Abstract
Background and Purpose
Monoamine releasers constitute a class of drugs that promote the release of dopamine (DA), serotonin (5-HT) and/or norepinephrine. Although some drugs in this class are well-known drugs of abuse (amphetamine, methamphetamine), others are thought to have reduced (3,4-methylenedioxy-N-methylamphetamine [MDMA]) or no (fenfluramine) abuse potential. The purpose of this study was to further elucidate the role of dopamine versus serotonin selectivity on expression of abuse-related effects produced by monoamine releasers in an assay of intracranial self-stimulation (ICSS) in rats.
Experimental Approach
This study evaluated effects produced in a frequency–rate ICSS procedure by 11 monoamine releasers that vary in selectivity to release DA versus 5-HT.
Key Results
Efficacy of monoamine releasers to facilitate ICSS correlated with DA-selectivity, such that DA-selective releasers exclusively facilitated ICSS, a 5-HT-selective releaser exclusively depressed ICSS, and mixed-action releasers both facilitated low ICSS rates and depressed high ICSS rates. Fixed-proportion mixtures of a DA-selective releaser and a 5-HT-selective releaser recapitulated effects of mixed-action releasers. Efficacy of monoamine releasers to facilitate ICSS also correlated with previously published data on efficacy to maintain self-administration in rhesus monkeys responding under a progressive-ratio schedule of reinforcement.
Conclusions and Implications
These data support the importance of selectivity for DA versus 5-HT in determining abuse potential of monoamine releasers and demonstrate a novel correlation between rat ICSS and nonhuman primate self-administration measures of abuse-related effects. Taken together, these results support the use of ICSS in rats as an experimental tool to study the expression and pharmacological determinants of abuse-related effects of monoamine releasers.
Keywords: dopamine, serotonin, ICSS, amphetamine, fenfluramine, methylenedioxymethylamphetamine
Introduction
Monoamine releasers constitute a class of drugs that function as substrates for monoamine transporters and promote the release of dopamine, serotonin and/or norepinephrine from presynaptic terminals (Parada et al., 1988; Rothman et al., 2007). This pharmacological class includes medications such as amphetamine that are used clinically for attention deficit hyperactivity disorder, obesity and narcolepsy (Bray, 1993; Nishino, 2007; Setlik et al., 2009), and monoamine releasers are also under consideration as candidate maintenance medications for the treatment of stimulant abuse (Grabowski et al., 2004). However, the therapeutic use of amphetamine and several other monoamine releaser medications is limited in part by high abuse liability (Jasinski and Kovacević-Ristanović, 2000). Moreover, many illicit monoamine releasers function as established or emerging drugs of abuse [e.g. 3,4-methylenedioxy-N-methylamphetamine (MDMA); cathinone derivatives] (Leung and Cottler, 2008; Winder et al., 2012). Preclinical assays of drug self-administration have played a key role in research on the expression and determinants of the abuse-related effects of monoamine releasers (Balster and Schuster, 1973; Griffiths et al., 1976; Ator and Griffiths, 2003; O'Connor et al., 2011). For example, monoamine releasers vary in their pharmacological selectivity to release dopamine, serotonin and norepinephrine, and one series of studies found that efficacy to maintain drug self-administration under a progressive-ratio schedule in rhesus monkeys varied as function of pharmacological selectivity to release dopamine (DA) versus serotonin (5-HT) (Wee et al., 2005). Thus, releasers with high selectivity to release DA versus 5-HT (e.g. amphetamine) functioned as more efficacious reinforcers than releasers with lower selectivity for DA release (e.g. the experimental compound PAL-313, para-methylamphetamine) or than 5-HT-selective releasers (e.g. fenfluramine). These results agree with other data to suggest that dopamine-selective monoamine releasers are readily self-administered (Götestam and Andersson, 1975; McKenna and Ho, 1980), serotonin-selective releasers are not (Woods and Tessel, 1974; Dahl and Götestam, 1989) and serotonergic effects of monoamine releasers can oppose and limit dopamine-mediated abuse-related effects (Rothman and Baumann, 2006; Wee and Woolverton, 2006; Howell and Kimmel, 2008; Baumann et al., 2011).
Intracranial self-stimulation (ICSS) offers an alternative approach to abuse liability assessment (Kornetsky and Esposito, 1979; Wise, 1996; Vlachou and Markou, 2011). In ICSS, subjects are implanted with chronic indwelling intracranial electrodes that target brain areas such as the medial forebrain bundle. Pulses of brain stimulation delivered via the electrode are made contingent on a behavioural response such as pressing a lever. Rates of ICSS can be controlled by manipulating the frequency or amplitude of electrical pulses, and many drugs of abuse increase (‘facilitate’) low rates of ICSS maintained by low frequencies or amplitudes of electrical stimulation. Consequently, drug-induced facilitation of ICSS is often interpreted as an effect that may be related to reinforcing effects in assays of drug self-administration and abuse potential in human cultural contexts (Carlezon and Chartoff, 2007). Previous studies with monoamine releasers generally support this proposition. For example, the DA-selective and abused monoamine releasers amphetamine and methamphetamine facilitated ICSS in rats (Elder et al., 1965; Esposito et al., 1980), whereas fenfluramine, a 5-HT-selective releaser with low abuse liability, only decreased ICSS (Olds and Yuwiler, 1992). (+/–)MDMA, which is relatively non-selective for DA versus 5-HT, has been shown to produce a mix of abuse-limiting effects (decreases in maximum rate) along with abuse-related effects (decreases in threshold) (Hubner et al., 1988; Lin et al., 1997). Taken together, these studies support the general proposition that ICSS can stratify abuse-related drug effects, and that selectivity to promote DA versus 5-HT release influences expression of abuse-related effects of monoamine releasers. However, previous studies used a variety of different ICSS procedures, and the pharmacological determinants of monoamine releaser effects on ICSS have not been systematically examined or directly compared to effects in more widely used assays of drug self-administration.
The purpose of the present study was to evaluate sensitivity of ICSS to differences in abuse-related effects produced by a series of 11 monoamine releasers that varied across a >8000-fold range in their pharmacological selectivity to promote release of DA versus 5-HT and that have been tested previously in nonhuman primate assays of drug self-administration (Table 1). The work was designed to test two related hypotheses. First, we hypothesized that efficacy to facilitate ICSS would correlate with pharmacological selectivity to promote release of DA versus 5-HT. This hypothesis was also examined by evaluating effects of drug mixtures that contained various proportions of a DA-selective and a 5-HT-selective releaser. Second, we hypothesized that efficacy to facilitate ICSS in rats would correlate with previously published efficacy to maintain drug self-administration in nonhuman primates responding under a progressive-ratio schedule (Wee et al., 2005; Wang and Woolverton, 2007). Confirmation of these hypotheses would clarify pharmacological determinants of the abuse-related effects of monoamine releasers and support the use of ICSS in both basic research on the neuropharmacology of drug abuse and in abuse liability assessment.
Table 1.
Previously published data on pharmacological selectivity and reinforcing efficacy of monoamine releasers
| Drug | DA | 5-HT | Selectivity (5-HT/DA) | Max. injection per session in NHP SA |
|---|---|---|---|---|
| m-fluroamphetamine (PAL-353) | 24.2 ± 1.1 | 1,937 ± 202 | 803 | 11.9 ± 1.14 |
| (+)-amphetamine (Amphetamine) | 24.8 ± 3.5 | 1,765 ± 94 | 711 | 13.8 ± 0.64 |
| (+)-phenmetrazine (Phenmetrazine) | 87.4 ± 7.8 | 3,246 ± 263 | 372 | Not Tested |
| S-(+)-methamphetamine (Methamphetamine) | 24.5 ± 2.1 | 736 ± 45 | 301 | 13 ± 15 |
| m-methylamphetamine (PAL-314) | 33.3 ± 1.3 | 218 ± 22 | 6.54 | 11.6 ± 1.24 |
| p-methylamphetamine (PAL-313) | 44.1 ± 2.6 | 53.4 ± 4.1 | 1.24 | 8.9 ± 1.74 |
| (+)-3,4-methylenedioxymethamphetamine ((+)MDMA) | 142 ± 4.0 | 74 ± 3.0 | 0.525 | 10 ± 15 |
| naphthylisopropylamine (PAL-287) | 12.6 ± 0.4 | 3.4 ± 0.2 | 0.273 | Not Tested |
| (+/–)-3,4-methylenedioxymethamphetamine ((+/–)MDMA) | 376 ± 16 | 56.6 ± 2.1 | 0.155 | 9 ± 15 |
| (–)-3,4-methylenedioxymethamphetamine ((–)MDMA) | 3,700 ± 100 | 340 ± 20 | 0.095 | 4.7 ± 0.85 * |
| (+/–)-fenfluramine (Fenfluramine) | >10,000 | 79.3 ± 11.5 | <0.011 | ** |
These data were correlated with ICSS data generated in the present study (Figure 6).
Maintained responding in fewer than half of the monkeys tested.
Does not maintain self-administration16.
Citations: 1) Rothman et al., 2001; 2) Rothman et al., 2002; 3) Rothman et al., 2005; 4) Wee et al., 2005; 5) Wang and Woolverton, 2007.
