Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is typified by the accumulation of fluid-filled cysts and abnormalities in renal epithelial cell function. The disease is principally caused by mutations in the gene encoding polycystin-1, a large basolateral plasma membrane protein expressed in kidney epithelial cells. Our studies reveal that, in normal kidney cells, polycystin-1 forms a complex with the adherens junction protein E-cadherin and its associated catenins, suggesting a role in cell adhesion or polarity. In primary cells from ADPKD patients, the polycystin-1/polycystin-2/E-cadherin/β-catenin complex was disrupted and both polycystin-1 and E-cadherin were depleted from the plasma membrane as a result of the increased phosphorylation of polycystin-1. The loss of E-cadherin was compensated by the transcriptional upregulation of the normally mesenchymal N-cadherin. Increased cell surface N-cadherin in the disease cells in turn stabilized the continued plasma membrane localization of β-catenin in the absence of E-cadherin. The results suggest that enhanced phosphorylation of polycystin-1 in ADPKD cells precipitates changes in its localization and its ability to form protein complexes that are critical for the stabilization of adherens junctions and the maintenance of a fully differentiated polarized renal epithelium.
INTRODUCTION
The main functions of the kidney—filtration, reabsorption and secretion of blood components—depend on the normal morphogenesis and function of the epithelial cells, lining the kidney tubules. One of the most common genetic diseases in humans, autosomal dominant polycystic kidney disease (ADPKD), is characterized by defects in the polarized phenotype and function of renal epithelial cells, leading to the extensive growth of numerous fluid-filled cysts in the kidneys. The disease is as yet incurable and causes serious renal failure, prompting intensive research to understand its pathogenesis.
The PKD1 gene mutated in the majority of ADPKD cases encodes the 450,000-460,000 MW transmembrane protein polycystin-1 (Consortium, 1995). The protein is predicted to consist of multiple transmembrane domains, a large extracellular domain mediating cell-cell and cell-extracellular matrix binding and a multifunctional carboxy-terminal region (Hughes et al., 1995; Gallagher et al., 2000; Arnaout, 2001). Regulated basolateral plasma membrane localization and developmental expression serve to implicate polycystin-1 in kidney morphogenesis and epithelial differentiation (Ward et al., 1996; Ibraghimov-Beskrovnaya et al., 1997; Van Adelsberg et al., 1997). Yet, the mechanism whereby mutant polycystin-1 leads to alterations in ADPKD cell polarity and function remains unclear.
The cytoplasmic carboxy-terminal domain of polycystin-1 has demonstrated importance in the regulation of kidney epithelial cell proliferation and morphogenesis (Sutters et al., 2001; Nickel et al., 2002). Specific protein-protein interactions and participation in multiple intracellular signaling pathways govern these regulatory functions of polycystin-1. Wnt, protein kinase C, cAMP, JAK-STAT, and G-protein-coupled signaling pathways as well as possible Ca2+ signaling regulation via its interaction with polycystin-2 have all been implicated to interface with polycystin-1 in the downstream control of kidney epithelial cell proliferation and differentiation (Arnould et al., 1998; Parnell et al., 1998; Kim et al., 1999; Li et al., 1999; Bhunia et al., 2002). However, the relative contributions of each pathway and mechanisms of upstream activation still remain to be elucidated in most cases. The control of calcium flux is considered an important upstream control mechanism brought about by a direct interaction between polycystin-1 and -2 to form a unique calcium-permeable channel (Hanaoka et al., 2000; Gonzalez-Perret et al., 2001). The disruption of the polycystin-1/polycystin-2 complex, through disease-specific mutations in the genes encoding either of the polycystins, is postulated to impair intracellular calcium signaling and hence polycystin-1 function (Wilson, 2001). The recent demonstration that polycystin-1 forms a complex with the essential epithelial cell adhesion molecule E-cadherin and its associated cytoplasmic catenins suggests that cell-cell adhesion may provide another important regulatory cue in the control of polycystin-1 function (Huan and van Adelsberg, 1999). Alternatively, proper polycystin-1/polycystin-2-mediated calcium signaling may be upstream of E-cadherin-associated Wnt and β-catenin signaling (Kim et al., 1999; van Adelsberg, 2000). Therefore, it is of interest to resolve the nature and conditions of the polycystin-1/E-cadherin/β-catenin associations.
Cadherin-mediated adhesion is critical during embryogenesis, morphogenesis, and in the normal function of epithelial tissues. As the central component of adherens junctions, E-cadherin promotes Ca2+-dependent cell-cell adhesion through homophilic interactions with E-cadherin on neighboring epithelial cells (Gumbiner, 1996; Angst et al., 2001). Via its carboxy-terminal cytoplasmic tail, E-cadherin associates with β- and α-catenin to promote interaction with the actin cytoskeleton (Yap et al., 1997; Angst et al., 2001; Gottardi et al., 2001). The E-cadherin/β-catenin complex plays a dual role. On the one hand, it stabilizes cellular adhesion through cytoskeletal attachment and on the other hand, the protein complex modulates signal transduction and consequently imparts gene expression and cell cycle control (Gumbiner, 1996; St. Amand and Klymkowsky, 2001). β-catenin is regarded as the key component of adherens junctions that mediates Wnt/Wingless signal transduction and thus regulates proliferation, apoptosis, and tumorigenesis (Gumbiner, 1996; Polakis, 1999). Activation by Wnt signaling causes β-catenin to dissociate from E-cadherin and translocate to the nucleus where it interacts with the DNA-binding TCF/LEF family proteins to activate Wnt responsive genes (St. Amand and Klymkowsky, 2001). Thus, excessive proliferation and metastasis are often directly correlated with reduced E-cadherin expression, resulting in an inability to sequester and regulate the transcriptional activity of β-catenin (Gottardi et al., 2001; Simcha et al., 2001; Stockinger et al., 2001).
The central importance attributed to the E-cadherin/β-catenin complex in the control of epithelial cell growth and tissue morphogenesis makes the evaluation E-cadherin/β-catenin and polycystin-1 interactions in normal and ADPKD cells of particular interest. The functional importance of adherens junction components in kidney epithelial function is underscored by the massive polycystic kidney disease in transgenic mice caused by expression of a dominant active β-catenin (Saadi-Kheddouci et al., 2001). A disruption of E-cadherin-mediated adhesion in ADPKD was initially suggested by the plasma membrane depletion and endosomal sequestration of E-cadherin observed in primary cultured ADPKD cells (Charron et al., 2000b).
In the present study we consider the possibility that expression of mutant polycystins in primary ADPKD cells leads to the disruption of a polycystin-1/E-cadherin/β-catenin complex, which in turn contributes to the alterations in renal epithelial cell function associated with disease pathogenesis.
MATERIALS AND METHODS
Reagents
All reagents were from Sigma Chemical Co. (St. Louis, MO) unless otherwise specified.
Cells and Cell Culture
Normal kidney cells and ADPKD cells were obtained from previously healthy individuals and ADPKD patients. Normal kidney cells were derived from the normal margins of surgical biopsies or from kidneys not suited for transplant. ADPKD cells were derived from kidneys removed for clinically indicated reasons. The ADPKD samples were judged to be PKD1 based on the clinical history. Sequencing of the PKD1 gene in our primary cell samples is in progress in Dr. Harris' laboratory to identify the germline mutation. Because of the complexity of this analysis, these results will be reported in a separate article. Three different normal kidney and three different ADPKD cell samples were used for all experiments. In addition, all experiments were performed at least three times to ensure all data reflect only consistently reproduced results. Representative images are shown of one normal kidney sample and two or three ADPKD samples to highlight the phenotypic range of results obtained. Primary epithelial cells were isolated from the cortex of normal kidney or the cysts of ADPKD kidneys as described (Carone et al., 1989; Charron et al., 2000b). Cells were cultured in renal epithelial cell growth media (Clonetics, Walkersville, MD) containing low serum, 10 μg/ml epidermal growth factor, 0.5 mg/ml epinephrine, 5 mg/ml insulin, 6.5 μg/ml triiodothyronine, and 10 mg/ml transferrin. The change of growth media did not affect our biochemical and immunostaining results, relative to published results (Charron et al., 2000b), but did improve the growth of primary kidney cells in culture.
Antibodies
A polyclonal antibody against polycystin-1, NM005, was raised by immunizing rabbits with a distal carboxy-terminal fragment of polycystin-1 (amino acids 4070-4302) as described (Ward et al., 1996). The NM005 antibody was purified using DE52, a DEAE-matrix (Whatman Ltd., Kent, England). Mouse monoclonal antibodies against human E-cadherin, β-catenin, and N-cadherin were obtained from Transduction Laboratories (Lexington, KY). A rabbit polyclonal antibody directed against human N-cadherin was obtained from Calbiochem (San Diego, CA) and rabbit polyclonal antibody against polycystin-2 was purchased from Zymed Laboratories (South San Francisco, CA). Horseradish peroxidase-labeled secondary antibodies were purchased from Amersham Life Science (Piscataway, NJ). Rhodamine-conjugated donkey anti-rabbit, FITC-conjugated donkey anti-mouse and Cy5-conjugated donkey anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) were used for immunofluorescence microscopy studies.
Immunofluorescence Microscopy
Cells grown on microscope coverglasses were fixed with 3% paraformaldehyde and permeabilized with 0.1% Triton X-100. After quenching and blocking they were incubated in primary antibodies for 1 h and subsequently, in fluorophore-conjugated secondary antibodies for 40-60 min at room temperature. Normal human kidney (NK) from the normal margins of surgical biopsies or from tissue not suited for transplant and ADPKD cystic tissue sections from kidneys removed for clinically indicated reasons were OCT-frozen and cryosectioned. Two different patient samples of each type were analyzed. Cryosections were fixed in 3% paraformaldehyde and permeabilized with 25 μg/ml digitonin/DMSO in Pipes buffer (pH 6.8). After quenching and blocking the sections were incubated in primary antibodies at 4°C overnight and subsequently in appropriate fluorophore-conjugated secondary antibodies for 2 h at room temperature. TRITC-conjugated asparagus and FITC-conjugated peanut lectin staining was performed following the immunostaining procedures, by incubating for 2 h at room temperature.
