Abstract
Background and Purpose
Recreational users report that mephedrone has similar psychoactive effects to 3,4-methylenedioxymethamphetamine (MDMA). MDMA induces well-characterized changes in body temperature due to complex monoaminergic effects on central thermoregulation, peripheral blood flow and thermogenesis, but there are little preclinical data on the acute effects of mephedrone or other synthetic cathinones.
Experimental Approach
The acute effects of cathinone, methcathinone and mephedrone on rectal and tail temperature were examined in individually housed rats, with MDMA included for comparison. Rats were killed 2 h post-injection and brain regions were collected for quantification of 5-HT, dopamine and major metabolites. Further studies examined the impact of selected α-adrenoceptor and dopamine receptor antagonists on mephedrone-induced changes in rectal temperature and plasma catecholamines.
Key Results
At normal room temperature, MDMA caused sustained decreases in rectal and tail temperature. Mephedrone caused a transient decrease in rectal temperature, which was enhanced by α1-adrenoceptor and dopamine D1 receptor blockade, and a prolonged decrease in tail temperature. Cathinone and methcathinone caused sustained increases in rectal temperature. MDMA decreased 5-HT and/or 5-hydroxyindoleacetic acid (5-HIAA) content in several brain regions and reduced striatal homovanillic acid (HVA) levels, whereas cathinone and methcathinone increased striatal HVA and 5-HIAA. Cathinone elevated striatal and hypothalamic 5-HT. Mephedrone elevated plasma noradrenaline levels, an effect prevented by α-adrenoceptor and dopamine receptor antagonists.
Conclusions and Implications
MDMA and cathinones have different effects on thermoregulation, and their acute effects on brain monoamines also differ. These findings suggest that the adverse effects of cathinones in humans cannot be extrapolated from previous observations on MDMA.
Keywords: cathinone, methcathinone, MDMA, mephedrone, 5-HT, dopamine, plasma noradrenaline, α-adrenoceptors, dopamine receptors, thermoregulation
Introduction
Cathinone, the β-keto analogue of amphetamine, is the main psychoactive ingredient in the leaves of the Khat shrub (Catha edulis) which are chewed daily by over 20 million people in the Arabian Peninsula and East Africa, and by up to 80% of Somali immigrants in the UK (Feyissa and Kelly, 2008). Although synthetic cathinone does not appear to be used recreationally, a range of synthetic cathinone derivatives have gained popularity in recent years (McElrath and O'Neill, 2011; Schifano et al., 2011). While methcathinone has some history of recreational use in the former Soviet Union during the 1970s and in the USA during the 1990s (Emerson and Cisek, 1993), the recreational use of 4-methylmethcathinone (mephedrone) was largely unheard of prior to 2007 when the extensive internet marketing of this compound as a ‘legal high’ began. This period was accompanied by decreased availability and purity of alternative illicit drugs, which is believed to have stimulated the rapid increase in mephedrone use (McElrath and O'Neill, 2011; Winstock et al., 2011a). Indeed, it was estimated that, by 2009, mephedrone had become one of the most common recreational drugs in UK, at least among individuals associated with the dance music scene (Winstock et al., 2011a). Despite the recent classification of synthetic cathinones as controlled substances in many European countries (EMCDDA, 2011), there is evidence that mephedrone remains widely available for illicit use and is still the drug of choice among certain populations (Wood et al., 2012).
Recreational mephedrone users report that the drug causes general stimulation, elevated mood, euphoria, enhanced music appreciation, decreased hostility, increased sociability and talkativeness, feelings of closeness and mild sexual stimulation (EMCDDA, 2011; Schifano et al., 2011). These stimulant, euphoric and empathogenic effects appear similar to those of cocaine, amphetamine and 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’), and some users even consider mephedrone superior to MDMA (Vardakou et al., 2011; Winstock et al., 2011b).
Hyperthermia is arguably the major acute adverse event that can follow ingestion of MDMA by recreational users (Green et al., 2003; Docherty and Green, 2010; Halpern et al., 2011; Parrott, 2012), and is particularly prevalent in young persons who have ingested the drug at dance clubs or parties where the ambient temperature is high. These individuals sometimes also present with medical problems related to hyperthermia, including rhabdomyolysis, myoglobinuria, renal failure, liver damage and disseminated intravascular coagulopathy. Such problems can be fatal and are identical to those seen in persons suffering from heatstroke (Kalant, 2001). While administration of higher doses of MDMA to rats usually causes hyperthermia, MDMA can also cause hypothermia in rodents, particularly following a low dose or when animals are housed singly or at a cool ambient temperature (Docherty and Green, 2010). Nevertheless, both MDMA-induced hyperthermia and hypothermia result primarily from monoamine release in the brain (Docherty and Green, 2010).
Mephedrone, like MDMA, has recently received intense media interest because of its possible role in a number of sudden fatalities and while mephedrone appears to be the sole cause of death in very few cases (e.g. Gustavsson and Escher, 2009; Maskell et al., 2011), its combination with other drugs is currently implicated in at least 48 deaths in the UK (Schifano et al., 2011). The self-reported side effects of mephedrone include uncomfortable changes in body temperature and cold or blue fingers (ACMD, 2010), but because of uncertainties over both purity and dose in a recreational setting, it remains unknown as to whether mephedrone and other related synthetic cathinones produce similar temperature effects to MDMA or to each other. There are also little preclinical data on the acute effects of these drugs on body temperature (Hadlock et al., 2011; Baumann et al., 2012). The first part of the present study examined the acute effects of cathinone, methcathinone and mephedrone on core body (rectal) temperature and peripheral (tail) temperature in the rat, with MDMA included for comparison. These measures were selected to examine drug-induced effects on central thermoregulation (rectal temperature), and to provide an indication of any change in peripheral vascular tone (tail temperature). In the current study, rats were housed singly at normal ambient temperature to allow comparison with previous behavioural studies (Macerola et al., 2011; Shortall et al., 2011; 2012), and under these conditions, MDMA induced hypothermia. We also measured brain regional levels of 5-HT, dopamine and their major metabolites 2 h after administration of MDMA and the cathinones. This time-point was chosen to maximize the likelihood of detecting acute effects of the cathinones on monoamine release and reuptake (Pehek et al., 1990; Gygi et al., 1996).
These initial studies examined two doses of the drugs under investigation, based in part on doses of MDMA considered to be of translational relevance. In the rat, an MDMA dose of 4 mg kg−1 produces a peak plasma concentration comparable to that induced by human recreational doses of the drug (Green et al., 2012). However, the rapid metabolism of MDMA in rats compared to humans means that the exposure time to a pharmacologically active dose is short in rats compared to humans (Green et al., 2012). In order to deal with this problem, we also administered a 10 mg kg−1 dose to provide a longer duration of action (Baumann et al., 2009). Because of the modest differences in the molecular weights of MDMA and the selected cathinones, it was logical to compare the same doses of all four compounds, particularly given the paucity of comparative pharmacokinetic information on the cathinones in rats and humans. In addition, previous studies with mephedrone have shown that the doses selected were high enough to elicit pharmacological and behavioural activity (Kehr et al., 2011; Meng et al., 2012) but low enough to avoid the possibility of neurotoxicity (Hadlock et al., 2011; Baumann et al., 2012).
To further elucidate the pharmacological mechanisms of 10 mg kg−1 mephedrone-induced temperature change, subsequent experiments examined the effect of several drugs that have been shown to influence MDMA-induced changes in rectal temperature. These were the α1-adrenoceptor antagonist prazosin, the α2A-adrenoceptor antagonist BRL 44408, the dopamine D1 receptor antagonist SCH 23390 and the dopamine D2 receptor antagonist L-741626. Doses of these compounds were selected from previous in vivo research in this laboratory with the dopamine receptor antagonists (Watson et al., 2012) and studies in which prazosin (Sprague et al., 2003; Vanattou-Saïfoudine et al., 2010) and SCH 23390 (Mechan et al., 2002), but not L-741626 (Shioda et al., 2008), prevented MDMA-induced hyperthermia and BRL 44408 prolonged MDMA-induced hypothermia (Bexis and Docherty, 2006). Plasma catecholamine levels were also measured in this study as an index of the effect of mephedrone on the sympathetic regulation of peripheral vascular tone (noradrenaline) and adrenal medullary function (adrenaline) which could influence thermoregulation, since MDMA increases both noradrenaline and adrenaline levels (Hysek et al., 2012a,b,c).
