Abstract
PURPOSE
An osteopontin (OPN; SPP1) gene promoter polymorphism modifies disease severity in Duchenne muscular dystrophy, and we hypothesized that it might also modify muscle phenotypes in healthy volunteers.
METHODS
Gene association studies were carried out for OPN (rs28357094) in the FAMuSS cohort (n=752; age 23.7±5.7 yrs). Phenotypes studied included muscle size (MRI), strength, and response to supervised resistance training. We also studied 147 young adults that had carried out a bout of eccentric elbow exercise (age 24.0 ± 5.2 yrs). Phenotypes analyzed included strength, soreness, and serum muscle enzymes.
RESULTS
In the FAMuSS cohort, the G allele was associated with 17% increase in baseline upper arm muscle volume only in women (F=26.32; p=5.32 × 10−7), explaining 5% of population variance. In the eccentric damage cohort, weak associations of the G allele were seen in women with both baseline myoglobin, and elevated CK. Sexually dimorphic effects of OPN on muscle were also seen in OPN null mice. Five of seven muscle groups examined showed smaller size in OPN null female mice, whereas two were smaller in males. Query of OPN gene transcription after experimental muscle damage in mice showed rapid induction within 12 hrs (100-fold increase from baseline), followed by sustained high level expression through 16 days of regeneration before falling to back to baseline.
CONCLUSION
OPN is a sexually dimorphic modifier of muscle size in normal humans and mice, and responds to muscle damage. The OPN gene is known to be estrogen responsive, and this may explain the female-specific genotype effects in adult volunteers.
Keywords: osteopontin (OPN), secreted phosphoprotein 1 (SPP1), genetic polymorphism, estrogen, hypertrophy, MRI
Introduction
Muscle tissue is both highly adaptable, remodeling to different activity patterns, as well as highly polymorphic, where individuals may be predisposed to endurance or strength, or large or small muscle volume. Inter-individual differences is a combination of genetics and training, with the gene polymorphisms influencing muscle phenotypes often called ‘genetic modifiers’. A polymorphism in the α-actinin 3 gene (ACTN3) is the best characterized and validated genetic modifier of muscle in healthy populations and athletes (7, 8, 37). This common nonsense (loss-of-function) polymorphism (R577X) is broadly distributed worldwide. In general, individuals with the intact ACTN3 gene are predisposed to greater strength and speed, whereas individuals with the loss-of-function allele have greater endurance (37).
Duchenne muscular dystrophy (DMD) is the most common muscle disease, causing progressive weakness and early death in about 1 in 3,500 males worldwide. The disease results from the loss of dystrophin, a membrane cytoskeletal protein normally present at the myofiber sarcolemma (16). Dystrophin deficiency causes myofiber membrane instability during muscle contraction and chronic bouts of muscle degeneration and regeneration. Patients with DMD show variability in the severity of their disease, both with regards to age of onset and disease progression, and this led to the search for genetic modifiers of disease severity. We recently reported a study of 29 candidate genes that had been previously shown to be correlated with muscle phenotypes in healthy volunteers (including ACTN3), and gene loci associated with metabolic syndrome and cardiovascular disease phenotypes (32). We also took a disease-centric approach, where archival muscle biopsies were accessed from particularly severe or mild DMD patients, and then mRNA profiled to identify potential biomarkers of later disease severity (31). In these studies, two of the 29 polymorphisms studied showed an association with disease severity in two independent DMD cohorts; ACTN3 and OPN. ACTN3 had been chosen as a candidate due to its association with muscle phenotypes in healthy volunteers and athletes, and the nonsense (loss-of-function) allele was associated with less severe disease. The OPN polymorphism studied was chosen due to the observation of high mRNA levels in muscle biopsies from particularly severe DMD patients, and due to the well-characterized nature of the rs28357094 SNP on the gene promoter and mRNA expression (13, 14, 19). The OPN polymorphism was particularly strongly associated with disease severity of DMD in two independent cohorts, with the more common TT genotype (60–70% of subjects) showing a milder disease progression compared to the GT/GG combined genotypes (p = 0.003 in one DMD cohort, and p = 0.0003 in a second independent DMD cohort). The OPN rs28357094 SNP is the most robust genetic modifier of DMD identified to date (32), and as the rarer G allele has been shown to reduce OPN gene expression in reporter constructs in HeLa cells (13), the data suggest that less OPN protein causes greater DMD severity.
Given that the ACTN3 polymorphism is a modifier of muscle function in both health and disease, we hypothesized that the OPN polymorphism might also influence muscle traits in normal volunteers. OPN is a protein that is increasingly studied as a key inflammatory cytokine involved in tissue remodeling. OPN is actively secreted by both inflammatory cells and remodeling tissues, and the extracellular protein is extensively yet variably processed by O-linked glycosylation, phosphorylation, and transglutamination (6, 20). OPN also shows multiple proteolytic cleavage sites for proteases such as thrombin and MMPs (mmp-2, -7, and -9), which cleave the protein into different biologically active isoforms (38). One of the key roles for OPN is to interact with at least nine integrins and CD44 at the cell surface, modulating cell-cell interactions, and cell-matrix interactions. OPN has been shown to be a key factor in cancer progression and metastasis in multiple tumor types, including prostrate (12), breast (22), and esophageal cancers (21), and hepatocellular carcinoma (5).
The different products of OPN have different roles in inflammatory cell recruitment and chemotaxis. At the sites of tissue damage, thrombin cleaves OPN to create an N-terminal fragment that is chemotactic for neutrophils (38). In addition, transglutaminase 2 at sites of tissue damage acts upon OPN resulting in polymerized OPN (polyOPN), and polyOPN is also highly chemotactic for neutrophils (29). The chemotactic activity of N-terminal fragment and polyOPN are directly through interactions with α9β1 integrin on leukocytes (38). However, the role of OPN is not only restricted to recruitment of neutrophils. There are numerous studies that support a role for OPN in the accumulation of macrophages at the sites of tissue injury and pathology (23, 29).
Given that OPN was a validated genetic modifier of muscle disease, we sought to determine if the same OPN polymorphism might modify muscle phenotypes in healthy young adult volunteers. To study this, we carried out sex-stratified genotype/phenotype associations in two previously described cohorts: a unilateral upper arm resistance training intervention in 752 healthy Caucasian volunteers (FAMuSS cohort; 34), and a unilateral upper arm eccentric challenge in 147 young adults (8, 33). The eccentric challenge was studied as OPN is a cytokine frequently associated with tissue damage.
Methods
Subjects: Unilateral resistance training cohort (FAMuSS)
The study population was derived from a multicenter, NIH funded study designed to identify genetic factors that dictate baseline bone, muscle and fat volume and the variability in response to exercise training. The study design protocol has been described in detail elsewhere (34), and we have previously published genetic associations with muscle strength and size, response to exercise and metabolic syndrome markers (7). Briefly, 1,300 men and women, average age 24 yrs were recruited by one of the 8 centers (University of Massachusetts Amherst, University of Connecticut, Dublin University (Ireland), Florida Atlantic University, Hartford Hospital, University of Central Florida, West Virginia University, Central Michigan University). The study was approved by the Children's National Medical Center Institutional Review Board (protocol #2449), and was in compliance with the Helsinki Declaration, and included written informed consent.