Each row shows (from left to right) drug name, in vitro IC50 values (nM ± SD) to promote release of dopamine (DA) or serotonin (5-HT), pharmacological selectivity expressed as the ratio of IC50 for 5-HT release ÷ IC50 for DA release, and maximum number of injections per session maintained in nonhuman primates responding under a progressive-ratio schedule of drug self-administration (Max injection per session in NHP SA).
Methods
Subjects
Thirty-eight adult male Sprague–Dawley rats (Harlan, Frederick, MD) were used. All rats had free access to water and were housed individually on a 12 h light–dark cycle (lights on from 6:00 a.m. to 6:00 p.m.) in a facility accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All rats also had free access to food and weighed between 314 and 375 g at the time of surgery. Animal maintenance accorded with The National Institutes of Health guidelines on care and use of animal subjects in research (National Academy of Science, 1996). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 2010). Experimental protocols were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Assay of ICSS
Surgery
Subjects were anesthetized with 2.5% isoflurane gas until unresponsive to toe-pinch. A sterotaxic device (Kopf, Tujunga, CA) was used to insert the cathode (0.25 mm diameter) of a bipolar electrode into the left medial forebrain bundle at the level of the lateral hypothalamus (2.8 mm posterior to Bregma, 1.7 mm lateral to the midsaggital line and 8.8 mm ventral to the skull). Three screws were placed in the skull, and the anode (0.125 mm diameter, un-insulated) was wrapped around one of the screws to act as a ground. Dental acrylic was used to secure the electrode to the screws (and thus to the skull). Ketoprofen (5 mg kg−1) was used as a post-operative analgesic immediately and 24 h after surgery. Animals were allowed to recover for at least 5 days before beginning ICSS training.
Apparatus
Operant chambers consisted of sound-attenuating boxes containing modular acrylic and metal test chambers (29.2 × 30.5 × 24.1 cm). Each chamber had a response lever (4.5 cm wide, 2.0 cm deep, 3.0 cm off the floor), three stimulus lights (red, yellow and green) centered 7.6 cm above the response lever, a 2 W house light and an ICSS stimulator (Med Associates, St. Albans, VT). Bipolar cables routed through a swivel-commutator connected the stimulator to the electrode (Model SL2C, Plastics One, Roanoke, VA). Med-PC IV computer software controlled all programming parameters and data collection (Med Associates).
Training
The behavioural procedure was similar to that described previously (Negus et al., 2010; Altarifi and Negus, 2011). In brief, the house light was illuminated during behavioural sessions, and lever press responding under a fixed-ratio 1 (FR 1) schedule produced delivery of a 0.5 s train of square-wave cathodal pulses (0.1 ms per pulse). Stimulus lights over the lever were illuminated, and responding had no scheduled consequences, during delivery of the intracranial stimulus. During initial 60 min training sessions, stimulation intensity was set at 150 μA, and stimulation frequency was set at 126 Hz. Stimulation intensity was then individually manipulated in each rat to identify an intensity that maintained a high rate of reinforcement (>30 stimulations min−1). Once an appropriate intensity was identified, changes in frequency were introduced during sessions consisting of three consecutive 10 min components, each of which contained ten 60 s trials. The stimulation frequency was 158 Hz for the first trial of each component, and frequency decreased in 0.05 log unit steps during the subsequent nine trials to a final frequency of 56 Hz. Each trial began with a 10 s time-out period, during which responding had no scheduled consequences, and five non-contingent stimulations at the designated frequency were delivered at 1 s intervals during the last 5 s of the time-out. During the remaining 50 s of each trial, responding produced both intracranial stimulation at the designated frequency and illumination of the lever lights under a FR 1 schedule as described above. Training continued until frequency-rate curves were not statistically different over three days of training as indicated by lack of a significant effect of ‘day’ in a two-way anova with frequency and day as the two variables (see data analysis). All training was completed within 5 weeks of surgery.
Testing
For dose–effect studies, test sessions consisted of three consecutive ‘baseline’ components followed first by a 20 min time-out period and then by three consecutive ‘test’ components. A single dose of a single drug was administered at the beginning of the time-out, immediately after the baseline components and before the test components. All test drugs are listed in Table 1. Also tested were mixtures of PAL-353 and fenfluramine in proportions of 1:1, 1:3 and 1:10 (PAL-353 : fenfluramine). These proportions were based on the relative potencies of PAL-353 and fenfluramine to alter ICSS and were intended to permit assessment of PAL-353 in combination with relatively low, intermediate and high proportions of fenfluramine. Time course studies were also conducted with the highest dose of each individual compound (with the exception that the penultimate dose was tested with PAL-287 because the highest dose produced lethality in some rats). Time course test sessions consisted of three consecutive baseline components followed immediately by drug injection and then by pairs of consecutive test components initiated 10, 30 and 100 min after drug injection. In some cases, additional pairs of test components were initiated 300 min or 24 h after drug injection. Due to the different durations of action of PAL-353 and fenfluramine, time courses were not determined for the fixed-proportion mixtures. Test sessions were usually conducted on Tuesdays and Fridays, and three-component training sessions were conducted all other weekdays. The order of drug dose was varied across subjects using a Latin-Square design. Experiments with any one compound were completed before progressing to another experimental manipulation, and order of drug testing was irregular across rats. Tests with different compounds with a given rat were separated by at least 1 week, and during this inter-drug interval, a saline/vehicle test session was conducted to assure that injections did not alter ICSS measures.
Data analysis
The primary dependent measure was the reinforcement rate in stimulations/trial. Raw reinforcement rates were normalized to the maximum control rate (MCR) for each subject on each day, where MCR was defined as the mean of the maximal rates observed during the second and third ‘baseline’ components for that day. Therefore, %MCR was equal to (response rate during a frequency trial) / (maximum control rate) × 100. Data for each frequency were averaged across test components for each rat and then across rats to yield a ‘frequency-rate’ curve for each experimental manipulation. Two-way anova was used to compare frequency–rate curves, with ICSS frequency as one variable and dose or time as the second factor. A Holm–Sidak post hoc test followed all significant anovas, and P-values less than 0.05 were considered significant.
Two additional dependent measures were calculated to summarize data from frequency–rate curves. First, ICSS thresholds were calculated where possible using linear regression through data on the linear portion of the frequency-rate curve as described previously (Elmer et al., 2010; Do Carmo et al., 2009), with the exception that ‘threshold’ was defined as the x-intercept rather than as frequency maintaining 50% of control rates, because the x-intercept is less vulnerable to perturbation by changes in maximal response rates (Miliaressis et al., 1986; Carlezon and Chartoff, 2007). For most ICSS curves, the linear portion of the curve was operationally defined as all data between 20% and 80% MCR, as well as the first point below 20% and the first point above 80% MCR. If peak asymptotic ICSS rates fell between 50% and 80% MCR, then the regression included only data up to the first point of the asymptote, defined operationally as the point at which further increases in stimulation frequency produced ≤10% increase in ICSS rate. To further protect against perturbations in threshold associated with global changes in response rates, regressions were not calculated, and thresholds were not determined if ICSS rates failed to go below 20% MCR or above 50% MCR across the entire frequency range (e.g. see Carlezon and Chartoff, 2007). Thresholds were determined before and after drug administration on each day, and drug effects were expressed as % reduction in threshold relative to baseline thresholds for that day. As a second summary measure of ICSS, the total number of stimulations delivered across all frequencies within a component was summed. The mean number of total stimulations per component was determined before and after drug administration on each day, and drug effects were expressed as the % baseline number of stimulations per component for that day. Because this second summary measure did not rely on fitting data to an equation, it did not require data selection or modification, and it could be applied to results from all treatment conditions.
Finally, correlations were evaluated between maximum facilitation of ICSS produced by each drug in this study and (a) in vitro selectivity to promote release of dopamine versus serotonin (Rothman et al., 2001; 2002; 2005; Wee et al., 2005; Wang and Woolverton, 2007), and (b) maximal break point maintained under a progressive-ratio schedule of reinforcement in rhesus monkeys (Wee et al., 2005; Wang and Woolverton, 2007) (Table 1). Maximal facilitation of ICSS was defined using each of the two summary measures as either (a) the maximal decrease in threshold or (b) the maximal increase in total stimulations produced by any dose of each drug. Maximal drug effects on threshold and total stimulations were also correlated with each other. Data were analysed by linear regression and a Pearson correlation test. A P-value <0.05 was determined to be significant for both the slope of the regression line and for the Pearson correlation test.
Drugs
(+)-Amphetamine sulfate, S-(+)-methamphetamine HCl and (–)-cocaine HCl were provided by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). (±)-Fenfluramine HCl was purchased from Sigma Chemical Co. (St. Louis, MO). All other compounds were synthesized as the fumarate salts by Dr Bruce Blough (Research Triangle Park, NC). All compounds were prepared in sterile saline and administered i.p. Doses are expressed in terms of the salt forms above.
Results
Under baseline conditions, electrical brain stimulation maintained a frequency-dependent increase in ICSS rates (e.g. Figure 1, open circles). The average ± SEM MCR for these studies was 58.6 ± 1.34 stimulations per trial. In general, low rates of ICSS were maintained by low stimulation frequencies (1.75–1.90 log Hz). ICSS rates increased at intermediate stimulation frequencies (1.90–2.05 log Hz), and high, asymptotic ICSS rates were maintained by high stimulation frequencies (2.05–2.20 log Hz). The mean ± SEM baseline threshold was 74.0 ± 0.8 Hz (1.87 log Hz). The mean ± SEM number of total stimulations earned during baseline components was 276.09 ± 9.4 stimulations per component.
