Abstract
Background and Purpose
The guinea pig trachea (GPT) is commonly used in airway pharmacology. The aim of this study was to define the expression and function of EP receptors for PGE2 in GPT as there has been ambiguity concerning their role.
Experimental Approach
Expression of mRNA for EP receptors and key enzymes in the PGE2 pathway were assessed by real-time PCR using species-specific primers. Functional studies of GPT were performed in tissue organ baths.
Key Results
Expression of mRNA for the four EP receptors was found in airway smooth muscle. PGE2 displayed a bell-shaped concentration–response curve, where the initial contraction was inhibited by the EP1 receptor antagonist ONO-8130 and the subsequent relaxation by the EP2 receptor antagonist PF-04418948. Neither EP3 (ONO-AE5-599) nor EP4 (ONO-AE3-208) selective receptor antagonists affected the response to PGE2. Expression of COX-2 was greater than COX-1 in GPT, and the spontaneous tone was most effectively abolished by selective COX-2 inhibitors. Furthermore, ONO-8130 and a specific PGE2 antibody eliminated the spontaneous tone, whereas the EP2 antagonist PF-04418948 increased it. Antagonists of other prostanoid receptors had no effect on basal tension. The relaxant EP2 response to PGE2 was maintained after long-term culture, whereas the contractile EP1 response showed homologous desensitization to PGE2, which was prevented by COX-inhibitors.
Conclusions and Implications
Endogenous PGE2, synthesized predominantly by COX-2, maintains the spontaneous tone of GPT by a balance between contractile EP1 receptors and relaxant EP2 receptors. The model may be used to study interactions between EP receptors.
Keywords: PGE2, airway smooth muscle, trachea, spontaneous tone, EP1, EP2, EP3, EP4, guinea pig
Introduction
PGE2 is a central messenger molecule with diverse biological effects. The action of non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit the COX reaction that catalyses its biosynthesis, have implicated PGE2 as a mediator of pain and inflammation (Flower, 2006). Nevertheless, PGE2 is continuously released in the airways (Brink et al., 1981; Selg et al., 2009) where it is involved in protective and anti-inflammatory responses. For example, refractoriness to repeated bouts of exercise-induced bronchoconstriction appears to depend upon local formation of PGE2 (Manning et al., 1993), and accordingly, inhalation of PGE2 will inhibit bronchoconstriction evoked by exercise, as well as several other triggers of asthma attacks (Pavord and Tattersfield, 1995). Furthermore, the inhalation of PGE2 is also associated with cough (Pavord and Tattersfield, 1995). The multiple effects of PGE2 are obviously explained by the presence of several EP receptors that may mediate different and sometimes opposing responses (Coleman et al., 1984).
The first observations of contractile and relaxant effects of PGE2 in the airways were made in the isolated guinea pig trachea (GPT) (Anggard and Samuelsson, 1965; Coleman and Kennedy, 1980; Gardiner and Collier, 1980) where the cumulative concentration–response curve for PGE2 was found to be biphasic (Coleman and Kennedy, 1980). In fact, experiments using this standard preparation for airway pharmacology provided early data for the general classification of prostanoid receptors (Coleman and Kennedy, 1980; 1985; Gardiner and Collier, 1980; Kennedy et al., 1982). Along with the observations that aspirin and other NSAIDs (Orehek et al., 1973), as well as the PG antagonist SC-19220 (Farmer et al., 1974), caused relaxation of GPT basal tone, it was assumed that endogenous PGs in this preparation predominantly acted on receptors that mediated contractions. Initially, it was thought that PGF2α or thromboxane (TX) A2 were the contractile compounds responsible for maintaining the smooth muscle tone (Farmer et al., 1974; Raeburn et al., 1987), but more recent evidence favours PGE2 as responsible for this role in GPT by activation of contractile EP1 receptors (Ndukwu et al., 1997).
Research on the mechanisms involved in the actions of PGE2 in the airways has however been limited by the low selectivity and potency of the pharmacological tools available. Although experiments using the previous generation of drugs support a general concept where bronchoconstriction is mediated by EP1 and EP3 receptors, and airway relaxation by EP2 and EP4 receptors, (Jones et al., 2009; Buckley et al., 2011), it has not so far been possible to simultaneously assess the role of each EP receptor in any airway preparation. For example, a role for the EP1 receptor in maintaining the basal tone of the GPT was implied by experiments using the compound AH6809 (Ndukwu et al., 1997), an unselective antagonist with similar affinities for EP1, EP2, EP3, DP1 and TP receptors (Abramovitz et al., 2000).
More selective agonists and antagonists for the EP receptors have recently become available (Aihara et al., 2007; Forselles et al., 2011). In this study, we have used a new generation of subtype-selective EP receptor antagonists to establish which receptors mediate the contractions and relaxations of GPT to exogenous PGE2. Intervention with antagonists for each of the EP receptors has to the best of our knowledge not been investigated simultaneously in any airway preparation, although some of the new antagonists have been tested individually (Buckley et al., 2011). The present study in particular examined the role of the different EP receptors in controlling the basal tone of the preparation. As there are considerable species differences regarding responsiveness to prostanoids (Martin et al., 1988; Held et al., 1999; Ressmeyer et al., 2006), one intended outcome of this study was to demonstrate the usefulness of the much employed GPT model for future research on PGE2 in the airways and in general.
On the basis of experiments with one selective COX-2 inhibitor, it has been proposed that PGE2 in GPT is generated by COX-2 (Charette et al., 1995). In order to clarify the pathways for PGE2 formation, we used several isotype selective COX inhibitors in this study. Another objective of the present investigation was to establish whether PGE2 alone is the critical COX product that maintains basal tone of the preparation, or if other COX products are involved. We therefore assessed the effects of all known prostanoid receptor antagonists, as well a highly specific PGE2 antibody (Mnich et al., 1995), on GPT basal tone.
In an attempt to provide a stronger molecular framework behind the observed functional responses, the present study for the first time uses real time-PCR with guinea pig-specific primers to document the expression of mRNA for the four EP receptors in different parts of the guinea pig lung and aorta. Although the study is focused on the characterization of EP receptors, we took advantage of the methodology for construction of species specific primers and also explored the mRNA expression of COX and PGE synthase (PGES) enzymes in the pathway for biosynthesis of PGE2 in the same tissues used for mapping of the receptors.
Methods
Tissue preparation and organ culture
All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). The study was approved by the regional animal experimentation ethical review board (N63/07 and N257/09). Trachea and aorta from male albino guinea pigs (Dunkin–Hartley; 350–600 g) and trachea from male albino rats (Sprague–Dawley; 300–350 g) were prepared as previously described (Adner et al., 2002; Morin et al., 2005; Larsson et al., 2007) with some minor changes (Supplementary methods).
Tissue organ bath
Intact guinea pig tracheal and aorta or rat tracheal segments cut as rings were set up in 5 mL organ baths with Tyrode's buffer (Supplementary methods). Changes in smooth muscle force were detected using an isometric force displacement transducer linked to a Grass polygraph. The response was displayed using the IOX data acquisition system (EMKA, Paris, France). During an equilibration period of 60 min with washes every 15 min, the resting force was adjusted to either 30 mN for the guinea pig trachea (GPT), 8 mN for guinea pig aorta and 10 mN for the rat trachea. As a control of guinea pig tracheal reactivity, histamine (0.1 nM to 0.3 mM) was cumulatively added, whereas for the guinea pig aorta and rat trachea, 60 mM KCl was applied. Before the pharmacological studies, a second wash period and a further 30–45 min equilibration period was completed. In aorta, the presence of an intact endothelium was assessed at the end of the experiment by relaxation to acetylcholine (0.1–10 μM) after pre-contraction with phenylephrine (10 μM).
To investigate the involvement of COX-activity on the spontaneous tone of the trachea, unselective COX inhibitors indomethacin, diclofenac and ibuprofen, selective COX-1 inhibitors FR-122047 and SC-560, selective COX-2 inhibitors lumiracoxib and etoricoxib, monoclonal PGE2 antibody (2B5), selective DP1 antagonist BWA868c, EP1 receptor antagonist ONO-8130, EP2 antagonist PF-04418948, FP antagonist AL-8810, IP antagonist CAY10441 or TP antagonist SQ-29548 were given either as a single concentration or by cumulative dosing subsequent to the second equilibration period. At the end of these experiments, a single concentration of theophylline (1 mM) or a combination of papaverine (0.1 mM) and sodium nitroprusside (0.1 mM) was given as a reference for the maximal relaxation. To study the action of PGE2 and other agonists, these were given in a cumulative manner either during spontaneous tone or after 30 min incubation with indomethacin (3 μM). Antagonists were added a minimum of 45 min prior to the agonists. When investigating contractile properties, the experiments were finished by adding histamine (1 mM), acetylcholine (1 mM) and KCl (60 mM) as a reference for the maximal contraction and contractile responses presented as a percentage of this maximum. In cases where maximal contractibility was not obtainable, the contraction was measured as absolute force in Newtons. Histamine was excluded from experiments using rat tissue. Relaxation was studied in segments exposed to carbachol (0.3 μM), to give a stable pre-contraction, prior to addition of the agonists. These experiments were ended with a single concentration of theophylline (1 mM) or a combination of papaverine (0.1 mM) and sodium nitroprusside (0.1 mM) as a reference for the maximal relaxation.