Exercise Training Program and Strength Assessments
Unilateral resistant training was performed with the non-dominant arm. The protocol was described elsewhere (34). Briefly it consisted of two 45 – 60 minute sessions per week for 12 weeks. Each session included dumbbell biceps curls, dumbbell biceps preacher curls, and incline dumbbell biceps curls, overhead dumbbell triceps extension, and dumbbell triceps kickbacks. The amount of weight was aggressively increased during the 12 weeks. Strength assessments were isometric biceps strength (maximum voluntary contraction; MVC), and one repetition maximum weight (1RM) before and after training. Details of the strength measurement protocols have been described previously (34).
MRI assessment
MRI assessment of both upper arms (trained and untrained), before and after the 12 wk resistance training intervention has been previously described (34). Entry MRI was done 24 to 48 h before the isometric or1RM (1 repetition maximum) test. Scans post-training MRI was performed 48 – 96 h after the last training session. Fifteen 16 mm contiguous axial slices from each arm were taken from each arm independently. Scans for both arms were taken by Fast Spoiled Gradient Recalled and Fast Spin Echo with TE 1.8/TR 200 msec. All eight centers submitted the MRI data to the Center for Genetic Medicine Research at Children's National Medical Center (CNMC) in Washington, D.C, via e-mail or DAT disc, and all scans were integrated into the study SQL database. The complete MRI data set with both pre- and post-measures of high quality images for both arms was n=752.
For the volumetric analysis of the MRI images, we used Rapidia (INFINITT Inc, Seoul, Korea), a PC based software that allows the semi-automatic quantification of muscle, bone and subcutaneous fat. The software was optimized to provide automated position adjustment, and to distinguish muscle, fat, and bone, with automatic edge detection, user modification of ambiguous edges, and automated propagation of defined tissue boundaries when possible. Volume measures were taken using an anatomical landmark (metaphyseal-diaphyseal junction of the humerus) as our starting point and assayed the six 1 cm slices proximal to it. Reliability of measures was determined using the untrained arm, with muscle volume measured at a 12 week interval, showing that our quantitation of muscle volume was highly reliable and sensitive (R2 = 0.968). Measurement values were automatically written and saved in a SQL database together with anthropomorphic and genotyping data.
Subjects: Eccentric challenge cohort
Data for this study were derived from a larger clinical trial assessing the efficacy of a topical analgesic on delayed onset muscle soreness, and genotype associations have been previously reported for this cohort (8, 17). Markers of muscle damage included muscle function, muscle soreness, serum creatine kinase (CK), and myoglobin (Mb) levels. Study protocols were approved by the University of Massachusetts Human Subjects Review committee, and included written informed consent.
Testing Schedule
During visit 1, subjects were screened for inclusion/exclusion criteria and signed informed consent documents. Within 1 wk following visit 1, subjects were seen by the study physician, height and weight were recorded, and subjects had blood drawn for baseline analyses (visit 2). Visit 3 occurred 2 days following visit 2, during which pre-exercise soreness and strength were assessed, followed by the eccentric exercise bout, followed immediately by reassessment of strength. Twelve hours after visit 3, subjects returned for treatment with topical analgesic (visit 4). Strength recovery was assessed at 3 (visit 5), 4 (visit 6), 7 (visit 7), and 10 days post exercise (visit 8). Blood was drawn at 4, 7, and 10 days post-exercise.
Strength testing
Maximal voluntary isometric strength of the elbow flexors was assessed before and immediately after the exercise. Strength was assessed on a modified preacher bench, with the elbow flexed at 90°. A strain gauge (model 32628CTL; Lafayette Instrument, Lafayette, IN) was used to measure the force produced. Three trials were averaged, with 1 min of rest between sets.
Exercise
The single exercise bout consisted of 50 maximal eccentric (muscle lengthening) contractions of the elbow flexor muscles of the non-dominant arm. Two sets of 25 contractions were separated by a 5-min rest period. Each contraction was 3 s long, followed by 12 s of rest.
DNA extraction and genotyping
DNA was extracted from blood samples obtained by phlebotomy before starting the exercise training using Qiagen Gentra PureGene kits. Genotyping was done using the TaqMan allele discrimination assay that employs the 5' nuclease activity of Taq polymerase to detect a fluorescent reporter signal generated during PCR reactions.
Statistical analyses
Hardy-Weinberg equilibrium was determined for each SNP using a χ2 test to compare the observed genotype frequencies to those expected under H-W equilibrium.
In the FAMuSS cohort, 12 quantitative phenotypes (baseline and 12 week change in MVC and 1-RM strength, whole muscle, cortical bone, and total bone volume, and whole muscle cross-sectional area) were analyzed as continuous quantitative traits. In the eccentric challenge cohort, muscle strength, soreness, serum CK and Mb were analyzed as continuous traits. Normality of each quantitative trait was confirmed using the Shapiro-Wilk normality test. Bivariate correlation analyses of each quantitative measurement showed several significant correlations with age and baseline mass, therefore, associations between each SNP and quantitative traits were assessed using analysis of covariance (ANCOVA) methods. Due to large gender differences in baseline values and the response to training, all analyses were performed separately for men and women.
Initially, co-dominant genetic models were tested, and all significant associations from the main ANCOVA model were subjected to pair-wise statistical tests among each of the three genotype groups for each SNP. Positive associations with the co-dominant model were then tested with both recessive and dominant models. Linear tests were performed between each of the genotype groups to determine which genotype groups were significantly different from one another. The resulting p values from these linear tests were adjusted for multiple comparisons using the Sidak post-hoc multiple comparison test. For all models, linear regression analysis, including likelihood ratio tests between full (containing genotype and covariates) and constrained (containing covariates only) models, were performed to estimate the proportion of variance in volumetric measurements attributable to each SNP's genotype. In order to avoid false positives, the p-value for significance was set at 0.002 to account for multiple testing (i.e. 12 phenotypes in males and females for a total of 24 tests in the FAMuSS cohort).
OPN (Spp1) KO mice
Six male and six female homozygous B6.129S6(Cg)-Spp1tm1Blh/J osteopontin (Spp1) knockout mice at 5–7 weeks of age were purchased from and housed at The Jackson Laboratory (Bar Harbor, ME, USA; http://jaxmice.jax.org/strain/004936.html) (24). Age- and gender-matched C57BL/6J mice served as controls. Animals were housed with food and water ad libitum in a 12-h light/dark cycle environment. Animals were euthanized via CO2 at 8–10 weeks of age, as approved by The Jackson Laboratory Institutional Animal Care and Use Committee.
Muscle groups were isolated immediately after euthanization. Muscles isolated from the left distal leg were the soleus, plantaris, gastrocnemius (G, which includes both the medial and lateral gastrocnemius), tibialis anterior (TA), and extensor digitorum longus (EDL). In the left proximal arm, the biceps bracchii longus and triceps brachii longus were isolated. All muscles were trimmed of excess tendon and weighed, and sex-stratified comparisons of wild-type and knock out animals using Student’s T test.
