained clCyt18 and clCASP8 in a punctate pattern, intermingled with intact and fragmented nuclei (Fig. 5A and B).
Figure 5.
Regions of fibrin-containing fibrinoid contain remnants of trophoblast apoptosis. Villous tissue was co-stained for E-cadherin (green), clCyt18 (red), and DNA (blue). Shown are (A and B) maximal pixel value projection of confocal Z-stacks of two different regions.
Characterization of apoptosis in cytotrophoblasts
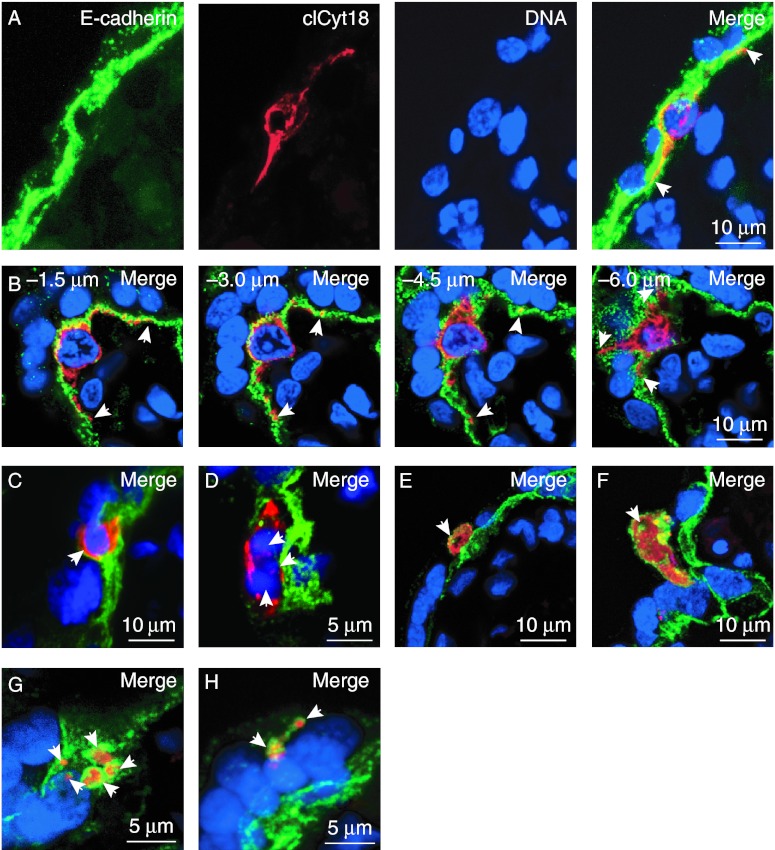
We next determined if there were distinct patterns of expression of markers of caspase activation in cytotrophoblasts. Rapidly fixed, freshly harvested villous tissues and villi from explant cultures were co-stained for, E-cadherin, clCyt18, and DNA and observed by Z-stack confocal microscopy. Except for regions with perivillous fibrin-containing fibrinoid, as noted above, all regions expressing clCyt18 were outlined by E-cadherin, indicating they were derived from cytotrophoblasts. The clCyt18 was observed in a filamentous pattern (Fig. 6A; Supplementary Movie 4A, see section on supplementary data given at the end of this article), typical of early stages of apoptosis in other cell systems (Caulin et al. 1997, Schutte et al. 2004, Ndozangue-Touriguine et al. 2008). This pattern of clCyt18 localization predominated in both control and staurosporine-exposed explants, in which apoptosis was induced during the culture, and was less frequent in the freshly fixed tissue. Over 95% of cells with filamentous clCyt18 contained intact nuclear DNA and the clCyt18 was present in stellate processes that typically extended for tens of microns along the basement membrane (Fig. 6A and B; Supplementary Movie 4A and B). When visualized en face, the size and complexity of these processes was striking (Fig. 6B; Supplementary Movie 4B). clCyt18-positive cytotrophoblasts with a rounded morphology were also observed (Fig. 6C; Supplementary Movie 5A, see section on supplementary data given at the end of this article), suggesting detachment from the basement membrane, possibly due to anoikis (Kroemer et al. 2009). Interestingly, this pattern of clCyt18 staining was more prominent in freshly fixed tissue than in the explants. Some rounded cells displayed filamentous clCyt18, but most displayed punctate or diffuse clCyt18, typical of later stage apoptosis (Caulin et al. 1997, Schutte et al. 2004, Ndozangue-Touriguine et al. 2008). Many of these cells contained intact nuclear DNA (Fig. 6C), but some contained fragmented nuclear DNA (Fig. 6D; Supplementary Movie 5B). We also identified clCyt18 in ∼7–10 μm diameter vesicles and in ∼2–5 μm diameter vesicles that lacked detectable nuclear DNA and that were outlined by E-cadherin (Fig. 6E and F; Supplementary Movie 6A and B, see section on supplementary data given at the end of this article). The smaller vesicles were typically present in clusters of two to five that appeared to be within the cytoplasm of the syncytium and that always lacked detectable nuclear DNA (Fig. 6G and H; Supplementary Movie 6C and D). These latter two patterns of clCyt18 were more abundant in freshly fixed tissue than in the explant tissue.
Figure 6.
Patterns of clCyt18 in apoptotic regions of villi. Villous tissue was co-stained for E-cadherin (green), clCyt18 (red), and DNA (blue). (A) Confocal Z-stack image showing side view of apoptotic cytotrophoblast with filamentous clCyt18. (B) Selected images from a confocal Z-stack of the depths indicated showing en face view of an apoptotic cytotrophoblast expressing filamentous clCyt18. (C–H) Maximal Z-stack pixel value projections of further stages of cytotrophoblast apoptosis, as discussed in the text. Regions with surrounding E-cadherin that were clCyt18 positive were identified that contained (A and B) filamentous clCyt18 and DNA that is (C) intact or (D) fragmented (arrowheads), (E and F) diffuse clCyt18 in large vesicles (arrowheads) that lacked DNA, and (G and H) diffuse clCyt18 in 2–5 small vesicles (arrowheads) that lacked DNA. Arrowheads in A and B indicate ends of stellate processes of cytotrophoblasts that express clCyt18.
