Abstract
Objective
To estimate whether continuous OCP (oral contraceptive pills) will result in more pain relief in primary dysmenorrhea patients than cyclic OCP, which induces withdrawal bleeding with associated pain and symptoms.
Material and Methods
We conducted a double-blind, randomized controlled trial comparing continuous to a cyclic 21/7 OCP regimen (gestodene 0.075 mg and ethinyl estradiol 20 mcg) for 6 months in 38 primary dysmenorrhea patients. The primary outcome was the difference in subjective perception of pain as measured by the Visual Analog Scale (VAS) over the period of 6 months.
Results
Twenty-nine patients completed the study. In both groups, pain reduction measured by VAS declined over time and was significant at 6 months compared to baseline with no difference between groups. Continuous regimen was superior to cyclic regimen after one month (mean difference: -27.3; 95% CI: (-40.5,-14.2); p<0.001) and 3 months (mean difference: -17.8; 95% CI: (-33.4,-2.1); p=0.03) of treatment. Secondary outcomes noted no difference between groups in terms of menstrual distress as measured by the Moos Menstrual Distress Questionnaire. After 6 months, there was an increase in weight and decrease in systolic blood pressure in continuous compared with the cyclic group.
Conclusions
Both regimens of OCP are effective in the treatment of primary dysmenorrhea. Continuous OCP outperforms cyclic OCP in the short term, but this difference is lost after 6 months.
INTRODUCTION
Primary dysmenorrhea (PD) is the most common cause of pelvic pain in women. It is estimated that 50–90% of the female population suffers from this condition, and 10% are severely affected for 1–3 days a month as a consequence [1]. This has both psychological and economic impact, as the resulting absenteeism causes severe economic loss each month. In one study dysmenorrhea accounted for 600 million lost work hours and $2 billion annually in the U.S. [2].
PD is most commonly attributable to excess prostaglandin production at the time of menstruation. Prostaglandin overproduction causes abnormal uterine contractions and increased intrauterine pressure, vasoconstriction of small uterine vessels leading to decreased uterine blood flow, increased sensitivity of pain receptors and ischemia of the uterine muscle, which consequently contributes to pelvic pain [3, 4]. During uterine contractions, endometrial blood flow decreases, indicating that ischemia due to the hypercontractility is the primary cause of the pain [3, 5]. Preventing menstruation thus may be a viable treatment option.
Oral contraceptives (OCP) are frequently prescribed for this condition, but are not always effective [6]. We theorized that cyclic OCP which induces withdrawal bleeding occasionally allows the production of prostaglandins and persistent pain. Continuous OCP has not been studied as a primary treatment of PD, although recent studies show its superiority to cyclic regimens in the treatment of endometriosis [7–9]. We hypothesized that continuous administration of an OCP will result in more pain relief than a cyclic 21 days active pills and 7 days placebo pills administration in PD patients. We hypothesized secondarily that the differences in uterine artery pulsatility index (PI) measured by color Doppler ultrasound would be significant between treatment groups at the end of the trial period, compared to the beginning of the trial; and that the patient self-evaluation of menstrual symptoms as determined by a standardized questionnaire would be more favorable in the continuous group compared to the cyclic 21/7 regimen group.
MATERIAL AND METHODS
The study was approved by the Institutional Review Board at Penn State Hersey Medical Center and by the Ethics Comittee at Nova Gradiska General Hospital, in Croatia, where it was conducted. Subjects were recruited from the Ob/Gyn department at Nova Gradiska General Hospital, from January 2007 to May 2010. A number of women who participated in the study were presenting for their annual screening and complaining about dysmenorrhea, while others were refered to the clinic for evaluation of dymenorrhea. All subjects gave written informed consent.
We conducted a a double-blind, randomized controlled trial of two treatment regimens of a monophasic OCP (gestodene 0.075 mg /ethinyl estradiol 20 mcg) in 38 PD patients with an allocation ratio of 1:1. The randomization list was created by our biostatistician at Penn State (ARK), who send the list to the certified reasearch pharmacy in Croatia (Magdis, Sveta Nedjelja), who overencapsulated the OCP to blind the treatment, and then sent it to research site. In order to preserve the blinding, we overencapsulated the commercially purchased intact gestodene 0.075 mg /ethinyl estradiol 20 mcg OCP, and identical-appearing placebo pills. Adherence was assessed with pill counts confirmed at each visit. The research pharmacy also kept the randomization list. Both participants and care providers in the study were blinded to the treatment. The patients were randomized into the study group of continuous OCP and control group of cyclic OCP based on a randomization table with block size of four known only by the biostatistician. Patients in the continuous group received 28 active pills (0.075 mg gestodene/20 µg EE, a monophasic OCP), while patients in the cyclic OCP group received 21 active pills and 7 placebo pills. Gestodene-containing oral contraceptives are currently not available in United States; however they are marketed in Croatia where the study was conducted (and we choose this as we wanted a low dose EE pill and this was the only 20 µg EE available in Croatia).
Inclusion Criteria
Subjects were in good health, their age was 18–35, and they had a history of PD (onset < 3 years after menarche). Subjects had regular (25–31 day) menstrual cycles for the three-month period preceding enrollment, with symptoms of moderate to severe PD during those cycles. The pain associated with PD was abdominal or pelvic, could radiate to the back and along the thighs, could begin up to one day before menses and last for the first three days of bleeding. The pain could be accompanied with systemic symptoms including nausea, vomiting, diarrhea, headache, fatigue, nervousness and dizziness.