Murine degeneration/regeneration time series
We have previously reported a 27 time point wild-type mouse muscle degeneration/regeneration time series mRNA profiling data set (39,40). Briefly, the gastrocnemii of adult mice were caused to undergo a complete round of staged degeneration and regeneration using a 10 needle manifold and injection of cardiotoxin. The muscles were then harvested at defined time points after the injection, with two muscles analyzed in each of 27 times point. mRNA profiling data was generated using Affymetrix U74v2 microarrays from the 54 murine gastrocnemii, and the dCHIP mismatch model probe set algorithm utilized to normalize array and generate signal intensities. This data set is accessible for dynamic queries via a public website visualization tool we developed (http://pepr.cnmcresearch.org) (4), and data for OPN shown in this current publication is taken from this web site analytical and graphic tool.
Results
OPN rs28357094 is associated with baseline muscle size in young women
Associations of rs28357094 genotypes (TT vs. GG/GT) with 12 quantitative phenotypes in sex-stratified cohort were evaluated (baseline isometric strength, absolute difference in isometric strength, % change in isometric strength, baseline 1-RM strength, difference in 1-RM strength, % change in 1-RM strength, baseline whole muscle volume, difference in whole muscle volume, baseline total bone volume, difference in total bone volume, baseline cortical bone volume, difference in cortical bone volume). Bone phenotypes were included due to the known role of osteopontin in regulation of bone remodeling. Muscle phenotypes were chosen due to the association of the rs28357094 genotypes with muscle strength and disease progression in Duchenne muscular dystrophy. Normality of each phenotype was confirmed, genotype data was in Hardy-Weinberg equilibrium, and genotype/phenotype associations were adjusted for multiple testing. MVC strength, 1-RM strength and whole muscle phenotypes were adjusted for age and weight, bone phenotypes were adjusted for age and height.
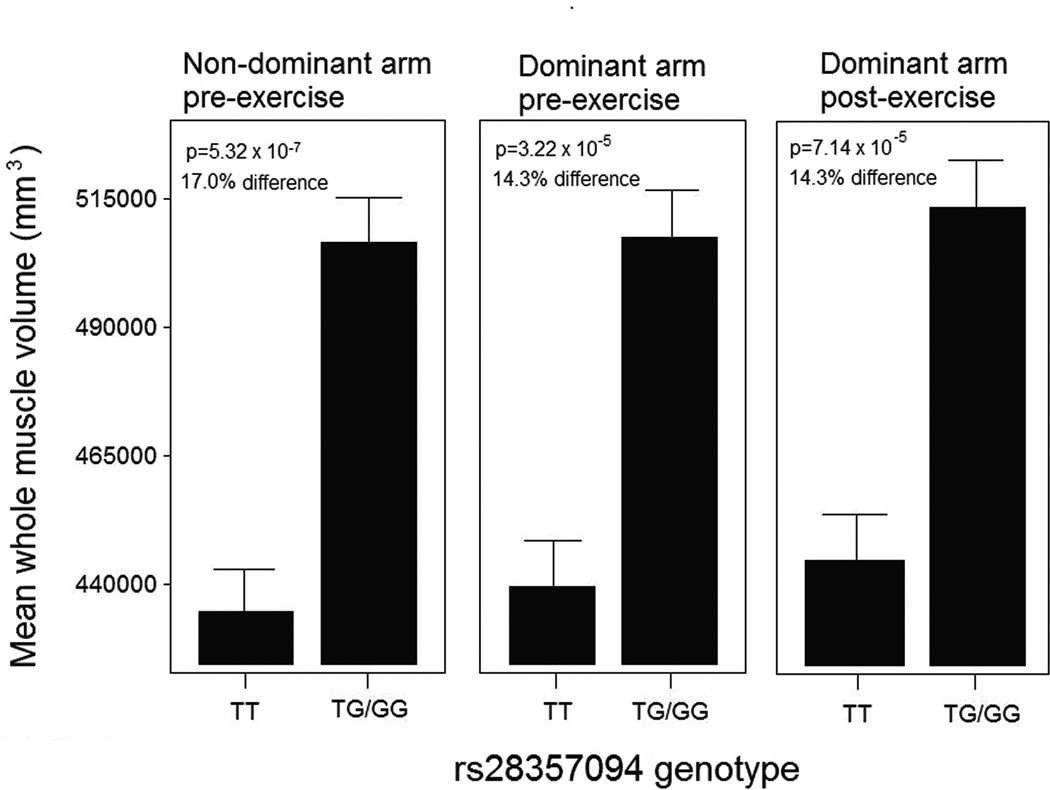
We found a strong association between OPN genotype and baseline muscle volume in females (Figure 1) (n=389). The other 11 phenotypes tested showed no significant genotype-phenotype associations after adjustment for multiple testing. Given the strong association with muscle volume in females (Figure 1), we further queried additional data fields in our FAMuSS data set. We had three measures of ‘baseline’ muscle volume: the dominant arm at study entry, the dominant (unexercised) arm at study exit, and the non-dominant arm at study entry (prior to exercise). However, these three measures are not independent measures, as there is a strong co-linear relationship between an individual’s dominant (untrained) pre- and post-intervention measures, and the non-dominant (trained) pre-intervention measure. Using a dominant inheritance model (TT, vs. GT/GG), all three measures of untrained (baseline) size showed a striking association of larger muscle size with the G allele (14.3% – 17.0% increase in size) (Figure 1). Considering the dominant arm at baseline, women carrying the G allele showed significantly larger muscle size (TT, N=167, 433910±9344 mm3; TG/GG, N=122, 507835 ± 10938 mm3) (F-test = 13.1; p = 5.3 × 10−7). The percentage of population variation for muscle size explained by this genotype was 5.1% (LRT p < 0.001), making this one of the largest effect sizes for any genetic modifier of muscle identified to date. This association was specific to females, with no association of baseline muscle volume in males. Tests of other traits such as baseline strength, or response to resistance training (size or strength) showed no associations, in either males or females.
Figure 1. OPN genotype is associated with untrained upper arm muscle size in young adult female volunteers.
Young adult (average age 24 yrs) volunteers enrolled in a unilateral upper arm resistance training study were genotyped for the OPN polymorphism, and males and females separately assessed for associations of OPN genotype with baseline and trained muscle strength and size. Upper arm total muscle volume measured by volumetric MRI showed strong association with OPN genotype in females, but not males, where the GT/GG genotype muscle volume was 15–17% larger than the TT genotype carriers. The untrained, dominant arm, showed similar associations before and after the 12 wks training bout, whereas the trained, non-dominant arm, showed association only at baseline. Standard errors are shown.
rs28357094 G allele is associated with increased serum myoglobin at baseline in females
Young adult volunteers undertook a bout of eccentric exercise, with phenotypes then measured as a function of time after the bout. Phenotypes included muscle strength loss, muscle soreness, and serum CK and Mb, tested in males (n=74) and females (n=78) separately. Using the same dominant inheritance model used in the muscle size data, we found an association of the GG/GT genotypes with increased serum myoglobin levels at baseline in females only (TT, N=61; 26.96 ± 0.61 IU/L); GT/GG, N=16, 29.91 ± 1.20) (Table 1). However, the limit of detection of the serum myoglobin assay used was 23 ng/ml, and many subjects in both genotype groups were at or below the limit of detection, and these were set at 23 ng/ml.