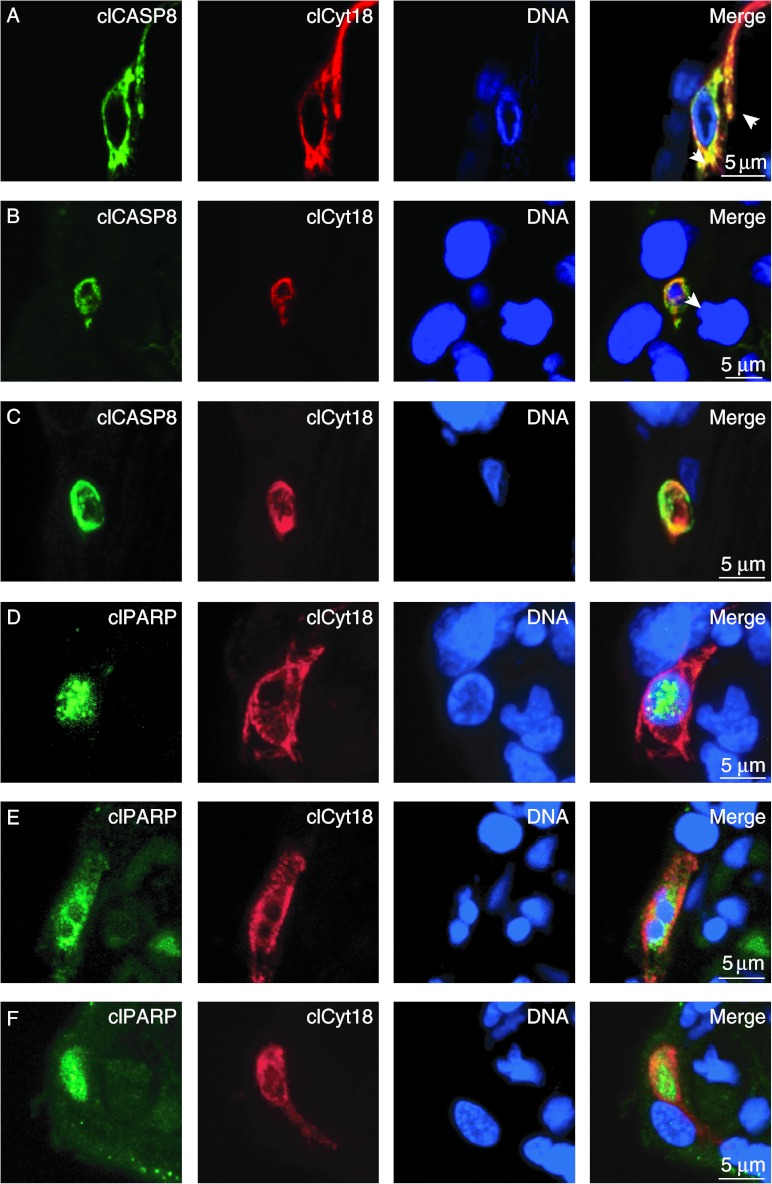
We next examined colocalization of markers of caspase activation in cytotrophoblasts. Most cytotrophoblasts with filamentous clCyt18 co-expressed clCASP8, which was present in a filamentous pattern that was largely superimposed on the clCyt18 (Fig. 7A; Supplementary Movie 7A, see section on supplementary data given at the end of this article). Cytotrophoblasts with punctate or diffuse clCyt18 showed diffuse localization of clCASP8 in cells with (Fig. 7B; Supplementary Movie 7B) or without (Fig. 7C; Supplementary Movie 7C) nuclear DNA. clPARP showed a similar colocalization with clCyt18, as did clCASP8: clPARP was often, but not always, present in the nuclei of cytotrophoblasts with filamentous clCyt18 (Fig. 7D; Supplementary Movie 7D), and was present in >90% of clCyt18-positive regions that contained fragmented nuclear DNA (Fig. 7E; Supplementary Movie 7E) or that lacked nuclear DNA (Fig. 7F; Supplementary Movie 7F).
Figure 7.
Apoptotic cytotrophoblasts co-express clCyt18 with clCASP8 and clPARP. Villous tissue was co-stained for (A–C) clCyt18, clCASP8, and DNA or (D–F) clCyt18, clPARP, and DNA. (A) clCASP8 colocalized with filamentous clCyt18 (arrowheads). (B and C) clCASP8 was co-expressed with clCyt18 in cells with (B) fragmented DNA or (C) vesicles lacking DNA. clPARP was present in (D) the nuclei of cells with filamentous clCyt18 and intact DNA, (E) cells with clCyt18 and fragmented DNA, and (F) vesicles with clCyt18 that lacked DNA. Shown are maximal pixel value projections of confocal Z-stacks.
Discussion
The data show that high-resolution confocal microscopy, with immunofluorescence detection of the plasma-membrane protein, E-cadherin, is required to distinguish cytotrophoblasts from syncytiotrophoblast in term villi. Using this approach, we found that one-third of the cytotrophoblasts in term villi were interdigitated into the syncytium. In placental explants and in villi from normotensive term pregnancies, we detected apoptotic cytotrophoblasts by three markers of caspase-mediated apoptosis, with apoptosis occurring in both interdigitated and non-interdigitated cytotrophoblasts. Because apoptotic cytotrophoblasts were often interdigitated into the syncytium, and because apoptotic cytotrophoblasts formed vesicles within the syncytiotrophoblast that often lacked DNA, studies of villous trophoblast apoptosis without using a plasma membrane marker may yield the misinterpretation of these apoptotic cytotrophoblasts as representing focal regions of apoptosis in the syncytiotrophoblast. Importantly, all regions that express markers of caspase-mediated apoptosis were outlined by E-cadherin, indicating they were derived from cytotrophoblasts, and we never detected evidence for apoptosis in a localized region of the syncytium in the absence of fibrin-containing fibrinoid. This observation indicates that caspase-mediated apoptosis in the syncytium is rare or absent, or that the products of the apoptosis of syncytial components are very rapidly released into the intervillous space and thus are not detectable by immunostaining. Although most villous regions with fibrin-containing fibrinoid expressed all three markers of caspase activation, as discussed later, we suggest that apoptosis of these regions occurs only after regions of syncytiotrophoblast have been isolated from the main syncytium of the villi. Our data have important implications for villous trophoblast organization, paracellular routes of transfer, turnover of the two villous trophoblast phenotypes, and the shedding of trophoblast microparticles and fragments into the peripheral circulation of pregnancies with or without complications. The data allow us to propose a model for the progression of apoptosis in villous cytotrophoblasts.
A continuous layer of cuboidal cytotrophoblast underlies the syncytiotrophoblast in first trimester human placental villi and then undergoes a morphological transition to form a non-cuboidal, discontinuous layer of cells with long stellate processes that cover most, but not all, of the trophoblast basement membrane shared with the syncytiotrophoblast (Mori et al. 2007, Jones et al. 2008). We confirmed findings from previous ultrastructural studies that showed ∼90% of villous trophoblast nuclei are in the syncytium with the remaining 10% in cytotrophoblasts. Notably, we found ∼30% of cytotrophoblasts at term are interdigitated into the syncytiotrophoblast layer. Compared with non-interdigitated cytotrophoblasts, the interdigitated cytotrophoblasts did not differ in nuclear volume or in the stage of the cell cycle, suggesting the two cytotrophoblast populations do not differ in their state of differentiation. We speculate that the extent of interdigitation into the syncytiotrophoblast by cytotrophoblasts is a dynamic and reversible process, rather than a static process reflecting the state of differentiation.
