Abstract
Background and Purpose
Although it is established that the receptor activity modifying proteins (RAMPs) can interact with a number of GPCRs, little is known about the consequences of these interactions. Here the interaction of RAMPs with the glucagon-like peptide 1 receptor (GLP-1 receptor), the human vasoactive intestinal polypeptide/pituitary AC-activating peptide 2 receptor (VPAC2) and the type 1 corticotrophin releasing factor receptor (CRF1) has been examined.
Experimental Approach
GPCRs were co-transfected with RAMPs in HEK 293S and CHO-K1 cells. Cell surface expression of RAMPs and GPCRs was examined by elisa. Where there was evidence for interactions, agonist-stimulated cAMP production, Ca2+ mobilization and GTPγS binding to Gs, Gi, G12 and Gq were examined. The ability of CRF to stimulate adrenal corticotrophic hormone release in Ramp2+/– mice was assessed.
Key Results
The GLP-1 receptor failed to enhance the cell surface expression of any RAMP. VPAC2 enhanced the cell surface expression of all three RAMPs. CRF1 enhanced the cell surface expression of RAMP2; the cell surface expression of CRF1 was also increased. There was no effect on agonist-stimulated cAMP production. However, there was enhanced G-protein coupling in a receptor and agonist-dependent manner. The CRF1 : RAMP2 complex resulted in enhanced elevation of intracellular calcium to CRF and urocortin 1 but not sauvagine. In Ramp2+/– mice, there was a loss of responsiveness to CRF.
Conclusions and Implications
The VPAC2 and CRF1 receptors interact with RAMPs. This modulates G-protein coupling in an agonist-specific manner. For CRF1, coupling to RAMP2 may be of physiological significance.
Keywords: receptor activity-modifying proteins (RAMPs), RAMP1, RAMP2, RAMP3, VPAC2 receptor, CRF1 receptor, Ramp2+/− mice, G-protein coupling, biased agonism
Introduction
Receptor activity-modifying proteins (RAMPs) consist, in mammals, of three single-pass transmembrane proteins, first identified as essential components of calcitonin gene-related peptide (CGRP) and adrenomedullin receptors (McLatchie et al., 1998). They associate in the endoplasmic reticulum with a GPCR known as calcitonin receptor-like receptor (CLR). The RAMPs and CLR by themselves have poor abilities to reach the cell surface and cannot bind any known endogenous ligand. However, the complexes are translocated to the cell surface where they respond to CGRP, adrenomedullin and adrenomedullin 2 via CGRP, AM1 and AM2 receptors (Poyner et al., 2002; Wootten et al., 2010; Hong et al., 2011). The trafficking of adrenomedullin receptors is also influenced by the RAMPs (Bomberger et al., 2005).
RAMPs have subsequently been shown to interact with a wider range of GPCRs. The best characterized of these interactions is with the calcitonin receptor (CTR), where the RAMPs do not alter receptor cell surface expression but instead change ligand binding and G-protein coupling to give amylin receptors (Hay et al., 2006; Morfis et al., 2008). RAMPs 1 and 3 are needed for cell surface expression of the calcium sensing receptor, a glutamate-like/family C GPCR (Bouschet et al., 2005). However, most interest has focussed on secretin-like/family B GPCRs. By monitoring the ability of GPCRs to increase the cell surface expression of RAMPs, it has been shown that the vasoactive intestinal polypeptide (VIP)/pituitary AC-activating peptide 1 receptor (VPAC1) could interact with all three RAMPs, the parathyroid hormone PTH1 receptor could interact with RAMP2, the parathyroid hormone PTH2 receptor could interact with RAMP3 and the glucagon receptor could interact with RAMP2. No evidence was found for interactions between RAMPs and the VPAC2 or either the GLP-1 or GLP-2 receptors (Christopoulos et al., 2003). Only in the case of the VPAC1 was there any characterization of the effects of RAMP association. The complex with RAMP2 had normal expression and pharmacology for activation of AC, but the maximum stimulation of phosphoinositide turnover in response to VIP was increased (Christopoulos et al., 2003). It would seem unlikely that there is no consequence of RAMP association with these different receptors. Indeed, recent studies of Ramp2+/– knockdown mice has shown there was a wide range of phenotypic changes that go far beyond what would be expected for effects mediated by peptides acting through CLR and CTR/RAMP complexes (Kadmiel et al., 2011).
In this study, the ability of three family B GPCRs, the type 1 corticotrophin releasing factor receptor (CRF1), the GLP-1 receptor and the VPAC2 receptor to interact with all three RAMPs have been examined in HEK 293S and CHO-K1 cells. These receptors are of potential therapeutic interest. In evolutionary terms, the CRF1 is the closest family B GPCR to the CTR and CLR (Fredriksson et al., 2003), but its ability to interact with RAMPs has not previously been investigated. The VPAC2 and GLP-1 receptor are on the other two main branches that make up secretin-like/family B GPCR family. The results show that the VPAC2 can interact with all three RAMPs, and the CRF1 can interact with RAMP2. These interactions differentially modulate G-protein coupling; in the case of the CRF1, it is shown that this alters the pattern of calcium signalling and furthermore, in Ramp2+/– mice, the physiological effects of CRF on adrenal corticotrophic hormone (ACTH) release are reduced.
Methods
Materials
Peptides were from Bachem (St. Helens, UK). Unless otherwise specified, chemicals were from Sigma or Fisher (Loughborough, UK). Cell culture reagents were from Gibco BRL (Paisley, Renfrewshire, UK) or Sigma. Monoclonal anti-HA, mouse clone HA-7, monoclonal mouse anti-FLAG, clone M2 and monoclonal goat anti-mouse antibody containing a conjugated HRP were purchased from Sigma. G-protein antibodies (Gs, Gq/11, G12/13, Gi/o/t/z) were from Santa Cruz (Santa Cruz, CA).
Expression constructs
Plasmid DNA was extracted from the cultures using a Wizard-Prep DNA extraction kit according to the manufacturer's instructions (Promega, Southampton, UK). The plasmid DNA was eluted in 100 μL sterile distilled water and stored at −20°C. For all receptors, a pcDNA3.1– template vector was produced containing a T8 signal peptide and a HA-Tag (cloned in using NotI and EcoRI). The mature protein sequence for each receptor was then cloned in using EcoRI and HindIII. The EcoRI site between the tag, and the receptor sequence was removed using Quikchange. The RAMPs were modified with a FLAG tag inserted just before residue 24 (RAMP1), 42 (RAMP2) and 25 (RAMP3). A pcDNA3.1+ template vector was produced containing a CD33 signal peptide and a FLAG-Tag (cloned in using HindIII and EcoRI). The mature protein sequence for each RAMP was then cloned in using EcoRI and XhoI. The EcoRI site between the tag, and the RAMP sequence was removed using site mutagenesis with a Quikchange kit (Stratagene, Leicester, UK). The untagged constructs were in pcDNA3.1– and were either gifts from AstraZeneca (receptors) or Dr Steve Foord, GSK-Wellcome (RAMPs). The CRF1 receptor was isoform 1, which includes residues 147–176.
Cell culture and transfection
Cells were cultured in DMEM supplemented with 10% (v/v) FBS and 5 % (v/v) penicillin/streptomycin in a humidified 95% air/5% CO2 atmosphere. For transfection, the cells were plated onto either 12- and 48-well plates or 100 mm dishes. Cells were transfected using a mixture (per 1 μg DNA) of 6 μL 10 mM polyethyleneimine and 45 μL 5% glucose solution incubated for 30 min at room temperature and added to an appropriate final volume of full media. Twelve- and 48-well plates were treated with 1 μg DNA per well, and 100 mm dishes were treated with 10 μg DNA per dish. The ratio of RAMP to receptor cDNA was 1:1 unless otherwise stated; where receptor or RAMP alone was transfected, the balance to 1 or 10 μg was made up with empty pcDNA3.1– vector. Characterization of expressed receptors was performed 48–72 h after transfection.
Real-time quantitative PCR
Cells from confluent flasks were washed briefly with 1 mL cold PBS, detached with versene and pelleted by spinning at 350× g for 5 min. Total cellular RNA was isolated using the RNeasy kit and Qiashredder columns from Qiagen (Crawley, UK) following the manufacturer's recommendations. RNA isolated 48 h post transfection from HEK-293S cells and CHO-K1 cells transfected with cDNAs for human RAMP1, RAMP2 and RAMP3 were used as positive controls. RNA concentration was calculated based on the absorbance at 260 nm. cDNA was generated from 5 μg of total RNA by using the Promega Reverse Transcription System. Quantification of RAMP1, RAMP2 and RAMP3 expression was performed by a real-time PCR Roche lightcycler (Burgess Hill, UK) and the SYBER green I PCR kit for the lightcycler following the manufacturer's protocol. RAMP expression was standardized to the expression of the housekeeping gene GADPH.
