Abstract
Background and Purpose
Phosphodiesterase 4 (PDE4) inhibitors produce potent antidepressant-like and cognition-enhancing effects. However, their clinical utility is limited by the major side effect of emesis, which appears to be PDE4 isoform-specific. Although PDE4D subtype plays the pivotal role in these therapeutic profiles, it is also the primary subtype responsible for emesis. Therefore, the aim of present research was to investigate whether long-form PDE4D variants mediate antidepressant-like and cognition-enhancing effects, but are irrespective with emesis.
Experimental Approach
In mice microinfused with lentiviral vectors that contained shRNA-mir hairpin structure targeting long-form PDE4Ds into bilateral prefrontal cortices, the tail-suspension and forced-swim tests were used to measure antidepressant-like effects; novel object recognition and Morris water-maze tasks were used to determine cognition-enhancing effects. The emetic potential was assessed by alpha2 adrenergic receptor-mediated anaesthesia, a surrogate measure of emesis. Intracellular cAMP signalling was analysed by time-resolved FRET immunoassay and Western-blot. Dendritic complexity was assessed by Golgi staining.
Key Results
Microinfusions of lentiviral PDE4D-shRNA down-regulated PDE4D4 and PDE4D5, and imitated the antidepressant-like and cognition-enhancing effects of the prototypical PDE4 inhibitor rolipram. The behavioural effects were related to dendritic complexity and mediated by the increased cAMP signalling. In addition, these effects were not enhanced in the presence of rolipram. Finally, while rolipram shortened the duration of combined anaesthesia, RNA interference-mediated PDE4D knock-down in the prefrontal cortex did not.
Conclusion and Implications
These data suggest that long-form PDE4Ds, at least PDE4D4 and PDE4D5, may be the promising targets for the development of PDE4 variant-selective inhibitors as the new pharmacotherapies for depressive disorders and neurodegenerative diseases involving memory deficits.
Keywords: phosphodiesterase 4D, RNA interference, antidepressant, cognition, cAMP, dendritic complexity
Introduction
Phosphodiesterase-4 (PDE4), a major PDE isozyme specifically hydrolysing cAMP, has been the potential therapeutic target for depression (O'Donnell and Zhang, 2004; Houslay et al., 2005; Zhang, 2009) and memory deficits (Zhang and O'Donnell, 2000; Zhang et al., 2004; Bourtchouladze et al., 2003). Rolipram, a prototypical selective inhibitor of PDE4, has been shown to exert antidepressant activity (O'Donnell and Zhang, 2004; Zhang, 2009) and memory enhancement (Barad et al., 1998; Rutten et al., 2008; Reneerkens et al., 2009) by elevating concentrations of intracellular cAMP, which activates the downstream signalling cascade, including cAMP-dependent response element-binding protein (CREB) (Li et al., 2009; 2011) and brain-derived neurotrophic factor (BDNF) (Fujimaki et al., 2000; Itoh et al., 2004), and subsequently improves neuroplasticity (Fujioka et al., 2004; Smith et al., 2009), the neurobiological basis of antidepressant activity (Manji et al., 2001) and memory enhancement (Dumitriu et al., 2010). Unfortunately, no PDE4 inhibitors to date have been approved for clinical utility for these therapeutic profiles due to the concomitant side effects, in particular emesis (Robichaud et al., 2001; Spina, 2008), which appears to be PDE4 isoform-specific (Robichaud et al., 2002b).
PDE4 has four isoforms (PDE4A-D) with at least 25 splice variants (Conti et al., 2003; O'Donnell and Zhang, 2004; Zhang, 2009). Studies have implicated that PDE4D may play a pivotal role in the antidepressant-like and cognition-enhancing effects of PDE4 inhibitor (Zhang et al., 2002; Burgin et al., 2010; Bruno et al., 2011; Li et al., 2011). However, PDE4D has been shown to be responsible for emesis, the major side effect of PDE4 inhibitors (Robichaud et al., 2002a; Li et al., 2011), leading to the difficulty in disassociating antidepressant and memory-enhancing properties from emesis induced by PDE4D inhibition. Therefore, it was of particular interest to know the roles of PDE4D variants in antidepressant-like and cognition-enhancing effects, as well as in emetic responses; although, to date, it has been difficult to target individual PDE4D variants because of their high homologies.
The 25 PDE4 variants are divided into four categories based on the upstream conserved regions 1 and 2 (UCR1 and UCR2): long form, short form, super-short form and dead-short form (Houslay, 2001; O'Donnell and Zhang, 2004; Zhang, 2009). Long-form PDE4Ds, which are characterized by the presence of the intact UCR1 and UCR2 domains, have greater capacity for cAMP degradation (Oki et al., 2000; Zhang, 2009). Given the role of cAMP signalling in antidepressant-like and cognition-enhancing effects of PDE4 inhibitors as described earlier, long-form PDE4Ds may be more important for antidepressant-like and cognition-enhancing effects. The latter has been demonstrated by our recent study showing that knock-down of long-form PDE4Ds in the hippocampus produces memory enhancement in mice (Li et al., 2011).
The purpose of this study was to determine the key roles of long-form PDE4Ds in antidepressant-like and cognition-enhancing effects in mice. The RNA interference (RNAi) technique was applied to knock-down long-form PDE4D given the lack of selective inhibitors of individual PDE4D variants. More specifically, lentiviral shRNA-mir hairpin structure targeting long-form PDE4Ds (Li et al., 2011) were microinfused into the prefrontal cortex (PFC), a brain region with high expression of PDE4D (Perez-Torres et al., 2000; Lakics et al., 2010) and, importantly, involved in depression (Soares and Mann, 1997) and cognition (Romanski, 2004). Animals were then examined for antidepressant-like and memory-enhancing behaviours, cAMP signalling and dendritic morphology in the brain. The results provided promising demonstration that knock-down of long-form PDE4D in the PFC produced antidepressant-like and cognition-enhancing effects on behaviours by up-regulating cAMP signalling and dendritic complexity.
Methods
Animals
Adult male Institute for Cancer Research mice initially weighing 22–24 g (Vital River, Beijing, China) were housed at room temperature with a humidity of 50–60% and a 12-h light/dark cycle. Food and water were available ad libitum. All procedures followed the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications no. 80-23) and were approved by the Animal Care and Use Committees of Beijing Institute of Pharmacology and Toxicology. Efforts were made to minimize suffering and to reduce the number of animals used.
Materials
Ketamine, xylazine and rolipram were all purchased from Sigma-Aldrich (St. Louis, MO, USA). Lentiviral vectors containing the non-targeting negative control (NC) sequence or shRNA sequence in a miRNA scaffold (Li et al., 2011) targeting long-form PDE4D (4DmiR) were provided by Dr. Steven Wilson of University of South Carolina and Dr. Han-Ting Zhang of West Virginia University. The vectors contained enhanced green fluorescence protein (EGFP) as a reporter for tracking lentivirus-mediated expression.
Experimental design
Animals were randomly divided into four groups: (i) NC + vehicle (Veh; 2.5% DMSO); (ii) NC + rolipram (Rol, 1.25 mg·kg−1); (iii) 4DmiR + Veh; and (iv) 4DmiR + Rol. The dose of rolipram was chosen based on our previous studies (Li et al., 2009; 2011). 4DmiR or NC was microinfused into bilateral prefrontal cortices on day 0. Rolipram or vehicle was administered (i.p.) once a day from day 0 to day 42, as illustrated in Figure 1. Training or testing was conducted 1 h after rolipram treatment. All behavioural studies were performed by two observers blind to the treatment conditions; the data were pooled for statistical analysis.
Figure 1.
Experimental design of the test order. Lentiviral vectors (4 × 106 TU·μL−1, 1 μL·side−1) harbouring the NC sequence or 4DmiR were microinfused into bilateral prefrontal cortices of mice following the 1-week acclimatization. Rolipram (1.25 mg·kg−1) or its vehicle (saline containing 2.5% DMSO) was injected (i.p.) once daily, beginning from 6 h after the viral infusions (day 0) and continuing until the end of behavioural tests. Behavioural experiments were performed 21 days after the PFC microinfusions and continued till d42 when the animals were sacrificed for biochemical assays. SAC, sacrifice.