Measurements of mediator release
PGE2 was measured by enzyme immunoassay (EIA, Cayman Chemical, Ann Arbor, MI, USA; Supplementary methods).
RNA preparation and real-time PCR
Guinea pig RNA was isolated using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and real-time PCR, employing primers designed towards available guinea pig sequences, was performed using Power SYBR® Green PCR Master Mix (ABI, Foster City, CA) according to the manufacturers' instructions (Supplementary methods).
Calculations and statistics
All data are presented as mean ± SEM. For agonists, a non-linear regression with a variable slope fit was used to calculate Emax, pEC50 and Hill slope. Statistical analysis was performed using the one-way anova test, followed by Bonferroni's multiple comparisons test and the Mann–Whitney U-test for comparisons between two groups using GraphPad Prism 5.01 software (GraphPad Software Inc., San Diego, CA).
For the antagonist assay, agonist concentration–response curves were globally fitted to the modified Gaddum/Schild model using GraphPad Prism 5.01 (Supplementary methods).
Drugs and suppliers
Stock solutions and dilutions were performed according to manufacturers and suppliers instructions (Supplementary methods).
Results
Expression of mRNA for COX-1, COX-2, mPGES-1, mPGES-2, cPGES and EP1–4 in guinea pig ASM, airway epithelium, aorta and lung parenchyma
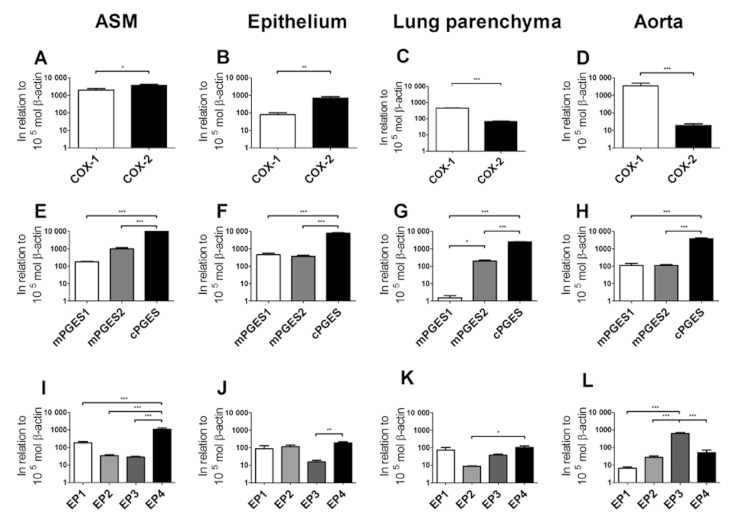
There was expression of mRNA for all the studied proteins (Figure 1: Supplementary results, Figure S1 and Table S1). For the initial step in the biosynthesis of PGE2, semi-quantitative analysis revealed a significantly higher expression of COX-2 compared with COX-1 in ASM and airway epithelium (P < 0.05; Figure 1A–B). In contrast, the expression of COX-1 was significantly higher than COX-2 in lung parenchyma and aorta (P < 0.05; Figure 1C–D).
Figure 1.
Real-time PCR expression of guinea pig mRNA for COX-1, COX-2, mPGES-1, mPGES-2, cPGES and EP1–4 in airway smooth muscle (A, E and I), airway epithelium (B, F and J), lung parenchyma (C, G and K) and aorta (D, H and L). All values are represented as mean ± SEM (n ≥ 5) mol in relation to 105 mol β-actin *, P < 0.05; **, P < 0.01; ***, P < 0.001 denotes significance between selected groups.
For the enzymes catalysing the isomerization of PGH2 to PGE2, there was a similar expression pattern in all investigated tissues; viz. the expression of cPGES was significantly greater than mPGES-1 and mPGES-2 (P < 0.05; Figure 1E–H). The expression of mRNA for mPGES-1 and mPGES-2 was similar in epithelium and aorta, whereas mPGES-2 was numerically higher than mPGES-1 in ASM and significantly higher (P < 0.05; Figure 1G) in the lung parenchyma.
Tissue-specific patterns of expression were observed for PGE2 receptors. Thus, the expression of mRNA for EP4 was significantly higher in ASM compared with EP1, EP2, and EP3 (P < 0.05; Figure 1I). The expression of mRNA for EP1, EP2 and EP4 receptors was similar in the airway epithelium, whereas the expression of the EP3 receptor was lower (P < 0.05; Figure 1J). In the lung parenchyma, the expression of mRNA for EP1, EP3 and EP4 receptors was similar, whereas the expression of EP2 receptors was lower (P < 0.05; Figure 1K). The pattern of mRNA expression for the EP receptors was however different in the aorta with the expression of EP3 being significantly greater than that of EP1, EP2 and EP4 (P < 0.05; Figure 1L).
Influence of indomethacin on the concentration–response curve to PGE2 in GPT
After the wash and resting period, following the initial standard assessment of histamine responsiveness, tracheal segments develop a spontaneous contractile tone that stabilizes within 30 min. This spontaneous tone could be relaxed by administration of indomethacin (3 μM). In line with previous observations (Coleman and Kennedy, 1980), exogenous PGE2 (0.1–10 000 nM) produced a bell-shaped concentration–response curve, and this response was observed both in absence and presence of indomethacin. Moreover, the peak contraction reached the same amplitude (29.4 ± 3.9 mN and 30.3 ± 2.2 mN) at the same concentration of PGE2 (100 nM), irrespective of whether or not the concentration–response curve was raised in the presence of indomethacin. The pEC50-values of PGE2 for the contractile part (8.2 ± 0.2 and 8.0 ± 0.1, respectively) and the relaxant part (6.7 ± 0.1 and 6.3 ± 0.3, respectively) were similar for both untreated segments and those relaxed by indomethacin.
PGE2 mediates contraction through the EP1 receptor in GPT
To characterize the receptors involved in the PGE2 response, initial experiments were performed using the selective EP1 antagonist ONO-8130. For PGE2, ONO-8130 caused a concentration-dependent reduction of the peak contraction response concomitant with a rightwards shift. Concentrations of ONO-8130 above 10 nM abolished the contractile response to exogenous PGE2 (Figure 2A). To further investigate the action of PGE2 on the EP1 receptor and to be able to estimate the potency of ONO-8130, experiments were performed using the EP1/EP3 receptor agonist 17-phenyl trinor PGE2. In these experiments, ONO-8130 caused a parallel shift to the right of concentration-response curve (no changes in Emax or Hill slopes). Schild plot analysis resulted in a slope of 1.04 [95% confidence interval (95% CI): 0.88–1.19] not different from unity (Figure 2B). Constraining the Schild slope to unity resulted in a pKB value of 8.93 for ONO-8130 (95% CI: 8.83–9.04; Supporting Figure S3A).
Figure 2.