Paracellular transfer of molecules into the fetal vasculature can occur at regions of denudation of the villi, and paracellular routes through an intact syncytiotrophoblast layer has been proposed (Edwards et al. 1993, Kertschanska & Kaufmann 1993, Kertschanska et al. 1995), which would allow direct transfer of molecules between the fetal and maternal circulations. Our high-resolution confocal microscopy studies show that there is always syncytiotrophoblast cytoplasm over even the most extensively interdigitated cytotrophoblasts, indicating that despite close approximation of some cytotrophoblasts with the intervillous space, they do not create paracellular channels. Notably, the markedly attenuated syncytial cytoplasm over deeply interdigitated cytotrophoblasts may enhance maternal–fetal exchange between these cytotrophoblasts and the maternal circulation.
Confocal microscopy and staining for E-cadherin revealed that cytotrophoblasts from term villi readily undergo caspase-mediated apoptosis. About 1% of cytotrophoblasts in villi rapidly fixed after collection from normotensive pregnancies at term were apoptotic, as indicated by expression of three markers for caspase activation: clCyt18, clCASP8, and clPARP. Explants exposed to vehicle control had an eightfold higher level of cytotrophoblast apoptosis than freshly fixed tissue and, in staurosporine-exposed explants, about one quarter of the cytotrophoblasts were apoptotic.
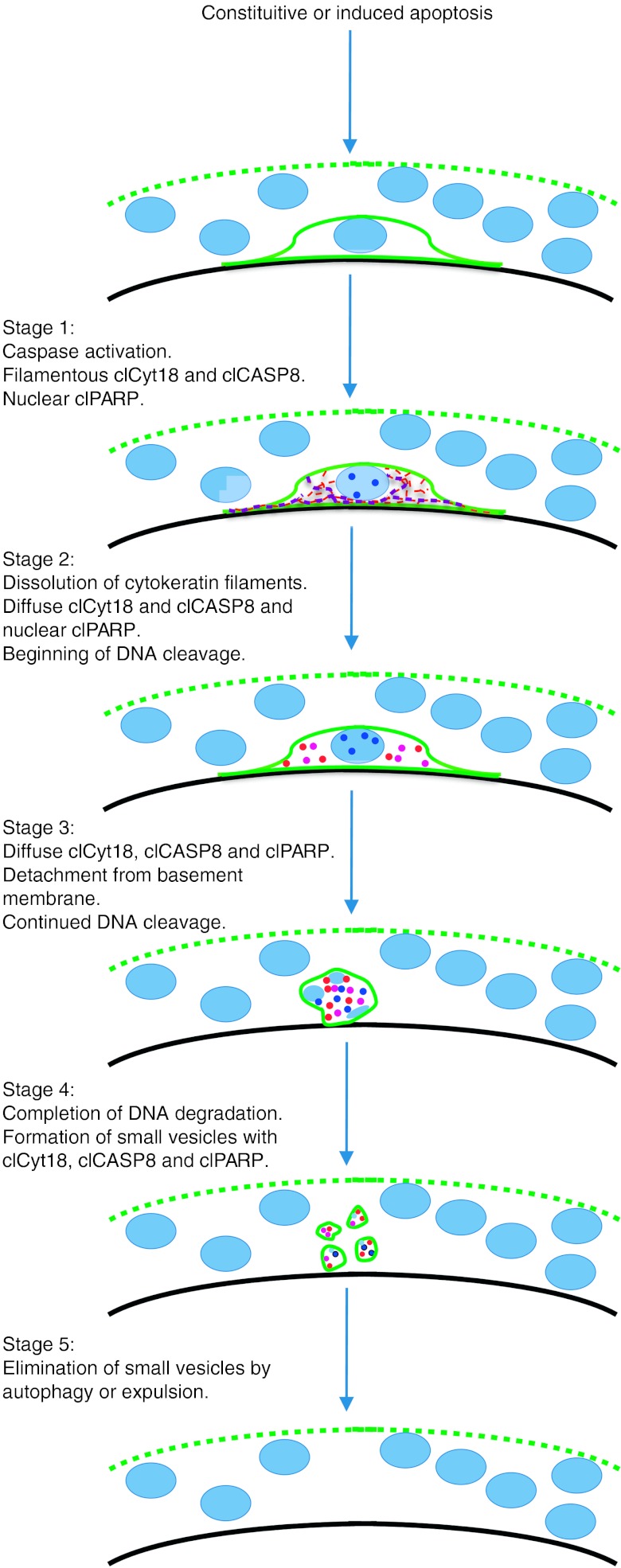
The patterns of clCyt18, clCASP8, and clPARP in apoptotic cytotrophoblasts suggest a model for apoptosis progression in these cells (Fig. 8) that is consistent with that of other epithelial cell types (Caulin et al. 1997, Schutte et al. 2004, Ndozangue-Touriguine et al. 2008). In stage 1, caspase activation occurs, either in response to endogenous stimuli such as reactive oxygen species (ROS) from mitochondrial respiration or from exogenous insults such as underperfusion-associated hypoxia and/or reperfusion-associated re-oxygenation that occurs in placentas of complicated pregnancies. In stage 1, cytokeratin 18 is cleaved at Asp396, but remains in filaments, with cleavage of nuclear DNA not yet begun. At this stage, confocal microscopy shows the clCASP8 co-aligns with the clCyt18 filaments, suggesting a physical interaction. In other epithelial cells, interactions of clCyt18 with active caspases is mediated by death effector domain containing DNA-binding protein (Schutte et al. 2006) and may regulate caspase activity (MacFarlane et al. 2000, Lee et al. 2002, Dinsdale et al. 2004, Schutte et al. 2006, Ndozangue-Touriguine et al. 2008). In stage 2, cytokeratin 18 is cleaved at a second site in the L1-2 linker region (Caulin et al. 1997, Schutte et al. 2004), resulting in filament disassembly and appearance of clPARP in nuclei, indicating that DNA cleavage has begun (He et al. 2009). In stage 3, cleavage of integrins (Werner et al. 2007) results in rounded cells and continued DNA cleavage yields fragmented DNA. In stage 4, DNA is further degraded and the cytotrophoblasts are fragmented into small vesicles surrounded by E-cadherin. These vesicular remnants of apoptotic cytotrophoblasts appear microscopically to be entirely within the cytoplasm of the syncytiotrophoblast, and may represent apoptotic bodies (Nunez et al. 2010). In stage 5, the syncytiotrophoblast may engulf such remnants by autophagy, as is also possible for the removal of apoptotic cytotrophoblast fragments in first trimester syncytiotrophoblast (Burton et al. 2003) and in apoptotic regions of perivillous fibrin (Nelson 1996). Alternatively, we propose that these vesicles are eliminated by extrusion (Andrade & Rosenblatt 2011, Wang et al. 2011) through the apical membrane of the syncytium into the maternal circulation. Our model for cytotrophoblast apoptosis in term villi is consistent with a previous EM study on apoptosis of villous cytotrophoblasts during the first trimester (Burton et al. 2003), which found that apoptotic cytotrophoblasts lose contact with the basement membrane, undergo degradation of the nuclear DNA, and ultimately form membrane-bound vesicles within the syncytiotrophoblast cytoplasm.
Figure 8.