Lentiviral transduction
HEK 293 cells were cultured in DMEM (Gibco, Carlsbad, CA, USA) supplemented with 10% FBS and maintained in a humidified incubator at 37°C with 5% CO2. HEK 293 cells were plated into 6-well plates and cultured for 24 h before the transduction of lentiviral vectors (2.0 × 104 TU·μL−1). The medium was replaced 48 h later, and cells were further incubated until 72 h or longer for up to 96 h as required.
Quantitative real-time PCR (qRT-PCR)
This was performed following the procedures described previously (Zhang et al., 2009; Zhang and Li, 2010). Total RNA was extracted by Trizol and first-strand cDNA was generated using the moloney murine leukaemia virus reverse transcriptase system (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol. qRT-PCR was performed using ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) and SYBR Green PCR master mix (Toyobo, Osaka, Japan). The sequences of primers and annealing temperatures were listed in Table 1. Experiment was performed in triplicate and the negative controls lacking template cDNA were included in each experiment. Data were analysed using the 2−ΔΔCT method (Livak and Schmittgen, 2001) with normalization to GAPDH.
Table 1.
Primer sequences and optimal annealing temperatures used for SYBR green-based quantitative real-time polymerase chain reaction
| Gene | Accession | Primer sequence (5′-3′) | Ta (°C) | |
|---|---|---|---|---|
| PDE4A | AF208021 | Fwd | ACACTGTCAGAGGAGACATGCCA | 60.0 |
| Rev | ACCTGGTTTCCTGACCTGCTCATT | |||
| PDE4B | AF208023 | Fwd | ATTTCCAGGCCAACCACACTACCT | 65.0 |
| Rev | ATGAAGTACCAGTCCCGACGAAGA | |||
| PDE4D | NM_011056 | Fwd | AGGGTCTGGGCTGATTCTC | 60.0 |
| Rev | GTTGATGGATGGTTGGTTGC | |||
| PDE4D3 | NM_001113332 | Fwd | CTCAGCAGACGACAAGCC | 63.5 |
| Rev | CCGCAGGGATTTCACAAG | |||
| PDE4D4 | AF031373 | Fwd | CATTCAACTGTTCTTGTGG | 64.5 |
| Rev | AATCTTGTCCTAGTTCTTGG | |||
| PDE4D5 | EF102484 | Fwd | AGCGAGGAAATCCGTTTCTCCCAA | 60.0 |
| Rev | TCCCGCTGATGTGCCATTGT | |||
| GAPDH | GU214026 | Fwd Rev | CGCTGAGTACGTCGTGGAG GAGGAGTGGGTGTCGCTGTT | 60.0 |
Ta, optimal annealing temperature; Fwd, forward; Rev, reverse primers; PDE4, phosphodiesterase 4.
Lentiviral microinfusions
Bilaterally prefrontal cortical infusions were performed via a 10-μL Hamilton microsyringe with a 30-G needle fitted to the arm of the stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). An incision was made in the scalp and a hole was drilled in the skull over the injection site following the coordinates (Nagai et al., 2007): anterior–posterior (AP, from Bregma), +1.5 mm, medial–lateral (ML, from midline), ±0.5 mm and dorsal–ventral (DV, from dura) −1.2 mm. Lentiviral vectors containing NC or 4DmiR (4 × 106 TU·μL−1, 1 μL·side−1) were infused at a rate of 0.2 μL·min−1 using a UMP3 microsyringe injector and Micro4 controller (World Precision Instruments, Sarasota, FL, USA) and the needle was slowly retracted after an additional 5 min. The microinjection sites were observed under a fluorescence microscope after the frozen coronal sections (30 μm) preparation as described previously (Li et al., 2009).
Western-blotting
This was performed as described previously (Wang et al., 2010; 2012) with minor modifications. Punched prefrontal cortical tissues (3 mm in diameter around the injection site on both sides) were extracted by RIPA plus protease inhibitor and phosphatase inhibitor cocktail (Thermo Scientific Pierce, Rockford, IL, USA). Proteins were separated by electrophoresis and transferred to nitrocellulose membranes, which were then incubated overnight with rabbit anti-pCREB (Ser133) IgG, anti-CREB IgG (both 1:500; Cell Signaling Technology, Danvers, MA, USA), anti-PDE4A IgG (1:500), anti-PDE4B IgG (1:500), anti-PDE4D IgG (all 1:500; FabGennix, Frisco, TX, USA) or anti-β-actin IgG (1:3000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C. After washing and incubating with secondary antibodies, the specific bands were detected and quantified using Gel-Pro Analyzer software (Media Cybernetics, Bethesda, MD, USA).
cAMP assay
The punched tissues were extracted by RIPA plus protease inhibitor and phosphatase inhibitor cocktail, and 0.5 mM isobutyl-methyl-xanthine. The assays were performed according to the manufacturer's instruction of Lance cAMP kit (PerkinElmer, Waltham, MA, USA). Counts at 665 nm obtained in cAMP standard curves allowed the quantitative determination of the cAMP concentration in samples. The signal at 615 nm is useful to identify dispensing or quenching problems.
Golgi impregnation
Golgi staining was performed according to manufacturer's instructions of FD Rapid GolgiStain™ kit (FD NeuroTechnologies, Ellicott City, MD, USA). Briefly, the fresh brains were impregnated into the solution containing potassium dichromate, mercuric chloride and potassium chromate for 2 weeks at room temperature in the dark. Tissues were then transferred into the cryoprotectant solution and stored at 4°C for 3 days in the dark. Coronal sections of 100-μm thickness from the PFC (±0.25 mm from the coronal section through the injection site in rostro-caudal direction, a total of four to five sections per animal) were cut on a freezing microtome (Leica, Wetzlar, Germany) and collected on clean gelatin-coated microscope slides. Following drying, sections were rehydrated, reacted in the developing solution (an ammonium hydroxide-based solution), and dehydrated with 50, 75, 95, and 100% ethanol, respectively. Finally, sections were cleared in xylene and coverslipped with resinous mounting media.
Dendrite analysis
The criteria used to select neurons for reconstruction have been fully described previously (Flores et al., 2005; Martinez-Tellez et al., 2005). Briefly, only fully impregnated pyramidal neurons located in the region of infection without obviously truncated dendrites were included in our analysis. Cells were digitally reconstructed and traced using Image-Pro Plus software (Media Cybernetics, Bethesda, MD, USA).
A Sholl analysis (Sholl, 1953) was then performed to determine the basal dendritic length and the number of branching points, which reflected dendritic complexity (Wang et al., 2008). Equidistant (10 μm apart) concentric rings were placed over the dendritic tree tracings using the centre of the soma as the reference point (Figure 5B). The basal dendritic length was calculated by multiplying the number of intersections of each ring by 10 μm and the number of dendritic branching points was counted in each successive concentric zone. For quantitative analysis, three to five neurons from each hemisphere per section, three to four sections per animal and four animals per group (n = 96–120 neurons per group) were analysed.
Figure 5.
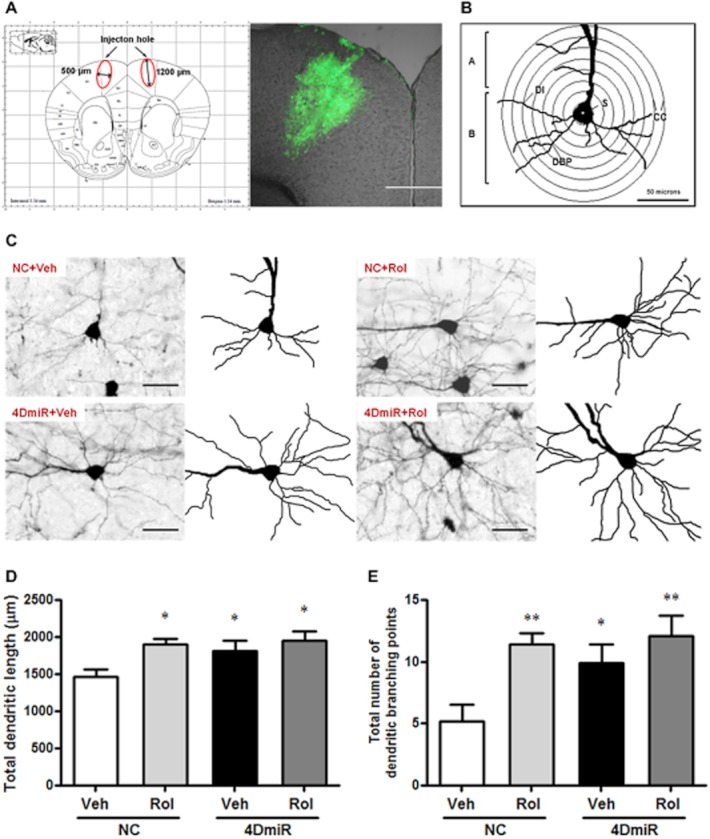
Effects of 4DmiR and/or rolipram (1.25 mg·kg−1) on the dendritic morphology of cortical neurons in the region of infection. (A) The brain region of infection was indicated by the red ellipse corresponding to the region with green fluorescent protein expression. Profile map was modified from Paxinos and Franklin (2001). Scale bar = 500 μm. (B) Diagram of a reconstructed dendritic tree and equidistant (10 μm apart) concentric circles that were used for quantitative analysis. A, apical dendrites; B, basal dendrites; DBP, dendritic branching points; DI, dendritic intersections; CC, concentric circles; S, soma. Scale bar = 50 μm. (C) Magnified (20 × objective) images of Golgi-impregnated cortical pyramidal neurons in the region of infection. Scale bar = 50 μm. (D) Similar to the effect of chronic treatment with rolipram, a significant increase in the total basal dendritic length was observed in mice treated with 4DmiR, which was not altered in the presence of rolipram. (E) Rolipram or 4DmiR alone or in combination significantly increased the total dendritic branching points. Values shown are means ± SEM; n = 96–120 neurons from four animals per group; *P < 0.05, **P < 0.01 versus the control (NC + Veh).