Membrane preparation
Cells from 100 mm dishes were washed briefly with 1 mL cold PBS. They were detached with versene and pelleted by spinning at 350× g for 5 min at room temperature. The pellet was washed in ice-cold homogenization buffer (100 mM NaCl, 10 mM MgCl2, 50 mM HEPES, pH 7.4), homogenized and then spun at 1700× g at 4°C for 10 min to pellet the nuclei. The supernatant was then respun at 40 000× g at 4°C for 90 min. The pellet was resuspended in homogenization buffer and stored at −80°C.
GTPγS binding
The binding reaction was set up on ice in 1.5 mL tubes in a final volume of 500 μL of assay buffer (10 mM NaCl, 10 mM MgCl2, 0.2% BSA, 20 mM HEPES, pH 7.4) containing 150 μg membrane, 0.01% saponin and either 1 μM (for Gs) or 0.1 μM GDP (G12/13, Gq/11 and Gi/o/t/z). After a 60 min preincubation at 30°C, 200 pM [35S]-GTPγS was added and incubated for a further 30 min at 30°C. The reaction was terminated by adding 1 mL ice-cold assay buffer and centrifuged in a refrigerated microfuge at full speed for 6 min. The pellet was washed with 100 μL of ice-cold solubilization buffer (assay buffer with 1.25% NP40) and left on ice for 30 min to solubilise; 2 μg of the appropriate anti-Gα subunit antibody was added and incubated overnight at 4°C; 50 μL of 30% slurry of protein-A (pre-equilibrated in assay buffer) was added and left at 4°C for 90 min. After washing twice with 500 μL of cold 1× solubilization solution, the pellet was resuspended with 100 μL of solubilization buffer supplemented with 0.2% SDS and measured by scintillation counting.
Assay of cAMP production
Growth medium was removed from the cells and replaced with DMEM containing 500 μM isobutyl methyl xanthine for 30 min. Peptides in the range 10 pM to 1 μM were added for a further 15 min. Ice-cold ethanol (95–100% v/v) was used to extract cAMP, which was subsequently measured by radio-receptor assay as previously described (Poyner et al., 1992). Data were normalized with respect to addition of 10 μM forskolin.
Measurements of intracellular Ca2+
Transfected cells were seeded in growth medium at 5 × 104 cells per well in black, clear-bottomed, 96-well plates and incubated overnight. Cells were washed with PBS, loaded with 100 μL per well of loading buffer (1X HBSS/20 mM HEPES/2 mM CaCl2, pH 7.4) containing 5 mM probenecid and incubated at 37°C for 1 h. Fluorescence was determined using a FlexStation (Molecular Devices, Sunnyvale, CA) immediately after peptide ligand addition with excitation wavelength 485 nm and emission wavelength to 520 nm. Peak magnitude was determined using five-point smoothing, followed by correction against basal fluorescence.
Analysis of cell-surface expression by elisa
Cells in 12-well plates were transiently transfected with receptors and RAMPs as appropriate. Details were as described previously (Conner et al., 2005). The cells were treated with 250 μL of primary antibody (mouse, anti-HA antibody HA-7 or mouse anti-FLAG M2 [Sigma] diluted 1:2000 in PBS with 5% BSA) for 1 h.
Measurement of ACTH
Ramp2+/– mice on an isogenic 129S6/SvEv genetic background have been previously described ((Dackor et al., 2007; Kadmiel et al., 2011). Ramp2+/– (n = 6) and wild-type control (n = 8) mice were injected i.p. with CRF, (40 μg kg−1, #H-2435, Bachem, Torrance, CA.) to stimulate release of ACTH. Blood samples were collected via sub-mandibular bleed after 2 h. An ultra-sensitive chemiluminescence elisa kit (#MBS580004, MyBioSource; San Diego, CA) was used according to the manufacturer's protocol to measure plasma levels of ACTH. Briefly, plasma samples were incubated a goat polyclonal antibody and a mouse monoclonal antibody to ACTH. One antibody is biotinylated and binds only the C-terminal of ACTH 34–39. The other antibody is labelled with HRP and binds only the mid-region and N-terminal of ACTH 1–24. The samples were then incubated with an enzyme-labelled antibody and a biotin coupled antibody in a streptavidin-coated microplate and analysed by addition of a luminal substrate. Concentrations of ACTH present in the controls and samples are determined from a standard curve and plotted as plasma ACTH (pg mL−1). Experimental animals were 6–8 months of age, and control animals were wild-type, age- and gender-matched littermates. Animals were fed ad libitum and housed in standard 12 h/12 h light/dark cycle. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). All experiments were approved by the Institutional Animal Care and Use Committee of the University of North Carolina at Chapel Hill.
Data analysis
Curve fitting was done with PRISM GraphPad 4 (GraphPad Software Inc., San Diego, CA). The data from each concentration–response curve were fitted to a sigmoidal concentration–response curve to obtain the maximum response and –logEC50 (pEC50). pEC50, basal and maximal responses were compared by paired Student's t-test or by one-way anova followed by Dunnett's test when multiple comparisons were made. Data from elisas were compared by the Mann–Whitney test. n-values refer to the number of independent experiments.
Results
Characterization of cell lines and receptors
As the effects of RAMPs are heavily influenced by cell line background (Tilakaratne et al., 2000; Udawela et al., 2006a), HEK 293S and CHO-K1 cells were both used in this study. They were first examined for expression of endogenous RAMPs by RT-PCR. The highest endogenous expression was that for RAMP2 in HEK 293S cells (5.1 ± 1.1% of GAPDH expression); in all other cases, endogenous RAMP expression was not more than 2.25% of GAPDH. As an added check, both cell lines were transfected with CLR alone and challenged with either CGRP or adrenomedullin at concentrations from 0.01 to 1000 nM. In no case was there any detectable increase in cAMP. Thus, there was insufficient endogenous RAMP expression to generate CGRP, AM1 or AM2 receptors (Supporting Information Figures S1 and S2).
The pharmacology of N-terminal, HA-tagged CRF1, CTR, GLP-1 receptor and VPAC2 were compared with the untagged receptors, by measuring the ability of CRF, calcitonin, GLP-1(7–36)amide (GLP1) or VIP to stimulate cAMP production in cells transfected with their cognate receptor. In no case was there any difference in pEC50, maximal or basal responses between the tagged and untagged receptors (Supporting Information Figure S3). Similarly, the properties of N-terminal FLAG-tagged RAMP1, 2 and 3 were compared with untagged RAMPs by co-transfecting with CLR and measuring the ability of either CGRP or adrenomedullin to stimulate cAMP at the resulting CGRP or adrenomedullin receptors. Again, there was no difference between the responses of the tagged and untagged RAMPs (Supporting Information Figure S4).
Cell surface expression of receptor–RAMP complexes
To investigate potential interactions between receptors and RAMPs, the ability of interacting receptors to increase cell surface RAMP expression was examined (Table 1) (Christopoulos et al., 2003). As expected, cell surface expression of RAMPs transfected on their own was poor in both HEK 293S and CHO-K1 cells, but it was enhanced by co-transfection of the CTR. Co-transfection with GLP-1 receptor had no effect on RAMP expression. By contrast, VPAC2 co-transfection significantly enhanced the expression of all three RAMPs in both cell lines; the largest change was seen for RAMP1 whereas the increase with RAMP3 was much smaller. Co-transfection with CRF1 enhanced cell surface expression of RAMP2 alone. In all cases, the enhancement of RAMP cell surface expression was greatest with the HEK 293S cells.
Table 1.
Cell surface expression of RAMPs in the presence or absence of receptors
| RAMP | HEK 293S | CHO-K1 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTR | VPAC2 | GLP-1R | CRF1 | pcDNA3 | CTR | VPAC2 | GLP-1R | CRF1 | pcDNA3 | |
| 1 | 100 | 69 ± 5* | 6 ± 1 | 2 ± 2 | 3 ± 2 | 100 | 45 ± 4* | 2 ± 2 | 5 ± 2 | 2 ± 2 |
| 2 | 100 | 62 ± 6* | 24 ± 3 | 57 ± 4* | 22 ± 6 | 100 | 36 ± 7* | 17 ± 3 | 39 ± 4* | 15 ± 4 |
| 3 | 100 | 49 ± 5* | 30 ± 8 | 27 ± 5 | 36 ± 4 | 100 | 32 ± 5* | 13 ± 6 | 22 ± 6 | 19 ± 3 |
RAMPs were detected by cell surface elisa of their FLAG tag. Expression was normalized (100%) to that seen when each RAMP was co-expressed with CTR. To determine receptor-independent expression, cells were co-transfected with the appropriate RAMP and empty vector (pcDNA3). Values are means ± SEM, n > 3.