Table 1.
Associations of OPN promoter polymorphism with phenotypes in an eccentric exercise study.
| Measurement | Gender | F- test |
P- value |
N; adjusted mean ± SEM | P-value of significantly different means |
% variability attributable to genotype; LRT p-value * |
|---|---|---|---|---|---|---|
| Myoglobin – baseline (Visit 2) | Female | 4.73 | 0.0329 | TT (N=61; 26.96 ± 0.61 ng/ml) TG/GG (N=16; 29.91 ± 1.20 ng/ml) 1 |
N/A | 6.1%; 0.0268 |
| CK – 10 days post bout (Visit 8) | Female | 5.65 | 0.0052 | GG (N=2; 1071.05 ± 232.17 IU/L)2 TG (N=14; 237.61 ± 88.40 IU/L) TT (N=62; 323.99 ± 41.53 IU/L) |
N/A | N/A |
| Difference in CK (10 days to baseline) | Female | 6.02 | 0.0038 | GG (N=2; 986.58 ± 227.33 IU/L)2 TG (N=14; 143.57 ± 86.56 IU/L) TT (N=62; 239.54 ± 40.66 IU/L) |
N/A | N/A |
The lower limit of detection of the serum myoglobin assay was 23 ng/ml, and many subjects in all genotype groups were at or below the limit of detection.
The p value for both CK – 10 days (Visit 8) and Difference in CK (10 days to baseline) was driven by a single GG homozygote subject who showed very high CKs (see Figure 2 and text).
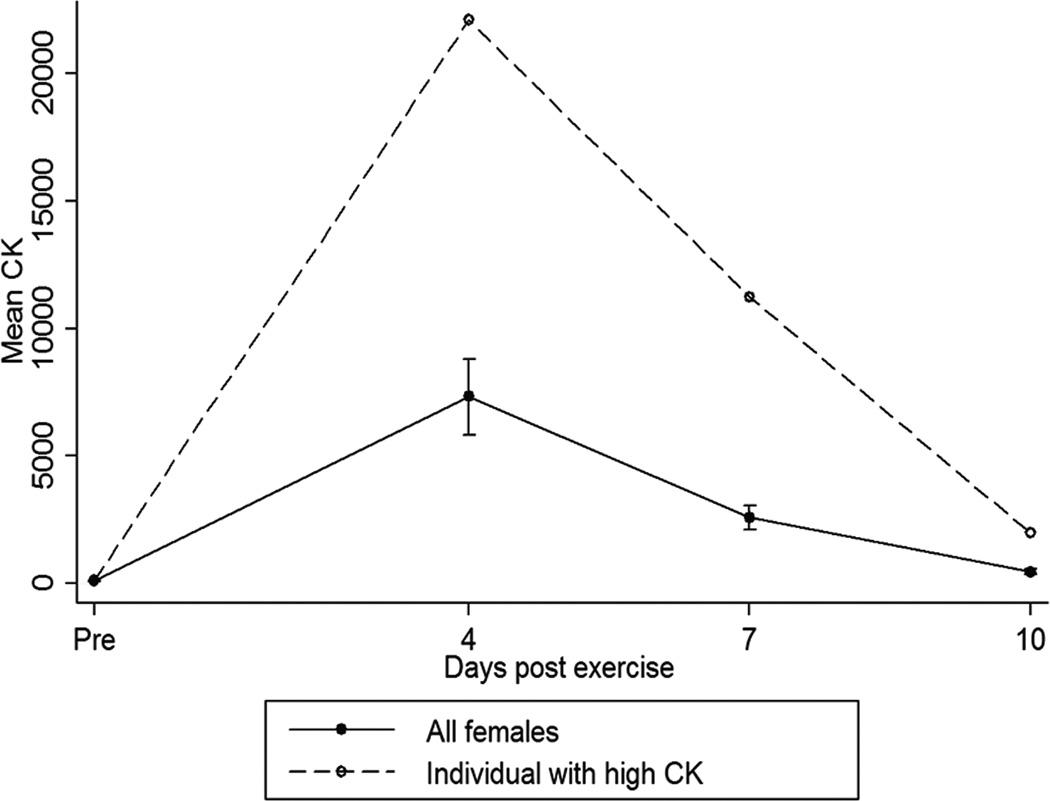
There were two female homozygotes for the rare GG allele, and one of these showed unusually high increase creatine kinase post-exercise (22,099, 11,257 and 1,986 IU/L compared to an average of 5,265 ± 6,687, 1,930 ± 2,410 and 306 ± 297 IU/L in other females at 4, 7 and 10 days respectively) (Figure 2). This led to significant associations of the GG genotype with both CK at 10 days, and difference between day 0 and day 10 CK, but this was driven by the single subject coupled with low variance of all other subjects in the day 0 and day 10 time points (Table 1).
Figure 2. A single OPN GG homozygote female shows evidence of unusual muscle damage following a challenge of upper arm eccentric activity.
Young adults were given a challenge of elbow eccentric contractions, then blood sampled at 4, 7 and 10 days after the bout of eccentric activity. There were two GG homozygote females in the 147 volunteer cohort, and one of these showed extraordinary elevations of serum creatine kinase (CK), reflective of muscle damage, at 7 days post-bout (green data points). The average elevation of CK in females was 500 IU/L at 7 days, whereas the GG female showed 25,000 IU/L. Standard errors are shown.
It is important to note that the eccentric exercise association data were not corrected for multiple testing, and there were many comparisons run in both males and females (soreness, strength, myoglobin and CK at multiple time points). Thus, myoglobin and CK associations are tentative and require validation in additional cohorts.