Model for progression of apoptosis in villous cytotrophoblasts. Light blue ovals and dots: nuclear DNA. Dotted green line: apical membrane of syncytiotrophoblast. Solid green line: plasma membrane of cytotrophoblast. Black line: basement membrane. Red lines and dots: clCyt18. Purple lines and dots: clCASP8. Dark blue dots: clPARP. See text for details.
In striking contrast to cytotrophoblasts, caspase-mediated apoptosis was not detected in the syncytiotrophoblast in term villi in vivo or in villous explants even after exposure to a harsh stimulus (staurosporine). We never detected regions of syncytia, defined as E-cadherin outlining apical and basal surface membranes containing multiple nuclei that expressed clCyt18, clCASP8, or clPARP. In agreement with these results, syncytiotrophoblasts that form in primary cultures are more resistant to both constitutive and stimulus-induced apoptosis, compared with cytotrophoblasts (Crocker et al. 2001, Yusuf et al. 2002, Hu et al. 2006, Chen et al. 2010). It is likely that at least part of the relative resistance of syncytiotrophoblast to apoptosis is due to downregulation of the p53 pro-apoptotic pathway (Hu et al. 2006, Chen et al. 2010) and of caspase activity (Yusuf et al. 2002). Together, these observations suggest that turnover of syncytiotrophoblast does not occur by spontaneous apoptosis and release of apoptotic fragments from an intact syncytiotrophoblast, but occurs only secondary to insult-induced injury and apoptosis of an isolated region of syncytiotrophoblast, as described later.
Previous studies (Smith et al. 1997, Mayhew et al. 1999), including our own (Levy et al. 2000), contrast with the results described here, as these studies suggested localized apoptosis occurs in the syncytium. Nuclear condensation has been used as an indicator of localized apoptosis within the syncytium, but this can result from histone phosphorylation or acetylation, and thus cannot be used as a definitive indicator of apoptosis (Burton & Jones 2009). Assessment of apoptosis by TUNEL staining (to assess DNA cleavage) by immunohistochemistry or by staining for clCyt18 or other markers of caspase activation have been used previously (Ishihara et al. 2002, Levy et al. 2002, Huppertz et al. 2003, Straszewski-Chavez et al. 2005, Heazell et al. 2007, 2008b, 2011, Roje et al. 2011, Tomas et al. 2011). However, our confocal microscopy results indicate that high-resolution microscopy with co-staining for a plasma membrane marker would be required to unambiguously identify a region with TUNEL-positive nuclei or markers for caspase activation to be within a region of the syncytium, as opposed to within one or more cytotrophoblasts. The vesicular remnants of apoptotic cytotrophoblasts, with or without nuclear DNA, or the stellate processes of apoptotic cytotrophoblasts, could easily be inappropriately scored as a localized region of apoptosis in the syncytiotrophoblast cytoplasm. In support of this idea, Burton et al. (2003) used the gold standard of electron microscopy to study apoptosis in first trimester placenta villi (Burton et al. 2003, Burton & Jones 2009) and also concluded that there was a potential for mis-scoring apoptotic cytotrophoblasts as localized regions of apoptosis within the syncytium.
The syncytiotrophoblast–cytotrophoblast phenotypes may be analogous to apoptosis in muscle fibers. Both systems contain multinucleated syncytia with adjacent mononucleated precursor cells, called satellite cells in muscle. Early studies suggested apoptosis occurred in localized areas of the muscle fiber, but recent high-resolution confocal microscopy studies indicate that apoptosis rarely, if ever, occurs in the syncytia of muscle (Bruusgaard & Gundersen 2008, Gundersen & Bruusgaard 2008, Bruusgaard et al. 2010). Instead, apoptosis is confined to the associated satellite and stromal cells. Approximately tenfold higher level of apoptosis occurs in cultured mononucleated myoblasts, whether exposed to apoptosis-inducing or constitutive conditions, compared with the multinucleated myotubes. Indeed, the myotubes express increased levels of proteins that inhibit caspase activity (Xiao et al. 2011). Our data, combined with these, indicate that there is a conserved resistance to apoptosis in syncytia, compared with the mononucleated precursor cells.
Our data (Nelson et al. 1990), and that of others (Austgulen et al. 2002), indicate that regions of villi with fibrin-containing fibrinoid contain the cleavage products of caspase-mediated apoptosis, including clCyt18, clCASP8, and fragmented nuclear DNA. One possibility is that a localized region of apoptosis in the syncytium yields a denuded region of the villous, which would then have fibrin deposited on the trophoblast basement membrane exposed to the maternal blood in the intervillous space. However, our data argue against this model, as we find no detectable apoptosis in the syncytium in any regions in the absence of fibrin. Work from others show that mechanical forces can damage endothelial and epithelial surfaces, such as cardiac and skeletal muscle, the epithelia of the gut, and in the endothelial vasculature, with resealing of the ruptures in the plasma membrane occurring by active processes (McNeil & Kirchhausen 2005). We suggest that mechanical forces from blood flow over villi, which occurs with elevated force in pregnancy pathologies (Burton et al. 2009), can disrupt the syncytial plasma membrane, which then reseals, resulting in the physical isolation of a syncytiotrophoblast fragment from the rest of the syncytium on the villous surface. Apoptosis of this isolated region of syncytium may then occur, as indicated by the presence of markers of apoptosis in these regions, and the apoptotic fragments may be released into the intervillous space. Indeed, shedding of fragments of syncytial cytoplasm, nuclei, and microparticles into the maternal blood is well recognized and increased during complicated pregnancies (Hung et al. 2002, Redman & Sargent 2008, Scifres & Nelson 2009, Sharp et al. 2010).
Materials and Methods
Tissue procurement and fixation
The Institutional Review Board of the Washington University School of Medicine approved this study. Placentas were obtained from term singleton gestations with normotensive pregnancies. Within 20 min of delivery, from each placenta four equidistant and random ∼4 mm diameter samples of villous tissue were taken midway between the maternal and fetal surfaces of and midway between the periphery and center. Specimens were fixed at 23 °C in 10% neutral buffered formalin for 24 h and embedded in paraffin.
Cell and explant culture
HeLa cells were obtained from American Type Culture Center (ATCC, Manassas, VA, USA). PHTs were isolated from normal term placentas, as described previously (Chen et al. 2010). Cells were cultured in a 5% CO2, air environment at 37 °C in DMEM supplemented with 10% fetal bovine serum (Invitrogen), 20 mm HEPES (Sigma) pH 7.4, 100 μ/ml penicillin, and 100 μg/ml streptomycin (Sigma) for 24 h followed by 4 h exposure to DMSO (as vehicle control: Sigma) or staurosporine (Sigma). Explants of villous tissue from term, normotensive pregnancies were cultured in a 5% CO2, air environment at 37 °C for 4 h in DMEM, as described earlier. Culture medium was replaced with medium containing DMSO or staurosporine and incubated for 12 h, followed by fixation with formalin.