Behavioural procedures
Open-field (OF) test
This test was performed as described previously (Li et al., 2009). Mice were placed individually in a white Plexiglas box (60 × 60 × 16 cm) with the floor divided into16 equal-sized squares. Line crossings (with all four paws placed into a new square) and rears (with both front paws raised from the floor) were recorded in a 5-min period. Apparatus was thoroughly cleaned using 10% ethanol after each animal.
Novel object recognition (NOR) test
This was performed as described previously (Li et al., 2011). One day, after the 5-min habituation to the testing box, mice were individually exposed to two identical objects placed in two corners of the box for 5 min. After 24 h, mice were placed in the same apparatus to start the second 5-min trial, during which the animals were exposed to a familiar object and a novel object, placing in the same positions. The accumulative time exploring each object (Tf and Tn for familiar and novel objects, respectively) was recorded for determination of the recognition index [RI = Tn/(Tn + Tf)]. Exploration was defined as initiatively facing, sniffing or touching the object (within 2 cm from the object).
Tail-suspension test (TST)
Behavioural despair was induced by the TST according to the procedure described previously (Zhang et al., 2002). Briefly, mice were suspended individually 40 cm above the floor by adhesive tapes without acoustical or visual interference. The tape attachment was approximately 1 cm from the tip of the tail. The total time spent immobile during the 6-min test period was scored. Immobility was defined as the absence of any limb or body movement.
Forced-swim test (FST)
The test was performed in mice as described previously (Li et al., 2009). In brief, mice were individually placed into an open glass cylinder (20 cm diameter, 45 cm height) filled with water (23 ± 1°C) to a depth of 28 cm. Animals were allowed to swim for 6 min, and the cumulative immobility time in the last 4 min was recorded. Immobility was defined as floating motionlessly in the water without additional movement except that necessary to keep its head above the water.
Morris water-maze (MWM) task
MWM task was performed as previously described (Li et al., 2011) with minor modifications. A circular water tank (100 cm diameter, 35 cm depth) was filled with 21 ± 1°C water (up to 17 cm depth), which was made opaque with powdered milk. The pool was conceptually divided into four identical quadrants. A platform was fixedly located in the centre of one of the quadrants and submerged 1 cm under the water surface. Mice were trained to escape by swimming and climbing onto the platform during the acquisition trials, which were carried out for three consecutive days (6 trials × 2 days plus 4 trials × 1 day; 16 trials in total). The escape latency for each mouse was recorded. Mice failing to find the platform within 90 s were guided to the platform and allowed to stay on it for 30 s. Twenty-four hours after the last acquisition training session, the probe trial was conducted in the absence of the platform. The number of entries and the time spent in the target quadrant were recorded with the cut-off time 60 s.
Alpha2 adrenergic receptor-mediated anaesthesia
This test was performed as described previously (Robichaud et al., 2002a,b). In brief, animals were injected (i.p.) with a combination of xylazine (10 mg·kg−1) and ketamine (80 mg·kg−1). Fifteen minutes later, rolipram or vehicle was injected (i.p.) and the animal was placed on its back. The end point of anaesthesia duration was determined by the restoration of the righting reflex when the animal spontaneously turned itself from the supine position to the prone position.
Statistical analysis
Data shown are expressed as means ± SEM and analysed with GraphPad Prism software (GraphPad, San Diego, CA, USA). All data were analysed using one-way anova except for the data from the acquisition training of the MWM and body weight, which were analysed using two-way anova. Bonferroni's tests were used for post hoc multiple treatment comparisons. A P-value of less than 0.05 was considered to be significant.
Results
4DmiR down-regulates PDE4D4/5 in vitro and in vivo
Fluorescent microscopy showed that 4DmiR and NC were well expressed in HEK 293 cells, as indicated by EGFP-positive cells (green) (Figure 2A). 4DmiR-treated cells displayed a significant decrease in PDE4D mRNA expression, when compared with NC-treated cells (P < 0.05); by contrast, the expressions of PDE4A and PDE4B were not changed (Figure 2B). Among the variants of long-form PDE4D, the mRNA levels of PDE4D4 and PDE4D5 were reduced by 65.1% (P < 0.01) and 68.3% (P < 0.01), respectively; PDE4D3 mRNA tended to be decreased, but it was not statistically significant.
Figure 2.
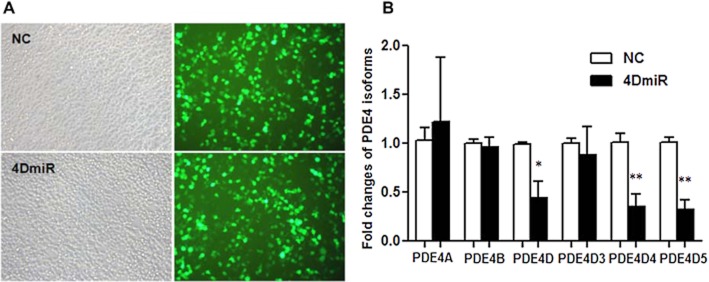
Effects of lenti-PDE4D-shRNA on expression of PDE4 isoforms and variants in transfected HEK293 cells measured by quantitative real-time PCR. (A) 4DmiR and NC were well expressed in HEK293 cells as indicated by EGFP (green) observed under a fluorescence microscope. Scale bar = 100 μm. (B) The values of densitometric analysis were calculated by the 2−ΔΔCT method and normalized by the level of GAPDH. The mean ± SEM of three independent experiments is shown; *P < 0.05, **P < 0.01 versus corresponding NC.
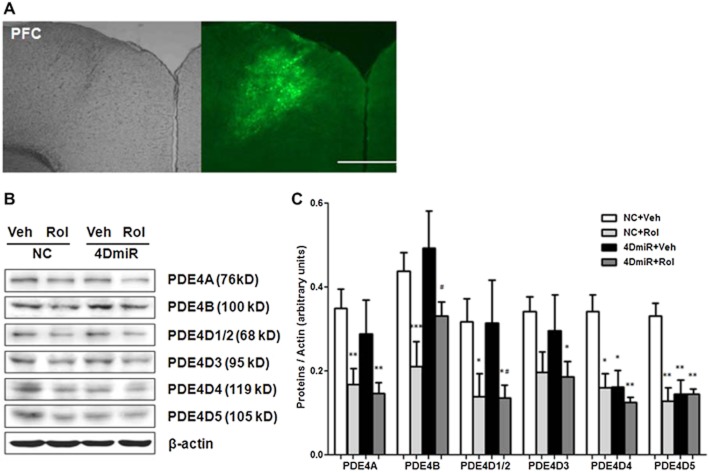
The lentivirus-mediated transduction in vivo was traced by the high and specific expression of EGFP (Figure 3A). The expressions of PDE4D4 and PDE4D5 were significantly reduced [F(3,12) = 2.271; P < 0.05 and P < 0.01, respectively;] compared with the control (NC plus vehicle) (Figure 3B and C), but those of other PDE4 isoforms (i.e. PDE4A and PDE4B), short-form PDE4Ds (PDE4D1/2) and PDE4D3 were not significantly changed. In addition, rolipram did not alter 4DmiR-induced decreases in expression of PDE4D4 and 4D5.