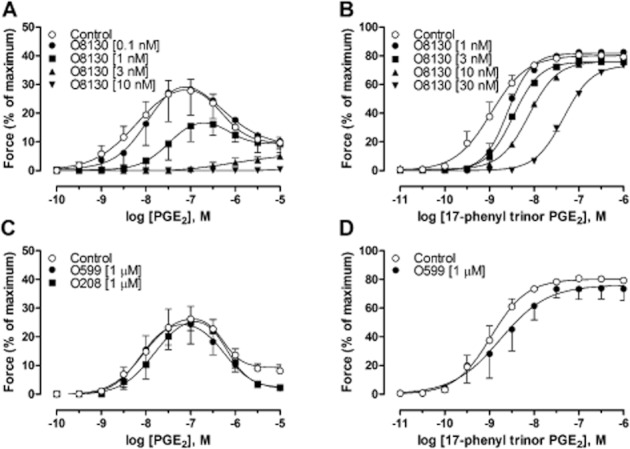
(A) Concentration–response curves to PGE2 in GPT in the presence of the selective EP1 receptor antagonist ONO-8130 (O8130) at different concentrations. (B) Contraction induced by cumulative concentrations to 17-phenyl trinor PGE2 in guinea pig tracheal segments in the presence of ONO-8130 (O8130) at different concentrations (1–30 nM). Schild plot analysis yielding a pA2 value of 8.9 with a slope of 1.0. (C) Concentration–response curves to PGE2 in GPT in the presence or absence of the selective EP3 receptor antagonist ONO-AE5-599 (O599), or selective EP4 receptor antagonist ONO-AE3-208 (O208). (D) Contraction induced by cumulative concentrations to 17-phenyl trinor PGE2 in the presence or absence of ONO-AE5-599 (O599). The contraction of each segment in all experiments was calculated as percentage of maximal contraction (histamine (1 mM), acetylcholine (1 mM) and KCl (60 mM)) in relation to maximal relaxation (theophylline (1 mM) or a combination of papaverine (0.1 mM) and sodium nitroprusside (0.1 mM)). All experiments were performed in the presence of 3 μM indomethacin (3 μM). Data represent mean ± SEM (n = 4–11).
The possible involvement of EP3 and EP4 receptors on the responses to PGE2 were investigated using the EP3 receptor antagonist ONO-AE5-599, or the EP4 receptor antagonist ONO-AE3-208 (1 μM). Neither ONO-AE5-599 nor ONO-AE3-208 changed the maximal contractile response or the potency, for either the contractile (7.9 ± 0.1 for both) or the relaxant part (6.2 ± 0.1 and 6.1 ± 0.0, respectively) of the concentration–response curve for PGE2, compared with controls (8.2 ± 0.2 for the contraction and 6.2 ± 0.1 for the relaxation; Figure 2C).
The absence of any effect attributable to the EP3 receptor in the GPT was further supported by the observation that the EP1/EP3 receptor agonist sulprostone did not induce either a contraction or relaxation of the GPT in the presence of ONO-8130 (100 nM; n = 7). Moreover, ONO-AE5-599 failed to antagonize the contraction generated by 17-phenyl trinor PGE2 (9.1 ± 0.2 and 8.7 ± 0.3, with and without antagonist respectively; Figure 2D).
PGE2 mediates relaxation through the EP2 receptor in GPT
To examine the role of the apparent relaxant receptor, the new selective EP2 receptor antagonist PF-04418948 (Forselles et al., 2011) was used. PF-04418948 caused a concentration-dependent increase of the peak contraction induced by PGE2, concomitant with a rightwards shift of only the relaxation part of the concentration–response curve (Figure 3A). In segments pre-contracted with carbachol (0.3 μM) in the presence of ONO-8130 (10 nM) and SQ-29548 (1 μM), PGE2 caused a relaxation with a parallel rightwards shift of the concentration–response curves with increasing concentrations of PF-04418948 (Figure 3B). There was no difference in Hill slopes and Emin. Performing a Schild plot analysis revealed a slope of 1.04 (95% CI: 0.97–1.11), which was not different from unity. Constraining the Schild slope to unity resulted in a pKB value of 7.48 for PF-04418948 (95% CI: 7.4–7.6; Supporting Figure S3B).
Figure 3.
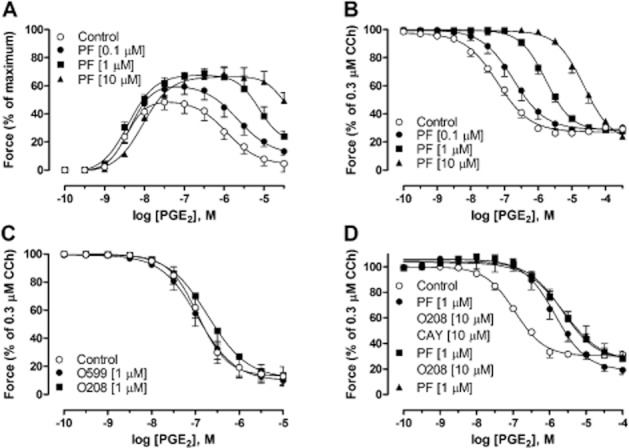
Concentration–response curves to PGE2 in GPT. (A) In the presence of the selective EP2 receptor antagonist PF-04418948 (PF) at different concentrations. (B) In segments pre-contracted with 0.3 μM carbachol (CCh) in the presence of ONO-8130 (100 nM) and SQ-29 548 (1 μM) and after treatment with PF-04418948 (PF) at different concentrations (0.1–10 μM). (C) In segments pre-contracted with 0.3 μM CCh after treatment with the selective EP3 receptor antagonist ONO-AE5-599 (O599) or the selective EP4 receptor antagonist ONO-AE3-208 (O208). (D) In segments pre-contracted with CCh after treatment with PF-04418948 (PF) together with the EP4 receptor antagonist ONO-AE3-208 (O208) and selective IP receptor antagonist CAY 10441 (CAY), either alone or in combination. The contraction of each segment in all experiments was calculated as percentage of maximal contraction (histamine (1 mM), acetylcholine (1 mM) and KCl (60 mM)) or 0.3 μM carbachol in relation to maximal relaxation [theophylline (1 mM) or a combination of papaverine (0.1 mM) and sodium nitroprusside (0.1 mM)]. All experiments were performed in the presence of indomethacin (3 μM). Data represent mean ± SEM (n = 4–6).
Further investigation of EP receptors revealed that PGE2 induced a concentration-dependent relaxation with a pEC50 of 6.9 ± 0.1 and a maximal effect of 87.1 ± 6.9% that was neither affected by the EP3 receptor antagonist ONO-AE5-599, nor the EP4 receptor antagonist ONO-AE3-208 (Figure 3C). Experiments intended to achieve a combined blockade of the EP2 receptor together with EP4 and IP receptors using PF-04418948, ONO-AE3-208 and CAY10441, respectively, did not provide evidence for the presence of a relaxant PGE2 effect, possibly mediated by EP4 and IP receptors (Figure 3D).
The antagonistic effect of ONO-AE5-599 and ONO-AE3-208 was verified in assays displaying EP3- and EP4-mediated responses
Since no antagonistic effect was found for ONO-AE5-599 and ONO-AE3-208 in GPT, these antagonists were tested in assays known to display effects mediated by EP3 and EP4 receptors (Lydford and McKechnie, 1994; Jones and Woodward, 2011). As a positive control for the EP3 receptor antagonist, it was shown that ONO-AE5-599 concentration-dependently antagonized the response to sulprostone in segments from endothelium-intact guinea pig aorta. These segments were pre-treated with ONO-8130 to abolish the EP1 component of the response to sulprostone (Figure 4A). The selectivity of the EP4 receptor antagonist was assessed in rat tracheal rings pre-contracted with carbachol. In this preparation, the relaxation induced by PGE2 shifted to the right by more than two orders of magnitude, and the maximal relaxation was 54.3 ± 3.3% in the presence of ONO-AE3-208, as compared with 79.6 ± 3.8% in its absence (Figure 4B).
Figure 4.
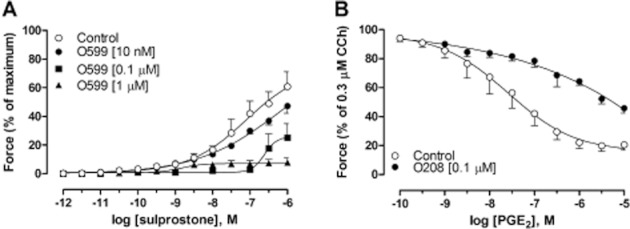
(A) Concentration–response curves to the selective EP1/EP3 receptor agonist sulprostone in endothelium-intact guinea pig aorta after treatment with the selective EP3 receptor antagonist ONO-AE5-599 (O599). (B) Concentration–response curves to PGE2 in rat tracheal segments pre-contracted with 0.3 μM CCh and treated with the EP4 receptor antagonist ONO-AE3-208 (O208). All experiments were performed in the presence of 3 μM indomethacin and in panel A; 10 nM of the EP1 receptor antagonist ONO-8130 was also added. Data represent mean ± SEM (n = 4–8).