Immunoprecipitations
Cells were scraped from the dishes or from filter inserts, in lysis buffer (500 and 250 μl, respectively), containing 1% (vol/vol) TX-100, 150 mM NaCl, 10 mM Tris-HCl, pH 7.4, and a protease inhibitor cocktail. The proteins were immunoprecipitated using protein A Sepharose beads. Cell lysates (1000-1500 μl) were used for each immunoprecipitation. The final immunoprecipitates were resuspended in an equal (40 μl) volume of 2× SDS sample buffer. In some experiments, the cell lysates were preincubated with protein A Sepharose, as a control for any nonspecific binding to protein A. To assure the equal protein loading in our experiments, the amount of cells in lysates was counted, and the equal amount of cell lysates or immunoprecipitates (20 μl) was loaded. The lysates of normal kidney cells and the cells from all ADPKD patients were prepared from ∼5 × 106 cells; 15 × 106 cells were used for each immunoprecipitation experiment. For immunoblot analyses the proteins were separated on 4, 6, or 10% SDS polyacrylamide gels. Two high-molecular-weight calibration markers ranging from 67,000 to 669,000 MW (Amersham Pharmacia Biotech, Piscataway, NJ) and from 10,000 to 250,000 MW (Bio-Rad, Hercules, CA) were used.
Labeling of Cultured Cells with [32P]orthophosphate
Cultured NK and ADPKD (patient 3) cells were cultivated in phosphate-free medium for 1 h at 37o and subsequently radiolabeled with [32P]orthophosphate (0.5 mCi/ml, for 2 h at 37°C). Radiolabeled and unlabeled control cell lysates were immunoprecipitated with a specific antibody against polycystin-1 and subjected to SDS PAGE in parallel. The acrylamide gel with the 32P-labeled samples was dried and subjected to phosphoimage analysis on a Molecular Dynamics (Sunnyvale, CA) system and was subsequently exposed to film. The unlabeled immunoprecipitates were transferred to nitrocellulose and probed with the antibodies against polycystin-1, E-cadherin, and β-catenin and compared with the phosphorylated polycystin-1-associated proteins.
Cell Surface Biotinylation
Cells were grown on cell culture filter inserts with 0.4-μm pores (Falcon, Becton Dickinson, Franklin Lakes, NJ), and incubated with Sulfo-NHS-LC-biotin (Pierce, Rockford, IL) at 0.6 mg/ml. Both apical and basolateral surfaces were biotinylated twice for 15 min each. The reaction was terminated by 50 mM NH4Cl. All procedures were performed on ice. The cell lysate was subjected to immunoprecipitation or to streptavidin affinity precipitation with 30 μl of streptavidin agarose (Pierce). Quantitative analysis of precipitated proteins was performed using densitometry and the Molecular Dynamics ImageQuant software. The relative amounts of immunoprecipitated polycystin-1, E-cadherin, and β-catenin, from a single immunoprecipitate were compared in order to calculate the ratio of each protein coprecipitated as a part of the complex. The relative ratios of proteins within the complex formed at the plasma membrane were calculated from the biotinylation experiments. Ratios were calculated based on immunoprecipitation with the antibody against polycystin-1 or calculated from values obtained from separate β-catenin immunoprecipitation experiments. The ratio of polycystin-1 coprecipitated with E-cadherin was determined, and the average ratios were plotted as % values with SEM from three to six independent experiments using GraphPad Prism software (San Diego, CA).
Affymetrix Gene Chip Assays
Primary human epithelial cells isolated from normal (two patients) and ADPKD (three patients) kidneys were grown on 10-cm dishes and RNA was isolated using a Qiagen DNA/RNA isolation minikit (Valencia, CA). The high quality of the RNA was established using an Agilent system before proceeding. Individual RNA samples were processed for chip hybridization according to manufacturer's (Affymetrix, Inc., Santa Clara, CA) instruction. The microarray experiments and analyses were performed using facilities in the Keck-UNM Genomics Resource and the UNM Cancer Research and Treatment Center. Initial data analysis was performed using Affymetrix Microarray Suite v5.0 software, setting the scaling of all probe sets to a constant value of 500 for each Gene Chip, and all scaling factors were <20. Additional data analysis was performed using GeneSpring (Silicon Genetics, Redwood City, CA). The data for the cadherin genes were normalized to the average of the two normal kidney samples and fold expression data were plotted for three different ADPKD patient samples. In cases where the Affymetrix chips include multiple oligonucleotide probes for a given gene, the values were plotted separately.
RESULTS
Polycystin-1 Is Associated with E-cadherin and β-catenin Both at the Cell Membrane and Intracellularly
It was previously reported that polycystin-1, E-cadherin, and β-catenin form a multiprotein complex in human pancreatic cells (Huan and van Adelsberg, 1999) and that E-cadherin was depleted from the plasma membranes of ADPKD cells (Charron et al., 2000b). Therefore we undertook a more detailed analysis of the polycystin-1/E-cadherin/β-catenin complex in normal primary kidney epithelial cells and the cells from ADPKD patients.
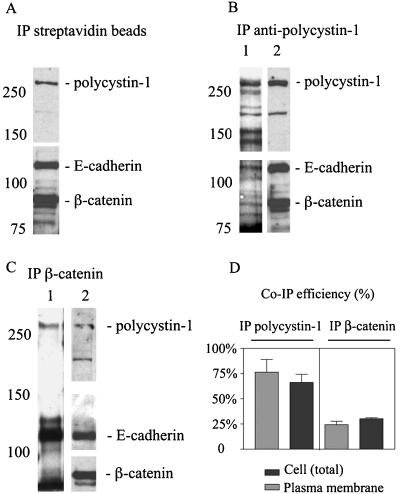
To address the question whether these proteins in fact associate at the plasma membrane, cell surface biotinylation experiments were conducted on normal kidney cells. Biotinylated cell surface proteins from normal kidney cell lysates were recovered with streptavidin-agarose, and the precipitated proteins were immunoblotted with antibodies against polycystin-1, E-cadherin, and β-catenin. These experiments confirmed the presence of all three proteins at the plasma membrane of normal kidney cells and demonstrated the utility of the biotinylation experiments in detecting the cell surface-expressed protein complexes (Figure 1A). The recovery of β-catenin in the biotinylated fraction reflects its tight association and coprecipitation with biotinylated E-cadherin rather than any direct biotinylation (see Figure 1, B and C, compare lanes 1 and 2). The fact that β-catenin was present in both samples, but not biotinylated demonstrates the specificity of our biotinylation assay in labeling only the cell surface-exposed epitopes. Similar lysates from biotinylated normal kidney cells were also immunoprecipitated with antibodies against polycystin-1 (Figure 1B) and β-catenin (Figure 1C). Half of the immunoprecipitated protein sample was then immunoblotted with HRP-conjugated streptavidin to identify the cell surface-exposed proteins (Figure 1, B and C, lane1). The remainder of the immunoprecipitated protein sample was immunoblotted in parallel to identify those biotinylated proteins that corresponded to polycystin-1, E-cadherin, or β-catenin (Figure 1, B and C, lane 2). The latter sample also provided an estimate of the total amount of each protein recovered in the immunoprecipitates, reflecting the combined cell surface and intracellular protein pools. As expected, a prominent biotinylated protein corresponding to polycystin-1 was recovered in samples immunoprecipitated with an antibody against polycystin-1 (Figure 1B). Conversely, biotinylated polycystin-1 was also recovered in samples immunoprecipitated with an antibody against β-catenin (Figure 1C), demonstrating its association with adherens junction components. Biotinylated E-cadherin was recovered in the samples immunoprecipitated with antibodies against polycystin-1 as well as in the positive control samples immunoprecipitated with β-catenin. These data demonstrate that polycystin-1 is present in a complex with E-cadherin and β-catenin and that the complex is present at the plasma membrane.
Figure 1.
Polycystin-1 forms a complex with E-cadherin and β-catenin both at the cell membrane and in cytoplasm. Lysates from cell surface-biotinylated cells were precipitated with (A) streptavidin agarose, (B) antibodies against polycystin-1, or (C) antibodies against β-catenin. The proteins recovered in the immunoprecipitated fractions were probed with streptavidin-horseradish peroxidase to visualize the biotinylated plasma membrane proteins associated with polycystin-1 (B, lane 1) and β-catenin (C, lane 1). In parallel the streptavidin precipitates or the immunoprecipitates were also probed with the antibodies against polycystin-1, E-cadherin, and β-catenin to identify these proteins in the biotinylated samples (A, lane 2 in B and C). (D) The association of E-cadherin and polycystin-1 in multiprotein complexes was quantified and plotted using Prism software. Light bars represent quantification of the efficiency of coimmunoprecipitation of the two proteins in the cell surface complexes as shown in the lane 1 of the B and C. Dark bars represent quantification of the efficiency of coimmunoprecipitation of E-cadherin and polycystin-1 in the total cellular complexes as shown in the lane 2 of B and C. The values reflect the fraction of E-cadherin coprecipitated with polycystin-1 (lefthand bars) using the antibody against polycystin-1 or the fraction of polycystin-1 coprecipitated with E-cadherin (righthand bars) using and antibody against β-catenin for immunoprecipitation (mean, SE; n = 3). The ratio of E-cadherin coprecipitated with β-catenin was 97% and served to validate this approach.