Figure 3.
Effects of 4DmiR and/or rolipram (1.25 mg·kg−1) on expression of PDE4 isoforms and variants in the PFC of mice. (A) Microinjection sites (left panels) and high, specific expressions of EGFP (green; right panels) in the PFC observed under fluorescence microscopy. Scale bars = 500 μm. (B) Representative immunoblots of PDE4 isoforms and variants. The prefrontal cortical tissues of 3 mm in diameter around the injection site were punched out for Western-blotting analysis. (C) The histogram represents semi-quantitative results of Western-blotting analysis. The values of densitometric analysis were normalized by the level of β-actin. Values shown are means ± SEM; n = 4–5; *P < 0.05, **P < 0.01, ***P < 0.001 versus the corresponding controls (NC + Veh); #P < 0.05 versus NC + 4DmiR.
4DmiR activates the cAMP signalling pathway
As shown in Figure 4, mice treated with 4DmiR displayed increases in cAMP [F(3,12) = 5.890; P < 0.05], phosphorylated CREB (pCREB) [F(3,12) = 5.099; P < 0.05] and BDNF [F(3,12) = 6.343; P < 0.05] in the frontal cortex, similar to the mice treated with rolipram. In addition, the effects of 4DmiR on the cAMP signalling were not changed in the presence of rolipram. These results suggest that long-form PDE4Ds are important in rolipram-induced activation of cAMP signalling.
Figure 4.
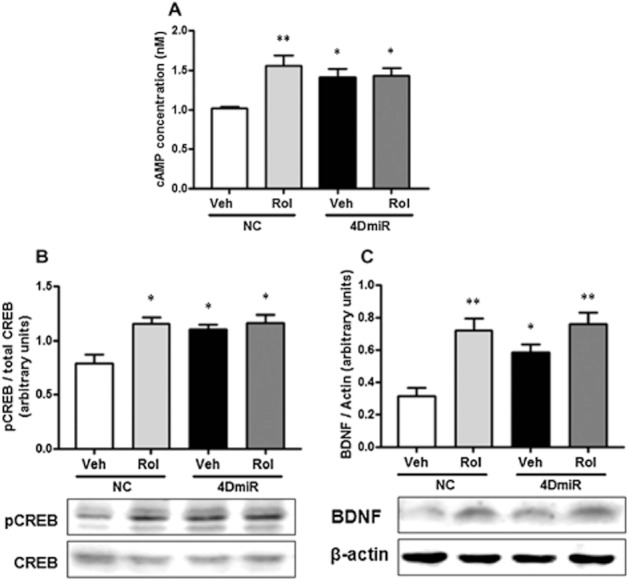
Effects of 4DmiR and/or rolipram (1.25 mg·kg−1) on the level of cAMP, phosphorylation of CREB, and expression of BDNF in the prefrontal cortices of mice. The prefrontal cortical tissues of 3 mm in diameter around the injection site were dissected for cAMP and Western-blotting assays. (A) Rolipram and/or 4DmiR treatment increased cAMP. (B, C) Rolipram and/or 4DmiR treatment increased pCREB (B) and BDNF (C). Lower panels are representative immunoblots detected by Western-blotting and upper panels are the corresponding quantifications. The values of densitometric analysis were normalized by the level of total CREB and β-actin, respectively. Values shown are means ± SEM; n = 4–5; *P < 0.05, **P < 0.01 versus the control (NC + Veh).
4DmiR increases the dendritic complexity
Golgi staining showed the pyramidal neurons located in the region of infection (Figure 5C). Significant increases of total basal dendritic length [F(3,416) = 4.046; P < 0.05] and branching points [F(3,416) = 5.567; P < 0.05] were found in mice treated with 4DmiR, similar to the mice treated with rolipram. In addition, the effects of 4DmiR on the dendritic complexity were not changed in the presence of rolipram (Figure 5D and E). These findings suggest that long-form PDE4Ds are important in rolipram-induced increases in dendritic complexity.
4DmiR microinjections elicit antidepressant-like behaviours
The TST and FST results depicted in Figure 6 showed that 4DmiR microinjections statistically reduced the duration of immobility in the TST [F(3,48) = 2.520; P < 0.05] and FST [F(3,48) = 4.550; P < 0.05], when compared with the control. Similar effects were observed in mice treated with rolipram. Furthermore, rolipram treatment did not significantly alter 4DmiR-induced antidepressant-like effects in either test. These results suggest that 4DmiR microinfusions produce antidepressant-like effects and long-form PDE4Ds, in particular PDE4D4 and PDE4D5, are important in rolipram-induced antidepressant activity.
Figure 6.
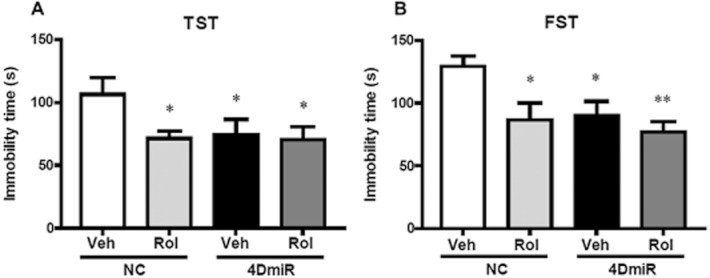
Effects of 4DmiR and/or rolipram on antidepressant-like behaviour in mice. Rolipram (1.25 mg·kg−1) administration and/or long-form PDE4D knock-down in the prefrontal cortices significantly decreased immobility in the TST (A) and FST (B). Values shown are means ± SEM; n = 12–14; *P < 0.05, **P < 0.01 versus the control (NC + Veh).
4DmiR microinjections elicit cognition-enhancing behaviours
In the NOR task, results revealed that the increased recognition index (Figure 7D) and duration in exploring novel object during the test session (Figure 7B and C) in mice treated with 4DmiR or rolipram alone or in combination [F(3,48) = 3.169; P < 0.05 for all] compared with the control. Consistent with this, in the MWM task, 4DmiR and/or rolipram-treated mice displayed robust increases in both duration [F(3,48) = 3.438; P < 0.01] and entries [F(3,48) = 4.346; P < 0.01] in the target quadrant, as shown in Figure 7F and G. Notably, during the 3-day acquisition training, significant changes in mice treated with 4DmiR alone or in combination with rolipram were shown, when compared with the control (Figure 7E). These results suggest that long-form PDE4Ds, in particular PDE4D4 and PDE4D5, are important in rolipram-induced cognition-enhancing effects.
Figure 7.
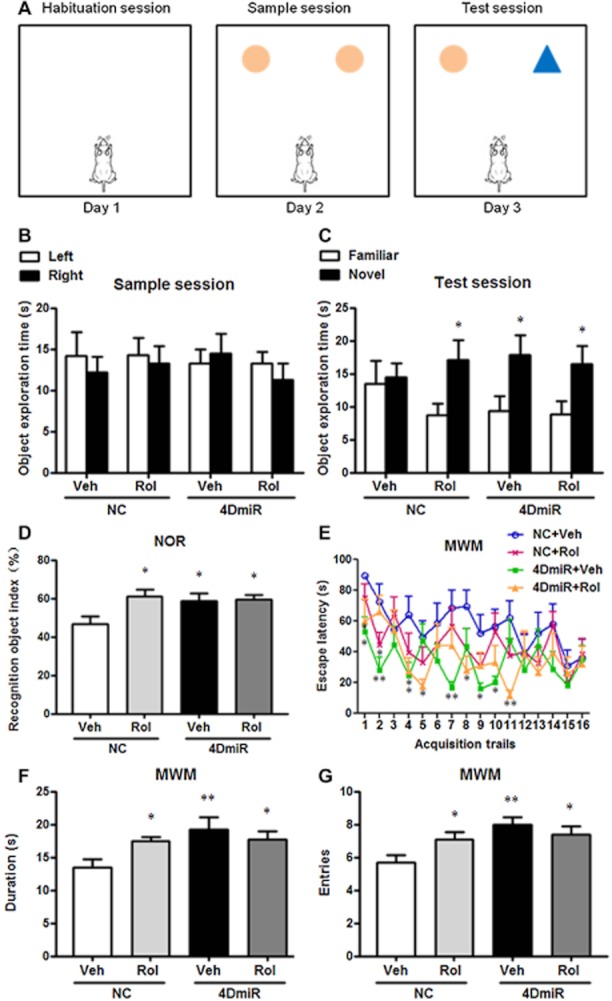
Effects of 4DmiR and/or rolipram (1.25 mg·kg−1) on cognition-enhancing behaviour in mice. (A) During the habituation session in the object recognition test, mice were released into the empty arena for 5 min of exploration and habituation; during the sample session, mice were allowed to explore two identical objects within 5 min; during the test session, one of the objects was replaced with a novel one and mice were reintroduced to the arena for another 5 min. (B, C) The duration of mice in exploring novel and familiar objects during the sample and test sessions. (D) Increased recognition index in the object recognition test in mice treated with 4DmiR and/or rolipram. The recognition index was the exploration time in the novel object (Tn) divided by the total exploration time (i.e. Tn plus Tf, the exploration time for the familiar object). (E) Escape latency during the acquisition trials (6 trials × 2 days plus 4 trials × 1 day) in the water-maze test in mice treated with 4DmiR and/or rolipram. (F, G) Increased duration and entries in the target quadrant in the probe trial of the water-maze test in mice treated with 4DmiR and/or rolipram. Values shown are means ± SEM; n = 12–14; *P < 0.05 versus the exploration time for the familiar object for Figure 7C; *P < 0.05, **P < 0.01 versus the control (NC + Veh) for Figure 7D–G.