Finally, we investigated the effect of group housing on 10 mg kg−1 mephedrone-induced changes in body temperature and plasma catecholamines, since group housing alters the rectal temperature response of rats to MDMA (Docherty and Green, 2010).
Methods
Animals
Adult male Lister hooded rats (190–390 g) were obtained from Charles River (UK) or the University of Nottingham Biomedical Services Unit (derived from Charles River stock). They were housed in groups of 3–4 on a 12 h light–dark cycle (lights on at 07:00 h) with constant environmental conditions (temperature 19–23°C, relative humidity 45–65%) and with food and water freely available. Experiments were conducted during the light phase, between 09:00 h and 15:00 h, at an ambient temperature of 19–22°C. All procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act (1986), with approval of the University of Nottingham Local Ethical Review Committee, and conform to the ARRIVE guidelines (Kilkenny et al., 2010; McGrath et al., 2010).
Drugs
(-)-Cathinone HCl, (±)-2-(methylamino)propiophenone HCl (methcathinone), (±)-3,4-methylenedioxymethamphetamine HCl (MDMA), 1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylcarbonyl)piperazine HCl (prazosin) and (+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine HCl (SCH 23390) were obtained from Sigma Aldrich (Poole, UK). (±)-4-Methylmethcathinone hydrochloride (mephedrone) was purchased from Ascent Scientific (Bristol, UK) and 2-[(4,5-dihydro-1H-imidazol-2-yl)methyl]-2,3-dihydro-1-methyl-1H-isoindole maleate (BRL 44408) was purchased from Tocris Bioscience (Bristol, UK). L-741626 was a gift from Institut de Recherches Servier (Croissy sur Seine, France). The cathinones and MDMA were dissolved in 0.154 M saline for administration, and all other compounds were dissolved in lactic acid and saline then adjusted to pH 6.5 with sodium hydroxide. The nomenclature used in this manuscript conforms to that provided in the Guide to receptors and channels (Alexander et al., 2011).
Effect of cathinones and MDMA on rectal and tail temperature, and brain monoamine levels in individually housed rats
Rats were placed in individual Perspex arenas and initial temperature measurements were made to allow habituation to the recording procedures; animals were gently handled by the tail, while core body temperature was measured with a rectal probe (Portec Instrumentation, Bedfordshire, UK), and peripheral temperature assessed by transient application of a MicroFlo DSP Digital Laser Perfusion Monitor (Oxford Optronix, Oxford, UK) against the tail. The rectal probe was inserted approximately 6.5 cm, after allowing any visible faeces to pass out of the rectum. Rectal and tail temperature devices gave stable readings within 20 s. After a 40 min interval baseline temperatures were obtained, and rats received a single i.p. injection of saline vehicle (1 mL kg−1), cathinone, methcathinone, mephedrone or MDMA (4 or 10 mg kg−1 of the hydrochloride salt, which is equivalent to 3.21-3.37 or 8.04–8.41 mg kg−1 of the free base; n = 5–6 per group). Temperatures were measured at 20 min intervals for the next 2 h, when rats were killed by concussion and immediately decapitated. The brains were rapidly removed and the hypothalamus, frontal cortex, hippocampus and striatum were dissected at 4°C on a refrigerated table (BC72: Osborne Refrigeration, Sussex, UK), snap frozen in liquid nitrogen and stored at −80°C for subsequent quantification of monoamine neurotransmitters and metabolites using HPLC with electrochemical detection (HPLC-ED).
HPLC-ED was performed using previously described methods (King et al., 2009). In summary, samples were thawed, weighed and sonicated (Soniprep 150: MSE Scientific Instruments, Crawley, UK) for 30 s in 800 μL of 0.05 M perchloric acid containing 1 μM sodium metabisulphite, then centrifuged (17 400× g, 4°C for 20 min; Harrier 18/80: MSE Scientific Instruments) and the supernatant filtered through a 0.45 μm syringe tip filter (Kinesis Ltd., St Neots, UK). Monoamines were separated using a PerkinElmer Series 200 autosampler and Targa C18 3 μm column (100 mm × 2.1 mm: Presearch, Basingstoke, UK), and detected at a potential of +0.59 V by an Antec Intro amperometric detector (Zoeterwoude, The Netherlands). Mobile phase was delivered at 0.2 mL min−1 by an isocratic pump (Dionex P680) and consisted of 50 mM citric acid, 50 mM phosphoric acid, 8 mM potassium chloride, 0.1 mM disodium EDTA, 0.15 mM octanesulphonic acid and 10% v/v methanol adjusted to pH 3.8–4. Quantification was achieved using Galaxie (version 1.8) software (Agilent Technologies, Berkshire, UK).
Effect of α1- and α2A-adrenoceptor antagonists, dopamine D1 and D2 receptor antagonists and group housing on mephedrone-induced changes in body temperature and plasma catecholamine levels
For antagonist studies, rats were placed in individual test arenas as described earlier, and for the group-housed study, cages were transferred to the procedure room in which individually housed experiments were performed, but rats remained within their home-cage groups of three. In each case, rats underwent initial temperature measurements 40 min prior to the start of the experiment to allow habituation to recording procedures. Individually housed rats then received an i.p. injection of vehicle (1 mL kg−1), 0.2 mg kg−1 prazosin HCl, 1 mg kg−1 BRL 44408 maleate, 2 mg kg−1 SCH 23390 HCl or 0.63 mg kg−1 L-741626, followed 30 min later by vehicle (1 mL kg−1) or 10 mg kg−1 mephedrone HCl (n = 6–7 per group). Group-housed rats received a single i.p. injection of vehicle or 10 mg kg−1 mephedrone HCl (n = 6 per group), with all rats within a cage receiving the same treatment. Rectal temperature (all rats) and tail temperature (group-housed rats only) were measured immediately prior to injection and then at 20 min intervals for the next 2 h, when rats were killed by concussion and immediately decapitated. Mixed arteriovenous trunk blood was immediately collected into lithium heparin blood tubes, on ice, each containing 7.5 μL of 250 mM EGTA, 195 mM glutathione per millilitre of whole blood. Samples were centrifuged (1000× g, 5 min; Centaur 2: MSE Scientific Instruments) and the plasma was removed and stored on ice and then at −80°C.
Extraction and HPLC were performed using methods adapted from Forster and Macdonald (1999). In summary, plasma was thawed, mixed and centrifuged (as previously) then 100 μL of sample added to 100 μL of 0.05 μM 3,4-dihydroxybenzylamine HCl (internal standard). This was followed by addition of 250 μL ammonia buffer (2 M ammonium chloride adjusted to pH 8.8 with 2 M ammonium hydroxide, and containing 22 mM diphenylboric acid ethanolamine complex 13 mM disodium EDTA) and 1 mL heptane mixture (heptane containing 6 mM tetraoctylammonium bromide and 1% v/v octanol). The resultant two-layer mixture was vortexed then centrifuged (as previously). A volume of 750 μL was removed from the organic top layer, to which 380 μL octan-1-ol and 40 μL of 400 mM acetic acid were added. This was vortexed for 3 min (Multi Tube Vortex Mixer: Labnet International, Edison, NJ, USA) then centrifuged (as previously). A volume of 30–35 μL was removed from the acid droplet at the base of the tube and stored at −80°C. Catecholamines were separated using a Spark-Holland Triathlon autosampler and HyperClone C18 3 μm ODS column (100 mm × 2 mm: Phenomenex, Macclesfield, UK), and detected at a potential of +0.65 V by an Antec Intro amperometric detector. Mobile phase was delivered at 0.18 mL min−1 (Merck Hitachi L-7110, Tokyo, Japan) and consisted of 110 mM sodium acetate, 110 μM disodium EDTA, 347 μM sodium dodecyl sulphate and 20% v/v methanol adjusted to pH 5.2. Quantification was achieved manually (ChromJet Integrator: Thermo Separation Products, Waltham, MA, USA) using an internal standard to establish recovery. The mean (±SEM) recovery was 80 ± 1% and the estimated limit of detection for noradrenaline and adrenaline was 1.2–1.4 nmol L−1.