Figure 1.
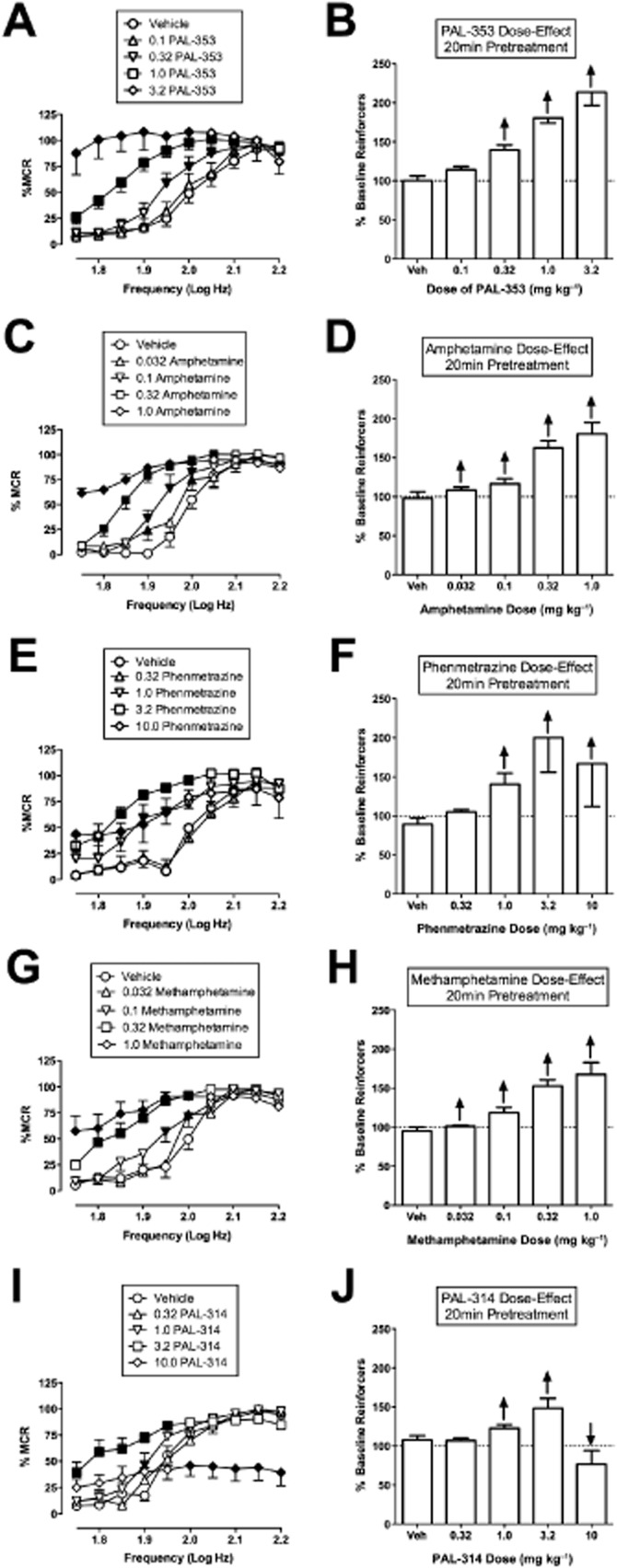
Effect of PAL-353, amphetamine, phenmetrazine, methamphetamine and PAL-314 on ICSS. Left panels (A, C, E, G, I) show drug effects on full ICSS frequency–rate curves. Abscissae: Frequency of electrical brain stimulation in Log Hz. Ordinates: Percent maximum control reinforcement rate (%MCR). Drug name and doses are indicated in legends. Filled points represent frequencies at which reinforcement rates were statistically different from vehicle rates as determined by a two-way anova followed by a Holm–Sidak post hoc test, P < 0.05. Right panels (B, D, F, H, J) show summary ICSS data expressed as percent pre-drug baseline number of reinforcers delivered across all frequencies of brain stimulation. Abscissae: drug dose in mg kg−1. Ordinates: Percent pre-drug baseline number of reinforcers. The drug and pretreatment time are shown for each panel. Upward/downward arrows indicate significant drug-induced increase/decrease in ICSS relative to vehicle for at least one brain stimulation frequency as determined by analysis of full frequency–rate curves. All data show mean ± SEM for five to seven rats (except for 3.2 mg kg−1 PAL-353, n = 4). Statistical results for data in left panels are as follows: (A) PAL-353 0.1–1.0 mg kg−1 (n = 7): significant main effect of frequency [F(9,54) = 88.6, P < 0.001], dose [F(3,18) = 38.8, P < 0.001] and significant interaction [F(27,162) = 7.9, P = < 0.001]. PAL-353 3.2 mg kg−1 (n = 4): significant main effect of frequency [F(9,27) = 17.1, P < 0.001], dose [F(1,3) = 12.0, P = 0.04] and significant interaction [F(9,27) = 18.8, P < 0.001]. (C) Amphetamine (n = 6): significant main effect of frequency [F(9,45) = 144.2, P < 0.001], dose [F(4,20) = 55.4, P < 0.001] and significant interaction [F(36,180) = 10.5, P = < 0.001]. (E) Phenmetrazine (n = 5): significant main effect of frequency [F(9,36) = 22.8, P < 0.001], dose [F(4,16) = 4.9, P < 0.009] and significant interaction [F(36,144) = 3.8, P = < 0.001]. (G) Effect of methamphetamine (n = 5): significant main effect of frequency [F(9,36) = 56.2, P < 0.001], dose [F(4,16) = 40.4, P < 0.001] and significant interaction [F(36,144) = 6.6, P = < 0.001]. (I) PAL-314 (n = 6): significant main effect of frequency [F(9,45) = 67.9, P < 0.001], dose [F(4,20) = 6.0, P = 0.003] and significant interaction [F(36,180) = 11.8, P = < 0.001].
Figure 1A–H shows that the DA-selective monoamine releasers robustly facilitated ICSS across a broad range of doses. In this and all subsequent figures, drugs are ordered from top to bottom in order of decreasing selectivity for DA versus 5-HT. Left panels show drug effects on full frequency-rate curves, right panels show summary data expressed as drug effects on the percent baseline number of total stimulations per component. As shown in Table 1, PAL-353, amphetamine, phenmetrazine and methamphetamine each have ≥30-fold selectivity for promoting release of DA versus 5-HT, and all four of these drugs produced exclusively rate-increasing effects across a 10- to 30-fold range of doses. Amphetamine and methamphetamine were the highest potency compounds, (significant facilitation of ICSS at doses ≥0.032 mg kg−1), followed by PAL-353 (≥0.32 mg kg−1) and phenmetrazine (≥1.0 mg kg−1). All drugs produced maximal increases in ICSS to least 160% of the baseline number of reinforcers. Only phenmetrazine showed evidence of asymptotic stimulation across the dose range tested, with 10 mg kg−1 producing a lower mean increase in ICSS than the lower dose of 3.2 mg kg−1. PAL-314 (Figure 1I, J), which has 6.5-fold selectivity to release DA versus 5-HT (Table 1), produced only rate-increasing effects at lower doses (1.0 and 3.2 mg kg−1); however, a higher dose of 10 mg kg−1 PAL-314 decreased higher rates of ICSS maintained by higher frequencies of brain stimulation (significant at 2.0–2.2 log Hz). Table 2 shows that these drugs all decreased ICSS threshold values. However, thresholds could not be calculated at higher drug doses, because at these doses, ICSS rates exceeded 20% MCR at all brain stimulation frequencies.
Table 2.
Percent reduction in threshold produced by each dose of each drug relative to baseline
| Drug | Veh | 0.032 | 0.1 | 0.32 | 1.0 | 3.2 | 10.0 | Δmax Θ0 |
|---|---|---|---|---|---|---|---|---|
| PAL-353 | 4.2 | 1.5 | 7.8 | 24.5 | NC* | NC* | – | 24.5 |
| Amphetamine | 4.3 | 8.3 | 7.4 | 32.1 | NC* | – | – | 32.1 |
| Phenmetrazine | −3.4 | – | – | −7.2 | 29.4 | NC* | NC* | 29.4 |
| Methamphetamine | 5.5 | 1.0 | 15.6 | NC | NC | – | – | 15.6 |
| PAL-314 | 3.7 | – | −0.1 | 11.2 | 20.6 | NC | NC | 20.6 |
| PAL-313 | −10.3 | – | – | 4.9 | 22.1 | 31.8 | – | 31.8 |
| (+)MDMA | −10.6 | – | 0.3 | 7.7 | NC* | NC* | – | 7.7 |
| PAL-287 | 2.5 | – | – | 11.6 | 0.4 | 17.5 | NC** | 17.5 |
| (+/–)MDMA | 0.1 | – | 4.4 | 17.5 | 14.9 | NC* | – | 17.5 |
| (−)MDMA | −0.1 | – | – | 12.2 | 16.6 | 28.3 | 27.4 | 28.3 |
| Fenfluramine | 2.9 | – | – | −2.6 | −13.7 | NC** | – | – |
No points fell below 20% of baseline.