An estimate of the extent of polycystin-1/E-cadherin complex formation at the cell surface (Figure 1, B and C, lane 1) or in the total cell samples (Figure 1, B and C, lane 2) was obtained by quantifying the amounts of E-cadherin and polycystin-1 in the precipitates and expressing the efficiency of coprecipitation as a fractional ratio (Figure 1D). When the multiprotein complex was precipitated with the antibody against polycystin-1, the efficiency of E-cadherin recovery was 76% for the cell surface complexes and 67% for the total cellular complexes (n = 3). When the complex was recovered with antibodies against β-catenin the efficiency of polycystin-1 recovery was 24% for the cell surface complexes and 30% for the total cellular complexes (n = 3). These data indicate a significant fraction of polycystin-1 is complexed with E-cadherin at the plasma membrane. The fact that this fraction is constant for both the total and cell surface samples suggests the complex may also exist in intracellular pools, though this point requires further evaluation. Pulse chase experiments were not tractable with the number of primary cells available. The reduced efficiency of polycystin-1 coprecipitation with E-cadherin complexes suggests that the polycystin-1 may play a regulatory role rather than a structural role in adherens junction stability or assembly because its association with E-cadherin appears to be substoichiometric in comparison to ∼1:1 (equal to 0.97) E-cadherin/β-catenin ratios determined in our experiment.
It is noteworthy that polycystin-1 associated not only with the adherens junction proteins, but also appeared to complex with additional cell surface proteins outside of the adherens junction. This was evidenced by the presence of a number of unidentified biotinylated proteins in Figure 1B, lane 1, that were not evident in the β-catenin immuoprecipitates in Figure 1C, lane 1. Future studies will be directed toward identifying these proteins.
The Levels of Polycystin-1 Protein Are Similar in Normal Kidney and ADPKD Cells
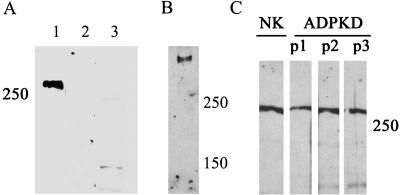
Cyst-lining ADPKD epithelia may continue to express polycystin-1 protein in situ without significant alteration in molecular weight (Ong et al., 1999; Rossetti et al., 2002). However, in light of other reports that ADPKD may result from a loss of heterozygosity and a loss of polycystin-1 expression (4), it was crucial to establish if polycystin-1 was expressed and/or significantly altered in our patient samples. Immunoblotting experiments performed with an antibody directed against polycystin-1 recognized a high-molecular-weight protein that was not detected by preimmune serum and blocked by preincubation with the antigen (Figure 2A). Using a 4% polyacrylamide gel and low current, we achieved greater separation of high-molecular-weight proteins by SDS-PAGE and could demonstrate that only a single protein with a molecular weight > 400,000 was detected with our antibody (Figure 2B). Immunoblot analyses of lysates prepared from primary normal kidney and ADPKD epithelia revealed similar expression levels of polycystin-1 in the normal and disease cells (Figure 2C). No significant alteration in the molecular weight of the proteins was noted, in agreement with previously published in situ data.
Figure 2.
Polycystin-1 expression levels are similar in normal kidney and ADPKD cells. (A, lanes 1-3) The specificity of our rabbit polyclonal antibody NM005 raised against a distal fragment of the polycystin-1 carboxy-terminus is demonstrated. Normal kidney cell lysates were immunoblotted with (1) NM005 antibody, directed against polycystin-1, (2) NM005 preincubated with antigen, and (3) preimmune serum. (B) The proteins from the normal kidney cell extract were separated on 4% polyacrylamide gel for 4 h, until only 150 and 250 MW prestained protein standard bands were left in the gel at the end of the electrophoresis. (C) Total cellular expression of polycystin-1 was evaluated in cell lysates prepared from the cells from normal kidney (NK) and three ADPKD patients (ADPKD P1, P2, and P3).
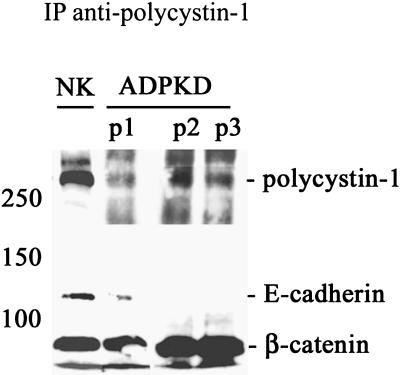
The Polycystin-1/E-cadherin/β-catenin Complex Is Disrupted in ADPKD Cells
The continued expression of polycystin-1 in the patient samples enabled us to address if the protein was still able to form functional complexes with the adherens junction proteins (Figure 3). Polycystin-1 protein complexes were evaluated by immunoprecipitation of cell lysates with our anti-polycystin-1 antibody. Very little or no E-cadherin was coimmunoprecipitated with polycystin-1 in experiments using ADPKD cell lysates from three different patients. In contrast, robust levels of E-cadherin were readily coprecipitated with polycystin-1 from normal kidney cell samples. These findings suggested that in ADPKD cells E-cadherin did not form a stable complex with polycystin-1. Interestingly, β-catenin remained associated with polycystin-1 in ADPKD cells.
Figure 3.
A normal multiprotein complex between polycystin-1, E-cadherin, and β-catenin is disrupted in ADPKD cells. Cell lysates prepared from normal kidney (NK) and three ADPKD patients (ADPKD P1, P2, and P3) were immunoprecipitated (IP) with our antipolycystin antibody under nondenaturing conditions. Immunoprecipitates were immunoblotted with antibodies directed against polycystin-1, E-cadherin, and β-catenin to determine expression of these proteins in the immunoprecipitates.
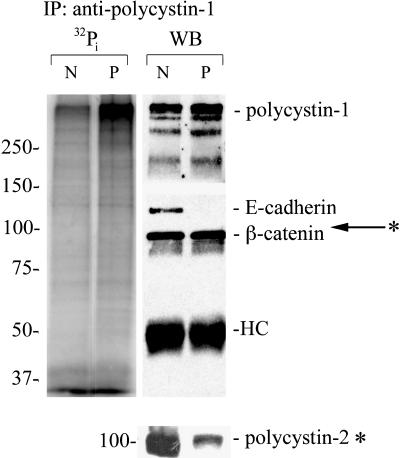
Polycystin-1 Is Highly Phosphorylated in ADPKD Cells
Both polycystin-1 and E-cadherin have been shown to be regulated by their phosphorylation status (Geng et al., 2000). Therefore, we tested whether or not changes in protein phosphorylation might contribute to their altered association. Cultured normal kidney and ADPKD cells were labeled with [32P]orthophosphate, and the cell lysates were immunoprecipitated with a specific antibody directed against polycystin-1. The phosphorylated proteins in the immunoprecipitates were identified by autoradiography (Figure 4, left lanes) and immunoblotting (Figure 4, right lanes). Phosphorylation of polycystin-1 was dramatically higher in ADPKD cells as compared with the normal kidney cells (Figure 4, left lanes). However, [32P]phosphate incorporation into other polycystin-1-associated proteins, including polycystin-2 and β-catenin, was not significantly different in the normal and disease cells. E-cadherin was associated with polycystin-1 only in the normal kidney cells, and its phosphorylation was not prominent. It is noteworthy that there also appeared to a be a lower recovery of polycystin-2 in the immunoprecipitates from the ADPKD than that in the normal control, suggesting that the normal association between polycystin-1 and -2 might also be compromised in ADPKD cells.
Figure 4.
Polycystin-1 is highly phosphorylated in ADPKD cells. The [32P]orthophosphate labeled samples (two left lanes) and unlabeled samples (right lanes) from normal kidney (N) and ADPKD (P) cells were immunoprecipitated (IP) with antipolycystin antibody under nondenaturing conditions. Immunoprecipitates were immunoblotted with antibodies directed against polycystin-1, polycystin-2, E-cadherin, and β-catenin to confirm their presence or absence in the immunoprecipitates and establish the identity of the 32P-labeled proteins. The relative migration of polycystin-2 is indicated with an arrow. Polycystin-2 was not significantly phosphorylated. Because polycystin-2 has a molecular weight just above beta-catenin this immunoblot is shown separately. HC, IgG heavy chain.
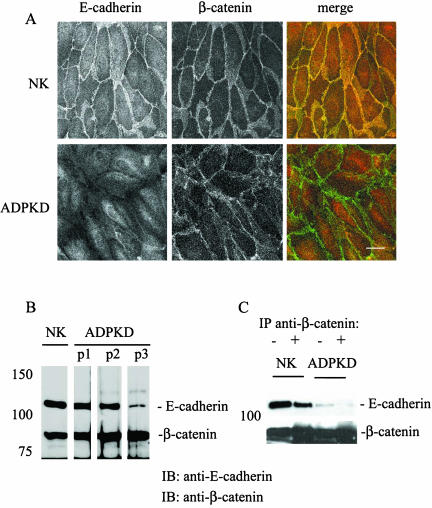
The Normal E-cadherin/β-catenin Complex Is Disrupted in ADPKD Cells
The previously described experiment showed that in ADPKD cells β-catenin remained in a complex with polycystin-1 in the absence of E-cadherin. These results raised questions as to the fate of the E-cadherin/β-catenin complex in ADPKD cells. Therefore, we compared the interaction of the two proteins in normal kidney and ADPKD primary epithelial cells within the adherens junction complex.