PGE2 acting at EP1 and EP2 receptors maintains spontaneous tone in GPT
As described above, addition of the unselective COX-inhibitor indomethacin inhibited the spontaneous tone. This treatment resulted in a prompt relaxation with the force decreasing by 93.1 ± 1.6% in relation to the maximal relaxation induced by theophylline (Figure 5A–B). The relaxant effect of indomethacin was reproduced with two other non-selective COX-inhibitors, ibuprofen and diclofenac (n = 4 and 5 respectively). Addition of the selective EP1 receptor antagonist ONO-8130, at concentrations of 10 nM and 1 μM, decreased the spontaneous tone (91.3 ± 1.2% and 90.4 ± 2.2%, respectively) to the same level as indomethacin (Figure 5A–B). The onset of the effect was more rapid than that of indomethacin for the highest concentration of the EP1 receptor antagonist. In contrast, treatment with 1 and 10 μM of the EP2 receptor antagonist PF-04418948 resulted in a concentration-dependent increase in spontaneous tone, reaching 64.5 ± 3.7% and 75.5 ± 3.9% respectively, as compared with the maximal tissue contractility (Figure 5B).
Figure 5.
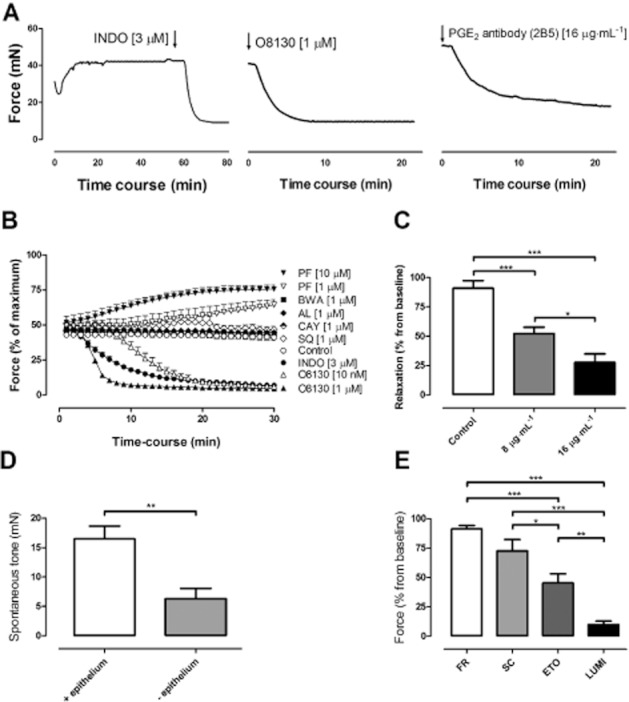
(A) Experimental trace showing the relaxation of tone in guinea pig trachea (GPT) induced by treatment with indomethacin (INDO), ONO-8130 (O8130) or monoclonal PGE2 antibody (2B5) added at the arrow. (B) Time course of change in spontaneous tone in GPT after addition of the selective DP1 receptor antagonist BWA868c (BWA), EP1 receptor antagonist ONO-8130 (O8130), EP2 receptor antagonist PF-04418948 (PF), FP receptor antagonist AL-8810 (AL), IP receptor antagonist CAY10441 (CAY), TP receptor antagonist SQ-29548 (SQ) or COX inhibitor INDO. (C) Relaxation of spontaneous tone in GPT subsequent to addition of monoclonal PGE2 antibody (2B5) in the absence of indomethacin. (D) Removal of spontaneous tone in guinea pig tracheal segments with or without tracheal epithelium, treated with 3 μM indomethacin. (E) Relaxation of spontaneous tone in guinea pig trachea subsequent to addition of FR-122047 (FR; 1 μM), SC-560 (SC; 1 μM), etoricoxib (ETO; 1 μM) or lumiracoxib (LUMI; 1 μM). Data represent mean ± SEM (n = 4–6).
To assess whether COX products other than PGE2 might contribute to the spontaneous tone, segments were treated with the DP1 receptor antagonist BWA868c, FP receptor antagonist AL-8810, IP receptor antagonist CAY10441 or TP receptor antagonist SQ-29548 under similar conditions. However, the relaxant effect of these four antagonists was negligible, 11.3 ± 4.3%, 6.4 ± 9.9%, 9.8 ± 4.7% and 12.3 ± 4.5, respectively, with no difference from the spontaneous decline observed in untreated segments (5.3 ± 5.1%) over the same time period (Figure 5B).
The claim that PGE2 controls the spontaneous tone of these preparations obtained strong support when it was found that addition of a high-affinity neutralizing monoclonal antibody against PGE2 relaxed the preparations (Figure 5A). The antibody concentration-dependently decreased the spontaneous tonus by 47.7 ± 5.5% (8 μg·mL−1) and 72.5 ± 7.4% (16 μg·mL−1), compared with untreated controls that relaxed 9.2 ± 6.5% during the same time (1 h) (P < 0.05; Figure 5C).
PGE2 is mainly produced by the epithelium in the GPT
Evidence was obtained to support that a significant part of the biosynthesis of PGE2, that maintains tone, originate from the tracheal epithelium. In preparations where the epithelium had been denuded, the spontaneous increase in active tone did not reach the same level as in segments with an intact epithelium. This attenuated active tone also resulted in a significantly reduced relaxant effect towards indomethacin, compared with segments with an intact epithelium (P < 0.05; Figure 5D). Thus, the effect of 3 μM indomethacin was only 40% of that observed in control preparations.
COX-2 is the major enzyme catalysing the formation of PGE2 in the GPT
In an attempt to investigate which COX isoenzyme that mediated the response, the selective COX-1 inhibitors FR-122047 (1 μM) and SC-560 (1 μM), as well as the selective COX-2 inhibitors etoricoxib (1 μM) and lumiracoxib (1 μM) were applied during the spontaneous tone. It was found that both COX-2 inhibitors were superior in relaxing the spontaneous tone compared with COX-1 inhibitors. Lumiracoxib was the most efficient, inducing a reduction of 89.5 ± 3.1%, followed by etoricoxib causing a reduction of 54.7 ± 7.7%, SC-560 of 27.4 ± 9.6% and FR-122047 of 8.7 ± 3% (Figure 5E).
Homologous desensitization of the contractile, but not the relaxant response to PGE2 in the GPT
To assess the how GPT responses are influenced by the endogenous production of PGE2 over a longer time period, the tracheal segments were incubated in tissue culture for 4 days. After the incubation, it was found that the tracheal segments had lost responsiveness to the contractile effect of PGE2. In contrast, when indomethacin was included in the medium during the incubation period, the responsiveness to exogenous PGE2 was retained, and also showed an increase in potency compared with fresh GPT (P < 0.05; Figure 6A). The tracheal segments incubated in the presence of indomethacin responded identically to untreated segments, with respect to the relaxation induced by PGE2, with an increased potency compared with fresh segments (P < 0.05; Figure 6B). The contraction evoked by histamine was not altered by indomethacin treatment during culture (Figure 6C). Since the decrease of the EP1 receptor-mediated effect could be due to homologous desensitization, the levels of PGE2 in the culture medium were measured. Indeed, very high levels of PGE2 (5–20 ng·mL−1) were found during the 4 days of incubation, which were almost abolished after incubation with indomethacin (Figure 6D).
Figure 6.
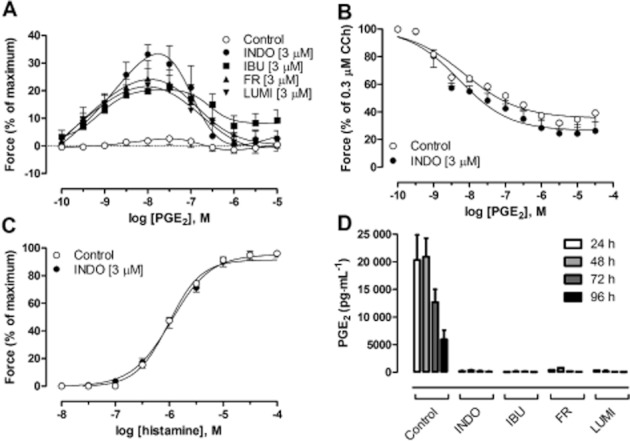
(A) Concentration–response curves to PGE2 in GPT following culture for 4 days in the absence (Control) or presence of indomethacin (INDO; 3 μM), ibuprofen (IBU; 3 μM), FR-122047 (FR; 3 μM) or lumiracoxib (LUMI; 3 μM). (B) Concentration–response curves to PGE2 in cultured guinea pig tracheal segments in the absence or presence of INDO (3 μM) and pre-contracted with 0.3 μM CCh. (C) Concentration–response curves to histamine in cultured guinea pig tracheal segments in the absence or presence of INDO (3 μM). (D) PGE2 immunoreactivity measured in culture media from tracheal segments cultured for up to 96 h (n = 4–17).