OPN null mice show sexually dimorphic differences in muscle size relative to wild-type mice
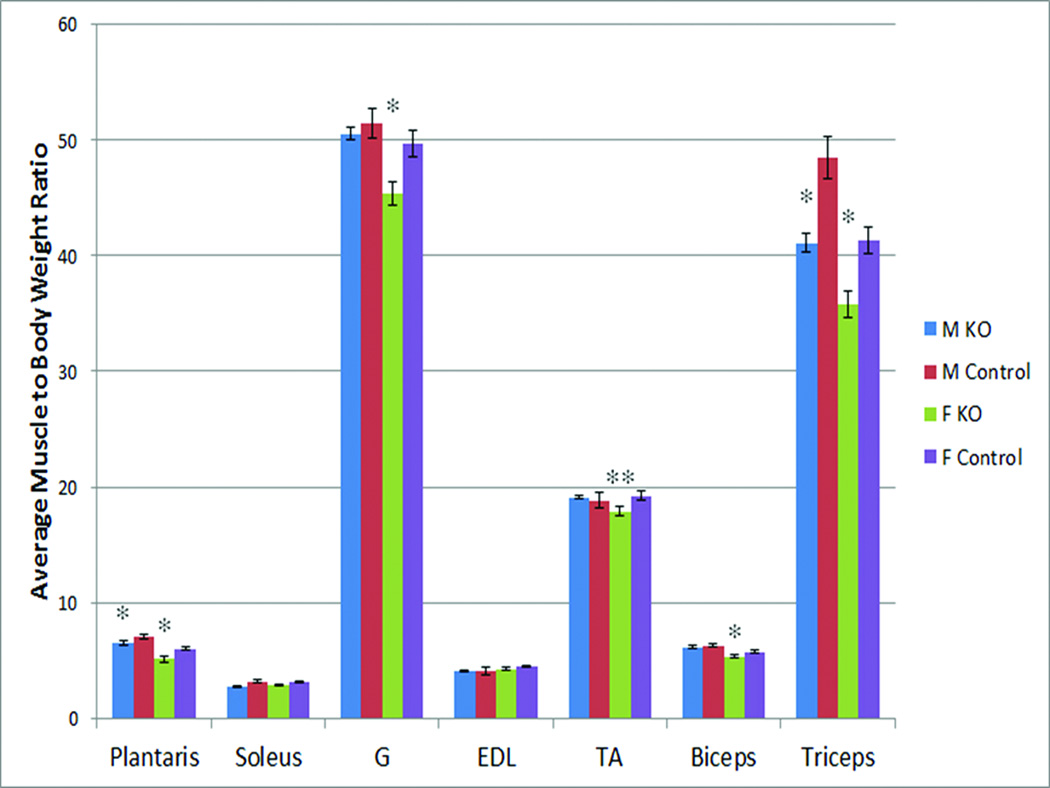
Given the female-specific association of muscle size with the OPN polymorphism in young adult females, we hypothesized that mouse knock-outs for the OPN gene might also show sex-specific alterations in muscle weights. The weights of seven dissected muscle groups were compared between OPN null mice and wild-type controls, in both males and females (n = 6 per group; 24 mice total). Weights were normalized to body weight, and sex-specific statistical comparisons of each muscle group done between the OPN and wild-type mice (Figure 3). OPN female mice showed significant reductions in muscle weight in five of the seven muscle groups analyzed, whereas male mice showed reductions in two of the seven groups. Murine female biceps and triceps were both significantly reduced in weight, and these correspond to the muscle groups analyzed by MRI in the young adult female volunteers (Figure 1).
Figure 3. Muscle weights normalized to body weight in OPN null mice and wild-type controls.
Shown is the average and standard error for males and females in the indicated muscle groups (n = 6 per group). OPN null female mice showed significant reduction in normalized muscle weights for five of the seven muscle groups examined, whereas male mice showed significant reduction in two of the 10 groups. Muscle weight to body weight ratio is calculated as (milligram muscle/gram body weight) × 10. * p< 0.05; ** p<0.01
OPN expression in muscle is associated with experimental muscle degeneration and regeneration
mRNA profiling has been used to study changes in the muscle transcriptome following different interventions and disease states, and we have developed a public access resource to query many of these data sets (http://pepr.cnmcresearch.org) (4). Using this interface, we queried the response of OPN mRNA accumulation after: experimental muscle damage in mouse (cardiotoxin induced degeneration/regeneration; 27 time point series) (39,40) (Figure 4).
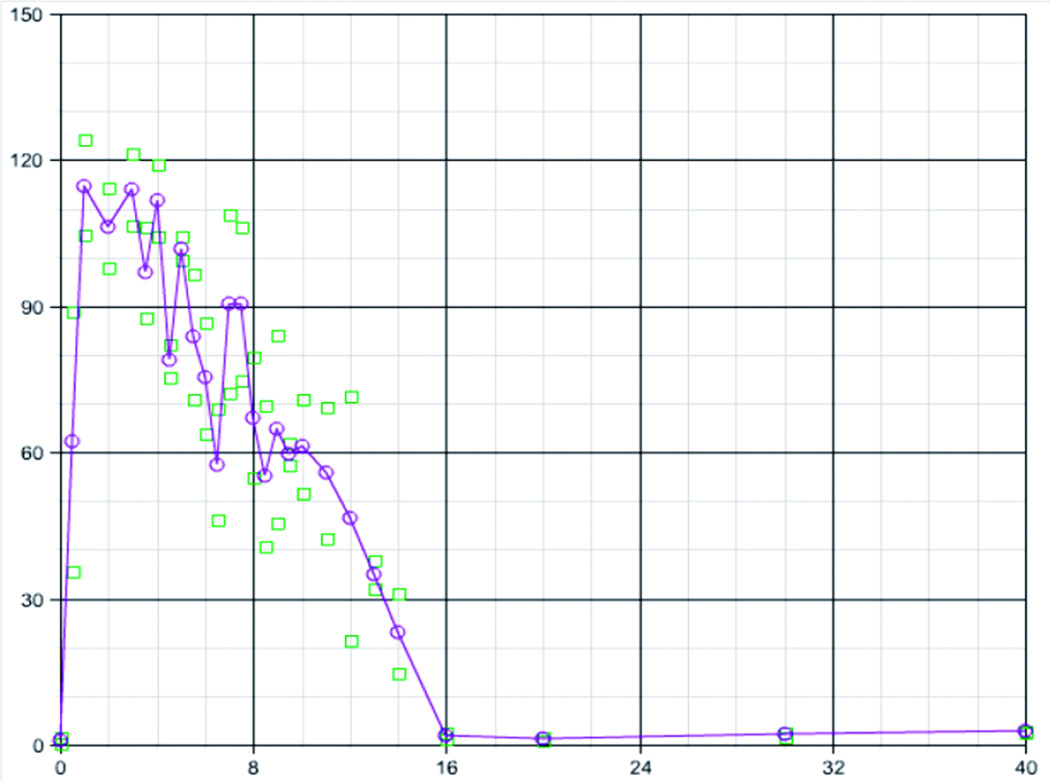
Figure 4. OPN is strongly induced by experimental muscle degeneration and regeneration.
Shown is mRNA expression profile data from 54 mice at defined time points during staged degeneration and regeneration. Time 0 is prior to injection, and the days of harvest of muscle are shown through 40 days (X axis). Shown is the fold-change in OPN mRNA expression in each mouse muscle relative to baseline (time 0) (Y axis). OPN shows low baseline expression at time 0, but then is rapidly induced to about 100-times baseline levels by 1 day following experimental muscle damage. High level OPN is sustained throughout the time period of muscle regeneration, falling back to baseline levels after 16 days when regeneration is largely complete. Shown are fold-change relative to averaged baseline (time 0) for each muscle (two per time point; squares), and the average of the two muscles (circles and line).
OPN mRNA showed low baseline expression in uninjured muscle (baseline; time 0), but was then rapidly and strongly induced following cardiotoxin-induced muscle degeneration, with a 120-fold increase in levels by 12 – 24 hrs (Figure 4). High levels of OPN were maintained in regenerating muscle through day 14, whereupon levels fell to baseline by day 16.
Discussion
The ACTN3 polymorphism has been identified as a genetic modifier of muscle function in both healthy volunteers and athletes (7, 8, 37), as well as in Duchenne muscular dystrophy (30). We sought to determine if an OPN gene promoter polymorphism we previously associated with disease severity in Duchenne dystrophy was also associated with muscle phenotypes in healthy young adult volunteers. The G allele of the rs28357094 polymorphism is a 5 bp upstream of the transcription start site, alters a SP1 transcription factor binding site, and reduces OPN gene transcription. This G allele was associated with more severe disease in DMD, suggesting that less OPN led to poor muscle remodeling in the context of continuous degeneration/regeneration (muscular dystrophy). We did not have expectations regarding the effect of the G allele in normal volunteer groups, and the effects on normal muscle (baseline, trained, or eccentric challenge) are quite different than the severe and chronic remodeling seen in muscular dystrophy.
