Abstract
CD8+ T cells eliminate intracellular infections through two contact-dependent effector functions: cytolysis and antiviral cytokine secretion. Here, we identify an additional function for memory CD8+ T cells persisting at frontline sites of microbial exposure: as local sensors of previously encountered antigens that precipitate innate-like alarm signals and draw circulating memory CD8+ T cells into the tissue. When memory CD8+ T cells residing in the female reproductive tract encountered cognate antigen, they expressed interferon-γ (IFN-γ), potentiated robust local inflammatory chemokine expression and induced rapid recruitment of circulating memory CD8+ T cells. Anamnestic responses in frontline tissues are thus an integrated collaboration between frontline and circulating populations of memory CD8+ T cells, and vaccines should establish both populations to maximize rapid responses.
Introduction
Most infections are initiated at vulnerable body surfaces, such as the mucosae. CD8+ T cells, which are endowed with potent effector functions, are typically required for the elimination of viral infections. These effector functions, cytotoxic elimination of infected host cells and the secretion of cytokines that interfere with viral replication or promote inflammation, operate locally and require direct interaction with viral antigen-bearing cells1-4. Thus, CD8+ T cells must be present in sufficient quantity at sites of infection to examine each host cell for the presence of viral antigens. The critical challenge is that prior to a primary infection, pathogen-specific CD8+ T cells are exceedingly rare and only patrol secondary lymphoid organs (SLOs)1,3,5. Thus in naïve individuals, the non-specific innate immune system, which is constitutively distributed throughout the host, must hold pathogen replication in check until more potent CD8+ T cells can proliferate and migrate to infected tissues5-7. This lag in CD8+ T cell responses provides a critical window of opportunity for pathogen replication.
In the event that the pathogen is cleared, hosts retain expanded populations of pathogen-specific memory CD8+ T cells that patrol SLOs3,6-15 and discrete populations that patrol non-lymphoid tissues2,3,8-15. Those memory T cells that patrol SLOs, referred to as central memory T cells (TCM), routinely recirculate between blood, lymph and various SLOs2,3. Non-lymphoid memory T cells, referred to as effector memory T cells (TEM), constitutively recirculate between blood, lymph and non-lymphoid tissues2,3,9-14,16,17. This model has recently been amended. Memory T cells do not undergo demonstrable recirculation through many non-lymphoid anatomic compartments, including the small intestine epithelium, skin epidermis, lung, salivary gland and central nervous system9-14,16-18. Rather, T cells are capable of migrating into many non-lymphoid compartments for only a brief time after antigen-stimulation, and then differentiate into organ-specific resident non-recirculating memory T cells in situ11,12,15,18. These sessile front-line populations have been referred to as resident memory T cells (TRM or resTEM).
Current models suggest that memory T cells positioned within non-lymphoid tissues versus SLOs make anatomically and temporally discrete contributions to secondary responses, only becoming functionally active when their respective tissues encounter cognate antigen. CD8+ T cells that populate SLOs recognize antigen draining from the site of infection before proliferating, differentiating into effectors that respond to inflammatory chemokines, and migrating to the frontline infected tissue. The contribution of this reserve force of the response, while potent, is delayed by several days. In contrast, resident memory CD8+ T cells that are positioned at sites of infection at the time of a pathogen re-exposure event are anatomically poised for immediate recognition and response14,15,17,19-21. Their role in protective immunity has been likened to an occupying force that operates at frontline sites of infection independently of recirculating memory T cells9,13,19,22. It has recently been demonstrated that mice that possess recirculating, but not resident, memory CD8+ T cell populations (rather than possessing both populations) exhibit significantly delayed control against viral re-challenges at frontline tissues9,13,22,23.
Here, we define a new function for resident memory CD8+ T cells: as sentinels of pathogen-derived antigens. Local adaptive immune recognition was far more potent and sensitive than the innate immune system in inducing local chemokine expression. Importantly, when resident memory T cells within the female reproductive tract recognized cognate antigen in situ, they precipitated rapid and abundant recruitment of unstimulated memory T cells to this tissue that is not under routine immunosurveillance by recirculating memory T cells. Thus, resident memory T cells are more than just an occupying force, as they orchestrate migration and thus serve to recruit reinforcements to fight infections.