Exclusion Criteria
Exclusion criteria were contraindications to OCP therapy (as described in the drug label), known or suspected secondary dysmenorrhea (major abdominal or pelvic surgery, endometriosis, pelvic inflammatory disease (PID), ovarian cysts, pathological vaginal secretion, chronic abdominal pain, inflammatory bowel disease, irritable bowel syndrome), concomitant treatment with OCPs, GnRH agonists and antagonists, antiandrogens, gonadotropins, and anti-obesity drugs, the use of contraceptive implants, injectable contraceptives or intrauterine devices (the washout period on all these medications was three months), smoking (because we were uncertain about the risk benefit of continuous OCP use in this population), migraine, increased severity of headaches, depression requiring hospitalization or associated with suicidal ideation during previous estrogen or OCP use, and known or suspected hypersensitivity to trial drug. Patients enrolled simultaneously into other investigative studies that require medications, or otherwise prevent compliance with the protocol were also excluded.
Study Visits
Patients had a screening visit, where inclusion and exclusion criteria were determined and informed consent signed; a randomization visit, and three study visits (after 1 month, 3 months and 6 months). At the screening visit, patients were asked to define pain they were feeling for the last three cycles, on a categorical scale presented, as none, mild, moderate or severe. They were allowed to participate in the study if they rated their pain as moderate or severe. The first two OCP boxes were dispensed at the randomization visit, and patients were advised to take one pill daily, and two pills if one day was missed, starting on first day of their next period.
At first study visit, scheduled between days 25–28 of the study, VAS scale and Moos Menstrual Distress Questionnaire (MMDQ) were administered for current bleeding, if it happened, and for previous bleeding, at days 1–4 of the study. Other visit were scheduled at days 53–56, 81–84, and 165–168 of the study. Subjects kept vaginal bleeding diaries that were reviewed at each study visit. On each page of the bleeding diary patients had three pictures of sanitary pads and three pictures of tampons (for analysis they were named spotting, moderate, and heavy bleeding), indicating the amount of blood staining. They were ask to make a mark for each pad or tampon they have used, that had bloodstaining that is similar to that shown in the picture, underneath the appropriate picture and across from the correct date. Color Doppler ultrasound was also performed at each study visit, using an Aloka 2000 color Doppler ultrasound with 7.5 MHz vaginal transducer. The scanning technique was as follows: after excluding uterine and ovarian pathology with the conventional ultrasound, uterine arteries were visualized laterally in the transverse section of the cervicocorporeal junction. Measurements of the pulsatility index (PI= maximal systolic flow−minimal diastolic flow/meanflow) were made after at least three consecutive blood flow velocity waveforms were analyzed.
The primary outcome was the difference in subjective perception of pain as measured by the Visual Analog Scale (VAS) over the period of six months, while the secondary outcomes were the differences in menstrual related complaints as determined by MMDQ and in ultrasound parameters (endometrial thickness, uterine artery PI). The VAS is a validated pain scale [10], used widely in clinical trials assessing the intensity of pain, especially in trials with patients suffering from PD. The VAS uses an analog linear scale to assess pain intensity. The scale is 100 mm long; the extremes of the scale are to the left “no pain” and to the right “worst pain I have ever felt”. A score itself is determined by measuring the distance from the left side of the scale to the point that the patient marked.
At baseline, at 3 months and 6 months, patients completed the MMDQ [11], a validated questionnaire of menstrual related complaints which is often used in the dysmenorrhea trials. In a questionnaire, patients are asked to quantify their symptoms related to the cycle, for four days before the current bleeding, for the time of the bleeding, and the rest of the cycle, describing it as 0 – No experience of symptom, 1 – Present, mild, 2 – Present, moderate, 3 – Present, strong, and 4 – Present, severe.
Sample Size and Data Analysis
A modified intention-to-treat (mITT) approach, defined prior to study initiation, was used for efficacy analyses. The pre-defined mITT approach included for analysis all subjects who took the medication they were randomized to and who recorded at least one primary outcome score post-dose. Baseline values were not imputed or carried forward. All analyses were performed using SAS statistical software (SAS Institute Inc., Cary, NC).
Linear mixed-effects models were fit to the data to assess between-group (treatment regimen) and within-group differences over time for all continuous outcomes [12]. The independent variables for these models were treatment regimen, time (month), and the interaction of treatment regimen and time. From these linear mixed-effects models that included the two main effects and their interaction, contrasts were constructed to assess changes from baseline to each specific post-randomization month to compare within and between the treatment regimens. The linear mixed-effects models used a spatial power law covariance structure to account for unequally spaced repeated measurements (i.e., visits) over time [13]. Poisson regression models were fit to assess differences in treatment regimens with respect to bleeding day outcomes, where bleeding days were represented as the total number of bleeding days over the 6 month study time interval. Effect sizes from the Poisson regression models were quantified using rate ratios [14] and 95% confidence intervals.
We estimated that 38 subjects would be needed to detect a 30 mm difference in VAS between the continuous and cyclic 21/7 OCP treatment regimens with 90% power using a two-sided test having a significance level of 0.05. The power calculation assumed a VAS standard deviation of 25 mm and factored in an anticipated 15% subject drop-out for the trial.