In normal kidney cells, both E-cadherin and β-catenin were expressed at the plasma membrane and colocalized at the lateral plasma membrane (Figure 5, top panels). Although E-cadherin was not expressed at the cell surface of ADPKD cells, β-catenin continued to outline cell borders of ADPKD cells analogous to normal kidney cells (Figure 5A, bottom panels). However, in ADPKD cells the plasma membrane β-catenin was notably less discretely linear and cytoplasmic staining was evident. No colocalization of E-cadherin and β-catenin was observed in ADPKD cells, even intracellularly (Figure 5A, bottom panel merge).
Figure 5.
E-cadherin is not expressed at the lateral plasma membrane and does not form a complex with β-catenin in ADPKD cells. (A) Confluent monolayers of primary normal kidney (NK) and ADPKD (patient 2) were immunostained for E-cadherin (red) and β-catenin (green). Samples were imaged on a Zeiss LSM510 (Thornwood, NY), and 0.5-μm sections are shown of each of the individual protein distributions (black and white images) as well as a merge (color) to show any colocalization in yellow. Bar, 20 μm. (B) Total cellular expression levels of E-cadherin and β-catenin were evaluated by immunoblotting samples from normal kidney (NK) and three ADPKD patients (ADPKD P1, P2, and P3). (C) The association of E-cadherin and β-catenin was monitored in confluent normal kidney (NK) and ADPKD cells by coimmunoprecipitation studies. Cell lysates prepared under nondenaturing conditions were immunoprecipitated with a specific mAb directed against β-catenin. Cell lysates (-) and immunoprecipitates (+) were immunoblotted (IB) with antibodies directed against E-cadherin and β-catenin.
The total cellular expression levels of E-cadherin in PKD cells were variable between individual ADPKD patient samples as reported previously (Charron et al., 2000a), whereas β-catenin levels were apparently invariant in normal and ADPKD cells (Figure 5B). E-cadherin was coimmunoprecipitated with β-catenin from normal kidney cell samples, but the two proteins were not coimmunoprecipitated from ADPKD cell samples (Figure 5C). Thus, both morphological and biochemical evaluations demonstrated that the E-cadherin/β-catenin complex was disrupted in ADPKD cells. The level of E-cadherin expression in ADPKD patient 3 was lower than in normal kidney cells (Figure 5B) and is likely due to the partial lysosomal degradation of the protein in the disease cells (Charron et al., 2000b).
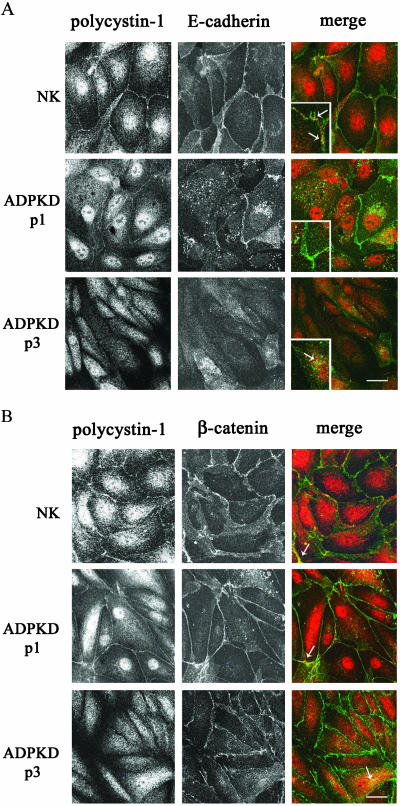
Plasma Membrane Expression of Polycystin-1 Is Diminished in ADPKD Cells
The observed interaction of polycystin-1 and β-catenin, even in the absence of cell surface E-cadherin, together with the continued cell surface expression of β-catenin raised the possibility that only E-cadherin was mislocalized in the disease cells. To address this issue, the subcellular localization of polycystin-1 relative to adherens junction proteins was evaluated in dual immunofluorescence staining experiments. Immunostaining for polycystin-1 showed that in normal kidney epithelial cells polycystin-1 was localized to the plasma membrane (Figure 6A, NK). A fraction of polycystin-1 was also consistently detected in the nucleus as has been observed by other investigators (Peters et al., 1999). In ADPKD cells from one of three patients polycystin-1 was faintly present at the plasma membrane; however, the polycystin-1 staining in these cells was more diffuse than that in normal kidney cells (Figure 6, A and B, ADPKD P1). In ADPKD cells from two other ADPKD patients, polycystin-1 was undetectable at the plasma membrane and instead was present in small intracellular vesicles under all conditions tested (Figure 6A, ADPKD P3; patient 2 is not shown). Interestingly, nuclear staining of polycystin-1 was most pronounced when the protein was detected at the plasma membrane, and nuclear staining was not evident when polycystin-1 exhibited a diffuse cytoplasmic localization. Thus, primary ADPKD cells exhibit a deficiency in their ability to express polycystin-1 at the plasma membrane and possibly in the nucleus.
Figure 6.
Comparison of polycystin-1, E-cadherin, and β-catenin localizations in normal kidney and ADPKD cells. Confluent normal kidney (NK) and ADPKD cells were double-labeled with polyclonal NM005 antibody directed against polycystin-1 and monoclonal antibodies directed against (A) E-cadherin and (B) β-catenin and imaged as in Figure 5. Shown are one NK sample and two ADPKD patient samples (P1 and P3). Black and white panels show individual localizations of polycystin-1, E-cadherin, and β-catenin as indicated. Dual staining is presented in the merged images where polycystin-1 is in the red channel, and (A) E-cadherin and (B) β-catenin are in the green channel. Colocalization sites of (A) polycystin-1 and E-cadherin and (B) polycystin-1 and β-catenin are seen as a yellow overlap pattern. Insets in the merged images (A) show a twofold enlarged detail of sites where the two proteins are colocalized. Bar, 20 μm.
In normal kidney cells polycystin-1 (red) and E-cadherin (green) were partially colocalized at the plasma membrane (Figure 6A, NK panels). Interestingly, the colocalization of the two proteins at the plasma membrane was not continuous, but was instead restricted to localized sites of cell-cell contacts (Figure 6A, NK, merge; see insets), further suggesting the polycystin-1/E-cadherin association may be regulatory in nature, rather than structural. In ADPKD patient 1, where polycystin-1 was weakly expressed at the plasma membrane, E-cadherin was largely sequestered in large cytoplasmic vesicles that are most likely endosomes (Le et al., 1999). In the occasional ADPKD cells where cell surface E-cadherin could still be observed, the staining appeared diffuse and disorganized and colocalization with polycystin-1 was not apparent (Figure 6A, ADPKD P1, merge inset). In the other two ADPKD patient samples neither polycystin-1 nor E-cadherin were expressed at the plasma membrane of the disease cells and only diffuse cytoplasmic expression was evident. Representative images from patient 3 are shown in Figure 6A, ADPKD P3 panels. Generally, little to no colocalization of the two proteins was seen in the cytoplasm, although in rare cells (usually in one cell per visual field) some intracellular colocalization of polycystin-1 and E-cadherin was observed (Figure 6A, ADPKD P3, merge inset).
Given the continued association of polycystin-1 with β-catenin observed in coimmunoprecipitation experiments, it was also of interest to examine the cellular localization of polycystin-1 relative to β-catenin. In normal kidney cells both polycystin-1 (red) and β-catenin (green) were very obviously colocalized at the plasma membrane (Figure 6B, NK panels). As was the case for E-cadherin, the colocalization was not continuous, but was seen in focal patches at sites of cell-cell contacts. The colocalization of the two proteins was more prominent than polycystin-1/E-cadherin colocalization (Figure 6B, NK, merge). In the ADPKD cells from patient 1 where polycystin-1 could still be detected at the plasma membrane, polycystin-1 exhibited some colocalization with β-catenin at the plasma membrane (Figure 6B, ADPKD P1 panels). Despite a noticeable increase in cytosolic β-catenin in the APDKD cells, an increase in nuclear β-catenin was not readily evident. However, this does not preclude the increased nuclear activity of β-catenin and would require further testing. Polycystin-1 and β-catenin were also partially colocalized intracellularly. In the absence of any polycystin-1 expression at the plasma membrane, as in patient 3, the β-catenin staining was more diffuse and was found colocalized with polycystin-1 in the cytoplasm of these cells (Figure 6B, ADPKD P3, merge). These data suggest there may be an important interaction between polycystin-1 and β-catenin that is distinct from the E-cadherin association.
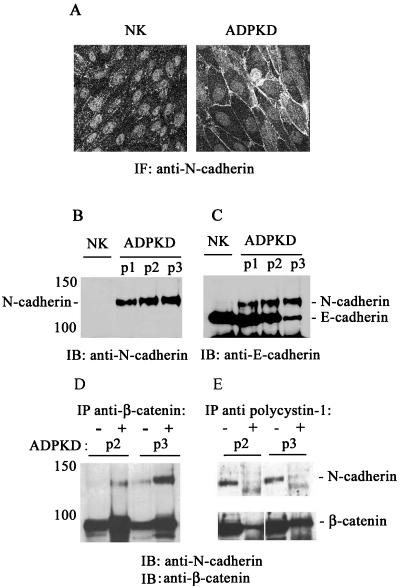
N-cadherin Replaces E-cadherin at the Plasma Membrane of ADPKD Cells and Is Not Significantly Associated with Polycystin-1
The observation that β-catenin was still expressed at the plasma membrane of disease cells, despite the depletion of E-cadherin from the membrane was quite surprising, because β-catenin is a cytosolic cadherin-associated protein and its plasma membrane localization requires association with an integral plasma membrane protein (usually a cadherin family member protein). Because polycystin-1 was also largely depleted from the plasma membrane of the disease cells, the observed association between β-catenin and polycystin-1 could not account for the plasma membrane disposition of β-catenin.