Results
Rapid local CD8 T cell recall response
To visualize virus-specific CD8+ T cells in the female reproductive tract (FRT), we transferred Thy1.1+ naive LCMV-specific P14 CD8+ T cells (which recognize the gp33 epitope of lymphocytic choriomeningitis virus, LCMV) into naïve mice. We then infected these recipients with 2 × 105 p.f.u. LCMV Armstrong i.p. and identified LCMV-specific memory CD8+ T cells two months later by Thy1.1 staining. These mice will be referred to as P14 immune chimeras. Ratios of P14 CD8+ T cells to nucleated cells were determined by computerized image analyses of Thy1.1 and DAPI stained sections (Methods and Supplementary Fig. 1). To test the dynamics of the CD8+ T cell recall response upon local antigen re-exposure, we transcervically (t.c.) challenged P14 immune chimeras with a recombinant vaccinia virus expressing gp33 (VV-gp33) by depositing virus directly into the cervical canal and uterine lumen23,24. Local recall infection elicited a rapid increase in the number of P14 T cells in the reproductive tract within two days (Fig. 1 and Supplementary Fig. 1). Infection with vaccinia virus expressing an irrelevant antigen, chicken ovalbumin (VV-OVA) elicited little accumulation of memory P14 T cells within the tissue, indicating that memory CD8+ T cell recognition of cognate antigen was required. To test if infection-induced inflammation was necessary for antigen-specific T cell accumulation within the FRT, we injected cognate gp33 peptide transcervically. Gp33 peptide, without infection, was sufficient to recapitulate the increase in the number of P14 T cells within the tissue seen upon local VV-gp33 re-challenge (Fig. 1). Control SIINFEKL peptide, which is not recognized by P14 T cells, had little effect. These data show that cognate antigen recognition by memory CD8+ T cells precipitates a rapid accumulation of memory CD8+ T cells in the reproductive tract. This response was recapitulated without the use of transgenic T cells (Supplementary Fig. 2).
Figure 1. Local antigen re-challenge precipitates rapid accumulation of antigen-specific CD8+ T cells within the female reproductive tract (FRT).
Widely disseminated Thy1.1+ memory P14 CD8+ T cells were established after LCMV infection. Immune mice were challenged transcervically (t.c.) with 4×105 p.f.u. VV-gp33 or VV-OVA, 50μg gp33 or SIINFEKL peptide, or left untreated. (a) 48 h later, P14 cells in FRT were enumerated relative to total nucleated cells. (b) Representative images show α-Thy1.1 (red) and DAPI (blue) staining. Scale bars = 100 μm. n = 6, one representative of three experiments. n = 6, pooled from 2 experiments. *, P < 0.05, **, P <0.001, unpaired two-tailed t-test, mean±SEM.
Antigen recognition by T cells induced chemokine expression
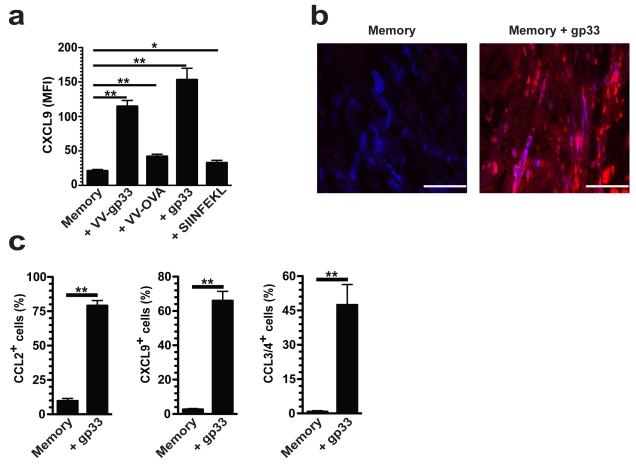
Chemokines are necessary for T cell extravasation from blood to tissues18,24,25. For example, CXCL9 induces effector T cell migration to the FRT11,12,18,25. To determine if memory CD8+ T cell reactivation induced chemokine expression within the FRT, P14 immune chimeras were rechallenged with VV-gp33 t.c. Within 12h, VV-gp33 induced a 5.5-fold increase in CXCL9 expression on the CD31+ vasculature (Fig. 2a,b and Supplementary Fig. 3). In comparison, challenge with VV-OVA t.c. induced little CXCL9 induction, demonstrating that cognate antigen recognition by T cells potentiated almost immediate local chemokine expression (Fig. 2). Antigen recognition without the context of infection was sufficient to drive this chemokine gradient as t.c. gp33 peptide inoculation recapitulated CXCL9 expression on CD31+ cells (Fig. 2a,b). Further analysis revealed that reactivation of memory T cells induced a general inflammatory chemokine response. For example, CCL2 and CXCL9 were expressed among tissue CD11c+ MHCII+ dendritic cells, CCL3 and CCL4 were expressed by memory P14 CD8+ T cells, and CXCL10 and CX3CL1 were present on CD31+ vasculature within 12 h (Fig. 2c and Supplementary Fig. 4). These data demonstrate that cognate antigen recognition by memory CD8+ T cells rapidly induces tissue chemokine expression, and to much higher concentrations at this early time point than achieved by innate recognition of vaccinia virus infection.