Statistical analysis
All data were checked for normality using Shapiro–Wilk or Kolmogorov–Smirnov tests before parametric analyses were applied. Temperature data exhibited homogeneous variance and were analysed by two-way repeated measures anova with Bonferroni's multiple comparison post hoc test. In the case of methcathinone, mephedrone and MDMA, the different doses were assessed on separate days. Vehicle data for the two days have been pooled for clarity of presentation (after confirming the lack of any between-group difference; two-way repeated measures anova with Bonferroni's multiple comparison post hoc test); however, statistical comparisons relate to the relevant vehicle control for each day, not the pooled values.
Monoamine levels were analysed by one-way anova with Bonferroni's multiple comparison post hoc test (homogeneous variance between groups) or Tamhane's post hoc test (heterogeneous variance between groups). The highest dose of methcathinone was evaluated on a separate day so changes in monoamine levels from the relevant vehicle control group were determined using unpaired Student's t-tests (homogeneous variance between groups). These analyses were applied separately to each neurotransmitter or metabolite in each brain region. For clarity of presentation, vehicle data for each brain region have been pooled (after confirming the lack of any between-group difference; one-way anova with Bonferroni's multiple comparison post hoc test), but, again, statistical comparisons relate to the relevant vehicle control group and not the pooled values.
Plasma catecholamine levels exhibited homogeneous variance and were analysed by two-way anova with Bonferroni's multiple comparison post hoc test (antagonist studies) or unpaired Student's t-tests (group-housed mephedrone study). All analyses were performed using Prism (version 5.03; GraphPad, La Jolla, CA, USA) or SPSS (version 16; IBM, Hampshire, UK). Data are presented as mean ± SEM and P < 0.05 was considered significant.
Results
Immediately prior to dosing the mean (±SEM) baseline rectal temperature across all studies was 39.6 ± 0.1°C and the tail temperature across studies was 30.4 ± 0.2°C. These values are consistent with previous findings (Green et al., 2005). There were no significant between-group differences in either rectal or tail temperature prior to dosing.
Effect of cathinones and MDMA on rectal and tail temperature, and brain monoamine levels in individually housed rats
MDMA caused a marked and sustained decrease in rectal temperature following administration of both the lower (4 mg kg−1) and higher (10 mg kg−1) dose (Figure 1A), which was significant over the entire 2 h monitoring period (P < 0.05 to P < 0.001). Tail temperature was decreased by the higher dose only from 20 to 60 min post-injection (Figure 1E; P < 0.05 to P < 0.01). Although both doses of mephedrone also produced a hypothermic response, the effect on rectal temperature was statistically significant only at the 20 min time-point following the lower dose and from 20 to 40 min following the higher dose (Figure 1B; P < 0.01 to P < 0.001), whereas the reduction in tail temperature was evident from 40 min onwards when rectal temperature had returned to baseline (Figure 1F). In contrast, the higher dose of both cathinone and methcathinone caused a sustained increase in rectal temperature, with cathinone having a significant effect from 40 to 80 min (Figure 1C; P < 0.05 to P < 0.001) and methcathinone from 40 to 120 min (Figure 1D; P < 0.01 to P < 0.001). These alterations in rectal temperature were not accompanied by any significant concomitant change in tail temperature (Figure 1G,H).
Figure 1.
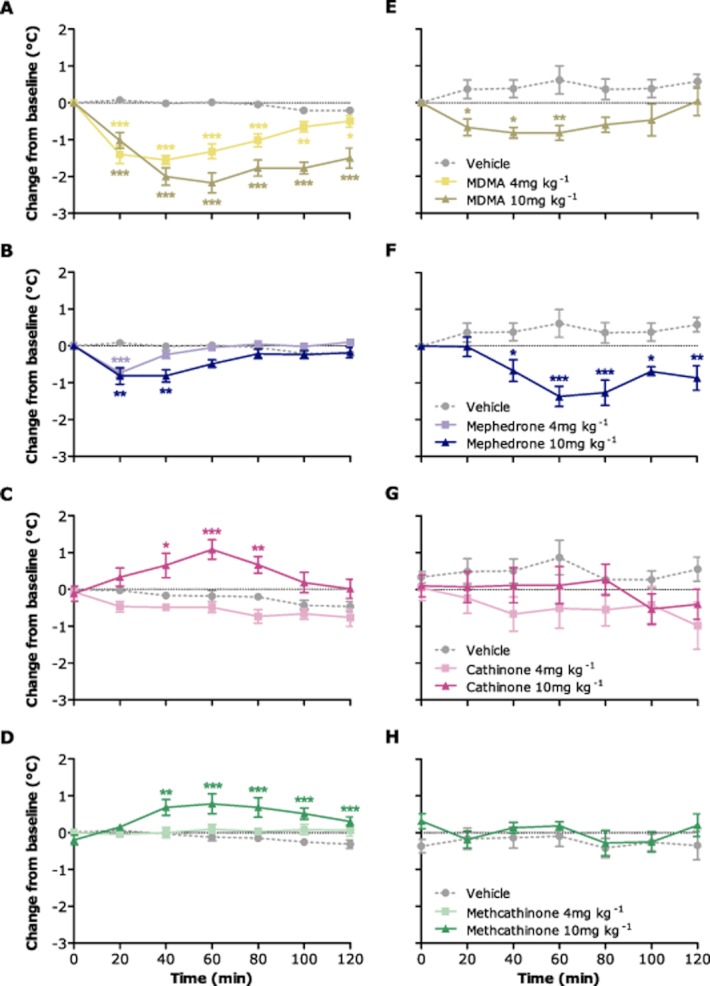
Effect of MDMA (A, E), mephedrone (B, F), cathinone (C, G) and methcathinone (D, H) on rectal (A–D) and tail (E–H) temperature in individually housed male Lister hooded rats (n = 5–6 per group). Compounds (4 or 10 mg kg−1 HCl salt) or saline vehicle (1 mL kg−1) were injected i.p. at 0 min and temperature assessed at 20 min intervals for the next 2 h. Data are expressed as change in temperature (°C, mean ± SEM) from the baseline reading taken at the time of injection. *P < 0.05; **P < 0.01; ***P < 0.001 versus vehicle control (two-way repeated measures anova with Bonferroni's post hoc test). Where results of multiple experiments are shown on the same graph, vehicle data have been pooled for clarity of presentation (P > 0.05 between studies; two-way repeated measures anova). However, all statistical comparisons shown relate to the original control for each experiment (n = 5–6), not the pooled values.
At the 2 h post-injection time-point, the lower dose of MDMA significantly decreased tissue 5-hydroxyindoleacetic acid (5-HIAA) (P < 0.05) levels in the hippocampus, by 26% (data not shown), but did not cause any other significant alterations in tissue monoamine levels. The higher MDMA dose produced a marked decrease in both 5-HT and 5-HIAA within the hippocampus, and a significant decrease in either 5-HT (frontal cortex) or 5-HIAA content (striatum and hypothalamus) in the other brain regions examined (Table 1). The higher dose of MDMA also produced a near significant (P = 0.08) increase in dopamine and a significant decrease in homovanillic acid (HVA) (P < 0.01) within the striatum (Table 2). Neither dose of mephedrone had any significant effect on levels of 5-HT, dopamine or their major metabolites in any brain region studied. Both cathinone and methcathinone increased 5-HIAA in the striatum following the higher dose, and the higher dose of cathinone also increased 5-HT levels in the striatum and hypothalamus (Table 1). The only significant effects of the cathinones on striatal dopamine were an increase in HVA levels following the lower dose of methcathinone (33% increase; P < 0.05, data not shown) and the higher dose of both cathinone and methcathinone (Table 2).
Table 1.