RESULTS
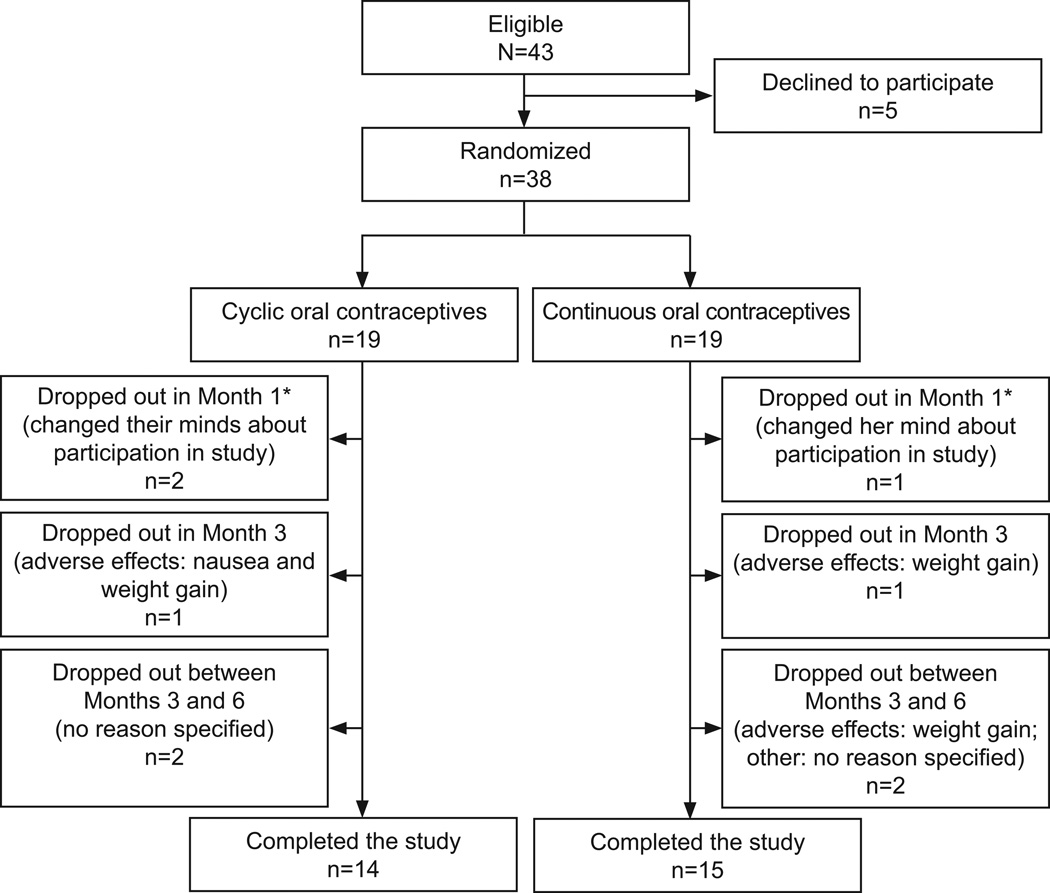
Forty three women were screened, 38 randomized, and 29 patients completed the study (Figure 1). Three of the 38 randomized women never returned for a post-dose visit assessment and therefore were excluded in the mITT analysis. Baseline descriptives are presented in Table 1. There were no significant changes from baseline between the two groups after 6 months of treatment for any of the following blood tests: bilirubin, alkaline phosphatase, ALT, glucose, total cholesterol, LDL, HDL, triglycerides, TSH, T3, T4, BUN, creatinine, and complete blood count.
Figure 1.
Flow of participants through the study.
Table 1.
Baseline Characteristics of Patients by Treatment Regimen
| Continuous | Cyclic | |||
|---|---|---|---|---|
| n | Mean(SD) | n | Mean(SD) | |
| Biometric | ||||
| Age (y) | 18 | 20.7 (3.9) | 17 | 21.1 (4.3) |
| Weight (kg) | 18 | 57.9 (6.8) | 17 | 55.9 (5.2) |
| BMI (kg/m2) | 18 | 20.7 (2.5) | 17 | 20.3 (1.8) |
| Systolic blood pressure (mm Hg) | 18 | 107.2 (6.7) | 17 | 101.8 (8.1) |
| Diastolic blood pressure (mm Hg) | 18 | 65.3 (6.1) | 17 | 60.6 (7.5) |
| Liver profile | ||||
| Bilirubin (umol/L) | 17 | 12.9 (3.8) | 15 | 14.0 (9.2) |
| Alkaline phosphates (J/L) | 16 | 57.4 (15.7) | 14 | 52.9 (21.7) |
| ALT (J/L) | 17 | 14.7 (4.7) | 15 | 13.4 (4.4) |
| Glucose (nmol/L) | 17 | 4.8 (0.4) | 15 | 5.2 (0.9) |
| Lipid profile | ||||
| Total Cholesterol (nmol/L) | 16 | 4.2 (1.1) | 15 | 4.1 (0.9) |
| LDL (nmol/L) | 16 | 2.5 (1.0) | 13 | 2.1 (0.9) |
| HDL (nmol/L) | 16 | 1.4 (0.3) | 13 | 1.7 (0.4) |
| Triglycerides (nmol/L) | 16 | 0.8 (0.2) | 15 | 0.8 (0.3) |
| Renal profile | ||||
| BUN (umol/L) | 13 | 246.2 (33.7) | 15 | 202.2 (77.5) |
| Creatinine (umol/L) | 17 | 81.7 (8.0) | 15 | 80.9 (9.9) |
| CBC | ||||
| White blood cell (x109/L) | 17 | 6.9 (2.2) | 15 | 6.4 (1.9) |
| Hemoglobin (g/L) | 18 | 134.1 (8.9) | 15 | 129.8 (9.8) |
| Hematocrit (L/L) | 18 | 0.4 (0.2) | 15 | 0.4 (0.0) |
| Platelet count (x109/L) | 18 | 262.3 (45.9) | 15 | 244.4 (41.0) |
| Ultrasound parameters | ||||
| Endometrial thickness (mm) | 16 | 7.2 (2.4) | 14 | 6.8 (3.1) |
| Total ovarian volume (cm3) | 16 | 11.1 (5.4) | 14 | 12.1 (7.0) |
SD, standard deviation; BMI, body mass index; ALT, alanine transminase; LDL, low-density lipoprotein; HDL, high-density lipoprotein; BUN, blood urea nitrogen; CBC, complete blood count.