Antibodies and immunoblotting
Primary and secondary antibodies used are described in Table 2. Protein isolation and immunoblotting was done as described previously (Chen et al. 2010).
Table 2.
Antibodies used in this study.
| Antigen | Species | Company (cat#) | Dilution (IF; IB) | Block (IB) |
|---|---|---|---|---|
| Actin, native | G | SCBT (Ac-1616) | ND; 1:3000 | NFDM |
| Cytokeratin 7, native | M | Invitrogen (18-00234) | 1:300; 1:3000 | NFDM |
| Cytokeratin 18, native | M | Invitrogen (18-0158Z) | 1:100; 1:1000 | NFDM |
| Cytokeratin 18, cleaved | M | Roche (12140322001) | 1:100; 1:1000 | NFDM |
| Caspase 8, cleaved | Rb | CST (9496) | 1:100; 1:1000 | BSA |
| E-cadherin, native | Rb | Abcam (ab40772) | 1:300; 1:3000 | NFDM |
| E-cadherin, native | M | Invitrogen (18-0223) | 1:100; ND | ND |
| hCG | Rb | Gift from Irv Boime | 1:100; ND | ND |
| Ki67 (MIB1) | M | Dako (M7240) | 1:100; ND | ND |
| PARP1, cleaved | Rb | CST (9541) | 1:100; 1:1000 | NFDM |
| Anti-rabbit, Alexa 488 | G | Invitrogen (A-21206) | 1:200; NA | NA |
| Anti-mouse, Alexa 555 | G | Invitrogen (A-11030) | 1:200; NA | NA |
G, goat; M, mouse; Rb, rabbit; NA, not applicable; ND, not done; CST, Cell Signaling Technology; SCBT, Santa Cruz Biotechnology, Inc.; IF, immunofluorescence; IB, immunoblotting. Block, indicates if 5% BSA or 5% non-fat dry milk (NFDM) in 1× PBS was used for blocking before immunoblotting. For immunofluorescence, 5% BSA was used for blocking.
Immunofluorescence
Ten micrometer thick tissue sections were placed on coated slides (Leica, Richmond, IL, USA) and paraffin removed using a graded series of alcohols. Antigen retrieval was in 10 mM sodium citrate, pH 6.0, for 15 min at 95 °C followed by 15 min at 23 °C. Slides were washed in PBS, incubated with 0.1% NP40 (octylphenoxypolyethoxyethanol; Sigma) in PBS for 5 min, and then for 1 h at 23 °C with 5% BSA (Sigma) in PBS. Primary antibodies were diluted in 5% BSA in PBS and incubated with tissues overnight at 4 °C. Slides were washed with PBS, incubated for 2 h at 23 °C with secondary antibodies and DRAQ5 (Biostatus Unlimited, Leicestershire, UK), washed with PBS, and mounted using Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA, USA). Rabbit anti-E-cadherin was used except when double staining used another rabbit-derived antibody, when mouse anti-E-cadherin antibody was used. Control experiments verified no signal in tissues incubated with secondary antibodies in the absence of primary antibodies.
Confocal microscopy
Confocal imaging was done with a Nikon E800 Eclipse C1 microscope with a 60× objective, 488, 543, and 633 nm lasers and emission filters of 515/30, 590/50, and a 650 nm long-pass filter. Image optimization was done using an optical section from the middle of the tissue sample and conditions adjusted to obtain maximal signal intensity without saturation. Typical image stacks were acquired with 15–20 Z-sections using a spacing of 0.5 μm, an image size of 1024× 1024 px and a zoom of 3×, capturing a region of 70.7×70.7 μm. Images were adjusted in ImageJ (rsbweb.nih.gov/ij/) using only linear adjustments and the 12-bit images were then converted to CMYK format.
Cytotrophoblast interdigitations into syncytiotrophoblast
Cytotrophoblasts were assigned as interdigitated if the nucleus overlapped the nearest syncytiotrophoblast nucleus by ≥50%. Cytotrophoblasts not fulfilling this criterion were assigned as non-interdigitated.
Determination of nuclear volume
Tissue sections were co-stained for E-cadherin and DNA, Z-stacks of cytotrophoblasts were obtained, and cells assigned to the interdigitated or non-interdigitated group. We verified the Z-stack encompassed the entire nucleus of interest, generated a cumulative pixel value projection, and measured the minimal and maximal nuclear diameters using ImageJ. Nuclear volumes were obtained by assuming nuclei have the shape of a prolate spheroid (volume of 4/3Πa2b) with (a) the minimal and (b) the maximal diameter of the nucleus.
Scoring of caspase activation
The data shown in Table 1 were obtained as follows. Tissues were co-stained for E-cadherin, DNA, and clCyt18. To avoid bias, images were obtained and scored as follows. Focus was obtained using transmitted light, and a confocal Z-stack was acquired. E-cadherin staining was observed first followed by scoring for clCyt18. The slide was moved one field of view in the same piece of tissue and the procedure repeated until >150 cytotrophoblasts had been imaged. This was repeated for the other three pieces of tissue, resulting in >600 cytotrophoblasts (with ∼6000 syncytiotrophoblast nuclei) being scored per placenta. Tissues co-stained for DNA, clCyt18, and clCASP8 were used to determine the coincidence of clCyt18 and clCASP8, scoring for clCyt18 followed by scoring for clCASP8 or scoring for clCASP8 followed by scoring for clCyt18. Tissues co-stained for DNA, clCyt18, and clPARP were used to determine the coincidence of clCyt18 and clPARP, scoring for clCyt18 followed by scoring for clPARP. In these cases, 20 cells/placenta were scored that were immunopositive for each of the first markers.
Statistical analysis
Ki67 expression was compared by the χ2 test. All other data were compared by the Student's t-test with P<0.05 for significance.
Supplementary data
This is linked to the online version of the paper at http://dx.doi.org/10.1530/REP-11-0340.
Acknowledgements
We thank Irv Boime for the generous gift of hCG antibodies and Fred Krause for helpful discussions.
Declaration of interest
The authors declare there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This study was supported by the National Institutes of Health grant RO1-HD29190 (D M Nelson) and by the BJH Foundation, St Louis, MO.
References
- Allaire AD, Ballenger KA, Wells SR, McMahon MJ, Lessey BA. Placental apoptosis in preeclampsia. Obstetrics and Gynecology. 2000;96:271–276. doi: 10.1016/S0029-7844(00)00895-4. [DOI] [PubMed] [Google Scholar]
- Andrade D, Rosenblatt J. Apoptotic regulation of epithelial cellular extrusion. Apoptosis. 2011;16:491–501. doi: 10.1007/s10495-011-0587-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin JD, Jones CJ, Harris LK. Adhesion molecules in human trophoblast – a review. I. Villous trophoblast. Placenta. 2009;30:293–298. doi: 10.1016/j.placenta.2008.12.001. [DOI] [PubMed] [Google Scholar]
- Austgulen R, Chedwick L, Saksen CV, Vatten L, Craven C. Trophoblast apoptosis in human placenta at term as detected by expression of a cytokeratin 18 degradation product of caspase. Archives of Pathology and Laboratory Medicine. 2002;126:1480–1486. doi: 10.5858/2002-126-1480-TAIHPA. [DOI] [PubMed] [Google Scholar]
- Benirschke K, Kaufmann P & Baergen R (Eds) 2006 Pathology of the Human Placenta, 5th edn. New York: Springer.