Effect of MDMA, mephedrone, cathinone and methcathinone on levels of 5-HT and its metabolite 5-HIAA in the frontal cortex, hippocampus, striatum and hypothalamus
| Frontal cortex | Hippocampus | Striatum | Hypothalamus | |
|---|---|---|---|---|
| 5-HT | ||||
| MDMA | 35 ± 6** | 42 ± 4*** | 86 ± 8 | 101 ± 24 |
| Mephedrone | 76 ± 13 | 100 ± 8 | 110 ± 9 | 94 ± 15 |
| Cathinone | 96 ± 17 | 99 ± 8 | 121 ± 4* | 122 ± 5* |
| Methcathinone | 131 ± 18 | 109 ± 22 | 104 ± 7 | 64 ± 15 |
| 5-HIAA | ||||
| MDMA | 81 ± 32 | 68 ± 3*** | 70 ± 4** | 55 ± 9** |
| Mephedrone | 87 ± 9 | 109 ± 5 | 96 ± 3 | 85 ± 12 |
| Cathinone | 112 ± 15 | 97 ± 6 | 142 ± 3*** | 108 ± 4 |
| Methcathinone | 154 ± 20 | 97 ± 16 | 132 ± 7** | 99 ± 7 |
5-HT and 5-HIAA levels were measured 2 h after i.p. injection of test compounds (10 mg kg−1 of HCl salt) or saline vehicle (1 mL kg−1) to individually housed male Lister hooded rats (n = 5–6 per group). Because different compounds were assessed in separate experiments, data are expressed as a mean percentage (± SEM) of the 5-HT or 5-HIAA level in the relevant vehicle control group (100%) for clarity. The pooled 5-HT levels (pmol mg−1 wet tissue weight) across control groups were: frontal cortex 3.7 ± 0.3, hippocampus 4.5 ± 0.3, striatum 3.1 ± 0.2 and hypothalamus 7.3 ± 0.3. The respective values for 5-HIAA were: 3.3 ± 0.3, 6.9 ± 0.3, 4.6 ± 0.2 and 7.6 ± 0.3.
P < 0.05;
P < 0.01;
P < 0.001 versus levels (pmol mg−1 wet tissue weight) in the relevant vehicle control group (one-way anova with Bonferroni's or Tamhane's post hoc tests, or unpaired Student's t-test for occasions when a single drug dose was evaluated on a separate test day).
Table 2.
Effect of MDMA, mephedrone, cathinone and methcathinone on levels of dopamine and its metabolites HVA and DOPAC in the striatum
| Dopamine | HVA | DOPAC | |
|---|---|---|---|
| MDMA | 133 ± 7 | 61 ± 2** | 61 ± 3 |
| Mephedrone | 86 ± 10 | 78 ± 15 | 70 ± 9 |
| Cathinone | 129 ± 6 | 143 ± 9*** | 115 ± 9 |
| Methcathinone | 117 ± 7 | 176 ± 18** | 92 ± 8 |
Dopamine, HVA and 3,4-dihydroxyphenylacetic acid (DOPAC) levels were measured 2 h after i.p. injection of test compounds (10 mg kg−1 of HCl salt) or saline vehicle (1 mL kg−1) to individually housed male Lister hooded rats (n = 5–6 per group). Because different compounds were assessed in separate experiments, data are expressed as a mean percentage (±SEM) of the dopamine, HVA or DOPAC level in the relevant vehicle control group (100%) for clarity. The pooled levels (pmol mg−1 wet tissue weight) across control groups were: dopamine 48.7 ± 3.0, HVA 7.6 ± 0.5 and DOPAC 9.3 ± 0.7.
*P < 0.05;
P < 0.01;
P < 0.001 versus level (pmol mg−1 wet tissue weight) in the relevant vehicle control group (one-way anova with Bonferroni's or Tamhane's post hoc tests, or unpaired Student's t-test for occasions when a single drug dose was evaluated on a separate test day).
Effect of α1- and α2A-adrenoceptor and dopamine D1 and D2 receptor antagonists on mephedrone-induced changes in body temperature
Pretreatment of individually housed rats with prazosin (Figure 2A), BRL 44408 (Figure 2B) or L-741626 (Figure 2D) had no effect on rectal temperature when followed by subsequent vehicle treatment (P > 0.05 vs. the vehicle + vehicle group in each case), and the only significant effect of SCH 23390 pretreatment in vehicle-treated rats was a significant (P < 0.05) decrease in rectal temperature at the 100 min time-point (Figure 2C). In individually housed vehicle-pretreated rats, 10 mg kg−1 mephedrone caused a transient significant decrease in rectal temperature (Figure 2), consistent with that observed in our previous experiments (Figure 1B). Of note, the duration of the mephedrone-induced hypothermia was prolonged in the presence of prazosin, reaching statistical significance from 20 to 60 min post-mephedrone (P < 0.001 vs. both vehicle + vehicle and prazosin + vehicle groups), compared to 20 min only in rats that received mephedrone alone (P < 0.05 vs. vehicle + vehicle; Figure 2A). Pretreatment with SCH 23390 also prolonged the mephedrone-induced hypothermia, causing it to reach statistical significance from 20 to 80 min post-mephedrone (P < 0.001 vs. vehicle + vehicle and vs. SCH 23390 + vehicle from 20–40 min, P < 0.05 vs. SCH 23390 + vehicle at 60 min), compared to 20–40 min in rats that received mephedrone alone (P < 0.001). In addition, the extent of the rectal temperature decrease was potentiated by the combination of SCH 23390 and mephedrone compared to mephedrone alone (P < 0.05). This effect was significant from 40 to 60 min post-mephedrone (Figure 2C). In contrast, mephedrone-induced hypothermia was unaffected by pretreatment with either BRL 44408 (Figure 2B) or L-741626 (Figure 2D).
Figure 2.
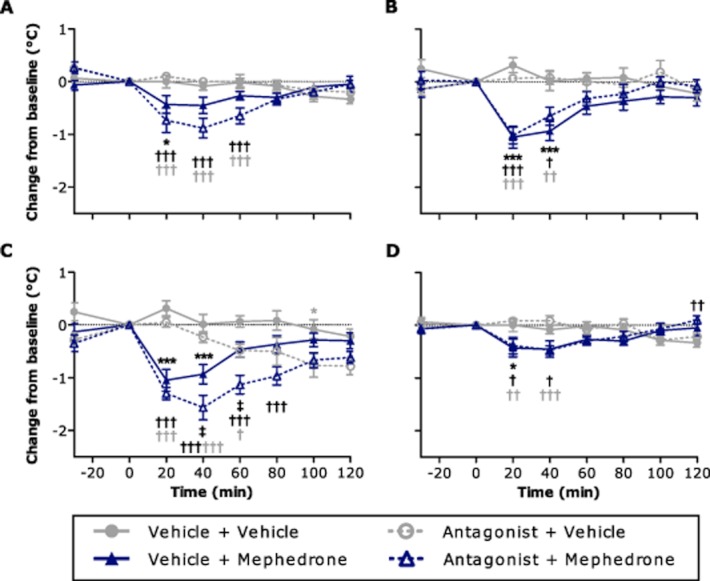
Effect of the α1-adrenoceptor antagonist prazosin (A), α2A-adrenoceptor antagonist BRL 44408 (B), dopamine D1 receptor antagonist SCH 23390 (C) and dopamine D2 receptor antagonist L-741626 (D) on the mephedrone-induced rectal temperature decrease in individually housed male Lister hooded rats (n = 6–7 per group). Saline vehicle (1 mL kg−1), prazosin HCl (0.2 mg kg−1), BRL 44408 maleate (1 mg kg−1), SCH 23390 HCl (2 mg kg−1) or L-741626 (0.63 mg kg−1) were injected i.p. at −30 min, and saline vehicle (1 mL kg−1) or mephedrone HCl (10 mg kg−1) were injected i.p. at 0 min. Rectal temperature was measured at −30 min and at 20 min intervals from 0 to 120 min. Data are expressed as change in temperature (°C, mean ± SEM) from the reading taken at 0 min.  P < 0.05 antagonist + vehicle versus vehicle + vehicle, *P < 0.05; ***P < 0.001 vehicle + mephedrone versus vehicle + vehicle, †P < 0.05; †††P < 0.001 antagonist + mephedrone versus vehicle + vehicle,
P < 0.05 antagonist + vehicle versus vehicle + vehicle, *P < 0.05; ***P < 0.001 vehicle + mephedrone versus vehicle + vehicle, †P < 0.05; †††P < 0.001 antagonist + mephedrone versus vehicle + vehicle,  P < 0.05;
P < 0.05;  P < 0.01;
P < 0.01;  P < 0.001 antagonist + mephedrone versus antagonist + vehicle, ‡P < 0.05 vehicle + mephedrone versus antagonist + mephedrone (two-way repeated measures anova with Bonferroni's post hoc test).
