SUMMARY
Setting
Adult HIV clinic in Tanzania.
Objective
In Africa, 10% of HIV infected adults starting antiretroviral therapy (ART) die within the first year, and tuberculosis (TB) is the leading cause of death. In this study, we investigated the predictors of ART-associated TB.
Design
In this nested case-control study, adults starting ART were screened for TB according to the WHO protocol and those not diagnosed with TB were observed for six months. Patients diagnosed with tuberculosis were defined as cases, and controls were selected from among patients who did not develop tuberculosis using incidence density matching.
Results
Among the 2514 HIV positive adults in our cohort, 72 (3%) were diagnosed with TB during the first six months of ART. By multivariate analysis, baseline characteristics predictive of TB were cough, fever, and night sweats and 76% (55/72) of cases had at least one of these symptoms at the time of starting ART.
Conclusion
75% of patients who developed TB during the first six months of ART had symptoms of TB at time of starting ART but the symptoms were sub-diagnostic. Improved TB diagnostics and/or better strategies for empirical anti-TB are needed for patients with symptoms of TB at ART initiation.
Keywords: HIV, mortality, cohort
INTRODUCTION
Ten percent of HIV-infected individuals who initiate antiretroviral therapy (ART) in sub-Saharan Africa will die within the first year.1 Many of these early deaths are likely related to tuberculosis (TB).2,3 The incidence of TB among HIV-infected individuals is highest in the first six months after the initiation of ART, most probably due to a combination of missed diagnosis and immune reconstitution inflammatory syndrome, and declines thereafter.2–4 TB occurring during the first six months after the initiation of ART is associated with higher mortality when compared to tuberculosis diagnosed before the initiation of ART.5
Because of the high incidence and mortality of TB after the initiation of ART, the World Health Organization (WHO) recommends careful screening for TB among all HIV-infected individuals starting ART.6 The utility of this diagnostic algorithm has been hampered by the fact that HIV-infected individuals starting ART in Africa often have a very low CD4+ T-cell count (CD4 count) and therefore present with atypical symptoms and chest radiograph findings and negative sputum smears for acid-fast bacilli (AFB).7–8
In this study, we examined a large African cohort of HIV-infected individuals who had all been screened for TB at the time of ART initiation by history and physical examination as well as by chest radiograph. We identified all cases of TB that were diagnosed within the first 6 months after ART initiation and conducted a nested case-control study to identify the clinical factors, present at the time of ART initiation, that were predictive of TB diagnosis.
STUDY POPULATION AND METHODS
Setting
This study was conducted in the outpatient HIV clinic of the Bugando Medical Centre in Mwanza, Tanzania. Bugando Medical Centre (BMC) is the zonal referral hospital for the Lake Victoria region in northwest Tanzania, and it serves a population of approximately 13 million people. Our outpatient HIV clinic has initiated ART in over 4000 patients. Patients are referred to Bugando from surrounding, community-based voluntary counseling and testing centers in the city of Mwanza.
Patients satisfying WHO criteria for ART are started on therapy and seen monthly or bi-monthly at the BMC clinic. At the time of the study, WHO criteria for starting ART included clinical stage three disease with CD4 count less than 350cells/μL, stage four disease regardless of CD4 count, or CD4 count less than 200cells/μL. At the time of the study, the first-line ART regimen was either zidovudine or stavudine + lamivudine + nevirapine or efavirenz. Cotrimoxazole prophylaxis was prescribed for all patients with CD4 counts of ≤ 350 cells/μL and/or WHO clinical stage III or IV, including all patients with TB, in accordance with Tanzanian national guidelines.9
Before initiation of ART, all patients at our clinic are screened for TB by history, physical examination, and chest X-ray. They also have a hemoglobin level measured. In accordance with the WHO and Tanzanian national guidelines, two AFB smears are performed and empiric anti-TB therapy should be considered for all patients with one or more of the following symptoms: productive cough for ≥2 weeks, fever for ≥2 weeks, night sweats for ≥2 weeks, hemoptysis, or weight loss.9,10 Culture for Mycobacterium tuberculosis was not available at our center at the time of the study. Any patient with suspected or proven TB is initiated on anti-TB treatment before the initiation of ART.