Treatment with another unselective COX inhibitor, ibuprofen, during the incubation period, mimicked the effect of indomethacin, causing both a maintained contractile response to PGE2 (Figure 6A) and an inhibition of PGE2 biosynthesis (Figure 6D). In order to assess which pathway that was responsible for the biosynthesis of PGE2 during the incubation, experiments were performed in the presence of the selective COX-1 inhibitor FR-122047 or the selective COX-2 inhibitor lumiracoxib during the 4 days of incubation. Under these conditions, both drugs somewhat unexpectedly caused complete inhibition of PGE2 production (Figure 6D) and maintained the PGE2 contractions (Figure 6A).
Discussion and conclusions
Using guinea pig-specific primers, we found mRNA expression of all four EP receptors in GPT. However, using new and selective pharmacological antagonists, we could only find evidence for functionally active contractile EP1 receptors and relaxant EP2 receptors. Furthermore, there was expression of mRNA for both COX-enzymes and experiments using a range of COX inhibitors suggest that both enzymes contribute to the biosynthesis of endogenous PGE2, although the action of COX-2 seems to predominate. Epithelial denudation suggested that PGE2 originates both from the smooth muscle and epithelial cells. Moreover, the experiments with COX-inhibitors, selective receptor antagonists for all prostanoids, and a specific antibody against PGE2, together provided strong evidence that the level of spontaneous tone in the tracheal segments is maintained by PGE2. Because of the relaxant effects of COX inhibitors, and previous data using unselective antagonists, it has been assumed that the basal tone is solely mediated by EP1 receptors (Ndukwu et al., 1997). This view seems to be in line with the observation that initial part of the concentration–response curve to PGE2 is contractile, suggesting that relaxations only occur at higher and perhaps un-physiological concentrations of PGE2. In the present study, we were able to test the new EP2 antagonist PF-04418948 (Forselles et al., 2011). It was revealed that basal tone immediately increased after EP2 blockade. This leads to the new understanding that basal tone is maintained by a balance of the effects of PGE2, and that both EP1 and EP2 receptors are active simultaneously.
This is the first report of mRNA expression for key enzymes and receptors in the PGE2 pathway, analysed in a study that also examined functional responses of the same tissues, and furthermore, used primers that were designed based on actual guinea pig genetic sequence. The characteristics of the in-house designed primers were excellent (Supporting Figure S1), whereas we failed to get homogeneous melting curve data for previously published primers targeting human EP receptor mRNA (Rehal et al., 2009). As described below, there were not only correlations between the mRNA data and functional experiments but also certain discrepancies suggesting that expression of the enzymes and receptors is regulated at several levels.
The expression of mRNA for EP1–4 receptors, in both tracheal smooth muscle and epithelium, suggested a possible involvement in functional responses. In line with previous observations (Coleman and Kennedy, 1980), PGE2 produced a bell-shaped concentration–response curve with contraction at low, and relaxation at high concentrations, indicating activation of multiple signalling pathways. In the present study, ONO-8130 shifted the contractile part of the bell-shaped concentration–response curve for exogenous PGE2 to the right and depressed the peak contractions at higher concentrations. The decrease in maximal effect was presumably due to simultaneous antagonism of the EP1 receptor, in combination with PGE2-mediated relaxation. When the antagonistic properties of ONO-8130 were further investigated using the selective EP1/EP3 receptor agonist, 17-phenyl trinor PGE2, the analysis demonstrated a pKB of 8.93 with a Schild slope not different from unity indicating a competitive effect at a single receptor site. The potency of ONO-8130 is in accordance with the earlier established binding affinity (Ki) of 1.9 nM, and antagonist activity (pIC50) of 9.3 nM for the mouse EP1 receptor (Data on file, Ono Pharmaceuticals Corp), which also showed that ONO-8130 exerts a more than 1000-fold selectivity for EP1 compared with the other EP receptors. Thus, ONO-8130 is a far more potent and selective EP1 receptor antagonist than the earlier used AH6809 with estimated pA2 values of 6.4–7.0 for the EP1 receptor and similar affinities for EP2, EP3, DP1 and TP receptors (Abramovitz et al., 2000). Further evidence in support of the PGE2-induced contraction being solely mediated via EP1 receptors was obtained since neither the EP3 antagonist ONO-AE5-599 (Aihara et al., 2007) nor the EP4 antagonist ONO-AE3-208 (Ohinata et al., 2006) had any effect on the concentration–response curves to PGE2 or 17-phenyl trinor PGE2. Accordingly, using the selective EP1 receptor antagonist ONO-8130, we were able to conclusively confirm and extend the previous suggestions obtained using AH6809 (Ndukwu et al., 1997).
The recently developed selective EP2 receptor antagonist PF-04418948 (Forselles et al., 2011) made it possible to characterize the EP-receptor-mediated relaxation. PF-04418948 did not antagonize the contractile part of the PGE2-induced bell-shaped response. Instead, the contraction was further increased, due to PF-04418948 shifting the relaxant part of the PGE2 induced bell-shaped curve to the right. In segments pre-contracted with carbachol in presence of both EP1 and TP receptor antagonists, increasing concentrations of PF-04418948 caused a parallel rightwards shift of the concentration–response curve for PGE2-induced relaxation. The Schild plot slope, which was not different from unity, indicated a competitive effect at a single receptor site and resulted in a pKB value of 7.5. Interestingly, this value is lower compared with the pKB value of 8.3 and 8.9 found in human myometrium and mouse trachea, respectively (Forselles et al., 2011), suggesting species differences. Furthermore, the selective EP2 receptor agonist ONO-AE1-259 relaxed carbachol pre-treated segments in a concentration dependent manner that was unaffected by the combination EP1, EP3 and EP4 receptor antagonists (Supporting Figure S2). The conclusion that the EP2 receptor is the only relaxant receptor for PGE2 in GPT was confirmed by the current findings, since neither the potency nor the maximal relaxation was affected by selective EP3, EP4 and IP receptor antagonists for exogenously applied PGE2. Interestingly, in pre-contracted preparations, the relaxations occurred at lower PGE2 concentrations than observed for the relaxant part of the bell-shaped concentration–response curve, suggesting an overlap in concentrations for the contractile and relaxant effects mediated by activation of EP1 and EP2 receptors, respectively.
To confirm the activity of ONO-AE5-599 and ONO-AE3-208, these compounds were tested in tissues known to express EP3 and EP4 receptors. In the presence of the EP1 antagonist ONO-8130, ONO-AE5-599 concentration-dependently antagonized the contractions of guinea pig aorta induced by the EP1/EP3 receptor agonist sulprostone. This confirms and extends the evidence for functional EP3 receptors in guinea pig aorta, previously identified using selective receptor antagonists (Jones et al., 1998; 2011). Furthermore, we found high expression of the EP3 receptor in guinea pig aorta. Moreover, in line with EP4 receptors mediating relaxation of the rat trachea (Lydford and McKechnie, 1994; Buckley et al., 2011), ONO-AE3-208 potently displaced the concentration–response curve for exogenous PGE2 in rat trachea, a feature also observed in experiments using human bronchus (Buckley et al., 2011). Thus, despite expression of mRNA for both EP3 and EP4 receptors in the smooth muscle, neither seems to be involved in contraction or relaxation of the GPT. As there are no guinea pig-specific antibodies for EP3 and EP4 available, we were not able to examine protein expression by Western Blot. The EP3 receptor is implicated in the modulation of neural responses (Maher et al., 2009) and activation has been linked to inhibitory responses on parasympathetic nerves innervating the GPT (Clarke et al., 2004), whereas the EP4 receptor has been reported to modulate secretory responses and cell growth (Pelletier et al., 2001; Okuyama et al., 2002; Rao et al., 2007; Yao et al., 2009). Future experiments are required to examine the functional relevance of the relatively high levels of EP4 expression.