Expression significantly different from pcDNA3, P < 0.05, Mann–Whitney.
With both CLR and the calcium sensing receptor, there is a reciprocal interaction, whereby RAMPs also enhance the cell surface expression of the receptor (McLatchie et al., 1998; Bouschet et al., 2005). Accordingly, the ability of RAMPs to traffic the receptors to the cell surface was also examined (Table 2). Only RAMP2 had any effects, increasing the delivery of CRF1. Consistent with the data on RAMP expression, the effect was larger in HEK 293S cells compared with CHO-K1 cells.
Table 2.
Cell surface expression of receptors in the presence or absence of RAMPs
| Receptor | HEK 293S | CHO-K1 | ||||||
|---|---|---|---|---|---|---|---|---|
| pcDNA3 | RAMP1 | RAMP2 | RAMP3 | pcDNA3 | RAMP1 | RAMP2 | RAMP3 | |
| CTR | 100 | 104 ± 8 | 106 ± 7 | 108 ± 6 | 100 | 98 ± 5 | 107 ± 8 | 104 ± 9 |
| VPAC2 | 100 | 105 ± 5 | 92 ± 3 | 83 ± 6 | 100 | 102 ± 6 | 95 ± 9 | 100 ± 6 |
| GLP-1R | 100 | 103 ± 4 | 98 ± 3 | 81 ± 5 | 100 | 101 ± 5 | 92 ± 7 | 79 ± 9 |
| CRF1 | 100 | 94 ± 6 | 208 ± 16* | 97 ± 7 | 100 | 95 ± 5 | 125 ± 7* | 99 ± 11 |
Receptors were detected by cell surface elisa of their HA tag. Expression was normalized (100%) to that seen in the absence of any RAMP [determined by cotransfecting with the appropriate receptor and empty vector (pcDNA3)]. Values are means ± SEM, n > 3.
Expression significantly different from pcDNA3, P < 0.05, Mann–Whitney.
Effects on receptor pharmacology
The effects of RAMP expression on the pharmacology of the VPAC2 and CRF1 were investigated by examining the ability of a range of agonists to stimulate cAMP production. For the VPAC2, the receptor was co-expressed with all three RAMPs in HEK 293S and CHO-K1 cells and challenged with VIP, BAY 55-9837, PACAP-27 and PHM-27 (Figure 1). These are all agonists at VIP/PACAP receptors with good potency against VPAC2 receptors; BAY 55-9837 is selective for this subtype (Tsutsumi et al., 2002). In no case was there any difference in the response of the receptors to the agonists. HEK 293S cells appeared to express an endogenous PAC1 receptor, as the untransfected cells showed a good response to PACAP-27 (Figure 1B, pEC50 8.74 ± 0.09, Emax 97 ± 2%) and PHM-27 (Figure 1D; pEC50 8.60 ± 0.12, Emax 62 ± 2%). However, for VIP and BAY 55-9837 (Figure 1A, C) in these cells as well as for all four agonists in the CHO-K1 cells (Figure 1E–H), there was little response in untransfected cells so the lack of effect of the RAMPs was clear. There was a modest increases in basal cAMP production for the VPAC1 receptor expressed with RAMP1 (23.5 ± 2.5%) and a slight decrease with RAMP3 (−9.0 ± 3.5%) compared with VPAC1 alone (8.0 ± 2.1%) (Figure 1B, P < 0.01 and 0.05, respectively, Dunnett's test following one-way anova). For the CRF1, the receptor was co-expressed with RAMP2 in HEK 293S cells and challenged with CRF, urocortin 1, and sauvagine (all agonists at the CRF1 receptor) (Dautzenberg et al., 2001). In addition, for CRF challenge, the receptor was also expressed with RAMP1 and RAMP3 (Figure 2). In no case was there any difference in the response of the receptors to the agonists. The experiments were also repeated in CHO-K1 cells with identical results (Supporting Information Figures S5 and S6).
Figure 1.
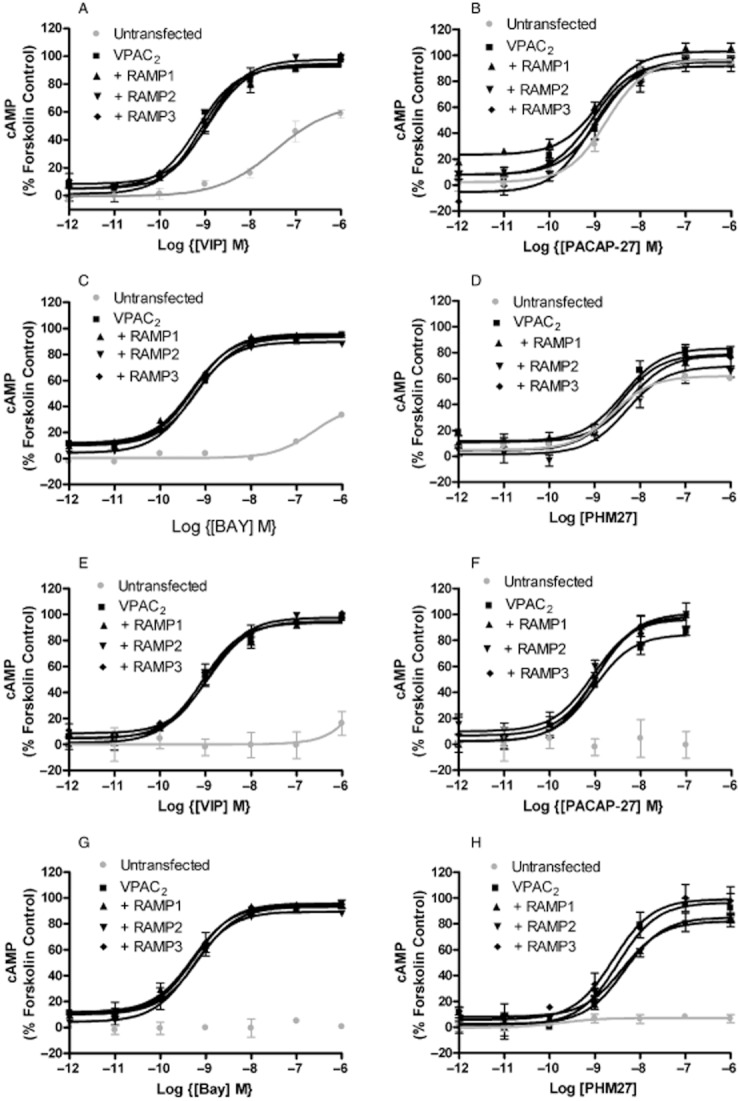
Effects of RAMP co-transfection on the pharmacology of the VPAC2 expressed in HEK 293S (A–D) and CHO-K1 cells (E–H). Cells were transiently transfected with either VPAC2 + pcDNA3, VPAC2 + RAMP1, VPAC2 + RAMP2, VPAC2 + RAMP3 or pcDNA3 alone. Values are normalized to the maximum response to forskolin when applied to the receptor alone. Values are means ± SEM, n > 3.
Figure 2.