All patients diagnosed with TB at our clinic are treated with the standard six-month regimen of fixed-dose combination pills. This entails two months of isoniazid, rifampicin, pyrazinamide and ethambutol followed by four months of isoniazid and rifampicin. Efavirenz-based ART regimens were used for patients on anti-TB therapy. Isoniazid prophylaxis was not part of the national guidelines at the time of the study.
Population
In this study, we included all adult (≥13 years), HIV-positive, ART treatment-naive patients who started ART at BMC between January 2006 and June 2009 who were not either diagnosed with active TB or receiving treatment for TB at the time of initiating ART. All patients underwent monthly evaluation for TB symptoms for the first six months, and dates of events occurring within those six months were recorded. For the diagnosis of pulmonary TB, we used the case definition of the American Thoracic Society. Diagnosis of TB required either: (1) symptoms consistent with TB and microbiologic confirmation of disease, or (2) symptoms and chest radiograph findings consistent with TB, plus a positive response to anti-TB therapy.5,11 The diagnosis of extrapulmonary TB was based on microbiologic confirmation and/or histopathological findings.
Patients who did not have a diagnosis of TB at the time of ART initiation and who were subsequently diagnosed with TB within the first six months of ART were defined as cases. Controls were randomly selected from among enrolled patients who were not diagnosed with TB within the first six months of ART. Controls were matched one-to-one with cases using incidence density matching to ensure that controls had similar lengths of follow-up as cases at the time of TB diagnosis.12
Design and data collection
We performed a cohort study with a nested case-control component. Data were collected by the investigator from the patient’s medical records and recorded on a standardized form. All ART enrollment characteristics of patients were recorded including demographics, clinical symptoms/signs, WHO clinical stage, tuberculosis status, body mass index (BMI), CD4 count, hemoglobin and chest radiograph findings. Chest radiographs were interpreted independently by the investigator and another physician who were both blinded to patients’ diagnoses. Any differences in interpretations of radiographs were resolved by the hospital radiologist.
Data analysis
Data were analyzed using Stata, Version 10 (Stata Corporation, College Station, Texas). The primary study outcome was diagnosis of TB during the first six months of ART. Categorical variables were presented as proportions and continuous variables as medians with interquartile ranges (IQRs). The χ2 test or Fisher’s exact test was used to compare proportions and the Wilcoxon rank-sum test was used to compare medians. All statistical tests were two-sided with α=0.05. Variables which were significant on the univariate analyses were included in the multivariate analyses. Logistic regression with a stepwise selection method was used to determine predictors of TB diagnosed during the first six months of ART.
Ethics
Ethical clearance was obtained from institutional review boards of both BMC and Weill Cornell Medical College.
RESULTS
Enrollment
Between January 2006 and June 2009, 2787 patients ≥13 years old were started on first-line ART at the BMC HIV clinic. Of these, 273 (10%) were receiving treatment for TB at the time of initiating ART. A total of 2514 HIV-positive adults who started first-line ART at the clinic between January 2006 and June 2009 and who were not receiving anti-TB therapy at the time of initiating ART were included in this study.
Study cohort
Of the 2514 HIV-positive adults included in the cohort: 65% were female, the median age was 36 years (IQR 31–43), the median baseline CD4 count was 106 cells/μL (IQR 47–165), and the median BMI was 20 kg/m2 (IQR 18–23). During the first 6 months of follow-up, 107 (4%) died and 263 (10%) were lost to follow-up. The mortality rate in this cohort was 108/1000 person-years in the first three months of ART compared to 37/1000 person-years in months 4–6. The loss-to-follow-up rate, defined as three consecutive missed appointments and failure to return to clinic thereafter, was 223/1000 person-years in the first six months.