Maximum responding did not reach 50% of baseline.
The last column shows the maximum decrease in threshold produced by each drug.
NC, not calculated.
Figure 2A–H shows the time courses of selected doses of each compound. PAL-353 (1.0 mg kg−1), amphetamine (1.0 mg kg−1) and phenmetrazine (3.2 mg kg−1) produced peak facilitation at the earliest time point (10 min), and facilitation of ICSS was no longer apparent after 100 min (Figure 2A–F). Methamphetamine (1.0 mg kg−1) facilitated ICSS with a similarly rapid rate of onset but a longer duration of action, and methamphetamine effects were no longer apparent after 300 min (Figure 2G, H). PAL-314 (10 mg kg−1) produced only rate-decreasing effects after 10 min, but this transitioned to mixed rate-increasing and rate-decreasing effects after 30 min and exclusive rate-increasing effects after 100 min before effects fully dissipated by 300 min (Figure 2I, J).
Figure 2.
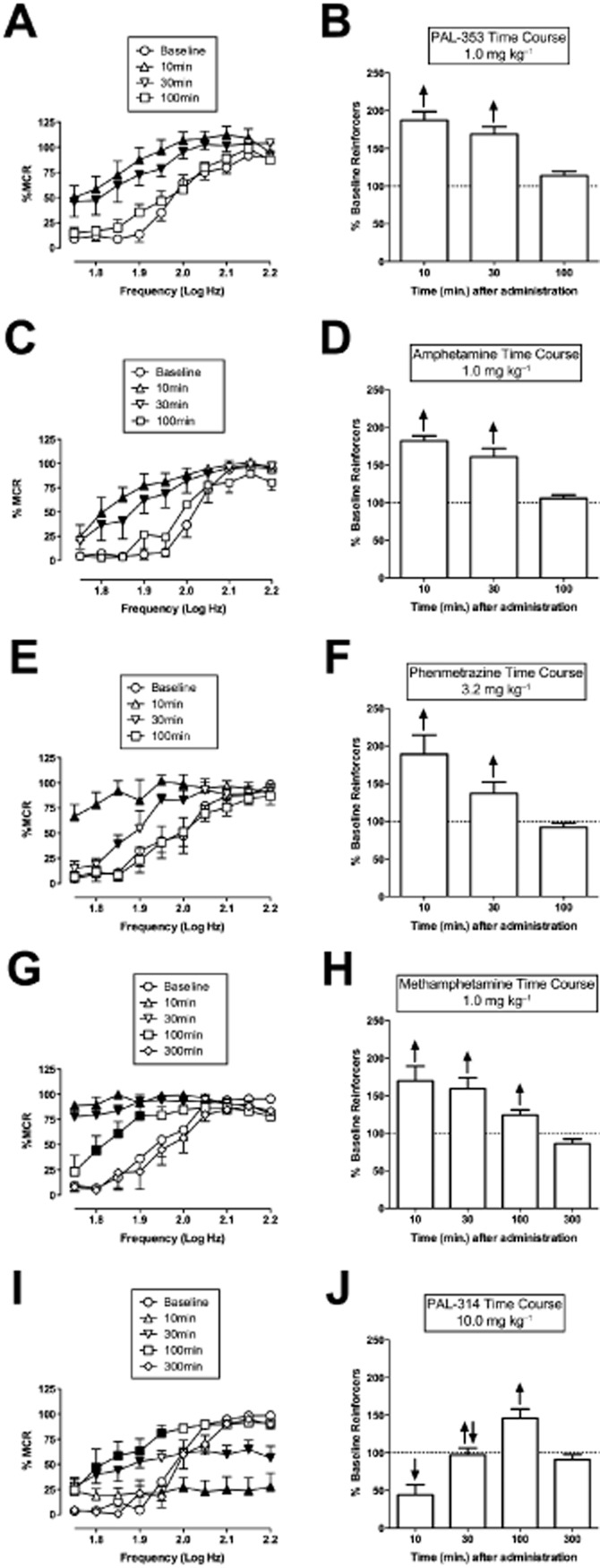
Time courses of PAL-353, amphetamine, phenmetrazine, methamphetamine and PAL-314. Left panels (A, C, E, G, I) show drug effects on full ICSS frequency-rate curves. Right panels (B, D, F, H, J) show summary ICSS data expressed as percent pre-drug baseline number of reinforcers delivered across all frequencies. Other details as in Figure 1. All data show mean ± SEM for five to seven rats. Statistical results for data in left panels are as follows: (A) PAL-353 (n = 7): significant main effect of frequency [F(9,54) = 40.6, P < 0.001], time [F(5,30) = 29.5, P < 0.001] and significant interaction [F(45,270) = 3.3, P < 0.001]. (C) Amphetamine (n = 5): significant main effect of frequency [F(9,36) = 63.9, P < 0.001], time [F(4,16) = 48.2, P < 0.001] and significant interaction [F(36,144) = 3.5, P < 0.001]. (E) Phenmetrazine (n = 5): significant main effect of frequency [F(9,36) = 23.4, P < 0.001], time [F(3,12) = 18.6, P < 0.001] and significant interaction [F(27,108) = 4.5, P < 0.001]. (G) Methamphetamine (n = 5): significant main effect of frequency [F(9,36) = 36.4, P < 0.001], time [F(4,16) = 14.9, P < 0.001] and significant interaction [F(36,144) = 10.0, P < 0.001]. I. PAL-314 (n = 5): significant main effect of frequency [F(9,36) = 47.8, P < 0.001], time [F(4,16) = 14.9, P < 0.001] and significant interaction [F(36,144) = 9.5, P < 0.001].
Figure 3A–H shows that releasers with lower selectivity to release DA versus 5-HT produced weaker facilitation of ICSS across a narrower range of doses than did more DA-selective releasers. Specifically, PAL-313, (+)MDMA, PAL-287 and (–)MDMA produced maximal stimulation of ICSS to levels less than 130% of baseline, and none of these releasers produced exclusive facilitation of ICSS across more than a 3.2-fold range of doses before rate-decreasing effects emerged. The 5-HT-selective releaser, fenfluramine, produced exclusively rate-decreasing effects at a dose of 3.2 mg kg−1 (Figure 3I, J). Table 2 shows that all these drugs except fenfluramine decreased ICSS thresholds. Again, though, thresholds could not be calculated at high doses of some drugs either because ICSS rates exceeded 20% MCR at all frequencies [(+)MDMA] or because rates failed to achieve a minimum of 50% MCR at any frequency (PAL-287, fenfluramine).
Figure 3.
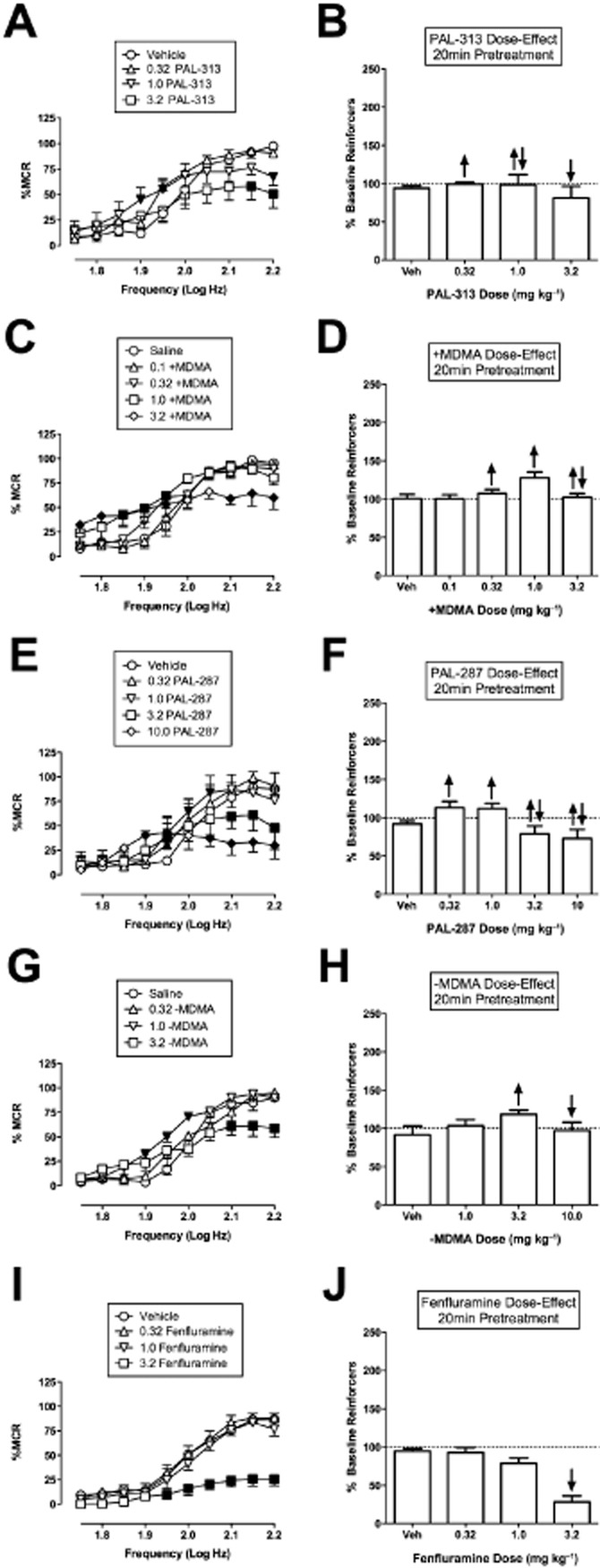
Effect of PAL-313, (+)MDMA, PAL-287, (–)MDMA and fenfluramine on ICSS. Left panels (A, C, E, G, I) show drug effects on full ICSS frequency–rate curves. Right panels (B, D, F, H, J) show summary ICSS data expressed as percent pre-drug baseline number of reinforcers delivered across all frequencies. Other details as in Figure 1. All data show mean ± SEM for five to seven rats (except for 10.0 mg kg−1 PAL-287, n = 3). Statistical results for data in left panels are as follows: (A) PAL-313 (n = 5): significant main effect of frequency [F(9,36) = 44.9, P < 0.001] but not dose [F(3,12) = 2.9, P = 0.079]. There was a significant interaction [F(27,108) = 3.8, P = < 0.001]. C. (+)MDMA: significant main effect of frequency [F(9,36) = 51.0, P < 0.001], dose [F(4,16) = 3.6, P = 0.028] and significant interaction [F(36,144) = 4.0, P < 0.001]. (E) PAL-287 0.32–3.2 mg kg−1 (n = 5): significant main effect of frequency [F(9,36) = 71.6, P < 0.001], dose [F(3,12) = 4.7, P < 0.021] and significant interaction [F(27,108) = 3.9, P = < 0.001]. PAL-287 10.0 mg kg−1 (n = 3): significant main effect of frequency [F(9,18) = 27.0, P < 0.001] but not dose [F(1,2) = 3.1, P = 0.222]. There was a significant interaction [F(9,18) = 21.7, P = < 0.001]. Additional rats were not tested at 10 mg kg−1 due to lethality. (G) (–)MDMA: significant main effect of frequency [F(9,36) = 72.4, P < 0.001] but not dose [F(3,12) = 3.4, P = 0.052]. There was a significant interaction [F(27,108) = 3.8, P < 0.001]. (I) Fenfluramine (n = 7): significant main effect of frequency [F(9,54) = 70.3, P < 0.001], dose [F(3,18) = 33.5, P < 0.001] and significant interaction [F(27,162) = 9.1, P = < 0.001].