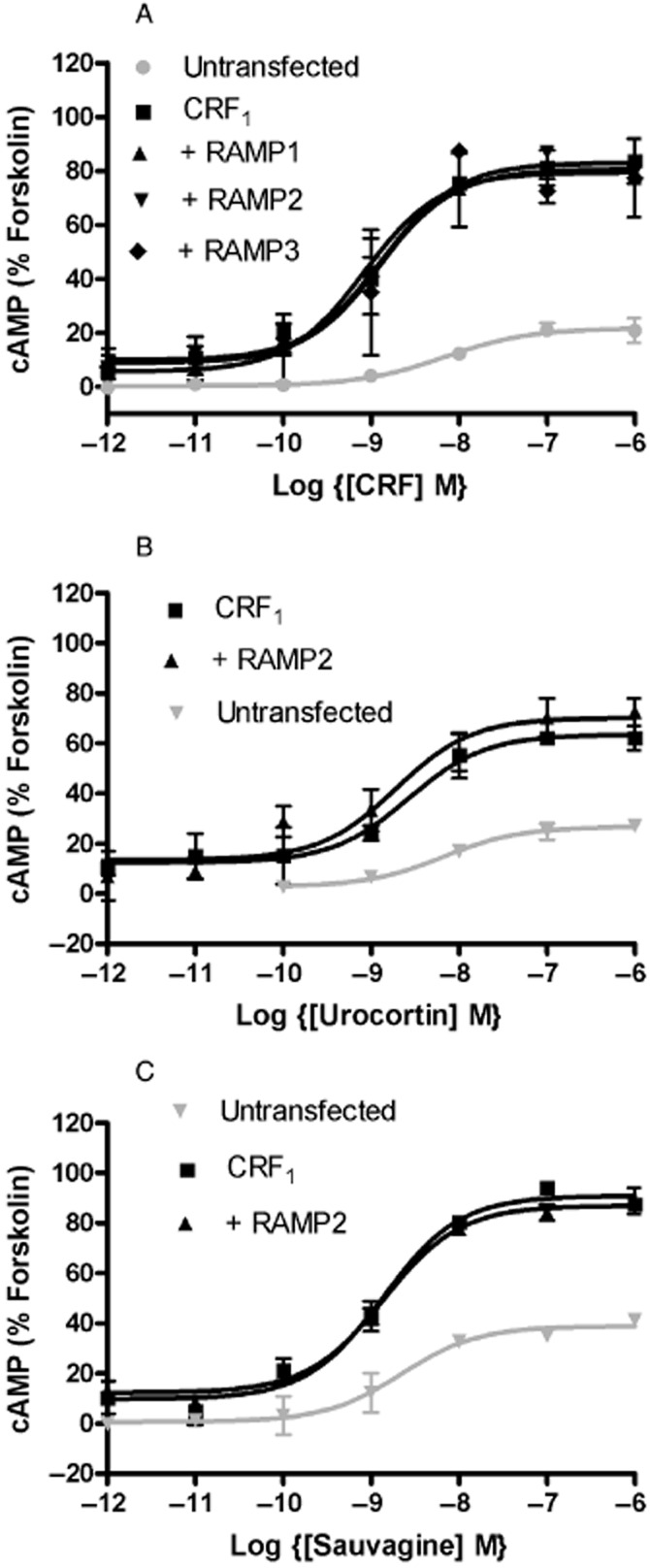
Effects of RAMP co-transfection on the pharmacology of the CRF1 expressed in HEK 293S cells. Cells were transiently transfected with either CRF1 + pcDNA3, CRF1 + RAMP1, CRF1 + RAMP2, CRF1 + RAMP3 or pcDNA3 alone. Values are normalized to the maximum response to forksolin when applied to the receptor alone. Values are means ± SEM, n > 3.
GTPγS binding
To investigate if the RAMPs could modulate G-protein coupling, agonist-stimulated GTPγS binding to different G-proteins was investigated. For the VPAC2, there was no effect on VIP-stimulated GTPγS binding to Gs (Figure 3). However, RAMP1 and RAMP2 significantly increased basal coupling to Gi/o/t/z in both cell lines (Table 3, Figure 4). RAMP1 in HEK 293S cells also gave a small increase in potency for VIP at stimulating this increase (Table 3, Figure 4A). RAMP3 had no effect on coupling to Gi/o/t/z in either cell line. There was no evidence of coupling of the VPAC2 to either Gq/11 or G12/13 in HEK 293S cells in the absence or presence of any of the three RAMPs (Supporting Information Figure S7).
Figure 3.
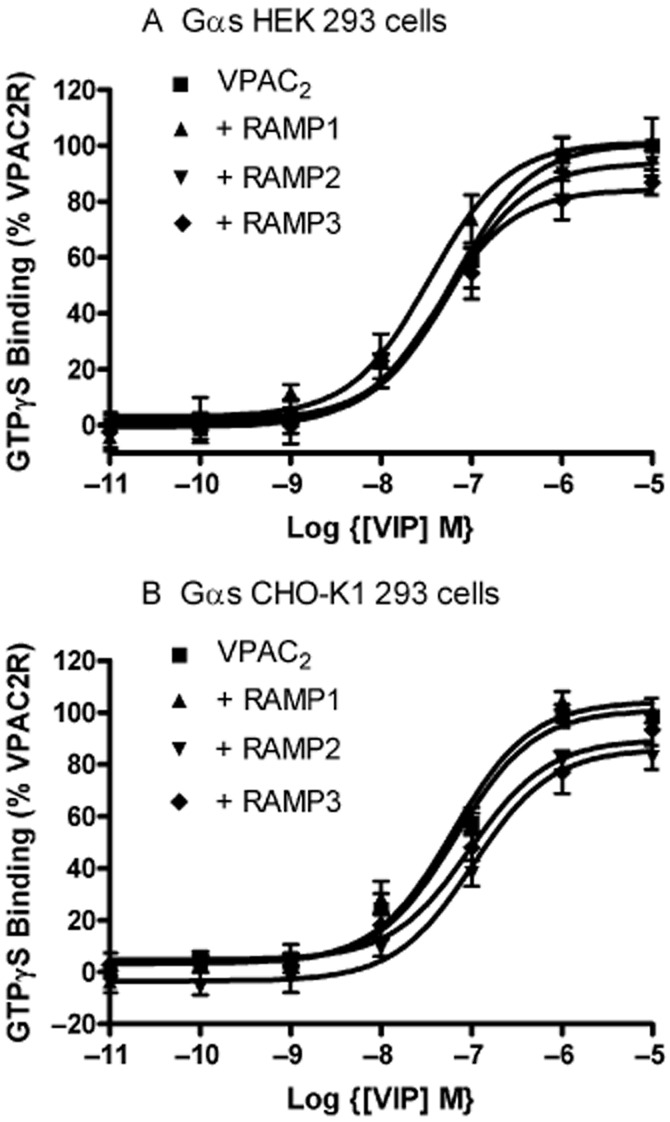
VIP-stimulated GTPγS binding to Gαs in (a) HEK 293S and (b) CHO-K1 cells following transient transfection of either VPAC2 + pcDNA3, VPAC2 + RAMP1, VPAC2 + RAMP2 or VPAC2 + RAMP3. Values are means ± SEM, n > 3.
Table 3.
Chief effects of RAMPs on the coupling of the VPAC2 receptor to G-proteins
| G-protein | Cells | RAMP | pEC50 | Emax | Basal |
|---|---|---|---|---|---|
| Gi/o/t/z | HEK293S | No RAMP | 6.79 ± 0.06 | 102.7 ± 2.8 | 1.3 ± 1.6 |
| Gi/o/t/z | HEK293S | RAMP 1 | 7.37 ± 0.12* | 106.6 ± 3.8 | 27.9 ± 2.7** |
| Gi/o/t/z | HEK293S | RAMP 2 | 7.45 ± 0.13* | 123.0 ± 4.7 | 15.6 ± 3.7* |
| Gi/o/t/z | CHO-K1 | No RAMP | 7.01 ± 0.16 | 104.2 ± 4.2 | -1.2 ± 2.8 |
| Gi/o/t/z | CHO-K1 | RAMP 1 | 7.29 ± 0.09 | 110.3 ± 2.9 | 21.7 ± 2.1** |
| Gi/o/t/z | CHO-K1 | RAMP 2 | 7.05 ± 0.16 | 108.6 ± 6.1 | 17.9 ± 4.0** |
Values are means ± SEM, n > 3.
*,**, P > 0.05, 0.01 or 0.001, relative to the parameter measured in the absence of a RAMP in the same cell line. Values compared using Dunnett's test following one-way anova.
Figure 4.
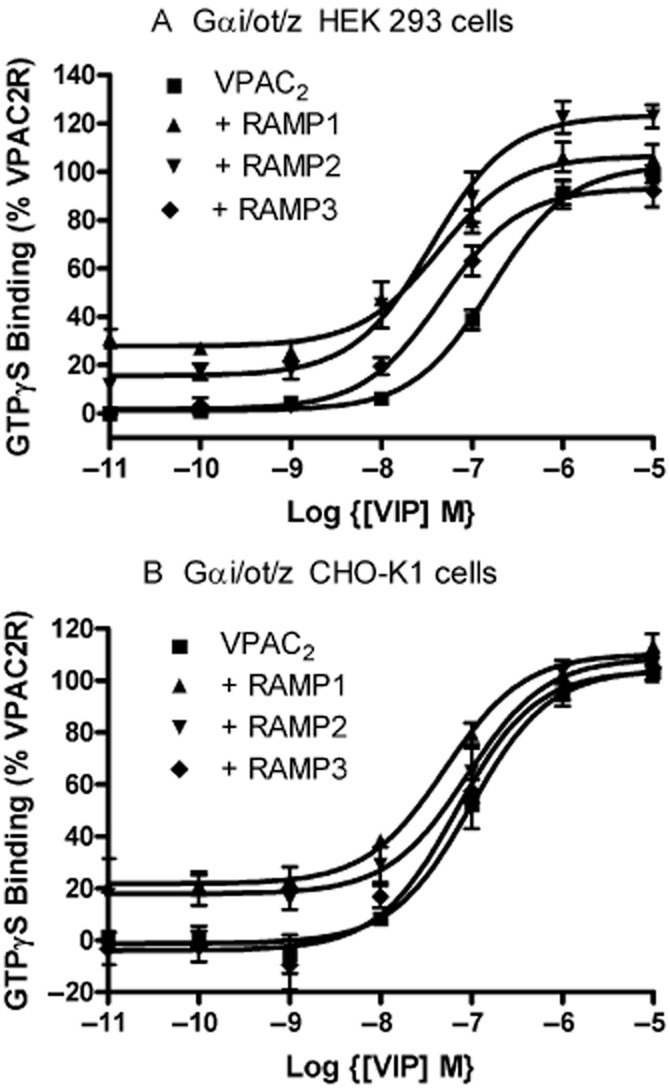
VIP-stimulated GTPγS binding to Gαi/o/t/z in (a) HEK 293S and (b) CHO-K1 cells following transient transfection of either VPAC2 + pcDNA3, VPAC2 + RAMP1, VPAC2 + RAMP2 or VPAC2 + RAMP3.Values are means ± SEM, n > 3.