Cases
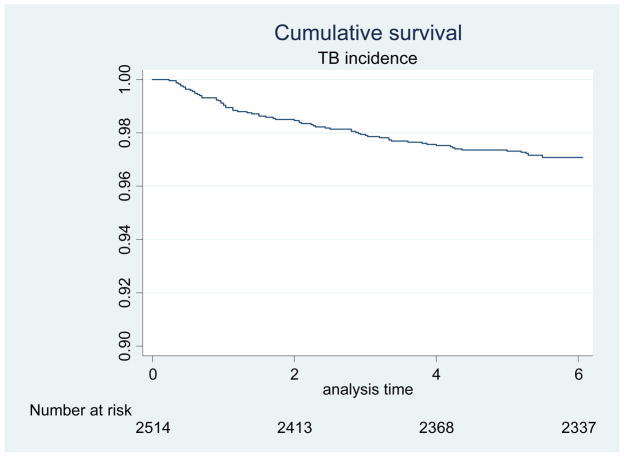
Among 2514 patients who had not been diagnosed with TB at the time of starting ART, 72 (3%) were diagnosed with TB within six months of ART for an incidence rate of 48/1000 person-years. Fifty-three of these 72 cases were diagnosed in months 1–3 of ART, making the incidence of TB diagnosis in this time period 70/1000 person-years, as compared to 27/1000 person-years in months 4–6 of ART (p-value for difference <0.001). This is in contrast to a reported national incidence of 31/1000 for HIV-infected adults not on ART and 4/1000 for those on ART.13 A Kaplan Meier curve for time to incident TB is shown in Figure 1.
Figure 1.
Kaplan-Meier Curve of Time to Incidence of Tuberculosis in the First Six Months after Initiation of Antiretroviral Therapy.
Among our 72 cases of TB, 66 (92%) were diagnosed with pulmonary TB and six (8%) were diagnosed with extrapulmonary TB: two with TB lymphadenopathy and four with pleural TB. Of the cases with pulmonary TB, 11/66 (17%) were sputum smear-positive and 55/66 (83%) were smear-negative.
Nested case-control study
For the 72 cases, we selected 72 controls without tuberculosis. We used incidence density matching to ensure that controls were at risk, and followed, for the same amount of time that TB cases were when they were diagnosed with TB. The characteristics of the 72 cases and 72 controls, as well as univariate analysis for predictors of development of TB, are shown in Table 1. Cases and controls were similar with regard to gender, age, BMI, CD4 count and WHO clinical stage at the time of starting ART. We observed a trend among cases toward higher rates of death or loss to follow-up in the first six months than in incidence density-matched controls (13% versus 4%, p=0.07).
Table 1.
Baseline Characteristics of 72 Cases of TB Diagnosed During the First 6 Months of ART and 72 Matched Controls.
| Cases (n=72) | Controls (n=72) | p-value | |
|---|---|---|---|
| Gender | |||
| Female | 42 (58%) | 43 (60%) | 0.87 |
| Age (Y) | |||
| Median (IQR) | 36 (29–42) | 40 (31–45) | 0.13 |
| BMI (kg/m2) | |||
| Median (IQR) | 19.9 (18.4–21.6) | 19.9 (17.5–23.1) | 0.23 |
| CD4 Count (cells/uL) | |||
| Median (IQR) | 106 (43–173) | 121 (50–192) | 0.14 |
| WHO Clinical Stages | |||
| Stage 1 | 9 (13%) | 11 (15%) | |
| Stage 2 | 20 (28%) | 21 (29%) | |
| Stage 3 | 29 (40%) | 27 (38%) | |
| Stage 4 | 14 (19%) | 13 (18%) | 0.95 |
| Hemoglobin (g/dL) | |||
| Median (IQR) | 10.4 (8.3–12.2) | 10.0 (9.0–12.9) | 0.43 |
| Symptoms | |||
| Symptomatic | 57 (79%) | 13 (18%) | <0.001* |
| Fever | 37 (51%) | 9 (13%) | <0.001* |
| Cough | 44 (61%) | 7 (10%) | <0.001* |
| Weight Loss | 16 (22%) | 5 (7%) | 0.009* |
| Night Sweats | 9 (13%) | 0 | 0.002* |
| Diarrhea | 10 (14%) | 1 (1%) | 0.005* |
| Signs | |||
| Adenopathy | 2 (3%) | 0 | 0.15 |
| Ascites | 1 (1%) | 0 | 0.32 |
| Outcomes | |||
| Death | 6 (8%) | 2 (3%) | 0.15 |
| Lost to Follow-up | 3 (4%) | 1 (1%) | 0.31 |
| Death + Lost | 9 (13%) | 3 (4%) | 0.07 |
Statistically significant by univariate analysis.