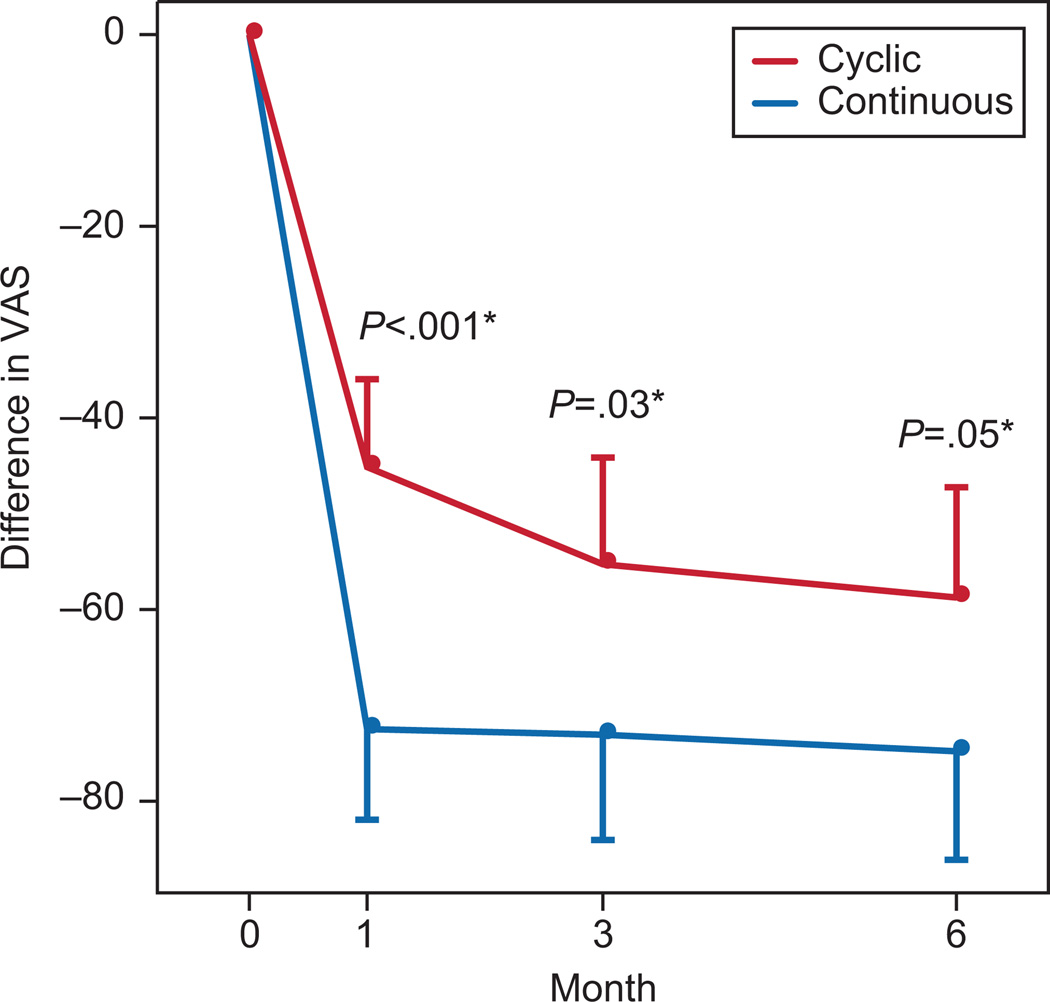
Mean VAS score (SD) in the cyclic regimen was 64.4 (31.9) at screening visit, 19.0 (24.8) after one month, and 5.2. (9.5) at the end of the study. Mean VAS score in the continuous group was 75.6 (16.7) at screening visit, 3.1 (5.8) after one month, and 0.8 (1.4) at the end of the study. In both groups pain reduction measured by VAS was significant compared to baseline (Table 2). The continuous regimen was superior to the cyclic regimen when comparing pain reduction after one and three months of treatment, and had marginal statistical significance after six months of treatment (Figure 2).
Table 2.