- Brownbill P, Edwards D, Jones C, Mahendran D, Owen D, Sibley C, Johnson R, Swanson P, Nelson DM. Mechanisms of alphafetoprotein transfer in the perfused human placental cotyledon from uncomplicated pregnancy. Journal of Clinical Investigation. 1995;96:2220–2226. doi: 10.1172/JCI118277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownbill P, Mahendran D, Owen D, Swanson P, Thornburg KL, Nelson DM, Sibley CP. Denudations as paracellular routes for alphafetoprotein and creatinine across the human syncytiotrophoblast. American Journal of Physiology. Regulatory, Integrative and Comparative Physiology. 2000;278:R677–R683. doi: 10.1152/ajpregu.2000.278.3.R677. [DOI] [PubMed] [Google Scholar]
- Bruusgaard JC, Gundersen K. In vivo time-lapse microscopy reveals no loss of murine myonuclei during weeks of muscle atrophy. Journal of Clinical Investigation. 2008;118:1450–1457. doi: 10.1172/JCI34022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruusgaard JC, Johansen IB, Egner IM, Rana ZA, Gundersen K. Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining. PNAS. 2010;107:15111–15116. doi: 10.1073/pnas.0913935107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Oxidative stress. Best Practice & Research. Clinical Obstetrics & Gynaecology. 2011;25:287–299. doi: 10.1016/j.bpobgyn.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton GJ, Jones CJP. Syncytial knots, sprouts, apoptosis, and trophoblast deportation from the human placenta. Taiwan Journal of Obstetrics and Gynecology. 2009;48:28–37. doi: 10.1016/S1028-4559(09)60032-2. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Skepper JN, Hempstock J, Cindrova T, Jones CJ, Jauniaux E. A reappraisal of the contrasting morphological appearances of villous cytotrophoblast cells during early human pregnancy; evidence for both apoptosis and primary necrosis. Placenta. 2003;24:297–305. doi: 10.1053/plac.2002.0882. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulin C, Salvesen GS, Oshima RG. Caspase cleavage of keratin 18 reorganization of intermediate filaments during epithelial cell apoptosis. Journal of Cell Biology. 1997;38:1379–1394. doi: 10.1083/jcb.138.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B, Longtine MS, Sadovsky Y, Nelson DM. Hypoxia downregulates p53 but induces apoptosis and enhances expression of BAD in cultures of human syncytiotrophoblasts. American Journal of Physiology. Cell Physiology. 2010;299:C968–C976. doi: 10.1152/ajpcell.00154.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corless CL, Matzuk MM, Ramabhadran TV, Krichevsky A, Boime I. Gonadotropin beta subunits determine the rate of assembly and the oligosaccharide processing of hormone dimer in transfected cells. Journal of Cell Biology. 1987;104:1173–1181. doi: 10.1083/jcb.104.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker IP, Barratt S, Kaur M, Baker PN. The in vitro characterization of induced apoptosis in placental cytotrophoblasts and syncytiotrophoblasts. Placenta. 2001;22:822–830. doi: 10.1053/plac.2001.0733. [DOI] [PubMed] [Google Scholar]
- Crocker IP, Cooper S, Ong SC, Baker PN. Differences in apoptotic susceptibility of cytotrophoblasts and syncytiotrophoblasts in normal pregnancy to those complicated with preeclampsia and intrauterine growth restriction. American Journal of Pathology. 2003;162:637–643. doi: 10.1016/S0002-9440(10)63857-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker IP, Tansinda DM, Jones CJ, Baker PN. The influence of oxygen and tumor necrosis factor-alpha on the cellular kinetics of term placental villous explants in culture. Journal of Histochemistry and Cytochemistry. 2004;52:749–757. doi: 10.1369/jhc.3A6176.2004. [DOI] [PubMed] [Google Scholar]
- Crocker IP, Arthur P, Heazell AE, Baker PN. The mitotic manipulation of cytotrophoblast differentiation in vitro. Placenta. 2007;28:408–411. doi: 10.1016/j.placenta.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Daoud G, Amyot M, Rassart E, Masse A, Simoneau L, Lafond J. ERK1/2 and p38 regulate trophoblasts differentiation in human term placenta. Journal of Physiology. 2005;566:409–423. doi: 10.1113/jphysiol.2005.089326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desoye G, Hauguel-de Mouzon S. The human placenta in gestational diabetes mellitus. The insulin and cytokine network. Diabetes Care. 2007;30(Suppl 2):S120–S126. doi: 10.2337/dc07-s203. [DOI] [PubMed] [Google Scholar]
- Dinsdale D, Lee JC, Dewson G, Cohen GM, Peter ME. Intermediate filaments control the intracellular distribution of caspases during apoptosis. American Journal of Pathology. 2004;164:395–407. doi: 10.1016/S0002-9440(10)63130-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards D, Jones CJ, Sibley CS, Nelson DM. Paracellular permeability pathways in the human placenta: a quantitative and morphological study of maternal–fetal transfer of horseradish peroxidase. Placenta. 1993;14:63–73. doi: 10.1016/S0143-4004(05)80249-8. [DOI] [PubMed] [Google Scholar]
- Genbacev O, McMaster MT, Fisher SJ. A repertoire of cell cycle regulators whose expression is coordinated with human cytotrophoblast differentiation. American Journal of Pathology. 2000;157:1337–1351. doi: 10.1016/S0002-9440(10)64648-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen K, Bruusgaard JC. Nuclear domains during muscle atrophy: nuclei lost or paradigm lost? Journal of Physiology. 2008;586:2675–2681. doi: 10.1113/jphysiol.2008.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Lu N, Zhou Z. Cellular and nuclear degradation during apoptosis. Current Opinion in Cell Biology. 2009;21:9000–9012. doi: 10.1016/j.ceb.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heazell AE, Moll SJ, Jones CJ, Baker PN, Crocker IP. Formation of syncytial knots is increased by hyperoxia, hypoxia and reactive oxygen species. Placenta. 2007;28(Suppl A):S33–S40. doi: 10.1016/j.placenta.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Buttle HR, Baker PN, Crocker IP. Altered expression of regulators of caspase activity within trophoblast of normal pregnancies and pregnancies complicated by preeclampsia. Reproductive Sciences. 2008a;15:1034–1043. doi: 10.1177/1933719108322438. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Lacey HA, Jones CJ, Huppertz B, Baker PN, Crocker IP. Effects of oxygen on cell turnover and expression of regulators of apoptosis in human placental trophoblast. Placenta. 2008b;29:175–186. doi: 10.1016/j.placenta.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Heazell AE, Sharp AN, Baker PN, Crocker IP. Intra-uterine growth restriction is associated with increased apoptosis and altered expression of proteins in the p53 pathway in villous trophoblast. Apoptosis. 2011;16:135–144. doi: 10.1007/s10495-010-0551-3. [DOI] [PubMed] [Google Scholar]