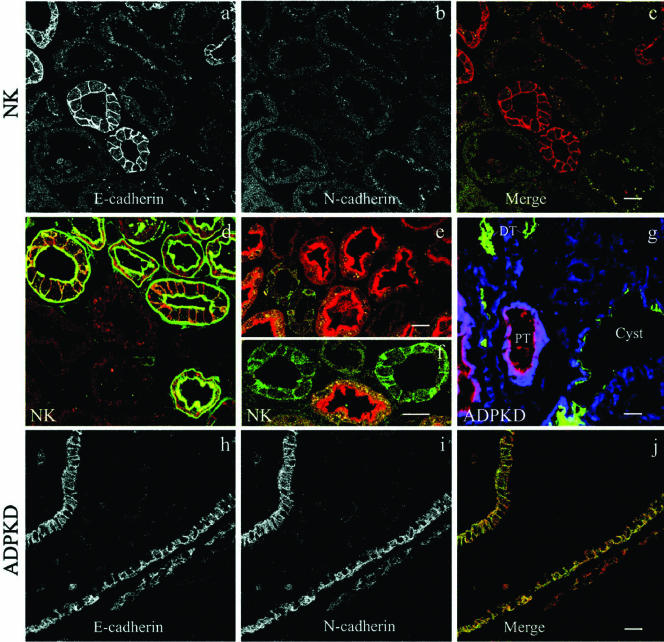
Immunofluorescence staining of the disease cells with a pan-cadherin antibody revealed the presence of an alternate cadherin family member at the plasma membrane (unpublished data). A series of immunostaining experiments were conducted to identify the alternate cadherin expressed in ADPKD cells. The kidney cadherin, K-cadherin/cadherin 6 was excluded because it was present at similarly low levels in both normal kidney and ADPKD cells (Charron et al., 2000b). Therefore, other candidates were considered with particular attention to N-cadherin. Switches in N-cadherin/E-cadherin expression occur during normal embryonic development and tissue morphogenesis (Vleminckx and Kemler, 1999; Angst et al., 2001; Pla et al., 2001) as well as in disease states such as oncogenic cell dedifferentiation (Islam et al., 1996; Tomita et al., 2000; Wheelock et al., 2001; Cavallaro et al., 2002). ADPKD cells lacking plasma membrane E-cadherin exhibited strong plasma membrane immunostaining with an antibody specific for N-cadherin (Figure 7A, right panel).
Figure 7.
N-cadherin replaces E-cadherin at the plasma membrane of ADPKD cells and forms a complex with β-catenin. (A) N-cadherin was visualized by immunofluorescence staining of normal kidney (NK) and ADPKD cells using a mouse mAb directed against N-cadherin. Bar, 20 μm. (B) The total cellular expression of N-cadherin was analyzed in normal kidney cell lysates (NK) and cell lysates from three ADPKD patients (ADPKD P1, P2, and P3) by immunoblotting (IB). (C) The same blot was subsequently probed with an antibody directed against E-cadherin to reveal the total levels of E-cadherin expression in the same samples. (D and E) The presence of N-cadherin/β-catenin complexes and their association with polycystin-1 were assessed by coimmunoprecipitation experiments. Cell lysates from two ADPKD patients (ADPKD P2, P3) were immunoprecipitated (IP) with an antibody directed against (D) β-catenin and (E) against polycystin-1. The cell lysates (-) and immunoprecipitates (+) were immunoblotted (IB) with a polyclonal antibody directed against N-cadherin and subsequently immunoblotted with an antibody directed against β-catenin.
In normal kidney epithelial cells N-cadherin was expressed at low abundance and not detected at the plasma membrane (Figure 7A, left panel). Immunoblot analysis revealed significant N-cadherin expression in ADPKD cells, whereas N-cadherin expression in normal kidney cells was very low (Figure 7B), in agreement with the immunofluorescence staining data. N-cadherin expression increased proportionately as the levels of E-cadherin decreased in ADPKD cells (Figure 7C). Immunoprecipitation experiments revealed that N-cadherin was significantly coimmunoprecipitated with β-catenin from ADPKD cell lysates (Figure 6D), indicating that the two proteins form a complex in ADPKD cells and explaining the continued presence of β-catenin at the plasma membrane of disease cells, despite the depletion of plasma membrane E-cadherin and polycystin-1.
Given the existence of an N-cadherin/β-catenin complex in ADPKD cells, we evaluated whether polycystin-1 might be associated with this complex in ADPKD cells. Polycystin-1 was consistently detected in a complex with β-catenin (see also data in Figure 3B), but was not apparently associated with mature N-cadherin in the ADPKD cell lysates (Figure 7E). A faint lower-molecular weight band seen in the immunoprecipitates (Figure 7E, + lanes) may represent an association with an extremely minor pool of nonglycosylated (Balsamo and Lilien, 1990) or nonphosphorylated (Wahl et al., 2003) newly synthesized N-cadherin. However, the higher-molecular-weight, cell surface-expressed mature form of N-cadherin, which predominated in cell lysates (Figure 7E, - lanes), was clearly not coimmunoprecipitated with polycystin-1. Immunofluorescence experiments also did not show any significant colocalization between polycystin-1 and N-cadherin in ADPKD cells (unpublished data). Thus, in ADPKD cells N-cadherin replaces E-cadherin at the plasma membrane and forms a complex with β-catenin, but is not significantly associated with polycystin-1 at the cell surface. Whether or not there is a minor intracellular pool of N-cadherin associated with polycystin-1 and its significance remains open for future investigation.
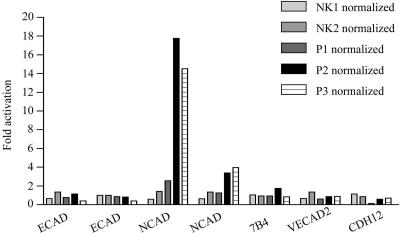
N-cadherin RNA Expression Is Increased in ADPKD Cells
Microarray assays were used to determine the differences in gene expression profiles in the normal kidney epithelial cells and the cells from ADPKD patients. The cells from two different kidney samples and three ADPKD patients (P1, P2, and P3) were analyzed. RNA was prepared and used for the synthesis of hybridization probes, which were hybridized to Affimetrix Gene Chips in the UNM KUGR facility. The data were analyzed using GeneSpring, enabling parallel evaluation of the RNA expression levels for E-cadherin, N-cadherin, vascular epithelial cadherin 7B4, vascular epithelial cadherin 2 (or protocadherin) VECAD2, and neuronal N-cadherin precursor protein CDH12 in normal vs. ADPKD samples. N-cadherin RNA expression was significantly upregulated in ADPKD cells compared with normal kidney cells (Figure 8), suggesting that the increased protein expression observed biochemically and by immunofluorescence results from changes in gene expression. The RNA expression levels for E-cadherin as well as for several other cadherins was not significantly different in all samples examined. This is consistent with our previous findings that the biosynthetic rate of E-cadherin was similar in normal and ADPKD cells with primarily the de novo insertion and plasma membrane stability of the protein being altered in the disease cells (Charron et al., 2000b).
Figure 8.
The level of N-cadherin RNA expression is significantly elevated in ADPKD cells. Total RNA was prepared from the cultured primary epithelial cells from two different normal kidney (NK1 and NK2) and three ADPKD samples (P1, P2, and P3) and used for hybridization to Affymetrix Gene Chips. The data were normalized against the median value of the normal kidney samples. The fold RNA expression values were plotted using Prism Software. Plotted are E-cadherin (ECAD), N-cadherin (NCAD), vascular epithelial cadherin (7B4), vascular epithelial cadherin 2 or protocadherin (VECAD2), and neuronal N-cadherin precursor protein (CDH12). The hybridization to two different E- and N-cadherin oligonucleotides on the chips is graphed separately.
ADPKD Cyst-lining Epithelia Express Both E- and N-cadherin In Situ
Immunofluorescence staining of normal and cystic kidney tissue slices was performed to analyze the E- and N-cadherin expression in normal kidney tubules and cysts. As reported by a number of authors (Leussink et al., 2001; Dahl et al., 2002) and shown in our experiments; the expression of these two adherens junction proteins in normal kidney epithelia is quite distinct and restricted to specific parts of the nephron (see Figure 9 below). Proximal and distal tubule markers were used to distinguish the origin of primary kidney epithelial cells, tubules, and cysts.
Figure 9.
ADPKD cysts express both N- and E-cadherin. Tissue sections of (a-f) normal kidney cortex and (g-j) ADPKD cystic tissue were double- or triple-labeled with (a, c, d-f, h, and j) polyclonal antibodies directed against E-cadherin, (b, c, g, i, and j) a mAb against N-cadherin, and (d-g) tubule-specific lectins. (a-c) E-cadherin (red) and N-cadherin (green) are expressed in distinct normal kidney tubules. (d) E-cadherin (red) is colocalized with peanut lectin (green) specific for distal parts of the nephron (distal tubules and collecting duct). (e and f) E-cadherin (green) exhibits a distinct distribution from asparagus lectin (red), a marker for the proximal tubules. (g) N-cadherin is overexpressed in early cysts. ADPKD tissue triple-labeled with an mAb directed against N-cadherin (blue), with lectins for proximal tubules (PT, red) and distal tubules (DT, green). (h-j) E-cadherin (red) and N-cadherin (green) exhibit overlapping distributions in ADPKD cysts. Bars, 20 μm.
In the normal kidney cortex E-cadherin was exclusively expressed in distal segments of the nephron (Figure 9, a-f). The distal tubules were definitively identified by their reactivity with FITC-labeled peanut lectin (Figure 9d), whereas proximal tubules were identified by the strong brush border staining produced by TRITC-labeled asparagus lectin (Figure 9, e and f). The E-cadherin was stained with TRITC- or FITC-conjugated secondary antibodies to determine its colocalization with the lectin probes (Figure 9, d-f). The E-cadherin was clearly arrayed along the basolateral membranes of only distal tubules, as evidenced by the sharp outlining of each cell in the tubule. N-cadherin (Figure 9, a-c) and K-cadherin/cadherin-6 (unpublished data) exhibited weak expression limited to proximal tubules. Neither N-cadherin nor K-cadherin were ever seen at the plasma membrane of the proximal tubules and only diffuse or punctate intracellular staining was observed (Figure 9b).