Figure 2. Local antigen re-challenge precipitates rapid inflammatory chemokine expression within the female reproductive tract (FRT).
Widely disseminated Thy1.1+ memory P14 CD8+ T cells were established after LCMV infection. Immune mice were challenged transcervically (t.c.) with 4×105 p.f.u. VV-gp33 or VV-OVA, 50μg gp33 or SIINFEKL peptide, or left untreated. (a) 48 h later, mean fluorescence intensity (MFI) of CXCL9 expression on CD31+ vessels was determined 12 h after t.c. challenge. (b) Representative images show α-CXCL9 (red) and α-CD31 (blue). Scale bars = 100 μm. (c) 12 h after t.c. gp33 challenge, CCL2, CXCL9 and CCL3/4 expression was evaluated on the indicated cell populations by flow cytometry. (a,b) n = 6, one representative of three experiments. (c) n = 6, pooled from 2 experiments. *, P < 0.05, **, P < 0.001, unpaired two-tailed t-test, mean±SEM.
Reactivated memory T cells recruited resting memory T cells
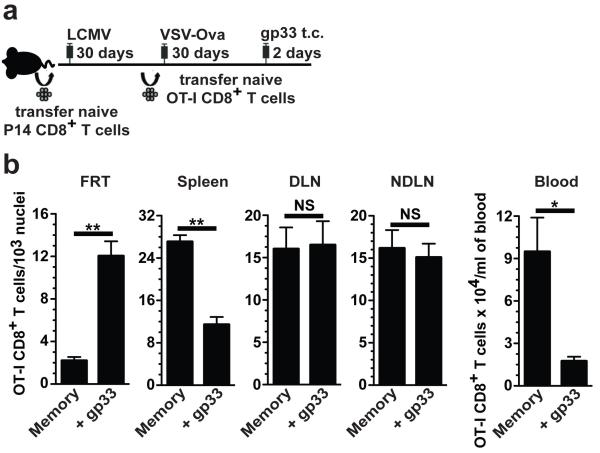
We next questioned whether memory CD8+ T cell accumulation was dependent on in situ proliferation of local memory CD8+ T cells, or alternatively, on migration of either antigen-reactivated, canonical effector CD8+ T cells, or resting memory CD8+ T cells from outside of the tissue. To test this, naïve CD45.1+ OT-I CD8+ T cells (specific for the SIINFEKL epitope within the ovalbumin protein) were transferred into P14 immune chimeras, which were then infected with recombinant vesicular stomatitis virus expressing ovalbumin (VSV-OVA). This experimental design permitted the visualization of two memory CD8+ T cell populations with distinct specificities: Thy1.1+ gp33-specific P14 and CD45.1+ SIINFEKL-specific OT-I cells. These mice were either left untreated or challenged t.c. with gp33 peptide to reactivate the P14 memory CD8+ T cell population (Fig. 3a). Two days later, OT-I CD8+ T cells were enumerated. Transcervical administration of gp33 peptide induced a 5.5-fold increase in the number of memory OT-I cells within the FRT, which was coupled with a 2.4-fold reduction in the spleen and a 5.4-fold reduction in blood (Fig. 3b). Interestingly, the number of memory OT-I CD8+ T cells did not change in either the draining or non-draining lymph nodes. These data demonstrate that bystander memory CD8+ T cells of irrelevant specificities accumulate within the FRT in response to memory CD8+ T cell reactivation, indicating that local CD8+ T cell amassment is not dependent on in situ proliferation or antigen-mediated effector differentiation, but rather it is inversely correlated with dispersion of resting memory CD8+ T cells from spleen and blood.