By multivariate analysis, baseline characteristics predictive of TB were cough (OR 10.0 [95% CI 3.5–28.2], p<0.001), fever (OR 4.0 [1.4–11.0], p=0.01), and night sweats. Results of the multivariate analysis are shown in Table 2.
Table 2.
Risk Factors (Present at Time of ART Initiation) for Incident TB During the First 6 Months of ART by Multivariate Analysis.
| Risk Factor | p-value | Odds Ratio | 95% Confidence Interval |
|---|---|---|---|
| Cough | <0.001* | 10.006 | 3.554 – 28.174 |
| Fever | 0.008* | 3.964 | 1.426 – 11.024 |
| Diarrhea | 0.071 | 7.779 | 0.838 – 72.195 |
| Weight Loss | 0.490 | 0.604 | 0.144 – 2.528 |
Statistically significant by multivariate analysis.
Night sweats was excluded from our multivariate model since all 9 patients with night sweats in our case-control population developed incident TB. For this reason, an odds ratio could not be calculated for night sweats.
Of the 72 cases diagnosed with TB during the first six months of ART, 55/72 (76%) had at least one of these three symptoms (either cough, fever, or night sweats) at the time of initiating ART compared to 12/72 (17%) of controls (p <0.001). Also, 28/72 (39%) of cases had ≥ two of these symptoms compared with only 4/72 (6%) of controls (p <0.001). Cases with CD4 counts <100 cells/μL were just as likely to have symptoms of TB at the time of starting ART as cases with CD4 counts >100 cells/μL.
Analysis of chest radiographs
For all patients beginning ART at Bugando, chest radiographs were routinely performed at the time of ART initiation. Of note, the hospital radiology unit was damaged during a flood after the study period and a portion of the chest radiographs were destroyed; we therefore could not review these radiographs. Thus we have radiographs at the time of ART initiation on 45 patients who subsequently developed TB.
For these 45 cases with available chest radiographs, we selected a second control patient using incidence density matching so that X-rays were matched 2:1. The characteristics of the 45 chest radiographs for cases and 90 chest radiographs for controls are shown in Table 3. Significant predictors of TB by chest X-ray included any abnormality, diffuse opacities and hilar adenopathy.
Table 3.
Baseline chest radiograph (CXR) findings among of 45 Cases of TB Diagnosed During the First 6 Months of ART and 90 Matched Controls.
| CXR Findings | Cases | Controls | p-value |
|---|---|---|---|
| Cavitations | 0 | 2 (2.2%) | 0.55 |
| Upper lobe opacity | 1 (2.2%) | 2 (2.2%) | 0.71 |
| Lower lobe opacity | 6 (13.3%) | 5 (5.6%) | 0.18 |
| Diffuse opacities | 13 (28.9%) | 12 (13.3%) | 0.04* |
| Hilar adenopathy | 8 (17.8%) | 3 (3.3%) | 0.007* |
| Any CXR abnormality | 26 (57.8%) | 23 (25.6%) | <0.001* |
Statistically significant by univariate analysis.
DISCUSSION
This study explored baseline predictors of incident TB in patients starting ART and found that more than three-fourths of patients (76%) diagnosed with TB in the first six months after ART initiation had classic symptoms of TB (fever, cough or night sweats) at the time of initiating ART compared to only 17% of controls. These findings, gathered from a large cohort of HIV-infected adults starting ART in sub-Saharan Africa, lend evidence to the hypothesis that most HIV-infected individuals who are diagnosed with tuberculosis during the first six months of ART actually have sub-diagnostic TB at the time of ART initiation. TB was present, but was below the threshold for the diagnosis of TB and initiation of TB therapy. These results have important implications for the management of HIV-infected adults in sub-Saharan Africa as TB is a major contributor to the 10% mortality rate seen during the first year after initiation of ART.1,14,15
In addition to symptoms, we also found that abnormalities of the chest radiograph at the time of ART initiation were predictive of subsequent TB. To the best of our knowledge, this is the first study to look at the utility of abnormal chest radiograph findings at the time of ART initiation to predict the development of TB within the first six months of ART. The abnormalities that were significantly associated with TB (diffuse opacities and hilar adenopathy) are not particularly subtle findings and, in resource-limited settings, could presumably be identified by a clinician even without the input of a radiologist.