Immunofluorescence staining of cystic kidney tissue from ADPKD patient showed an abundance of cysts of distal tubule or collecting duct origin based on their reactivity with peanut lectin (green; Figure 9g and supplementary material Figure 1). Proximal tubule derived cysts positive for asparagus lectin (red) were observed only occasionally, possibly because of the destruction of the proximal tubule epithelial brush border. Based on lectin staining of our primary epithelial, both our normal and ADPKD cells were of distal nephron origin (supplementary material).
Analyses of the cadherin distributions in ADPKD tissue revealed that, unlike the normal kidney tubules, the cystlining epithelia simultaneously expressed both E- and N-cadherin (Figure 9, h-j). These results are in agreement with our biochemical experiments showing both E-cadherin and N-cadherin reciprocally expressed in ADPKD cells (Figure 7, B and C). N-cadherin expression levels in cysts were markedly increased relative to the normal kidney tubule samples, and N-cadherin was plasma membrane associated, though an intracellular pool was also apparent (compare Figure 9i to 9b). E-cadherin was only partially plasma membrane localized. Significant intracellular staining was evident by the fuzzy appearance of the cell borders compared with the sharp lateral cell staining seen in the normal kidney samples (contrast Figure 9, a and h). Thus, the observed expression of N-cadherin with decreased E-cadherin expression in the primary ADPKD cells (Figure 7) accurately reflects what is observed in cyst lining epithelia in situ.
The apparent switch in cadherin expression during the ADPKD cyst development or progression is apparent in Figure 9g. In this ADPKD kidney section, noncystic distal tubules (DT, green) are negative for N-cadherin (blue). However, even in relatively small cysts of distal tubule origin (green), the epithelium is positive for N-cadherin expression (blue). Two proximal tubules (PT, red) displayed stronger N-cadherin staining than observed in the normal kidney sample and may represent small proximal tubule-derived cysts at an early stage of cyst formation. At later stages such proximal tubule-derived cysts are difficult to detect and may reflect the loss of such cysts during disease progression. The cumulative results show that cystic epithelia of distal origin reexpress N-cadherin together with E-cadherin even at early stages of cyst formation. The expression of N-cadherin in ADPKD cysts is also demonstrated in Figure 2 of the supplementary material. Thus, the loss of normal cadherin expression in ADPKD cyst-lining epithelia appears to be an important event in disease pathogenesis and likely contributes to impaired effectiveness of cell-cell adhesion.
DISCUSSION
The present study identified a disruption of the normal polycystin-1 complex with the adherens junction proteins, E-cadherin and β-catenin, in primary kidney epithelial cells from ADPKD patients. Polycystin-1 was largely absent from the plasma membrane of ADPKD cells, as was E-cadherin, and the two proteins were not associated in the disease cells. Dramatically increased phosphorylation of polycystin-1 in the disease cells most likely reflects changes in intracellular signaling cascades and suggests that the protein complex might be regulated by changes in phosphorylation. Quite unexpectedly, a significant fraction of the β-catenin remained at the cell surface because of an interaction with N-cadherin expressed at the plasma membrane of ADPKD cells and cyst-lining epithelium in situ. N-cadherin could not apparently substitute for E-cadherin and failed to complex with polycystin-1. Increased N-cadherin expression was transcriptionally regulated, whereas E-cadherin was modulated at the protein level. Thus, ADPKD kidney epithelial cells are typified by a transition toward a partially dedifferentiated state, in part characterized by reexpression of mesenchymal N-cadherin. We suggest the expression of mutant polycystin-1 in ADPKD cells, which fails to be cell surface-expressed and assemble into multiprotein complexes with E-cadherin, results in altered cell-cell adhesion and polycystin-1 signaling, thereby triggering dedifferentiation.
Loss of Plasma Membrane Polycystin-1 in ADPKD Cells and Cysts
It is of interest to consider the possible causes contributing to the loss of plasma membrane localized polycystin-1 in ADPKD cells and cysts. In our biochemical analyses, polycystin-1 protein expression levels were remarkably similar in both normal kidney cells and primary ADPKD cells from several patients and there was no evidence of any obvious molecular weight change in the disease cells. These data concur with in situ studies of polycystin-1 protein expression in cysts (Ward et al., 1996). Sequence analysis the PKD1 and PKD2 alleles in our primary ADPKD cells is in progress. Sequencing of the complete PKD2 gene (except for exon 1) revealed no mutations. Sequencing of nearly two thirds of the PKD1 gene (exons 2-11, 15-21, and 33-46) in two of the three patient samples (P1 and P2) and one quarter of the PKD1 gene (exons 15-21) in the third patient sample (P3) has not yet identified disease-specific mutations. Because of the complexity of the undertaking and the fact that no mutations are found in 30% of all patients screened, complete molecular analyses will require more detailed study. All together the data support the postulate that secondary mutations in the remaining normal PKD1 allele, thought to incite cyst formation in ADPKD, frequently do not lead to loss of polycystin-1 protein or gross changes in the expressed protein. Therefore, the loss of plasma membrane polycystin-1 reflects changes in protein localization and function rather than changes in expression level.
The suggestion that changes in polycystin-1 localization are closely coupled to disease status is further supported by the published literature. For example in an in vitro model, polycystin-1 was cell surface localized in MDCK cells during tubulogenesis, but entirely intracellular under conditions promoting cystogenesis (Bukanov et al., 2002). The loss of polycystin-1 cell surface expression precipitated by its intracellular accumulation has also been implicated to contribute to tuberous sclerosis, another renal cystic disorder (Kleymenova et al., 2001; Kugoh et al., 2002). Thus, we propose that the loss of cell surface polycystin-1 expression in ADPKD cells, possibly precipitated by changes in its phosphorylation status, serves as an important trigger in altered epithelial cell differentiation and morphology.
Hyperphosphorylation of Polycystin-1 and Impaired Plasma Membrane Expression of E-cadherin in ADPKD Cells
Increased phosphorylation of polycystin-1 in ADPKD cells suggests that the complexes between polycystin-1 and its associated proteins may be disrupted through altered phosphorylation. It is well known that protein-protein interactions are very often dependent on the phosphorylation status of the complexed proteins. In the case of the adherens junction proteins, tyrosine phosphorylation leads to a loss of β-catenin/E-cadherin association and decreases adhesion efficiency, whereas dephosphorylation of β-catenin increases cadherin-mediated cell-cell adhesion (Taddei et al., 2002). Phosphotyrosine immunoblots failed to reveal any significant differences in E-cadherin or β-catenin phosphorylation status in normal kidney compared with ADPKD cells (unpublished data), suggesting this was not the cause of the observed changes in E-cadherin-mediated adhesion. Instead, polycystin-1 proved to be hyperphosphorylated in ADPKD cells. Polycystin-1 multiprotein complexes are known to be modified by tyrosine phosphorylation, with inhibition of phosphorylation resulting in enhanced polycystin-1/E-cadherin association (Geng et al., 2000). Taken together, it appears likely that hyperphosphorylated state of polycystin-1 contributes to the disruption of the polycystin-1/E-cadherin complex in ADPKD cells.
Increased phosphorylation of polycystin-1 in ADPKD cells could also account for the observed decrease in its association with polycystin-2 and would be expected to impact calcium flux across the plasma membrane. Polycystin-1 is well known to form a complex with polycystin-2 and thus modulate and stabilize polycystin-2 calcium-permeable channel activity (Tsiokas et al., 1997; Xu et al., 2003). The conductivity of a number of other ion channels (such as CFTR Cl-, Ca2+, and ROMK1) is regulated and often inhibited by the phosphorylation (Barbar et al., 2003; Raghuram et al., 2003; Zeng et al., 2003). Consequently, we speculate that diminished or impaired Ca2+ influx via polcystin-1/2 channel could result as a consequence of polycystin-1 hyperphosphorylation and/or the disruption of their association in ADPKD cells.
The loss of cell surface E-cadherin expression in ADPKD cells is most likely closely linked to failed plasma membrane stabilization rather than to problems with expression or de novo insertion. In the present study we show that E-cadherin RNA levels are not dramatically different in normal and ADPKD cells. This finding agrees with our previous work where it was shown that E-cadherin biosynthesis rates and catenin assembly were relatively normal, whereas there was a twofold reduction in plasma membrane delivery of newly synthesized molecules (Charron et al., 2000b). Reduced delivery alone cannot account for the complete depletion of cell surface E-cadherin observed at a steady state. Therefore it is interesting that E-cadherin in ADPKD cells was noticeably accumulated in punctate endosomal-like structures, distinct from the Golgi complex. Internalization and recycling of E-cadherin is known to influence plasma membrane expression levels and has emerged as a tightly regulated process (Le et al., 1999; Fujita et al., 2002).
E-cadherin stability at the plasma membrane may also depend on appropriate local calcium concentrations or calcium-dependent signaling dictated by proper polycystin-1/polycystin-2 association and channel function. The importance of calcium is supported by the observation that high exogenous calcium promotes the association of E-cadherin and polycystin-1 in human collecting tubule cells (Geng et al., 2000). Thus, alterations in E-cadherin stabilization may be set in motion by changes in the plasma membrane distribution of polycystin-1 that in turn impact polycystin-2 association and calcium channel activity. Therefore, analyses of how the phosphorylation status and Ca2+ channel activity of the polycystins impact E-cadherin recycling are considered important topics for further study.