- Hoshina M, Boothby M, Boime I. Cytological localization of chorionic gonadotropin alpha and placental lactogen mRNAs during development of the human placenta. Journal of Cell Biology. 1982;93:190–198. doi: 10.1083/jcb.93.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Smith SD, Pang L, Sadovsky Y, Nelson DM. Enhanced basal apoptosis in cultured term human cytotrophoblasts is associated with a higher expression and physical interaction of p53 and Bak. Placenta. 2006;27:978–983. doi: 10.1016/j.placenta.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Hung TH, Skepper JN, Charnock-Jones DS, Burton GJ. Hypoxia-reoxygenation: a potent inducer of apoptotic changes in the human placenta and possible etiological factor in preeclampsia. Circulation Research. 2002;90:1274–1281. doi: 10.1161/01.RES.0000024411.22110.AA. [DOI] [PubMed] [Google Scholar]
- Huppertz B, Kingdom J, Caniggia I, Desoye G, Black S, Korr H, Kaufmann P. Hypoxia favours necrotic versus apoptotic shedding of placental syncytiotrophoblasts into the maternal circulation. Placenta. 2003;24:181–190. doi: 10.1053/plac.2002.0903. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Matsuo H, Murakoshi H, Laoag-Fernandez JB, Samoto T, Maruo T. Increased apoptosis in the syncytiotrophoblast in human term placentas complicated by either preeclampsia or intrauterine growth retardation. American Journal of Obstetrics and Gynecology. 2002;186:158–166. doi: 10.1067/mob.2002.119176. [DOI] [PubMed] [Google Scholar]
- Jones CJ, Harris LK, Whittingham J, Aplin JD, Mayhew TM. A re-appraisal of the morphophenotype and basal lamina coverage of cytotrophoblasts in human term placenta. Placenta. 2008;29:215–219. doi: 10.1016/j.placenta.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Kadyrov M, Kaufmann P, Huppertz B. Expression of a cytokeratin 18 neo-epitope is a specific marker for trophoblast apoptosis in human placenta. Placenta. 2001;22:44–48. doi: 10.1053/plac.2000.0616. [DOI] [PubMed] [Google Scholar]
- Kay HH, Nelson DM & Wang Y (Eds) 2011 The Placenta: From Development to Disease, 1st edn. Oxford: John Wiley & Sons.
- Kertschanska S, Kaufmann P. Morphological evidence for the existence of transtrophoblastic channels in human placental villi. Placenta. 1993;13:A33. doi: 10.1016/0143-4004(92)90128-G. [DOI] [Google Scholar]
- Kertschanska S, Kosanke G, Kaufmann P. Is there morphological evidence for transtrophoblastic channels in human placental villi? Trophoblast Research. 1995;8:581–596. [Google Scholar]
- Kliman HJ, Nestler JE, Sermasi E, Sanger JM, Strauss JF. Purification, characterization, and in vitro differentiation of cytotrophoblasts from human term placentae. Endocrinology. 1986;118:1567–1582. doi: 10.1210/endo-118-4-1567. [DOI] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Vandenabeele P, Abrams J, Alnemri ES, Baehrecke EH, Blagosklonny MV, El-Deiry WS, Golstein P, Green DR, et al. Classification of cell death: recommendations of the Nomenclature Committee on Cell Death 2009. Cell Death and Differentiation. 2009;16:3–11. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Liao J, Omary MB. Apoptosis generates stable fragments of human type I keratins. Journal of Biological Chemistry. 1997;272:33197–33203. doi: 10.1074/jbc.272.52.33197. [DOI] [PubMed] [Google Scholar]
- Lecuit M, Nelson DM, Smith SD, Khun H, Huerre M, Vacher-Lavenu MC, Gordon JI, Cossart P. Targeting and crossing of the human maternofetal barrier by Listeria monocytogenes: role of internalin interaction with trophoblast E-cadherin. PNAS. 2004;101:6152–6157. doi: 10.1073/pnas.0401434101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JC, Schickling O, Stegh AH, Oshima RG, Dinsdale D, Cohen GM, Peter ME. DEDD regulates degradation of intermediate filaments during apoptosis. Journal of Cell Biology. 2002;158:1051–1066. doi: 10.1083/jcb.200112124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leers MP, Kolgen W, Bjorklund V, Bergman T, Tribbick G, Persson B, Bjorkland P, Ramaekers FCS, Bjorklund B, Nap M, et al. Immunocytochemical detection and mapping of a cytokeratin 18 neo-epitope exposed during early apoptosis. Journal of Pathology. 1999;197:567–572. doi: 10.1002/(SICI)1096-9896(199904)187:5%3C567::AID-PATH288%3E3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Levy R, Smith SD, Chandler K, Sadovsky Y, Nelson DM. Apoptosis in human cultured trophoblasts is enhanced by hypoxia and diminished by epidermal growth factor. American Journal of Physiology. Cell Physiology. 2000;278:C982–C988. doi: 10.1152/ajpcell.2000.278.5.C982. [DOI] [PubMed] [Google Scholar]
- Levy R, Smith SD, Yusuf K, Huettner PC, Kraus FT, Sadovsky Y, Nelson DM. Trophoblast apoptosis from pregnancies complicated by fetal growth restriction is associated with enhanced p53 expression. American Journal of Obstetrics and Gynecology. 2002;186:1056–1061. doi: 10.1067/mob.2002.122250. [DOI] [PubMed] [Google Scholar]
- Longtine MS, Nelson DM. Placental dysfunction and fetal programming: the importance of placental size, shape, histopathology, and molecular composition. Seminars in Reproductive Medicine. 2011;29:187–196. doi: 10.1055/s-0031-1275515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacFarlane M, Merrison W, Dinsdale D, Cohen GM. Active caspases and cleaved cytokeratins are sequestered into cytoplasmic inclusions in TRAIL-induced apoptosis. Journal of Cell Biology. 2000;148:1239–1254. doi: 10.1083/jcb.148.6.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Simpson RA. Quantitative evidence for the spatial dispersal of trophoblast nuclei in human placental villi during gestation. Placenta. 1994;15:837–844. doi: 10.1016/S0143-4004(05)80185-7. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Wadrop E, Simpson RA. Proliferative versus hypertrophic growth in tissue subcompartments of human placental villi during gestation. Journal of Anatomy. 1994;184:535–543. [PMC free article] [PubMed] [Google Scholar]
- Mayhew TM, Leach L, McGee R, Ismail WW, Myklebust R, Lammiman MJ. Proliferation, differentiation and apoptosis in villous trophoblast at 13–41 weeks of gestation (including observations on annulate lamellae and nuclear pore complexes) Placenta. 1999;20:407–422. doi: 10.1053/plac.1999.0399. [DOI] [PubMed] [Google Scholar]