Figure 3. Unstimulated memory CD8+ T cells redistribute when other memory CD8+ T cells are reactivated.
(a) Two populations of memory CD8+ T cells with different specificities, Thy1.1+ P14 cells (gp33-specific) and CD45.1+ OT-I cells (SIINFEKL-specific), were established. (b) Mice were challenged with gp33. 48 h later, OT-I cells were enumerated in the FRT, spleen, blood, draining lymph node (DLN) and non-draining lymph node (NDLN). n ≥ 5, *, P < 0.05, **, P < 0.001, unpaired two-tailed t-test, mean±SEM.
Local memory CD8+ T cells orchestrate recruitment
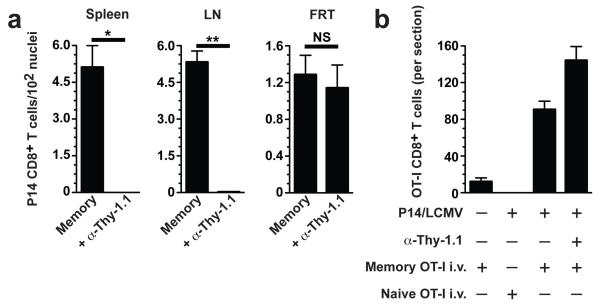
We next asked whether antigen-dependent reactivation of memory CD8+ T cells within prototypical inductive sites of immune responses, such as lymph nodes and spleen, were required to precipitate resting memory CD8+ T cell migration to the FRT. To this end, we developed a model whereby memory P14 cells could be removed from SLOs but preserved in the FRT by injecting P14 immune chimeras with 3 μg of complement fixing Thy1.1-specific antibody (Fig. 4a). Depleted mice were then injected i.v. with memory OT-I CD8+ T splenocytes and challenged with gp33 t.c. Two days later, OT-I CD8+ T cells were enumerated, revealing that frontline memory CD8+ T cells, in the absence of SLO and blood memory, were able to recruit memory CD8+ T cells from outside the tissue (Fig. 4b). However, naïve OT-I CD8+ T cells were excluded from the FRT, indicating that only antigen-experienced T cells migrated to local sites of T cell reactivation (Fig. 4b).
Figure 4. Memory CD8+ T cells within nonlymphoid tissue orchestrate rapid recruitment of unstimulated memory, but not naïve, CD8+ T cells to the site of antigen re-exposure.
(a) Thy1.1+ memory P14 cells were depleted from recirculating compartments but preserved in the FRT via administration of complement fixing α-Thy1.1 Ab. P14 cells were evaluated in tissues 72 h after Ab treatment. (b) Naïve mice, P14 immune chimeras, and P14 immune chimeras depleted of circulating P14 cells received i.v. transfers of naïve or memory OT-I CD8+ T cells, as indicated. 24 h after OT-I transfer, mice were challenged t.c. with gp33 and transferred OT-I cells were enumerated 48 h later within 7 μm coronal sections through the entire FRT. n = 3, representative of 2-3 independent experiments. *, P < 0.01, **, P <0.001, unpaired two-tailed t-test, mean±SEM.