Figure 4A–J shows the time courses of 3.2 m kg−1 of each compound. For all drugs except fenfluramine, rate-decreasing effects were significant and tended to predominate at early time points, whereas rate-increasing effects were significant and tended to predominate at later time points. With the serotonin-selective releaser fenfluramine, the greatest decrease in responding was seen at 30 min, and this effect was nearly gone by 24 h (Figure 4I, J). Fenfluramine did not facilitate ICSS at any dose or pretreatment time at any frequency of brain stimulation.
Figure 4.
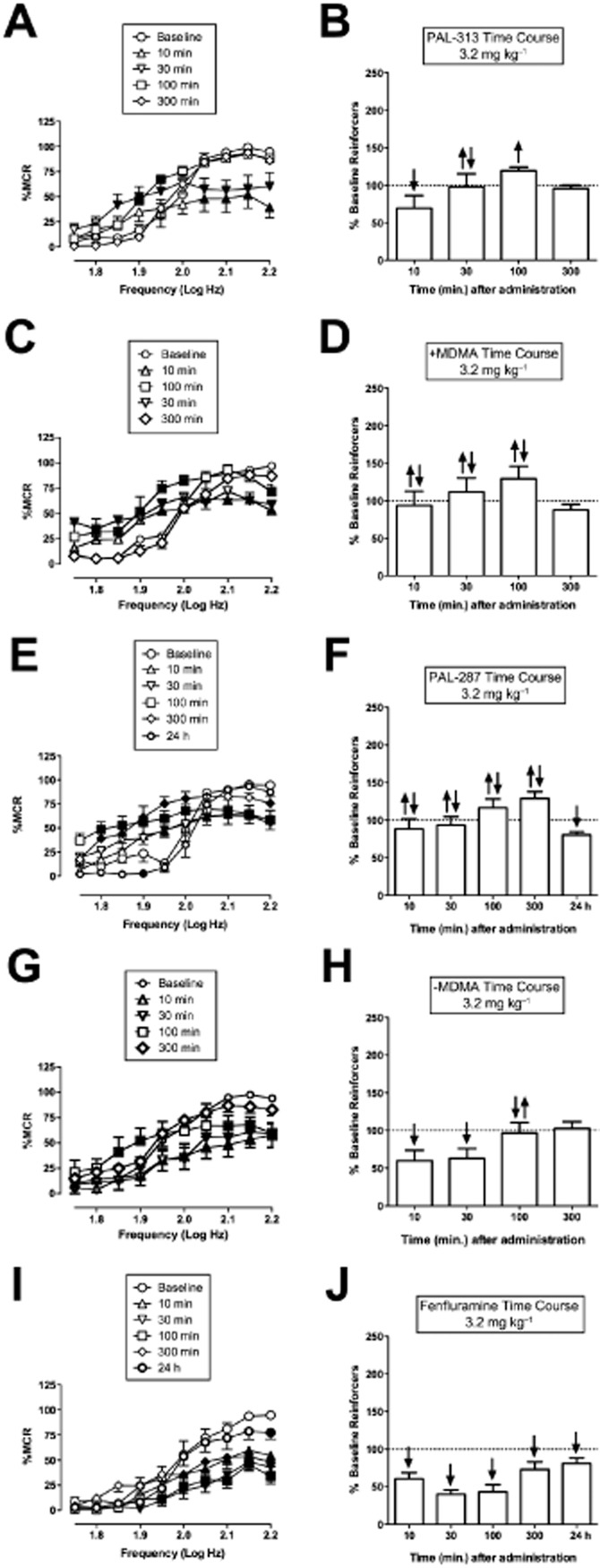
Time courses of PAL-313, (+)MDMA, PAL-287, (–)MDMA and fenfluramine. Left panels (A, C, E, G) show time course of drug effects on full ICSS frequency–rate curves. Right panels (B, D, F, H) show summary ICSS data expressed as percent pre-drug baseline number of reinforcers delivered across all frequencies. Other details as in Figure 1. All data show mean ± SEM for five to seven rats. Statistical results for data in left panels are as follows: (A) PAL-313 (n = 6): significant main effect of frequency [F(9,45) = 62.9, P < 0.001], time [F(4,20) = 3.6, P < 0.001] and significant interaction [F(36,180) = 8.0, P < 0.001]. C. (+)MDMA (n = 5): significant main effect of frequency [F(9,36) = 36.6, P < 0.001], time [F(4,16) = 3.733, P = 0.025] and significant interaction [F(36,144) = 7.803, P < 0.001]. (E) PAL-287 (n = 5): significant main effect of frequency [F(9,36) = 85.0, P < 0.001], time [F(5,20) = 6.5, P < 0.001] and significant interaction [F(45,180) = 11.0, P < 0.001]. (G) (–)MDMA (n = 6): significant main effect of frequency [F(9,45) = 24.2, P < 0.001], time [F(4,20) = 5.5, P = 0.004] and significant interaction [F(36,180) = 3.6, P < 0.001]. (I) Fenfluramine (n = 7): significant main effect of frequency [F(9,54) = 41.1, P < 0.001], time [F(5,30) = 14.5, P < 0.001] and significant interaction [F(45,270) = 3.8, P < 0.001].
(+/–)MDMA (0.15-fold selective for DA versus 5-HT; Table 1) was also tested and produced rate-increasing effects at 1.0 m kg−1 and both rate-increasing and rate-decreasing effects at 3.2 mg kg−1. A time course of 3.2 mg kg−1 showed mixed rate-increasing and rate-decreasing effects at 10 and 30 min, exclusively rate-increasing effects at 100 min, and no effect at 300 min (detailed data not shown, summary data included in Figure 6 and Table 2).
Figure 6.
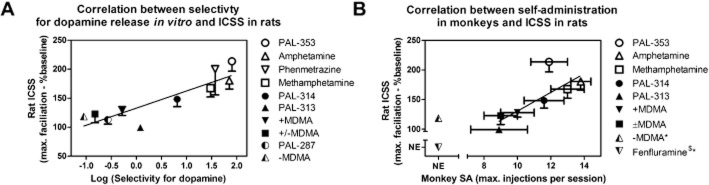
Correlation of ICSS facilitation in rats with (A) in vitro selectivity to promote DA versus 5-HT release and (B) break points maintained under a progressive-ratio schedule of drug self-administration in rhesus monkeys. (A) Abscissa: Log selectivity to release DA versus 5-HT expressed as the log of selectivity values shown in Table 1. Ordinate: Maximum facilitation of ICSS expressed as the maximum increase produced by any drug dose in percent pre-drug baseline number of reinforcers delivered across all brain stimulation frequencies (from Figures 1 and 3, right panels). Fenfluramine was excluded from this figure because it did not facilitate ICSS at any dose or time and because precise selectivity could not be quantified due to low potency to release dopamine. (B) Abscissa: Maximum break point maintained by any drug dose under a progressive-ratio schedule of drug self-administration in rhesus monkeys. Ordinate. Maximum facilitation of ICSS as in panel A. (–)MDMA and fenfluramine were excluded from the correlation because they did not facilitate ICSS in rats and/or did not reliably maintain self-administration in monkeys (self-administration by <50% of monkeys tested). PAL-287 and phenmetrazine were also excluded, because they have not been tested by Woolverton and colleagues under the progressive-ratio schedule of drug self-administration in rhesus monkeys.