Linear Mixed-Effects Model-Based Differences in Key Outcomes of Treatment Compared to Baseline by Treatment Group and Difference Between Groups
| Continuous | Cyclic | Difference (Continuous-Cyclic) |
|||||
|---|---|---|---|---|---|---|---|
| Month | Mean Change (95% CI) |
P | Mean Change (95% CI) |
P | Mean Change (95% CI) |
P | |
| Visual Analog Scale | |||||||
| Degree of dysmenorrhea | 1 | -72.5 (-81.9,-63.0) | <.001 | -45.1 (-54.3,-36.0) | <.001 | -27.3 (-40.5,-14.2) | <.001 |
| 3 | -73.1 (-84.0,-62.1) | <.001 | -55.3 (-66.4,-44.1) | <.001 | -17.8 (-33.4,-2.1) | 0.03 | |
| 6 | -74.8 (-86.1,-63.5) | <.001 | -58.7 (-70.3,-47.2) | <.001 | -16.0 (-32.2,0.1) | 0.05 | |
| MOOS Menstrual Distress Questionnaire | |||||||
| T-Score Pain Scale (menstrual) | 1 | -8.0 (-16.7,0.7) | 0.07 | -4.5 (-12.9,4.0) | 0.29 | -3.5 (-15.6,8.6) | 0.57 |
| 3 | -21.0 (-32.4,-9.7) | <.001 | -18.5 (-30.0,-6.9) | 0.002 | -2.6 (-18.8,13.7) | 0.76 | |
| 6 | -23.0 (-35.4,-10.5) | <.001 | -18.4 (-31.0,-5.9) | 0.004 | -4.5 (-22.2,13.2) | 0.61 | |
| T-Score Pain Scale (premenstrual) | 1 | -2.5 (-8.0,2.9) | 0.35 | 0.4 (-4.9,5.6) | 0.89 | -2.9 (-10.5,4.7) | 0.45 |
| 3 | -6.2 (-14.1,1.6) | 0.12 | -3.4 (-11.3,4.5) | 0.40 | -2.8 (-14.0,8.3) | 0.61 | |
| 6 | -6.6 (-15.8,2.7) | 0.16 | -0.6 (-9.9,8.7) | 0.89 | -5.9 (-19.1,7.2) | 0.37 | |
| T-Score Pain Scale (intermenstrual) | 1 | -6.1 (-12.9,0.8) | 0.08 | -5.7 (-12.3,1.0) | 0.09 | -0.4 (-9.9,9.2) | 0.93 |
| 3 | -13.0 (-22.7,-3.3) | 0.009 | -10.5 (-20.4,-0.6) | 0.04 | -2.5 (-16.4,11.4) | 0.72 | |
| 6 | -14.3 (-25.7,-2.9) | 0.01 | -7.1 (-18.6,4.3) | 0.22 | -7.2 (-23.4,9.0) | 0.38 | |
Figure 2.
Comparisons between the two groups in Visual Analogue Scores (VAS) at varying time points in the study.
The changes in MMDQ scores over time were the same for the continuous and cyclic group, with no changes between the groups (Table 2). We did note significant increase in weight and BMI, and decrease in systolic blood pressure at 6 months between the study groups (all P < 0.05) (Table 3). There were no significant changes from baseline between the two groups after 6 months of treatment for ovarian volume and endometrial thickness, nor for uterine artery resistance and pulsatility indices (data not shown). Within both groups, there was a signficant decrease in endometrial thickness after 6 months of treatment [continuous -3.7 (-5.4,-2.0), p<.0001, and cyclic -3.4 (-5.2,-1.7), p=0.0002] with no difference between treatments. We observed more bleeding and spotting days in continuous compared to cyclic regimen over the period of 6 months, as shown in Table 4.
Table 3.
Linear Mixed-Effects Model-Based Differences in Safety Outcomes of Treatment Compared to Baseline and Difference Between Groups*
| Continuous | Cyclic | Difference (Continuous-Cyclic) |
||||
|---|---|---|---|---|---|---|
| Mean Change (95% CI) |
P | Mean Change (95% CI) |
P | Mean Change (95% CI) |
P | |
| Weight (kg) | 2.1 (1.1,3.1) | <.001 | -0.2 (-1.3,0.8) | 0.67 | 2.3 (0.8,3.8) | 0.003 |
| BMI (kg/m2) | 0.8 (0.4,1.1) | <.001 | 0.0 (-0.4,0.4) | 0.92 | 0.7 (0.2,1.3) | 0.01 |
| Systolic blood pressure (mm Hg) | 2.4 (-2.8,7.6) | 0.35 | 10.5 (5.1,15.8) | <.001 | -8.1 (-15.5,-0.6) | 0.04 |
| Diastolic blood pressure (mm Hg) | 3.1 (-2.6,8.9) | 0.27 | 11.0 (5.1,16.9) | <.001 | -7.9 (-16.1,0.4) | 0.06 |
| Alkaline phosphatase (J/L) | -9.3 (-17.1,-1.5) | 0.02 | -7.6 (-15.9,0.7) | 0.07 | -1.7 (-13.1,9.7) | 0.76 |
| ALT (J/L) | 2.8 (0.0,5.6) | 0.05 | 1.7 (-1.5,4.8) | 0.28 | 1.2 (-3.0,5.4) | 0.57 |
| Total cholesterol (nmol/L) | 0.4 (0.2,0.7) | 0.003 | 0.4 (0.1,0.7) | 0.007 | 0.0 (-0.4,0.4) | 0.96 |
| HDL (nmol/L) | 0.2 (0.0,0.3) | 0.05 | 0.2 (0.1,0.4) | 0.007 | -0.1 (-0.3,0.1) | 0.45 |
| Triglycerides (nmol/L) | 0.3 (0.1,0.5) | 0.006 | 0.2 (-0.0,0.4) | 0.07 | 0.1 (-0.2,0.4) | 0.49 |
Only parameters with a significant difference compared to baseline or between groups. BMI, body mass index; ALT, alanine transminase; HDL, high-density lipoprotein.