4DmiR microinjections have no effects on locomotor activity and body weight
In the OF test, no significant alterations of line crossings and rears were observed in mice treated with 4DmiR or rolipram alone or in combination (Figure 8A and B), indicating that 4DmiR and/or rolipram did not affect locomotor activity of mice and the antidepressant-like and memory-enhancing effects were not due to potential locomotor activity changes.
Figure 8.
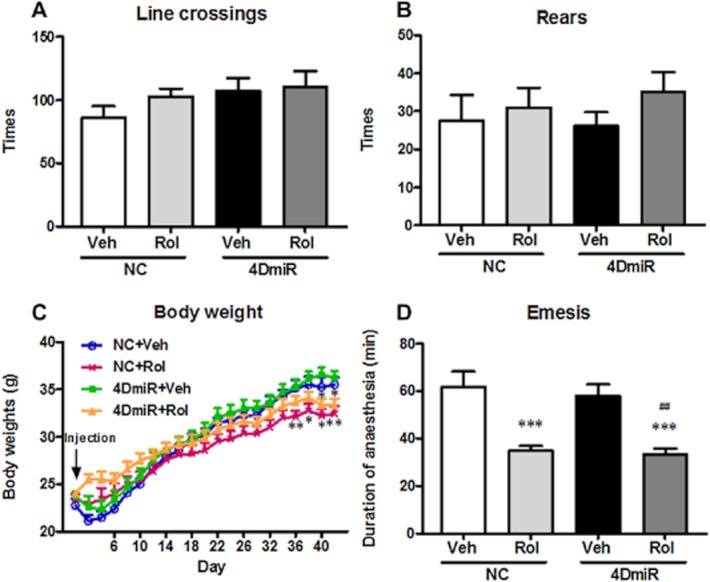
Effects of 4DmiR and/or rolipram (1.25 mg·kg−1) on locomotor activity, α2 adrenergic receptor-mediated anaesthesia and body weight in mice. (A, B) The times of line crossings (A; with all four paws placed into a new square) and rears (B; standing on rear paws) were recorded in a 5-min period in the open-field test. None of the treatments altered locomotor activity of animals. (C) Chronic administration of rolipram significantly decreased the body weight, but 4DmiR treatment alone did not. (D) Rolipram alone or plus 4DmiR significantly decreased the duration of anaesthesia induced by xylazine/ketamine, but 4DmiR treatment alone did not. Values shown are means ± SEM; n = 12–14. *P < 0.05, **P < 0.01, ***P < 0.001 versus the control (NC + Veh); ##P < 0.01 versus NC + 4DmiR.
While rolipram alone or in combination with 4DmiR significantly disrupted the normal gain in body weight as compared with the control starting from d36 [rolipram alone: F(3,30) = 3.807, P < 0.01 for d36, F(3,30) = 4.096, P < 0.05 for d38, F(3,30) = 5.830, P < 0.05 for d40, F(3,30) = 5.656, P < 0.01 for d42; rolipram plus 4DmiR: F(3,30) = 4.096, P < 0.01 for d38, F(3,30) = 5.830, P < 0.01 for d40; Figure 8C], 4DmiR alone did not affect the body weight gain. This result indicates that chronic administration of rolipram may affect the appetite whereas long-form PDE4D knock-down in the PFC does not.
Emetic potential is not associated with long-form PDE4D
As shown in Figure 8D, the duration of ketamine/xylazine-induced anaesthesia was robustly reduced in mice treated with rolipram alone or in combination with 4DmiR, when compared with the control [F(3,30) = 11.26; both P < 0.001]. In contrast, long-form PDE4D knock-down in the PFC had no effect on anaesthesia duration. As compared with mice treated with vehicle plus 4DmiR, the duration of anaesthesia was also shortened by a combination of rolipram and 4DmiR (P < 0.01). This result suggests that down-regulation of long-form PDE4Ds, at least PDE4D4 and PDE4D5, in the PFC may not cause emesis.
Discussion and conclusions
It has been demonstrated that PDE4D is important in the mediation of antidepressant activity and memory (Zhang et al., 2002; Burgin et al., 2010; Li et al., 2011). However, the lack of selective inhibitors of individual PDE4D variants and the complexity of the PDE4 family (Conti et al., 2003; Houslay et al., 2005) have made it impossible to use conventional pharmacological approaches or gene knockout techniques to identify the role of PDE4D variants in these properties. Using RNAi-mediated gene silencing, we were able to knock-down long-form PDE4Ds in the PFC in mice, by which we demonstrated that knock-down of long-form PDE4Ds, in particular the PDE4D4 and PDE4D5, is sufficient to produce antidepressant-like and cognition-enhancing effects. The latter is consistent with our recent study (Li et al., 2011).
Given the difficulty in targeting individual PDE4D variants because of their high homologies, we designed the shRNA sequence targeting nucleotides 642–662 of the PDE4D coding sequence, which correspond to amino acids 214–221 within the UCR1 domain of long-form PDE4Ds (Li et al., 2011). The lentivirus was selected as the vector for the purpose of stable and long-term gene suppression (Schratt et al., 2006). Our data revealed that lentiviral vector-delivered shRNA was well expressed in the PFC. While the short-form PDE4Ds such as 4D1 and 4D2 were not changed, long-form PDE4Ds such as 4D4 and 4D5 were significantly down-regulated and 4D3 also tended to be decreased. The other long-form PDE4Ds (i.e. 4D7-9 and 4D11) were not examined due to the unavailability of specific antibodies, but their roles cannot be excluded. In other words, the alteration of behaviours, cAMP signalling and dendritic morphology should still be ascribed to the group of long-form PDE4Ds. The role of long-form PDE4D4 and PDE4D5 was merely identified in the present study. Further studies are required to identify the potential contribution of the other PDE4Ds.
Down-regulation of long-form PDE4Ds, at least PDE4D4 and PDE4D5, produced antidepressant-like effects, as indicated by decreased immobility in the TST and FST. Furthermore, we compared the antidepressant-like effect of 4DmiR with that of combined rolipram and 4DmiR. If long-form PDE4Ds were not important, inhibition of the remained PDE4 subtypes and variants by rolipram should have further enhanced this effect. However, this was not the case; the antidepressant-like effects of 4DmiR did not differ from those of combined rolipram and 4DmiR, supporting the important role of long-form PDE4Ds in the mediation of antidepressant activity. These results not only are consistent with antidepressant responses in PDE4D knockout mice (Zhang et al., 2002), but also extended the findings for the first time to the role of PDE4D variants.