Replacement of E-cadherin by N-cadherin in ADPKD Cells and in Cysts
The loss of E-cadherin was offset by an induction of N-cadherin gene and protein expression in ADPKD cells, causing β-catenin to be largely retained at the plasma membrane. ADPKD patient P3 was somewhat exceptional in this regard, exhibiting both cell surface and cytoplasmic expression of β-catenin. The persistent association of a fraction of β-catenin with polycystin-1 even in the disease cells implies that their interaction may be independent of E-cadherin, and it will be important to examine whether or not the polycystin-1/β-catenin complex has any role in regulating proliferation. In general, it appears that retention of the large β-catenin pool at the plasma membrane, through an association with N-cadherin, may slow ADPKD progression and explain the continued retention of a partially polarized phenotype. The loss of plasma membrane polycystin-1 and E-cadherin with continued plasma membrane retention of β-catenin reported here for ADPKD cells is analogous to findings obtained using a rat model of tuberous sclerosis, another cystic kidney disease (Kugoh et al., 2002). However, in this case N-cadherin expression was not examined. In tuberous sclerosis defects in trafficking machinery components result in the intracellular retention of polycystin-1 in the Golgi complex (Kleymenova et al., 2001). Taken together the data stress the close coupling between the membrane localization of polycystin-1 and E-cadherin in the maintenance of proper renal epithelial cell function and polarity.
The upregulation and expression of mesenchymal N-cadherin in ADPKD cells both in vivo and in situ supports the growing consensus that ADPKD is associated with a dedifferentiated phenotype (Calvet, 1998). Increased N-cadherin expression is transcriptionally regulated and may be a compensatory event resulting either directly from the decreased E-cadherin plasma membrane expression, or later, as a consequence of the partial cell depolarization/dedifferentiation. N-cadherin overexpression shifts the balance away from E-cadherin expression, which is critical for the proper tubular morphology and function. It also reflects a reversal of the normal process of mesenchyme-to-epithelium transition during kidney organogenesis (Horster et al., 1999), during which time the mesenchymal N-cadherin is replaced by E-cadherin. An imbalance in cadherins is associated with increased epithelial cell motility, because of a preference for forming homophilic cadherin interactions despite any obvious differences in the binding affinities of heterotypic complexes (Niessen and Gumbiner, 2002). Thus, the abnormal cells expressing both N- and E-cadherin may be sorted out from the normal E-cadherin-expressing cells on account of an increased proliferative and migratory potential, thereby resulting in the formation of an abnormal tubule and contributing to cyst formation.
SUMMARY
The present study establishes a correlation between the expression of wild-type polycystin-1 and the formation of stable E-cadherin-based adherens junction complexes in kidney epithelial cells. The interaction between normal polycystin-1 and adherens junction components is likely regulatory in nature, with mutant forms of polycystin-1 disrupting the normal maintenance of epithelial polarity in ADPKD cells through alterations in polycystin-1-phosphorylation, localization, and association with polycystin-2. The inadequate assembly and stabilization of E-cadherin-containing adherens junctions and reexpression of N-cadherin consequently results in impairment of cell-cell adhesion in the kidney epithelia that may contribute directly to cyst formation.
Supplementary Material
Acknowledgments
We are indebted to the anonymous patients and their families who donated tissue to make this research possible. We gratefully acknowledge Elsa Romero and Melanie Lenhart for expert technical assistance and Valorie Bivins for administrative support. We thank Dr. Rebecca Lee for expert technical support in the maintenance and operation of the Cancer Center Microscopy Facility. The gene expression experiments were performed in the Keck-UNM Genomics Resource, a facility supported by a grant from the W. M. Keck Foundation as well as State of New Mexico and University of New Mexico Cancer Research and Treatment Center, with the expert assistance of Marilee Morgan. Images in this article were generated in the Fluorescence Microscopy Facility, which received support from National Center for Research Resources Grants P20 RR11830, S10 RR14668, and S10 RR016918; National Science Foundation Grants MCB9982161; National Cancer Institute Grant R24 CA88339, the University of New Mexico Health Sciences Center, and the University of New Mexico Cancer Center. We acknowledge NDRI for normal human kidney tissue. This work was supported by National Institute for Diabetes, Digestive and Kidney Diseases Grants R01 50141, Polycystic Kidney Diseases Foundation Grant 12(A-C) 2R, and American Heart Association Grant 0040211N to A.W.N. and subcontracts to R.L.B. T.R. is supported by a National Kidney Foundation Fellowship F758.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03-05-0296. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0296.
Abbreviations used: ADPKD, autosomal polycystic kidney disease; NK, normal kidney; PKD1, polycystic kidney disease locus 1.
Online version of this article contains supplementary materials. Online version is available at www.molbiolcell.org.
References
- Angst, B.D., Marcozzi, C., and Magee, A.I. (2001). The cadherin superfamily: diversity in form and function. J. Cell Sci. 114, 629-641. [DOI] [PubMed] [Google Scholar]
- Arnaout, M.A. (2001). Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu. Rev. Med. 52, 93-123. [DOI] [PubMed] [Google Scholar]
- Arnould, T., Kim, E., Tsiokas, L., Jochimsen, F., Gruning, W., Chang, J.D., and Walz, G. (1998). The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273, 6013-6018. [DOI] [PubMed] [Google Scholar]
- Balsamo, J., and Lilien, J. (1990). N-cadherin is stably associated with and is an acceptor for a cell surface N-acetylgalactosaminylphosphotransferase. J. Biol. Chem. 265, 2923-2928. [PubMed] [Google Scholar]
- Barbar, E., Rola-Pleszczynski, M., Payet, M.D., and Dupuis, G. (2003). Protein kinase C inhibits the transplasma membrane influx of Ca2+ triggered by 4-aminopyridine in Jurkat T lymphocytes. Biochim. Biophys. Acta 1622, 89-98. [DOI] [PubMed] [Google Scholar]
- Bhunia, A.K., Piontek, K., Boletta, A., Liu, L., Qian, F., Xu, P.N., Germino, F.J., and Germino, G.G. (2002). PKD1 induces p21(waf1) and regulation of the cell cycle via direct activation of the JAK-STAT signaling pathway in a process requiring PKD2. Cell 109, 157-168. [DOI] [PubMed] [Google Scholar]
- Bukanov, O.N., Husson, H., Dackowski, W.R., Lawrence, B.D., Clow, P.A., Roberts, B.L., Klinger, K.W., and Ibraghimov-Beskrovnaya, O. (2002). Functional polycystin-1 expression is developmentally regulated during epithelial morphogenesis in vitro: downregulation and loss of membrane localization during cystogenesis. Hum. Mol. Genet. 11, 923-936. [DOI] [PubMed] [Google Scholar]
- Calvet, J.P. (1998). Molecular genetics of polycystic kidney disease. J. Nephrol. 11, 24-34. [PubMed] [Google Scholar]
- Carone, F.A., Nakamura, S., Schumacher, B.S., Punyarit, P., and Bauer, K.D. (1989). Cyst-derived cells do not exhibit accelerated growth or features of transformed cells in vitro. Kidney Int. 35, 1351-1357. [DOI] [PubMed] [Google Scholar]
- Cavallaro, U., Schaffhauser, B., and Christofori, G. (2002). Cadherins and the tumour progression: is it all in a switch? Cancer Lett. 176, 123-128. [DOI] [PubMed] [Google Scholar]
- Charron, A.J., Bacallao, R.L., and Wandinger-Ness, A. (2000a). ADPKD: a human disease altering Golgi function and basolateral exocytosis in renal epithelia. Traffic 1, 675-686. [DOI] [PubMed] [Google Scholar]
- Charron, A.J., Nakamura, S., Bacallao, R., and Wandinger-Ness, A. (2000b). Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J. Cell Biol. 149, 111-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consortium, I.P.K.D. (1995). Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell 81, 289-298. [DOI] [PubMed] [Google Scholar]
- Dahl, U., Sjodin, A., Larue, L., Radice, G.L., Cajander, S., Takeichi, M., Kemler, R., and Semb, H. (2002). Genetic dissection of cadherin function during nephrogenesis. Mol. Cell. Biol. 22, 1474-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita, Y., Krause, G., Scheffner, M., Zechner, D., Leddy, H.E., Behrens, J., Sommer, T., and Birchmeier, W. (2002). Hakai, a c-Cbl-like protein, ubiquitinates and induces endocytosis of the E-cadherin complex. Nat. Cell. Biol. 4, 222-231. [DOI] [PubMed] [Google Scholar]
- Gallagher, A.R., Obermuller, N., Cedzich, A., Gretz, N., and Witzgall, R. (2000). An ever-expanding story of cyst formation. Cell Tissue Res. 300, 361-371. [DOI] [PubMed] [Google Scholar]