resTEM-derived IFN-γ is required for recruitment
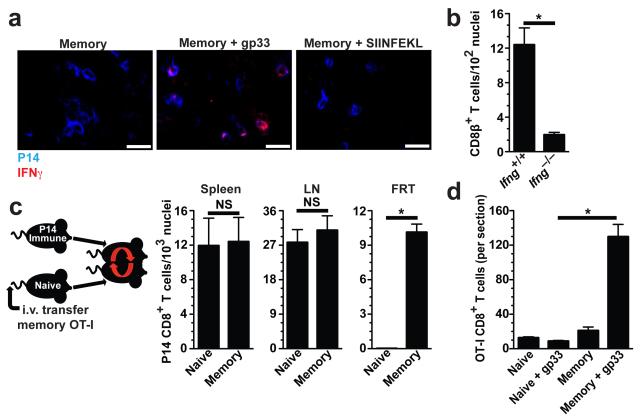
It remained unclear how local memory CD8+ T cells communicated an antigen-recognition event in order to precipitate peripheral T cell recruitment. IFN-γ is a secreted cytokine that signals ‘danger’ or microbial infection to the host. Antigen-specific memory T cells within the FRT rapidly expressed IFN-γ in vivo after local antigen rechallenge (Fig. 5a). To test whether IFN-γ expression was important for inducing rapid CD8+ T cell recruitment to the FRT, we generated C57BL/6 mice that contained either wild-type or Ifnγ−/− memory OT-I CD8+ T cells after immunization with VV-OVA. Mice were then challenged t.c. with SIINFEKL peptide to reactivate memory OT-I cells. CD8β+ T cells and CXCL9 expression were then quantified (Fig. 5b and Supplementary Fig. 5), revealing that the alarm function of sensitized memory CD8+ T cells was dependent upon their ability to express IFN-γ.
Figure 5. IFN-γ and resident memory CD8+ T cells are required for rapid recruitment of memory T cells to the site of antigen reexposure.
(a) OT-I immune chimeras were challenged t.c. with SIINFEKL. 12 h later, OT-I cells and IFN-γ were visualized by immunofluorescence microscopy. (b) OT-I.Ifng+/+ and OT-I.Ifng−/− immune chimeras were challenged t.c. with SIINFEKL. 48 h later, CD8β+ cells were enumerated in FRT. (c) CD45.1+ memory OT-I cells were transferred to naïve recipients, which were then conjoined to Thy1.1+ P14 immune chimeric mice via parabiosis. 14-16 days after surgery, memory P14 cells were enumerated in both conjoined parabionts. (d) Parabionts (as in c) were challenged with gp33 t.c. and 48 h later OT-Is were quantified within 7 μm coronal sections through the entire FRT. Scale bars = 50 μm.. Data pooled from two independent experiments totaling 5-6 mice per group. *, P <0.001, unpaired two-tailed t-test, mean±SEM.
In short-term homing assays, effector but not memory CD8+ T cells migrated to intestinal epithelium, salivary gland or FRT9,11-13,18,22. These data support a model whereby effector T cells seed mucosal tissues shortly after antigen stimulation, then differentiate into resident memory T cells that persist in situ without recirculating5,9,12,13,22,26. To further interrogate this hypothesis, we conjoined P14 immune chimeras by parabiosis surgery with naïve mice that had received an i.v. transfer of OT-I memory CD8+ T cells. 14-16 days later, P14 memory CD8+ T cells had equilibrated between the SLOs, but not FRTs, of both parabionts, indicating that memory T cells in the FRT were not recirculating (Fig. 5c). When both parabionts were challenged with gp33 t.c., rapid OT-I accumulation occurred only in mice that contained P14s within the FRT (Fig. 5d). These data demonstrate that resident memory T cells at the site of antigen exposure are required for inciting rapid local amassment of unstimulated memory T cells, thus defining a new function for cell-mediated immunity positioned at initial sites of infection.
Discussion
Upon primary infection, the innate immune system responds to microbial-derived “danger” signals, resulting in the expression of inflammatory chemokines and recruitment of leukocytes to the affected tissue5,26-28. Although this response is often deemed “immediate” because the innate immune system is pre-positioned in tissues, clearance of most pathogens requires the specificity and more potent effector mechanisms of the adaptive immune system. However, primary adaptive responses are slow to develop because rare pathogen-specific lymphocytes must proliferate and migrate from SLOs to sites of infection, providing a temporal window of opportunity for continued pathogen replication and disease.