Figure 5 shows the effects of 1:1, 1:3 and 1:10 mixtures of the DA-selective releaser PAL-353 and the 5-HT-selective releaser fenfluramine. The 1:1 mixture produced exclusive and dose-dependent rate-increasing effects over a 10-fold dose range and increased the % baseline reinforcers to a maximum of 151% (Figure 5A, B). The 1:3 mixture produced only rate-increasing effects at the lower two doses and a maximum facilitation of ICSS to 150% baseline, but both rate-increasing and rate-decreasing effects were observed at the highest dose (Figure 5C, D). The 1:10 mixture produced only rate-increasing effects at the lowest dose to a maximum of 120% baseline, both rate-increasing and rate-decreasing effects at the middle dose, and only rate-decreasing effects at the highest dose (Figure 5E, F). Thus, increasing proportions of fenfluramine were associated with decreases in the maximum facilitation of ICSS and decreases in the range of doses across which facilitation was observed.
Figure 5.
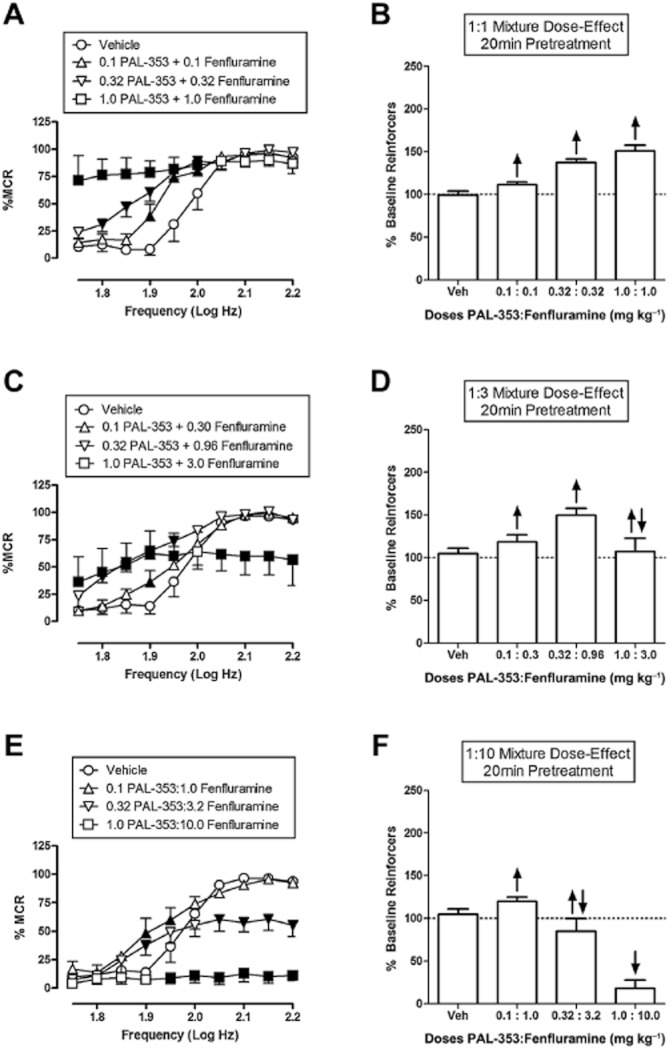
Effect of PAL-353/fenfluramine mixtures on ICSS. Left panels (A, C, E) show drug effects on full ICSS frequency–rate curves. Right panels (B, D, F) show summary ICSS data expressed as percent pre-drug baseline number of reinforcers delivered across all frequencies. Other details as in Figure 1. All data show mean ± SEM for five to six rats. Statistical results for data in left panels are as follows: (A) 1:1 PAL-353/Fenfluramine (n = 5): significant main effect of frequency [F(9,36) = 66.5, P < 0.001], dose [F(3,12) = 43.2, P < 0.001] and significant interaction [F(27,108) = 10.4, P < 0.001]. (C) 1:3 PAL-353/Fenfluramine (n = 6): significant main effect of frequency [F(9,45) = 65.8, P < 0.001], dose [F(3,15) = 7.0, P < 0.001] and significant interaction [F(27,135) = 8.1, P < 0.001]. (E) 1:10 PAL-353/Fenfluramine (n = 6): significant main effect of frequency [F(9,45) = 54.2, P < 0.001], dose [F(3,15) = 21.6, P < 0.001] and significant interaction [F(27,135) = 10.3, P < 0.001].
Figure 6A shows the correlation between maximal facilitation of ICSS (expressed as maximal increase in total stimulations) and previously published data regarding in vitro selectivity to release DA versus 5-HT (Table 1). The slope of the regression line was 29.6 ± 5.2 and was significantly different from zero (P ≤ 0.0001). A Pearson correlation test showed a significant correlation (Pearson r = 0.89, R2 = 0.78, P = 0.0006). Fenfluramine was excluded from this figure because it did not facilitate ICSS at any dose or time and because precise selectivity could not be quantified due to low potency to release dopamine. Figure 6B shows the correlation between maximal facilitation of ICSS and in vivo efficacy to main drug self-administration under a progressive-ratio procedure in rhesus monkeys (Table 1). The slope of the regression line was 15.8 ± 3.0 and was significantly different from zero (P ≤ 0.0001). A Pearson correlation test also showed a significant correlation (Pearson r = 0.80, R2 = 0.63, P = 0.0320). (–)MDMA and fenfluramine were excluded from the correlation because they did not facilitate ICSS in rats and/or did not reliably maintain self-administration in monkeys (self-administration by <50% of monkeys tested). PAL-287 and phenmetrazine were also excluded, because they have not been tested by Woolverton and colleagues under the progressive-ratio schedule of drug self-administration in rhesus monkeys.
Correlations were also determined using maximal reductions in ICSS thresholds from Table 2. However, drug-induced changes in thresholds did not correlate significantly with either in vitro selectivity to release DA versus 5-HT (P = 0.32) or with progressive-ratio break points in rhesus monkeys (P = 0.67), and they also did not correlate with drug-induced changes in total stimulations (P = 0.98).
Discussion
This study evaluated effects of 11 monoamine releasers on ICSS in rats. There were two main findings. First, maximal degrees of ICSS facilitation correlated with in vitro selectivity to promote release of DA versus 5-HT. Moreover, addition of a selective 5-HT releaser (fenfluramine) to a selective DA releaser (PAL-353) produced a proportion-dependent attenuation of ICSS facilitation produced by the DA-selective releaser. These results suggest that serotonergic effects of monoamine releasers are sufficient to oppose and limit DA-mediated facilitation of ICSS. The second major finding of this study was that maximal degrees of ICSS facilitation in rats correlated with maximal break points maintained under a progressive-ratio schedule of reinforcement in rhesus monkeys. This latter correlation suggests that ICSS may be useful not only for qualitative identification of drugs with abuse potential but also for quantitative stratification of relative abuse liability across different drugs. Taken together, these results support the use of ICSS in rats as an experimental tool to study the expression and pharmacological determinants of abuse-related effects of monoamine releasers.
Effects of monoamine releasers on ICSS
Results of the present study confirm and extend previous studies that have examined effects of monoamine releasers on intracranial self-stimulation in rats across a heterogeneous range of conditions. In agreement with the present findings, both amphetamine (Esposito et al., 1980; Kling-Petersen et al., 1994; Wise and Munn, 1995; Lin et al., 2000) and methamphetamine (Elder et al., 1965) have been shown to facilitate ICSS. For example, amphetamine (0.5–2.0 mg kg−1, i.p.) decreased the threshold of medial forebrain bundle stimulation required to maintain ICSS in a frequency–rate procedure in rats similar to the procedure used here, although this earlier study did not fully characterize amphetamine potency or time course (Wise and Munn, 1995). Also in agreement with the present study, fenfluramine has been shown previously to depress ICSS (Olds and Yuwiler, 1992; Olds, 1995). In these earlier studies with fenfluramine, stable baseline rates of ICSS were maintained by a single magnitude of brain stimulation (i.e. a single frequency and intensity of stimulation), and 20 mg kg−1 (i.p.) fenfluramine decreased ICSS dramatically for at least 13 h; however, other doses of fenfluramine were not studied, and fenfluramine effects on low rates of ICSS maintained by low stimulation magnitudes were not assessed to evaluate the potential for fenfluramine to facilitate ICSS. Finally, (+/–)MDMA (0.5–4 mg kg−1, i.p.) was studied under an intensity-rate ICSS procedure (i.e. with manipulation of brain stimulation intensity rather than frequency), and similar to the present study, (+/–)MDMA produced a mixture of decreases in peak response rates maintained by high stimulation amplitudes (rate-decreasing effects) and increases in low rates of responding maintained by low stimulation amplitudes (rate-increasing effects) (Lin et al., 1997).