PDE4D is also involved in the mediation of memory. Inhibition or deficiency of PDE4D enhances synaptic plasticity and memory (Burgin et al., 2010; Bruno et al., 2011; Li et al., 2011). Most recently, we further demonstrated that knock-down of long-form PDE4Ds in the hippocampus enhances memory (Li et al., 2011). Here we extended the study to the PFC, a brain region important for mediating cognition associated with depression (Kerestes et al., 2012). Knock-down of long-form PDE4D in the PFC enhanced object recognition memory, which was not altered in the presence of rolipram, indicating a predominant role of long-form PDE4D in the mediation of cognition. In addition, long-form PDE4D knock-down in the PFC enhanced spatial memory in the MWM task, although the MWM was initially used for testing hippocampus-dependent spatial memory (Morris et al., 1982). Given that specific down-regulation of long-form PDE4D in the PFC did not cause any changes in the hippocampus including PDE4D expression, cAMP signalling and neurogenesis (data not shown), the PFC should be the main region responsible for the cognition-enhancing effects, although the crosstalk of cortico-parahippocampal-hippocampal connectivity may not be excluded (Witter et al., 2000).
It should be pointed out that the infusion sites using the coordinates (AP +1.5 mm, ML ± 0.5 mm, DV-1.2 mm; Nagai et al., 2007) in the present study, which were verified by EGFP (Figure 3A), are the secondary motor areas (M2) based on the mouse brain atlas (Paxinos and Franklin, 2001). In fact, M2 belongs to the PFC, rather than the motor cortex (Uylings et al., 2003). The term M2 identified by Paxinos and Franklin (2001) is the same to the term frontal area 2 (Fr2) introduced by Zilles (Zilles, 1985) or the medial precentral area (PrCm) defined by Krettek and Price (Krettek and Price, 1977). M2 (or Fr2, or PrCm) is one of the prefrontal subregions (Uylings et al., 2003). Therefore, it is not surprising that manipulation of cAMP signalling in the PFC altered object recognition memory. Regardless, locomotor activity of mice was not affected by knock-down of long-form PDE4Ds in the PFC, as illustrated by Figure 8A and B, suggesting that the observed behavioural differences, including antidepressant activity and cognition enhancement, were not due to potential locomotor activity changes.
Studies have shown that the particular role of PDE4 in the therapeutic intervention of depression and memory deficit is determined by the importance of PDE4 for mediating cAMP signalling (Zhang et al., 2000; 2004; Houslay, 2001; Li et al., 2009; 2011). Here we found that, similar to PDE4 inhibition by rolipram, silence of long-form PDE4D induced the activation of cAMP signalling that was not altered by rolipram, which is in agreement with the observations of antidepressant-like and cognition-enhancing effects on behaviour. The lack of synergistic effects of rolipram on cAMP in the presence of long-form PDE4D knock-down may be due to that long-form PDE4Ds, in particular PDE4D4 and PDE4D5, play a dominant role in cAMP hydrolysis (Oki et al., 2000; Zhang, 2009). Therefore, inhibition of the rest PDE4Ds with rolipram may exhibit limited effects on cAMP. Another possibility is the lack of sufficient expression of PDE4Ds rather than PDE4D4 and PDE4D5 in the frontal cortex. For instance, the frontal cortex only exhibits a background level of PDE4D3 mRNA across the layers by in situ hybridization histochemistry (Miro et al., 2002), although this was not verified in the present study probably because of the high titer of PDE4D3 antibodies. Thus, these findings suggest that long-form PDE4Ds, at least PDE4D4 and PDE4D5, are important for the regulation of cAMP signalling, the molecular basis mediating antidepressant-like (Newton et al., 2002; Thome et al., 2002) and cognition-enhancing responses (Mozzachiodi et al., 2008; Schutsky et al., 2011). The notion is supported by our previous studies (Zhang et al., 2002; Li et al., 2011).
While it is relatively well established that cAMP signalling is involved in the mediation of memory, the reports on its role to date are inconsistent. One hypothesis is that overactive cAMP signalling impairs working memory in the aged PFC (Ramos et al., 2003), whereas another hypothesis presumes that activation of the cAMP signalling in the frontal cortex is necessary for working memory (Aujla and Beninger, 2001). The explanations for this discrepancy may include: (i) activation of cAMP signalling within the PFC shows an inverted U-shape dose–response on working memory (Arnsten et al., 2005) and memory requires an optimum range of cAMP rather than an overmuch or scanty production; (ii) the continuous and dynamic updating of cAMP levels occurs at the different time-course of memory formation (Perez-Garcia and Meneses, 2008); (iii) cAMP activation might be beneficial for working memory under conditions that require hippocampal–PFC interactions (Aujla and Beninger, 2001). Given the complexity of cAMP-dependent responses and studies of the PFC are far behind those of hippocampus, much work remains to be done to verify the influences of cAMP on functions of the PFC at behavioural and cellular levels.
PDE4-mediated cAMP signalling is essential for the regulation of neuronal plasticity (Sakamoto et al., 2011; Antoni, 2012), which is associated with processes of emotion (Manji et al., 2001) and memory (Dumitriu et al., 2010). Consistent with this, chronic administration with rolipram increases the dendritic complex in the newborn neurons of the hippocampus, which are accompanied by the increased levels of pCREB (Fujioka et al., 2004). Here, our data provided evidence that mature neurons in the PFC from mice treated with rolipram or silence of long-form PDE4D displayed the increased dendritic complex, paralleling changes in levels of pCREB. This finding was in accordance with our previous report that newborn neurons in the dentate gyrus of the hippocampus expressed pCREB, whereas almost all mature neurons in the PFC expressed pCREB in response to rolipram administration (Li et al., 2009). The role of CREB is supported by the finding that rolipram administration fails to increase the dendritic complex in transgenic mice expressing a dominant-negative mutant of CREB (Fujioka et al., 2004). In addition, the regulation of dendritic complex by long-form PDE4D was not altered by rolipram, indicating a predominant role of long-form PDE4D in this property.
Another finding was the lack of emetic-like responses in mice with long-form PDE4Ds down-regulated in the PFC. In the α2 adrenergic receptor-mediated anaesthesia test, chronic treatment with rolipram alone or in combination with 4DmiR decreased the duration of anaesthesia, indicating emetic-like behaviour. By contrast, 4DmiR alone did not shorten the duration of anaesthesia. In addition, rolipram had the potential to suppress the appetite, but 4DmiR-treated mice were not significantly different from the controls. These results suggest that down-regulation of long-form PDE4Ds in the PFC may not cause nausea and emesis, the major side effects of PDE4 inhibitors, consistent with our recent study (Li et al., 2011). Our results are also supported by the findings that selective PDE4D allosteric modulators incompletely inhibit PDE4 activity and enhance memory, but have reduced potential to cause emesis (Burgin et al., 2010).
Notably, α2 adrenergic receptor-mediated anaesthesia provides only a surrogate assay for measuring emesis in species without emetic responses (Robichaud et al., 2002a,b). We could not rule out the possibility of a direct effect of long-form PDE4D down-regulation in vomiting species. In addition, the present study was also limited by the region-specific silence, given that the PFC is not involved in vomiting, although it is the depression-correlative brain region. Further studies on the role of long-form PDE4Ds in mediating emesis in other brain regions, in particular the area postrema and nucleus of solitary tract that are critical for emetic responses (Ariumi et al., 2000; Perez-Torres et al., 2000), are required to address these concerns.
Taken together, using RNAi-induced gene silencing in vivo, we demonstrated for the first time that: (i) long-form PDE4Ds were the pivotal variants responsible for antidepressant-like effects of PDE4 inhibitors in a cAMP signalling-dependent manner; (ii) knock-down of long-form PDE4D in the PFC produced a cognition-enhancing effect; and (iii) down-regulation of long-form PDE4Ds, at least PDE4D4 and PDE4D5, in the PFC did not result in the emetic potential. These findings may facilitate the development of PDE4 subtype- or variant-selective inhibitors with high therapeutic indices for treatment of depression and other diseases involving cognition deficits.
Acknowledgments
This work was supported by research grants from the National Natural Science Foundation of China (30973515, 30973516, 81072624, 81173036, 81202507), the National Basic Research Program of China (2007CB512307), the National Key New Drug Creation Program (2009ZX09103-025, 2012ZX09J12201-004), NARSAD (YI 2006, 2008), and NIA (AG031687).