Our study defines an additional functional role for non-lymphoid tissue resident memory CD8+ T cells: the use of the highly sensitive lymphocyte antigen receptor to precipitate much faster and more robust tissue chemokine expression than accomplished by the innate immune system, and the ability to initiate rapid recruitment of pre-formed memory CD8+ T cells to sites of antigen recognition. This feature of the adaptive immune response likely allows numerical amassment of antigen-experienced T cells to sites of re-infection before the protracted process of SLO-dependent reactivation and subsequent migration.
These results do not exclude other roles for frontline memory CD8+ T cells during anamnestic responses. Recent reports indicate that resident memory CD8+ T cells can initiate proliferation in situ in response to local antigen stimulation27-29. It has also been shown that CD4+ T cell reactivation in lung potentiates IFN-γ-independent inflammatory innate-like cytokine production18,29. Perhaps this process could facilitate recruitment of various leukocyte subsets, including monocytes, neutrophils and natural killer cells. A recent study showed that topical vaginal applications of CXCL9 and CXCL10 enhance effector T cell migration to the FRT, but fail to recruit resting memory T cells18,23. We show that reactivation of local resident memory, which was established during a prior effector response, is sufficient to drive recruitment of unstimulated memory T cells from outside the tissue. In other words, the exclusion of memory T cells from recirculating through restricted non-lymphoid compartments is context dependent, and can be overcome when local memory T cells sound the alarm in response to antigen-recognition within the tissue. This process likely provides a means to recruit pathogen-experienced T cells to sites of reinfection more rapidly; by bypassing the delay inherent in waiting for antigen dissemination from infected sites, followed by TCR-recognition and effector differentiation.
These observations highlight the importance of establishing memory CD8+ T cells at frontline sites of pathogen exposure to provide sensitive detection of antigens previously recognized in a pathogenic context. But in addition, optimal rapid responses in tissues will require recruitable memory CD8+ T cell populations from outside the affected tissue.
Methods
Mice and infections
All mice were used in accordance with the guidelines of the Institutional Animal Care and Use Committees at the University of Minnesota. C57BL/6J mice were purchased from The Jackson Laboratory. Thy1.1+ P14, CD45.1+ OT-I, and CD45.1+ mice were fully backcrossed to C57BL/6J mice and maintained in our animal colony. OT-I.Ifng−/− mice were generously provided by M. Mescher (University of Minnesota), and were generated as follows: B6.129S7-Ifngtm1Ts/J mice, deficient for IFN-γ (Ifng), were obtained from The Jackson Laboratory and bred with OT-I mice. P14 immune chimeras were generated by transferring 5 × 104 naive transgenic Thy1.1+ P14 T cells into naive C57BL/6J mice and infecting with 2 × 105 p.f.u. LCMV Armstrong the next day. Memory OT-I cells were generated by transferring 5 × 104 naïve transgenic CD45.1+ OT-I T cells into naive C57BL/6J mice or into memory P14 chimeras. The next day, recipients were infected with either 1 × 106 p.f.u. VSV-OVA or 2 × 106 p.f.u. VV-OVA i.v. For local re-challenge experiments, 50 μg of the indicated peptides or 4 × 105 p.f.u. VV-gp33 or VV-OVA was delivered t.c. as described13,23 in a volume of 35 μl delivered by modified gel loading pipet. For depleting circulating memory P14 CD8+ T cells, mice were injected with 3 μg anti-Thy1.1 (clone HIS51) into the peritoneal cavity. Depletion was confirmed by Thy1.1 (clone OX-7) and H-2Db/gp33 MHC I tetramer staining.
Parabiosis surgery
Parabiosis surgeries were performed as described13,30. Briefly, mice were shaved along opposite lateral flanks. Skin was then wiped clean of fur with alcohol prep pads, and further cleaned with betadine solution and 70% alcohol. Mirrored incisions were made on lateral aspects of both mice and 5-0 vicryl thread was used to suture skin to conjoin the mice. Additional sutures were placed through the olecranon and knee joints to secure the legs. Conjoined mice were then rested for 14 to 16 days before experiments.
