Abstract
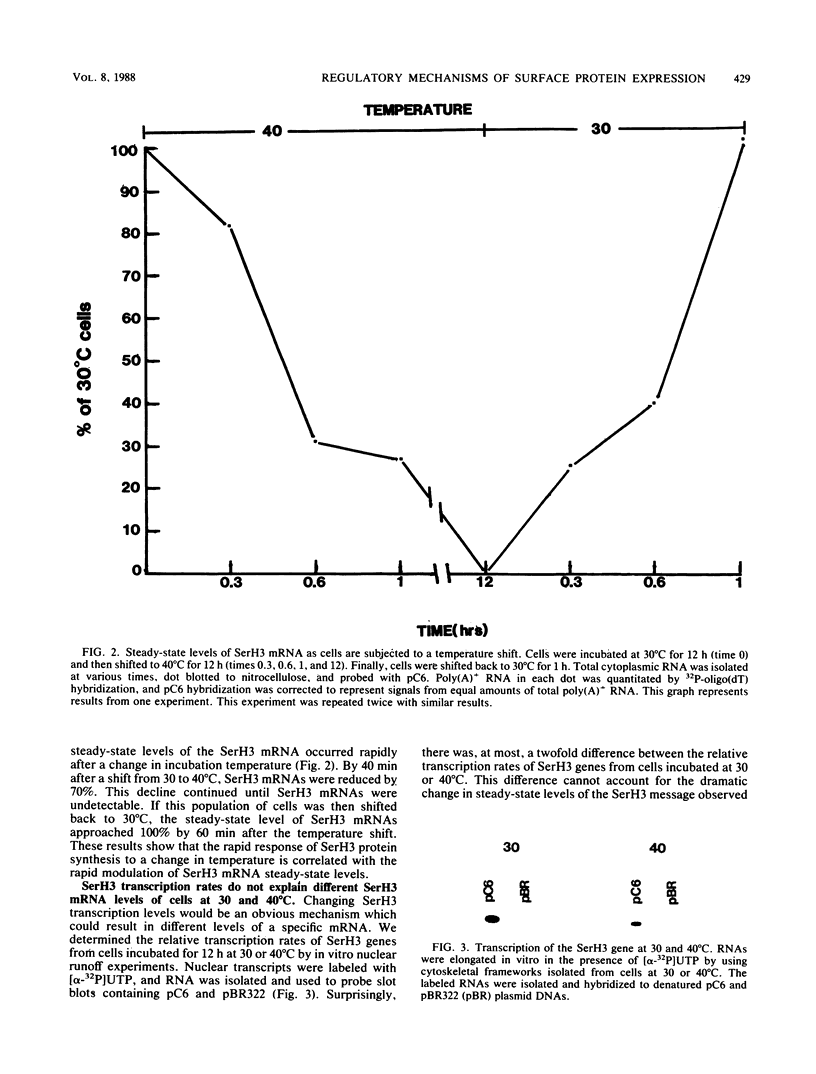
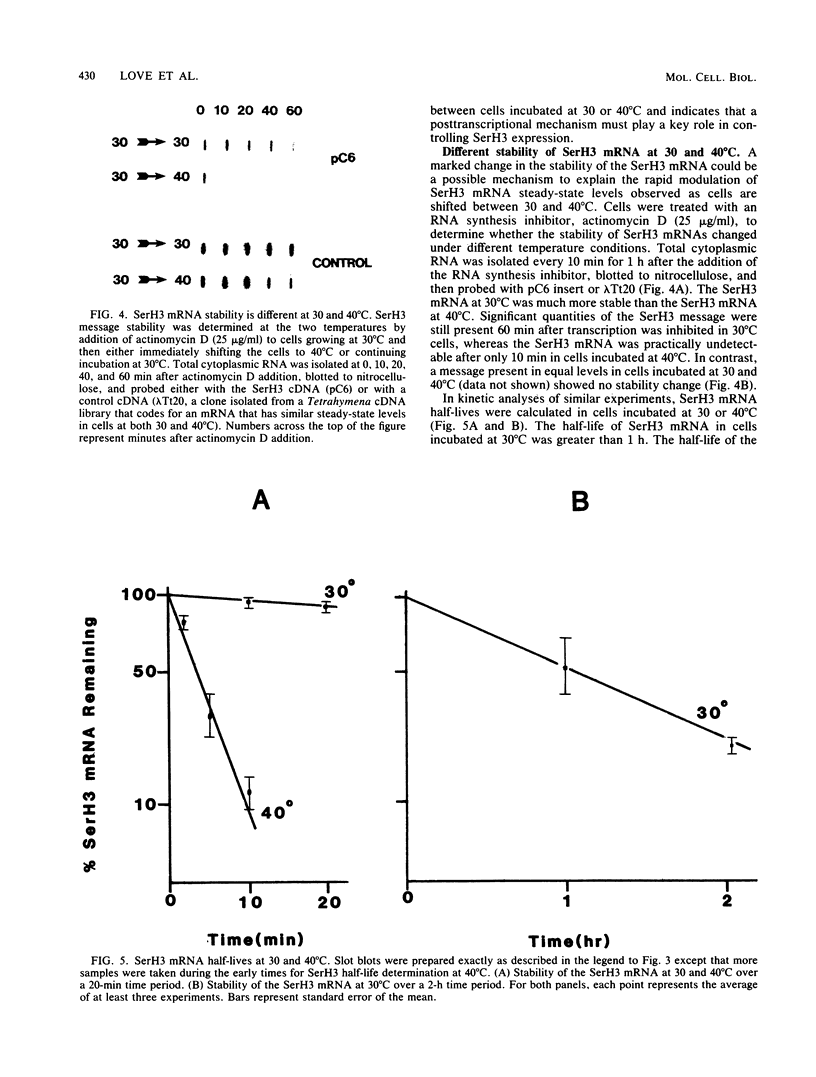
Synthesis of the serotype H3 (SerH3) surface antigen is temperature dependent and responds within 1 h to a change in incubation conditions (G.A. Bannon, R. Perkins-Dameron, and A. Allen-Nash, Mol. Cell. Biol. 6:3240-3245, 1986). Recently, a Tetrahymena thermophila cDNA clone (pC6; D.W. Martindale and P.J. Bruns, Mol. Cell. Biol. 3:1857-1865, 1983) has been shown to be homologous to a portion of the SerH3 mRNA (F.P. Doerder and R.L. Hallberg, personal communication), and it was shown that the cellular levels of this RNA rapidly decreased when cells were shifted from 30 to 41 degrees C (R.L. Hallberg, K.W. Kraus, and R.C. Findly, Mol. Cell. Biol. 4:2170-2179, 1984). These observations indicate that synthesis of the SerH3 protein is highly regulated in response to temperature and led us to initiate studies to determine the mechanism(s) by which SerH3 gene expression is controlled. Using pC6 as a hybridization probe for the SerH3 mRNA, we have determined that (i) the level of SerH3 protein synthesis is directly correlated with the amount of SerH3 message available for translation; (ii) there is, at most, a twofold difference between the relative transcription rates of SerH3 genes at 30 and 40 degrees C; (iii) the SerH3 mRNA half-life in cells incubated at 30 degrees C is greater than 1 h, whereas the half-life in cells incubated at 40 degrees C is only approximately 3 min. These results demonstrate that Tetrahymena SerH3 surface protein expression is regulated by mRNA abundance. Furthermore, the major mechanism controlling mRNA abundance is a dramatic temperature-dependent change in SerH3 mRNA stability.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bannon G. A., Bowen J. K., Yao M. C., Gorovsky M. A. Tetrahymena H4 genes: structure, evolution and organization in macro- and micronuclei. Nucleic Acids Res. 1984 Feb 24;12(4):1961–1975. doi: 10.1093/nar/12.4.