- McNeil PL, Kirchhausen T. An emergency response team for membrane repair. Nature Reviews. Molecular Cell Biology. 2005;6:499–505. doi: 10.1038/nrm1665. [DOI] [PubMed] [Google Scholar]
- Mori M, Ishikawa G, Luo SS, Mishima T, Goto T, Robinson JM, Matsubara S, Takeshita T, Kataoka H, Takizawa T. The cytotrophoblast layer of human chorionic villi becomes thinner but maintains its structural integrity during gestation. Biology of Reproduction. 2007;76:164–172. doi: 10.1095/biolreprod.106.056127. [DOI] [PubMed] [Google Scholar]
- Ndozangue-Touriguine O, Hamelin J, Breard J. Cytoskeleton and apoptosis. Biochemical Pharmacology. 2008;76:11–18. doi: 10.1016/j.bcp.2008.03.016. [DOI] [PubMed] [Google Scholar]
- Nelson DM. Apoptotic changes occur in syncytiotrophoblast of human placental villi where fibrin type fibrinoid is deposited at discontinuities in the villous trophoblast. Placenta. 1996;17:387–391. doi: 10.1016/S0143-4004(96)90019-3. [DOI] [PubMed] [Google Scholar]
- Nelson DM, Crouch EC, Curran EM, Farmer DR. Trophoblast interaction with fibrin matrix. Epithelialization of perivillous fibrin deposits as a mechanism for villous repair in the human placenta. American Journal of Pathology. 1990;136:855–865. [PMC free article] [PubMed] [Google Scholar]
- Nunez R, Sancho-Martinez SM, Novoa JM, Lopez-Hernandez FJ. Apoptotic volume decrease as a geometric determinant for cell dismantling into apoptotic bodies. Cell Death and Differentiation. 2010;17:1665–1671. doi: 10.1038/cdd.2010.96. [DOI] [PubMed] [Google Scholar]
- Rampersad R, Nelson DM. Trophoblast biology, responses to hypoxia and placental dysfunction in preeclampsia. Frontiers in Bioscience. 2007;12:2447–2456. doi: 10.2741/2246. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Circulating microparticles in normal pregnancy and pre-eclampsia. Placenta. 2008;29(Suppl A):S73–S77. doi: 10.1016/j.placenta.2007.11.016. [DOI] [PubMed] [Google Scholar]
- Redman CW, Sargent IL. Placental stress and pre-eclampsia: a revised view. Placenta. 2009;30(Suppl A):S38–S42. doi: 10.1016/j.placenta.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Roje D, Tomas SZ, Prusac IK, Capkun V, Tadin I. Trophoblast apoptosis in human term placentas from pregnancies complicated with idiopathic intrauterine growth retardation. Journal of Maternal–Fetal and Neonatal Medicine. 2011;24:745–751. doi: 10.3109/14767058.2010.526158. [DOI] [PubMed] [Google Scholar]
- Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. Journal of Cellular Physiology. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3%3C311::AID-JCP1%3E3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Schutte B, Henfling M, Kolgen W, Bouman M, Meex S, Leers MP, Nap M, Bjorklund V, Bjorklund P, Bjorklund B, et al. Keratin 8/18 breakdown and reorganization during apoptosis. Experimental Cell Research. 2004;297:11–26. doi: 10.1016/j.yexcr.2004.02.019. [DOI] [PubMed] [Google Scholar]
- Schutte B, Henfling M, Ramaekers FC. DEDD association with cytokeratin filaments correlates with sensitivity to apoptosis. Apoptosis. 2006;11:1561–1572. doi: 10.1007/s10495-006-9113-0. [DOI] [PubMed] [Google Scholar]
- Scifres CM, Nelson DM. Intrauterine growth restriction, human placental development and trophoblast cell death. Journal of Physiology. 2009;587:3453–3458. doi: 10.1113/jphysiol.2009.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp AN, Heazell AEM, Crocker IP, Mor G. Placental apoptosis in health and disease. American Journal of Reproductive Immunology. 2010;64:159–164. doi: 10.1111/j.1600-0897.2010.00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson RA, Mayhew TM, Barnes PR. From 13 weeks to term, the trophoblast of human placenta grows by the continuous recruitment of new proliferative units: a study of nuclear number using the disector. Placenta. 1992;13:501–512. doi: 10.1016/0143-4004(92)90055-X. [DOI] [PubMed] [Google Scholar]
- Smith SC, Baker PN, Symonds EM. Increased placental apoptosis in intrauterine growth restriction. American Journal of Obstetrics and Gynecology. 1997;177:1395–1401. doi: 10.1016/S0002-9378(97)70081-4. [DOI] [PubMed] [Google Scholar]
- Straszewski-Chavez SL, Abrahams VM, Mor G. The role of apoptosis in the regulation of trophoblast survival and differentiation during pregnancy. Endocrine Reviews. 2005;26:877–897. doi: 10.1210/er.2005-0003. [DOI] [PubMed] [Google Scholar]
- Tabata T, McDonagh S, Kawakatsu H, Pereira L. Cytotrophoblasts infected with a pathogenic human cytomegalovirus strain dysregulate cell–matrix and cell–cell adhesion molecules: a quantitative analysis. Placenta. 2007;28:527–537. doi: 10.1016/j.placenta.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Tao G-Z, Li DH, Toivola DM, Strnad P, Sandersara N, Cheung RC, Hong A, Omary MB. Monitoring of epithelial cell caspase activation via detection of durable keratin fragment formation. Journal of Pathology. 2008;215:164–174. doi: 10.1002/path.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomas SZ, Prusac IK, Roje D, Tadin I. Trophoblast apoptosis in placentas from pregnancies complicated by preeclampsia. Gynecologic and Obstetric Investigation. 2011;71:250–255. doi: 10.1159/000320289. [DOI] [PubMed] [Google Scholar]
- Wang F, Wang F, Zou Z, Liu D, Wang J, Su Y. Active deformation of apoptotic intestinal epithelial cells with adhesion-restricted polarity contributes to apoptotic clearance. Laboratory Investigation. 2011;91:462–471. doi: 10.1038/labinvest.2010.182. [DOI] [PubMed] [Google Scholar]
- Werner ME, Chen F, Moyano JV, Yehiely F, Jones JC, Cryns VL. Caspase proteolysis of the integrin beta4 subunit disrupts hemidesmosome assembly, promotes apoptosis, and inhibits cell migration. Journal of Biological Chemistry. 2007;282:5560–5569. doi: 10.1074/jbc.M603669200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao R, Ferry AL, Dupont-Versteegden EE. Cell death-resistance of differentiated myotubes is associated with enhanced anti-apoptotic mechanisms compared to myoblasts. Apoptosis. 2011;16:221–234. doi: 10.1007/s10495-010-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusuf K, Smith SD, Sadovsky Y, Nelson DM. Trophoblast differentiation modulates the activity of caspases in primary cultures of term human trophoblasts. Pediatric Research. 2002;52:411–415. doi: 10.1203/00006450-200209000-00018. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.