RAMP2 had no effect on coupling of the CRF1 to Gs in the presence of CRF. However, there was an enhanced coupling to Gi/o/t/z, Gq/11 and G12/13 (Table 4, Figure 5). The differences depended on the G-protein. For Gi/o/t/z (Figure 5B), there was an enhancement of basal GTPγS binding and an increase in CRF-stimulated maximum response. For Gq/11, the main effect was an increase in the size of the CRF-stimulated maximum response (Figure 5B). For G12/13, the main effect was to increase the potency of CRF (Figure 5D). A very similar pattern was seen for urocortin (Figure 5E–H, Table 4).
Table 4.
Effects of RAMP 2 on the coupling of the CRF1 receptor to G-proteins
| Agonist | G-protein | pEC50 | Emax | Basal | pEC50 | Emax | Basal |
|---|---|---|---|---|---|---|---|
| No RAMP | RAMP 2 | ||||||
| CRF | Gs | 7.28 ± 0.13 | 98.1 ± 5.0 | 6.1 ± 5.0 | 7.12 ± 0.15 | 92.0 ± 4.3 | 9.5 ± 6.3 |
| Urocotin | Gs | 7.56 ± 0.16 | 109.9 ± 6.4 | −8.2 ± 5.3 | 7.45 ± 0.14 | 113.7 ± 5.9 | −9.8 ± 4.7 |
| CRF | Gi/o/t/z | 6.97 ± 0.08 | 98.8 ± 4.7 | 3.8 ± 2.7 | 7.29 ± 0.31 | 269.1 ± 26.2** | 65.3 ± 17.6* |
| Urocortin | Gi/o/t/z | 7.25 ± 0.16 | 96.4 ± 5.7 | −0.6 ± 4.1 | 7.41 ± 0.14 | 288.1 ± 10.7*** | 67.0 ± 8.3*** |
| CRF | Gq | 7.48 ± 0.90 | 82.5 ± 27.4 | 4.2 ± 21.8 | 7.56 ± 0.26 | 270.4 ± 28.2** | −3.7 ± 22.4 |
| Urocortin | Gq | 6.99 ± 0.35 | 105.2 ± 14.5 | 5.9 ± 9.4 | 7.23 ± 0.13 | 291.3 ± 12.4*** | 38.7 ± 8.8 |
| CRF | G12 | 5.93 ± 0.19 | 107.4 ± 16.4 | 6.40 ± 3.2 | 7.57 ± 0.24** | 138.4 ± 12.8 | −3.4 ± 9.4 |
| Urocortin | G12 | 6.24 ± 0.29 | 105.6 ± 15.0 | 6.1 ± 3.1 | 7.57 ± 0.14* | 176.4 ± 7.3* | 17.6 ± 6.1 |
Values are means ± SEM, n > 3.
*,**,***P > 0.05, 0.01 or 0.001, relative to the parameter measured in the absence of RAMP 2 in the same cell line. Values compared using by Student's t-test.
Figure 5.
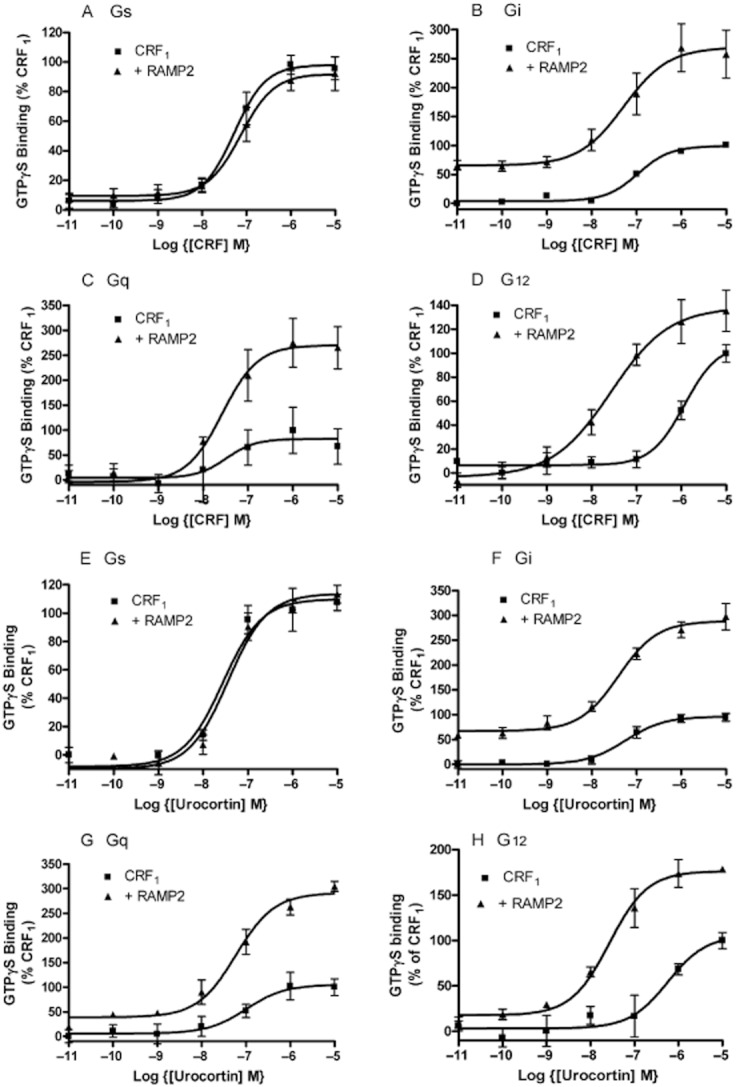
CRF- (a-d) and urocortin- (e-h) stimulated GTPγS binding to (A and E) Gαs, (B and F) Gαi, (C and G) Gαq and (D and H) Gα12 in HEK 293S cells following transfection with CRF1+ pcDNA3 and CRF1 + RAMP2. Values are means ± SEM of n = 3.
As RAMP2 increases CRF1 expression at the cell surface, there was a possibility that the enhanced GTPγS binding could be secondary to an increase in receptor number. Initially, the RAMP to receptor ratio was varied. If, instead of transfecting each well with 1 μg of plasmid containing DNA for the CRF1, 0.6 μg was used, receptor expression was virtually reduced back to control values in the absence of RAMP2 (112 ± 6% of the expression of CRF1 seen in the absence of RAMP2 measured by elisa). The lower CRF1 expression made little difference to the enhanced coupling seen to Gi/o/t/z in the presence of RAMP2 (Supporting Information Figure S8).
Enhanced Ca2+ elevation for CRF1/RAMP2
The increased coupling seen to Gq/11 and Gi/o/t/z suggested that RAMP2 should enhance CRF1-mediated intracellular calcium mobilization. In HEK 293S cells there was a small elevation of intracellular Ca2+ in response to agonists in untransfected cells, suggesting low-level expression of an endogenous CRF receptor. However, the response became substantially larger following transfection with the CRF1 and agonist potency also increased (Figure 6, Supporting Information Table S1). Transfection with RAMP2 enhanced the maximum response seen to CRF by 81 ± 2% and to urocortin by 64 ± 2% but had no effect on the response to sauvagine.
Figure 6.