The present study extends on these earlier findings in three ways. First, this study used a single procedure to evaluate effects produced by multiple doses of 11 monoamine releasers that varied along a continuum of pharmacological selectivity to promote release of DA and 5-HT. This permitted direct comparison of dose-effect curves for a range of compounds under a standard set of experimental conditions. For example, both amphetamine and (+)MDMA produced abuse-related facilitation of ICSS, but amphetamine produced facilitation across a broader range of doses and to a higher maximal degree than (+)MDMA. This in turn permitted correlation of ICSS data to in vitro biochemical data, and there was a significant correlation between maximal facilitation of ICSS and biochemical selectivity to release DA versus 5-HT. Second, this study compared effects of manipulating DA/5-HT selectivity of individual compounds with effects of manipulating proportion of DA- or 5-HT-selective releasers in fixed-proportion mixtures of compounds. Results with mixtures supported results with individual compounds in suggesting that promotion of 5-HT release is sufficient to oppose and limit dopaminergically mediated ICSS facilitation. Although pharmacokinetic interactions could contribute to the net effects of PAL-353/fenfluramine by altering the potency of either drug (Sills et al., 1999), these interactions alone are not sufficient to explain the qualitative difference between effects of the mixtures versus either drug alone. Finally, this study evaluated time course of all compounds. Most drugs displayed comparable onsets of action (within 10 min) and durations of action (100–300 min), although consistent with earlier ICSS studies (Olds and Yuwiler, 1992; Olds, 1995), fenfluramine had a slightly slower onset and longer duration of action than the other releasers. Time course studies also permitted comparison of the duration of rate-increasing and rate-decreasing effects produced by weakly selective compounds (e.g. PAL-314, PAL-313, PAL-287 and the MDMA enantiomers). For all these compounds, rate-decreasing effects required higher doses and had shorter durations than rate-increasing effects. As a result, the highest levels of ICSS facilitation with these compounds were observed early after administration of low doses but later after administration of higher doses. The limited ability of these compounds to facilitate ICSS soon after their administration may be related to their weaker reinforcing efficacy in assays of drug self-administration (see below), because early-onset effects of drugs or other consequent stimuli play a stronger role than delayed effects as determinants of reinforcement (Lattal, 2010; Woolverton et al., 2012).
Correlation of monoamine releaser effects in assays of ICSS and drug self-administration
One rationale for selecting the test compounds for this study was that most had been tested previously in rhesus monkeys responding under a progressive-ratio schedule of drug self-administration (Wee et al., 2005; Wang and Woolverton, 2007). Drug self-administration by rhesus monkeys is a strong predictor of abuse liability in humans, and it has historically played an important role in preclinical abuse-liability assessment for regulatory purposes (Balster and Bigelow, 2003). Moreover, progressive-ratio schedules of reinforcement may be especially useful in stratifying the relative reinforcing efficacy of different drugs (Richardson and Roberts, 1996; Stafford et al., 1998; Stoops, 2008), and the monoamine releasers studied here maintained a wide range of different breakpoints in rhesus monkeys responding under a progressive-ratio schedule (Wee et al., 2005; Wang and Woolverton, 2007). Consequently, these compounds afforded an opportunity to compare drug effects on ICSS in rats with effects of the same drugs in a well-established nonhuman primate assay of preclinical abuse liability assessment. The correlation of results across assays was significant, suggesting that ICSS in rats may function as a good predictor of reinforcing effects of monoamine releasers in monkeys, and by extension, of abuse liability in humans. Previous studies have provided qualitative evidence to suggest that ICSS procedures are predictive of abuse liability (Wise, 1996; Vlachou and Markou, 2011). This study extends on this earlier work by demonstrating a quantitative relationship between abuse-related effects in ICSS and drug self-administration procedures. Two of the 11 drugs tested in ICSS (phenmetrazine and PAL-287) were not included in the comparison across assays because they have not been tested under the progressive-ratio schedule of self-administration in rhesus monkeys. However, phenmetrazine produced relatively high maximal facilitation of ICSS and also maintained self-administration in nonhuman primates under fixed-ratio schedules of reinforcement (Griffiths et al., 1976; Corwin et al., 1987), whereas PAL-287 produced weaker maximal facilitation of ICSS and did not reliably maintain self-administration in rhesus monkeys under a fixed-ratio schedule of reinforcement (Rothman et al., 2005). Thus, results with these compounds also support the general correspondence of results from rat ICSS and monkey self-administration procedures.
Comments on data analysis
Two approaches were used in this study to generate summary measures of drug effects on ICSS for correlation with other neurochemical and behavioural data. One approach used regression analysis to determine drug-induced changes in threshold frequencies required to maintain ICSS. The second approach evaluated drug-induced changes in the total number of stimulations delivered across all frequencies, and this latter measure integrated both drug-induced increases in low rates of ICSS maintained by low stimulation frequencies and drug-induced decreases in high rates of ICSS maintained by high stimulation frequencies. Both approaches agreed in showing that all drugs except fenfluramine facilitated ICSS. However, only the total-stimulations approach yielded a measure of ICSS efficacy that correlated with both in vitro selectivity to release DA versus 5-HT and in vivo efficacy to maintain self-administration in rhesus monkeys responding under a progressive-ratio schedule. By contrast, threshold measures did not correlate with in vitro pharmacological selectivity or in vivo reinforcing efficacy, nor did they correlate with the total-stimulations measure of efficacy to facilitate ICSS. This dissociation likely reflects constraints on the range of conditions across which threshold analysis can be applied. For example, in the present study, thresholds could often not be calculated after administration of high drug doses because ICSS rates were greater or less than criterion rates across the entire frequency range. Such drug-induced ‘vertical’ shifts in ICSS frequency–rate curves are often interpreted as evidence of motor effects that alter response capability, as opposed to lateral shifts often interpreted as hedonic effects that selectively alter reward-related effects produced by stimulating brain reward substrates (Miliaressis et al., 1986; Carlezon and Chartoff, 2007). The constraints imposed by threshold analysis have provided useful boundaries that protect against confounding motor effects in research using ICSS to examine hedonic effects of experimental manipulations on brain reward substrates. However, the present results suggest that these constraints may pose an obstacle to quantitative prediction of rewarding effects and abuse liability, perhaps because expression of rewarding effects and abuse liability involves an integration of both hedonic and motor effects. The significant correlation of the total-stimulation measure of efficacy to facilitate ICSS with both in vitro pharmacological selectivity and in vivo efficacy in non-human primate self-administration assays suggests that this metric could be useful in predicting expression and pharmacological determinants of the abuse liability of existing and novel monoamine releasers. The utility of this measure for predicting reinforcing effects and abuse liability of drugs from other pharmacological classes remains to be determined.
Acknowledgments
A special thanks to Robert O'Connell and Crystal Young for technical support. These studies were supported by National Institute of Health grants R01-DA026946 (SSN) and R01-DA012790 (BEB).
Conflicts of interest
SSN and BEB have funding from the NIH and no other conflicts of interest. CTB and MLB have no conflicts of interest.