ONO-8130 was found to reduce the spontaneous tone of the GPT, and at the highest concentration (1 μM), the response to ONO-8130 was more rapid than that caused by indomethacin. This would suggest a mechanism involving the immediate blockade of receptors rather than a gradually diminished biosynthesis caused by indomethacin. In contrast, blocking other prostaglandin receptors (DP1, FP, IP and TP receptors) did not affect spontaneous tone. Since EP3 receptors are expressed in the trachea and have a possible neuronal link, this receptor could theoretically also be involved in the response to PGE2. However, the cholinergic tone is essentially abolished by vagotomy or ganglionic blockade, suggesting that it is dependent upon ongoing pre-ganglionic input arising from the CNS (Kesler and Canning, 1999) and thus is not relevant in our preparation. Unexpectedly, selectively blocking the EP2 receptor induced a concentration-dependent increase of the spontaneous tone. This new and interesting finding clearly suggests that endogenously released PGE2 also activates this relaxant receptor. The final piece of evidence that PGE2 maintains spontaneous tone was shown by the concentration-dependent decrease in tone caused by addition of a specific high-affinity PGE2 antibody, which has been shown to selectively block PGE2 responses in cell models (Mnich et al., 1995). Taken together, our experiments show that the tone in the GPT is mediated via endogenous PGE2, and depends on the balance between EP1-mediated contraction and EP2-mediated relaxation. The finding that addition of either indomethacin or ONO-8130 sometimes did not produce complete 100% relaxation might suggest there is a small non-prostanoid mediator contributing to basal tension, or that there are small compensatory changes in length–tension relationship within the smooth muscle during the course of the experiment (Gunst et al., 1995).
Using the optimized primers designed for this study, both COX-1 and COX-2 were expressed in all four tissues studied. COX-2 was predominantly expressed in the tracheal epithelium and smooth muscle, whereas COX-1 was more abundant than COX-2 in the lung parenchyma and thoracic aorta. Previous reports of COX-1 and COX-2 expression in the guinea pig lung have been conflicting. A Northern blot study using guinea pig-specific cDNA revealed constitutive expression of both enzymes in lung homogenates. This was confirmed by Western blot analysis, although the antibodies used were primarily raised against other species (Oguma et al., 2002). An immunoblot study using rabbit antibodies detected COX-2 but not COX-1 in the smooth muscle and cartilage of GPT, and one selective COX-2 inhibitor had the same effect as indomethacin on spontaneous tone (Charette et al., 1995). However, selective COX-1 inhibition was not tested in that study. We found that two different, but potent, COX-1 inhibitors (FR-122047 and SC-560) had a smaller effect on spontaneous tone compared with two selective COX-2 inhibitors (etoricoxib and lumiracoxib), confirming that COX-2 appears to be the quantitatively dominating enzyme catalysing formation of the PGE2 that maintains tone under tissue bath conditions. Our study has the limitation that concentration–effect relations for the inhibitors not have been established in the tissue bath setting; however, the compounds were used in concentrations that in other models have shown high degree of selectivity (Ochi et al., 2000; Esser et al., 2005).
Since the epithelium has been suggested to be an important source of PGE2 (Hay et al., 1988), experiments were performed comparing the active tension between intact and epithelium-denuded segments. The active tension was lower in denuded segments than in intact segments, indicating that the epithelium is a major source of PGE2. However, in line with the high expression of COX-2 in both smooth muscle and epithelial cells, the airway smooth muscle is also likely to contribute to the basal release of PGE2.
Another aim of the current study was to examine how the endogenous production of PGE2 affects the responses of EP1 and EP2 receptors over a longer period of time. We found that 4 days of culture resulted in a completely abolished contraction, yet maintained relaxation towards exogenously added PGE2. This difference in response to PGE2 is consistent with the described homologous desensitization of the EP1 receptor, which has not been observed for the EP2 receptor (Illes and Knoll, 1975; Vermue et al., 1987; Penn et al., 2001). Furthermore, the observed desensitization did not occur when the endogenous biosynthesis of PGE2 was inhibited by indomethacin, ibuprofen, FR-122047 or lumiracoxib during the culture period. We cannot explain why under these conditions the inhibitors did not show selectivity. Both compounds (FR-122047 and lumiracoxib) were used at concentrations that in other models are very selective (Ochi et al., 2000; Esser et al., 2005); therefore, a lack of selectivity at the level of COX inhibition is an unlikely explanation of our results. It may also be that the kinetics of enzyme inhibition is altered during long-term incubation, enabling an inhibitor with a low potency to induce an effect. The mechanism underlying this phenomenon requires further investigation. Interestingly, after culturing, both the contractile and relaxant response towards PGE2 was increased in potency. This type of hyperreactivity has been described previously for other excitatory and inhibitory stimuli in this culture model and is thought to be due to an enhanced coupling efficiency of the effectors involved (Morin et al., 2005).
To put our main findings in perspective, using new, potent and selective pharmacological tools, we have not only confirmed that the EP1 receptor is involved in the spontaneous tone of the GPT, but also discovered that the endogenous biosynthesis of PGE2 simultaneously mediates relaxation through EP2 receptors. We show for the first time that constitutively released PGE2 induces a basal tone in GPT that depends upon the balance between the actions of these two opposing receptor functions. As the response of human airways towards prostanoids is known to be complex (Coleman and Kennedy, 1980; Brink et al., 1981; Muccitelli et al., 1987; Ressmeyer et al., 2006; Canning and Chou, 2008; Ricciardolo et al., 2008), and constitutively released PGE2 can induce both contraction and relaxation, we suggest that PGE2 may also play a similar regulatory role in human airways. Clearly, the net effect of PGE2 regulation in human bronchi appears to be opposite to that in guinea pig airways, with indomethacin increasing tone, at least under tissue bath conditions (Bjorck and Dahlen, 1993; Coleman et al., 1996; Watson et al., 1997). Neither is the relative contribution of COX-1 or COX-2 to formation of PGE2 in human bronchi known, whereas COX-1 appears to dominate in the upper airways (Harrington et al., 2008). Further indications of species differences are the findings that in human bronchi relaxant EP4 receptors appear to have a significant role (Buckley et al., 2011; Benyahia et al., 2012), although it remains to examine the effect of EP2 receptor antagonism in the human tissue. The desensitization of EP1 receptors by endogenous PGE2 is also an observation with potential implications of relevance to human disease. One may speculate that this mechanism is important in situations where the local release of PGE2 is increased, for example during chronic airway inflammation. This would change the balance between the contractile and relaxant response to PGE2 in favour of a protective relaxation. In fact, there is good evidence that PGE2 has a protective function in asthmatic airways (Melillo et al., 1994; Pavord and Tattersfield, 1995; Gauvreau et al., 1999).
Acknowledgments
The authors would like to express their gratitude to Melinda Verriere for animal care and Ingegerd Larsson for PCR methodological support during the course of the study. We would also like to acknowledge the support from the Swedish Medical Research Council, the Swedish Heart-Lung Foundation, the Swedish Society of Medicine, Vinnova Chronic Inflammation – Diagnostic and Therapy (CIDaT), Swedish Foundation for Strategic Research (SSF), The Swedish Society for Medical Research, the Stockholm County Council Research Funds (ALF) and Karolinska Institutet.
Glossary
- cPGES
cytosolic PGE synthase
- GPT
guinea pig trachea
- mPGES
microsomal PGE synthase
- NSAID
non-steroidal anti-inflammatory drug
- PGI2
prostacyclin
- TX
thromboxane
Conflict of interest
Kirk Maxey is employed by Cayman Chemicals from which we received the monoclonal PGE2 antibody.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Target primers were designed in-house towards available sequences from the Ensemble database and the NCBI Genebank Sequences from the second scaffold of the guinea pig genome comprised by Broad Institute (Feb. 2008 Cavia porcellus draft assembly cavPor3). Validation included a melt curve analysis and dilution series of cDNA expressed as a slope regression line.
Figure S2 Concentration–response curves to selective EP2 receptor agonist ONO-AE1-259 in guinea pig tracheal segments pre-contracted with 0.3 μM carbachol (CCh) and treated with selective receptor antagonists; ONO-8130 (O8130), ONO-AE5-599 (O599) and ONO-AE3-208 (O208). All experiments are performed in the presence of indomethacin (3 μM). Data represent mean ± SEM (n = 4–6).
Figure S3 (A) Schild plot derived from Figure 2B revealed a slope of 1.04 (95% CI: 0.88–1.19), which was not different from unity. Constraining the Schild slope to unity resulted in a pKB value of 8.93 for ONO-8130 (95% CI: 8.83–9.04). (B) Schild plot derived from Figure 3B revealed a slope of 1.07 (95% CI: 0.97–1.11), which was not different from unity. Constraining the Schild slope to unity resulted in a pKB value of 7.48 for PF-04418948 (95% CI: 7.4–7.6).
Table S1 Real-time PCR expression of mRNA in guinea pig tissue in relation to 105 mol·mol−1 β-actin.