Table 4.
Differences in Vaginal Bleeding (Days) by Treatment Group Based on Poisson Regression Models
| Continuous | Cyclic | Continuous Compared With Cyclic |
||
|---|---|---|---|---|
| Mean (95% CI) | Mean (95% CI) | Rate Ratio (95% CI) |
P | |
| All bleeding (days) | 58.2 (43.8,77.2) | 33.7 (23.6,47.9) | 1.7 (1.1,2.7) | 0.02 |
| Moderate-to-heavy bleeding (days) | 16.7 (10.5,26.5) | 17.6 (11.5,27.0) | 0.9 (0.5,1.8) | 0.87 |
| Spotting (days) | 41.5 (29.3,58.8) | 16.0 (9.4,27.3) | 2.6 (1.4,4.9) | 0.003 |
DISCUSSION
This is a randomized trial of continuous versus cyclic OCP in the treatment of primary dysmenorrhea. Our results show that in healthy young women both regimens of OCP are effective in the treatment of PD at 6 months, although the continuous OCP regimen is superior in pain relief to cyclic OCP in the short term (up to 3 months), with no differences in quality of life as determined by the Moos MDQ between groups. Further we noted in the continuous group a greater weight gain, more bleeding/spotting day, but a decrease in systolic blood pressure compared to the cyclic group.
Dysmenorrhea has been succesfully treated with OCP. Davis et al [15] assessed whether a low-dose OCP is more effective than placebo treatment for dysmenorrhea pain in adolescents, and reported that the mean MMDQ pain score was lower (less pain) in the OCP group than the placebo group, comparable to our findings in both groups. Our findings are also consistent with other studies of other gynecologic disorders with chronic pain. For instance, several studies addressed the use of a continuous OCP regimen in patients with endometriosis, which is associated with pelvic pain, dysmenorrhea, dyspareunia and infertility [7–9]. They found less endometriosis-associated dysmenorrhea in the continuous contraceptive groups, which is comparable to our findings. Kwiecien et al. [16] in a study of bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose OCP, looked into symptoms of menstrual pain. At the conclusion of the study, subjects completed a satisfaction questionnaire, and significantly less menstrual pain was noted in the continuous group.
Under social, cultural, and religious influences, women have traditionally been prescribed OCP in a pattern of 21 days of active pills with seven days of inactive pills as a way of mimicking the natural menstrual cycle [17]. However, in dysmenorrhea patients, this may attenuate the full pain benefit of OCP. The continuous administration may reduce dysmenorrhea by eliminating withdrawal bleeding and associated uterine contractions, as well as by preventing rebound ovarian function during the pill free interval which may stimulate the growth of the endometrium [18]. Previous studies in other populations have shown greater satisfaction and quality of life with extended cycle regimens. [19–21]. A multicenter randomized trial [22] of an extended cycle OCP assessed patient satisfaction with the continuous regimen. Patients reported a preference for the reduced frequency of menstrual periods, and that they would prefer to have fewer menstrual periods following completion of the study.
We found no or only modest differences between the groups in premenstrual and intermenstrual symptoms that constitute the MMDQ, which is different from other studies [18, 23]. However, some studies did not find that OCP use alter the incidence or severity of premenstrual change in MMDQ [24]. The reason for this may be our small sample size, but also the subjective nature of the questionnaire.
Our patients in the continuous group gained weight, while patients in the cyclic group did not. We did not note this in our previous trial [18], and moreover a recent metaanalysis [25] did not find evidence to support a large weight change on OCP. Though this reflect improved appetite due to decreased pelvic pain, this may be a chance finding due to our small sample size, but also likely the consequence of weighing women when the two groups were in a different endocrinologic millieu [in the continuous group always on active pills, in the cyclic group on placebo pills (i.e.the last four days of each pill cycle)]. Women in the cyclic group had an increase in both systolic and diastolic blood pressure, which is expected, as OCP is associated with a small but significant increase in blood pressure. Recent studies [26, 27] confirmed this, but while this may be true for cyclic regimen of OCP, continuous regimens, as in our study, may not change blood pressure to the same degree [18, 28].
Although ultrasound has been the most important diagnostic tool in gynecology in the past decades, it has little value in the diagnosis of PD. Its main contribution in such patients is in excluding the patients with secondary dysmenorrhea by positively identifying endometriomas, uterine leiomyomas, adenomyosis, and other abnormalities. This was confirmed in this study, because the only significant change we observed was a change in endometrial thickness for both groups compared to baseline after six months of treatment, a common change in these studies [29, 30].
Women in our study reported more beeding days and more spotting on continuous regimen than previously reported [16]. However this was likely a minor distraction, without involvement of the myometrium and prostaglandin production, given the signficant reduction in VAS and the lack of difference in the quality of life as reported on the MMDQ. Further, there was no significant difference in the total number of moderate/heavy bleeding days in our study between regimens, although previously we noted fewer days [18]. However we were studying normal women and using a different OCP formulation.