References
- Altarifi AA, Negus SS. Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharm. 2011;22:663–673. doi: 10.1097/FBP.0b013e32834aff54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ator NA, Griffiths RR. Principles of drug abuse and liability assessment in laboratory animals. Drug Alcohol Depend. 2003;70(Suppl. 3):S55–S72. doi: 10.1016/s0376-8716(03)00099-1. [DOI] [PubMed] [Google Scholar]
- Balster RL, Bigelow GE. Guidelines and methodological reviews concerning drug abuse liability assessment. Drug Alcohol Depend. 2003;70(Suppl. 5):S13–S40. doi: 10.1016/s0376-8716(03)00097-8. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. A comparison of d-amphetamine, l-amphetamine, and methamphetamine self-administration in rhesus monkeys. Pharmacol Biochem Behav. 1973;1:67–71. doi: 10.1016/0091-3057(73)90057-9. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. JPET. 2011;337:218–225. doi: 10.1124/jpet.110.176271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray GA. Use and abuse of appetite-suppressant drugs in the treatment of obesity. Ann Intern Med. 1993;119:707–713. doi: 10.7326/0003-4819-119-7_part_2-199310011-00016. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Chartoff EH. Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc. 2007;2:2987–2995. doi: 10.1038/nprot.2007.441. [DOI] [PubMed] [Google Scholar]
- Corwin RL, Woolverton WL, Schuster CR, Johanson CE. Anorectics: effects on food intake and self-administration in rhesus monkeys. Alcohol Drug Res. 1987;7:351–361. [PubMed] [Google Scholar]
- Dahl CB, Götestam KG. Lack of self-administration of different fenfluramine isomers in rats. Addict Behav. 1989;14:239–247. doi: 10.1016/0306-4603(89)90055-5. [DOI] [PubMed] [Google Scholar]
- Do Carmo GP, Folk JE, Rice KC, Chartoff E, Carlezon WA, Jr, Negus SS. The selective non-peptidic delta opioid agonist SNC80 does not facilitate intracranial self-stimulation in rats. Eur J Pharmacol. 2009;604:58–65. doi: 10.1016/j.ejphar.2008.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elder ST, Noel PM, Merrill MR. Effects of Food Deprivation and Methamphetamine on Fixed-Ratio Schedules of Intracranial Self-Stimulation. Psychol Rep. 1965;16:1225–1233. [Google Scholar]
- Elmer GI, Pieper JO, Hamilton LR, Wise RA. Qualitative differences between C57BL/6J and DBA/2J mice in morphine potentiation of brain stimulation reward and intravenous self-administration. Psychopharmacology (Berl) 2010;208:309–321. doi: 10.1007/s00213-009-1732-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito RU, Perry W, Kornetsky C. Effects of d-amphetamine and naloxone on brain stimulation reward. Psychopharmacology. 1980;69:187–191. doi: 10.1007/BF00427648. [DOI] [PubMed] [Google Scholar]
- Götestam KG, Andersson BE. Self-administration of amphetamine analogues in rats. Pharmacol Biochem Behav. 1975;3:229–233. doi: 10.1016/0091-3057(75)90152-5. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–1464. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Griffiths RR, Winger G, Brady JV, Snell JD. Comparison of behavior maintained by infusions of eight phenylethylamines in baboons. Psychopharmacology (Berl) 1976;50:251–258. doi: 10.1007/BF00426841. [DOI] [PubMed] [Google Scholar]
- Howell LL, Kimmel HL. Monoamine transporters and psychostimulant addiction. Biochem Pharmacol. 2008;75:196–217. doi: 10.1016/j.bcp.2007.08.003. [DOI] [PubMed] [Google Scholar]
- Hubner CB, Bird M, Rassnick S, Kornetsky C. The threshold lowering effects of MDMA (ecstasy) on brain-stimulation reward. Psychopharmacology (Berl) 1988;95:49–51. doi: 10.1007/BF00212765. [DOI] [PubMed] [Google Scholar]
- Jasinski DR, Kovacević-Ristanović R. Evaluation of the abuse liability of modafinil and other drugs for excessive daytime sleepiness associated with narcolepsy. Clin Neuropharmacol. 2000;23:149–156. doi: 10.1097/00002826-200005000-00004. [DOI] [PubMed] [Google Scholar]
- Kling-Petersen T, Ljung E, Svensson K. The preferential dopamine autoreceptor antagonist (+)-UH232 antagonizes the positive reinforcing effects of cocaine and d-Amphetamine in the ICSS paradigm. Pharmacol Biochem Behav. 1994;49:345–351. doi: 10.1016/0091-3057(94)90432-4. [DOI] [PubMed] [Google Scholar]
- Kornetsky C, Esposito RU. Euphorigenic drugs: effects on the reward pathways of the brain. Fed Proc. 1979;38:2473–2476. [PubMed] [Google Scholar]
- Lattal KA. Delayed reinforcement of operant behavior. J Exp Anal Behav. 2010;93:129–139. doi: 10.1901/jeab.2010.93-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung KS, Cottler LB. Ecstasy and other club drugs: a review of recent epidemiologic studies. Curr Opin Psychiatry. 2008;21:234–241. doi: 10.1097/YCO.0b013e3282f9b1f1. [DOI] [PubMed] [Google Scholar]
- Lin D, Koob GF, Markou A. Time-Dependent Alterations in ICSS Thresholds Associated With Repeated Amphetamine Administrations. Pharmacol Biochem Behav. 2000;65:407–417. doi: 10.1016/s0091-3057(99)00213-0. [DOI] [PubMed] [Google Scholar]
- Lin HQ, Jackson DM, Atrens DM, Christie MJ, McGregor IS. Serotonergic modulation of 3,4-methylenedioxymethamphetamine (MDMA)-elicited reduction of response rate but not rewarding threshold in accumbal self-stimulation. Brain Res. 1997;744:351–357. doi: 10.1016/S0006-8993(96)01210-3. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, Kilkenny C, Wainwright C. Guidelines for reportingexperiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna ML, Ho BT. The role of dopamine in the discriminative stimulus properties of cocaine. Neuropharmacology. 1980;19:297–303. doi: 10.1016/0028-3908(80)90153-7. [DOI] [PubMed] [Google Scholar]
- Miliaressis E, Rompre PP, Laviolette P, Philippe L, Coulombe D. The curve-shift paradigm in self-stimulation. Physiol Behav. 1986;37:85–91. doi: 10.1016/0031-9384(86)90388-4. [DOI] [PubMed] [Google Scholar]
- National Academy of Science. Guide for the Care and Use of Laboratory Animals. 7th edn. Washington, DC: National Academy Press; 1996. [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology. 2010;210:149–159. doi: 10.1007/s00213-009-1770-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino S. Clinical and neurobiological aspects of narcolepsy. Sleep Med. 2007;8:373–399. doi: 10.1016/j.sleep.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor EC, Chapman K, Butler P, Mead AN. The predictive validity of the rat self-administration model for abuse liability. Neurosci Biobehav Rev. 2011;35:912–938. doi: 10.1016/j.neubiorev.2010.10.012. [DOI] [PubMed] [Google Scholar]
- Olds ME. Dopamine agonists prevent or counteract the suppression of brain stimulation reward by fenfluramine. Pharmacol Biochem Behav. 1995;50:41–48. doi: 10.1016/0091-3057(94)00240-j. [DOI] [PubMed] [Google Scholar]
- Olds ME, Yuwiler A. Effects of acute and chronic fenfluramine on self-stimulation and its facilitation by amphetamine. Eur J Pharmacol. 1992;216:363–372. doi: 10.1016/0014-2999(92)90432-4. [DOI] [PubMed] [Google Scholar]
- Parada M, Hernandez L, Schwartz D, Hoebel BG. Hypothalamic infusion of amphetamine increases serotonin, dopamine and norepinephrine. Physiol Behav. 1988;44:607–610. doi: 10.1016/0031-9384(88)90325-3. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Balance between dopamine and serotonin release modulates behavioral effects of amphetamine-type drugs. Ann N Y Acad Sci. 2006;1074:245–260. doi: 10.1196/annals.1369.064. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Baumann MH. Dual dopamine/serotonin releasers as potential medications for stimulant and alcohol addictions. AAPS J. 2007;9:1–10. doi: 10.1208/aapsj0901001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Katsnelson M, Vu N, Partilla JS, Dersch CM, Blough BE, et al. Interaction of the anorectic medication, phendimetrazine, and its metabolites with monoamine transporters in rat brain. Eur J Pharmacol. 2002;447:51–57. doi: 10.1016/s0014-2999(02)01830-7. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Blough BE, Woolverton WL, Anderson KG, Negus SS, Mello NK, et al. Development of a rationally designed, low abuse potential, biogenic amine releaser that suppresses cocaine self-administration. JPET. 2005;313:1361–1369. doi: 10.1124/jpet.104.082503. [DOI] [PubMed] [Google Scholar]
- Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics. 2009;124:875–880. doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- Sills TL, Greenshaw AJ, Baker GB, Fletch PJ. Acute fluoxetine treatment potentiates amphetamine hyperactivity and amphetamine-induced nucleus accumbens dopamine release: possible pharmacokinetic interaction. Psychopharmacology. 1999;141:421–427. doi: 10.1007/s002130050852. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stoops WW. Reinforcing effects of stimulants in humans: sensitivity of progressive-ratio schedules. Exp Clin Psychopharmacol. 2008;16:503–512. doi: 10.1037/a0013657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachou S, Markou A. Intracranial self-stimulation. Anim Models Drug Addict. 2011;53:3–56. [Google Scholar]
- Wang Z, Woolverton WL. Estimating the relative reinforcing strength of (±)-3,4-methylenedioxymethamphetamine (MDMA) and its isomers in rhesus monkeys: comparison to (+)-methamphetamine. Psychopharmacology. 2007;189:483–488. doi: 10.1007/s00213-006-0599-5. [DOI] [PubMed] [Google Scholar]
- Wee S, Woolverton WL. Self-administration of mixtures of fenfluramine and amphetamine by rhesus monkeys. Pharmacol Biochem Behav. 2006;84:337–343. doi: 10.1016/j.pbb.2006.05.022. [DOI] [PubMed] [Google Scholar]
- Wee S, Anderson KG, Baumann MH, Rothman RB, Blough BE, Woolverton WL. Relationship between the serotonergic activity and reinforcing effects of a series of amphetamine analogs. JPET. 2005;313:848–854. doi: 10.1124/jpet.104.080101. [DOI] [PubMed] [Google Scholar]
- Winder GS, Stern N, Hosanagar A. Are ‘Bath Salts’ the next generation of stimulant abuse? J Subst Abuse Treat. 2012 doi: 10.1016/j.jsat.2012.02.003. In Press. Available at: http://dx.doi.org/10.1016/j.jsat.2012.02.003 (accessed 19 October 2012) [DOI] [PubMed] [Google Scholar]
- Wise RA. Addictive drugs and brain stimulation reward. Annu Rev Neurosci. 1996;19:319–340. doi: 10.1146/annurev.ne.19.030196.001535. [DOI] [PubMed] [Google Scholar]
- Wise RA, Munn E. Withdrawal from chronic amphetamine elevates baseline intracranial self-stimulation thresholds. Psychopharmacology. 1995;117:130–136. doi: 10.1007/BF02245178. [DOI] [PubMed] [Google Scholar]
- Woods JH, Tessel RE. Fenfluramine: amphetamine congener that fails to maintain drug-taking behavior in the rhesus monkey. Science. 1974;185:1067–1069. doi: 10.1126/science.185.4156.1067. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Freeman KB, Myerson J, Green L. Suppression of cocaine self-administration in monkeys: effects of delayed punishment. Psychopharmacology. 2012;220:509–517. doi: 10.1007/s00213-011-2501-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