References
- Abramovitz M, Adam M, Boie Y, Carrière M-C, Denis D, Godbout C, et al. The utilization of recombinant prostanoid receptors to determine the affinities and selectivities of prostaglandins and related analogs. Biochim Biophys Acta Mol Cell Biol Lipids. 2000;1483:285–293. doi: 10.1016/s1388-1981(99)00164-x. [DOI] [PubMed] [Google Scholar]
- Adner M, Rose AC, Zhang Y, Sward K, Benson M, Uddman R, et al. An assay to evaluate the long-term effects of inflammatory mediators on murine airway smooth muscle: evidence that TNF[alpha] up-regulates 5-HT2A-mediated contraction. Br J Pharmacol. 2002;137:971–982. doi: 10.1038/sj.bjp.0704928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aihara E, Nomura Y, Sasaki Y, Ise F, Kita K, Takeuchi K. Involvement of prostaglandin E receptor EP3 subtype in duodenal bicarbonate secretion in rats. Life Sci. 2007;80:2446–2453. doi: 10.1016/j.lfs.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Anggard E, Samuelsson B. Biosynthesis of prostaglandins from arachidonic acid in guinea pig lung. Prostaglandins and related factors. 38. J Biol Chem. 1965;240:3518–3521. [PubMed] [Google Scholar]
- Benyahia C, Gomez I, Kanyinda L, Boukais K, Danel C, Leseche G, et al. PGE(2) receptor (EP(4)) agonists: potent dilators of human bronchi and future asthma therapy? Pulm Pharmacol Ther. 2012;25:115–118. doi: 10.1016/j.pupt.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Bjorck T, Dahlen SE. Leukotrienes and histamine mediate IgE-dependent contractions of human bronchi: pharmacological evidence obtained with tissues from asthmatic and non-asthmatic subjects. Pulm Pharmacol. 1993;6:87–96. doi: 10.1006/pulp.1993.1012. [DOI] [PubMed] [Google Scholar]
- Brink C, Duncan PG, Douglas JS. Histamine, endogenous prostaglandins and cyclic nucleotides in the regulation of airway muscle responses in the guinea pig. Prostaglandins. 1981;22:729–738. doi: 10.1016/0090-6980(81)90212-4. [DOI] [PubMed] [Google Scholar]
- Buckley J, Birrell MA, Maher SA, Nials AT, Clarke DL, Belvisi MG. EP4 receptor as a new target for bronchodilator therapy. Thorax. 2011;66:1029–1035. doi: 10.1136/thx.2010.158568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canning BJ, Chou Y. Using guinea pigs in studies relevant to asthma and COPD. Pulm Pharmacol Ther. 2008;21:702–720. doi: 10.1016/j.pupt.2008.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charette L, Misquitta C, Guay J, Reindeau D, Jones TR. Involvement of cyclooxygenase 2 (COX-2) in intrinsic tone of isolated guinea pig trachea. Can J Physiol Pharmacol. 1995;73:1561–1567. doi: 10.1139/y95-215. [DOI] [PubMed] [Google Scholar]
- Clarke DL, Giembycz MA, Patel HJ, Belvisi MG. E-ring 8-isoprostanes inhibit ACh release from parasympathetic nerves innervating guinea-pig trachea through agonism of prostanoid receptors of the EP3-subtype. Br J Pharmacol. 2004;141:600–609. doi: 10.1038/sj.bjp.0705648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Kennedy I. Contractile and relaxant actions of prostaglandins on guinea-pig isolated trachea. Br J Pharmacol. 1980;68:533–539. doi: 10.1111/j.1476-5381.1980.tb14569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Kennedy I. Characterisation of the prostanoid receptors mediating contraction of guinea-pig isolated trachea. Prostaglandins. 1985;29:363–375. doi: 10.1016/0090-6980(85)90096-6. [DOI] [PubMed] [Google Scholar]
- Coleman RA, Humphrey PPA, Kennedy I, Lumley P. Prostanoid receptors – the development of a working classification. Trends Pharmacol Sci. 1984;5:303–306. [Google Scholar]
- Coleman RA, Nials AT, Rabe KF, Vardey CJ, Watson N. Isolated, electrically-stimulated airway preparations – their use in determining beta-adrenoceptor agonist activity. Pulm Pharmacol. 1996;9:107–117. doi: 10.1006/pulp.1996.0012. [DOI] [PubMed] [Google Scholar]
- Esser R, Berry C, Du Z, Dawson J, Fox A, Fujimoto RA, et al. Preclinical pharmacology of lumiracoxib: a novel selective inhibitor of cyclooxygenase-2. Br J Pharmacol. 2005;144:538–550. doi: 10.1038/sj.bjp.0706078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer JB, Farrar DG, Wilson J. Antagonism of tone and prostaglandin-mediated responses in a tracheal preparation by indomethacin and SC-19220. Br J Pharmacol. 1974;52:559–565. doi: 10.1111/j.1476-5381.1974.tb09724.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flower RJ. Prostaglandins, bioassay and inflammation. Br J Pharmacol. 2006;147(Suppl. 1):S182–S192. doi: 10.1038/sj.bjp.0706506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forselles KA, Root J, Clarke T, Davey D, Aughton K, Dack K, et al. In vitro and in vivo characterisation of PF-04418948, a novel, potent and selective prostaglandin E(2) receptor-2 (EP(2)) antagonist. Br J Pharmacol. 2011;164:1847–1856. doi: 10.1111/j.1476-5381.2011.01495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiner PJ, Collier HO. Specific receptors for prostaglandins in airways. Prostaglandins. 1980;19:819–841. doi: 10.1016/0090-6980(80)90116-1. [DOI] [PubMed] [Google Scholar]
- Gauvreau GM, Watson RM, O'Byrne PM. Protective Effects of Inhaled PGE2 on Allergen-induced Airway Responses and Airway Inflammation. Am J Respir Crit Care Med. 1999;159:31–36. doi: 10.1164/ajrccm.159.1.9804030. [DOI] [PubMed] [Google Scholar]
- Gunst SJ, Meiss RA, Wu MF, Rowe M. Mechanisms for the mechanical plasticity of tracheal smooth muscle. Am J Physiol. 1995;268:C1267–C1276. doi: 10.1152/ajpcell.1995.268.5.C1267. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Lucas R, McMaster SK, Moreno L, Scadding G, Warner TD, et al. COX-1, and not COX-2 activity, regulates airway function: relevance to aspirin-sensitive asthma. FASEB J. 2008;22:4005–4010. doi: 10.1096/fj.08-107979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay DW, Muccitelli RM, Horstemeyer DL, Raeburn D. Is the epithelium-derived inhibitory factor in guinea-pig trachea a prostanoid? Prostaglandins. 1988;35:625–637. doi: 10.1016/0090-6980(88)90036-6. [DOI] [PubMed] [Google Scholar]
- Held HD, Martin C, Uhlig S. Characterization of airway and vascular responses in murine lungs. Br J Pharmacol. 1999;126:1191–1199. doi: 10.1038/sj.bjp.0702394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Illes P, Knoll J. Specific desensitition to PGE1 and PGE2 in guinea-pig ileum; evidence for a common receptor site. Pharmacol Res Commun. 1975;07:37–47. doi: 10.1016/s0031-6989(75)80028-2. [DOI] [PubMed] [Google Scholar]
- Jones RL, Woodward DF. Interaction of prostanoid EP and TP receptors in guinea-pig isolated aorta: contractile self-synergism of 11-deoxy-16,16-dimethyl PGE. Br J Pharmacol. 2011;162:521–531. doi: 10.1111/j.1476-5381.2010.01039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Qian YM, Chan KM, Yim AP. Characterization of a prostanoid EP3-receptor in guinea-pig aorta: partial agonist action of the non-prostanoid ONO-AP-324. Br J Pharmacol. 1998;125:1288–1296. doi: 10.1038/sj.bjp.0702189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Giembycz MA, Woodward DF. Prostanoid receptor antagonists: development strategies and therapeutic applications. Br J Pharmacol. 2009;158:104–145. doi: 10.1111/j.1476-5381.2009.00317.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RL, Woodward DF, Wang JW, Clark RL. Roles of affinity and lipophilicity in the slow kinetics of prostanoid receptor antagonists on isolated smooth muscle preparations. Br J Pharmacol. 2011;162:863–879. doi: 10.1111/j.1476-5381.2010.01087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy I, Coleman RA, Humphrey PP, Levy GP, Lumley P. Studies on the characterisation of prostanoid receptors: a proposed classification. Prostaglandins. 1982;24:667–689. doi: 10.1016/0090-6980(82)90036-3. [DOI] [PubMed] [Google Scholar]