P < 0.001 antagonist + mephedrone versus antagonist + vehicle, ‡P < 0.05 vehicle + mephedrone versus antagonist + mephedrone (two-way repeated measures anova with Bonferroni's post hoc test).
Effect of mephedrone on plasma noradrenaline and adrenaline levels, and the influence of α1- and α2A-adrenoceptor and dopamine D1 and D2 receptor antagonists on these changes
Mephedrone (10 mg kg−1) produced a significant increase in plasma noradrenaline levels (P < 0.05 vehicle + mephedrone vs. vehicle + vehicle; Figure 3) which was completely abolished by pretreatment with prazosin (Figure 3A), BRL 44408 (Figure 3B) and SCH 23390 (Figure 3C). The increase in plasma noradrenaline just failed to reach significance in L-741626 pretreated rats (Figure 3D). Mephedrone appeared to increase plasma adrenaline in a similar manner (vehicle + vehicle 25.6 ± 3.9 nmol L−1, vehicle + mephedrone 39.8 ± 5.4 nmol L−1; P = 0.044, n = 12), but this effect just failed to reach statistical significance in each of the individual drug studies (data not shown).
Figure 3.
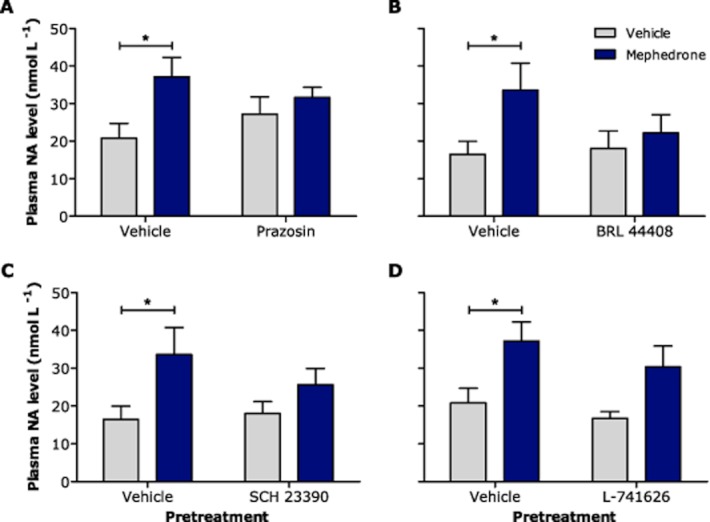
Effect of the α1-adrenoceptor antagonist prazosin (A), α2A-adrenoceptor antagonist BRL 44408 (B), dopamine D1 receptor antagonist SCH 23390 (C) and dopamine D2 receptor antagonist L-741626 (D) on mephedrone-induced increases in plasma noradrenaline levels in individually housed male Lister hooded rats (n = 6–7 per group). Arteriovenous trunk blood was collected 2.5 h after i.p. administration of saline vehicle (1 mL kg−1), prazosin HCl (0.2 mg kg−1), BRL 44408 maleate (1 mg kg−1), SCH 23390 HCl (2 mg kg−1) or L-741626 (0.63 mg kg−1), and 2 h after i.p. administration of saline vehicle (1 mL kg−1) or mephedrone HCl (10 mg kg−1). *P < 0.05 versus vehicle + vehicle group (two-way anova with Bonferroni's post hoc test).
Effect of group housing on mephedrone-induced changes in body temperature and plasma catecholamine concentration
Group housing (n = 3 per cage) completely prevented both the mephedrone-induced rectal and tail temperature decreases (P > 0.05 vs. vehicle across the entire 2 h period in each case; data not shown) and the mephedrone-induced increase in plasma noradrenaline (vehicle 17.1 ± 3.6 nmol L−1, mephedrone 21.8 ± 4.0 nmol L−1) and adrenaline (vehicle 19.8 ± 5.7 nmol L−1, mephedrone 23.4 ± 6.0 nmol L−1) levels.
Discussion
The major findings of this study were, firstly, that the acute effects of cathinone and methcathinone on thermoregulation and brain monoamine metabolism in the rat differed from those of mephedrone and MDMA. Secondly, mephedrone-induced hypothermia differed from MDMA-induced hypothermia in both its temporal profile and sensitivity to α-adrenoceptor and dopamine receptor antagonists and, finally, mephedrone produced a marked elevation in plasma noradrenaline.
Simmler et al. (2012) have recently performed a detailed pharmacological characterization of the cathinones and classified them into three separate groups on the basis of their relative potencies for inhibition of monoamine reuptake and stimulation of monoamine release. Mephedrone and MDMA both have a substituted phenyl ring and, consistent with this structural homology (Carvalho et al., 2012), mephedrone was found to be a non-selective monoamine reuptake inhibitor which also stimulates 5-HT release, whereas cathinone and methcathinone (which lack the phenyl ring substitution) were selective catecholamine reuptake inhibitors and releasers. This subdivision matches our observation during the first part of the current study that the effects of cathinone and methcathinone on body temperature differed qualitatively from those of mephedrone and MDMA. The temperature response of rats to MDMA is complex, involving both central monoamine-mediated effects and also changes in peripheral blood flow and thermogenesis (Docherty and Green, 2010). While the majority of studies have reported a hyperthermic response to MDMA administration in the rat, there are also several reports of hypothermia, particularly when administered to individually housed rats under normal ambient temperatures, and importantly at doses that produce plasma levels akin to those reported with illicit use in man (Docherty and Green, 2010; Green et al., 2012). The current study again found that acute MDMA administration (4 or 10 mg kg−1) under these conditions induced hypothermia, as indicated by the sustained decrease in rectal temperature. The accompanying decrease in tail temperature, which is indicative of peripheral vasoconstriction and should aid heat conservation, may be mediated via a direct effect on α2A-adrenoceptors (Bexis and Docherty, 2006; Simmler et al., 2012) known to regulate tail blood flow and heat loss in the rat (Redfern et al., 1995). However, the fact that the MDMA-induced decrease in tail temperature was both short in duration and modest in size compared to the long lasting and major decrease in rectal temperature demonstrates that heat conservation mechanisms appear to be disrupted after this single dose of MDMA.
Under the same conditions of individual housing and normal ambient temperature, mephedrone also produced hypothermia, but the small and short lasting decrease in rectal temperature was followed by a prolonged decrease in tail temperature which therefore differed from the temporal profile of MDMA-induced hypothermia. The rapid onset and short duration of the mephedrone-induced rectal temperature change following a single acute injection matched the time course of locomotor hyperactivity we observed in separate experiments (Shortall et al., 2011; 2012). The speed of onset is consistent with very high brain permeability (Simmler et al., 2012) and the duration may indicate a short plasma half-life, especially since one recent study revealed peak plasma levels (of 1206 ng mL−1) within 15 min of a 5.6 mg kg−1 s.c. dose in the rat with the majority of the drug cleared within 2 h (Miller et al., 2012). Interestingly, mephedrone is a more potent inhibitor of both the dopamine transporter and dopamine release and a less potent inhibitor of the serotonin transporter than MDMA (Simmler et al., 2012), which may contribute to the different pharmacodynamic effects. The decrease in tail temperature following mephedrone is consistent with its affinity for both α1- and α2A-adrenoceptors (Simmler et al., 2012), the recently reported hypertension produced in rats (Meng et al., 2012), and the side effects of cold or blue fingers that feature among the adverse events experienced by recreational users (ACMD, 2010; Schifano et al., 2011; Winstock et al., 2011a). However, the mismatch between the transient core and prolonged tail temperature changes in the current study suggests that a metabolite, rather than the parent compound, may be responsible for these peripheral vascular effects. Mephedrone metabolites include nor-mephedrone, nor-dihydro mephedrone, hydroxytolyl mephedrone and nor-hydroxytolyl mephedrone (Meyer et al., 2010), but, at present, there is no information on their pharmacological activity.