The two normal volunteer groups studied were a resistance training intervention cohort, and an eccentric bout group. The resistance training group was meant to study a 12 wk training effect, whereas the eccentric group was meant to study acute responses to a single bout of muscle damage from lengthening contractions. In the resistance training group, we found a strong association of the OPN polymorphism with baseline muscle size in females, but not males (Figure 1). Women with the rare allele (GT and GG) showed 17% increase in muscle volume of the upper arm by MRI volume compared to TT (p=5.3 × 10−7), and this finding was similar in both arms prior to the exercise intervention, and in the control unexercised dominant arm following the unilateral resistance training of the non-dominant arm (Figure 1). This single genetic locus explained approximately 5% of population variance (LRT p<0.001) in the females in FAMuSS. This was a surprising large proportion of variance, and is quite unusual for any single genetic polymorphism in any population-based trait. A factor that may contribute to the large effect size is the highly objective and quantitative nature of the MRI phenotype studied here. As stated by Long and Langley (1994), “an increase in the variance attributable to a quantitative trait locus is seen with the careful definition of the phenotype so that any variation in phenotype is less likely due to other factors” (25). The cohort studied was also relatively homogenous in age and gender, and phenotypes were adjusted for age, weight and stratified by gender. Each of these would be expected to reduce variance, and thus augment our sensitivity in detecting gene × phenotype (MRI) associations. Finally, our cohort was quite young, and we have previously shown that young cohorts may augment effect sizes in genetic association studies (10). This is because age-associated phenotypes (such as sarcopenia with aging) would serve as confounding (hidden) variables and contribute to greater variance in older cohorts (10). This more robust nature of the MRI phenotype may also explain the lack of association with muscle strength, as the latter has a central effort component.
A correlation of a gene polymorphism with muscle size might be expected to similarly be correlated with muscle strength. However, studies of the relationship of size and strength typically show correlation coefficients of only r = 0.7 (1, 26). In addition, strength has a central component (effort) that likely contributes to increased variance, whereas MRI measures of size have no such central component. Further studies of the relationship of size and strength and the role of OPN in muscle are required.
The association with muscle size was not seen in males, and there was no association of strength or size changes upon resistance training in either males or females. It is important to validate genetic association studies in additional cohorts, however we were unable to identify similar large cohorts of young adults with volumetric MRI of muscle. Thus, independent validation must await future studies.
In an eccentric contraction (muscle damage) group, we found weak associations with females with baseline myoglobin, and serum CK at 7 days (Table 1; Figure 2). The associations with CK were in a recessive model and were driven by one of two female homozygotes for the rare allele (GG) showed striking elevations of CK 10 days following the exercise challenge (Figure 2). These findings were not corrected for multiple testing, and are subject to false positive associations.
The sex-related effects of OPN on muscle size were validated in our study of mouse knock-outs (null) for OPN (Figure 3). OPN null female mice showed significant reductions in five of seven muscle groups examined, including the biceps and triceps muscles (the same muscle groups studied by MRI in the healthy young adult female volunteers in FAMuSS). Male mice showed significant reductions in two of the seven muscle groups. Thus, OPN expression appears related to muscle size in mice as well as humans, and that the effect is also largely sex-specific to females. We also showed that OPN is strongly expressed during muscle remodeling in mice subjected to a bout of muscle degeneration and regeneration (Figure 4). This suggests that OPN likely plays a role in repair of muscle damage, with expression elevated until the muscle is fully regenerated at about 14 days post-insult.
Our findings indicate that the OPN polymorphism is a genetic modifier of muscle in both health (young adult volunteers), and disease (DMD). Thus, both ACTN3 and OPN polymorphisms modulate both healthy volunteer muscle, as well as dystrophic muscle. That said, our study raises a question regarding the sex-specificity of our findings. DMD is an X-linked disorder that affects young boys, yet our significant genetic association findings in healthy volunteers were restricted to females. OPN has gene promoter elements that have been shown to be responsive to estrogen (9, 27), and other disease-related association studies with the same polymorophism have also found sex-specific effects (2, 15). It is challenging to draw parallels between pre-pubertal DMD boys, and the likely estrogen-sensitive gene effects we have observed in the post-pubertal volunteer women studied. However, sex-specific effects of gene polymorphisms in other loci have also been reported (7, 19, 31, 32).
The association of the rare allele of the OPN promoter SNP with larger muscle size in females and more severe disease in DMD boys is challenging to reconcile into a single unifying gene/phenotype association. Our finding that mice with loss of OPN (knock-out) show sexually dimorphic effects on muscle size is supportive of sex-modified effects and the apparent importance of OPN in muscle tissue, but again does not aid in defining a unifying model. We also showed that mouse muscle shows very low levels of OPN at baseline, but it is rapidly and extensively induced by muscle damage (is at quite low levels in normal muscle, and is strongly induced upon muscle damage (120-fold increase in levels by 12 – 24 hrs). Taken together, our data suggests that OPN likely plays a role in response of muscle to damage (as a tissue remodeling cytokine), and may also have a role in normal development. With regards to muscle damage, it is important to note that OPN is expressed in both infiltrating macrophages and in regenerating myoblasts, and different isoforms of the protein have different effects on initiation of inflammation (e.g. transglutaminated form and neutrophil chemotaxis) (29, 38), and resolution of inflammation (e.g. macrophage polarization towards a tissue repair IL-10 producing phenotype) (3). Future research should focus on mechanistic studies of specific OPN protein isoforms and their role in muscle inflammation and remodeling, in both healthy muscle and dystrophic muscle. The effect of the promoter polymorphism on OPN regulation in muscle, and the modulatory effect of estrogen, also requires further study.
Acknowledgements
We thank Dr. Kitipong Uaesoontrachoon for helpful revisions of the manuscript, and the assistance of Amy Kearns and Monica Hubal in carrying out the eccentric exercise study. Disclosure of funding sources: Funded by the National Institutes of Health (2RO1AR55100, 1U54HD053177, 5R24HD050846), the United States Army Research Institute of Environmental Medicine, and Medivona Inc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Professional relationships with companies or manufacturers who will benefit from the results of the present study:
Eric P. Hoffman: No conflict of interest
Heather Gordish-Dressman: No conflict of interest
Virginia D. McLane: No conflict of interest
Joseph M. Devaney: No conflict of interest
Paul Thompson: No conflict of interest
Paul Visich: No conflict of interest
Paul M. Gordon: No conflict of interest
Linda Pescatello: No conflict of interest
Robert F. Zoeller: No conflict of interest
Niall Moyna: No conflict of interest
Theodore J. Angelopoulos: No conflict of interest
Elena Pegoraro: No conflict of interest
Gregory A. Cox: No conflict of interest
Priscilla Clarkson: No conflict of interest
Conflicts of interest: No authors have any conflicts of interest related to the described studies. The results of the present study do not constitute endorsement by ACSM.