- Kesler BS, Canning BJ. Regulation of baseline cholinergic tone in guinea-pig airway smooth muscle. J Physiol. 1999;518(Pt 3):843–855. doi: 10.1111/j.1469-7793.1999.0843p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson AK, Fumagalli F, DiGennaro A, Andersson M, Lundberg J, Edenius C, et al. A new class of nitric oxide-releasing derivatives of cetirizine; pharmacological profile in vascular and airway smooth muscle preparations. Br J Pharmacol. 2007;151:35–44. doi: 10.1038/sj.bjp.0707214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydford SJ, McKechnie K. Characterization of the prostaglandin E2 sensitive (EP)-receptor in the rat isolated trachea. Br J Pharmacol. 1994;112:133–136. doi: 10.1111/j.1476-5381.1994.tb13042.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher SA, Birrell MA, Belvisi MG. Prostaglandin E2 mediates cough via the EP3 receptor: implications for future disease therapy. Am J Respir Crit Care Med. 2009;180:923–928. doi: 10.1164/rccm.200903-0388OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning PJ, Watson RM, O'Byrne PM. Exercise-induced refractoriness in asthmatic subjects involves leukotriene and prostaglandin interdependent mechanisms. Am Rev Respir Dis. 1993;148:950–954. doi: 10.1164/ajrccm/148.4_Pt_1.950. [DOI] [PubMed] [Google Scholar]
- Martin TR, Gerard NP, Galli SJ, Drazen JM. Pulmonary responses to bronchoconstrictor agonists in the mouse. J Appl Physiol. 1988;64:2318–2323. doi: 10.1152/jappl.1988.64.6.2318. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melillo E, Woolley KL, Manning PJ, Watson RM, O'Byrne PM. Effect of inhaled PGE2 on exercise-induced bronchoconstriction in asthmatic subjects. Am J Respir Crit Care Med. 1994;149:1138–1141. doi: 10.1164/ajrccm.149.5.8173753. [DOI] [PubMed] [Google Scholar]
- Mnich SJ, Veenhuizen AW, Monahan JB, Sheehan KC, Lynch KR, Isakson PC, et al. Characterization of a monoclonal antibody that neutralizes the activity of prostaglandin E2. J Immunol. 1995;155:4437–4444. [PubMed] [Google Scholar]
- Morin C, Proteau S, Rousseau E, Brayden J. Organ-cultured airway explanst: a new model of airway hyperresponsiveness. Exp Lung Res. 2005;31:719–744. doi: 10.1080/01902140500248613. [DOI] [PubMed] [Google Scholar]
- Muccitelli RM, Tucker SS, Hay DW, Torphy TJ, Wasserman MA. Is the guinea pig trachea a good in vitro model of human large and central airways? Comparison on leukotriene-, methacholine-, histamine- and antigen-induced contractions. J Pharmacol Exp Ther. 1987;243:467–473. [PubMed] [Google Scholar]
- Ndukwu IM, White SR, Leff AR, Mitchell RW. EP1 receptor blockade attenuates both spontaneous tone and PGE2-elicited contraction in guinea pig trachealis. Am J Physiol Lung Cell Mol Physiol. 1997;273:L626–L633. doi: 10.1152/ajplung.1997.273.3.L626. [DOI] [PubMed] [Google Scholar]
- Ochi T, Motoyama Y, Goto T. The analgesic effect profile of FR122047, a selective cyclooxygenase-1 inhibitor, in chemical nociceptive models. Eur J Pharmacol. 2000;391:49–54. doi: 10.1016/s0014-2999(00)00051-0. [DOI] [PubMed] [Google Scholar]
- Oguma T, Asano K, Shiomi T, Fukunaga K, Suzuki Y, Nakamura M, et al. Cyclooxygenase-2 expression during allergic inflammation in guinea-pig lungs. Am J Respir Crit Care Med. 2002;165:382–386. doi: 10.1164/ajrccm.165.3.2103093. [DOI] [PubMed] [Google Scholar]
- Ohinata K, Suetsugu K, Fujiwara Y, Yoshikawa M. Activation of prostaglandin E receptor EP4 subtype suppresses food intake in mice. Prostaglandins Other Lipid Mediat. 2006;81:31–36. doi: 10.1016/j.prostaglandins.2006.06.008. [DOI] [PubMed] [Google Scholar]
- Okuyama T, Ishihara S, Sato H, Rumi MA, Kawashima K, Miyaoka Y, et al. Activation of prostaglandin E2-receptor EP2 and EP4 pathways induces growth inhibition in human gastric carcinoma cell lines. J Lab Clin Med. 2002;140:92–102. doi: 10.1067/mlc.2002.125784. [DOI] [PubMed] [Google Scholar]
- Orehek J, Douglas JS, Lewis AJ, Bouhuys A. Prostaglandin regulation of airway smooth muscle tone. Nat New Biol. 1973;245:84–85. doi: 10.1038/newbio245084a0. [DOI] [PubMed] [Google Scholar]
- Pavord ID, Tattersfield AE. Bronchoprotective role for endogenous prostaglandin E2. Lancet. 1995;345:436–438. doi: 10.1016/s0140-6736(95)90409-3. [DOI] [PubMed] [Google Scholar]
- Pelletier S, Dube J, Villeneuve A, Gobeil F, Jr, Yang Q, Battistini B, et al. Prostaglandin E(2) increases cyclic AMP and inhibits endothelin-1 production/secretion by guinea-pig tracheal epithelial cells through EP(4) receptors. Br J Pharmacol. 2001;132:999–1008. doi: 10.1038/sj.bjp.0703886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penn RB, Pascual RM, Kim YM, Mundell SJ, Krymskaya VP, Panettieri RA, Jr, et al. Arrestin specificity for G protein-coupled receptors in human airway smooth muscle. J Biol Chem. 2001;276:32648–32656. doi: 10.1074/jbc.M104143200. [DOI] [PubMed] [Google Scholar]
- Raeburn D, Hay DWP, Muccitelli RM, Dey RD, Fedan JS. The development of tone in the smooth muscle of guinea-pig isolated tracheal preparations may be influenced by prostanoids released from the adjacent airway cartilage. Prostaglandins. 1987;33:651–661. doi: 10.1016/0090-6980(87)90032-3. [DOI] [PubMed] [Google Scholar]
- Rao R, Redha R, Macias-Perez I, Su Y, Hao C, Zent R, et al. Prostaglandin E2-EP4 receptor promotes endothelial cell migration via ERK activation and angiogenesis in vivo. J Biol Chem. 2007;282:16959–16968. doi: 10.1074/jbc.M701214200. [DOI] [PubMed] [Google Scholar]
- Rehal S, Blanckaert P, Roizes S, von der Weid PY. Characterization of biosynthesis and modes of action of prostaglandin E2 and prostacyclin in guinea pig mesenteric lymphatic vessels. Br J Pharmacol. 2009;158:1961–1970. doi: 10.1111/j.1476-5381.2009.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressmeyer AR, Larsson AK, Vollmer E, Dahlen SE, Uhlig S, Martin C. Characterisation of guinea pig precision-cut lung slices: comparison with human tissues. Eur Respir J. 2006;28:603–611. doi: 10.1183/09031936.06.00004206. [DOI] [PubMed] [Google Scholar]
- Ricciardolo FL, Nijkamp F, De Rose V, Folkerts G. The guinea pig as an animal model for asthma. Curr Drug Targets. 2008;9:452–465. doi: 10.2174/138945008784533534. [DOI] [PubMed] [Google Scholar]
- Selg E, Andersson M, Lastbom L, Ryrfeldt A, Dahlen SE. Two different mechanisms for modulation of bronchoconstriction in guinea-pigs by cyclooxygenase metabolites. Prostaglandins Other Lipid Mediat. 2009;88:101–110. doi: 10.1016/j.prostaglandins.2008.11.003. [DOI] [PubMed] [Google Scholar]
- Vermue NA, Den Hertog A, Zaagsma J. Desensitization of PGE2 and PGI2 induced contractions in different smooth muscles of guinea-pig unmasking relaxing properties of prostanoids. Eur J Pharmacol. 1987;144:399–403. doi: 10.1016/0014-2999(87)90396-7. [DOI] [PubMed] [Google Scholar]
- Watson N, Magnussen H, Rabe KF. Inherent tone of human bronchus: role of eicosanoids and the epithelium. Br J Pharmacol. 1997;121:1099–1104. doi: 10.1038/sj.bjp.0701244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Sakata D, Esaki Y, Li Y, Matsuoka T, Kuroiwa K, et al. Prostaglandin E2-EP4 signaling promotes immune inflammation through Th1 cell differentiation and Th17 cell expansion. Nat Med. 2009;15:633–640. doi: 10.1038/nm.1968. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.