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bannon G. A., Perkins-Dameron R., Allen-Nash A. Structure and expression of two temperature-specific surface proteins in the ciliated protozoan Tetrahymena thermophila. Mol Cell Biol. 1986 Sep;6(9):3240–3245. doi: 10.1128/mcb.6.9.3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calzone F. J., Angerer R. C., Gorovsky M. A. Regulation of protein synthesis in Tetrahymena: isolation and characterization of polysomes by gel filtration and precipitation at pH 5.3. Nucleic Acids Res. 1982 Mar 25;10(6):2145–2161. doi: 10.1093/nar/10.6.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens M. J. A potential role for RNA transcribed from B2 repeats in the regulation of mRNA stability. Cell. 1987 Apr 24;49(2):157–158. doi: 10.1016/0092-8674(87)90553-8. [DOI] [PubMed] [Google Scholar]
- Doerder F. P., Berkowitz M. S. Purification and partial characterization of the H immobilization antigens of Tetrahymena thermophila. J Protozool. 1986 May;33(2):204–208. doi: 10.1111/j.1550-7408.1986.tb05590.x. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gorovsky M. A., Yao M. C., Keevert J. B., Pleger G. L. Isolation of micro- and macronuclei of Tetrahymena pyriformis. Methods Cell Biol. 1975;9(0):311–327. doi: 10.1016/s0091-679x(08)60080-1. [DOI] [PubMed] [Google Scholar]
- Gough N. M., Gough J., Metcalf D., Kelso A., Grail D., Nicola N. A., Burgess A. W., Dunn A. R. Molecular cloning of cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony stimulating factor. 1984 Jun 28-Jul 4Nature. 309(5971):763–767. doi: 10.1038/309763a0. [DOI] [PubMed] [Google Scholar]
- Horowitz S., Bowen J. K., Bannon G. A., Gorovsky M. A. Unusual features of transcribed and translated regions of the histone H4 gene family of Tetrahymena thermophila. Nucleic Acids Res. 1987 Jan 12;15(1):141–160. doi: 10.1093/nar/15.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelso A., Metcalf D. Characteristics of colony-stimulating factor production by murine T-lymphocyte clones. Exp Hematol. 1985 Jan;13(1):7–15. [PubMed] [Google Scholar]
- Martindale D. W., Bruns P. J. Cloning of abundant mRNA species present during conjugation of Tetrahymena thermophila: identification of mRNA species present exclusively during meiosis. Mol Cell Biol. 1983 Oct;3(10):1857–1865. doi: 10.1128/mcb.3.10.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer E., Caron F., Guiard B. Blocking of in vitro translation of Paramecium messenger RNAs is due to messenger RNA primary structure. Biochimie. 