Two recent studies report that binge dosing of mephedrone induces hyperthermia, both in individually housed rats at normal ambient temperatures (three doses of 3–10 mg kg−1 s.c.; Baumann et al., 2012) and in group-housed rats in a warm (≥27°C) environment (four doses of 1–25 mg kg−1 s.c.; Hadlock et al., 2011). This conversion from hypothermic effects of a single dose to hyperthermia after binge doses matches our recent observations with MDMA in individually housed rats, where acute administration (6 mg kg−1) at normal ambient temperature induced hypothermia, with hyperthermia only appearing after binge dosing (total administered dose: 18 mg kg−1; Rodsiri et al., 2011). However, a third recent study found that an elevated ambient temperature (30°C) prevented the hypothermia produced by mephedrone (1–5.6 mg kg−1 s.c.) at 20°C, but failed to convert this to the hyperthermic effect seen with an equivalent dose of MDMA under these conditions (Miller et al., 2012). Similarly, the current study revealed that group housing (n = 3 per cage) prevented the mephedrone-induced hypothermia but failed to convert this to the hyperthermia seen in group-housed MDMA-treated (3–20 mg kg−1 i.p.) rats at normal ambient temperature (Nash et al., 1988; Colado et al., 1993; Mechan et al., 2002). Taken together, these findings suggest that elevated ambient temperature and group housing, which predispose to hyperthermic effects of MDMA, do not appear to precipitate a hyperthermic response to mephedrone. The reasons for this apparent difference are yet to be elucidated, but crucially mephedrone-related adverse events reported in humans to date do not appear to include hyperpyrexia (Wood et al., 2010; 2011; Dargan et al., 2011) and this contrasts strongly with MDMA where hyperthermia can be a fatal event (Kalant, 2001).
In complete contrast to mephedrone and MDMA, both cathinone and methcathinone induced hyperthermia at the higher dose without any effect on tail temperature. The same dose of cathinone has been reported to induce hyperthermia and thermogenesis in urethane-anaesthetized rats (Tariq et al., 1989), and one study describes hyperthermia following intravenous infusion of high doses of methcathinone to individually restrained rats (Rockhold et al., 1997), but the current study is the first to demonstrate similar effects in awake freely-moving rats. The lack of any alteration in tail temperature during this study indicates that these compounds produced a centrally mediated increase in body temperature without causing accompanying peripheral vasoconstriction. This contrasts with MDMA where, when hyperthermia does occur, its extent appears to be further exacerbated by the combination of thermogenesis (Blessing et al., 2006) and peripheral vasoconstriction (Gordon et al., 1991; Pedersen and Blessing, 2001). The lack of peripheral vasoconstriction following cathinone is surprising given that it is metabolized to the sympathomimetic amines norephedrine and norpseudoephedrine (Brenneisen et al., 1986) and has slightly higher affinity than MDMA for human α2A-adrenoceptors, while methcathinone has almost identical affinity to mephedrone for human α1- and α2A-adrenoceptors (Simmler et al., 2012). It is noteworthy that cathinone causes coronary vasoconstriction in vitro through mechanisms other than indirect sympathomimetic and α1-adrenoceptor agonist activity, which may involve trace amine receptors (Broadley, 2010; Simmler et al., 2012). It is also possible that the concentrations of cathinone used herein were too low to induce these effects in vivo, but because of the magnitude of rectal temperature increase produced by cathinone and methcathinone, we did not feel able to examine higher doses of these compounds.
Consistent with previous reports (Green et al., 2003), MDMA administration at the higher dose produced an acute decrease in tissue 5-HT and/or 5-HIAA at the 2 h post-injection time-point in each brain region studied, presumably reflecting monoamine release and/or inhibition of reuptake. The effects of MDMA on striatal dopamine metabolism were also entirely consistent with previous reports of increased neurotransmitter and decreased metabolite levels in the first few hours after administration (Green et al., 2003). In marked contrast, cathinone actually increased tissue 5-HT in the striatum and hypothalamus, and both cathinone and methcathinone increased 5-HIAA and HVA levels in the striatum alone. The increase in tissue HVA is consistent with previous findings at the same time-point (Gygi et al., 1996), although it is unclear at present how these effects are mediated, especially since the cathinones inhibit monoamine oxidase B (Osorio-Olivares et al., 2004), the isoenzyme primarily responsible for dopamine metabolism. There are several possible reasons why the changes in monoamine metabolism observed following administration of the cathinone compounds did not mimic those seen after MDMA, despite reports that the cathinones do enhance 5-HT and dopamine release during in vivo microdialysis studies (Pehek et al., 1990; Gygi et al., 1997; Hadlock et al., 2011; Kehr et al., 2011; Baumann et al., 2012). Firstly, tissue levels are a ‘snapshot’ at a single time-point of a dynamic situation involving changes in neurotransmitter release, synthesis, metabolism and elimination. Secondly, the short half-life of mephedrone, for example when compared to MDMA, will influence what change is seen 2 h after drug administration. Finally, and perhaps most importantly, cathinone and methcathinone have recently been reported to preferentially affect release and reuptake of catecholamines and not 5-HT (Simmler et al., 2012).
Further studies herein to elucidate the pharmacological mechanisms of mephedrone-induced hypothermia revealed several differences from those reported for the MDMA-induced temperature change. Thus, mephedrone-induced hypothermia was prolonged by the α1-adrenoceptor antagonist prazosin, but unaffected by the α2A-adrenoceptor antagonist BRL 44408 (which potentiates and prolongs MDMA-induced hypothermia; Bexis and Docherty, 2006). Furthermore, mephedrone-induced hypothermia was prolonged and potentiated by the dopamine D1 receptor antagonist SCH 23390 (which fails to affect MDMA-induced hypothermia in a cool environment (Green et al., 2005), but unaffected by dopamine D2 receptor blockade (which prevents MDMA-induced hypothermia; Green et al., 2005). This differential pharmacological sensitivity is surprising given that mephedrone and MDMA share similar affinities for the human α2A-adrenoceptor (Ki 11 and 15 μM, respectively) and possibly also the human D1 (Ki > 13.6 μM in each case) and D2 (Ki 25.2 and >30 μM, respectively) receptors (Simmler et al., 2012), although binding and functional activity at the rat receptors are unknown. Interestingly mephedrone, like MDMA (Hysek et al., 2012a,b,c), also increased plasma noradrenaline levels and, in the case of mephedrone, this effect was sensitive to α1-adrenoceptor, α2A-adrenoceptor and dopamine D1 receptor blockade.
The functional and neurochemical differences between the cathinones and MDMA do not appear to correlate with the modest differences in their molar doses. We have recently pointed out that there are problems in translating preclinical data on MDMA to functional effects of the drug in human recreational users (Green et al., 2012). This is primarily due to the very different pharmacokinetics of the drug in animals and humans. On the basis of information on plasma levels of MDMA in rats and humans, we can state that the doses chosen in this study are not excessive; it is the half-life of the drug in humans versus rats that differs markedly. This allows the conclusion that mephedrone and the other cathinones have a pharmacodynamic profile that is distinct from that of MDMA, and that the adverse effects of the cathinones in humans cannot be extrapolated from previous observations on MDMA.
Acknowledgments
The authors acknowledge Ben Pointer-Gleadhill and Ian Topham for their technical assistance. This research was funded by the University of Nottingham School of Biomedical Sciences.
Glossary
- 5-HIAA
5-hydroxyindoleacetic acid
- HPLC-ED
HPLC with electrochemical detection
- HVA
homovanillic acid
- MDMA
3,4-methylenedioxymethamphetamine
Conflict of interest
The authors state no conflict of interest.