- Geng, L., Burrow, C.R., Li, H.P., and Wilson, P.D. (2000). Modification of the composition of polycystin-1 multiprotein complexes by calcium and tyrosine phosphorylation. Biochim. Biophys. Acta 1535, 21-35. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Perret, S. et al. (2001). Polycystin-2, the protein mutated in autosomal dominant polycystic kidney disease (ADPKD), is a Ca2+-permeable nonselective cation channel. Proc. Natl. Acad. Sci. USA 98, 1182-1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardi, C.J., Wong, E., and Gumbiner, B.M. (2001). E-cadherin suppresses cellular transformation by inhibiting beta-catenin signaling in an adhesion-independent manner. J. Cell Biol. 153, 1049-1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B.M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345-357. [DOI] [PubMed] [Google Scholar]
- Hanaoka, K., Qian, F., Boletta, A., Bhunia, A.K., Piontek, K., Tsiokas, L., Sukhatme, V.P., Guggino, W.B., and Germino, G.G. (2000). Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature 408, 990-994. [DOI] [PubMed] [Google Scholar]
- Horster, M.F., Braun, G.S., and Huber, S.M. (1999). Embryonic renal epithelia: induction, nephrogenesis, and cell differentiation. Physiol. Rev. 79, 1157-1191. [DOI] [PubMed] [Google Scholar]
- Huan, Y., and van Adelsberg, J. (1999). Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J. Clin. Invest. 104, 1459-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes, J., Ward, C.J., Peral, B., Aspinwall, R., Clark, K., San Millan, J.L., Gamble, V., and Harris, P.C. (1995). The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat. Genet. 10, 151-160. [DOI] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya, O. et al. (1997). Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc. Natl. Acad. Sci. USA 94, 6397-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam, S., Carey, T.E., Wolf, G.T., Wheelock, M.J., and Johnson, K.R. (1996). Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J. Cell Biol. 135, 1643-1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, E., Arnould, T., Sellin, L.K., Benzing, T., Fan, M.J., Gruning, W., Sokol, S.Y., Drummond, I., and Walz, G. (1999). The polycystic kidney disease 1 gene product modulates Wnt signaling. J. Biol. Chem. 274, 4947-4953. [DOI] [PubMed] [Google Scholar]
- Kleymenova, E., Ibraghimov-Beskrovnaya, O., Kugoh, H., Everitt, J., Xu, H., Kiguchi, K., Landes, G., Harris, P., and Walker, C. (2001). Tuberin-dependent membrane localization of polycystin-1: a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol. Cell 7, 823-832. [DOI] [PubMed] [Google Scholar]
- Kugoh, H., Kleymenova, E., and Walker, C.L. (2002). Retention of membrane-localized beta-catenin in cells lacking functional polycystin-1 and tuberin. Mol. Carcinog. 33, 131-136. [DOI] [PubMed] [Google Scholar]
- Le, T.L., Yap, A.S., and Stow, J.L. (1999). Recycling of E-cadherin: a potential mechanism for regulating cadherin dynamics. J. Cell Biol. 146, 219-232. [PMC free article] [PubMed] [Google Scholar]
- Leussink, B.T., Litvinov, S.V., de Heer, E., Slikkerveer, A., van der Voet, G.B., Bruijn, J.A., and de Wolff, F.A. (2001). Loss of homotypic epithelial cell adhesion by selective N-cadherin displacement in bismuth nephrotoxicity. Toxicol. Appl. Pharmacol. 175, 54-59. [DOI] [PubMed] [Google Scholar]
- Li, H.P., Geng, L., Burrow, C.R., and Wilson, P.D. (1999). Identification of phosphorylation sites in the PKD1-encoded protein C-terminal domain. Biochem. Biophys. Res. Commun. 259, 356-363. [DOI] [PubMed] [Google Scholar]
- Nickel, C., Benzing, T., Sellin, L., Gerke, P., Karihaloo, A., Liu, Z.X., Cantley, L.G., and Walz, G. (2002). The polycystin-1 C-terminal fragment triggers branching morphogenesis and migration of tubular kidney epithelial cells. J. Clin. Invest. 109, 481-489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen, C.M., and Gumbiner, B.M. (2002). Cadherin-mediated cell sorting not determined by binding or adhesion specificity. J. Cell Biol. 156, 389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ong, A.C., Harris, P.C., Davies, D.R., Pritchard, L., Rossetti, S., Biddolph, S., Vaux, D.J., Migone, N., and Ward, C.J. (1999). Polycystin-1 expression in PKD1, early-onset PKD1, and TSC2/PKD1 cystic tissue. Kidney Int. 56, 1324-1333. [DOI] [PubMed] [Google Scholar]
- Parnell, S.C., Magenheimer, B.S., Maser, R.L., Rankin, C.A., Smine, A., Okamoto, T., and Calvet, J.P. (1998). The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem. Biophys. Res. Commun. 251, 625-631. [DOI] [PubMed] [Google Scholar]
- Peters, D.J., van de Wal, A., Spruit, L., Saris, J.J., Breuning, M.H., Bruijn, J.A., and de Heer, E. (1999). Cellular localization and tissue distribution of polycystin-1. J. Pathol. 188, 439-446. [DOI] [PubMed] [Google Scholar]
- Pla, P., Moore, R., Morali, O.G., Grille, S., Martinozzi, S., Delmas, V., and Larue, L. (2001). Cadherins in neural crest cell development and transformation. J. Cell. Physiol. 189, 121-132. [DOI] [PubMed] [Google Scholar]
- Polakis, P. (1999). The oncogenic activation of beta-catenin. Curr. Opin. Genet. Dev. 9, 15-21. [DOI] [PubMed] [Google Scholar]
- Raghuram, V., Hormuth, H., and Foskett, J.K. (2003). A kinase-regulated mechanism controls CFTR channel gating by disrupting bivalent PDZ domain interactions. Proc. Natl. Acad. Sci. USA 100, 9620-9625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti, S. et al. (2002). The position of the polycystic kidney disease 1 (PKD1) gene mutation correlates with the severity of renal disease. J. Am. Soc. Nephrol. 13, 1230-1237. [DOI] [PubMed] [Google Scholar]
- Saadi-Kheddouci, S., Berrebi, D., Romagnolo, B., Cluzeaud, F., Peuchmaur, M., Kahn, A., Vandewalle, A., and Perret, C. (2001). Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20, 5972-5981. [DOI] [PubMed] [Google Scholar]
- Simcha, I., Kirkpatrick, C., Sadot, E., Shtutman, M., Polevoy, G., Geiger, B., Peifer, M., and Ben-Ze'ev, A. (2001). Cadherin sequences that inhibit beta-catenin signaling: a study in yeast and mammalian cells. Mol. Biol. Cell 12, 1177-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St. Amand, A.L., and Klymkowsky, M.W. (2001). Cadherins and catenins, Wnts and SOXs: embryonic patterning in Xenopus. Int. Rev. Cytol. 203, 291-355. [DOI] [PubMed] [Google Scholar]
- Stockinger, A., Eger, A., Wolf, J., Beug, H., and Foisner, R. (2001). E-cadherin regulates cell growth by modulating proliferation-dependent beta-catenin transcriptional activity. J. Cell Biol. 154, 1185-1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutters, M., Yamaguchi, T., Maser, R.L., Magenheimer, B.S., St. John, P.L., Abrahamson, D.R., Grantham, J.J., and Calvet, J.P. (2001). Polycystin-1 transforms the cAMP growth-responsive phenotype of M-1 cells. Kidney Int. 60, 484-494. [DOI] [PubMed] [Google Scholar]
- Taddei, M.L. et al. (2002). Beta-catenin interacts with low-molecular-weight protein tyrosine phosphatase leading to cadherin-mediated cell-cell adhesion increase. Cancer Res. 62, 6489-6499. [PubMed] [Google Scholar]
- Tomita, K., van Bokhoven, A., van Leenders, G.J., Ruijter, E.T., Jansen, C.F., Bussemakers, M.J., and Schalken, J.A. (2000). Cadherin switching in human prostate cancer progression. Cancer Res. 60, 3650-3654. [PubMed] [Google Scholar]
- Tsiokas, L., Kim, E., Arnould, T., Sukhatme, V.P., and Walz, G. (1997). Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc. Natl. Acad. Sci. USA 94, 6965-6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Adelsberg, J. (2000). Polycystin-1 interacts with E-cadherin and the catenins—clues to the pathogenesis of cyst formation in ADPKD? Nephrol. Dial. Transplant. 15, 1-2. [DOI] [PubMed] [Google Scholar]
- Van Adelsberg, J., Chamberlain, S., and D'Agati, V. (1997). Polycystin expression is temporally and spatially regulated during renal development. Am. J. Physiol. 272, F602-609. [DOI] [PubMed] [Google Scholar]
- Vleminckx, K., and Kemler, R. (1999). Cadherins and tissue formation: integrating adhesion and signaling. Bioessays 21, 211-220. [DOI] [PubMed] [Google Scholar]
- Wahl, J.K., 3rd, Kim, Y.J., Cullen, J.M., Johnson, K.R., and Wheelock, M.J. (2003). N-cadherin-catenin complexes form prior to cleavage of the proregion and transport to the plasma membrane. J. Biol. Chem. 278, 17269-17276. [DOI] [PubMed] [Google Scholar]
- Ward, C.J., Turley, H., Ong, A.C., Comley, M., Biddolph, S., Chetty, R., Ratcliffe, P.J., Gattner, K., and Harris, P.C. (1996). Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc. Natl. Acad. Sci. USA 93, 1524-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock, M.J., Soler, A.P., and Knudsen, K.A. (2001). Cadherin junctions in mammary tumors. J. Mammary Gland Biol. Neoplasia 6, 275-285. [DOI] [PubMed] [Google Scholar]
- Wilson, P.D. (2001). Polycystin: new aspects of structure, function, and regulation. J. Am. Soc. Nephrol. 12, 834-845. [DOI] [PubMed] [Google Scholar]
- Xu, G.M., Gonzalez-Perrett, S., Essafi, M., Timpanaro, G.A., Montalbetti, N., Arnaout, M.A., and Cantiello, H.F. (2003). Polycystin-1 activates and stabilizes the polycystin-2 channel. J. Biol. Chem. 278, 1457-1462. [DOI] [PubMed] [Google Scholar]
- Yap, A.S., Brieher, W.M., and Gumbiner, B.M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119-146. [DOI] [PubMed] [Google Scholar]
- Zeng, W.Z., Li, X.J., Hilgemann, D.W., and Huang, C.L. (2003). Protein kinase C inhibits ROMK1 channel activity via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. J. Biol. Chem. 278, 16852-16856. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.