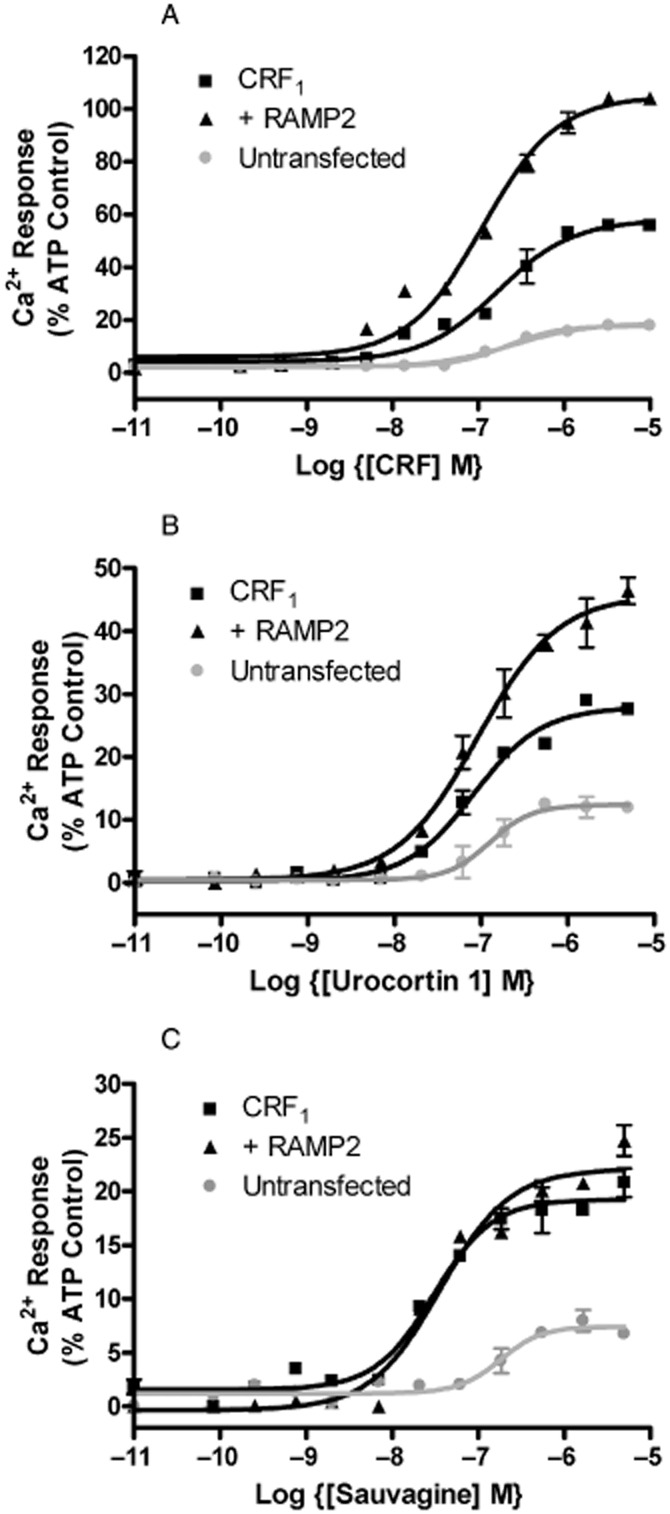
Effect of RAMP2 on (a) CRF-induced, (b) Urocortin1-induced and (c) sauvagine-induced Ca2+ mobilization in HEK 293S cells transfected with either CRF1 + pcDNA3, CRF1 + RAMP2 or pcDNA3 alone. Values are normalized to the maximum response produced by ATP. Values are means ± SEM, n = 3.
The mechanism responsible for the enhanced Ca2+ elevation was investigated by use of inhibitors (Table 5). In the absence of RAMP2, the elevated intracellular calcium appeared to be come entirely from an intracellular pool. Its release was blocked by the PLC inhibitor U73122, and the pool could be depleted by the CaATPase inhibitor thapsigargin. By contrast, in the presence of RAMP2, there was evidence for the use of extracellular Ca2+ in addition to this intracellular pool. Removal of extracellular Ca2+ reduced the response by about a one-third, and correspondingly, U73122 and thapsigargin only blocked about 2/3rd of the Ca2+ elevation. Pertussis toxin also inhibited the Ca2+ elevation by around a one-third. As its effects were additive with those of U73122 and thapsigargin but not with removal of extracellular Ca2+, it appears that the additional pool of extracellular Ca2+ is utilized via a Gi/o/t/z-mediated pathway.
Table 5.
Inhibition of Ca2+ mobilization in response to activation of the CRF1
| Inhibitors | % Inhibition of Ca2+ response following stimulation by 1 μM CRF | ||
|---|---|---|---|
| CRF1 | CRF1 + RAMP 2 | ||
| None | 0 | 0 | |
| + U73122 | 99 ± 2* | 76 ± 3* | |
| + Thapsigargin | 98 ± 3* | 72 ± 4* | |
| + PTx | 12 ± 8 | 32 ± 7* | |
| +PTx + U73122 | 100 ± 4 | 100 ± 1* | |
| + CTx | 9 ± 5 | 6 ± 3 | |
| – Ca2+ | 0 ± 5 | 31 ± 8* | |
| – Ca2+ + U73122 | 100 ± 1* | 100 ± 1* | |
| – Ca2+ + Thapsigargin | 100 ± 1* | 99 ± 4* | |
| – Ca2+ + PTx | 9 ± 2 | 41 ± 5* | |
Intracellular calcium was measured as described in Methods. Values are means ± SEM, n > 3.
Inhibition significantly different from 0%, P < 0.05, Mann–Whitney.
CRF function in Ramp2+/– mice
To further investigate whether there might be any physiological consequences of CRF1/RAMP2 interactions, the ability of CRF to increase plasma levels of ACTH was compared in normal and Ramp2+/– mice (Kadmiel et al., 2011). Two hours after CRF administration, the plasma ACTH concentration in the Ramp2+/– animals was significantly reduced to levels that were roughly 20% lower than similarly treated wild-type control animals (Figure 7).
Figure 7.
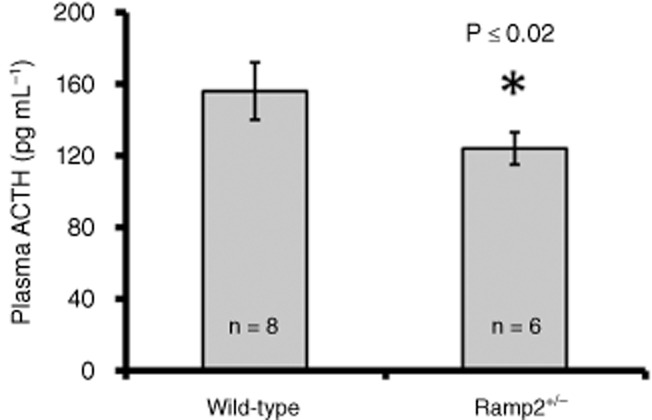
ACTH response to CRF in wild-type and Ramp2+/− mice. Male mice were injected with 40 mg kg−1 CRF and plasma was collected 2 h later. Plasma ACTH levels were measured by chemiluminescence elisa. Wild-type ACTH 156 ± 47 pg mL−1; Ramp2+/− ACTH 124 ± 22 pg mL−1. Error bars represent SEM, n = 6–8. Values were compared by Student's t-test.
Discussion and conclusions
This study has demonstrated the coupling of two family B GPCRs to RAMPs additional to those previously known. The VPAC2 can interact with all three RAMPs; for RAMPs 1 and 2, this leads to enhanced coupling to Gi/o/t/z. The CRF1 interacts with RAMP2 leading to enhanced CRF1 expression at the cell surface and increased coupling to Gi/o/t/z, Gq/11 and G12/13. The Gi/o/t/z coupling results in extracellular Ca2+ entry following challenge with CRF. RAMP2 association also reveals differences in the ability of CFR and urocortin on the one hand and sauvagine on the other to increase intracellular calcium.
This study provides further evidence of the importance of the cell line background in modulating the effects of RAMPs. In the current study, the responses resulting from RAMP interactions were consistently larger using HEK 293S compared with CHO-K1 cells. In addition, a previous study investigating RAMP interactions revealed no association with the VPAC2 (Christopoulos et al., 2003). Thus, care is needed in interpreting negative data when investigating RAMP effects. However, it is striking that the failure of the GLP1 receptor to associate with any RAMP has now been observed in three cell lines, consistently supporting the conclusion that it has very little, if any, ability to associate with these proteins.
The diversity of RAMP effects are clearly illustrated in this study. Only for CRF1 was there an effect on cell surface expression. A more general effect was to promote differential G-protein coupling. This has previously been observed, for the VPAC1 and the CTR (Christopoulos et al., 2003; Morfis et al., 2008). In the case of the CTR, RAMP2 enhanced Gs association whereas RAMP3 enhanced both Gs and Gq (Morfis et al., 2008). In the current study, it appears that the mechanism of enhancement depends on the individual RAMP and the G-protein. Enhanced coupling of Gi/o/t/z was observed to both the VPAC2 and the CRF1, and in all cases, the main effect was an increase in basal G-protein activation. This implies that the RAMP enhances baseline activity of both receptors. With RAMP2, for both receptors when expressed in HEK 293S cells, there was also an increase in the maximum response, implying a greater number of active receptor–Gi complexes; this mirrors the effect noted for the VPAC1 : RAMP2 complex on enhanced phosphoinositide breakdown (Christopoulos et al., 2003). The simplest interpretation is that by some mechanism, the RAMP increases the accessibility of the receptor to the G-protein (Morfis et al., 2003). This effect was cell line-dependent as it was not seen in the CHO-K1 cells. RAMP2 also enhanced coupling between the CRF1 and Gq/11 and G12/13, but the mechanisms appeared to be different. For Gq/11, the maximum response was increased, suggesting an increased number of receptor–Gq/11 complexes. For G12/13, the main effect was an increase in potency of both CRF and a second agonist, urocortin 1. This implies an increased affinity for the G-protein. A small increase in potency for VIP was noted with Gi/o/t/z in HEK 293S cells, although as there was also an increase in maximum response, this might in part at least be due to increased accessibility of the G-protein to the receptor. Regardless of this, it appears that there are multiple mechanisms by which RAMPs can modulate G-protein coupling.