References
- ACMD. 2010. Consideration of the cathinones. Available at: http://www.homeoffice.gov.uk/acmd1/acmd-cathinones-report-2010 (accessed 23/9/2010)
- Alexander SP, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 5th edition. Br J Pharmacol. 2011;164(Suppl. 1):S1–S324. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Zolkowska D, Kim I, Scheidweiler KB, Rothman RB, Huestis MA. Effects of dose and route of administration on pharmacokinetics of (±)-3,4-methylenedioxymethamphetamine (MDMA) in the rat. Drug Metab Dispos. 2009;37:2163–2170. doi: 10.1124/dmd.109.028506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, et al. The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology. 2012;37:1192–1203. doi: 10.1038/npp.2011.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bexis S, Docherty JR. Effects of MDMA, MDA and MDEA on blood pressure, heart rate, locomotor activity and body temperature in the rat involve α-adrenoceptors. Br J Pharmacol. 2006;147:926–934. doi: 10.1038/sj.bjp.0706688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blessing WW, Zilm A, Ootsuka Y. Clozapine reverses increased brown adipose tissue thermogenesis induced by 3,4-methylenedioxymethamphetamine and by cold exposure in conscious rats. Neuroscience. 2006;141:2067–2073. doi: 10.1016/j.neuroscience.2006.05.050. [DOI] [PubMed] [Google Scholar]
- Brenneisen R, Geisshüsler S, Schorno X. Metabolism of cathinone to (–)–norephedrine and (–)–norpseudoephedrine. J Pharm Pharmacol. 1986;38:298–300. doi: 10.1111/j.2042-7158.1986.tb04571.x. [DOI] [PubMed] [Google Scholar]
- Broadley KJ. The vascular effects of trace amines and amphetamines. Pharmacol Ther. 2010;125:363–375. doi: 10.1016/j.pharmthera.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Carvalho M, Carmo H, Costa VM, Capela JP, Pontes H, Remião F, et al. Toxicity of amphetamines: an update. Arch Toxicol. 2012;86:1167–1231. doi: 10.1007/s00204-012-0815-5. [DOI] [PubMed] [Google Scholar]
- Colado MI, Murray TK, Green AR. 5-HT loss in rat brain following 3,4-methylenedioxymethamphetamine (MDMA), p-chloroamphetamine and fenfluramine administration and effects of chlormethiazole and dizocilpine. Br J Pharmacol. 1993;108:583–589. doi: 10.1111/j.1476-5381.1993.tb12846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dargan PI, Sedefov R, Gallegos A, Wood DM. The pharmacology and toxicology of the synthetic cathinone mephedrone (4-methylmethcathinone) Drug Test Anal. 2011;3:454–463. doi: 10.1002/dta.312. [DOI] [PubMed] [Google Scholar]
- Docherty JR, Green AR. The role of monoamines in the changes in body temperature induced by 3,4-methylenedioxymethamphetamine (MDMA, ecstasy) and its derivatives. Br J Pharmacol. 2010;160:1029–1044. doi: 10.1111/j.1476-5381.2010.00722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMCDDA. 2011. Report on the risk assessment of mephedrone in the framework of the Council Decision on new psychoactive substances. Available at: http://www.emcdda.europa.eu/html.cfm/index116639EN.html (accessed October 2011)
- Emerson TS, Cisek JE. Methcathinone: a Russian designer amphetamine infiltrates the rural midwest. Ann Emerg Med. 1993;22:1897–1903. doi: 10.1016/s0196-0644(05)80419-6. [DOI] [PubMed] [Google Scholar]
- Feyissa AM, Kelly JP. A review of the neuropharmacological properties of khat. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1147–1166. doi: 10.1016/j.pnpbp.2007.12.033. [DOI] [PubMed] [Google Scholar]
- Forster CD, Macdonald IA. The assay of the catecholamine content of small volumes of human plasma. Biomed Chromatogr. 1999;13:209–315. doi: 10.1002/(SICI)1099-0801(199905)13:3<209::AID-BMC820>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Gordon CJ, Watkinson WP, O'Callaghan JP, Miller DB. Effects of 3,4-methylenedioxymethamphetamine on autonomic thermoregulatory responses of the rat. Pharmacol Biochem Behav. 1991;38:339–344. doi: 10.1016/0091-3057(91)90288-d. [DOI] [PubMed] [Google Scholar]
- Green AR, Mechan AO, Elliott JM, O'Shea E, Colado MI. The pharmacology and clinical pharmacology of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) Pharmacol Rev. 2003;55:463–508. doi: 10.1124/pr.55.3.3. [DOI] [PubMed] [Google Scholar]
- Green AR, O'Shea E, Saadat KS, Elliott JM, Colado MI. Studies on the effect of MDMA (‘ecstasy’) on the body temperature of rats housed at different ambient room temperatures. Br J Pharmacol. 2005;146:306–312. doi: 10.1038/sj.bjp.0706318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green AR, King MV, Shortall SE, Fone KC. Lost in translation: preclinical studies on MDMA provide information on mechanisms of action, but do not allow accurate prediction of adverse events in humans. Br J Pharmacol. 2012;166:1523–1536. doi: 10.1111/j.1476-5381.2011.01819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustavsson D, Escher C. Mephedrone – Internet drug which seems to have come and stay. Fatal cases in Sweden have drawn attention to previously unknown substance. Lakartidningen. 2009;106:2769–2771. [PubMed] [Google Scholar]
- Gygi MP, Gibb JW, Hanson GR. Methcathinone: an initial study of its effects on monoaminergic systems. J Pharmacol Exp Ther. 1996;276:1066–1072. [PubMed] [Google Scholar]
- Gygi MP, Fleckenstein AE, Gibb JW, Hanson GR. Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J Pharmacol Exp Ther. 1997;283:1350–1355. [PubMed] [Google Scholar]
- Hadlock GC, Webb KM, McFadden LM, Chu PW, Ellis JD, Allen SC, et al. 4-Methylmethcathinone (mephedrone): neuropharmacological effects of a designer stimulant of abuse. J Pharmacol Exp Ther. 2011;339:530–536. doi: 10.1124/jpet.111.184119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern P, Moskovich J, Avrahami B, Bentur Y, Soffer D, Peleg K. Morbidity associated with MDMA (ecstasy) abuse: a survey of emergency department admissions. Hum Exp Toxicol. 2011;30:259–266. doi: 10.1177/0960327110370984. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Brugger R, Simmler LD, Bruggisser M, Donzelli M, Grouzmann E, et al. Effects of the α2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther. 2012a;340:286–294. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Ineichen M, Grouzmann E, Hoener MC, Brenneisen R, et al. The norepinephrine transporter inhibitor reboxetine reduces stimulant effects of MDMA (‘ecstasy’) in humans. Clin Pharmacol Ther. 2012b;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- Hysek CM, Simmler LD, Nicola VG, Vischer N, Donzelli M, Krähenbühl S, et al. Duloxetine inhibits effects of MDMA (‘ecstasy’) in vitro and in humans in a randomized placebo-controlled laboratory study. Plos ONE. 2012c;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalant H. The pharmacology and toxicology of ‘ecstasy’ (MDMA) and related drugs. Can Med Assoc J. 2001;165:917–928. [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, et al. Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol. 2011;164:1949–1958. doi: 10.1111/j.1476-5381.2011.01499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG NC3Rs Reporting Guidelines Working Group. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King MV, Spicer CH, Sleight AJ, Marsden CA, Fone KC. Impact of regional 5-HT depletion on the cognitive enhancing effects of a typical 5-ht6 receptor antagonist, Ro 04-6790, in the Novel Object Discrimination task. Psychopharmacology. 2009;202:111–123. doi: 10.1007/s00213-008-1334-1. [DOI] [PubMed] [Google Scholar]
- McElrath K, O'Neill C. Experiences with mephedrone pre- and post-legislative controls: perceptions of safety and sources of supply. Int J Drug Policy. 2011;22:120–127. doi: 10.1016/j.drugpo.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Macerola AE, Swaby RTR, Jayson R, Korash C, King MV, Shortall SE, et al. Cathinone: the behavioural and neurotoxic effects in Lister Hooded rodents. J Psychopharmacol. 2011;25:A45. [Google Scholar]