1984 May;66(5):403–412. doi: 10.1016/0300-9084(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Murphy D., Brickell P. M., Latchman D. S., Willison K., Rigby P. W. Transcripts regulated during normal embryonic development and oncogenic transformation share a repetitive element. Cell. 1983 Dec;35(3 Pt 2):865–871. doi: 10.1016/0092-8674(83)90119-8. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott M. R., Westphal K. H., Rigby P. W. Activation of mouse genes in transformed cells. Cell. 1983 Sep;34(2):557–567. doi: 10.1016/0092-8674(83)90388-4. [DOI] [PubMed] [Google Scholar]
- Shaw G., Kamen R. A conserved AU sequence from the 3' untranslated region of GM-CSF mRNA mediates selective mRNA degradation. Cell. 1986 Aug 29;46(5):659–667. doi: 10.1016/0092-8674(86)90341-7. [DOI] [PubMed] [Google Scholar]
- Singh K., Carey M., Saragosti S., Botchan M. Expression of enhanced levels of small RNA polymerase III transcripts encoded by the B2 repeats in simian virus 40-transformed mouse cells. Nature. 1985 Apr 11;314(6011):553–556. doi: 10.1038/314553a0. [DOI] [PubMed] [Google Scholar]
- Spradling A. C., Digan M. E., Mahowald A. P., Scott M., Craig E. A. Two clusters of genes for major chorion proteins of Drosophila melanogaster. Cell. 1980 Apr;19(4):905–914. doi: 10.1016/0092-8674(80)90082-3. [DOI] [PubMed] [Google Scholar]
- Williams N. E., Doerder F. P., Ron A. Expression of a cell surface immobilization antigen during serotype transformation in Tetrahymena thermophila. Mol Cell Biol. 1985 Aug;5(8):1925–1932. doi: 10.1128/mcb.5.8.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe J. Cytoskeletal reorganization and plasma membrane fusion in conjugating Tetrahymena. J Cell Sci. 1985 Feb;73:69–85. doi: 10.1242/jcs.73.1.69. [DOI] [PubMed] [Google Scholar]
- Wong G. G., Witek J. S., Temple P. A., Wilkens K. M., Leary A. C., Luxenberg D. P., Jones S. S., Brown E. L., Kay R. M., Orr E. C. Human GM-CSF: molecular cloning of the complementary DNA and purification of the natural and recombinant proteins. Science. 1985 May 17;228(4701):810–815. doi: 10.1126/science.3923623. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]