Patients who were diagnosed with TB during the first six months after starting ART demonstrated a trend toward higher rates of death and loss to follow-up when compared to incidence density-matched controls. Our findings are consistent with a study from Haiti that showed high mortality rates among HIV-infected individuals who were diagnosed with TB after the initiation of ART.5
During our study period, in accordance with then-current WHO guidelines (10), patients with cough or fever only underwent workup for TB if the symptom had persisted for ≥2 weeks. New WHO guidelines recommend workup for TB in any HIV-infected adult with cough, fever, weight loss or night sweats of any duration.16 Since 75% of the incident TB cases in our cohort had at least one of these symptoms at the time of starting ART, these would have undergone more evaluation for TB before the initiation of ART. Our data provides support for the application of these new guidelines to this population.
While these new guidelines are a major improvement, the question still remains as to whether clinicians would have decided to treat for TB in these cases and whether further evaluation would have delayed ART in a setting where the only diagnostic tests available for TB are chest radiograph and sputum smear. Clearly, better diagnostic tests are needed. Several newly-emerging diagnostic tools show great promise in providing rapid diagnosis of smear-negative TB.17 A low-cost bedside urine assay for TB antigens has been recently shown to have >70% sensitivity in diagnosing smear-negative TB among HIV-infected adults starting ART at a median CD4 count of 106 cells/μL.18 In the meantime, a policy of empiric anti-TB therapy for patients with any symptom of TB at the time of ART initiation may be warranted. This may lead not only to reduced transmission and mortality, but also could serve as TB prophylaxis.19
Our study has several limitations. First, the use of 1:1 matching, rather than a greater ratio of controls to cases, decreased the study’s power to detect subtle predictors of TB but the major findings are highly significant even with 1:1 matching. Second, CXRs were missing for ~40% of cases. Third, certain clinical characteristics such as the duration of symptoms, use of antibiotics among controls, and objective measures or response to anti-TB therapy were not routinely recorded by clinicians in our HIV clinic and therefore could not be evaluated.
CONCLUSIONS
TB is common in the first six months after ART initiation and is associated with high mortality. In our case-control study, most patients who developed TB during the first six months of ART had symptoms of TB and/or abnormalities on the chest radiograph at the time of initiating ART but these symptoms were sub-diagnostic (i.e. below the threshold of current diagnostic standards and technologies). Rapid and specific diagnostic tests for TB are clearly needed. Meanwhile, strategies for empiric anti-TB should be considered.
Acknowledgments
Funding:
This project was supported by a grant from the United States National Institute of Health Fogarty International Center (TW 00018) and a scholarship program at Weill Cornell Medical College supported by Pfizer Inc. The sponsors were not involved in study design or preparation of the manuscript.
We would also like to thank Dr. Charles Majinge, Director of Bugando Medical Centre, for his support. We would also like to thank Ms. Lauren Webster for her assistance in data retrieval.