- McGrath JC, Drummond GB, McLachlan EM, Kilkenny C, Wainwright CL. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maskell PD, De Paoli G, Seneviratne C, Pounder DJ. Mephedrone (4-methylmethcathinone)-related deaths. J Anal Toxicol. 2011;35:188–191. doi: 10.1093/anatox/35.3.188. [DOI] [PubMed] [Google Scholar]
- Mechan AO, Esteban B, O'Shea E, Elliott JM, Colado MI, Green AR. The pharmacology of the acute hyperthermic response that follows administration of 3,4-methylenedioxymethamphetamine (MDMA, ‘ecstasy’) to rats. Br J Pharmacol. 2002;135:170–180. doi: 10.1038/sj.bjp.0704442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Cao J, Kang J, Ying X, Ji J, Reynolds W, et al. Mephedrone, a new designer drug of abuse, produces acute hemodynamic effects in the rat. Toxicol Lett. 2012;208:62–68. doi: 10.1016/j.toxlet.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Wilhelm J, Peters FT, Maurer HH. Beta-keto amphetamines: studies on the metabolism of the designer drug mephedrone and toxicological detection of mephedrone, butylone, and methylone in urine using gas chromatography-mass spectrometry. Anal Bioanal Chem. 2010;397:1225–1233. doi: 10.1007/s00216-010-3636-5. [DOI] [PubMed] [Google Scholar]
- Miller ML, Creehan KM, Angrish D, Barlow DJ, Houseknecht KL, Dickerson TJ, et al. Changes in ambient temperature differentially alter the thermoregulatory, cardiac and locomotor stimulant effects of 4-methylmethcathinone (mephedrone) Drug Alcohol Depend. 2012 doi: 10.1016/j.drugalcdep.2012.07.003. doi: 10.1016/j.drugalcdep.2012.07.003 Jul 23 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash JF, Jr, Meltzer HY, Gudelsky GA. Elevation of serum prolactin and corticosterone concentrations in the rat after the administration of 3,4-methylenedioxymethamphetamine. J Pharmacol Exp Ther. 1988;245:873–879. [PubMed] [Google Scholar]
- Osorio-Olivares M, Rezende MC, Sepúlveda-Boza S, Cassels BK, Fierro A. MAO inhibition by arylisopropylamines: the effect of oxygen substituents at the beta-position. Bioorg Med Chem. 2004;12:4055–4066. doi: 10.1016/j.bmc.2004.05.033. [DOI] [PubMed] [Google Scholar]
- Parrott AC. MDMA and temperature: a review of the thermal effects of ‘Ecstasy’ in humans. Drug Alcohol Depend. 2012;121:1–9. doi: 10.1016/j.drugalcdep.2011.08.012. [DOI] [PubMed] [Google Scholar]
- Pedersen NP, Blessing WW. Cutaneous vasoconstriction contributes to hyperthermia induced by 3,4-methylenedioxymethamphetamine (ecstasy) in conscious rabbits. J Neurosci. 2001;21:8648–8654. doi: 10.1523/JNEUROSCI.21-21-08648.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehek EA, Schechter MD, Yamamoto BK. Effects of cathinone and amphetamine on the neurochemistry of dopamine in vivo. Neuropharmacology. 1990;29:1171–1176. doi: 10.1016/0028-3908(90)90041-o. [DOI] [PubMed] [Google Scholar]
- Redfern WS, MacLean MR, Clague RU, McGrath JC. The role of alpha 2-adrenoceptors in the vasculature of the rat tail. Br J Pharmacol. 1995;114:1724–1730. doi: 10.1111/j.1476-5381.1995.tb14963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockhold RW, Carlton FB, Jr, Corkern R, Derouen L, Bennett JG, Hume AS. Methcathinone intoxication in the rat: abrogation by dextrorphan. Ann Emerg Med. 1997;29:383–391. doi: 10.1016/s0196-0644(97)70351-2. [DOI] [PubMed] [Google Scholar]
- Rodsiri R, Spicer C, Green AR, Marsden CA, Fone KC. Acute concomitant effects of MDMA binge dosing on extracellular 5-HT, locomotion and body temperature and the long-term effect on novel object discrimination in rats. Psychopharmacology. 2011;213:365–376. doi: 10.1007/s00213-010-1921-9. [DOI] [PubMed] [Google Scholar]
- Schifano F, Albanese A, Fergus S, Stair JL, Deluca P, Corazza O, et al. Mephedrone (4-methylmethcathinone; ‘meow meow’): chemical, pharmacological and clinical issues. Psychopharmacology. 2011;214:593–602. doi: 10.1007/s00213-010-2070-x. [DOI] [PubMed] [Google Scholar]
- Shioda K, Nisijima K, Yoshino T, Kuboshima K, Iwamura T, Yui K, et al. Risperidone attenuates and reverses hyperthermia induced by 3,4-methylenedioxymethamphetamine (MDMA) in rats. Neurotoxicology. 2008;29:1030–1036. doi: 10.1016/j.neuro.2008.07.005. [DOI] [PubMed] [Google Scholar]
- Shortall SE, Pillidge KE, King MV, Green AR, Fone KCF. Behavioural and neurochemical responses to chronic intermittent mephedrone administration in the rat. J Psychopharmacol. 2011;25:A38. [Google Scholar]
- Shortall SE, Macerola AE, Swaby RT, Jayson R, Korsah C, Pillidge KE, et al. Behavioural and neurochemical comparison of chronic intermittent cathinone, mephedrone and MDMA administration to the rat. Eur Neuropsychopharmacol. 2012 doi: 10.1016/j.euroneuro.2012.09.005. doi: 10.1016/j.euroneuro.2012.09.005 [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Simmler LD, Buser TA, Donzelli M, Schramm Y, Dieu LH, Huwyler J, et al. Pharmacological characterization of designer cathinones in vitro. Br J Pharmacol. 2012;168:458–470. doi: 10.1111/j.1476-5381.2012.02145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague JE, Banks ML, Cook VJ, Mills EM. Hypothalamic-pituitary-thyroid axis and sympathetic nervous system involvement in hyperthermia induced by 3,4-methylenedioxymethamphetamine (Ecstasy) J Pharmacol Exp Ther. 2003;305:159–166. doi: 10.1124/jpet.102.044982. [DOI] [PubMed] [Google Scholar]
- Tariq M, Islam MW, al-Meshal IA, el-Feraly FS, Ageel AM. Comparative study of cathinone and amphetamine on brown adipose thermogenesis. Life Sci. 1989;44:951–955. doi: 10.1016/0024-3205(89)90494-3. [DOI] [PubMed] [Google Scholar]
- Vanattou-Saïfoudine N, McNamara R, Harkin A. Mechanisms mediating the ability of caffeine to influence MDMA (‘Ecstasy’)-induced hyperthermia in rats. Br J Pharmacol. 2010;160:860–877. doi: 10.1111/j.1476-5381.2010.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardakou I, Pistos C, Spiliopoulou CH. Drugs for youth via Internet and the example of mephedrone. Toxicol Lett. 2011;201:191–195. doi: 10.1016/j.toxlet.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Watson DJ, Marsden CA, Millan MJ, Fone KC. Blockade of dopamine D3 but not D2 receptors reverses the novel object discrimination impairment produced by post-weaning social isolation: implications for schizophrenia and its treatment. Int J Neuropsychopharmacol. 2012;15:471–484. doi: 10.1017/S1461145711000435. [DOI] [PubMed] [Google Scholar]
- Winstock A, Mitcheson L, Ramsey J, Davies S, Puchnarewicz M, Marsden J. Mephedrone: use, subjective effects and health risks. Addiction. 2011a;106:1991–1996. doi: 10.1111/j.1360-0443.2011.03502.x. [DOI] [PubMed] [Google Scholar]
- Winstock AR, Mitcheson LR, Deluca P, Davey Z, Corazza O, Schifano F. Mephedrone, new kid for the chop? Addiction. 2011b;106:154–161. doi: 10.1111/j.1360-0443.2010.03130.x. [DOI] [PubMed] [Google Scholar]
- Wood DM, Davies S, Greene SL, Button J, Holt DW, Ramsey J, et al. Case series of individuals with analytically confirmed acute mephedrone toxicity. Clin Toxicol (Phila) 2010;48:924–927. doi: 10.3109/15563650.2010.531021. [DOI] [PubMed] [Google Scholar]
- Wood DM, Greene SL, Dargan PI. Clinical pattern of toxicity associated with the novel synthetic cathinone mephedrone. Emerg Med J. 2011;28:280–282. doi: 10.1136/emj.2010.092288. [DOI] [PubMed] [Google Scholar]
- Wood DM, Hunter L, Measham F, Dargan PI. Limited use of novel psychoactive substances in South London nightclubs. QJM. 2012;105:959–964. doi: 10.1093/qjmed/hcs107. [DOI] [PubMed] [Google Scholar]