Glossary
- 4DmiR
shRNA in a miRNA scaffold targeting long-form PDE4D
- AP
anterior–posterior
- BDNF
brain-derived neurotrophic factor
- CC
concentric circles
- CREB
cAMP response element-binding protein
- CT
threshold cycle
- DBP
dendritic branching points
- DI
dendritic intersections
- DMSO
dimethyl sulfoxide
- DV
dorsal–ventral
- EGFP
enhanced green fluorescence protein
- FST
forced-swim test
- miRNAs
microRNAs
- ML
medial–lateral
- MWM
Morris water-maze
- NC
negative control
- NOR
novel object recognition
- OF
open-field
- pCREB
phosphorylated cAMP response element-binding protein
- PDE4
phosphodiesterase-4
- PFC
prefrontal cortex
- qRT-PCR
quantitative real-time PCR
- RNAi
RNA interference
- Rol
rolipram
- SAC
sacrifice
- TR-FRET
time-resolved FRET
- TST
tail-suspension test
- UCR
upstream conserved region
- Veh
vehicle
Conflicts of interest
Han-Ting Zhang is the Scientific Consultant of Asubio Pharmaceuticals; he and James M. O'Donnell have received financial support for their research from Lundbeck Pharmaceuticals. Neither of the two companies sold any drugs or devices mentioned in the paper. The other authors do not have financial interests to disclose.
References
- Antoni FA. New paradigms in cAMP signalling. Mol Cell Endocrinol. 2012;353:3–9. doi: 10.1016/j.mce.2011.10.034. [DOI] [PubMed] [Google Scholar]
- Ariumi H, Saito R, Nago S, Hyakusoku M, Takano Y, Kamiya H. The role of tachykinin NK-1 receptors in the area postrema of ferrets in emesis. Neurosci Lett. 2000;286:123–126. doi: 10.1016/s0304-3940(00)01113-7. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Ramos BP, Birnbaum SG, Taylor JR. Protein kinase A as a therapeutic target for memory disorders: rationale and challenges. Trends Mol Med. 2005;11:121–128. doi: 10.1016/j.molmed.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Aujla H, Beninger RJ. Hippocampal-prefrontocortical circuits: PKA inhibition in the prefrontal cortex impairs delayed nonmatching in the radial maze in rats. Behav Neurosci. 2001;115:1204–1211. [PubMed] [Google Scholar]
- Barad M, Bourtchouladze R, Winder DG, Golan H, Kandel E. Rolipram, a type IV-specific phosphodiesterase inhibitor, facilitates the establishment of long-lasting long-term potentiation and improves memory. Proc Natl Acad Sci U S A. 1998;95:15020–15025. doi: 10.1073/pnas.95.25.15020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, Romashko D, et al. A mouse model of Rubinstein–Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A. 2003;100:10518–10522. doi: 10.1073/pnas.1834280100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno O, Fedele E, Prickaerts J, Parker LA, Canepa E, Brullo C, et al. GEBR-7b, a novel PDE4D selective inhibitor that improves memory in rodents at non-emetic doses. Br J Pharmacol. 2011;164:2054–2063. doi: 10.1111/j.1476-5381.2011.01524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin AB, Magnusson OT, Singh J, Witte P, Staker BL, Bjornsson JM, et al. Design of phosphodiesterase 4D (PDE4D) allosteric modulators for enhancing cognition with improved safety. Nat Biotechnol. 2010;28:63–70. doi: 10.1038/nbt.1598. [DOI] [PubMed] [Google Scholar]
- Conti M, Richter W, Mehats C, Livera G, Park JY, Jin C. Cyclic AMP-specific PDE4 phosphodiesterases as critical components of cyclic AMP signaling. J Biol Chem. 2003;278:5493–5496. doi: 10.1074/jbc.R200029200. [DOI] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, et al. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores G, Alquicer G, Silva-Gomez AB, Zaldivar G, Stewart J, Quirion R, et al. Alterations in dendritic morphology of prefrontal cortical and nucleus accumbens neurons in post-pubertal rats after neonatal excitotoxic lesions of the ventral hippocampus. Neuroscience. 2005;133:463–470. doi: 10.1016/j.neuroscience.2005.02.021. [DOI] [PubMed] [Google Scholar]
- Fujimaki K, Morinobu S, Duman RS. Administration of a cAMP phosphodiesterase 4 inhibitor enhances antidepressant-induction of BDNF mRNA in rat hippocampus. Neuropsychopharmacology. 2000;22:42–51. doi: 10.1016/S0893-133X(99)00084-6. [DOI] [PubMed] [Google Scholar]
- Fujioka T, Fujioka A, Duman RS. Activation of cAMP signaling facilitates the morphological maturation of newborn neurons in adult hippocampus. J Neurosci. 2004;24:319–328. doi: 10.1523/JNEUROSCI.1065.03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houslay MD. PDE4 cAMP-specific phosphodiesterases. Prog Nucleic Acid Res Mol Biol. 2001;69:249–315. doi: 10.1016/s0079-6603(01)69049-4. [DOI] [PubMed] [Google Scholar]
- Houslay MD, Schafer P, Zhang KY. Keynote review: phosphodiesterase-4 as a therapeutic target. Drug Discov Today. 2005;10:1503–1519. doi: 10.1016/S1359-6446(05)03622-6. [DOI] [PubMed] [Google Scholar]
- Itoh T, Tokumura M, Abe K. Effects of rolipram, a phosphodiesterase 4 inhibitor, in combination with imipramine on depressive behavior, CRE-binding activity and BDNF level in learned helplessness rats. Eur J Pharmacol. 2004;498:135–142. doi: 10.1016/j.ejphar.2004.07.084. [DOI] [PubMed] [Google Scholar]
- Kerestes R, Ladouceur CD, Meda S, Nathan PJ, Blumberg HP, Maloney K, et al. Abnormal prefrontal activity subserving attentional control of emotion in remitted depressed patients during a working memory task with emotional distracters. Psychol Med. 2012;42:29–40. doi: 10.1017/S0033291711001097. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. The cortical projections of the mediodorsal nucleus and adjacent thalamic nuclei in the rat. J Comp Neurol. 1977;171:157–191. doi: 10.1002/cne.901710204. [DOI] [PubMed] [Google Scholar]
- Lakics V, Karran EH, Boess FG. Quantitative comparison of phosphodiesterase mRNA distribution in human brain and peripheral tissues. Neuropharmacology. 2010;59:367–374. doi: 10.1016/j.neuropharm.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Li YF, Huang Y, Amsdell SL, Xiao L, O'Donnell JM, Zhang HT. Antidepressant- and anxiolytic-like effects of the phosphodiesterase-4 inhibitor rolipram on behavior depend on cyclic AMP response element binding protein-mediated neurogenesis in the hippocampus. Neuropsychopharmacology. 2009;34:2404–2419. doi: 10.1038/npp.2009.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YF, Cheng YF, Huang Y, Conti M, Wilson SP, O'Donnell JM, et al. Phosphodiesterase-4D knock-out and RNA interference-mediated knock-down enhance memory and increase hippocampal neurogenesis via increased cAMP signaling. J Neurosci. 2011;31:172–183. doi: 10.1523/JNEUROSCI.5236-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Manji HK, Drevets WC, Charney DS. The cellular neurobiology of depression. Nat Med. 2001;7:541–547. doi: 10.1038/87865. [DOI] [PubMed] [Google Scholar]
- Martinez-Tellez R, Gomez-Villalobos MJ, Flores G. Alteration in dendritic morphology of cortical neurons in rats with diabetes mellitus induced by streptozotocin. Brain Res. 2005;1048:108–115. doi: 10.1016/j.brainres.2005.04.048. [DOI] [PubMed] [Google Scholar]
- Miro X, Perez-Torres S, Puigdomenech P, Palacios JM, Mengod G. Differential distribution of PDE4D splice variant mRNAs in rat brain suggests association with specific pathways and presynaptical localization. Synapse. 2002;45:259–269. doi: 10.1002/syn.10100. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Mozzachiodi R, Lorenzetti FD, Baxter DA, Byrne JH. Changes in neuronal excitability serve as a mechanism of long-term memory for operant conditioning. Nat Neurosci. 2008;11:1146–1148. doi: 10.1038/nn.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai T, Takuma K, Kamei H, Ito Y, Nakamichi N, Ibi D, et al. Dopamine D1 receptors regulate protein synthesis-dependent long-term recognition memory via extracellular signal-regulated kinase 1/2 in the prefrontal cortex. Learn Mem. 2007;14:117–125. doi: 10.1101/lm.461407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton SS, Thome J, Wallace TL, Shirayama Y, Schlesinger L, Sakai N, et al. Inhibition of cAMP response element-binding protein or dynorphin in the nucleus accumbens produces an antidepressant-like effect. J Neurosci. 2002;22:10883–10890. doi: 10.1523/JNEUROSCI.22-24-10883.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Donnell JM, Zhang HT. Antidepressant effects of inhibitors of cAMP phosphodiesterase (PDE4) Trends Pharmacol Sci. 2004;25:158–163. doi: 10.1016/j.tips.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Oki N, Takahashi SI, Hidaka H, Conti M. Short term feedback regulation of cAMP in FRTL-5 thyroid cells. Role of PDE4D3 phosphodiesterase activation. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin KBJ. The Mouse Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 2001. p. 65. [Google Scholar]