The RAMP effects also depend on the nature of the agonist. With CRF1, the calcium response to CRF and urocortin 1 was enhanced whereas this was not observed with sauvagine. Differential enhancement of agonist potency has been observed at amylin receptors, where RAMP1 association selectively increases the potency of CGRP at stimulating AC (Udawela et al., 2006b). It has been established that distinct conformations of the CRF1 are involved in coupling to Gs and Gi (Berger et al., 2006) and bias in agonist-signalling has been observed at the CRF1 (Ruhmann et al., 1999; Grammatopoulos et al., 2000; Beyermann et al., 2007; Grammatopoulos, 2012). Thus, in HEK 293S cells, sauvagine and urocortin are equipotent at promoting GTPγS binding to Gs but urocortin is more potent on Gi/o/t/z. Furthermore, it is possible to discriminate between urocortin- and sauvagine-mediated increases in GTPγS binding using antagonists (Berger et al., 2006). Thus there is good evidence that the two agonists promote different conformations of the CRF1, and this could explain the different Ca2+ responses revealed after RAMP2 transfection.
The interactions between the VPAC2 and CRF1 and the different RAMPs are potentially of physiological importance. In the case of the CRF1 receptor, this study has shown that the alterations in G-protein coupling cause changes in the pattern of calcium mobilization in transfected cells and that in mice, a genetic reduction in RAMP2 reduces the ability of CRF to stimulate ACTH release. The ability of the CRF1 to increase intracellular calcium (and especially the role of extracellular calcium in that process) is known to be cell-type-dependent (Soares et al., 2005; Gutknecht et al., 2008); the presence of RAMP2 may be one factor behind this. CRF stimulation of ACTH release is of pivotal importance to the role of this hormone (Bale and Vale, 2004). The mechanism by which RAMP2 modulates this response remains to be established as this effect is normally considered to be mediated via cAMP (Reisine et al., 1985), and no effect was observed on this second messenger in the current study. As the effects of RAMPs are cell-line-dependent, this may be less of a paradox then first appears. Whatever the explanation, the observation of reduced CRF responsiveness is consistent with the RAMP2–CRF1 interaction being relevant in vivo. Given that the animals are only heterozygote for RAMP2, the reduction seen in ACTH levels may underestimate the real contribution of RAMP2 association to enhancing the response to CRF.
Comparison of the distribution of the VPAC2 and CRF1 receptors and the relevant RAMPs show there is potential for co-expression in vivo. For CRF1 mRNA, there is overlap with the reported distribution of RAMP2 mRNA in several rat brain structures including the dentate gyrus, the CA1 and 3 regions of the hippocampus, various regions of the amygdala, some cortical layers and the dorsomedial hypothalamus (Potter et al., 1994; Oliver et al., 2001). There is also reported to be co-expression of mRNA and/or protein in human adipocytes (Seres et al., 2004; Silaghi et al., 2007) and cerebral arteries, albeit based on a rat-human comparison (Oliver et al., 2002; Deussing et al., 2007). For the VPAC2, there are overlapping distributions of the receptor with RAMPs 1 and 2 in similar regions of rat brain to the CRF (Joo et al., 2004). In the periphery, there are common distribution patterns in many types of smooth muscle, especially vascular smooth muscle (Knutsson and Edvinsson, 2002). These studies are no more than suggestive; ultimately it will be necessary to show co-localization of the relevant components in the same cells. However, the current data suggest further work in this area would be useful.
In conclusion, this work demonstrates the interaction of RAMPs with two additional GPCRs. It suggests that RAMPs can enhance G-protein interactions. These interactions can have measurable consequences for cell signalling and for CRF in vivo responsiveness.
Acknowledgments
We would like to thank AZ for financial support, Britt-Marie Kihlberg for experimental assistance and Drs Dave Smith and John Simms for scientific discussions. Portions of this study were supported by US NIH grants HL091973 and HD060860 to KMC.
Glossary
- ACTH
adrenal corticotrophic hormone
- CGRP
calcitonin gene-related peptide
- CLR
calcitonin receptor-like receptor
- CTR
calcitonin receptor
- GLP-1
glucagon-like peptide 1
- HA
haemagglutinin
- RAMP
receptor activity modifying proteins
- PACAP
pituitary AC-activating peptide
- PHM-27
peptide histidine methionine-27
- VIP
vasoactive intestinal polypeptide
- WT
wild-type
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Failure of HEK 293 cells transfected with CLR alone to respond to CGRP or adrenomedullin (ADM). Values are means ± range of duplicate determinations from a single experiment.
Figure S2 Failure of CHO-K1 cells transfected with CLR alone to respond to CGRP or ADM. Values are means ± range of duplicate determinations from a single experiment.
Figure S3 Effects of epitope tags on the VPAC2, GLP-1, CRF1 and CT receptors transiently expressed in CHO-K1 cells. Values are means ± SD, n = 2.
Figure S4 Effects of epitope tags on the pharmacology of CGRP, AM1 or AM2 receptors expressed in HEK-293 cells. Number indicates the residue in the RAMP to which the FLAG tag was attached. Values are means ± SEM from three independent experiments experiment.
Figure S5 Pharmacology of VPAC2 receptor transfected with RAMPs in CHO-K1 cells. Values are means ± SEM from three independent experiments.
Figure S6 Pharmacology of CRF1 receptor transfected with RAMP2 in CHO-K1 cells. Values are means ± SEM determined from three independent experiments performed in duplicate.
Figure S7 GTPγS stimulation mediated by the VPAC2 in the presence or absence of RAMPs in HEK293 cells. Top; stimulation of binding to G12/13. Bottom, stimulation of binding to Gq/G11. A small signal was detected but this was also observed in a RAMP1 only transfection. Experiments are representative of three. Values are means ± SEM of three independent experiments performed in duplicate.
Figure S8 Effects of varying CRF1R cDNA concentration on CRF-stimulated GTPγS to Gi in HEK 293S cells. Values are normalized to the maximum response seen in cells transfected with CRF1R alone. Values are means ± SEM of three independent experiments performed in duplicate.
Table S1 Effects of RAMP2 on the CRF1-mediated elevation in intracellular calcium in HEK 293S cells.