Immunofluorescence microscopy and flow cytometry
For immunofluorescence microscopy, tissues were frozen in 2-methylbutane surrounded by dry ice. Frozen blocks were cut into 7 μm sections, fixed in acetone, blocked in a 5% bovine serum albumin PBS solution for 1 h, and stained with DAPI (Invitrogen) and antibodies specific for Thy1.1 (OX-7), CD31 (390) and CD8β (YTS156.7.7) (Biolegend), IFN-γ (XMG12 and CD45.1 (A20) (eBioscience), CXCL9 (goat polyclonal) and CXCL11 (goat polyclonal) (R&D), CX3CL1 (rabbit polyclonal), ERTR-7, and cytokeratin 8/18 (rabbit polyclonal) (Novus Biologicals), Collagen IV (rabbit polyclonal) (Acris), or CXCL10 (rabbit polyclonal) (Peprotech). IFN-γ-PE staining was amplified using rabbit aντı–PE (Novus Biologicals). Jackson Immunoresearch secondary antibodies conjugated to various fluorochromes were used to stain unconjugated antibodies. Tiled images were acquired with an automated Leica DM5500B microscope and analysis of coronal sections was performed with ImageJ and Adobe Photoshop. Cell isolations and flow cytometry were performed as previously described30.
Briefly, female reproductive tracts, including the vagina, cervix, uterine horns and ovaries, were excised and chopped into small pieces. Tissue pieces were then digested in 500 mg/L of Collagenase IV (Sigma) while stirring at 300 rpm for 1 h at 37°C with a 1 inch magnetic stir bar. Tissues were then homogenized using a gentleMACS (Miltenyi) and filtered. For chemokine staining by flow cytometry, cells were stained with antibodies specific for CD45.2 (104), CD11c (N418), CD3e (145-2C11) (Ebioscience) and Thy1.1 (HIS51) (BD). Chemokines were stained as previously described31.
Quantification of microscopy images
Individual Thy1.1 or DAPI images were loaded into ImageJ64 software as JPEG files. Color images were then converted into “binary” format, which transforms images into black (either DAPI or Thy1.1) and white pixels. Quantification was then performed on black pixels using the “analyze particle” function. Computer enumeration was confirmed by periodic manual counting. Fluorescence intensity analysis was performed on grayscale TIFF files. Briefly, CD31 and chemokine images were loaded into stacks. Polygon gates were placed around CD31+ vessels and then these gates were applied to matching chemokine images. Mean grey value was then measured using the “measure” function.
Statistical analysis
P values were determined by a two-tailed, unpaired Student’s t-test. Differences between groups were considered significant if P≤0.05.
Supplementary Material
Acknowledgments
We thank M. Mescher (University of Minnesota) for OT-I.Ifng−/− mice and S. Jameson (University of Minnesota) for helpful discussion. Supported by NIH R01AI084913-01 (D.M.), NIH T32AI997313 (J.M.S.), and by the Office Of The Director, NIH, under Award Number DP2OD006467 (D.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Author Contributions
J.M.S., V.V. and D.M. designed the experiments; J.M.S. and K.A.F. performed the experiments; J.M.S. and D.M. wrote the manuscript.