- Perez-Garcia G, Meneses A. Ex vivo study of 5-HT(1A) and 5-HT(7) receptor agonists and antagonists on cAMP accumulation during memory formation and amnesia. Behav Brain Res. 2008;195:139–146. doi: 10.1016/j.bbr.2008.07.033. [DOI] [PubMed] [Google Scholar]
- Perez-Torres S, Miro X, Palacios JM, Cortes R, Puigdomenech P, Mengod G. Phosphodiesterase type 4 isozymes expression in human brain examined by in situ hybridization histochemistry and [3H]rolipram binding autoradiography. Comparison with monkey and rat brain. J Chem Neuroanat. 2000;20:349–374. doi: 10.1016/s0891-0618(00)00097-1. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Birnbaum SG, Lindenmayer I, Newton SS, Duman RS, Arnsten AF. Dysregulation of protein kinase a signaling in the aged prefrontal cortex: new strategy for treating age-related cognitive decline. Neuron. 2003;40:835–845. doi: 10.1016/s0896-6273(03)00694-9. [DOI] [PubMed] [Google Scholar]
- Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: a promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–443. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Tattersall FD, Chan CC. PDE4 inhibitors induce emesis in ferrets via a noradrenergic pathway. Neuropharmacology. 2001;40:262–269. doi: 10.1016/s0028-3908(00)00142-8. [DOI] [PubMed] [Google Scholar]
- Robichaud A, Savoie C, Stamatiou PB, Lachance N, Jolicoeur P, Rasori R, et al. Assessing the emetic potential of PDE4 inhibitors in rats. Br J Pharmacol. 2002a;135:113–118. doi: 10.1038/sj.bjp.0704457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robichaud A, Stamatiou PB, Jin SL, Lachance N, MacDonald D, Laliberte F, et al. Deletion of phosphodiesterase 4D in mice shortens alpha(2)-adrenoceptor-mediated anesthesia, a behavioral correlate of emesis. J Clin Invest. 2002b;110:1045–1052. doi: 10.1172/JCI15506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanski LM. Domain specificity in the primate prefrontal cortex. Cogn Affect Behav Neurosci. 2004;4:421–429. doi: 10.3758/cabn.4.4.421. [DOI] [PubMed] [Google Scholar]
- Rutten K, Prickaerts J, Schaenzle G, Rosenbrock H, Blokland A. Sub-chronic rolipram treatment leads to a persistent improvement in long-term object memory in rats. Neurobiol Learn Mem. 2008;90:569–575. doi: 10.1016/j.nlm.2008.04.016. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116:1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schratt GM, Tuebing F, Nigh EA, Kane CG, Sabatini ME, Kiebler M, et al. A brain-specific microRNA regulates dendritic spine development. Nature. 2006;439:283–289. doi: 10.1038/nature04367. [DOI] [PubMed] [Google Scholar]
- Schutsky K, Ouyang M, Castelino CB, Zhang L, Thomas SA. Stress and glucocorticoids impair memory retrieval via beta2-adrenergic, Gi/o-coupled suppression of cAMP signaling. J Neurosci. 2011;31:14172–14181. doi: 10.1523/JNEUROSCI.2122-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholl DA. Dendritic organization in the neurons of the visual and motor cortices of the cat. J Anat. 1953;87:387–406. [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M. Reversal of long-term dendritic spine alterations in Alzheimer's disease models. Proc Natl Acad Sci U S A. 2009;106:16877–16882. doi: 10.1073/pnas.0908706106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares JC, Mann JJ. The anatomy of mood disorders – review of structural neuroimaging studies. Biol Psychiatry. 1997;41:86–106. doi: 10.1016/s0006-3223(96)00006-6. [DOI] [PubMed] [Google Scholar]
- Spina D. PDE4 inhibitors: current status. Br J Pharmacol. 2008;155:308–315. doi: 10.1038/bjp.2008.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome J, Henn FA, Duman RS. Cyclic AMP response element-binding protein and depression. Expert Rev Neurother. 2002;2:347–354. doi: 10.1586/14737175.2.3.347. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Groenewegen HJ, Kolb B. Do rats have a prefrontal cortex? Behav Brain Res. 2003;146:3–17. doi: 10.1016/j.bbr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- Wang JW, David DJ, Monckton JE, Battaglia F, Hen R. Chronic fluoxetine stimulates maturation and synaptic plasticity of adult-born hippocampal granule cells. J Neurosci. 2008;28:1374–1384. doi: 10.1523/JNEUROSCI.3632-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZZ, Li G, Chen XY, Zhao M, Yuan YH, Wang XL, et al. Chemokine-like factor 1, a novel cytokine, induces nerve cell migration through the non-extracellular Ca2+-dependent tyrosine kinases pathway. Brain Res. 2010;1308:24–34. doi: 10.1016/j.brainres.2009.10.047. [DOI] [PubMed] [Google Scholar]
- Wang ZZ, Zhang Y, Yuan YH, Chen NH. Developmental expression of chemokine-like factor 1, a novel member of chemokines family, in postnatal rat cerebral cortex. Neurosci Lett. 2012;519:51–55. doi: 10.1016/j.neulet.2012.05.019. [DOI] [PubMed] [Google Scholar]
- Witter MP, Naber PA, van Haeften T, Machielsen WC, Rombouts SA, Barkhof F, et al. Cortico-hippocampal communication by way of parallel parahippocampal-subicular pathways. Hippocampus. 2000;10:398–410. doi: 10.1002/1098-1063(2000)10:4<398::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Zhang HT. Cyclic AMP-specific phosphodiesterase-4 as a target for the development of antidepressant drugs. Curr Pharm Des. 2009;15:1688–1698. doi: 10.2174/138161209788168092. [DOI] [PubMed] [Google Scholar]
- Zhang HT, O'Donnell JM. Effects of rolipram on scopolamine-induced impairment of working and reference memory in the radial-arm maze tests in rats. Psychopharmacology (Berl) 2000;150:311–316. doi: 10.1007/s002130000414. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Crissman AM, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of cyclic AMP phosphodiesterase (PDE4) reverses memory deficits associated with NMDA receptor antagonism. Neuropsychopharmacology. 2000;23:198–204. doi: 10.1016/S0893-133X(00)00108-1. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Huang Y, Jin SL, Frith SA, Suvarna N, Conti M, et al. Antidepressant-like profile and reduced sensitivity to rolipram in mice deficient in the PDE4D phosphodiesterase enzyme. Neuropsychopharmacology. 2002;27:587–595. doi: 10.1016/S0893-133X(02)00344-5. [DOI] [PubMed] [Google Scholar]
- Zhang HT, Zhao Y, Huang Y, Dorairaj NR, Chandler LJ, O'Donnell JM. Inhibition of the phosphodiesterase 4 (PDE4) enzyme reverses memory deficits produced by infusion of the MEK inhibitor U0126 into the CA1 subregion of the rat hippocampus. Neuropsychopharmacology. 2004;29:1432–1439. doi: 10.1038/sj.npp.1300440. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Li PP. Shu-Gan-Liang-Xue Decoction, a Chinese herbal formula, down-regulates the expression of steroid sulfatase genes in human breast carcinoma MCF-7 cells. J Ethnopharmacol. 2010;127:620–624. doi: 10.1016/j.jep.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Qian RQ, Li PP. Shikonin, an ingredient of Lithospermum erythrorhizon, down-regulates the expression of steroid sulfatase genes in breast cancer cells. Cancer Lett. 2009;284:47–54. doi: 10.1016/j.canlet.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Zilles K. The Cortex of the Rat. A Stereotaxic Atlas. Berlin: Springer-Verlag; 1985. p. 121. [Google Scholar]