In conclusion, patients with PD may benefit from continuous administration of OCP compared to cyclic OCP by a more rapid achievement in pain reduction. However, since PD therapy is a long-term therapy, and continuous regimen could be associated with unpleasant side-effects such as weight gain, women should be counseled about this. In the clinical setting, they may be less tolerant to side effects, and thus discontinue the OCP. Further larger randomized trials are needed to establish the risk benefit ratio of longer use of extended cycle OCP regimens for PD.
Acknowledgments
Supported by the National Institutes of Health (NIH) grants RO3 TW007438 and K24 HD01476. The authors thank Martina Slisuric and Ivana Segedin from Nova Gradiska General Hospital for supervising this study, and Christina Stetter from the Department of Public Health Sciences Penn State College of Medicine for statistical programming support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Presented as oral presentation at the 67th Annual Meeting of the American Society for Reproductive Medicine in Orlando, Florida in October 15–18, 2011.
Financial Disclosure: Allen Kunselman owns stock in Merck & Co. Dr. Legro has received the following: 1) travel reimbursement from the British Fertility Society, The Association for Clinical Embryologists & Society for Reproductive & Fertility; First Affiliated Hospital, Heilongjiang University of Chinese Medicine; The Israel Fertility Society; American Society of Reproductive Medicine; University of Michigan; Weill Cornell Medical College; Reproductive Medicine Network; American Board of Obstetrics & Gynecology; Taiwan Society of Reproductive Medicine; Northwestern University; Specialized Cooperative Centers Program in Reproductive & Infertility Research; The Endocrine Society; V Conference Gynaecological Endocrinology; European Society of Human Reproduction & Embryology, Virginia Commonwealth University, Einstein Medical School, University of Sao Paulo, Southwestern Medical Center, National Institutes of Health (STEP), and the NIH Federal Drug Administration (Adelphi, MD); 2) honoraria for lectures from the Taiwan Society of Reproductive Medicine; Northwestern University, and Southwestern Medical Center; 3) honoraria for consulting from American Society of Reproductive Medicine; National Institute of Health, American Board of Obstetrics and Gynecology, National Institutes of Health Office of Extramural Research STEP Program, NIH Federal Drug Administration; 4) travel reimbursement from the University of Pennsylvania Medicine, University of Vermont College of Medicine, European Society of Human Reproduction and Embryology; 5) honoraria for lectures from the University of Vermont College of Medicine; and 6) honoraria for consulting from Deutsche Bank Securities Inc. The other author did not report any potential conflicts of interest.
REFERENCES
- 1.Andersch B, Milsom I. An epidemiologic study of young women with dysmenorrhea. Am J Obstet Gynecol. 1982;144(6):655–660. doi: 10.1016/0002-9378(82)90433-1. [DOI] [PubMed] [Google Scholar]
- 2.Dawood MY. Ibuprofen and dysmenorrhea. Am J Med. 1984;77(1A):87–94. doi: 10.1016/s0002-9343(84)80025-x. [DOI] [PubMed] [Google Scholar]
- 3.Hauksson A, Akerlund M, Melin P. Uterine blood flow and myometrial activity at menstruation, and the action of vasopressin and a synthetic antagonist. Br J Obstet Gynaecol. 1988;95(9):898–904. doi: 10.1111/j.1471-0528.1988.tb06577.x. [DOI] [PubMed] [Google Scholar]
- 4.Pulkkinen MO. Prostaglandins and the non-pregnant uterus. The pathophysiology of primary dysmenorrhea. Acta Obstet Gynecol Scand Suppl. 1983;113:63–67. doi: 10.3109/00016348309155200. [DOI] [PubMed] [Google Scholar]
- 5.Akerlund M. Vascularization of human endometrium. Uterine blood flow in healthy condition and in primary dysmenorrhoea. Ann N Y Acad Sci. 1994;734:47–56. doi: 10.1111/j.1749-6632.1994.tb21735.x. [DOI] [PubMed] [Google Scholar]
- 6.Wong CL, Farquhar C, Roberts H, Proctor M. Oral contraceptive pill for primary dysmenorrhoea. Cochrane Database Syst Rev. 2009;4:CD002120. doi: 10.1002/14651858.CD002120.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wiegratz I, Kuhl H. Long-cycle treatment with oral contraceptives. Drugs. 2004;64(21):244724–244762. doi: 10.2165/00003495-200464210-00006. [DOI] [PubMed] [Google Scholar]
- 8.Vercellini P, De Giorgi O, Mosconi P, Stellato G, Vicentini S, Crosignani PG. Cyproterone acetate versus a continuous monophasic oral contraceptive in the treatment of recurrent pelvic pain after conservative surgery for symptomatic endometriosis. Fertil Steril. 2002;77(1):52–61. doi: 10.1016/s0015-0282(01)02951-x. [DOI] [PubMed] [Google Scholar]
- 9.Vercellini P, Frontino G, De Giorgi O, Pietropaolo G, Pasin R, Crosignani PG. Continuous use of an oral contraceptive for endometriosis-associated recurrent dysmenorrhea that does not respond to a cyclic pill regimen. Fertil Steril. 2003;80(3):560–563. doi: 10.1016/s0015-0282(03)00794-5. [DOI] [PubMed] [Google Scholar]