References
- 1.Obar JJ, Khanna KM, Lefrançois L. Endogenous Naive CD8+ T Cell Precursor Frequency Regulates Primary and Memory Responses to Infection. Immunity. 2008;28:859–869. doi: 10.1016/j.immuni.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 3.Andrian, von UH, Mackay CR. T-cell function and migration. Two sides of the same coin. N. Engl. J. Med. 2000;343:1020–1034. doi: 10.1056/NEJM200010053431407. [DOI] [PubMed] [Google Scholar]
- 4.Seder RA, Ahmed R. Similarities and differences in CD4+ and CD8+ effector and memory T cell generation. Nat Immunol. 2003;4:835–842. doi: 10.1038/ni969. [DOI] [PubMed] [Google Scholar]
- 5.Akira S, Uematsu S. Pathogen recognition and innate immunity. Cell. 2006;240:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 7.Wherry EJ, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 8.Masopust D, Vezys V, Marzo AL, Lefrançois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 9.Gebhardt T, et al. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. 2009;10:524–530. doi: 10.1038/ni.1718. [DOI] [PubMed] [Google Scholar]
- 10.Wakim LM, Woodward-Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc. Natl. Acad. Sci. USA. 2010;107:17872–17879. doi: 10.1073/pnas.1010201107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann M, Pircher H. E-cadherin promotes accumulation of a unique memory CD8 T-cell population in murine salivary glands. Proc. Natl. Acad. Sci. USA. 2011;108:16741–16746. doi: 10.1073/pnas.1107200108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Masopust D, et al. Dynamic T cell migration program provides resident memory within intestinal epithelium. J. Exp. Med. 2010;207:553–564. doi: 10.1084/jem.20090858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang X, et al. Skin infection generates non-migratory memory CD8+ TRM cells providing global skin immunity. Nature. 2012;483:227–231. doi: 10.1038/nature10851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gebhardt T, Mueller SN, Heath WR, Carbone FR. Peripheral tissue surveillance and residency by memory T cells. Trends Immunol. 2013;34:27–32. doi: 10.1016/j.it.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Masopust D, Picker LJ. Hidden Memories: Frontline Memory T Cells and Early Pathogen Interception. J. Immunol. 2012;188:5811–5817. doi: 10.4049/jimmunol.1102695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teijaro JR, et al. Cutting Edge: Tissue-Retentive Lung Memory CD4 T Cells Mediate Optimal Protection to Respiratory Virus Infection. J. Immunol. 2011;187:5510–5514. doi: 10.4049/jimmunol.1102243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cauley LS, Lefrançois L. Guarding the perimeter: protection of the mucosa by tissue-resident memory T cells. Mucosal Immunol. 2013;6:14–23. doi: 10.1038/mi.2012.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shin H, Iwasaki A. A vaccine strategy that protects against genital herpes by establishing local memory T cells. Nature. 2012;491:463–467. doi: 10.1038/nature11522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bevan MJ. Memory T cells as an occupying force. Eur. J. Immunol. 2011;41:1192–1195. doi: 10.1002/eji.201041377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 21.Ariotti S, et al. Tissue-resident memory CD8+ T cells continuously patrol skin epithelia to quickly recognize local antigen. Proc. Natl. Acad. Sci. USA. 2012;109:19739–19744. doi: 10.1073/pnas.1208927109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mackay LK, et al. Long-lived epithelial immunity by tissue-resident memory T (TRM) cells in the absence of persisting local antigen presentation. Proc. Natl. Acad. Sci. USA. 2012;109:7037–7042. doi: 10.1073/pnas.1202288109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins MK, Tay C-S, Erlebacher A. Dendritic cell entrapment within the pregnant uterus inhibits immune surveillance of the maternal/fetal interface in mice. J. Clin. Invest. 2009;119:2062–2073. doi: 10.1172/JCI38714. doi:10.1172/JCI38714DS1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol. 2008;9:970–980. doi: 10.1038/ni.f.213. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi Y, Lu B, Gerard C, Iwasaki A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature. 2009;462:510–513. doi: 10.1038/nature08511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luster AD. The role of chemokines in linking innate and adaptive immunity. Curr. Opin. Immunol. 2002;14:129–135. doi: 10.1016/s0952-7915(01)00308-9. [DOI] [PubMed] [Google Scholar]
- 27.Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J. Immunol. 2008;181:5837–5841. doi: 10.4049/jimmunol.181.9.5837. [DOI] [PubMed] [Google Scholar]
- 28.Çuburu N, et al. Intravaginal immunization with HPV vectors induces tissue-resident CD8+ T cell responses. J. Clin. Invest. 2012;122:4606–4620. doi: 10.1172/JCI63287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strutt TM, et al. Memory CD4+ T cells induce innate responses independently of pathogen. Nat. Med. 2010;16:558–564. doi: 10.1038/nm.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casey KA, et al. Antigen-independent differentiation and maintenance of effector-like resident memory T cells in tissues. J. Immunol. 2012;188:4866–4875. doi: 10.4049/jimmunol.1200402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Eberlein J, et al. Comprehensive assessment of chemokine expression profiles by flow cytometry. J. Clin. Invest. 2010;120:907–23. doi: 10.1172/JCI40645. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.