References
- 1.Akagi R, Takai Y, Kato E, Fukuda M, Wakahara T, Ohta M, Kanehisa H, Kawakami Y, Fukunaga T. Relationships between muscle strength and indices of muscle cross-sectional area determined during maximal voluntary contraction in middle-aged and elderly individuals. J Strength Cond Res. 2009 Jul;23(4):1258–1262. doi: 10.1519/JSC.0b013e3181918a9b. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee A, Apte UM, Smith R, Ramaiah SK. Higher neutrophil infiltration mediated by osteopontin is a likely contributing factor to the increased susceptibility of females to alcoholic liver disease. J Pathol. 2006 Mar;208(4):473–485. doi: 10.1002/path.1917. [DOI] [PubMed] [Google Scholar]
- 3.Bollyky PL, Wu RP, Falk BA, Lord JD, Long SA, Preisinger A, Teng B, Holt GE,Standifer NE, Braun KR, Xie CF, Samuels PL, Vernon RB, Gebe JA, Wight TN, Nepom GT. ECM components guide IL-10 producing regulatory T-cell (TR1) induction from effector memory T-cell precursors. Proc Natl Acad Sci U S A. 2011;108:7938–7943. doi: 10.1073/pnas.1017360108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, Zhao P, Massaro D, Clerch LB, Almon RR, DuBois DC, Jusko WJ, Hoffman EP. The PEPR GeneChip data warehouse, and implementation of a dynamic time series query tool (SGQT) with graphical interface. Nucleic Acids Res. 2004;32:D578–D581. doi: 10.1093/nar/gkh003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen RX, Xia YH, Cui JF, Xue TC, Ye SL. Osteopontin, a single marker for predicting the prognosis of patients with tumor-node-metastasis stage I hepatocellular carcinoma after surgical resection. J Gastroenterol Hepatol. 2010;25:1435–1442. doi: 10.1111/j.1440-1746.2010.06277.x. [DOI] [PubMed] [Google Scholar]
- 6.Christensen B, Nielsen MS, Haselmann KF, Petersen TE, Sorensen ES. Post-translationally modified residues of native human osteopontin are located in clusters: identification of 36 phosphorylation and five O-glycosylation sites and their biological implications. Biochem J. 2005;390:285–292. doi: 10.1042/BJ20050341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarkson PM, Devaney JM, Gordish-Dressman H, Thompson PD, Hubal MJ, Urso M, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Seip RL, Hoffman EP. ACTN3 genotype is associated with increases in muscle strength in response to resistance training in women. J Appl Physiol. 2005a;99:154–163. doi: 10.1152/japplphysiol.01139.2004. [DOI] [PubMed] [Google Scholar]
- 8.Clarkson PM, Hoffman EP, Zambraski E, Gordish-Dressman H, Kearns A, Hubal M, Harmon B, Devaney JM. ACTN3 and MLCK genotype associations with exertional muscle damage. J Appl Physiol. 2005b;99:564–569. doi: 10.1152/japplphysiol.00130.2005. [DOI] [PubMed] [Google Scholar]
- 9.Craig AM, Denhardt DT. The murine gene encoding secreted phosphoprotein 1 (osteopontin): promoter structure, activity, and induction in vivo by estrogen and progesterone. Gene. 1991;100:163–171. doi: 10.1016/0378-1119(91)90362-f. [DOI] [PubMed] [Google Scholar]
- 10.Devaney JM, Thompson PD, Visich PS, Saltarelli WA, Gordon PM, Orkunoglu-Suer EF, Gordish-Dressman H, Harmon BT, Bradbury MK, Panchapakesan K, Khianey R, Hubal MJ, Clarkson PM, Pescatello LS, Zoeller RF, Moyna NM, Angelopoulos TJ, Kraus WE, Hoffman EP. The 1p13.3 LDL (C)-associated locus shows large effect sizes in young populations. Pediatr Res. 2011a;69:538–543. doi: 10.1203/PDR.0b013e3182139227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaney JM, Gordish-Dressman H, Harmon BT, Bradbury MK, Devaney SA, Harris TB, Thompson PD, Clarkson PM, Price TB, Angelopoulos TJ, Gordon PM, Moyna NM, Pesca LS, VIsich PS, Zoeller RF, Seip RL, Seo J, Kim BH, Tosi LL, Garcia M, Li R, Zmuda JM, Delmonico MJ, Lindsay RS, Howard BV, Kraus WE, Hoffman EP. AKT1 polymorphisms are associated with risk for metabolic syndrome. Hum Genet. 2011;129:129–139. doi: 10.1007/s00439-010-0910-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ding Z, Wu CJ, Chu GC, Xiao Y, Ho D, Zhang J, Perry SR, Labrot ES, Wu X, LisR, Hoshida Y, Hiller D, Hu B, Jiang S, Zheng H, Stegh AH, Scott KL, Signoretti S, Bardeesy N, Wang YA, Hill DE, Golub TR, Stampfer MJ, Wong WH, Loda M, Mucci L,Chin L, DePinho RA. SMAD4-dependent barrier constrains prostate cancer growth and metastatic progression. Nature. 2011;470:269–273. doi: 10.1038/nature09677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Giacopelli F, Marciano R, Pistorio A, Catarsi P, Canini S, Karsenty G, Ravazzolo R. Polymorphisms in the osteopontin promoter affect its transcriptional activity. Physiol Genomics. 2004;20:87–96. doi: 10.1152/physiolgenomics.00138.2004. [DOI] [PubMed] [Google Scholar]
- 14.Glas J, Seiderer J, Bayrle C, Wetzke M, Fries C, Tillack C, Olszak T, Beigel F, Steib C, Friedrich M, Diegelmann J, Czamara D, Brand S. The role of osteopontin (OPN/SPP1) haplotypes in the susceptibility to Crohn's disease. PLoS One. 2011;6:e29309. doi: 10.1371/journal.pone.0029309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Han S, Guthridge JM, Harley IT, Sestak AL, Kim-Howard X, Kaufman KM, Namjou B, Deshmukh H, Bruner G, Espinoza LR, Gilkeson GS, Harley JB, James JA, Nath SK. Osteopontin and systemic lupus erythematosus association: a probable gene-gender interaction. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001757. e0001757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffman EP, Brown RH, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- 17.Hubal MJ, Devaney JM, Hoffman EP, Zambraski EJ, Gordish-Dressman H, Kearns AK, Larkin JS, Adham K, Patel RR, Clarkson PM. CCL2 and CCR2 polymorphisms are associated with markers of exercise-induced skeletal muscle damage. J Appl Physiol. 2010;108:1651–1658. doi: 10.1152/japplphysiol.00361.2009. [DOI] [PubMed] [Google Scholar]
- 18.Hubal MJ, Urso ML, Clarkson PM. Genetic Aspects of Muscular Strength and Size. In: Pescatello L, Roth SM, editors. Exercise Genomics. New York: Humana Press; 2011. pp. 157–178. [Google Scholar]