- 10.Littman GS, Walker BR, Schneider BE. Reassessment of verbal and visual analog ratings in analgesic studies. Clin Pharmacol Ther. 1985;38(1):16–23. doi: 10.1038/clpt.1985.127. [DOI] [PubMed] [Google Scholar]
- 11.Markum RA. Assessment of the reliability of and the effect of neutral instructions on the symptom ratings on the Moos Menstrual Distress Questionnaire. Psychosom Med. 1976;38(3):163–172. doi: 10.1097/00006842-197605000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38(4):963–974. [PubMed] [Google Scholar]
- 13.Littell RCMG, Stroup WW, Wolfinger RD, Schabenberger O. SAS® for Mixed Models, Second Edition. Cary, NC: SAS Institute Inc; 2006. [Google Scholar]
- 14.Hilbe J. Negative Binomial Regression. Cambridge University Press: Cambridge; 2007. [Google Scholar]
- 15.Davis AR, Westhoff C, O'Connell K, Gallagher N. Oral contraceptives for dysmenorrhea in adolescent girls: a randomized trial. Obstet Gynecol. 2005;106(1):97–104. doi: 10.1097/01.AOG.0000165826.03915.65. [DOI] [PubMed] [Google Scholar]
- 16.Kwiecien M, Edelman A, Nichols MD, Jensen JT. Bleeding patterns and patient acceptability of standard or continuous dosing regimens of a low-dose oral contraceptive: a randomized trial. Contraception. 2003;67(1):9–13. doi: 10.1016/s0010-7824(02)00445-6. [DOI] [PubMed] [Google Scholar]
- 17.Thomas SL, Ellertson C. Nuisance or natural and healthy: should monthly menstruation be optional for women? Lancet. 2000;355(9207):922–924. doi: 10.1016/S0140-6736(99)11159-0. [DOI] [PubMed] [Google Scholar]
- 18.Legro RS, Pauli JG, Kunselman AR, Meadows JW, Kesner JS, Zaino RJ, et al. Effects of continuous versus cyclical oral contraception: a randomized controlled trial. J Clin Endocrinol Metab. 2008;93(2):420–429. doi: 10.1210/jc.2007-2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaunitz AM. Menstruation: choosing whether...and when. Contraception. 2000;62(6):277–284. doi: 10.1016/s0010-7824(00)00182-7. [DOI] [PubMed] [Google Scholar]
- 20.Loudon NB, Foxwell M, Potts DM, Guild AL, Short RV. Acceptability of an oral contraceptive that reduces the frequency of menstruation: the tri-cycle pill regimen. Br Med J. 1977;2(6085):487–490. doi: 10.1136/bmj.2.6085.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamerlynck JV, Vollebregt JA, Doornebos CM, Muntendam P. Postponement of withdrawal bleeding in women using low-dose combined oral contraceptives. Contraception. 1987;35(3):199–205. doi: 10.1016/0010-7824(87)90022-9. [DOI] [PubMed] [Google Scholar]
- 22.Anderson FD, Hait H. A multicenter, randomized study of an extended cycle oral contraceptive. Contraception. 2003;68(2):89–96. doi: 10.1016/s0010-7824(03)00141-0. [DOI] [PubMed] [Google Scholar]
- 23.Barbosa IC, Filho CI, Faggion D, Jr, Baracat EC. Prospective, open-label, noncomparative study to assess cycle control, safety and acceptability of a new oral contraceptive containing gestodene 60 microg and ethinylestradiol 15 microg (Minesse) Contraception. 2006;73(1):30–33. doi: 10.1016/j.contraception.2005.06.057. [DOI] [PubMed] [Google Scholar]
- 24.Ross C, Coleman G, Stojanovska C. Prospectively reported symptom change across the menstrual cycle in users and non-users of oral contraceptives. J Psychosom Obstet Gynaecol. 2003;24(1):15–29. doi: 10.3109/01674820309042797. [DOI] [PubMed] [Google Scholar]
- 25.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database Syst Rev. 9:CD003987. doi: 10.1002/14651858.CD003987.pub2. [DOI] [PubMed] [Google Scholar]
- 26.Boldo A, White WB. Blood pressure effects of the oral contraceptive and postmenopausal hormone therapies. Endocrinol Metab Clin North Am. 40(2):419–432. ix. doi: 10.1016/j.ecl.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 27.Hickson SS, Miles KL, McDonnell BJ, Yasmin Cockcroft JR, Wilkinson IB, et al. Use of the oral contraceptive pill is associated with increased large artery stiffness in young women: the ENIGMA study. J Hypertens. 29(6):1155–1159. doi: 10.1097/HJH.0b013e328346a5af. [DOI] [PubMed] [Google Scholar]
- 28.Machado RB, Fabrini P, Cruz AM, Maia E, da Cunha Bastos A. Clinical and metabolic aspects of the continuous use of a contraceptive association of ethinyl estradiol (30 microg) and gestodene (75 microg) Contraception. 2004;70(5):365–370. doi: 10.1016/j.contraception.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 29.Rabe T, Nitsche DC, Runnebaum B. The effects of monophasic and triphasic oral contraceptives on ovarian function and endometrial thickness. Eur J Contracept Reprod Health Care. 1997;2(1):39–51. doi: 10.1080/13625189709049933. [DOI] [PubMed] [Google Scholar]
- 30.Grow DR, Iromloo K. Oral contraceptives maintain a very thin endometrium before operative hysteroscopy. Fertil Steril. 2006;85(1):204–207. doi: 10.1016/j.fertnstert.2005.06.044. [DOI] [PubMed] [Google Scholar]