References
- 1.Braitstein P, Brinkhof MWG, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006;367:817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Bonnet MMB, Pinoges LLP, Varaine FFV, et al. Tuberculosis after HAART initiation in HIV-positive patients from five countries with a high tuberculosis burden. AIDS. 2006;20(9):1275–9. doi: 10.1097/01.aids.0000232235.26630.ee. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, Myer L, Bekker L-G, Wood R. Burden of tuberculosis in an antiretroviral treatment programme in sub-Saharan Africa: impact on treatment outcomes and implications for tuberculosis control. AIDS. 2006;20(12):1605–12. doi: 10.1097/01.aids.0000238406.93249.cd. [DOI] [PubMed] [Google Scholar]
- 4.Dembélé M, Saleri N, Carvalho ACC, et al. Incidence of tuberculosis after HAART initiation in a cohort of HIV-positive patients in Burkina Faso. Int J Tuberc Lung Dis. 2010;14(3):318–23. [PubMed] [Google Scholar]
- 5.Koenig SP, Riviere C, Leger P, et al. High mortality among patients with AIDS who received a diagnosis of tuberculosis in the first 3 months of antiretroviral therapy. Clin Infect Dis. 2009;48(6):829–31. doi: 10.1086/597098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. Interim policy on collaborative TB/HIV activities. Geneva, Switzerland: 2004. WHO/HTM/TB/2004.330. [Google Scholar]
- 7.Cain KP, McCarthy KD, Heilig CM, et al. An algorithm for tuberculosis screening and diagnosis in people with HIV. N Engl J Med. 2010;362(8):707–16. doi: 10.1056/NEJMoa0907488. [DOI] [PubMed] [Google Scholar]
- 8.Mtei L, Matee M, Herfort O, et al. High rates of clinical and subclinical tuberculosis among HIV-infected ambulatory subjects in Tanzania. Clin Infect Dis. 2005;40(10):1500–7. doi: 10.1086/429825. [DOI] [PubMed] [Google Scholar]
- 9.Tanzanian Ministry of Health. National Guidelines for Clinical Management of HIV/AIDS. Dar es Salaam; Tanzania: 2009. [Google Scholar]
- 10.World Health Organization. Improving the diagnosis and treatment of smear-negative pulmonary and extrapulmonary tuberculosis among adults and adolescents: recommendations for HIV-prevalent and resource-constrained settings. Geneva, Switzerland: 2007. WHO/HTM/2007.379 & WHO/HIV/2007.1.:1–36. [Google Scholar]
- 11.Blumberg HM, Burman WJ, Chaisson RE, et al. American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis. Am J Respir Crit Care Med. 2003;167(4):603–62. doi: 10.1164/rccm.167.4.603. [DOI] [PubMed] [Google Scholar]
- 12.Richardson DB. An incidence density sampling program for nested case-control analyses. Occup Environ Med. 2004 Dec;61(12):e59. doi: 10.1136/oem.2004.014472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somi G, van den Hombergh J, Todd J, Kilama B, Josiah R, RS High TB burden associated with five years (2004–2009) antiretroviral treatment for HIV/AIDS in Tanzania. 6th IAS Conference on HIV Pathogenesis, Treatment and Prevention; 2011. TUPE422-Poster Exhibition. Available from: http://pag.ias2011.org/Abstracts.aspx?AID=2329. [Google Scholar]
- 14.Lawn SD, Harries AD, Anglaret X, Myer L, Wood R. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22(15):1897–908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meintjes G, Lawn SD, Scano F, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–23. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.World Health Organization. Guidelines for intensified tuberculosis case-finding and isoniazid preventive therapy for people living with HIV in resource-constrained settings. Geneva, Switzerland: 2011. Available from: http://whqlibdoc.who.int/publications/2011/9789241500708_eng.pdf. [Google Scholar]
- 17.Lawn SD, Wood R. Tuberculosis in antiretroviral treatment services in resource-limited settings: addressing the challenges of screening and diagnosis. J Infect Dis. 2011;204 (Suppl 4):S1159–67. doi: 10.1093/infdis/jir411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawn SD, Kerkhoff AD, Vogt M, Wood R. Diagnostic accuracy of a low-cost, urine antigen, point-of-care screening assay for HIV-associated pulmonary tuberculosis before antiretroviral therapy: a descriptive study. Lancet Infect Dis. 2011;3099(11):1–9. doi: 10.1016/S1473-3099(11)70251-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lawn SD, Ayles H, Egwaga S, et al. Potential utility of empirical tuberculosis treatment for HIV-infected patients with advanced immunodeficiency in high TB-HIV burden settings. Int J Tuberc Lung Dis. 2011 Mar;15(3):287–95. [PubMed] [Google Scholar]