- 19.Kariuki SN, Moore JG, Kirou KA, Crow MK, Utset TO, Niewold TB. Age- and gender-specific modulation of serum osteopontin and interferon-alpha by osteopontin genotype in systemic lupus erythematosus. Genes Immun. 2009;10:487–494. doi: 10.1038/gene.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keykhosravani M, Doherty-Kirby A, Zhang C, Brewer D, Goldberg HA, Hunter GK, Lajoie G. 2005. Comprehensive identification of post-translational modifications of rat bone osteopontin by mass spectrometry. Biochemistry. 2005;44:6990–7003. doi: 10.1021/bi050109p. [DOI] [PubMed] [Google Scholar]
- 21.Kim SM, Park YY, Park ES, Cho JY, Izzo JG, Zhang D, Kim SB, Lee JH, Bhutani MS, Swisher SG, Wu X, Coombes KR, Maru D, Wang KK, Buttar NS, Ajani JA, Lee JS. Prognostic biomarkers for esophageal adenocarcinoma identified by analysis oftumor transcriptome. PLoS One. 2010;5:e15074. doi: 10.1371/journal.pone.0015074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kretschmer C, Sterner-Kock A, Siedentopf F, Schoenegg W, Schlag PM, Kemmner W. Identification of early molecular markers for breast cancer. Mol Cancer. 2011;10:15. doi: 10.1186/1476-4598-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kwon HJ, Won YS, Yoon WK, Nam KH, Kim DY, Kim HC. The role of osteopontin in d-galactosamine-induced liver injury in genetically obese mice. Toxicol Appl Pharmacol. 2010;242:344–351. doi: 10.1016/j.taap.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 24.Liaw L, Birk DE, Ballas CB, Whitsitt JS, Davidson JM, Hogan BL. Altered wound healing in mice lacking a functional osteopontin gene (spp1) J Clin Invest. 1998;101:1468–1478. doi: 10.1172/JCI1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Long AD, Langley CH. The power of association studies to detect the contribution of candidate genetic loci to variation in complex traits. Genome Res. 1999;8:720–731. [PMC free article] [PubMed] [Google Scholar]
- 26.Minotti JR, Pillay P, Oka R, Wells L, Christoph I, Massie BM. Skeletal muscle size: relationship to muscle function in heart failure. J Appl Physiol. 1993 Jul;75(1):373–381. doi: 10.1152/jappl.1993.75.1.373. [DOI] [PubMed] [Google Scholar]
- 27.Miyajima J, Hayashi T, Saito K, Iida S, Matsuoka K. The interaction between female sex hormone receptors and osteopontin in a rat hyperoxaluric model. Kurume Med J. 2010;57:73–80. doi: 10.2739/kurumemedj.57.73. [DOI] [PubMed] [Google Scholar]
- 28.Mori R, Shaw TJ, Martin P. Molecular mechanisms linking wound inflammation and fibrosis: knockdown of osteopontin leads to rapid repair and reduced scarring. J Exp Med. 2008;205:43–51. doi: 10.1084/jem.20071412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimichi N, Hayashita-Kinoh H, Chen C, Matsuda H, Sheppard D, Yokosaki Y. Osteopontin undergoes polymerization in vivo and gains chemotactic activity for neutrophils mediated by integrin alpha9beta1. J Biol Chem. 2011;286:11170–11178. doi: 10.1074/jbc.M110.189258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pegoraro E, Hoffman EP, Piva L, Gavassini BF, Cagnin S, Ermani M, Bello L, Soraru G, Pacchioni B, Bonifati MD, Lanfranchi G, Angelini C, Kesari A, Lee I, Gordish-Dressman H, Devaney JM, McDonald CM Cooperative International Neuromuscular Research Group. SPP1 genotype is a determinant of disease severity in Duchenne muscular dystrophy. Neurology. 2011;76:219–226. doi: 10.1212/WNL.0b013e318207afeb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sayer AA, Syddall H, O'Dell SD, Chen XH, Briggs PJ, Briggs R, Day IN, Cooper C. Polymorphism of the IGF2 gene, birth weight and grip strength in adult men. Age and ageing. 2002;31:468–470. doi: 10.1093/ageing/31.6.468. [DOI] [PubMed] [Google Scholar]
- 32.Schrager MA, Roth SM, Ferrell RE, Metter EJ, Russek-Cohen E, Lynch NA, Lindle RS, Hurley BF. Insulin-like growth factor-2 genotype, fat-free mass, and muscle performance across the adult life span. J Appl Physiol. 2004;97:2176–2183. doi: 10.1152/japplphysiol.00985.2003. [DOI] [PubMed] [Google Scholar]
- 33.Sewright KA, Hubal MJ, Kearns A, Holbrook MT, Clarkson PM. Sex differences in response to maximal eccentric exercise. Med Sci Sports Exerc. 2008;40:242–251. doi: 10.1249/mss.0b013e31815aedda. [DOI] [PubMed] [Google Scholar]
- 34.Thompson PD, Moyna N, Seip R, Price T, Clarkson P, Angelopoulos T, Gordon P, Pescatello L, Visich P, Zoeller R, Devaney JM, Gordish H, Bilbie S, Hoffman EP. Functional Polymorphisms Associated with Human Muscle Size and Strength. Med Sci Sports Exerc. 2004(36):1132–1139. doi: 10.1249/01.mss.0000132274.26612.23. [DOI] [PubMed] [Google Scholar]
- 35.Uaesoontrachoon K, Yoo HJ, Tudor EM, Pike RN, Mackie EJ, Pagel CN. Osteopontin and skeletal muscle myoblasts: association with muscle regeneration and regulation of myoblast function in vitro. Int J Biochem Cell Biol. 2008;40:2303–2314. doi: 10.1016/j.biocel.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 36.Wang KX, Denhardt DT. Osteopontin: role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008;19:333–345. doi: 10.1016/j.cytogfr.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Yang N, MacArthur DG, Gulbin JP, Hahn AG, Beggs AH, Easteal S, North K. ACTN3 genotype is associated with human elite athletic performance. Am J Hum Genet. 2003;73:627–631. doi: 10.1086/377590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokosaki Y, Matsuura N, Sasaki T, Murakami I, Schneider H, Higashiyama S, Saitoh Y, Yamakido M, Taooka Y, Sheppard D. The integrin alpha(9)beta(1) binds to a novel recognition sequence (SVVYGLR) in the thrombin-cleaved amino-terminal fragment of osteopontin. J. Biol. Chem. 1999;274:36328–36334. doi: 10.1074/jbc.274.51.36328. [DOI] [PubMed] [Google Scholar]
- 39.Zhao P, Iezzi S, Carver E, Dressman D, Gridley T, Sartorelli V, Hoffman EP. Slug is a novel downstream target of MyoD. Temporal profiling in muscle regeneration. J Biol Chem. 2002;277:30091–30101. doi: 10.1074/jbc.M202668200. [DOI] [PubMed] [Google Scholar]
- 40.Zhao P, Caretti G, Mitchell S, McKeehan WL, Boskey AL, Pachman LM, Sartorelli V, Hoffman EP. Fgfr4 is required for effective muscle regeneration in vivo. Delineation of a MyoD-Tead2-Fgfr4 transcriptional pathway. J Biol Chem. 2006;281:429–438. doi: 10.1074/jbc.M507440200. [DOI] [PMC free article] [PubMed] [Google Scholar]