References
- Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Berger H, Heinrich N, Wietfeld D, Bienert M, Beyermann M. Evidence that corticotropin-releasing factor receptor type 1 couples to Gs- and Gi-proteins through different conformations of its J-domain. Br J Pharmacol. 2006;149:942–947. doi: 10.1038/sj.bjp.0706926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyermann M, Heinrich N, Fechner K, Furkert J, Zhang W, Kraetke O, et al. Achieving signalling selectivity of ligands for the corticotropin-releasing factor type 1 receptor by modifying the agonist's signalling domain. Br J Pharmacol. 2007;151:851–859. doi: 10.1038/sj.bjp.0707293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomberger JM, Spielman WS, Hall CS, Weinman EJ, Parameswaran N. Receptor activity-modifying protein (RAMP) isoform-specific regulation of adrenomedullin receptor trafficking by NHERF-1. J Biol Chem. 2005;280:23926–23935. doi: 10.1074/jbc.M501751200. [DOI] [PubMed] [Google Scholar]
- Bouschet T, Martin S, Henley JM. Receptor-activity-modifying proteins are required for forward trafficking of the calcium-sensing receptor to the plasma membrane. J Cell Sci. 2005;118(Pt 20):4709–4720. doi: 10.1242/jcs.02598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulos A, Christopoulos G, Morfis M, Udawela M, Laburthe M, Couvineau A, et al. Novel receptor partners and function of receptor activity-modifying proteins. J Biol Chem. 2003;278:3293–3297. doi: 10.1074/jbc.C200629200. [DOI] [PubMed] [Google Scholar]
- Conner AC, Hay DL, Simms J, Howitt SG, Schindler M, Smith DM, et al. A key role for transmembrane prolines in calcitonin receptor-like receptor agonist binding and signalling: implications for family B G-protein-coupled receptors. Mol Pharmacol. 2005;67:20–31. [PubMed] [Google Scholar]
- Dackor R, Fritz-Six K, Smithies O, Caron K. Receptor activity-modifying proteins 2 and 3 have distinct physiological functions from embryogenesis to old age. J Biol Chem. 2007;282:18094–18099. doi: 10.1074/jbc.M703544200. [DOI] [PubMed] [Google Scholar]
- Dautzenberg FM, Py-Lang G, Higelin J, Fischer C, Wright MB, Huber G. Different binding modes of amphibian and human corticotropin-releasing factor type 1 and type 2 receptors: evidence for evolutionary differences. J Pharmacol Exp Ther. 2001;296:113–120. [PubMed] [Google Scholar]
- Deussing JM, Kuhne C, Putz B, Panhuysen M, Breu J, Stenzel-Poore MP, et al. Expression profiling identifies the CRH/CRH-R1 system as a modulator of neurovascular gene activity. J Cereb Blood Flow Metab. 2007;27:1476–1495. doi: 10.1038/sj.jcbfm.9600451. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Lagerstrom MC, Lundin LG, Schioth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- Grammatopoulos DK. Insights into mechanisms of corticotropin-releasing hormone receptor signal transduction. Br J Pharmacol. 2012;166:85–97. doi: 10.1111/j.1476-5381.2011.01631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grammatopoulos DK, Randeva HS, Levine MA, Katsanou ES, Hillhouse EW. Urocortin, but not corticotropin-releasing hormone (CRH), activates the mitogen-activated protein kinase signal transduction pathway in human pregnant myometrium: an effect mediated via R1alpha and R2beta CRH receptor subtypes and stimulation of Gq-proteins. Mol Endocrinol. 2000;14:2076–2091. doi: 10.1210/mend.14.12.0574. [DOI] [PubMed] [Google Scholar]
- Gutknecht E, Van der Linden I, Van Kolen K, Verhoeven KF, Vauquelin G, Dautzenberg FM. Molecular mechanisms of corticotropin releasing factor receptor-induced calcium signaling. Mol Pharmacol. 2008;75:648–657. doi: 10.1124/mol.108.050427. [DOI] [PubMed] [Google Scholar]
- Hay DL, Poyner DR, Sexton PM. GPCR modulation by RAMPs. Pharmacol Ther. 2006;109:173–197. doi: 10.1016/j.pharmthera.2005.06.015. [DOI] [PubMed] [Google Scholar]
- Hong Y, Hay DL, Quirion R, Poyner DR. The pharmacology of Adrenomedullin 2/Intermedin. Br J Pharmacol. 2011;166:110–120. doi: 10.1111/j.1476-5381.2011.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo KM, Chung YH, Kim MK, Nam RH, Lee BL, Lee KH, et al. Distribution of vasoactive intestinal peptide and pituitary adenylate cyclase-activating polypeptide receptors (VPAC1, VPAC2, and PAC1 receptor) in the rat brain. J Comp Neurol. 2004;476:388–413. doi: 10.1002/cne.20231. [DOI] [PubMed] [Google Scholar]
- Kadmiel M, Fritz-Six K, Pacharne S, Richards GO, Li M, Skerry TM, et al. Research resource: haploinsufficiency of receptor activity-modifying protein-2 (RAMP2) causes reduced fertility, hyperprolactinemia, skeletal abnormalities, and endocrine dysfunction in mice. Mol Endocrinol. 2011;25:1244–1253. doi: 10.1210/me.2010-0400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. NC3Rs Reporting Guidelines Working Group. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutsson M, Edvinsson L. Distribution of mRNA for VIP and PACAP receptors in human cerebral arteries and cranial ganglia. Neuroreport. 2002;13:507–509. doi: 10.1097/00001756-200203250-00030. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, et al. RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Morfis M, Christopoulos A, Sexton PM. RAMPs: 5 years on, where to now? Trends Pharmacol Sci. 2003;24:596–601. doi: 10.1016/j.tips.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Morfis M, Tilakaratne N, Furness SG, Christopoulos G, Werry TD, Christopoulos A, et al. Receptor activity-modifying proteins differentially modulate the G protein-coupling efficiency of amylin receptors. Endocrinology. 2008;149:5423–5431. doi: 10.1210/en.2007-1735. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Kane SA, Salvatore CA, Mallee JJ, Kinsey AM, Koblan KS, et al. Cloning, characterization and central nervous system distribution of receptor activity modifying proteins in the rat. Eur J Neurosci. 2001;14:618–628. doi: 10.1046/j.0953-816x.2001.01688.x. [DOI] [PubMed] [Google Scholar]
- Oliver KR, Wainwright A, Edvinsson L, Pickard JD, Hill RG. Immunohistochemical localization of calcitonin receptor-like receptor and receptor activity-modifying proteins in the human cerebral vasculature. J Cereb Blood Flow Metab. 2002;22:620–629. doi: 10.1097/00004647-200205000-00014. [DOI] [PubMed] [Google Scholar]
- Potter E, Sutton S, Donaldson C, Chen R, Perrin M, Lewis K, et al. Distribution of corticotropin-releasing factor receptor mRNA expression in the rat brain and pituitary. Proc Natl Acad Sci USA. 1994;91:8777–8781. doi: 10.1073/pnas.91.19.8777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Andrew DP, Brown D, Bose C, Hanley MR. Pharmacological characterization of a receptor for calcitonin gene-related peptide on rat, L6 myocytes. Br J Pharmacol. 1992;105:441–447. doi: 10.1111/j.1476-5381.1992.tb14272.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyner DR, Sexton PM, Marshall I, Smith DM, Quirion R, Born W, et al. International Union of Pharmacology. XXXII. The mammalian calcitonin gene-related peptides, adrenomedullin, amylin, and calcitonin receptors. Pharmacol Rev. 2002;54:233–246. doi: 10.1124/pr.54.2.233. [DOI] [PubMed] [Google Scholar]
- Reisine T, Rougon G, Barbet J, Affolter HU. Corticotropin-releasing factor-induced adrenocorticotropin hormone release and synthesis is blocked by incorporation of the inhibitor of cyclic AMP-dependent protein kinase into anterior pituitary tumor cells by liposomes. Proc Natl Acad Sci USA. 1985;82:8261–8265. doi: 10.1073/pnas.82.23.8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhmann A, Bonk I, Kopke AK. High-affinity binding of urocortin and astressin but not CRF to G protein-uncoupled CRFR1. Peptides. 1999;20:1311–1319. doi: 10.1016/s0196-9781(99)00136-9. [DOI] [PubMed] [Google Scholar]
- Seres J, Bornstein SR, Seres P, Willenberg HS, Schulte KM, Scherbaum WA, et al. Corticotropin-releasing hormone system in human adipose tissue. J Clin Endocrinol Metab. 2004;89:965–970. doi: 10.1210/jc.2003-031299. [DOI] [PubMed] [Google Scholar]
- Silaghi A, Achard V, Paulmyer-Lacroix O, Scridon T, Tassistro V, Duncea I, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am J Physiol Endocrinol Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- Soares SM, Thompson M, Chini EN. Role of the second-messenger cyclic-adenosine 5'-diphosphate-ribose on adrenocorticotropin secretion from pituitary cells. Endocrinology. 2005;146:2186–2192. doi: 10.1210/en.2004-1298. [DOI] [PubMed] [Google Scholar]
- Tilakaratne N, Christopoulos G, Zumpe ET, Foord SM, Sexton PM. Amylin receptor phenotypes derived from human calcitonin receptor/RAMP coexpression exhibit pharmacological differences dependent on receptor isoform and host cell environment. J Pharmacol Exp Ther. 2000;294:61–72. [PubMed] [Google Scholar]
- Tsutsumi M, Claus TH, Liang Y, Li Y, Yang L, Zhu J, et al. A potent and highly selective VPAC2 agonist enhances glucose-induced insulin release and glucose disposal: a potential therapy for type 2 diabetes. Diabetes. 2002;51:1453–1460. doi: 10.2337/diabetes.51.5.1453. [DOI] [PubMed] [Google Scholar]
- Udawela M, Christopoulos G, Morfis M, Christopoulos A, Ye S, Tilakaratne N, et al. A critical role for the short intracellular C terminus in receptor activity-modifying protein function. Mol Pharmacol. 2006a;70:1750–1760. doi: 10.1124/mol.106.024257. [DOI] [PubMed] [Google Scholar]
- Udawela M, Christopoulos G, Tilakaratne N, Christopoulos A, Albiston A, Sexton PM. Distinct receptor activity-modifying protein domains differentially modulate interaction with calcitonin receptors. Mol Pharmacol. 2006b;69:1984–1989. doi: 10.1124/mol.105.021915. [DOI] [PubMed] [Google Scholar]
- Wootten DL, Simms J, Hay DL, Christopoulos A, Sexton PM. Receptor activity modifying proteins and their potential as drug targets. Prog Mol Biol Transl Sci. 2010;91:53–79. doi: 10.1016/S1877-1173(10)91003-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


